Abstract
Classification and regression tree (CART) analysis was used to identify subpopulations with lower palliative care program (PCP) enrolment rates. CART analysis uses recursive partitioning to group predictors. The PCP enrolment rate was 72 percent for the 6,892 adults who died of cancer from 2000 and 2005 in two counties in Nova Scotia, Canada. The lowest PCP enrolment rates were for nursing home residents over 82 years (27 percent), a group residing more than 43 kilometres from the PCP (31 percent), and another group living less than two weeks after their cancer diagnosis (37 percent). The highest rate (86 percent) was for the 2,118 persons who received palliative radiation. Findings from multiple logistic regression (MLR) were provided for comparison. CART findings identified low PCP enrolment subpopulations that were defined by interactions among demographic, social, medical, and health system predictors.
Keywords: inequities, disparities, vulnerable populations, classification and regression tree analysis, palliative care, end-of-life care
INTRODUCTION
Variation in the proportion of persons accessing palliative care programs (PCPs) has been observed (1–14). In 2009, a literature review by Walshe et al. (14) classified predictors of PCP enrolment into demographic, social, and medical themes. There was limited commonality in the variables included. Some studies used linked administrative databases; others used interviews.
Demographic factors that Walshe et al. reviewed were age, sex, marital status, and ethnicity. Studies of ethnicity were predominantly from the United States. Marital status was frequently missing. The majority of studies showed no association with sex. Of those that did, males were less likely to be enrolled in a PCP. Advanced age was often associated with lower rates of PCP enrolment. Studies usually were from the U.S. when advanced age was associated with greater PCP access. In a separate systematic literature review in 2006 examining age in relation to PCP access for adults dying of cancer, Burt et al. (3) used different study inclusion criteria than Walshe et al. and concluded that all studies showed lower PCP use for older persons.
Social factors reviewed by Walshe et al. comprised carer and socioeconomic variables. Carer data was not included in the majority of studies. When it was, it related to either varying definitions of informal home support, or diverse forms of formal care provision often associated with the study location. Nursing home data was included in a couple of studies, and insurance status was identified in a few studies, typically those from the U.S. Socio-economic information was frequently based on the community where the person resided and not the persons themselves.
Medical factors identified by Walshe et al. were grouped by diagnosis and functional status. Diagnosis included time from diagnosis to death. A short time from diagnosis to death was less likely to be associated with PCP care. PCP access varied somewhat by cancer diagnoses but had no consistent pattern across studies. Only 9 of the 48 studies reviewed had a functional status measure; all 9 used different measures.
Walshe et al. recommended that future research emphasize the importance of context and the use of alternative services. The literature (3, 6, 14) also recommends that groups with lower rates of PCP enrolment should be investigated to help improve equity in access to palliative care.
In this paper, we use classification and regression tree (CART) analysis to identify subpopulations of persons who died of cancer with lower than average PCP enrolment. Low enrolment groups can then be assessed to discern whether interventions are needed to improve access. Demographic, social, and medical factors were included with a focus on health service intervention points: nursing home, oncology (radiotherapy) care, and geographic location.
This study uses administrative databases, thereby avoiding selection bias that often occurs in interview-based surveys (6, 15) but limiting the variables available for investigation. A primary purpose of this paper is to demonstrate the application and value of CART analysis in comparison to multivariate logistic regression (MLR).
CART is an analytic method that uses recursive partitioning (splitting) to classify or group predictors of a dependent variable (16, 17). It has been used in public health and healthcare to model clinical decision-making, predict health outcomes (18–22), and explore health service use (23–25). In this paper, the CART results provide binary PCP enrolment splits to produce a set of mutually exclusive, optimally homogenous groups that are reported as “terminal nodes” on a multi-level inverted “tree” (22).
METHODS
Study Population
This population-based retrospective study examined 6,892 adults 20 years or older who died of cancer from 2000 to 2005 in either Cape Breton or Halifax county in Nova Scotia, Canada. These two largely urban counties have a combined population of almost 500,000 people, which is approximately half the province’s population (26). The two counties are separated by more than a three-hour drive and each has had its own PCP for 20 years. The remainder of the province is more rural and is covered by seven smaller PCPs, each with its own database.
Study Setting
In Halifax, the PCP includes an in-patient unit, in-hospital consultation services, clinic follow-up for ambulatory patients, and home consultation services. Until 2004, home consultations were primarily provided to people living within 30 kilometres of the PCP. In Cape Breton, a similar range of services is provided, and home services are limited for those at a distance from the PCP site. Each PCP is located within a health service complex that includes a cancer treatment centre. More than 90 percent of those seen in these specialized PCPs during this study’s time period were dying of cancer. In addition to oncology referrals, PCP referrals come from hospital in-patient units, emergency departments, and family and specialist physicians. Public funding covers all hospital-based and physician services. Therefore, PCP services are fully funded and not dependent on insurance coverage. Payment for nursing home and home care services is on a sliding scale of financial need and is borne in part by individuals and/or private insurance (27). There were no free-standing hospices in Nova Scotia at the time of this study.
Data Preparation
The study subjects were identified through Nova Scotia Vital Statistics (VS). Using probabilistic record linkage, they were linked to enrolment databases of the Cape Breton PCP or the Capital Health Integrated Palliative Service in Halifax, as well as the Nova Scotia Oncology Patient Information System (OPIS). Variables extracted for each decedent from the VS data included sex, dates of birth and death, county of residence at death, postal code, and place of residence and of death. Nursing home residence was defined as having a nursing home address as the place of residence and/or death (8). PCP enrolment was defined as having a record of PCP assessment (Halifax) or referral (Cape Breton). PCP service ranged from a single contact to ongoing support. Postal codes of residence and PCP location were used to calculate the straight-fine distance from the decedent’s residence to the closest PCP. Radiotherapy (RT) was defined as being provided in the last nine months of life since that time period correlates with palliative radiotherapy (28). RT and date of cancer diagnosis were extracted from OPIS. Dates of birth and death were used to compute age at death. Dates of cancer diagnosis and death were used to compute time from cancer diagnosis to death. Year of death was obtained from date of death. These study variables were included in the analyses reported in the MLR table and CART figure herein.
In earlier analyses (29), five additional variables were created from the VS and OPIS data and entered into the CART and logistic regression analyses. These were medical oncology consultation, cancer site, and three community (ecological) measures derived from linking a study subject’s postal code of residence to census data - community median income, living in a community where a high percentage of people are living alone, and living in a community with a high percentage of French-speaking persons. Medical oncology consultation and community median income did not help define a CART subpopulation with low PCP enrolment. Therefore they were not studied further. The two remaining community variables identified small subpopulations with quite limited points of intervention and so were not retained, as the intent of the analyses reported herein was to identify key intervention points to improve access to PCP services. The findings from these community variables are briefly noted in the results. Cancer site did not identify subpopulations with particularly low PCP enrolment rates and was associated with other variables that were better able to define subpopulations for further investigation. Thus, cancer site was removed from the CART and MLR analyses.
Statistical Analysis
The CART (16, 17) method repeatedly splits a study population into smaller and smaller sub-populations based on the distribution of the outcome or dependent variable of interest, in this case PCP enrolment. Each branch is based on optimally selected split-points identified from across the set of independent variables. The CART algorithm partitions the data into increasingly more homogeneous, mutually exclusive subsets. Default stopping rules are used to limit the extent to which the algorithm continues to partition the data.
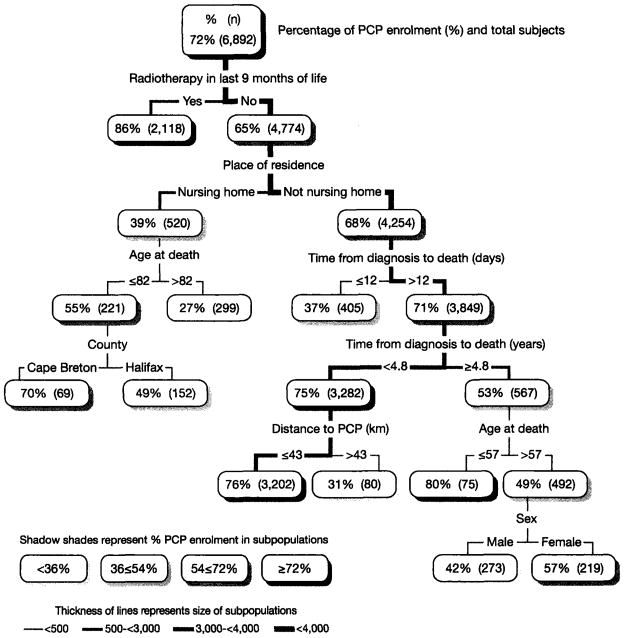
In the figure displaying the CART findings, the thickness of lines between boxes reflects the size of the subpopulation splits, with darker lines indicating larger population groups. The shading of the population boxes indicates the percent PCP enrolment for each population group. The CART results are discussed in terms of the end-point groups (terminal nodes), that is, the lowest population groups on the branches of the CART tree, the total of which represents the 6,892 study subjects.
Univariate logistic regression was used to determine the unadjusted odds ratios (OR). Stepwise multiple logistic regression (MLR) was then used to identify the set of factors significantly associated with PCP enrolment after controlling for the other independent variables. Adjusted odds ratios (aOR) and 95 percent confidence intervals (CI) are reported. Goodness-of-fit was assessed using the Hosmer-Lemeshow test (30). The MLR and CART analyses were carried out using SAS 9.1.3 and SAS Enterprise Miner 4.3, respectively.
RESULTS
Among the 6,892 adults who died of cancer from 2000 to 2005 in two counties in Nova Scotia, Canada, 47.5 percent were male, and 44.9 percent were 75 years or over. Nine percent were nursing home residents. Approximately two-thirds (68.9 percent) resided in Halifax County; the remainder were from Cape Breton County. Reflective of this largely urban population, only 5.2 percent lived 30 or more kilometres from a PCP.
The first level of the CART results in Figure 1 shows an overall PCP enrolment rate of 72 percent. The second level shows that the highest PCP enrolment rate observed was 86 percent for the 2,118 decedents who had radiation treatment (RT) in last the nine months of life. Two other sub-populations had PCP enrolment rates higher than the 72 percent average; they are in the sixth CART level. Both subpopulations did not receive RT, were not nursing home residents, and lived more than 12 days after their cancer diagnosis. One of the two subpopulations died less than 4.8 years after their cancer diagnosis and lived closer to the PCP (76 percent, n=3202). The other subpopulation lived longer and was younger (80 percent, n=75). Together, these three subpopulations (n=5395) with an above average PCP enrolment rate had a combined average PCP enrolment rate of 80 percent and comprised the majority (78.3 percent) of the total population.
Figure 1.
Classification and Regression Tree of Subpopulations Differentiated by PCP Enrolment for Cancer Decedents in Cape Breton and Halifax Counties, 2000–2005
In contrast, at the lowest end of the PCP enrolment rate continuum, two subpopulations representing 5.5 percent of the decedents (379 of 6,892) had enrolment rates of less than half of the average PCP enrolment rate, that is, rates below 36 percent. CART level four shows that the rate was 27 percent for the 299 nursing home residents over 82 years of age who did not have RT. CART level six shows a rate of 31 percent for the 80 decedents residing more than 43 kilometers from the PCP who did not have RT, but otherwise had favorable risk factors in that they were not nursing home residents and they lived more than 12 days but less than 4.8 years after their cancer diagnosis.
Three additional CART subpopulations representing 12 percent (n=830) of the decedents had PCP enrolment rates in the 36 percent to 54 percent range; all were below 50 percent PCP enrolment. CART level four shows that those who did not have RT and were not nursing home residents but only lived 12 days or less after their cancer diagnosis (n=405) had a 37 percent rate. CART level seven shows that males over 57 years who lived more than 4.8 years after their cancer diagnosis, did not have RT and were not nursing home residents (n=273) had a 42 percent rate (comparable females had a 57 percent rate). CART level five shows that the Halifax nursing home residents who did not have RT and were 82 years or younger (n=152) had a 49 percent PCP enrolment rate (Cape Breton had 70 percent).
The logistic regression analysis generated somewhat comparable results to those reported from the CART analysis. Table 1 findings show that across all the decedents, males had a slightly lower PCP enrolment rate (70.0 percent) than females (73.3 percent). The difference was marginally significant in the univariate logistic regression findings (OR=1.18, CI:1:06–1.31), and more substantive when adjusted for other variables (aOR=1.37, CI:1.22–1.53). In contrast to the logistic regression findings, the CART analysis revealed a subpopulation of males with a 42 percent PCP enrolment rate (n=273) to target for further investigation. These were males older than 57 years who died more than five years after diagnosis, who did not have RT, and who were not nursing home residents.
Table 1.
Logistic Regression Predictors of Palliative Care Program (PCP) Enrolment for Cancer Decedents, Cape Breton and Halifax Counties, 2000–2005 (n=6,892)
| Predictor variables | Study population n (%) | PCP enrolment rate (%) | Odds ratios (95% confidence intervals)
|
|
|---|---|---|---|---|
| univariate | adjusted | |||
|
| ||||
| Sex | ||||
| male | 3,622 (52.6) | 70.0 | 1 | 1 |
| female | 3,270 (47.5) | 73.3 | 1.18 (1.06, 1.31) | 1.37 (1.22, 1.53) |
|
| ||||
| Age (years) | ||||
| <65 | 1,959 (28.4) | 82.5 | 4.57 (3.86, 5.42) | 3.05 (2.54, 3.68) |
| 65–74 | 1,840 (26.7) | 75.3 | 2.94 (2.50, 3.46) | 2.24 (1.88, 2.68) |
| 75–84 | 2,090 (30.3) | 68.0 | 2.05 (1.76, 2.40) | 1.71 (1.45, 2.01) |
| 85+ | 1,003 (14.6) | 50.8 | 1 | 1 |
|
| ||||
| Radiation therapy in 9 months before death | ||||
| no | 4,774 (69.3) | 65.0 | 1 | 1 |
| yes | 2,118 (30.7) | 86.4 | 3.41 (2.97, 3.91) | 2.63 (2.27, 3.05) |
|
| ||||
| Place of residence | ||||
| nursing home | 621 (9.0) | 44.4 | 1 | 1 |
| not nursing home | 6,271 (91.0) | 74.3 | 3.61 (3.05, 4.27) | 2.87 (2.38, 3.45) |
|
| ||||
| Time from diagnosis to death (days) | ||||
| 0–60 | 1,528 (22.2) | 60.5 | 1 | 1 |
| 61–119 | 752 (10.9) | 80.1 | 2.62 (2.13, 3.22) | 2.44 (1.96, 3.04) |
| 120+ | 4,612 (66.9) | 73.9 | 2.49 (2.12, 2.92) | 1.69 (1.46, 1.90) |
|
| ||||
| Distance to closest PCP (km) | ||||
| 30+ | 358 (5.2) | 48.3 | 1 | 1 |
| <30 | 6,534 (94.8) | 72.8 | 2.87 (2.32, 3.56) | 3.34 (2.64, 4.23) |
|
| ||||
| County of residence | ||||
| Halifax | 4,727 (68.6) | 70.0 | 1 | 1 |
| Cape Breton | 2,165 (31.4) | 74.9 | 1.28 (1.14, 1.43) | 1.36 (1.20, 1.54) |
|
| ||||
| Year of death | ||||
| 2000 | 1,106 (16.1) | 69.2 | 1 | 1 |
| 2001 | 1,087 (15.8) | 69.0 | 0.99 (0.83, 1.19) | 1.01 (0.83, 1.23) |
| 2002 | 1,131 (16.4) | 70.0 | 1.04 (0.87, 1.25) | 1.04 (0.86, 1.27) |
| 2003 | 1,177 (17.1) | 71.5 | 1.12 (0.94, 1.34) | 1.14 (0.95, 1.39) |
| 2004 | 1,202 (17.4) | 73.6 | 1.24 (1.04, 1.49) | 1.32 (1.09, 1.61) |
| 2005 | 1,189 (17.3) | 75.6 | 1.38 (1.15, 1.66) | 1.53 (1.26, 1.87) |
In Table 1, age was inversely associated with PCP rates ranging from 82.5 percent for those less than 65 years to 50.8 percent for those 85 years or more. The adjusted odds ratios were less than the univariate odds ratio in part due to older decedents comprising a higher proportion of females. In contrast to the regression findings, CART findings revealed age split points for investigation in two subpopulations. One of these was nursing home residents over 82 years whose PCP enrolment rate was 27 percent. The other was persons over 57 years who had cancer for many years but no RT, who were not nursing home residents and, as previously noted, were males.
Both Table 1 and the CART figure report that the highest PCP enrolment rate (86 percent) was for decedents who received RT. The CART analysis removed those who received RT, revealing a low PCP rate of 39 percent for nursing home residents who did not receive RT. The logistic regression findings show that all nursing home residents had a similar, though slightly higher, PCP rate (44.4 percent) since all nursing home residents are included in the regression model. The difference between residents and non-residents in the regression findings was statistically significant even when adjusted for age and other factors (aOR: 2.87, CI:2.38–3.45).
Table 1 shows a U-curve relationship between PCP enrolment and time from diagnosis to death. The arbitrary regression analysis cut-off points of 60 and 120 days showed a smaller range of PCP rates (60.5 percent to 80.1 percent) than was observed for the data-derived split points in the CART findings of 12 days and 4.8 years for decedents who did not receive RT and were not nursing home residents (37 percent to 75 percent).
In Table 1, the cut-off point of 30 kilometres for the distance variable was statistically significant, with decedents living at a greater distance having lower PCP rates (48.3 percent versus 72.8 percent). The CART findings identified a lower enrolment (31 percent) subpopulation of persons living more than 43 kilometres from a PCP.
Among the entire population, Halifax PCP rates (70.0 percent) were significantly lower than Cape Breton rates (74.9 percent). CART findings revealed even more marked differences between the two areas among younger nursing home residents who did not receive RT: Halifax (49 percent) versus Cape Breton (70 percent).
PCP rates increased yearly from 69.2 percent in 2000 to 75.6 percent in 2005 (aOR=1.53, CI:1.26, 1.87) (Table 1) part of a decade-long steady increase that began in 1996. In the CART analysis, year did not help identify low PCP enrolment sub-populations for investigation.
From analyses using 2000 to 2003 data (29), cancer site was associated with RT in the last nine months of life, distance to treatment, and survival time. The lowest PCP enrolment rate for a subpopulation defined in part by a cancer site was 57 percent for 357 adults who lived longer than 12 days, were not nursing home residents, did not have RT in the last nine months of life, and died of a genito-urinary or head and neck cancer. Other subpopulations in part defined by a cancer site had higher than average PCP enrolment rates (77 percent to 92 percent). The objective of the analysis reported in this paper was to identify low PCP enrolment subpopulations. Cancer site proved to be of limited use and therefore was not included.
The 2000 to 2003 analysis also showed CART effects for two limited subpopulations that would be difficult to target for intervention, so were not included. Adults living fewer than 12 days after their cancer diagnosis who lived in Cape Breton in a community with 20 percent or more of residents living alone had a lower PCP referral rate (n=54; 35 percent) than those in a community with less than 20 percent living alone (n=23; 74 percent). Nursing home residents over 80 years who had been diagnosed more than seven months before death who were living in a community with less than 4 percent French-speaking people had lower PCP enrolment (n=97; 15 percent) than those who lived in a community with 4 percent or more French-speaking people (n=8; 83 percent).
DISCUSSION
The overall PCP enrolment rate (72 percent) for adults dying of cancer is relatively high compared to rates observed in most studies (2, 3, 6,10–14) and comparable to one Australian study (5). Even so, almost 30 percent of those dying of cancer in these two mainly urban areas of Nova Scotia were not enrolled in a PCP. Therefore, this study was designed to identify subpopulations with lower PCP enrolment rates so that these groups could be assessed to discern whether interventions are needed to improve PCP access.
CART analysis identified five mutually exclusive subpopulations (terminal nodes) of lower PCP enrolment. Each had a PCP enrolment rate of less than 50 percent. Combined, they represent almost one-fifth (17.5 percent) of the study subjects. All five subpopulations were among those who did not have RT in their last nine months of life (a proxy measure of palliative RT).
Unlike CART analysis, which segments the total population using interactions among the predictor variables to identify low PCP enrolment subpopulations, logistic regression produces univariate and confounder-controlled PCP enrolment rates across all study subjects for each predictor. The lowest univariate PCP rates from the logistic regression analysis were nursing home residents (44.4 percent) and persons living more than 30 kilometres from the PCP (48.3 percent).
Future analyses and literature reviews should look for combinations of factors that, together, define subpopulations with lower PCP rates than are revealed by MLR, which averages the effect of a variable across the entire population. Walshe et al. (14) state that we need to examine the complexities of service use and discern whether there are inequities in access to palliative care. The CART analysis shows that alternative health services (palliative RT, nursing home, geographic location) are associated with PCP enrolment for adults dying of cancer, and that one of the complexities of PCP access is the interaction among predictors of enrolment.
Health system changes in Nova Scotia appear to have contributed to steadily increasing PCP enrolment in the last decade. However, year was not important in the identification of CART low enrolment subpopulations. There has been increasing collaboration between the PCPs and oncology services. In Halifax, there was an increase in PCP in-patient beds from 6 to 10 in 1998, in PCP home consultant registered nurses from 3 to 5 in 1999, and in full-time PCP physicians from 3 to 5 between 1994 and 2003. Integration with provincial home care for palliative support occurred in 2003. At Dalhousie University, an academic Division of Palliative Medicine was created. Across the province, there was increased visibility due to expanded educational activities and advocacy. These system advances did not appear to overcome potential inequities (31) in the provision of care. This is consistent with previous analysis that found that as PCP enrolment increased, disparities across age groups remained (4).
An association between PCP enrolment and palliative RT was reported previously (7,9), so the fact the RT appeared as a CART split point was not unexpected. What was new was the importance of RT as an indicator of higher PCP enrolment. Receiving RT in the last nine months of life was the first factor (level one) in the CART findings. This highlighted the upstream potential of working collaboratively with cancer centres in investigating and addressing potential inequities in cancer survivor access to palliative care. In MLR analyses, palliative RT was just one of many factors relevant to consider, rather than a key point of potential intervention. Refinement of the definition of palliative RT, and investigation of oncology care that is supportive (versus curative), is recommended in future studies so that the impact of possible lapses in survivor contact, delayed contact, or lack of contact can be assessed.
Nursing home residence was observed as the second level of the CART analysis. An association between increasing age and lower PCP enrolment rates was observed previously (4, 7). A further analysis that included nursing home residence (8) revealed that nursing home residents were less likely to receive palliative RT and be enrolled in the PCPs in both Halifax and Cape Breton. Controlling for age, sex, time from cancer diagnosis to death, tumour type, medical oncology consultation, and place of death did not remove the significant association between being a nursing home resident and lower rates of both palliative RT and PCP enrolment (8). The CART analysis has revealed that it was nursing home residents over 82 years who experience lower PCP enrolment rates. Nursing home residents may have received their palliative RT and PCP care prior to being admitted to the nursing home. Thus, the use of RT and PCP may be even lower if the analysis were limited to their time in the nursing home. Nursing home policies and services, co-morbidities, and patient or family choice are factors requiring further attention (8). Studies are under way to better understand end-of-life care in nursing homes. Change is now occurring to improve access to family physician care and better integrate nursing homes into district health care.
The CART analysis revealed a difference between Halifax and Cape Breton for persons 82 years or younger who were nursing home residents at the time of their death. Beyond the CART findings reported herein, we also observed that the association between short survival time and low PCP enrolment appeared greatest in Halifax (29). Further investigation suggests that both of these differences may be due, at least in part, to the PCP’s definition of “enrolment”. In Halifax, “assessment” and the date of assessment were used. In Cape Breton, “referral” and the date of referral were used; the number of those referred who were actually assessed, and the time between referral and assessment, were not known. So, while the Cape Breton PCP appeared to provide improved access to care, this may not be the case. The need for common definitions across PCP databases became apparent. The Halifax PCP now records referrals and the dates of both referral and assessment. PCPs are encouraged to record both referral and assessment; this information is critical to document wait time and access issues.
The CART analysis identified an association between sex and lower PCP enrolment in a group of males over 57 years. They were not nursing home residents, did not have RT in their last nine months of life, and were diagnosed with cancer more than 4.8 years before death. These men may have received care at home by their wives (9); if marital status had been available in our data set, this may have helped interpret these findings. Grande et al. (1) report that as the age of the family caregiver increases, so does the need for palliative home care. Our data suggests a lower rather than higher level of support for them and so this is of concern.
The U-curve relationship between PCP enrolment and time from cancer diagnosis to death was observed previously (4, 7). The CART analysis provides split points rather than using more arbitrary time divisions. The CART findings point to two subpopulations at greater risk of low PCP enrolment: those dying within 12 days of their cancer diagnosis, and those living longer than about five years who were not nursing home residents and did not receive RT in their last nine months. Five years is the typical follow-up time by oncologists after a cancer diagnosis.
Since policies in both counties indicate that PCP services are for persons living less than 30 kilometres from the PCP, it was not surprising that relatively low PCP rates were observed for decedents who resided beyond these PCP catchment boundaries. Provision of home-based palliative care to persons living further from the care centres remains a challenge that is not limited to PCP enrolment. Another study shows that this problem also occurs for palliative RT consultation (28).
Benchmarks for optimal rates of palliative care enrolment do not exist. Thus, there is no adequate basis for concluding that the overall observed PCP enrolment rate of 72 percent was optimal. In the absence of a needs-based benchmark, the combined PCP enrolment rate of 80 percent for the three groups with a greater than average PCP enrolment rate, representing almost 80 percent of the decedents, might instead be an appropriate norm. Furthermore, the lower PCP rates reported herein do not necessarily mean poorer palliative care. Rather, the subpopulations need to be investigated to understand why their PCP rates are lower, and whether their palliative care needs are being adequately met. The need for palliative care from specialized programs may appropriately vary across subpopulations. As yet there are no standards for access to specialized palliative programs.
Comparison of CART with MLR
MLR has been widely used to assess associations between multiple predictors and outcomes of interest. Therefore, this parametric method is relatively familiar to palliative care researchers and journal readers. In accord with other generalized linear regression methods, MLR reports the overall effect of predictors. In MLR, continuous variables are often categorized intuitively rather than empirically. Analyses of interactions among predictors are laborious, and so are often not carried out. In contrast, CART analysis is a relatively new non-parametric statistical method that uses a binary recursive partitioning algorithm (17). It was developed and is used to uncover hidden patterns and identify, or classify, study subjects into optimally homogeneous subpopulations. In CART analyses, the data are examined and the model is displayed, with multilevel interactions presented as a decision tree. Paths are defined by covariates and optimally selected binary split points for continuous and ordered categorical variables or optimally selected binary partitions for categorical variables. Interaction terms are an automatic consequence of the modeling process and refer to the splitting on different covariates at different depths of the tree. CART analysis has been used to develop clinical guidelines. In this paper, it is used to identify health services intervention points that reveal potential inequities for future investigation.
While both MLR and CART models are amenable to validation by content experts who can check the model’s plausibility, CART findings are arguably more intuitively interpretable, as they replicate the in-clinic decision process in which physicians typically find themselves engaged. CART analysis more simply defines target subpopulations for investigation and intervention.
LIMITATIONS
The focus herein was on the interpretability of CART and MLR results. Although a comparison of classification errors from the two methods applying cost complexity analysis and cross validation would be an interesting investigation, this was beyond the scope of this paper.
The number of variables included in this study was limited due to the use of linked administrative data. Care needs, preferences for location of care, and informal carer characteristics are all important to include but were not available for inclusion in this study; this is a shortcoming of much of the research to date (14). However, by using this data source we avoided selection bias (6,15) since we included 100 percent of the deaths in the two locations studied. Further studies using CART analysis are needed to identify interactions among demographic, social, medical, and health system factors. The role of home care, hospital (including the emergency department), and physician coverage need to be investigated in future studies. Insurance status (12, 13) may also be important in some contexts.
CONCLUSION
This paper shows how CART analysis is useful for identifying multi-dimensional subpopulations with lower PCP enrolment rates. The two primary factors contributing to low PCP enrolment were not receiving RT in the last months of life and nursing home residence. Age, time from cancer diagnosis to death, distance to the PCP, and sex emerged as also being important in defining subpopulations. While the study herein is limited to cancer decedents and access to PCP, CART analysis can be applied to all persons with terminal chronic disease who could benefit from assessment to help determine possible unmet need for palliative care.
Acknowledgments
The assistance of Mohamed Abdolell in reviewing our CART interpretation is much appreciated. This study was funded by the Canadian Institutes of Health Research, Vulnerable Populations ICE Strategic Initiative grant # HOA-80067. Ethics approval was granted by the Capital Health and Cape Breton District Health Authority research ethics boards.
Contributor Information
Jun Gao, Health Canada, Centre for Vaccine Evaluation, Biologics and Genetic Therapies Directorate, Ottawa, Ontario, Canada.
Grace M. Johnston, School of Health Administration, Dalhousie University, and Surveillance and Epidemiology Unit, Cancer Care Nova Scotia, Bethune 568, 1276 South Street, Halifax, Nova Scotia, Canada B3H 2Y9
M. Ruth Lavergne, University of British Columbia, Centre for Health Services and Policy Research, Vancouver, British Columbia, Canada.
Paul McIntyre, Department of Medicine, Dalhousie University, and Palliative Medicine, Capital Health, Halifax, Nova Scotia, Canada.
References
- 1.Grande GE, Farquhar MC, Barclay SIG, et al. The influence of patient and carer age in access to palliative care services. Age Ageing. 2006;35(3):267–73. doi: 10.1093/ageing/afj071. [DOI] [PubMed] [Google Scholar]
- 2.Rosenwax LK, McNamara BA. Who receives specialist palliative care in Western Australia - and who misses out. Palliat Med. 2006;20(4):439–445. doi: 10.1191/0269216306pm1146oa. [DOI] [PubMed] [Google Scholar]
- 3.Burt J, Raine R. The effect of age on referral to and use of specialist palliative care services in adult cancer patients: a systematic review. Age Ageing. 2006;35(5):469–476. doi: 10.1093/ageing/afl001. [DOI] [PubMed] [Google Scholar]
- 4.Burge FI, Johnston G, Lawson B, et al. Population-based trends in referral of the elderly to a comprehensive palliative care programme. Palliat Med. 2002;16(3):255–256. doi: 10.1191/0269216302pm550xx. [DOI] [PubMed] [Google Scholar]
- 5.Hunt RW, Fazekas BS, Luke CG, et al. The coverage of cancer patients by designated palliative services: A population-based study, South Australia, 1999. Palliat Med. 2002;16(5):403–409. doi: 10.1191/0269216302pm571oa. [DOI] [PubMed] [Google Scholar]
- 6.Addington-Hall JM, Altmann D. Which terminally ill cancer patients in the United Kingdom receive care from community specialist palliative care nurses? J Adv Nurs. 2000;32(4):799–806. [PubMed] [Google Scholar]
- 7.Johnston GM, Gibbons L, Burge FI, et al. Identifying potential need for cancer palliation in Nova Scotia. CMAJ. 1998;158(13):1691–1698. [PMC free article] [PubMed] [Google Scholar]
- 8.O’Brien MB, Johnston G, Gao J, et al. End-of-life care for nursing home residents dying from cancer in Nova Scotia, Canada, 2000–2003. Support Care Cancer. 2007;15(9):1015–1021. doi: 10.1007/s00520-007-0218-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burge F, Lawson B, Johnston G. A population based study of age inequalities in access to palliative care among cancer patients. Medical Care. 2008;46(12):1203–1211. doi: 10.1097/MLR.0b013e31817d931d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fadul N, Elsayem A, Palmer JL, et al. Predictors of access to palliative care services among patients who died at a Comprehensive Cancer Center. J Palliat Med. 2007;10(5):1146–1152. doi: 10.1089/jpm.2006.0259. [DOI] [PubMed] [Google Scholar]
- 11.Keating NL, Herrinton LJ, Zaslavsky AM, et al. Variations in hospice use among cancer patients. J Natl Cancer Inst. 2006;98(15):1053–1059. doi: 10.1093/jnci/djj298. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy EP, Burns RB, Davis RB, et al. Barriers to hospice care among older patients dying with lung and colorectal cancer. J Clin Oncol. 2003;21(4):728–735. doi: 10.1200/JCO.2003.06.142. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy EP, Burns RB, Ngo-Metzger Q, et al. Hospice use among Medicare managed care and fee-for-service patients dying with cancer. JAMA. 2003;289(17):2238–2245. doi: 10.1001/jama.289.17.2238. [DOI] [PubMed] [Google Scholar]
- 14.Walshe C, Todd C, Caress A, et al. Patterns of access to community palliative care services: a literature review. J Pain Symptom Manag. 2009;37(5):884–912. doi: 10.1016/j.jpainsymman.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Lavergne R, Johnston GM, Gao J, et al. Exploring generalizability in a study of costs for community-based palliative care. J Pain Symptom Manag. 2011;41 (4):779–787. doi: 10.1016/j.jpainsymman.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breiman L. Classification and regression trees. New York: Chapman & Hall; 1984. [Google Scholar]
- 17.Zhang Heping, Singer Burton H. Recursive partitioning and applications. 2. New York: Springer; 2010. [Google Scholar]
- 18.Graham PL, Kuhnert PM, Cook DA, et al. Improving the quality of patient care using reliability measures: a classification tree approach. Stat Med. 2006;26(1):184–196. doi: 10.1002/sim.2461. [DOI] [PubMed] [Google Scholar]
- 19.Royall DR, Chiodo LK, Polk MJ. An empiric approach to level of care determinations: the importance of executive measures. J Gerontol A Biol Sci Med Sci. 2005;60(8):1059–1064. doi: 10.1093/gerona/60.8.1059. [DOI] [PubMed] [Google Scholar]
- 20.Anderson RT, Balkrishnan R, Camacho F. Risk classification of Medicare HMO enrollee cost levels using a decision-tree approach. Am J of Manag Care. 2004;10(2 Pt 1):89–98. [PubMed] [Google Scholar]
- 21.Rovlias A, Kotsou S. Classification and regression tree for prediction of outcome after severe head injury using simple clinical and laboratory variables. J Neurotrauma. 2004;21(7):886–893. doi: 10.1089/0897715041526249. [DOI] [PubMed] [Google Scholar]
- 22.Lemon SC, Roy J, Clark MA, et al. Classification and regression tree analysis in public health: methodological review and comparison with logistic regression. Ann of Behav Med. 2003;26(3):172–181. doi: 10.1207/S15324796ABM2603_02. [DOI] [PubMed] [Google Scholar]
- 23.Peters D, Chen C, Markson LE, et al. Using an asthma control questionnaire and administrative data to predict health-care utilization. Chest. 2006;129(4):918–924. doi: 10.1378/chest.129.4.918. [DOI] [PubMed] [Google Scholar]
- 24.Gregory KD, Korst LM, Gornbein JA, et al. Using administrative data to identify indications for elective primary cesarean delivery. Health Serv Res. 2002;37(5):1387–1401. doi: 10.1111/1475-6773.10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rakowski W, Clark MA. Do groups of women aged 50 to 75 match the national average mammography rate? Am J Prev Med. 1998;15(3):187–197. doi: 10.1016/s0749-3797(98)00048-8. [DOI] [PubMed] [Google Scholar]
- 26.Statistics Canada. Catalogue # 92-591-XWE. Author; 2006. 2006 community profiles. [Google Scholar]
- 27.Dumont S, Jacobs P, Fassbender K, et al. Costs associated with resource utilization during the palliative phase of care: a Canadian perspective. Palliat Med. 2009;23(8):708–717. doi: 10.1177/0269216309346546. [DOI] [PubMed] [Google Scholar]
- 28.Johnston GM, Boyd CJ, Joseph P, et al. Variation in delivery of palliative radiotherapy to persons dying of cancer in Nova Scotia, 1994 to 1998. J Clin Oncol. 2001;19(14):3323–3332. doi: 10.1200/JCO.2001.19.14.3323. [DOI] [PubMed] [Google Scholar]
- 29.Johnston G, Lawson B, Gao J, et al. Predictors of palliative care program enrolment in Nova Scotia, Canada using new analytic methods for improved application and understanding. Palliat Med. 2008;22(4 Supp 1):418. [Google Scholar]
- 30.Hosmer DW, Lemeshow S. Applied logistic regression. 2. New York: Wiley; 2000. [Google Scholar]
- 31.Asada Y. Vulnerability in palliative care: an application and extension of the risk chain model. Progress Palliat Care. 2010;18.2:72–78. [Google Scholar]