Review on the functional differences of immune cells in various anatomical compartments, and how tissue specific factors influence systemic and mucosal immunity.
Keywords: dendritic cell, microenvironment, microbiota
Abstract
Discovery of DCs and PRRs has contributed immensely to our understanding of induction of innate and adaptive immune responses. Activation of PRRs leads to secretion of inflammatory cytokines that regulate priming and differentiation of antigen-specific T and B lymphocytes. Pathogens enter the body via different routes, and although the same set of PRRs is likely to be activated, it is becoming clear that the route of immune challenge determines the nature of outcome of adaptive immunity. In addition to the signaling events initiated following innate-immune receptor activation, the cells of the immune system are influenced by the microenvironments in which they reside, and this has a direct impact on the resulting immune response. Specifically, immune responses could be influenced by specialized DCs, specific factors secreted by stromal cells, and also, by commensal microbiota present in certain organs. Following microbial detection, the complex interactions among DCs, stromal cells, and tissue-specific factors influence outcome of immune responses. In this review, we summarize recent findings on the phenotypic heterogeneity of innate and adaptive immune cells and how tissue-specific factors in the systemic and mucosal immune system influence the outcome of adaptive-immune responses.
Introduction
The immune system detects pathogen invasion, tissue damage, transformation, and malignancy and also participates in wound healing and tissue remodeling. As these events could happen anywhere in the organism, the immune system has to have a functional presence in every tissue and organ of the body. A hallmark of metazoans is the compartmentalization of their body, which unavoidably leads to the compartmentalization of the immune system. However, the cells of the immune system have the unique ability to travel between compartments and are influenced by the local environments in these compartments. Grossly, the immune system can be divided into three main compartments: the systemic immune system, which filters the blood and detects blood-borne infections; the mucosal immune system, which detects pathogens evading various mucosal surfaces, including the gastrointestinal tract, the respiratory tract, the urinary tract, and the urogenital tract; and the cutaneous immune system, which detects pathogens entering via the skin, because of breaches or insect bites [1]. Traditionally, we have gained most of our information about the immune system by analyzing cells from the spleen and LNs, as a result of the convenience of obtaining large numbers of cells. However, in recent years, it has become increasingly appreciated that the phenotype, differentiation status, longevity, turnover rate, function, and regulatory mechanisms of immune cells are drastically different and impacted significantly by the anatomical compartment in which they reside.
Each of the three immune compartments faces distinct challenges in generating effective antipathogen-immune responses, while maintaining homeostasis and avoiding overwhelming, self-damaging responses. As a result, immunity has to operate in a tissue-specific manner. The systemic immune system mounts immune responses to invading pathogens via the induction of robust proinflammatory and bactericidal programs that may have damaging consequences to the host. This is understandable, as the danger from the pathogen is very high when it gains access to the circulation. However, in the case of mucosal and cutaneous immune systems, maintaining organ integrity is the number one priority. Therefore, at the steady state, the immunity here has to be tightly controlled to allow peaceful coexistence with, rather than eradication of, the symbionts. The inability to maintain homeostasis results in immunopathology and tissue destruction, as seen in emphysema (uncontrolled activation of alveolar macrophages) and colitis (uncontrolled activation of T cells against commensals). As the same type of immune cells residing in different tissues originate from the same precursors prior to entering the tissue, it would be expected that organ-specific factors instruct regional immune responses. Consequently, each organ would have its own threshold for activating the immune system, which is correlated inversely with the relative sterility of the organ.
Specifically, the differences in each of the immune systems are determined by the type of immune-sentinel cells residing in them, the type of response they mount, the longevity and homing capacity of the activated immune cells they generate, and the mechanism they use to regulate the immune responses. In this review, we summarize the recent advances in the differential behavior of immune cells based on their location, with an emphasis on T cells, and discuss how their behavior is instructed by innate immune cells as well as stromal cells.
PHENOTYPIC AND FUNCTIONAL HETEROGENEITY OF DCs AND MACROPHAGES
DCs take up, process, and present antigens to T cells and play an important role in initiating adaptive immunity [2]. DCs also participate in controlling B cell responses [3]. Different DC subsets can be found in different sites of the body, and these diverse DC populations serve as a critical link between the immune microenvironment and adaptive immunity [2]. DCs can be classified into two main subsets: cDCs and pDCs. As pDCs do not present antigen as effectively as cDCs, we will not discuss these cells for the purpose of this review. cDCs can be classified further according to their location. DCs that reside in the blood and lymphoid organs are termed blood DCs, whereas DCs that reside in the peripheral tissues, such as the skin, mucosa, and solid organs, are termed tissue DCs [4].
Macrophages originate primarily from circulating monocyte precursors and complete their differentiation process after migrating into specific tissues [5]. Specialized tissue-resident macrophages, including osteoclasts (bone), alveolar macrophages (lung), histiocytes (intestinal connective tissue), Kupffer cells (liver), and microglia (brain), all exert distinct functions, depending on their resident tissue [6]. In the secondary lymphoid organs, macrophages are found primarily in the red pulp and marginal zone of the spleen, as well as the subcapsular region of the LNs. The function of macrophages is designed to benefit the tissue in which they reside. For instance, the primary function of marginal zone macrophages is to suppress innate and adaptive immunity to apoptotic cells [7]. The subcapsular sinus macrophages in the LNs are able to initiate humoral responses [8]. Macrophages, residing in immune-privileged sites such as the brain, eye, and testis, are mainly involved in tissue remodeling and homeostasis[6].
DCs in the spleen and LNs
Splenic DCs are also known as blood-derived DCs, as the majority of them differentiates from blood-borne precursors once they enter the spleen. Splenic DCs can be classified into three subsets: CD11b+CD4+CD8−DCs, CD11b−CD4−CD8+ DCs, and CD11b−CD4−CD8− DCs [9]. Splenic DCs are thought to reside at immature state and screen the blood for pathogens. In addition to the three DC subtypes found in the spleen (blood DCs), the LNs contain a substantial fraction of tissue-derived DCs, or migratory DCs, which resides originally in the peripheral tissue and subsequently migrates to the respective draining LNs, either constitutively or upon activation by pathogen infection [1, 10–13]. LN resident DCs may capture antigen indirectly from migratory DCs or directly from the draining LN [14]. The specific types of migratory DCs may differ between particular LNs. In the skin-draining LNs, LCs and two dDC subsets are found. The mLNs contain similar DC subtypes found in the intestinal mucosa. The interaction between resident and migratory DCs in the LNs has important outcomes on the resulting immune response.
DCs and macrophages in nonlymphoid tissues
Skin DCs.
Skin DCs are categorized based on their localization in different compartments of the skin tissue. LCs reside in the epidermis, whereas dDCs reside in the dermis. LCs are characterized by expression of Langerin (CD207) and epithelial CAM [15]. At least two distinct dDC subsets have been described, one being the classical Langerin− dDCs and the recently discovered Langerin+CD103+ dDCs [15]. The actual APCs for initiating cutaneous immunity seem to vary depending on the nature of immune challenge. LCs, LN-resident DCs [16], Langerin− dDCs [17, 18], and Langerin+ dDCs [18, 19] have all been implicated as APCs in different studies. Interestingly, skin-resident DCs appear to play distinct, sometimes opposing, roles in generating a T cell response. With the use of a Candida albicans skin infection model, Igyarto et al. [20] showed that LCs are necessary and sufficient for direct antigen presentation and generation of antigen-specific Th17 cells but not for a CTL response. In contrast, Langerin+ dDCs are required for the generation of antigen-specific CTL and Th1 responses. Additionally, Langerin+ dDCs inhibited the ability of LCs and Langerin− DCs to induce Th17 cell responses [20]. Several other studies have also determined Langerin+ dDCs as the major population mediating cross-presentation of viral and tissue antigens to CD8 T cells, whereas LCs play negligible roles [19, 21]. In contrast, the role of LCs seem rather controversial. In vitro and in vivo evidence suggests that LCs can be immunogenic or tolerogenic [22]. LCs were shown to be sufficient to induce contact hypersensitivity, independent of Langerin+ dDCs [23, 24]. They were also shown to be responsible for activating pathogenic CD8 T cells in a skin-specific autoimmunity model [25]. Conversely, using a cutaneous leishmaniasis model, LCs were shown to function as negative regulators of T cell immunity by driving the expansion and immigration of Tregs [26]. Consistently, LC-ablated mice develop enhanced contact hypersensitivity [27]. A similar tolerogenic effect of LCs was also observed in the context of protein immunization [28]. The induction of Tregs appears to be a unique function of LCs, as it cannot be observed with Langerin+ dDCs [29]. It is possible that the actual function of a LC in situ is determined by stimuli from the environment, as well as the skin commensals. In unperturbed skin, they are most likely associated with down-regulating T cell responses, whereas in the context of inflammation or tissue damage, LCs display immunogenic properties [22].
Gut DCs and macrophages.
DCs are present in all of the lymphoid organs associated with the intestines, including the PPs, ILFs, and the mLNs [30]. They are also abundant in the nonlymphoid tissues, such as the LP facing the lumen [31], the muscular layers, and the serosa facing the peritoneum [32]. When stimulated through TLRs, PP and LP DCs secrete much less IL-12 but more IL-10 [33, 34]. As a result, these DCs induce more IL-4 and IL-10 from naïve CD4 T cells [34, 35] compared with splenic DCs, suggesting tolerogenic functions of these DCs. DC subsets within mucosal tissues have unique immune-inductive capacities. The PPs harbor three DC subtypes: CD11b+CD8− DCs that localize in the subepithelial dome, CD11b−CD8+ DCs in the interfollicular regions, and CD11b−CD8− DCs at both sites, as well as the follicle-associated epithelium. The CD11b−CD8− DCs are much more abundant in the PPs and mLNs compared with the spleen and pLNs. The tolerogenic properties of PP DCs are restricted to CD11b+CD8− DCs, whereas CD11b−CD8+ and CD11b−CD8− DCs produce IL-12 and prime T cells for IFN-γ production [34].
In the LP, DCs (CD11chiMHCIIhi) can be classified according to their expression of CD103 and CD11b. Two major populations—CD11b+CD103+ and CD11b+CD103−—can be observed [36]. The CD11b+CD103+ DCs develop from common DC progenitors and pre-DCs under the control of Fms-like tyrosine kinase and GM-CSFR ligands and express little M-CSFR and CX3CR1. The CD11b+CD103− DCs, however, arise from monocytes under the control of M-CSFR ligands and express high levels of M-CSFR and CX3CR1. CD103+CD11b+ DCs are highly capable of migrating from the intestinal LP to the mLNs in a CCR7-dependent manner [37]. This process allows them to transport antigens from the gut to the mLNs, where they present soluble antigen to CD8+ and CD4+ T cells [38], induce gut-homing molecules on T cells [37, 39], and promote the conversion of naïve T cells into iTregs [40]. In contrast, CD11b+CD103− DCs are part of a nonmigratory, gut-resident population that does not enter the mLNs effectively [41]. It has been shown that CD11b+CD103− DCs are capable of extending their transepithelial dendrites into the intestinal lumen for antigen sampling in a way mediated by the CX3CR1 receptor [42]. Thus, CD11b+CD103− DCs may primarily function locally in the gut tissue. It is possible that CD11b+CD103+ DCs acquire antigen from the CD11b+CD103− DCs for T cell priming, although a recent study showed that CD103+ DCs patrol the intestinal epithelium while extending intraepithelial dendrites to phagocytose the bacteria in the lumen and subsequently activate CD8 T cells in mLNs [43]. Similar to the PPs, bifurcation of DC function in T cell priming is also seen between the two LP DC populations. In vitro experiments show that CX3CR1+ DCs preferentially supported Th1/Th17 CD4 T cell differentiation, whereas CD103+ DCs preferentially induce the differentiation of Foxp3-expressing Tregs [44].
Macrophages in the intestinal mucosa adapt to a microbe-rich environment by selectively shutting off their proinflammatory mediator production yet fully preserving phagocytic and bactericidal activities at the steady state [45]. Human intestinal macrophages lack some key receptors for proinflammatory immunity, such as the costimulatory molecules CD40, CD80, and CD86, receptors for IgA (CD89) and IgG (CD16, CD32, CD64), and the LPS coreceptor CD14 [46, 47]. Intestinal macrophages also down-regulate the production of TLR-induced cytokines, such as IL-1, IL-6, IL-8, TNF-α, IL-10, and RANTES. This results from reduced expression of key signaling molecules of the TLR pathway in these macrophages, including MyD88, Toll/IL-1R domain-containing adaptor-inducing IFN-β, and TRAF6, as well as enhanced expression of the NF-κB inhibitor IκBα [46, 48].
Lung DCs.
In the mouse lung, four cDC subsets have been described: a CD11b−CD103+ Langerin+ subset in the intraepithelial compartment lining the conducting airway, a CD11bhisignal-regulatory protein α+CX3CR1 subset immediately underneath in the LP, as well as CD11b+ and CD11b− subsets in the lung parenchyma [49]. During inflammation and infection, additional Ly6C+ monocyte-derived DCs can be observed. CD103+ DCs are specialized in cross-presentation of apoptotic cells and viral antigens to CD8+ T cells [50, 51]. In contrast, CD11b+ DCs are superior in producing inflammatory chemokines [52] and presenting soluble antigen to CD4+ T cells [53].
TISSUE-SPECIFIC REGULATION OF MACROPHAGE AND DC RESPONSES BY THE LOCAL MICROENVIRONMENT
Innate-immune cells at the barrier sites continuously receive instructive signals from the resident environment. Nonhematopoietic cells, namely ECs and stromal cells, have been shown to play important roles in conditioning the local microenvironment to confer tissue-specific immune regulation. For instance, blood monocytes cultured with conditioned media from intestinal stromal cells can gain features of gut macrophages and down-regulate proinflammatory cytokine production [46]. Similarly, when cultured with conditioned media from caco-2 cells (human intestinal EC line), human monocyte-derived DCs fail to undergo maturation or secrete IL-12 when stimulated with Salmonella typimurium but instead, became potent Th2 inducers [54]. The immune regulatory properties of nonhematopoietic cells can be amplified by locally resident immune cells once they are exposed to conditioning signals, thereby forming a positive-feedback loop.
Different organs take advantage of tissue-specific metabolites and soluble factors and membrane-bound molecules to establish tissue-specific means of immune modulation. The gut tissue is by far the most well-studied tissue with regard to how microenvironment factors influence behavior of resident cells. In the intestinal mucosa, TGF-β is produced by a number of different cells, including ECs, mast cells [55], Tregs [56], and stromal cells [46], to constantly dampen the inflammatory properties of resident macrophages. IL-10, produced by DCs themselves, as well as neighboring Tregs, ECs, and stromal cells, has also been shown to contribute to the reduced inflammatory properties of intestinal DCs [33]. In addition, stromal cells in the small intestinal LP constitutively express cyclooxygenase-2 and produce PGE2, which inhibit the production of type I IFNs by pDCs in the PPs [56] and reduce the activity of human cDCs. IECs constitutively release TSLP, which can inhibit maturation and IL-12 production by DCs in response to bacteria and favors Th2- over Th1-polarizing cells [54]. TSLP production by IECs seems to be independent of microbial stimulation or inflammatory signals, such as TNF-α or IL-1β, but requires IKKβ. Mice defective in IKKβ in the IECs express a reduced amount of TSLP when infected with the gut-dwelling parasite Trichuris. As a result, DCs from these animals express exacerbated IL-12p40, which leads to an increase in Th1 and Th17 responses but failure to develop Th2 response [57]. Membrane-bound molecules have also been implicated in regulating immune tolerance in the gut. Such molecules include 4-1BB, a TNFR family member. Ligation of 4-1BB promotes retinaldehyde dehydrogenases expression and activity in CD103+ gut DCs and enhances their ability to generate iTregs in the GALT when exposed to oral antigen [58]. In addition, DC-intrinsic mechanisms have been discovered to operate in tissue-specific fashions to promote immune tolerance in the gut. Mice lacking the Wnt signal transducer β-catenin, specifically in DCs, have a reduced percentage of Tregs in the gut, but not the spleen, suggesting that β-catenin in DCs is involved selectively in the tolerogenic function [59].
The lung microenvironment has also been shown to affect directly the function of immune cells. A recent study shows that TLR activation induced IDO in the lung tissue, where it inhibited Th2- and Th1-meditated lung inflammation by inhibiting T cell trafficking into the lung and by killing effector T cells infiltrating the lung tissues. In the spleen, however, such IDO up-regulation and enhanced T cell death are not seen [60]. Another study shows that alveolar macrophages express higher levels of the inhibitory receptor CD200R than macrophages from other tissues. Ligation of CD200R by CD200-expressing airway ECs suppressed the production of proinflammatory cytokines IL-6 TNF-α, as well as immunopathology associated with airway infection [61].
PHENOTYPIC AND FUNCTIONAL DIVERSITY OF LYMPHOCYTES
T cells that have finished the developmental program in the thymus enter the circulation and are able to enter the secondary lymphoid organs through the high endothelial venules. This process is mediated by recognition of the addressins by CD62L and the chemokines CCL21/CCL19 by CCR7 [62]. Naïve T cells circulate through the bloodstream, the spleen, and the LNs. The secondary lymphoid organs serve as reservoirs for naïve T and B cells, where these cells form organized structures to allow efficient activation. During activation by the APCs, T cells not only receive antigenic activation and costimulation that allow them to undergo clonal expansion but also receive cytokine cues that dictate their differentiation [63]. Importantly, T cells are also imprinted with a homing capacity that allows them to leave the secondary lymphoid organ and migrate to the peripheral tissues [64]. The ability of memory T cells to migrate selectively into different peripheral tissues is controlled by the expression of different combinations of adhesion molecules and chemokine receptors. The ability of T cells to express these specific combinations of surface molecules is “imprinted” during initial antigen priming and depends on molecular cues that are governed by the LN microenvironment [64].
Innate T cells at effector sites
The secondary lymphoid organs are the primary sites for the initiation of adaptive immunity and are therefore enriched with antigen-inexperienced T and B cells. The barrier sites, such as the skin and the mucosa, however, are directly facing the external environment and thus, the primary sites of pathogen entry. These sites depend on cells that can rapidly execute effector functions to fight against invading pathogens, facilitate wound repair, and assist barrier functions. As a result, lymphocytes at the barrier sites often exhibit innate-like features. Unconventional lymphocytes, such as γδT cells, NKT cells, and the recently discovered innate lymphoid cells, are found mostly in the nonlymphoid tissues [65–67]. Even among the conventional lymphocytes, those resident in the barrier surfaces bear a distinct antigen receptor repertoire compared with those in the systemic immune system. Within the TCRαβ+ subset, in addition to the conventional CD4+ and CD8αβ+ T cells, the IELs contain large numbers of CD8αα+TCRαβ+ T cells [68]. In contrast to conventional, foreign-reactive T cells, CD8αα+ IELs are selected primarily by high-affinity self-antigens [69–71]. These self-reactive CD8αα+ T cells are imprinted with activated/memory characteristics and have been shown to carry out regulatory function not seen in other IEL subsets [72]. Gut-resident effector/memory T cells differ from their splenic counterparts in a number of ways, including an initial dependence on CD40–CD40L interactions [73], high expression of CD69 [74], immediate effector function [73], and enhanced survival [75].
Memory T cell functions are determined by their location
Following initial clonal expansion, T cells undergo a contraction phase, where 90% of the T cells die via apoptosis, leaving behind a long-lived memory pool that can respond to antigen rechallenge more rapidly and more robustly than the primary response [76]. During the naïve-memory transition, several fundamental changes take place. Memory T cells in peripheral tissues are more resistant to apoptosis than their systemic counterparts [77, 78]. The activation threshold of T cells appears to be lowered, as they become less-dependent on costimulation [79]. They have also increased sensitivity to cytokine signals as a result of up-regulation of cytokine receptors. As a result, memory T cell reactivation can happen in the absence of antigen stimulation of the TCR, purely driven by cytokines. Several studies have demonstrated that IL-18, in combination with IL-12, IL-15, or IFN-β, can trigger reactivation of memory CD8 T cells in an antigen-independent manner [80, 81]. The memory T cell pool is not a homogeneous population but contains three subgroups that occupy distinct compartments of the body with different differentiation statuses and functions [82]. The secondary lymphoid organs (spleen and LNs) contain memory T cells that express CCR7 and CD62L, known as TCM, which circulate through the secondary lymphoid organs and undergo recall response there. TCM appear to be a relatively immature subset of memory T cells. Upon reactivation, these cells themselves produce limited amounts of signature cytokines of the respective effector lineage. Instead, they secrete large amounts of IL-2 and can undergo massive proliferation to give rise to a new pool of effector cells capable of effector cytokine production [83, 84]. A second type of memory T cells, termed TEM, have lost their ability to enter the secondary lymphoid organs and are, instead, found in the blood and peripheral tissues. These cells can enter the peripheral tissue temporarily and return to the circulation. These cells proliferate poorly upon secondary stimulation but elicit high effector functions, resulting in a rapid response at the site of infection that limits pathogen replication prior to the arrival of secondary effector T cells from the LNs [83, 84].
Most recently, a third memory T cell subset, the TRM, has been shown to reside solely in the peripheral tissue and does not enter the circulation within a significant period of time [85]. In contrast to TEM, TRM are locally restrained, self-sustained, long-term residents [86]. CD8 TRM were observed initially in the sensory ganglia and the skin of HSV-infected mice [86] and were later seen in the brain [87], the lung [88–90], the salivary glands [91], and the intestinal epithelium [92]. TRM isolated from the brain of VSV-infected mice fail to undergo recall expansion after i.v. transfer into VSV-infected recipients [87]. TRM express unique molecules to facilitate their retention in the tissue, such as CD103 and very late antigen 1, which are not present on circulating memory cells [86, 87]. In contrast to systemic memory cells, TRM undergo minimal homeostatic proliferation and respond poorly to survival factors for systemic memory cells, such as IL-7 and IL-15 [93]. Interestingly, in the same HSV infection model, TRM CD4 are more mobile, preferentially locate in the dermis as opposed to the epidermis, and express intermediate levels of CD103. Conversely, TRM CD8 are sequestered in the epidermis and have limited migratory capacity [94]. Similar uneven distribution of CD4 and CD8 T cells has also been observed in the gut mucosa, and CD4 T cells and CD8 T cells localize predominately in the LP and the epithelium, respectively [94].
It is important to consider the intended location of the resulting effector/memory cells for the design of vaccines against particular pathogens. Consideration of the route of immunization is important for inducing immunity against pathogens, particularly when aiming for T cell-mediated protection, as a robust or productive systemic effector/memory T cell response does not necessarily correlate with effective protection at sites for pathogen entry. One recent study has successfully used a “prime and pull” vaccination strategy to induce TRM that confer effective protection during rechallenge. Specifically, following s.c. vaccination with an attenuated strain of HSV-2 to elicit systemic T cell responses (prime), CXCL9 and CXCL10—ligands for CXCR3 specifically expressed by highly activated effector T cells—were administered vaginally to recruit activated, HSV-specific CD8+ T cells to the vagina (pull), where these T cells were retained locally and form a long-term resident memory pool to mediate protective immunity [95].
PRIMING MICROENVIRONMENTS AND ROUTES OF INFECTION DICTATE THE OUTCOME OF T CELL RESPONSES
It has long been noticed that the efficacy of vaccination is impacted greatly by the route of immunization. This phenomenon has been seen in vaccination against Mycobacterium, where intranasal immunization, compared with the s.c. or the i.v. route, with bacille Calmette-Guérin led to superior protection to airborne challenge with Mycobacterium tuberculosis [96, 97]. Similar observations have been made with Listeria pneumonitis, where mice vaccinated through the airborne route, but not i.v. or in the footpad, acquire more robust and prolonged resistance to airborne rechallenge [98]. The underlining mechanism is likely a result of the type, the longevity, and the homing pattern of the effectors generated by a different vaccination route.
One example showing the correlation between the route of infection and the resulting effectors comes from a study where mice were infected with Listeria monoytogenes through the i.v. or the intranasal route. Whereas i.v. infection elicited antigen-specific Th1 cells, intranasal infection elicited antigen-specific Th17 cells [99]. Moreover, i.v. infection-elicited Th1 cells persisted following the contraction phase, whereas the intranasal infection-elicited Th17 cells progressively declined. This short-lived phenotype of intranasal infection-elicited Th17 cells correlated with diminished CD27 expression, reduced homeostatic proliferation, and reduced IL-15R and Bcl2 (antiapoptotic factor) expression [99]. Therefore, the route of infection seems to imprint fundamental differences in the resulting effector cells, shaping their differentiation pathway and survival potential. Similarly, intranasal and i.v./s.c. inoculation of group A Streptococcus pyogenes induced antigen-specific Th17 and Th1 cells, respectively [100]. It has been demonstrated that IL-23 is produced in larger quantities by intestinal APCs, whereas IL-12 is more highly expressed by APCs from the periphery, such as the spleen [101]. The enrichment of Th17 cells in the mucosa-associated lymphoid tissue compared with other tissues is likely determined by the type of proinflammatory cytokines that resident APCs make. In the case of Francisella tularensis infection, the intranasal and intradermal routes of infection favor Th17 and Th1 responses, respectively. The skewed responses correlated with enhanced PGE2 production during intranasal infection, and upon blockade of its production, Th1 response was partially restored [102]. The route of infection also dictates which pathways provide protective immunity. With the use of Yersinia enterocolitica, DePaolo et al. [103] showed that oral infection promotes Th17 immunity, whereas systemic infection promotes Th1 immunity. Importantly, TLR1 was shown to be involved specifically in inducing Th17-mediated, protective immunity during oral infection, resulting from the TLR2/TLR1-induced IL-6/IL-23 and the presence of TGF-β in the gut environment. TLR2/TLR1 was, however, not required for Th1 immune responses during systemic infection [103].
It has been proposed that activated T cells preferentially traffic to the tissue in which they were primed initially [64]. Tissue-specific migration of T cells is dependent on differential expression of homing molecules on the cell surfaces. For instance, CD4 effector/memory cells activated in cutaneous LNs and mLNs preferentially up-regulate P-selectin ligand and α4β7, respectively [104]. The integrin α4β7 and the chemokine receptor CCR9 guide T cells to home to the GALT (mLN, PP, the small intestinal LP, the intestinal epithelium), via their interaction with the receptor mucosal vascular addressin cell adhesion molecule 1 and the chemokine CCL25 (thymus-expressed chemokine), respectively [105, 106]. In contrast, T cell homing to the skin is mediated by P- and E-selectin ligands, as well as CCR4 and CCR10 [107, 108]. Tissue-specific homing molecules, conditioned by the local microenvironment, play a critical role in the instruction of activated T cells to acquire proper tropism. Gut-resident DCs, particularly CD103+ DCs, have the unique ability to induce gut-homing molecules during T cell priming [35, 37, 109]. Likewise, priming with skin-derived DCs imprints skin tropism of T cells [110, 111]. The gut and skin use the vitamin A metabolite RA and the vitamin D-derived metabolite [1,25-(OH)2D3], respectively, to induce tissue-specific homing molecules specifically [64, 112, 113].
The inflammatory cytokines that are made by DCs also have a context-dependent role in shaping the effector T cell responses. For example, we have reported recently that IL-6 is differentially required in the systemic and mucosal immune systems for Th17 differentiation, in that it is critical for Th17 lineage priming in the skin and the mucosal tissue, such as the gut and the lung, but is completely dispensable for Th17 priming in the spleen (Fig. 1). These differential cytokine requirements for Th17 lineage commitment are guided by different populations of DCs resident in different tissues: CD103+ DCs that are present in the skin and LP but not in the spleen favor the generation of iTreg cells and suppress Th17 differentiation. IL-6 is required to overcome this effect [114]. The context-dependent role of cytokines is also observed to regulate lymphocyte trafficking. For example, the IL-12 induces and IL-4 suppresses the expression of the skin-homing molecule cutaneous lymphocyte-associated antigen on activated T cells [115], but these cytokines have the opposite effect on the skin-homing chemokine receptor CCR4, which is up-regulated by IL-4 and suppressed by IL-12 [116]. It has been observed that different effector T cells preferentially express distinct combinations of receptors [117]. Although the underlining mechanism and significance still remain to be investigated, it has been proposed that the coordinated expression of homing molecules by different T cells requires the coordinated activity of multiple cytokines, in the context of environmental cues, to allow tissue- and subset-dependent trafficking of effector cells to their proper sites [64].
Figure 1. Tissue-specific role of IL-6 in Th17 differentiation.
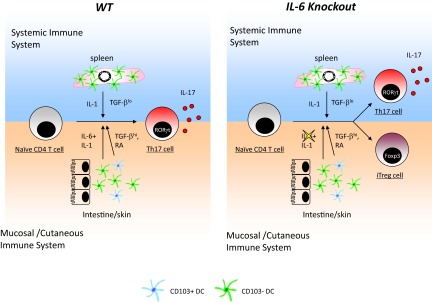
Systemic and mucosal/cutaneous immune systems have differential requirements of IL-6 for Th17 lineage differentiation. In the systemic immune system (left and right, upper), which lacks CD103+ DCs, IL-1 but not IL-6 is required for generating Th17. In the mucosal and cutaneous immune systems (left and right, lower), CD103+ DCs produce TFG-β and RA to inhibit Th17 differentiation and favor immune regulation. IL-6 is necessary to overcome the inhibitory effect of TGF-β and RA and is therefore required for Th17 differentiation in the mucosal and cutaneous immune systems. In the absence of IL-6 (right), the systemic immune system can still generate Th17 cells, but the mucosal/cutaneous immune systems fail to do so and instead, generate iTreg cells, as a result of TGF-β and RA signals from CD103+ DCs. RORγt, Thymus-specific retinoic acid receptor-related orphan receptor γ.
REGULATION OF T CELL RESPONSES BY TISSUE-SPECIFIC FACTORS AND THE COMMENSAL MICROBIOTA
Apart from thymically generated Tregs (nTreg), Tregs can also be induced in the periphery from naïve CD4 T cells [118]. The GALT is a preferential site for iTreg generation. GALT APCs (DCs and macrophages), particularly CD103+ DCs, are very efficient in inducing iTreg differentiation as a result of their ability to produce a high amount of TGF-β and RA [40, 119]. It is likely that the superior iTreg-inducing capacity of gut APCs is imposed by the local microenvironment, as human IEC-derived TSLP, TGF-β, and RA have been shown to condition monocyte-derived DCs to become iTreg-inducing tolerogenic DCs [120, 121]. Another study demonstrated that intestinal ECs synthesize RA from dietary retinol and that monocyte-derived DCs treated with RA can acquire characteristics of mucosal DCs, such as secretion of TGF-β, the ability to induce gut-homing molecules, and IgA production [122]. Gut iTregs are generated upon encounter with commensal microbiota. Sequencing of colonic Treg TCRs reveals a distinct TCR repertoire compared with Tregs from the spleen and LNs, with many of the iTreg TCRs specific for commensal bacteria-derived antigens. In contrast, effector T cells with the same specificities are pathogenic [123]. iTregs have been proposed to be important in maintaining immune homeostasis in the intestine by providing tolerance to the commensal microbiota [124]. This idea is also supported by the observation that mice lacking the TGF-β-activating integrin αvβ8 in DCs have reduced iTregs in the gut and develop colitis [125]. Mice lacking a particular region of the Foxp3 enhancer required for iTreg but not nTreg development acquire severe type 2 inflammation, specifically in the gut but not systemically [126]. Therefore, iTreg generation is required specifically at the mucosal surface to maintain immune tolerance. We have observed that IL-6-deficient mice lack Th17 cells but harbor elevated Tregs specifically in the intestine but not the spleen. These data support the notion that the reciprocal regulation of Th17 and iTreg cells is seen predominantly in mucosal tissues and is absent in the systemic immune system [114].
Intestinal ECs can also directly regulate intestinal T cells. IECs sense microbes and produce anti-inflammatory cytokines, such as TSLP, IL-25, TGF-β, and IL-10, to dampen proinflammatory immunity in the gut [127]. Gut DCs themselves have been shown to express TSLP in response to TLR ligation. TSLP is produced by the CD103+ subset and acts directly on T cells to reduce their capacity to produce IL-17 and promote the development of Tregs [128]. Commensal-dependent expression of the IL-17 family member IL-25 by IECs limits the expansion of Th17 cells in the intestine by repressing macrophage-derived IL-23 [129]. IECs also express surface molecules to deliver regulatory signals. One well-characterized example of these surface molecules is the MHC-I-like molecule, TL, the ligand for the CD8αα receptor [130]. CD8αα engagement by TL alters TCR signals, leading to reduced proliferation and cytotoxicity but elevated cytokine secretion, which allows protection without any expense to tissue integrity [72].
GALT DCs can also be immunogenic, as is seen in the case of pathogenic infections and colitis. The proinflammatory function of gut DCs could be carried out by a distinct DC subset, such as the recently reported E-cadherin-expressing population [131]. However, it is equally important to appreciate that the tolerogenic function of CD103+ DCs is dynamic and subject to change by the local microenvironment. In fact, under highly inflammatory conditions, CD103 DCs are impaired in their ability to produce TGF-β and RA and lose their ability to generate iTreg cells but instead, promote the induction of Th1 and Th17 cells [132]. Therefore, it is important to reassess the activity of CD103+ DCs in different contexts. This concept is supported by work showing that RA, in the context of intestinal inflammation, instead of being immunosuppressive, synergizes with IL-15 to promote celiac disease [133].
Conditioning of DCs by the neighboring environment is also seen in the skin. Skin-resident mast cells have been shown to confer tolerogenic capacities to skin-derived DCs in a skin graft model [134]. Similar to the intestine, the skin local microenvironment also uses tissue-specific metabolites to give tolerogenic properties to T cells. The skin synthesizes vitamin D3 upon exposure to sunlight, which is subsequently converted to [1,25-(OH)2D3], which has immunoregulatory activities [135–137]. Vitamin D3, together with dexamethasone, induced naïve CD4 T cells to become IL-10 producers with regulatory capacity [138].
In addition to tissue-specific factors of host origin, commensals play an important role in regulating the immune response at barrier sites. Gut commensals are able to limit trafficking of bacterial antigens to the MLNs by CX3CR1hi DCs, thereby constraining the intestinal immune response to avoid inflammation [139]. Certain bacterial spices (Clostridium) or bacterial products (Bacteroides fragillis polysaccharide A) have been implicated to promote iTreg generation [140, 141]. Incubation of ECs with the probiotic Lactobacillus paracasei B21060 results in the induction of immunomodulatory mediators by ECs, which limits the release of cytokines (reduced IL-12p70) and the capacity of DCs to drive the development of Th1 T cells in response to Salmonella [142]. Consistently, L. paracasei was shown to be protective in dextran sulfate sodium-induced colitis [142]. On the other hand, certain bacterial species or products are immunostimulatory. Segmented filamentous bacteria and bacteria-derived ATP have been shown to enhance Th17 cell generation in the gut [143, 144]. Bacterial DNA suppresses iTreg cell generation, while promoting Th1 and Th17 effector cells through TLR9 [145].
Although the gut and the skin are in close contact with microbial communities that are important for the development and maintenance of local immunity, it has been shown recently that the intestinal and skin microbiota only control their resident barrier sites but have no immunoregulatory effect on the other barrier system. Both compartments contain T cells that produce IFN-γ and IL-17A in a commensal-dependent manner. Mono-association of germ-free mice with a gut commensal, segmented filamentous bacteria restored cytokine production by intestinal T cells but failed to do so to skin T cells. Likewise, mono-association of germ-free mice by a skin commensal, Staphylococcus epidermidis, restored cytokine production by skin-resident but not intestinal T cells. IL-1α production induced by the skin commensal is selectively required for IL-17A production by dermal T cells, consistent with the notion that different immune compartments have evolved distinct pathways to control their local immune responses [146].
TISSUE-SPECIFIC REGULATION OF B CELL RESPONSES
A major hallmark of mucosal immune response is IgA production by B cells, and it is pertinent to the discussion here, as there is a large body of work that demonstrates that tissue-specific factors contribute to mucosal IgA responses. IgA is produced almost exclusively by the mucosal immune system. In contrast to IgG and IgE class-switching, which stringently rely on CD4 T cell help, IgA class-switching in the mucosa-associated lymphoid tissue can take place via TD and T cell-independent mechanisms (Fig. 2). IgA production in the mucosal immune system seems to use an alternative pathway compared with systemic antibody responses. For instance, mice deficient in CD28 have a compromised antibody response when challenged with a T-dependent antigen through the systemic route. However, these mice retain the ability to produce antigen-specific IgA when a T-dependent antigen is administered orally [147]. The mucosal microenvironment conditions DCs and T cells, such that the generation of IgA-producing B cells is favored. DCs play a central role in inducing intestinal IgA production. They can directly produce factors important for IgA class-switching and plasma cell differentiation/survival and also promote the generation of CD4 T cells with IgA-inducing effector functions.
Figure 2. Pathways for induction of IgA response in the intestine.
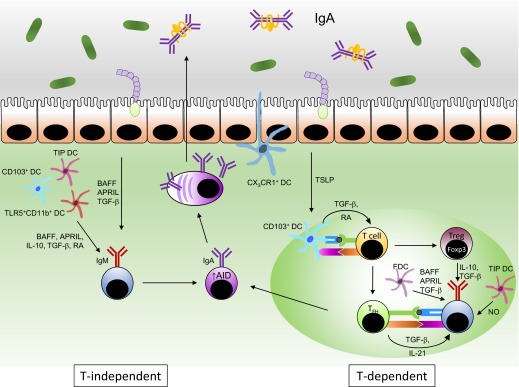
IgM+ B cells in the LP, PPs, ILFs, and mLNs undergo IgA class-switching upon exposure to antigens and IgA-inducing factors, including RA, BAFF, APRIL, IL-10, and TGF-β, provided by various cell types in the absence (left) or presence (right) of class-switching signal from follicular B helper T cells (TFH). Activated B cells up-regulate expression of AID, a prerequisite for CSR and somatic hypermutation, and subsequently, differentiate into plasma cells that produce IgA.
PPs, ILFs, and mLNs comprise the major IgA inductive site in the intestine. PPs develop in the fetus independently of the microbiota, but robust germinal center reaction depends on microbial signals, as mice depleted of intestinal bacteria have fewer and smaller germinal centers in the PPs [148]. In PPs, CX3CR1+ DCs are present underneath of the follicle-associated epithelium, suggesting that these DCs may be responsible for transporting antigens from the intestinal lumen to the PPs [42]. CD103+ DCs migrate from the LP to the interfollicular area of PPs and mLNs to present antigen to T cells [38, 41]. As they sample antigen from the gut, intestinal DCs receive “conditioning” signals from intestinal ECs, such as TSLP, that renders intestinal DCs, particularly CD103+ DCs, to release IL-10 and RA to promote the development of iTreg cells, as discussed before [54, 149]. Through the secretion of IL-10 and TGF-β1, iTregs, together with other TGF-β-producing cells, help to create an environment that favors IgA CSR [150, 151]. Furthermore, intestinal Tregs themselves are able to differentiate into follicular Th cells, which induce germinal center B cell differentiation as well as IgA CSR and production via CD40L, IL-21, and TGF-β1 [152]. TIP DCs, which are abundant in the GALT, enhance TD IgA responses by up-regulating the expression of the TGF-βR on follicular B cells from PPs via NO [153]. Additionally, FDCs in the PPs also promote IgA production through the release of BAFF, APRIL, and TGF-β upon conditioning by mucosal signals, such as commensal TLR ligands and RA. These factors enhance the IgA-inducing function of T cells in the PPs [154].
T-independent IgA class-switching takes place in the ILFs and the LP [155, 156]. DCs in the ILFs, similar to their PP counterparts, are also highly capable of producing the IgA-inducing factors TGF-β1, BAFF, and APRIL, which are enhanced by microbial TLR signals [155, 157, 158]. IgA CSR in the LP likely involves in situ activation of B cells by DCs [156]. Several LP DC subsets, including TIP DCs (via BAFF and APRIL) and TLR5-expressing CD11b+CD11c+ DCs (via RA and IL-6), have been shown to acquire IgA-inducing function upon microbial sensing [153, 156]. pDCs, activated by type I IFNs from intestinal stromal cells produce BAFF and APRIL, can also induce TI IgA class-switch [159]. IECs also secrete BAFF and APRIL upon TLR activation to induce IgA class-switching of LP B cells or do so indirectly by enhancing their production by DCs via TSLP [160, 161]. BAFF and APRIL trigger IgA CSR by engaging the CD40-related receptor TACI. Together with cytokine and TLR signals, TACI induces AID expression via NF-κB, followed by CSR, antibody production, and plasma cell differentiation [162, 163].
CONCLUDING REMARKS
In the past, the study of the immune system had been largely limited to primary and secondary lymphoid organs. This led to a wealth of information, leading to greater understanding of how T and B cells are selected in the thymus and bone marrow and how they differentiate into effector cells (or antibody-producing cells) in response to varied challenges. The secondary lymphoid organs, such as the spleen and pLNs, are largely sterile organs, unless there is a systemic infection. Thus, studies that introduce the pathogen directly into circulation to measure immune responses in the spleen and LNs miss the complexity of interactions that happen at the site of infection. Many pathogens enter the body via mucosal surfaces and interact and influence the behavior of resident cells. In addition, the skin and mucosal surfaces are in constant contact with a large and diverse population of commensal organisms, and this requires a balancing act that suppresses immune responses against commensals but maintains the ability to mount robust responses against virulent pathogens. One way in which this could be achieved is to set up different thresholds for activation [164]. For example, the intestinal immune system is in constant contact with the microflora, but overt immune responses that result in inflammation are absent. This is mainly because of two reasons: the commensal microflora are not virulent and do not attempt to cross the intestinal barrier, and the immune cells in the gut reside in a fairly immunosuppressive microenvironment. In the event of an infection with a pathogen, the virulent microbe will try to cross the intestinal barrier and in addition, will express additional ligands that will activate the innate-immune system, resulting in a higher state of inflammation. These signals will then have the ability to overcome the suppressive milieu and induce productive immune responses against the pathogen. In contrast, if a nonpathogenic commensal microbe is introduced into the systemic immune system, either by the i.p. or i.v. route, the splenic and LN microenvironment is not suppressive, and a productive immune response could be induced regardless of the pathogenic capacity of the microbe. The nature of resultant immune responses, when the pathogen enters by its natural route or when introduced by artificial route, will be different, as the primary interactions between the pathogen and the resident cells will be different. These interactions are dictated not only by the differences in cell populations that reside in particular tissues but also by tissue-specific factors that influence cellular behavior [165]. Despite recent important studies, there are several outstanding questions that remain to be addressed. For example, a productive immune response induced by a particular pathogen is not necessarily a protective one. If a Th17 response is necessary to eliminate a particular class of pathogen, and the immune system mounts a robust Th1 response, it is unlikely to be protective. We also do not clearly understand how engagement of multiple PRRs in vivo by a given pathogen shapes the nature of adaptive immunity. The role of tissue-specific factors in influencing development of effective memory also needs to be elucidated. Understanding the molecular and cellular basis of immune responses in the tissues will not only lead to important discoveries about the immune system but will also help us design and deliver a better vaccine and think of targeted therapies for organ-specific autoimmunity.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health grant AI082265 to C.P.
We thank Ty Troutman for critical reading of this manuscript.
Footnotes
- [1,25-(OH)2D3]
- 1,25-dihydroxyvitamin D3
- AID
- activation-induced cytidine deaminase
- APRIL
- a proliferation-inducing ligand
- BAFF
- B cell-activating factor
- CD40
- CD40 ligand
- cDC
- conventional DC
- CSR
- class-switch recombination
- dDC
- dermal DC
- EC
- epithelial cell
- FDC
- follicular DC
- Foxp3
- forkhead box p3
- HSV
- herpes simplex virus
- IEC
- intestinal epithelial cell
- IEL
- intraepithelial lymphocyte
- ILF
- isolated lymphoid follicle
- iTreg
- induced regulatory T cell
- LP
- lamina propria
- mLN
- mesenteric LN
- nTreg
- natural regulatory T cell
- pDC
- plasmacytoid DC
- pLN
- peripheral LN
- PP
- Peyer's patch
- RA
- retinoic acid
- TACI
- transmembrane activator and calcium-modulating cyclophilin ligand interactor
- TCM
- central memory T cell
- TD
- T cell-dependent
- TEM
- effector memory T cell
- TIP DC
- TNF and iNOS producing DC
- TL
- thymic leukemia antigen
- Treg
- regulatory T cell
- TRM
- resident memory T cell
- TSLP
- thymic stromal lymphopoietin
AUTHORSHIP
Both authors contributed to writing the review.
REFERENCES
- 1. Iwasaki A. (2007) Mucosal dendritic cells. Annu. Rev. Immunol. 25, 381–418 [DOI] [PubMed] [Google Scholar]
- 2. Steinman R. M. (2012) Decisions about dendritic cells: past, present, and future. Annu. Rev. Immunol. 30, 1–22 [DOI] [PubMed] [Google Scholar]
- 3. Cerutti A., Puga I., Cols M. (2012) New helping friends for B cells. Eur. J. Immunol. 42, 1956–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Itano A. A., Jenkins M. K. (2003) Antigen presentation to naive CD4 T cells in the lymph node. Nat. Immunol. 4, 733–739 [DOI] [PubMed] [Google Scholar]
- 5. Geissmann F., Manz M. G., Jung S., Sieweke M. H., Merad M., Ley K. (2010) Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murray P. J., Wynn T. A. (2011) Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11, 723–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McGaha T. L., Chen Y., Ravishankar B., van Rooijen N., Karlsson M. C. (2011) Marginal zone macrophages suppress innate and adaptive immunity to apoptotic cells in the spleen. Blood 117, 5403–5412 [DOI] [PubMed] [Google Scholar]
- 8. Junt T., Moseman E. A., Iannacone M., Massberg S., Lang P. A., Boes M., Fink K., Henrickson S. E., Shayakhmetov D. M., Di Paolo N. C., van Rooijen N., Mempel T. R., Whelan S. P., von Andrian U. H. (2007) Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 450, 110–114 [DOI] [PubMed] [Google Scholar]
- 9. Shortman K., Liu Y. J. (2002) Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2, 151–161 [DOI] [PubMed] [Google Scholar]
- 10. Henri S., Vremec D., Kamath A., Waithman J., Williams S., Benoist C., Burnham K., Saeland S., Handman E., Shortman K. (2001) The dendritic cell populations of mouse lymph nodes. J. Immunol. 167, 741–748 [DOI] [PubMed] [Google Scholar]
- 11. Villadangos J. A., Schnorrer P. (2007) Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat. Rev. Immunol. 7, 543–555 [DOI] [PubMed] [Google Scholar]
- 12. Kamath A. T., Henri S., Battye F., Tough D. F., Shortman K. (2002) Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood 100, 1734–1741 [PubMed] [Google Scholar]
- 13. Geissmann F., Dieu-Nosjean M. C., Dezutter C., Valladeau J., Kayal S., Leborgne M., Brousse N., Saeland S., Davoust J. (2002) Accumulation of immature Langerhans cells in human lymph nodes draining chronically inflamed skin. J. Exp. Med. 196, 417–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Heusch M., Blocklet D., Egrise D., Hauquier B., Vermeersch M., Goldman S., Moser M. (2007) Bidirectional MHC molecule exchange between migratory and resident dendritic cells. J. Leukoc. Biol. 82, 861–868 [DOI] [PubMed] [Google Scholar]
- 15. Nestle F. O., Di Meglio P., Qin J. Z., Nickoloff B. J. (2009) Skin immune sentinels in health and disease. Nat. Rev. Immunol. 9, 679–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Allan R. S., Smith C. M., Belz G. T., van Lint A. L., Wakim L. M., Heath W. R., Carbone F. R. (2003) Epidermal viral immunity induced by CD8α+ dendritic cells but not by Langerhans cells. Science 301, 1925–1928 [DOI] [PubMed] [Google Scholar]
- 17. Ritter U., Meissner A., Scheidig C., Korner H. (2004) CD8 α- and Langerin-negative dendritic cells, but not Langerhans cells, act as principal antigen-presenting cells in leishmaniasis. Eur. J. Immunol. 34, 1542–1550 [DOI] [PubMed] [Google Scholar]
- 18. Brewig N., Kissenpfennig A., Malissen B., Veit A., Bickert T., Fleischer B., Mostbock S., Ritter U. (2009) Priming of CD8+ and CD4+ T cells in experimental leishmaniasis is initiated by different dendritic cell subtypes. J. Immunol. 182, 774–783 [DOI] [PubMed] [Google Scholar]
- 19. Bedoui S., Whitney P. G., Waithman J., Eidsmo L., Wakim L., Caminschi I., Allan R. S., Wojtasiak M., Shortman K., Carbone F. R., Brooks A. G., Heath W. R. (2009) Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat. Immunol. 10, 488–495 [DOI] [PubMed] [Google Scholar]
- 20. Igyarto B. Z., Haley K., Ortner D., Bobr A., Gerami-Nejad M., Edelson B. T., Zurawski S. M., Malissen B., Zurawski G., Berman J., Kaplan D. H. (2011) Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity 35, 260–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Henri S., Poulin L. F., Tamoutounour S., Ardouin L., Guilliams M., de Bovis B., Devilard E., Viret C., Azukizawa H., Kissenpfennig A., Malissen B. (2010) CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J. Exp. Med. 207, 189–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Romani N., Brunner P. M., Stingl G. (2012) Changing views of the role of Langerhans cells. J. Invest. Dermatol. 132, 872–881 [DOI] [PubMed] [Google Scholar]
- 23. Honda T., Nakajima S., Egawa G., Ogasawara K., Malissen B., Miyachi Y., Kabashima K. (2010) Compensatory role of Langerhans cells and langerin-positive dermal dendritic cells in the sensitization phase of murine contact hypersensitivity. J. Allergy Clin. Immunol. 125, 1154.e2–1156.e2 [DOI] [PubMed] [Google Scholar]
- 24. Noordegraaf M., Flacher V., Stoitzner P., Clausen B. E. (2010) Functional redundancy of Langerhans cells and Langerin+ dermal dendritic cells in contact hypersensitivity. J. Invest. Dermatol. 130, 2752–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mayerova D., Parke E. A., Bursch L. S., Odumade O. A., Hogquist K. A. (2004) Langerhans cells activate naive self-antigen-specific CD8 T cells in the steady state. Immunity 21, 391–400 [DOI] [PubMed] [Google Scholar]
- 26. Kautz-Neu K., Noordegraaf M., Dinges S., Bennett C. L., John D., Clausen B. E., von Stebut E. (2011) Langerhans cells are negative regulators of the anti-Leishmania response. J. Exp. Med. 208, 885–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaplan D. H., Jenison M. C., Saeland S., Shlomchik W. D., Shlomchik M. J. (2005) Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity 23, 611–620 [DOI] [PubMed] [Google Scholar]
- 28. Shklovskaya E., O'Sullivan B. J., Ng L. G., Roediger B., Thomas R., Weninger W., Fazekas de St Groth B. (2011) Langerhans cells are precommitted to immune tolerance induction. Proc. Natl. Acad. Sci. USA 108, 18049–18054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schwarz A., Noordegraaf M., Maeda A., Torii K., Clausen B. E., Schwarz T. (2010) Langerhans cells are required for UVR-induced immunosuppression. J. Invest. Dermatol. 130, 1419–1427 [DOI] [PubMed] [Google Scholar]
- 30. Rescigno M. (2010) Intestinal dendritic cells. Adv. Immunol. 107, 109–138 [DOI] [PubMed] [Google Scholar]
- 31. Coombes J. L., Powrie F. (2008) Dendritic cells in intestinal immune regulation. Nat. Rev. Immunol. 8, 435–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Flores-Langarica A., Meza-Perez S., Calderon-Amador J., Estrada-Garcia T., Macpherson G., Lebecque S., Saeland S., Steinman R. M., Flores-Romo L. (2005) Network of dendritic cells within the muscular layer of the mouse intestine. Proc. Natl. Acad. Sci. USA 102, 19039–19044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Monteleone I., Platt A. M., Jaensson E., Agace W. W., Mowat A. M. (2008) IL-10-dependent partial refractoriness to Toll-like receptor stimulation modulates gut mucosal dendritic cell function. Eur. J. Immunol. 38, 1533–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Iwasaki A., Kelsall B. L. (2001) Unique functions of CD11b+, CD8 α+, and double-negative Peyer's patch dendritic cells. J. Immunol. 166, 4884–4890 [DOI] [PubMed] [Google Scholar]
- 35. Johansson-Lindbom B., Svensson M., Wurbel M. A., Malissen B., Marquez G., Agace W. (2003) Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J. Exp. Med. 198, 963–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bogunovic M., Ginhoux F., Helft J., Shang L., Hashimoto D., Greter M., Liu K., Jakubzick C., Ingersoll M. A., Leboeuf M., Stanley E. R., Nussenzweig M., Lira S. A., Randolph G. J., Merad M. (2009) Origin of the lamina propria dendritic cell network. Immunity 31, 513–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johansson-Lindbom B., Svensson M., Pabst O., Palmqvist C., Marquez G., Forster R., Agace W. W. (2005) Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J. Exp. Med. 202, 1063–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jaensson E., Uronen-Hansson H., Pabst O., Eksteen B., Tian J., Coombes J. L., Berg P. L., Davidsson T., Powrie F., Johansson-Lindbom B., Agace W. W. (2008) Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J. Exp. Med. 205, 2139–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Svensson M., Johansson-Lindbom B., Zapata F., Jaensson E., Austenaa L. M., Blomhoff R., Agace W. W. (2008) Retinoic acid receptor signaling levels and antigen dose regulate gut homing receptor expression on CD8+ T cells. Mucosal Immunol. 1, 38–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Coombes J. L., Siddiqui K. R., Arancibia-Carcamo C. V., Hall J., Sun C. M., Belkaid Y., Powrie F. (2007) A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J. Exp. Med. 204, 1757–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schulz O., Jaensson E., Persson E. K., Liu X., Worbs T., Agace W. W., Pabst O. (2009) Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J. Exp. Med. 206, 3101–3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Niess J. H., Brand S., Gu X., Landsman L., Jung S., McCormick B. A., Vyas J. M., Boes M., Ploegh H. L., Fox J. G., Littman D. R., Reinecker H. C. (2005) CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307, 254–258 [DOI] [PubMed] [Google Scholar]
- 43. Farache J., Koren I., Milo I., Gurevich I., Kim K. W., Zigmond E., Furtado G. C., Lira S. A., Shakhar G. (2013) Luminal bacteria recruit CD103(+) dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity 38, 581–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Niess J. H., Adler G. (2010) Enteric flora expands gut lamina propria CX3CR1+ dendritic cells supporting inflammatory immune responses under normal and inflammatory conditions. J. Immunol. 184, 2026–2037 [DOI] [PubMed] [Google Scholar]
- 45. Smith P. D., Smythies L. E., Shen R., Greenwell-Wild T., Gliozzi M., Wahl S. M. (2011) Intestinal macrophages and response to microbial encroachment. Mucosal Immunol. 4, 31–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Smythies L. E., Sellers M., Clements R. H., Mosteller-Barnum M., Meng G., Benjamin W. H., Orenstein J. M., Smith P. D. (2005) Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J. Clin. Invest. 115, 66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Smith P. D., Smythies L. E., Mosteller-Barnum M., Sibley D. A., Russell M. W., Merger M., Sellers M. T., Orenstein J. M., Shimada T., Graham M. F., Kubagawa H. (2001) Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for LPS- and IgA-mediated activities. J. Immunol. 167, 2651–2656 [DOI] [PubMed] [Google Scholar]
- 48. Smythies L. E., Shen R., Bimczok D., Novak L., Clements R. H., Eckhoff D. E., Bouchard P., George M. D., Hu W. K., Dandekar S., Smith P. D. (2010) Inflammation anergy in human intestinal macrophages is due to Smad-induced IκBα expression and NF-κB inactivation. J. Biol. Chem. 285, 19593–19604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Plantinga M., Hammad H., Lambrecht B. N. (2010) Origin and functional specializations of DC subsets in the lung. Eur. J. Immunol. 40, 2112–2118 [DOI] [PubMed] [Google Scholar]
- 50. GeurtsvanKessel C. H., Willart M. A., van Rijt L. S., Muskens F., Kool M., Baas C., Thielemans K., Bennett C., Clausen B. E., Hoogsteden H. C., Osterhaus A. D., Rimmelzwaan G. F., Lambrecht B. N. (2008) Clearance of influenza virus from the lung depends on migratory langerin+CD11b− but not plasmacytoid dendritic cells. J. Exp. Med. 205, 1621–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Desch A. N., Randolph G. J., Murphy K., Gautier E. L., Kedl R. M., Lahoud M. H., Caminschi I., Shortman K., Henson P. M., Jakubzick C. V. (2011) CD103+ pulmonary dendritic cells preferentially acquire and present apoptotic cell-associated antigen. J. Exp. Med. 208, 1789–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Beaty S. R., Rose C. E., Jr., Sung S. S. (2007) Diverse and potent chemokine production by lung CD11bhigh dendritic cells in homeostasis and in allergic lung inflammation. J. Immunol. 178, 1882–1895 [DOI] [PubMed] [Google Scholar]
- 53. Del Rio M. L., Rodriguez-Barbosa J. I., Kremmer E., Forster R. (2007) CD103− and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. J. Immunol. 178, 6861–6866 [DOI] [PubMed] [Google Scholar]
- 54. Rimoldi M., Chieppa M., Salucci V., Avogadri F., Sonzogni A., Sampietro G. M., Nespoli A., Viale G., Allavena P., Rescigno M. (2005) Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat. Immunol. 6, 507–514 [DOI] [PubMed] [Google Scholar]
- 55. Smythies L. E., Maheshwari A., Clements R., Eckhoff D., Novak L., Vu H. L., Mosteller-Barnum L. M., Sellers M., Smith P. D. (2006) Mucosal IL-8 and TGF-β recruit blood monocytes: evidence for cross-talk between the lamina propria stroma and myeloid cells. J. Leukoc. Biol. 80, 492–499 [DOI] [PubMed] [Google Scholar]
- 56. Contractor N., Louten J., Kim L., Biron C. A., Kelsall B. L. (2007) Cutting edge: Peyer's patch plasmacytoid dendritic cells (pDCs) produce low levels of type I interferons: possible role for IL-10, TGFβ, and prostaglandin E2 in conditioning a unique mucosal pDC phenotype. J. Immunol. 179, 2690–2694 [DOI] [PubMed] [Google Scholar]
- 57. Zaph C., Troy A. E., Taylor B. C., Berman-Booty L. D., Guild K. J., Du Y., Yost E. A., Gruber A. D., May M. J., Greten F. R., Eckmann L., Karin M., Artis D. (2007) Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature 446, 552–556 [DOI] [PubMed] [Google Scholar]
- 58. Lee S. W., Park Y., Eun S. Y., Madireddi S., Cheroutre H., Croft M. (2012) Cutting edge: 4-1BB controls regulatory activity in dendritic cells through promoting optimal expression of retinal dehydrogenase. J. Immunol. 189, 2697–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Manicassamy S., Reizis B., Ravindran R., Nakaya H., Salazar-Gonzalez R. M., Wang Y. C., Pulendran B. (2010) Activation of β-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science 329, 849–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hayashi T., Beck L., Rossetto C., Gong X., Takikawa O., Takabayashi K., Broide D. H., Carson D. A., Raz E. (2004) Inhibition of experimental asthma by indoleamine 2,3-dioxygenase. J. Clin. Invest. 114, 270–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Snelgrove R. J., Goulding J., Didierlaurent A. M., Lyonga D., Vekaria S., Edwards L., Gwyer E., Sedgwick J. D., Barclay A. N., Hussell T. (2008) A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat. Immunol. 9, 1074–1083 [DOI] [PubMed] [Google Scholar]
- 62. Forster R., Davalos-Misslitz A. C., Rot A. (2008) CCR7 and its ligands: balancing immunity and tolerance. Nat. Rev. Immunol. 8, 362–371 [DOI] [PubMed] [Google Scholar]
- 63. Lanzavecchia A. (1999) Three signals and a master switch in the regulation of T-cell immunity. Cold Spring Harb. Symp. Quant. Biol. 64, 253–257 [DOI] [PubMed] [Google Scholar]
- 64. Sigmundsdottir H., Butcher E. C. (2008) Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat. Immunol. 9, 981–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kronenberg M., Gapin L. (2002) The unconventional lifestyle of NKT cells. Nat. Rev. Immunol. 2, 557–568 [DOI] [PubMed] [Google Scholar]
- 66. Kaufmann S. H. (1996) γ/δ and other unconventional T lymphocytes: what do they see and what do they do? Proc. Natl. Acad. Sci. USA 93, 2272–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sonnenberg G. F., Artis D. (2012) Innate lymphoid cell interactions with microbiota: implications for intestinal health and disease. Immunity 37, 601–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Guy-Grand D., Cerf-Bensussan N., Malissen B., Malassis-Seris M., Briottet C., Vassalli P. (1991) Two gut intraepithelial CD8+ lymphocyte populations with different T cell receptors: a role for the gut epithelium in T cell differentiation. J. Exp. Med. 173, 471–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rocha B., von Boehmer H., Guy-Grand D. (1992) Selection of intraepithelial lymphocytes with CD8 α/α co-receptors by self-antigen in the murine gut. Proc. Natl. Acad. Sci. USA 89, 5336–5340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cruz D., Sydora B. C., Hetzel K., Yakoub G., Kronenberg M., Cheroutre H. (1998) An opposite pattern of selection of a single T cell antigen receptor in the thymus and among intraepithelial lymphocytes. J. Exp. Med. 188, 255–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Leishman A. J., Gapin L., Capone M., Palmer E., MacDonald H. R., Kronenberg M., Cheroutre H. (2002) Precursors of functional MHC class I- or class II-restricted CD8αα(+) T cells are positively selected in the thymus by agonist self-peptides. Immunity 16, 355–364 [DOI] [PubMed] [Google Scholar]
- 72. Leishman A. J., Naidenko O. V., Attinger A., Koning F., Lena C. J., Xiong Y., Chang H. C., Reinherz E., Kronenberg M., Cheroutre H. (2001) T cell responses modulated through interaction between CD8αα and the nonclassical MHC class I molecule, TL. Science 294, 1936–1939 [DOI] [PubMed] [Google Scholar]
- 73. Masopust D., Jiang J., Shen H., Lefrancois L. (2001) Direct analysis of the dynamics of the intestinal mucosa CD8 T cell response to systemic virus infection. J. Immunol. 166, 2348–2356 [DOI] [PubMed] [Google Scholar]
- 74. Kim S. K., Reed D. S., Heath W. R., Carbone F., Lefrancois L. (1997) Activation and migration of CD8 T cells in the intestinal mucosa. J. Immunol. 159, 4295–4306 [PubMed] [Google Scholar]
- 75. Kim S. K., Schluns K. S., Lefrancois L. (1999) Induction and visualization of mucosal memory CD8 T cells following systemic virus infection. J. Immunol. 163, 4125–4132 [PubMed] [Google Scholar]
- 76. Kaech S. M., Wherry E. J., Ahmed R. (2002) Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2, 251–262 [DOI] [PubMed] [Google Scholar]
- 77. Gupta S., Gollapudi S. (2007) Effector memory CD8+ T cells are resistant to apoptosis. Ann. N. Y. Acad. Sci. 1109, 145–150 [DOI] [PubMed] [Google Scholar]
- 78. Grayson J. M., Harrington L. E., Lanier J. G., Wherry E. J., Ahmed R. (2002) Differential sensitivity of naive and memory CD8+ T cells to apoptosis in vivo. J. Immunol. 169, 3760–3770 [DOI] [PubMed] [Google Scholar]
- 79. Croft M., Bradley L. M., Swain S. L. (1994) Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J. Immunol. 152, 2675–2685 [PubMed] [Google Scholar]
- 80. Berg R. E., Crossley E., Murray S., Forman J. (2003) Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J. Exp. Med. 198, 1583–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Soudja S. M., Ruiz A. L., Marie J. C., Lauvau G. (2012) Inflammatory monocytes activate memory CD8(+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity 37, 549–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sallusto F., Geginat J., Lanzavecchia A. (2004) Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22, 745–763 [DOI] [PubMed] [Google Scholar]
- 83. Sallusto F., Lenig D., Forster R., Lipp M., Lanzavecchia A. (1999) Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 [DOI] [PubMed] [Google Scholar]
- 84. Pepper M., Jenkins M. K. (2011) Origins of CD4(+) effector and central memory T cells. Nat. Immunol. 12, 467–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ariotti S., Haanen J. B., Schumacher T. N. (2012) Behavior and function of tissue-resident memory T cells. Adv. Immunol. 114, 203–216 [DOI] [PubMed] [Google Scholar]
- 86. Gebhardt T., Wakim L. M., Eidsmo L., Reading P. C., Heath W. R., Carbone F. R. (2009) Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 10, 524–530 [DOI] [PubMed] [Google Scholar]
- 87. Wakim L. M., Woodward-Davis A., Bevan M. J. (2010) Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc. Natl. Acad. Sci. USA 107, 17872–17879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Teijaro J. R., Turner D., Pham Q., Wherry E. J., Lefrancois L., Farber D. L. (2011) Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol. 187, 5510–5514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wakim L. M., Gupta N., Mintern J. D., Villadangos J. A. (2013) Enhanced survival of lung tissue-resident memory CD8(+) T cells during infection with influenza virus due to selective expression of IFITM3. Nat. Immunol. 14, 238–245 [DOI] [PubMed] [Google Scholar]
- 90. Purwar R., Campbell J., Murphy G., Richards W. G., Clark R. A., Kupper T. S. (2011) Resident memory T cells (T(RM)) are abundant in human lung: diversity, function, and antigen specificity. PLoS One 6, e16245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hofmann M., Pircher H. (2011) E-Cadherin promotes accumulation of a unique memory CD8 T-cell population in murine salivary glands. Proc. Natl. Acad. Sci. USA 108, 16741–16746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Masopust D., Choo D., Vezys V., Wherry E. J., Duraiswamy J., Akondy R., Wang J., Casey K. A., Barber D. L., Kawamura K. S., Fraser K. A., Webby R. J., Brinkmann V., Butcher E. C., Newell K. A., Ahmed R. (2010) Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 207, 553–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wakim L. M., Woodward-Davis A., Liu R., Hu Y., Villadangos J., Smyth G., Bevan M. J. (2012) The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J. Immunol. 189, 3462–3471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gebhardt T., Whitney P. G., Zaid A., Mackay L. K., Brooks A. G., Heath W. R., Carbone F. R., Mueller S. N. (2011) Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 477, 216–219 [DOI] [PubMed] [Google Scholar]
- 95. Shin H., Iwasaki A. (2012) A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 491, 463–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Anacker R. L., Barclay W. R., Brehmer W., Goode G., List R. H., Ribi E., Tarmina D. F. (1969) Effectiveness of cell walls of Mycobacterium bovis strain BCG administered by various routes and in different adjuvants in protecting mice against airborne infection with Mycobacterium tuberculosis strain H37Rv. Am. Rev. Respir. Dis. 99, 242–248 [DOI] [PubMed] [Google Scholar]
- 97. Larson C. L., Wicht W. C. (1962) Studies of resistance to experimental tuberculosis in mice vaccinated with living attenuated tubercle bacilli and challenged with virulent organisms. Am. Rev. Respir. Dis. 85, 833–846 [DOI] [PubMed] [Google Scholar]
- 98. Lefford M. J., Warner S., Amell L. (1979) Listeria pneumonitis: influence of route of immunization on resistance to airborne infection. Infect. Immun. 25, 672–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Pepper M., Linehan J. L., Pagan A. J., Zell T., Dileepan T., Cleary P. P., Jenkins M. K. (2010) Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat. Immunol. 11, 83–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Dileepan T., Linehan J. L., Moon J. J., Pepper M., Jenkins M. K., Cleary P. P. (2011) Robust antigen specific Th17 T cell response to group A Streptococcus is dependent on IL-6 and intranasal route of infection. PLoS Pathog. 7, e1002252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hue S., Ahern P., Buonocore S., Kullberg M. C., Cua D. J., McKenzie B. S., Powrie F., Maloy K. J. (2006) Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J. Exp. Med. 203, 2473–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Woolard M. D., Hensley L. L., Kawula T. H., Frelinger J. A. (2008) Respiratory Francisella tularensis live vaccine strain infection induces Th17 cells and prostaglandin E2, which inhibits generation of γ interferon-positive T cells. Infect. Immun. 76, 2651–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. DePaolo R. W., Kamdar K., Khakpour S., Sugiura Y., Wang W., Jabri B. (2012) A specific role for TLR1 in protective T(H)17 immunity during mucosal infection. J. Exp. Med. 209, 1437–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Campbell D. J., Butcher E. C. (2002) Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. J. Exp. Med. 195, 135–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Berlin C., Berg E. L., Briskin M. J., Andrew D. P., Kilshaw P. J., Holzmann B., Weissman I. L., Hamann A., Butcher E. C. (1993) α 4 β 7 Integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74, 185–195 [DOI] [PubMed] [Google Scholar]
- 106. Campbell D. J., Butcher E. C. (2002) Intestinal attraction: CCL25 functions in effector lymphocyte recruitment to the small intestine. J. Clin. Invest. 110, 1079–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Tietz W., Allemand Y., Borges E., von Laer D., Hallmann R., Vestweber D., Hamann A. (1998) CD4+ T cells migrate into inflamed skin only if they express ligands for E- and P-selectin. J. Immunol. 161, 963–970 [PubMed] [Google Scholar]
- 108. Reiss Y., Proudfoot A. E., Power C. A., Campbell J. J., Butcher E. C. (2001) CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J. Exp. Med. 194, 1541–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Stagg A. J., Kamm M. A., Knight S. C. (2002) Intestinal dendritic cells increase T cell expression of α4β7 integrin. Eur. J. Immunol. 32, 1445–1454 [DOI] [PubMed] [Google Scholar]
- 110. Dudda J. C., Lembo A., Bachtanian E., Huehn J., Siewert C., Hamann A., Kremmer E., Forster R., Martin S. F. (2005) Dendritic cells govern induction and reprogramming of polarized tissue-selective homing receptor patterns of T cells: important roles for soluble factors and tissue microenvironments. Eur. J. Immunol. 35, 1056–1065 [DOI] [PubMed] [Google Scholar]
- 111. Mora J. R., Cheng G., Picarella D., Briskin M., Buchanan N., von Andrian U. H. (2005) Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J. Exp. Med. 201, 303–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Iwata M., Hirakiyama A., Eshima Y., Kagechika H., Kato C., Song S. Y. (2004) Retinoic acid imprints gut-homing specificity on T cells. Immunity 21, 527–538 [DOI] [PubMed] [Google Scholar]
- 113. Sigmundsdottir H., Pan J., Debes G. F., Alt C., Habtezion A., Soler D., Butcher E. C. (2007) DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat. Immunol. 8, 285–293 [DOI] [PubMed] [Google Scholar]
- 114. Hu W., Troutman T. D., Edukulla R., Pasare C. (2011) Priming microenvironments dictate cytokine requirements for T helper 17 cell lineage commitment. Immunity 35, 1010–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Picker L. J., Treer J. R., Ferguson-Darnell B., Collins P. A., Bergstresser P. R., Terstappen L. W. (1993) Control of lymphocyte recirculation in man. II. Differential regulation of the cutaneous lymphocyte-associated antigen, a tissue-selective homing receptor for skin-homing T cells. J. Immunol. 150, 1122–1136 [PubMed] [Google Scholar]
- 116. Kim C. H., Nagata K., Butcher E. C. (2003) Dendritic cells support sequential reprogramming of chemoattractant receptor profiles during naive to effector T cell differentiation. J. Immunol. 171, 152–158 [DOI] [PubMed] [Google Scholar]
- 117. Kim C. H., Rott L., Kunkel E. J., Genovese M. C., Andrew D. P., Wu L., Butcher E. C. (2001) Rules of chemokine receptor association with T cell polarization in vivo. J. Clin. Invest. 108, 1331–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Horwitz D. A., Zheng S. G., Gray J. D. (2008) Natural and TGF-β-induced Foxp3(+)CD4(+) CD25(+) regulatory T cells are not mirror images of each other. Trends Immunol. 29, 429–435 [DOI] [PubMed] [Google Scholar]
- 119. Denning T. L., Wang Y. C., Patel S. R., Williams I. R., Pulendran B. (2007) Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat. Immunol. 8, 1086–1094 [DOI] [PubMed] [Google Scholar]
- 120. Iliev I. D., Spadoni I., Mileti E., Matteoli G., Sonzogni A., Sampietro G. M., Foschi D., Caprioli F., Viale G., Rescigno M. (2009) Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut 58, 1481–1489 [DOI] [PubMed] [Google Scholar]
- 121. Iliev I. D., Mileti E., Matteoli G., Chieppa M., Rescigno M. (2009) Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunol. 2, 340–350 [DOI] [PubMed] [Google Scholar]
- 122. Saurer L., McCullough K. C., Summerfield A. (2007) In vitro induction of mucosa-type dendritic cells by all-trans retinoic acid. J. Immunol. 179, 3504–3514 [DOI] [PubMed] [Google Scholar]
- 123. Lathrop S. K., Bloom S. M., Rao S. M., Nutsch K., Lio C. W., Santacruz N., Peterson D. A., Stappenbeck T. S., Hsieh C. S. (2011) Peripheral education of the immune system by colonic commensal microbiota. Nature 478, 250–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bilate A. M., Lafaille J. J. (2012) Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu. Rev. Immunol. 30, 733–758 [DOI] [PubMed] [Google Scholar]
- 125. Travis M. A., Reizis B., Melton A. C., Masteller E., Tang Q., Proctor J. M., Wang Y., Bernstein X., Huang X., Reichardt L. F., Bluestone J. A., Sheppard D. (2007) Loss of integrin α(v)β8 on dendritic cells causes autoimmunity and colitis in mice. Nature 449, 361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Josefowicz S. Z., Niec R. E., Kim H. Y., Treuting P., Chinen T., Zheng Y., Umetsu D. T., Rudensky A. Y. (2012) Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature 482, 395–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Taylor B. C., Zaph C., Troy A. E., Du Y., Guild K. J., Comeau M. R., Artis D. (2009) TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J. Exp. Med. 206, 655–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Spadoni I., Iliev I. D., Rossi G., Rescigno M. (2012) Dendritic cells produce TSLP that limits the differentiation of Th17 cells, fosters Treg development, and protects against colitis. Mucosal Immunol. 5, 184–193 [DOI] [PubMed] [Google Scholar]
- 129. Zaph C., Du Y., Saenz S. A., Nair M. G., Perrigoue J. G., Taylor B. C., Troy A. E., Kobuley D. E., Kastelein R. A., Cua D. J., Yu Y., Artis D. (2008) Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J. Exp. Med. 205, 2191–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Weber D. A., Attinger A., Kemball C. C., Wigal J. L., Pohl J., Xiong Y., Reinherz E. L., Cheroutre H., Kronenberg M., Jensen P. E. (2002) Peptide-independent folding and CD8 α α binding by the nonclassical class I molecule, thymic leukemia antigen. J. Immunol. 169, 5708–5714 [DOI] [PubMed] [Google Scholar]
- 131. Siddiqui K. R., Laffont S., Powrie F. (2010) E-Cadherin marks a subset of inflammatory dendritic cells that promote T cell-mediated colitis. Immunity 32, 557–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Laffont S., Siddiqui K. R., Powrie F. (2010) Intestinal inflammation abrogates the tolerogenic properties of MLN CD103+ dendritic cells. Eur. J. Immunol. 40, 1877–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. DePaolo R. W., Abadie V., Tang F., Fehlner-Peach H., Hall J. A., Wang W., Marietta E. V., Kasarda D. D., Waldmann T. A., Murray J. A., Semrad C., Kupfer S. S., Belkaid Y., Guandalini S., Jabri B. (2011) Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature 471, 220–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. De Vries V. C., Pino-Lagos K., Nowak E. C., Bennett K. A., Oliva C., Noelle R. J. (2011) Mast cells condition dendritic cells to mediate allograft tolerance. Immunity 35, 550–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Griffin M. D., Lutz W. H., Phan V. A., Bachman L. A., McKean D. J., Kumar R. (2000) Potent inhibition of dendritic cell differentiation and maturation by vitamin D analogs. Biochem. Biophys. Res. Commun. 270, 701–708 [DOI] [PubMed] [Google Scholar]
- 136. Piemonti L., Monti P., Sironi M., Fraticelli P., Leone B. E., Dal Cin E., Allavena P., Di Carlo V. (2000) Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J. Immunol. 164, 4443–4451 [DOI] [PubMed] [Google Scholar]
- 137. Penna G., Adorini L. (2000) 1 α,25-Dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J. Immunol. 164, 2405–2411 [DOI] [PubMed] [Google Scholar]
- 138. O'Garra A., Barrat F. J., Castro A. G., Vicari A., Hawrylowicz C. (2008) Strategies for use of IL-10 or its antagonists in human disease. Immunol. Rev. 223, 114–131 [DOI] [PubMed] [Google Scholar]
- 139. Diehl G. E., Longman R. S., Zhang J. X., Breart B., Galan C., Cuesta A., Schwab S. R., Littman D. R. (2013) Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature 494, 116–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Atarashi K., Tanoue T., Shima T., Imaoka A., Kuwahara T., Momose Y., Cheng G., Yamasaki S., Saito T., Ohba Y., Taniguchi T., Takeda K., Hori S., Ivanov I. I., Umesaki Y., Itoh K., Honda K. (2011) Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Round J. L., Lee S. M., Li J., Tran G., Jabri B., Chatila T. A., Mazmanian S. K. (2011) The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332, 974–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Mileti E., Matteoli G., Iliev I. D., Rescigno M. (2009) Comparison of the immunomodulatory properties of three probiotic strains of Lactobacilli using complex culture systems: prediction for in vivo efficacy. PLoS One 4, e7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Atarashi K., Nishimura J., Shima T., Umesaki Y., Yamamoto M., Onoue M., Yagita H., Ishii N., Evans R., Honda K., Takeda K. (2008) ATP drives lamina propria T(H)17 cell differentiation. Nature 455, 808–812 [DOI] [PubMed] [Google Scholar]
- 144. Ivanov I. I., Atarashi K., Manel N., Brodie E. L., Shima T., Karaoz U., Wei D., Goldfarb K. C., Santee C. A., Lynch S. V., Tanoue T., Imaoka A., Itoh K., Takeda K., Umesaki Y., Honda K., Littman D. R. (2009) Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Hall J. A., Bouladoux N., Sun C. M., Wohlfert E. A., Blank R. B., Zhu Q., Grigg M. E., Berzofsky J. A., Belkaid Y. (2008) Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity 29, 637–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Naik S., Bouladoux N., Wilhelm C., Molloy M. J., Salcedo R., Kastenmuller W., Deming C., Quinones M., Koo L., Conlan S., Spencer S., Hall J. A., Dzutsev A., Kong H., Campbell D. J., Trinchieri G., Segre J. A., Belkaid Y. (2012) Compartmentalized control of skin immunity by resident commensals. Science 337, 1115–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Gardby E., Wrammert J., Schon K., Ekman L., Leanderson T., Lycke N. (2003) Strong differential regulation of serum and mucosal IgA responses as revealed in CD28-deficient mice using cholera toxin adjuvant. J. Immunol. 170, 55–63 [DOI] [PubMed] [Google Scholar]
- 148. Cebra J. J., Periwal S. B., Lee G., Lee F., Shroff K. E. (1998) Development and maintenance of the gut-associated lymphoid tissue (GALT): the roles of enteric bacteria and viruses. Dev. Immunol. 6, 13–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Sun C. M., Hall J. A., Blank R. B., Bouladoux N., Oukka M., Mora J. R., Belkaid Y. (2007) Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 204, 1775–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Cazac B. B., Roes J. (2000) TGF-β receptor controls B cell responsiveness and induction of IgA in vivo. Immunity 13, 443–451 [DOI] [PubMed] [Google Scholar]
- 151. Defrance T., Vanbervliet B., Briere F., Durand I., Rousset F., Banchereau J. (1992) Interleukin 10 and transforming growth factor β cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J. Exp. Med. 175, 671–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Tsuji M., Komatsu N., Kawamoto S., Suzuki K., Kanagawa O., Honjo T., Hori S., Fagarasan S. (2009) Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science 323, 1488–1492 [DOI] [PubMed] [Google Scholar]
- 153. Tezuka H., Abe Y., Iwata M., Takeuchi H., Ishikawa H., Matsushita M., Shiohara T., Akira S., Ohteki T. (2007) Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature 448, 929–933 [DOI] [PubMed] [Google Scholar]
- 154. Suzuki K., Maruya M., Kawamoto S., Sitnik K., Kitamura H., Agace W. W., Fagarasan S. (2010) The sensing of environmental stimuli by follicular dendritic cells promotes immunoglobulin A generation in the gut. Immunity 33, 71–83 [DOI] [PubMed] [Google Scholar]
- 155. Tsuji M., Suzuki K., Kitamura H., Maruya M., Kinoshita K., Ivanov I. I., Itoh K., Littman D. R., Fagarasan S. (2008) Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity 29, 261–271 [DOI] [PubMed] [Google Scholar]
- 156. Uematsu S., Fujimoto K., Jang M. H., Yang B. G., Jung Y. J., Nishiyama M., Sato S., Tsujimura T., Yamamoto M., Yokota Y., Kiyono H., Miyasaka M., Ishii K. J., Akira S. (2008) Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat. Immunol. 9, 769–776 [DOI] [PubMed] [Google Scholar]
- 157. Bergqvist P., Stensson A., Lycke N. Y., Bemark M. (2010) T cell-independent IgA class switch recombination is restricted to the GALT and occurs prior to manifest germinal center formation. J. Immunol. 184, 3545–3553 [DOI] [PubMed] [Google Scholar]
- 158. Barone F., Patel P., Sanderson J. D., Spencer J. (2009) Gut-associated lymphoid tissue contains the molecular machinery to support T-cell-dependent and T-cell-independent class switch recombination. Mucosal Immunol. 2, 495–503 [DOI] [PubMed] [Google Scholar]
- 159. Tezuka H., Abe Y., Asano J., Sato T., Liu J., Iwata M., Ohteki T. (2011) Prominent role for plasmacytoid dendritic cells in mucosal T cell-independent IgA induction. Immunity 34, 247–257 [DOI] [PubMed] [Google Scholar]
- 160. He B., Xu W., Santini P. A., Polydorides A. D., Chiu A., Estrella J., Shan M., Chadburn A., Villanacci V., Plebani A., Knowles D. M., Rescigno M., Cerutti A. (2007) Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity 26, 812–826 [DOI] [PubMed] [Google Scholar]
- 161. Xu W., He B., Chiu A., Chadburn A., Shan M., Buldys M., Ding A., Knowles D. M., Santini P. A., Cerutti A. (2007) Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat. Immunol. 8, 294–303 [DOI] [PubMed] [Google Scholar]
- 162. Litinskiy M. B., Nardelli B., Hilbert D. M., He B., Schaffer A., Casali P., Cerutti A. (2002) DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 3, 822–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. He B., Santamaria R., Xu W., Cols M., Chen K., Puga I., Shan M., Xiong H., Bussel J. B., Chiu A., Puel A., Reichenbach J., Marodi L., Doffinger R., Vasconcelos J., Issekutz A., Krause J., Davies G., Li X., Grimbacher B., Plebani A., Meffre E., Picard C., Cunningham-Rundles C., Casanova J. L., Cerutti A. (2010) The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat. Immunol. 11, 836–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Raz E. (2007) Organ-specific regulation of innate immunity. Nat. Immunol. 8, 3–4 [DOI] [PubMed] [Google Scholar]
- 165. Blander J. M., Sander L. E. (2012) Beyond pattern recognition: five immune checkpoints for scaling the microbial threat. Nat. Rev. Immunol. 12, 215–225 [DOI] [PubMed] [Google Scholar]


