Abstract
Salmonella enterica serovar Typhimurium is a flagellated bacterium and one of the leading causes of gastroenteritis in humans. Bacterial flagellin is required for motility and also a prime target of the innate immune system. Innate immune recognition of flagellin is mediated by at least two independent pathways, TLR5 and Naip5-Naip6/NlrC4/Caspase-1. The functional significance of each of the two independent flagellin recognition systems for host defense against wild type Salmonella infection is complex, and innate immune detection of flagellin contributes to both protection and susceptibility. We hypothesized that efficient modulation of flagellin expression in vivo permits Salmonella to evade innate immune detection and limit the functional role of flagellin-specific host innate defenses. To test this hypothesis, we used Salmonella deficient in the anti-sigma factor flgM, which overproduce flagella and are attenuated in vivo. In this study we demonstrate that flagellin recognition by the innate immune system is responsible for the attenuation of flgM− S. Typhimurium, and dissect the contribution of each flagellin recognition pathway to bacterial clearance and inflammation. We demonstrate that caspase-1 controls mucosal and systemic infection of flgM− S. Typhimurium, and also limits intestinal inflammation and injury. In contrast, TLR5 paradoxically promotes bacterial colonization in the cecum and systemic infection, but attenuates intestinal inflammation. Our results indicate that Salmonella evasion of caspase-1 dependent flagellin recognition is critical for establishing infection and that evasion of TLR5 and caspase-1 dependent flagellin recognition helps Salmonella induce intestinal inflammation and establish a niche in the inflamed gut.
Introduction
Innate immune recognition of Salmonella is mediated by evolutionarily conserved receptors [pattern recognition receptors (PRRs)] that are capable of sensing conserved bacterial structures [pathogen associated molecular patterns (PAMPs)] that promote inflammatory and immune responses [1]. PRRs recognize a wide range of microbial ligands such as bacterial DNA, cell wall components (peptidoglycan and LPS) and flagellin. In this study, we focus on Salmonella flagellin recognition by the innate immune system. Bacterial flagellin has been studied for decades due to its importance in motility. Flagellin based motility and chemotaxis are important for the induction of acute colitis, and to compete with other microbiota for nutrients in the inflamed gut [2]; [3]. Flagellin is also a target of the innate immune system. There are at least two flagellin recognition pathways in mammals: i) cytosolic flagellin is detected by Naip5-Naip6/NlrC4/Caspase-1 and ii) extracellular flagellin is detected by Toll-like receptor 5 (TLR5) [4]–[11].
Mice detect a conserved site in the carboxy-terminus of flagellin using the intracellular receptors Naip5 and Naip6, leading to Naip5/6 association with Nlrc4 and the activation of caspase-1 [4]–[6], [8], [9]. Nlrc4 contains a caspase activating region domain (CARD) and can associate with caspase-1 in the absence of Asc, promoting caspase-1 autocleavage and cell death [4], [12]. Association of the Nlrc4 inflammasome with Asc leads to efficient processing and secretion of IL-1B and IL-18 [13]. Caspase-1 protects mice against oral infection with Salmonella, which is dependent on IL-1B and IL-18 production and mediated by the Nlrc4 and Nlrp3 inflammasomes [13]–[15]. The Nlrc4 inflammasome also controls intraperitoneal infection of mice from Salmonella that overexpress flagellin, where Nlrc4–mediated protection is dependent on pyroptosis and independent of IL-1B and IL-18 [16]. The relevance of caspase-1 dependent flagellin detection during Salmonella-induced enterocolitis has not been fully elucidated.
TLR5 recognizes a conserved surface in the D1 domain of monomeric flagellin, and activates NF-kB dependent signaling in epithelial and immune cells [7], [10], [11], [17], [18]. TLR5 stimulation has been associated with release of inflammatory mediators, such as CCL20, a strong DC chemoattractant; IL-6 and IL-12/IL23p40; Reg3γ, an antimicrobial peptide; IL-8-like chemokines; and IL-10, an immunosuppresor [19]–[22]. Alexopoulo and colleagues found that TLR5 protected mice against Salmonella infection, but that this function was largely masked by TLR4 [23]. Akira and colleagues demonstrated that TLR5 recognition of flagellin promoted Salmonella infection and systemic spread [24]. Gewirtz and colleagues demonstrated that loss of TLR5 led to an increased basal inflammatory state and non-specific resistance to Salmonella infection, suggesting that TLR5 interactions with the intestinal microbiota may promote Salmonella infection [25], [26]. Thus the role of TLR5 in mucosal Salmonella infection is complex, and involves both homeostatic interactions with gut commensals that influence microbial composition and immune status, and detection of flagellated pathogens.
Flagellar assembly is under complex and tight regulation, with multiple signals that converge on the operon for the master regulator flhDC, as well as additional transcriptional, translational and post-translational mechanisms that control the expression of class II and III genes, and the assembly of the flagellum [27]. FlgM inhibits FliA, the sigma factor required for the transcription of class III flagellar genes [27]. Because flagellar assembly is costly, complex regulation may help conserve energy for growth in certain environmental conditions [28], [29]. Salmonella selectively represses flagellin expression in systemic sites during infection, suggesting that active evasion of immune surveillance may also be critical for Salmonella to establish systemic infection [2], [30], [31]. During infection Salmonella has several mechanisms to sense the host environment and regulate gene expression. Some of these sensors, such as PhoPQ and ClpXP, down regulate flagellin synthesis [32]–[34]. Functional FlgM is also required to silence flagellin expression in vivo, and bacteria with deletions in flgM are attenuated during infection of mice in a flagellin-dependent manner [35]. We hypothesized that FlgM-dependent flagellin silencing is critical for Salmonella to evade innate immune detection, and that either TLR5 or caspase-1 are responsible for the attenuation of flgM− Salmonella.
Our studies demonstrate that TLR5 and caspase-1 have modest roles in host defense against wild type Salmonella infection, which is largely due to efficient FlgM-dependent silencing of flagellin expression. When flagellin production is dysregulated by deletion of flgM, TLR5 paradoxically limits cecal inflammation and promotes cecal colonization, but does not alter systemic dissemination. In contrast, flgM− Salmonella are efficiently cleared by the innate immune system in a caspase-1 dependent manner. In addition, caspase-1 is critical for limiting mucosal inflammation, and modulating the production of several cytokines and chemokines that may play cytoprotective roles in Salmonella-induced enterocolitis. Thus caspase-1 has ideal properties for innate immune defense at mucosal surfaces, and simultaneously limits bacterial dissemination and mucosal inflammation.
Methods
Ethics Statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All protocols were approved by the Institutional. Animal Care and Use Committee of the University of Washington (protocol: 4031-01, Macrophage Biology).
Bacterial Strains
The experiments were performed using wildtype Salmonella enterica serovar Typhimurium strain SL1344 (WT) (from Brad Cookson, University of Washington). The flagellin-deficient (fliC−/fljB−) strain in this same background was also a gift from Brad Cookson (University of Washington). A flgM deletion mutant was made in SL1344 using lambda-red technology [36]. The deletion was confirmed by PCR, and also transferred to the fliC−/fljB− mutant using P22 phage [37]. The deletion of flgM was once again confirmed by PCR. A constitutive GFP expressing plasmid (pDW5: ptetA::gfp; gfp downstream of tetA promoter in pBR322); reference PMID: 16803592) was introduced in Salmonella for fluorescent microscopy studies, the plasmid was a gift from Brad Cookson (University of Washington). Bacteria were grown in Luria broth at 37°C with aeration.
Mouse Infection
WT C57BL/6J (B6) mice were purchased from Jackson Labs and housed or bred in our facilities at the University of Washington. Caspase 1−/− [38] and Toll-like receptor 5 deficient mice (TLR5−/−) [24] on a B6 background were bred in our specific pathogen free (SPF) animal facilities. Animals were housed individually or in groups of up to five animals under standard barrier conditions in individually ventilated cages. 8–14 weeks old mice were used for infections throughout this study. One day before infection, food was withdrawn 4 h before oral gavage with 20 mg of streptomycin in 0.1 ml of PBS [39]. Afterwards, animals were supplied food ad libitum. At 20 h after streptomycin treatment, food was withdrawn again for 4 h before the mice were orally infected with 1000 CFU S. Typhimurium (delivered in 0.1 ml of PBS by gavage) or sterile PBS (control). The inoculum containing Salmonella was prepared by back-diluting an overnight culture 1∶50 in LB +50 µg/ml of streptomycin. After 4 hours, the concentration of bacteria was measured and diluted in cold PBS to a coentration of 1×104 CFU/ml, and CFU of the inoculum was verified by plating on LB agar plates with 50 µg/ml streptomycin. Food was replaced immediately after gavage. Five days post-infection, mice were sacrificed by CO2 asphyxiation, and blood and tissue (intestine, mesenteric lymph node, spleen, and liver) were promptly removed. Tissue samples from cecum and spleen were stored at −80°C for RNA extraction. Additional samples from all tissues were fixed in 10% formaldehyde, or snap frozen in OCT medium for histopathological analysis. For some infections, cecal samples were also placed in Karnovsky’ fixative for ultrastructural studies. Bacterial burden was assessed by weighing and homogenizing the tissues in PBS with 0.025% Triton X-100, and plating dilutions of the samples on MacConkey agar plates with streptomycin (50 ug/ml). For the homogenizing step, the ceca were scraped and blotted to remove fecal content.
Competitive Index Infection
The procedure for competitive index experiments followed the mouse infection protocol described above with the following exceptions: 1) the inoculum prepared contained equal numbers (1000 CFU) of WT and flgM− Salmonella, the inoculum was verified by plating bacteria on antibiotic specific LB plates (flgM− Salmonella were kanamycin and streptomycin resistant (kanr-strr) and wildtype Salmonella were streptomycin resistant only (kans-strr); 2) dilutions of the homogenized tissues (liver, spleen, MLN, and cecum) were plated on both kanamycin/streptomycin (50 µg/ml each) and streptomycin (50 µg/ml) MacConkey agar plates; and 3) the competitive index (C.I.) of flgM−/WT Salmonella was determined by the following equation:
C.I. = CFU kanr−strr/(CFU kans−strr − CFU kanr−strr).
Quantitative Histologic Assessment of Inflammation in Cecum, Small Intestine, Liver, Spleen and MLN
The formalin-fixed tissue was embedded in paraffin using standard protocols. 4 µm thick sections were stained with hematoxylin and eosin using standard procedures. A blinded pathologist (KDS) examined the slides and scored them according to the following criteria. Scores were assigned for changes to the cecum as follows: submucosal expansion (S) - 0 = no significant change, 1 = <25% of the wall, 2 = 25–50% of the wall, 3 = >50% of the wall; mucosal neutrophilic infiltrate (M) - 0 = no significant infiltrate, 1 = mild neutrophilic inflammation, 2 = moderate neutrophilic inflammation, 3 = severe neutrophilic inflammation; lymphoplasmacytosis (L) - 0 = no significant infiltrate, 1 = focal infiltrates (mild), 2 = multifocal infiltrates (moderate), 3 = extensive infiltrates involving mucosa and submucosa (severe); goblet cells (G) - 0 = >28/HPF, 1 = 11–28/HPF, 2 = 1–10/HPF, 3 = <1/HPF; epithelial integrity (E) - 0 = no significant change, 1 = desquamation (notable shedding of epithelial cells into the lumen), 2 = erosion (loss of epithelium with retention of architecture), 3 = ulceration (destruction of lamina propria).
Western Blot
Single colonies were grown overnight at 37°C with constant shaking, back diluted the next day 1∶50 and grown under similar conditions for 3 hrs. Equal numbers of cells were pelleted, the pellets and the supernatants were separated by SDS-PAGE, and transferred to Immobilon-P filters (Source, San Diego, CA). Filters were blocked with 5% non-fat milk, and Antibodies specific for Salmonella flagellin were used to detect the protein on the blot. The primary anti-FliC antibody was a monoclonal purified anti-FliC (Biolegend) at a concentration of 1∶1000 diluted in PBS (Invitrogen) plus 1% BSA (SIGMA). The secondary antibody was a peroxidase-conjugated affinipure Goat anti-mouse IgG (H+L) (Jackson laboratories) at a concentration of 1∶1000 diluted in PBS plus 1% BSA.
Motility Assay
Motility was tested by using motility plates (LB with 0.3% agar). The radius of growth was measured at different time points during culture at 37°C.
Macrophage Cytotoxicity Assay
Thioglycollate elicited peritoneal macrophages were plated in a 96-well plate at a concentration of 5×105 macrophages/well in RPMI 1640 medium with L-glutamine, 10% fetal bovine serum. S. Typhimurium was grown overnight in LB and backdiluted the next day 1∶50 in LB medium and grown for 3–4 hrs. The bacteria was centrifuged (1000 rpm, 5 minutes) and the pellet resuspended to the final desired concentration of bacteria/ml. Macrophages were infected with the desired multiplicity of infection, centrifuged at 250×g for 5 minutes (to ensure infection of macrophages), and the infection was allowed to progress for an hour. Gentamicin (50 ug/ml) was added after an hour to kill extracellular bacteria. After an additional hour the supernatants were removed, and cytotoxicity was measured using Cytotox 96 kit (Promega).
Real-time PCR Analysis of Gene Expression in Cecum
Tissue was collected immediately post-euthanization and stored frozen at −80°C. The tissue was later homogenized in Trizol reagent (Invitrogen, Carlsbad, CA), and total RNA was isolated according to manufacturer’s procedure. Genomic DNA was removed with DNA-free ™ (Ambion, Austin, TX), and cDNA was synthesized from 5 ug total RNA using Superscript III (Invitrogen, Carlsbad, CA). Multiplex real-time PCR was performed using primer probes sets for CCL20, CSCL1, CXCL2, IFNG, IL-12A, IL-12B, IL-17, IL-1B, IL-22, IL-23A, IL27, IL-6, LCN2, RG3G, TNF (all FAM labeled); the GAPDH control (VIC-labeled) was included in each reaction to normalize results. All reagents were from Applied Biosystems, and samples were analyzed on an ABI Prism 7900 using the fast protocol and reagents.
Measurements of Serum Cytokines by ELISA
Blood was collected from euthanized animals by cardiac puncture, and the serum was collected, centrifuged at 7000 g for 10 minutes and frozen at −20°C. ELISAs for TNF-α, IL-12, IL-6 and IL-1B were performed on serum samples according to manufacturer’s protocol (R&D systems, Minneapolis, MN).
Fluorescent Staining
Frozen tissue blocks collected from mice infected with Salmonella containing a GFP plasmid were cut in 4 µm sections using a cryostat. Sections were fixed using acetone (-20°C, Fisher Chemicals, Hanover Park, Il), washed with PBS (4°C) and blocked with blocking buster™ (Innovex Bioscience, Redmond, CA) for 10 minutes at room temperature. The sections were washed with cold PBS and exposed to the F4/80 antibody (Rat anti-mouse, Serotec Inc, Raleigh, NC; diluted in PBS +1% BSA 1∶200) or TROMA-1 antibody (Rat anti-mouse, Developmental Studies Hybridoma Bank at the University of Iowa. Iowa city, IA; diluted in PBS +1% BSA 1∶200) for 2 hours at room temperature. The sections were then washed with cold PBS and an Alexa fluor 594 secondary antibody (donkey anti-rat, Invitrogen, Carlsbad, CA) was used at a concentration of 1∶200 for 2 hrs. The sections were then washed with cold PBS and Vectashield™ mounting buffer with DAPI was added to cover all tissue (Vector labs, Burlingame, CA). Salmonella associated cells were quantified manually by counting the number of F4/80+ positive cells that co-localized with GFP-positive Salmonella; at least 100 cells were counted to determine the percentage of Salmonella carrying cells. The number of GFP-positive Salmonella associated with each F4/80+ cells was determined by counting the number of bacteria within the F4/80+ staining region for each infected cell (in WT cecum tissue, we counted the number of intracellular bacteria in 33 cells, in caspase-1−/− cecum tissue, we counted 48 cells).
Electron Microscopy (EM)
During dissection, the ceca of WT and caspase-1−/− mice orally infected with flgM− was fixed in 1/2 strength Karnovsky’s, post fixed in osmium tetroxide and then embedded in Eponate-12 by standard EM procedures. Tissue processing for EM by the Pathology Research Service at the University of Washington, Seattle. For detailed instructions please refer to [40], [41].
Statistics
Significance was obtained by using the software GraphPad Prism (San Diego, CA). The Mann-Whitney test was used for all data where significance is shown. In all graphs, significance was established and represented using the following system: * = p<0.05, ** = p<0.01, *** = p<0.001. One way ANOVA was used when comparing three groups or more, using either the Dunn’s (non-Guassian) or Bonferroni’s (Guassian) multiple comparisons test. Statistical significance was represented as above.
Results
TLR5 Promotes and Caspase-1 limits Salmonella Infection in Streptomycin-pretreated Mice
Flagellin recognition by the innate immune system is dependent on two pathways, the TLR5 pathway and a Naip5–6/Nlrc4/caspase-1 pathway. In order to establish the importance of each pathway, we infected TLR5−/− mice or Casp1 −/− mice with WT Salmonella. Caspase-1 protects against systemic dissemination of WT Salmonella during oral infection in mice (Fig. 1 A–D). WT Salmonella colonized the systemic organs of TLR5−/− and WT C57BL/6 mice equally well, suggesting that WT Salmonella efficiently evade TLR5 recognition of flagellin (Fig. 1 E–H). Paradoxically, the cecal bacterial load was decreased in TLR5−/− mice, indicating a role for TLR5 in the regulating cecal colonization by Salmonella (Fig. 1 E).
Figure 1. Wildtype Salmonella efficiently evades flagellin detection during acute mucosal infection in streptomycin-pretreated mice.
Bacterial burden of WT C57BL/6 (n = 11) and caspase-1−/− (n = 8) mice infected with 1000 cfu WT SL1344 Salmonella in the cecum (A), spleen (B), liver (C), MLN (D). Bacterial burden of WT C57BL/6 (n = 12) and TLR5−/− (n = 13) mice infected with 1000 cfu wildtype Salmonella in the cecum (E), spleen (F), liver (G), MLN (H). Figures A–D are the combined data from two independent experiments. Figures E–H are the combined data from three independent experiments. Mann-Whitney test. * = p<0.05. ** = p<0.01. *** = p<0.001.
Caspase-1 Mediated Protection is Dependent on Salmonella Flagellin
We next infected Casp1−/− mice with flagellin-deficient Salmonella to determine if flagellin is needed for enhanced virulence in Casp1−/− mice. Flagellin-deficient Salmonella colonized the cecum, spleen, liver and MLN of Casp1−/− and WT mice equally well, indicating that caspase-1 mediated control of Salmonella infection is dependent on flagellin expression by Salmonella (Fig. 2 A–D).
Figure 2. Flagellin detection accounts for the caspase-1 increased susceptibility during acute mucosal infection in streptomycin-pretreated mice.
Bacterial burden of WT C57BL/6 (n = 10) and caspase-1−/− (n = 16) mice infected with 1000 cfu flagellin− Salmonella in the cecum (A), spleen (B), liver (C), MLN (D). Bacterial burden WT C57BL/6 (n = 15) and TLR5−/− (n = 16) mice infected with 1000 cfu flagellin− Salmonella in the cecum (E), spleen (F), liver (G), MLN (H). Figures A–D are the combined data from two independent experiments. Figures E–H are the combined data from three independent experiments. Mann-Whitney test * = p<0.05. *** = p<0.001.
TLR5 Promotes Infection Independent of Salmonella Flagellin
To determine if flagellin is needed for the increased cecal colonization in WT relative to TLR5−/− mice, we infected these mice with flagellin-deficient bacteria. Flagellin-deficient Salmonella were even more attenuated than WT bacteria in the TLR5−/− mice, and fewer bacteria were recovered from all organs (Fig. 2 E–H). Thus TLR5 promotes Salmonella infection independently of flagellin expression by Salmonella.
In vitro Characterization of flgM-deficient Salmonella
O’Brien and colleagues determined that flgM is required for Salmonella virulence [35], [42], [43]. FlgM hypersecretion due to a mutation in fliD is also associated with decreased virulence [44]. The loss of virulence in FlgM defective Salmonella requires fliA and fliC [35], indicating that FlgM inhibition of FliA-dependent flagellin expression is necessary for virulence. To confirm these previous studies and extend these findings to Salmonella induced enterocolitis, we deleted the entire flgM gene from S. Typhimurium SL1344 by homologous recombination (see methods). The flgM deletion (referred to as flgM− throughout this paper) secreted more flagellin in vitro and had more cell-associated flagellin (Fig. S3 A), but did not affect growth in vitro (Fig. S3 B) or motility (Fig. S3 C). In addition, flgM− and wild type Salmonella killed macrophages equally well, and this was largely flagellin-dependent (Fig. 3 D). Finally, flgM− Salmonella produced more TLR5 stimulatory activity than WT Salmonella, consistent with greater flagellin production by this mutant (Fig. S3 E).
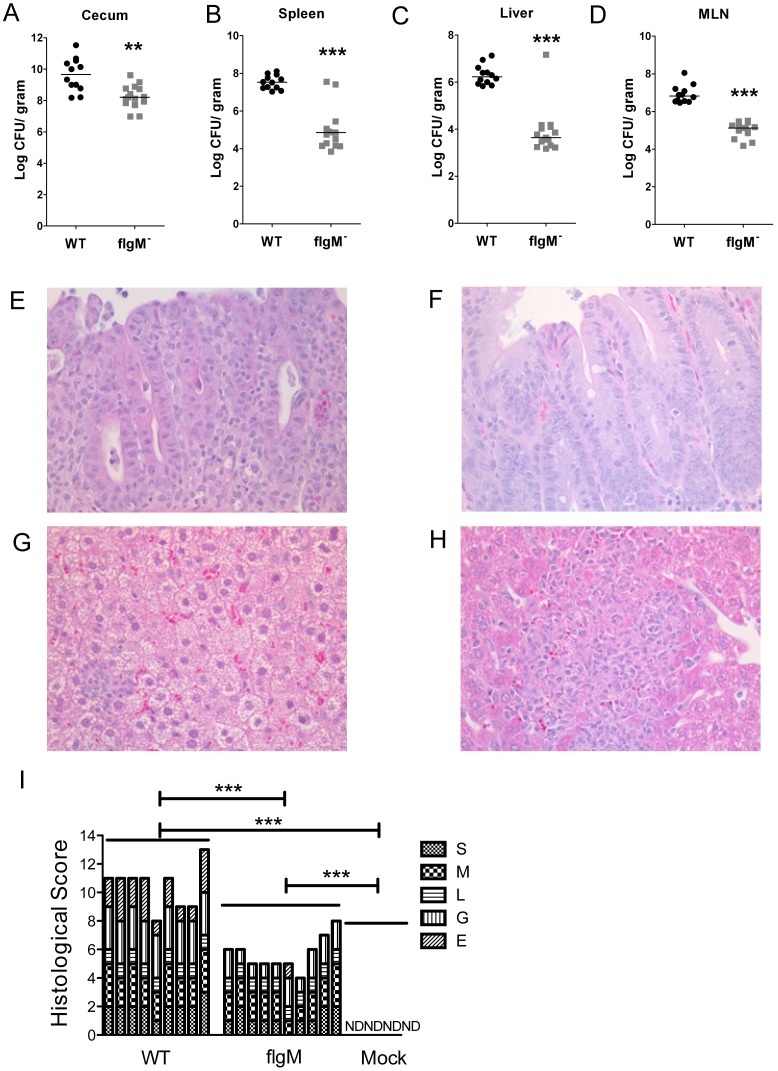
Figure 3. flgM− Salmonella has an attenuated in vivo phenotype.
C57BL/6 mice were infected with 1000 CFU of either WT SL1344 (n = 12) or flgM− (n = 15) Salmonella. (A) Bacterial burden in the cecum. (B) Spleen. (C) Liver. (D) MLN. Representative histology of the cecum (E+F) and liver (G+H) infected with WT (E+G) or flgM− Salmonella (F+H). (I) Histological scores for changes in the cecum. Submucosal expansion (S); mucosal neutrophilic infiltrate (M); lymphoplasmacytosis (L); goblet cells (G); epithelial integrity (E). Liver images (400X magnification), cecal images (200X magnification). Figures A–D are the combined data from three independent experiments. Mann-Whitney test. ** = p<0.01, *** = p<0.001.
Deletion of flgM Attenuates S. Typhimurium in vivo
Mice infected with flgM− had substantially and significantly less bacteria in their tissues, which was modest in the cecum (Fig. 3 A), and more pronounced in the spleen, MLN and the liver (Fig. 3 B–D). The ceca of WT and flgM− infected mice were thick walled, pale and small (Fig. S1 A). Histologically, WT Salmonella infected mice exhibited prominent epithelial injury, edema, and leukocytic infiltration (Fig. 3 E). In contrast, the ceca of flgM− infected mice showed moderate inflammation, characterized by edema and moderate leukocyte infiltration, with good preservation of the epithelium (Fig. 3 F). Quantification of cecal inflammation demonstrated that, flgM− Salmonella induced less inflammation relative to WT (Fig. 3 I). We measured gene expression in the cecal tissue from these mice. Compared to mock-infected animals both WT and flgM− infected mice demonstrated significantly increased expression of Cxcl1, Cxcl2, IL-1B, Reg3g, Lcn2 and TNF, and the flgM− infected mice also had increased levels of Ccl20, IFN-γ and IL-6 (Fig. S2 A). Mice infected with flgM− Salmonella had significantly increased Reg3g expression compared to WT infected mice (Fig. S2 A).
Systemically, flgM− infected mice had discrete foci of inflammation in the liver and spleen with no significant histologic evidence of hepatotoxicity (Fig. 3 H and data not shown), while WT infected mice exhibited severe hepatotoxicity, characterized by prominent vesicular change within the hepatocyte cytoplasm, and overall less inflammation (Fig. 3 G). Salmonella did not colocalize with hepatocytes suggesting that liver damage was indirect and not due to direct infection of hepatocytes (data not shown). Thus we hypothesized that the prominent hepatotoxicity was the result of a cytokine storm in the setting of systemic infection and sepsis [45]. Measurement of serum cytokines confirmed that WT infected mice had higher levels of pro-inflammatory cytokines such as TNF-α and IL-6 (Fig. S4 A, S4 C respectively). Serum levels of IL-1B were similar in all infected groups (Fig. S4 B). In contrast IL-12/23p40 was higher in flgM− infected mice (Fig. S4 D).
The Attenuated Phenotype of flgM− is Dependent on Flagellin
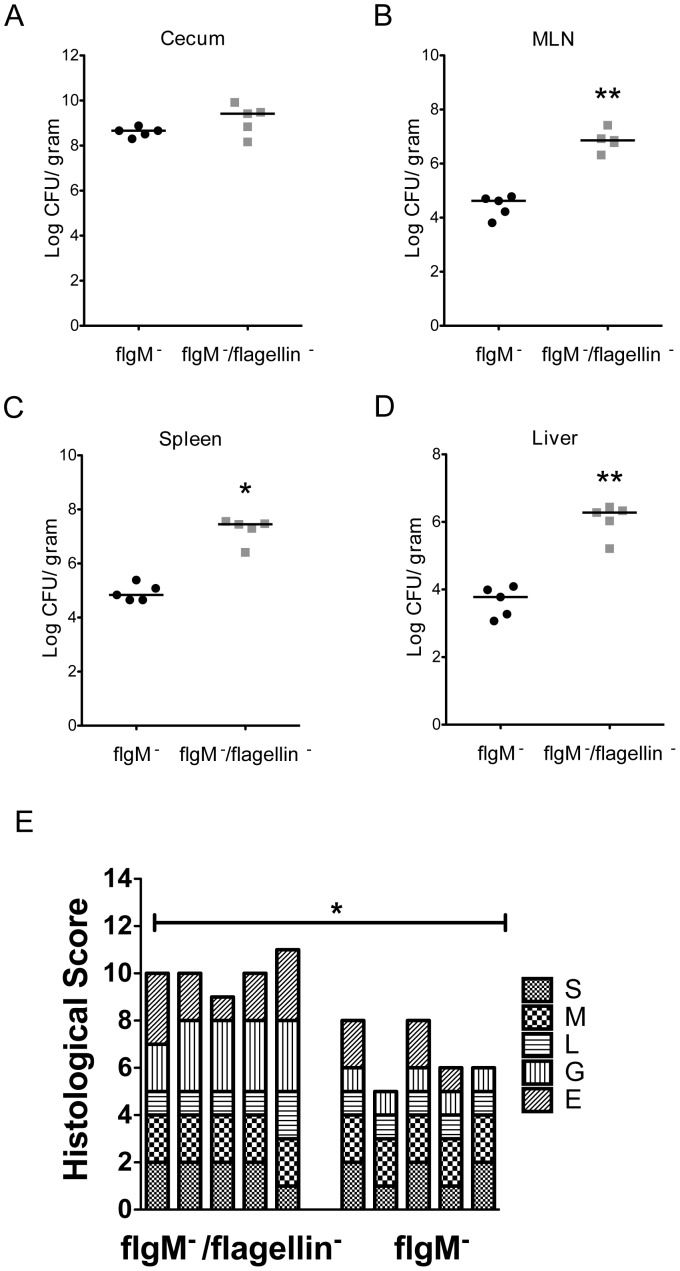
To verify that the decreased bacterial burden and inflammation in flgM− infected mice was dependent on flagellin, we deleted the flagellin genes, fljB and fliC, in the flgM− bacteria. In the cecum the bacterial burden of flgM/flagellin-deficient Salmonella was comparable to that of flgM− (Fig. 4 A). The failure of the flgM/flagellin-deficient Salmonella to regain the modest growth advantage in the cecum may be due to loss of motility [2]. Deletion of the flagellin genes restored virulence to flgM− bacteria as evidenced by increased CFU in the MLN, liver and spleen (Fig. 4 B–D). The flgM/flagellin-deficient strain also induced hepatotoxic changes as seen with WT Salmonella (data not shown). Deletion of the flagellin genes restored inflammation and injury in the cecal mucosa, which was confirmed by histologic quantification, demonstrating decreased goblet cells and loss of epithelial integrity (Fig. 4 E and fig. S1 B). Thus loss of flgM results in flagellin-dependent attenuation of S. Typhimurium, which is manifest as decreased mucosal inflammation, mucosal injury, hepatotoxicity and systemic dissemination of bacteria.
Figure 4. flgM− Salmonella attenuated phenotype is dependent on flagellin.
C57BL/6 WT mice were infected with 1000 CFU of either flgM−/flagellin− (n = 5) or flgM− (n = 5) Salmonella. Bacterial burden in the cecum (A). MLN (B). Spleen (C). Liver (D). (E) Histological scores for changes in the cecum in cecal pathology as described in Fig. 4. Figures A–D represent data from one experiment (flgM− n = 5; flgM−/flagellin− n = 5). Mann Whitney test. * = p<0.05, ** = p<0.01, *** = p<0.001.
Caspase-1 is Required for Attenuation of flgM-deficient Salmonella
We next investigated the role of caspase-1 in flagellin-dependent attenuation of flgM− Salmonella. The bacterial burden in the cecum of Casp1−/− mice was significantly increased compared to WT C57BL/6 mice (Fig. 5 A), and differences were even greater in the spleen, liver and MLN (Fig. 5 B, C, D respectively). Hepatotoxic changes were also observed in the liver of Casp1 −/− mice, while WT mice exhibited only mild changes characterized predominantly by discrete foci inflammation (Fig. 5 G, H). Once again, the hepatotoxic changes correlated with higher serum levels of TNF (Fig. S5 A). We also observed higher serum levels of IL-1B and IL-6 and lower IL12/IL23p40 in Casp1−/− mice (Fig. S5 B–D). The ceca of all infected mice were thick walled, small and pale (Fig. S1 C). However, compared to WT mice, Casp1 −/− mice had more severe inflammation and injury in the cecum (Fig. 3 E, F), which even exceeded the histologic changes seen in WT mice infected with WT Salmonella (Fig. 3 I) or flgM/flagellin-deficient Salmonella (Fig. 4 E). To further verify the importance of caspase-1 in the attenuation of flgM-deficient Salmonella, we performed competitive infection studies where mice were orally infected with equal numbers of WT and flgM-deficient Salmonella. The WT Salmonella outcompeted flgM-deficient Salmonella in the cecum, liver, spleen and MLN in WT mice, but not Casp1 −/− mice (Fig. S6). These data suggest that caspase-1 dependent flagellin recognition is primarily responsible for the attenuated phenotype of flgM− Salmonella.
Figure 5. Caspase-1 is required for attenuation of flgM-deficient Salmonella.
C57BL/6 WT (n = 9) and caspase 1−/− (n = 8) mice were infected with 1000 CFU flgM− Salmonella. Bacterial burden in the cecum (A). Spleen (B). Liver (C). MLN (D). Representative histology of the cecum of WT mice (E) or caspase-1−/− (F), and liver of WT mice (G) or caspase-1−/− (H). (I) Histological scores for changes in the cecal pathology as described in Fig. 4. Figures A–D represent data from two independent experiments. Liver images (200X magnification), cecal images (100X magnification). Mann-Whitney test, * = p<0.05, ** = p<0.01, *** = p<0.001.
Casp1 −/− Mice have Altered Gene Expression in the Cecum
Casp1 −/− mice infected with flgM− Salmonella had higher bacterial counts in the cecum and higher histologic inflammatory scores compared to WT Salmonella infections in WT mice (Fig. 5 I). To assess the importance of caspase-1 in mucosal immunity, we analyzed gene expression in ceca of WT and Casp1−/− mice infected with flgM−. Several genes that are involved in mucosal protection and immunity, such as Cxcl2 and IL-12A, retain levels of expression that are comparable to those observed in WT C57BL/6 mice and Lcn2, a gene that has been associated with gut inflammation [46], was detected at higher levels in Casp1 −/− mice (Fig. S2 C). Paradoxically, Casp1 −/− mice had significantly lower levels of Ccl20, Cxcl1, and Reg3γ (Fig. S2 C). This finding suggests that during Salmonella-induced enterocolitis caspase-1 selectively affects the expression of genes, of which some may be important for cytoprotection. Because Ccl20, Cxcl1 and Reg3γ are all known to be expressed by epithelial cells, and their expression is decreased in flgM-infected Casp1−/− mice but not flgM/flagellin- infected WT mice (Fig. S2 C), caspase-1 may also regulate cecal inflammatory responses in a flagellin-independent manner.
Caspase-1 Limits Intracellular Growth of Salmonella
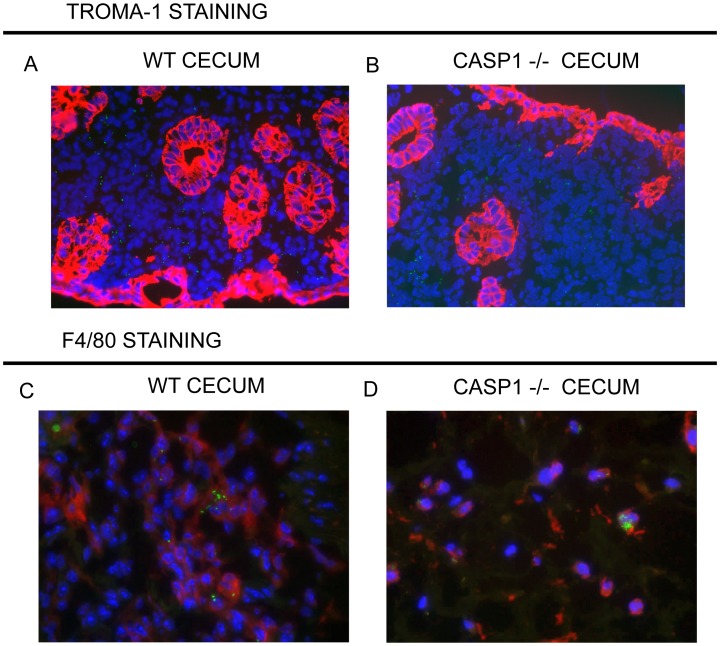
Using fluorescent microscopy we studied the location of Salmonella in the cecum and the contribution of caspase-1 in vivo. In the cecum, Salmonella does not reside in intestinal epithelial cells of WT mice as demonstrated by TROMA-1 staining and only rarely present in epithelial cells of Casp1 −/− mice (Fig. 6 A, B respectively). This observation was confirmed by electron microscopy, where no bacteria were seen in epithelial cells of WT mice, and only few cytoplasmic bacteria were detected in the epithelial cells of Casp1 −/− mice (Fig. S7). In addition these studies confirmed better preservation of the epithelium in WT compared to Casp1 −/− mice (Fig. S7). Salmonella colocalized predominantly with TROMA-1 negative cells in the mucosa and submucosa of WT and Casp1−/− mice. Most Salmonella colocalized with F4/80+ cells indicating that phagocytes were the main host cells for Salmonella in vivo (Fig. 6 C, D and Fig. S8 A, B). Quantification of Salmonella associated with F4/80+ cells revealed that Casp1−/− mice had significantly more infected cells and more bacteria per cell (Fig. S8 C). Electron microscopy studies confirmed this observation, and revealed that most of the bacteria in the lamina propria and submucosa of WT mice were extracellular and adjacent to cell debris, with only rare intracellular bacteria present within simple vesicles (Fig. S9). In contrast, the Casp1 −/− lamina propria cells often harbored numerous bacteria that were frequently associated with complex and heterogeneous vacuoles, and occasionally free in the cytoplasm (Fig. S8 D and Fig. S10). These data suggest that caspase-1 controls intracellular bacterial growth in vivo. Immunofluorescence microscopy analysis of the MLN, spleen and liver revealed that flgM− Salmonella were also present in F4/80+ cells in Casp1−/− mice; bacteria were not detected in these organs of WT mice (data not shown).
Figure 6. Salmonella reside within F4/80+ cells the cecum.
C57BL/6 WT (n = 5) (A+C) or Caspase-1−/− (n = 5) (B+D) mice were infected with 1000 cfu of flgM− Salmonella containing a stable GFP expressing plasmid. Intestinal epithelial cells were stained using TROMA (A+B), and phagocytes were stained using F4/80 (C+D) antibody. Red = TROMA-1 (A, B) and F4/80 (C, D); green = GFP (Salmonella). Shown are representative images from 10 mice examined.
The Innate Immune Receptor TLR5 Plays a Complex Role in the Attenuation of flgM−Salmonella
We hypothesized that innate immune detection of flagellin by TLR5 may also contribute to the attenuated phenotype of flgM− S. typhimurium. We first tested the role of TLR5 by infecting WT andTLR5 −/− mice with flgM− Salmonella. Similar to WT Salmonella infection (Fig. 1 E, F, G, H), bacterial burden in the spleen, liver and MLN was indistinguishable between WT and TLR5 −/− mice (Fig. 7 A, B, C, D), indicating that the attenuation mediated by flgM deficiency is not due to flagellin detection by TLR5. Similar to WT Salmonella infection (Fig. 1 E), TLR5 −/− mice also had lower bacterial burden in the cecum compared to WT mice (Fig. 8 A), consistent with our previous finding that TLR5 promotes cecal colonization of Salmonella. The ceca of all mice were inflamed, small and pale (Fig. S1 D). Histologically, the cecum (Fig. 7 E, F) and the liver (Fig. 7 G, H) of WT and TLR5 −/− mice were similar, although histologic quantification showed a modest but significant increase in cecal inflammation in TLR5 −/− mice (Fig. 7 E). Analysis of gene expression in the cecum of WT and TLR5 −/− mice showed no significant differences in all genes tested (Fig. S2 D). Serum cytokines in WT and TLR5 −/− showed similar levels of TNF-α, IL-1B, IL-12/23p40 in both groups; IL-6 was increased in TLR5−/− mice (Fig. S11). To confirm that TLR5 promotes cecal colonization of flgM− Salmonella independent of Salmonella’s flagellin; we infected WT and TLR5−/− mice with flgM−/flagellin- Salmonella. Once again, these flagellin-deficient bacteria were even more attenuated in the TLR5−/− mice (Fig. S12), providing further evidence that the host protection provided by TLR5-deficiency is independent of flagellin expression by the pathogen, S. Typhimurium.
Figure 7. The innate immune receptor TLR5 is dispensable in the attenuation of flgM−.
C57BL/6 WT (n = 14) and TLR5−/− (n = 16) mice were infected with 1000 CFU flgM− Salmonella. Bacterial burden in the cecum (A). Spleen (B). Liver (C). MLN (D). Representative histology of the cecum for WT mice (E) or TLR5−/− (F), and liver of WT mice (G) or TLR5−/− (H). Liver images (200X magnification), cecal images (100X magnification). Figures A–D represent data from three independent experiments. Mann-Whitney test * = p<0.05. ** = p<0.01.(I) Histological scores for changes in the cecal pathology as described in Fig. 4. Figures A–D represent data from three independent experiments. Mann-Whitney test *** = p<0.001.
Figure 8. Composite analysis of Salmonella WT, flagellin- and flgM− infections in WT, caspase-1−/− and TLR5−/− mice.
We combined the CFU data for all experiments using WT, flagellin deficient and flgM− S. Typhimurium SL1344, and the data was analyzed using two-way ANOVA and Bonferroni’s multiple comparisons post tests. * = P<0.05, ** = P<0.01, and *** = P<0.001. Cecum (A), Spleen (B), Liver (C), and MLN (D).
We compiled all of the CFU data from experiments that we performed using WT, TLR5−/− and Casp1−/− mice in infections with WT, flagellin-null and flgM− Salmonella (Fig. 8). This analysis reveals that dysregulated flagellin production profoundly attenuates Salmonella infection in all tissues in a caspase-1 dependent manner. In the absence of caspase-1, the flgM deletion has essentially no phenotype, and behaves nearly identical to wt Salmonella. Intriguingly, both wt and flgM− Salmonella are more virulent than flagellin- Salmonella in caspase-1 mice. The added virulence property that flagellin bestows upon Salmonella in the absence of caspase-1 may be motility, which is supported by experiments with motility and chemotaxis mutants [2], [47], or possibly some other functional interaction between flagellin and the host. The most parsimonious explanation for modest increase in virulence of flagellin- Salmonella relative to WT Salmonella in WT mice is that flagellin- Salmonella are evading inflammasome detection at the cost of losing a flagellin-dependent virulence property, such as motility. Inflammasome detection of flagellin has only been convincingly demonstrated for rodents. Because inflammasomes in humans and many other animals are incapable of flagellin detection [9], some host-restricted Salmonella strains, such as S. typhi, are not under the same selective pressures to repress flagellin expression during infection.
The composite data (Fig. 8) also illustrate that TLR5 does not contribute to the attenuation of flgM− Salmonella, and that loss of TLR5 leads to paradoxical protection against Salmonella infection, which is most evident in the cecum. These experiments illuminate the complexity of host-pathogen interactions, and how the complex interplay between the host, pathogen and microbiota influence the outcome of infection. A more precise understanding of host-pathogen interactions will need to take into account the microbiota, and control for differences in microbiota that influence host-pathogen interactions.
Discussion
In this study we demonstrate the importance of flagellin regulation by Salmonella and define the role of host flagellin recognition pathways in controlling systemic dissemination of bacteria, inflammation and tissue injury. Proper flagellin regulation is paramount for the infectious life cycle of Salmonella. Salmonella downregulates flagellin production in systemic sites in mice [30], [31] and disruption of flagellin regulation reduces bacterial burden and increases survival of mice [35]. Previous studies have demonstrated that deletion of flgM, the anti-sigma factor, leads to overproduction of flagellin and flagella in vitro, and attenuates Salmonella in vivo [35]. Our studies demonstrate that attenuation of flgM-deficient Salmonella in vivo is due to recognition of flagellin by the innate immune system, and predominantly by caspase-1 dependent pathways.
The role of TLR5 during Salmonella infection is controversial. TLR5 deficiency in mice has been associated with both increased and decreased susceptibility to WT Salmonella infections [23], [24], [26]. Our studies indicate that TLR5−/− mice have a complex phenotype. Similar to the findings of the Gewirtz’s and Akira’s groups [24], [25], we found that TLR5−/− mice have lower CFU in the cecum during WT Salmonella infection. Akira and others have demonstrated that TLR5 plays an important role in Salmonella trafficking by a subpopulation of dendritic cells from the cecum to the MLN, thus providing a potential explanation to our observations. More intriguingly, flagellin− Salmonella were even more attenuated in TLR5−/− mice. When the gut is bypassed by intraperitoneal infection, we have seen no difference between WT and TLR5−/− infected with either WT or aflagellate Salmonella (M. Lai, unpublished observations). This suggests that decreased cecal colonization is responsible for the decreased colonization in systemic organs. Vijay-Kumar et al. also reported increased survival of TLR5−/− mice infected with aflagellate Salmonella during conventional oral infection [25] . These data suggest that recognition of Salmonella’s flagellin by TLR5 is not required for enhanced virulence of Salmonella in WT mice compared to TLR5−/− mice. Vijay-Kumar et al. attributed the enhanced resistance of TLR5−/− mice to an increased basal inflammatory state of TLR5−/− mice. Consistent with this hypothesis, we found that infected TLR5−/− mice had increased histologic evidence of inflammation in the ceca. However, our gene expression data showed no significant differences between infected TLR5 sufficient and deficient mice and serum cytokine measurements revealed only a modest increase in IL-6 in the TLR5−/− mice (Fig. S2 D, Fig. S11 C). In addition, we have never observed spontaneous colitis in our TLR5−/− mouse colony. Thus similar to Vijay-Kumar et al, we find that TLR5-deficiency protects mice against Salmonella infection, and that this protection does not require flagellin expression by Salmonella. TLR5−/− mice have recently been demonstrated to have an altered gut microbiota that promotes metabolic syndrome [26], and it is possible that an altered microflora or altered interactions between the host and the gut microbiota in TLR5−/− mice could establish a more effective barrier against Salmonella infection. A more thorough investigation of the gut microbiota in our TLR5−/− mice may help define how TLR5-deficiency is protective against Salmonella infection. Thus Salmonella may capitalize on TLR5-dependent host responses in the gut to gain competitive advantages over the microflora [39], [48]. Further studies are required to elucidate how TLR5 influences Salmonella infection in the absence of flagellin expression by Salmonella.
Caspase-1 protected mice against WT Salmonella infection (Fig. 1), consistent with previous reports [14], [15]. The deletion of flgM substantially magnified the protective capacity of caspase-1 as evidenced by 100–10,000 fold greater tissue CFU in flgM− Salmonella infected Casp1−/− mice compared to WT mice (Fig. 5). Our results suggest that flagellin recognition by the inflammasome during WT Salmonella infection limits Salmonella spread to distant sites, and that flgM-dependent evasion of flagellin recognition by the inflammasome is necessary for Salmonella virulence.
Recently, murine TLR11 has been proposed as another flagellin receptor, and has been shown to protect mice against Salmonella infection and restrict S. typhi from growth in mice [9], [49,]. Interestingly, the virulence defect flgM− Salmonella is completely complemented by caspase-1 deficiency, suggesting that TLR11 does not contribute to the attenuation of flgM− Salmonella. Additional studies will be needed to clarify the role of TLR11 in this model system.
FlgM− Salmonella infections in mice demonstrate the importance of proper flagellin regulation for virulence, systemic dissemination and inflammation. Cecal inflammation was diminished in flgM− infections compared to WT indicating a role for flagellin recognition in regulating inflammation and injury at mucosal surfaces. It is possible that excess stimulation of TLR5 or the inflammasome during mucosal infection leads to the preservation of mucosal integrity, which is further enhanced by the better control of the bacterial infection. Casp1−/− mice had more severe inflammation and injury in the cecum and higher bacterial burden in all organs indicative of caspase-1 role in protecting mucosal surfaces and containing infection. Surprisingly, Casp1−/− mice had a selective decrease in pro-inflammatory gene expression in the cecum (Fig. S2 C), suggesting that caspase-1 also promotes inflammatory responses in the cecum that may be important for limiting tissue injury. Caspase-1, as well as Nlrc6 and Asc, limit mucosal injury in dextran sodium sulfate induced colitis through the modulation of the intestinal microbiota [9]. Our data indicate that caspase-1 also limits mucosal injury in Salmonella infection, and this may be mediated through caspase-1-dependent control of Salmonella growth, through caspase-1 regulation of mucosal inflammatory responses, or a combination of these factors. Further studies will be needed to determine which inflammasomes protect the gut during Salmonella infection, whether alterations in the gut microbiota contribute to inflammation and injury, and whether Asc-dependent IL-1 and IL-18 are required to protect against mucosal Salmonella infection and preserve epithelial integrity.
Using a heterologous Spi2 promoter to drive the expression of flagellin and FliS, the flagellin chaperone, Ed Miao and colleagues also observed a strong role for caspase-1 in sensing flagellin during intraperitoneal infections [16]. In their study, neither TLR5 nor IL-1/IL-18 played a significant role in limiting Salmonella growth, and attenuation of their Salmonella strain was dependent on Nlrc4/caspase-1 and pyroptosis [16]. It is likely that pyroptosis also contributed to attenuation of flgM-deficient Salmonella infection, and this is supported by the electron microscopy studies demonstrating extracellular Salmonella adjacent to dead cells and cellular debris within the tissue of WT mice.
It has been recently demonstrated that the Casp1 −/− mice used in this study are also deficient in Casp11 (also referred to as Casp4), and that Casp11 deficiency in the context of Casp1 deficiency is protective against Salmonella infection. The model predicts that Casp11 induced cell death in the absence of Casp1-dependent IL-1B and IL-18 production increases host susceptibility to Salmonella infection [50]. This is thought to be manifest by Casp11-mediated killing of phagocytes and release of Salmonella, without IL-1B mediated recruitment of neutrophils to limit Salmonella infection. We noted that many of the intracellular Salmonella seen in Casp1−/− (and Casp11−/−) mice were associated with complex phagosomes, suggesting that autophagy/xenophagy may be contributing to host defense in the combined absence of Casp1 and Casp11. Further studies will be needed to determine the interaction between these caspases and the autophagy pathway in the control of Salmonella infection.
Altogether, our data indicate that regulation of flagellin production by Salmonella is critical to evade innate immune detection, and in particular caspase-1 dependent responses. Caspase-1 is critical for controlling systemic dissemination, severe inflammation and the integrity of the mucosal barrier.
Supporting Information
Gross cecum inflammation was prevalent in all Salmonella infected mice. Representative gross anatomy pictures of C57BL/6 WT mice infected with 1000 cfu WT SL1344 or flgM− Salmonella (A). Gross anatomy pictures of C57BL/6 WT mice infected with 1000 cfu flgM− or flgM−/flagellin− Salmonella (B). Gross anatomy pictures of C57BL/6 WT or caspase-1−/− mice infected with 1000 cfu flgM− Salmonella (C). Gross anatomy pictures of C57BL/6 WT or TLR5−/− mice infected with 1000 cfu flgM− Salmonella (D). The bar represents 1 cm.
(TIF)
Flagellin and caspase-1 contribute to cytokine gene expression in the cecum. Gene expression in the cecum measured by RT-PCR for C57BL/6 mock-infected mice, or mice infected with SL1344 WT or flgM− Salmonella (A). Genes that are significantly induced in flgM− (*) or WT and flgM− (**) Salmonella infected mice compared to mock-infected mice are designated by the asterix below the x-axis (A); genes that are significantly higher in flgM− infected mice are designated by the symbol (#) above the bars (A). Gene expression in the cecum measured by RT-PCR for C57BL/6 WT for flgM vs flgM/flagellin Salmonella infected mice (B). Gene expression in the cecum measured by RT-PCR for C57BL/6 WT or caspase-1−/−mice infected with flgM− Salmonella (C). Gene expression in the cecum measured by RT-PCR for C57BL/6 WT or TLR5−/− mice infected with flgM− Salmonella (D). ns = no statistical significance. Gene expression was normalized to GAPDH, and comparisons were made using the one-way ANOVA and Bonferroni’s multiple comparisons test. Genes with significant differences (P<0.05) are designated by either symbols (* or #) above the bars.
(TIF)
FlgM − Salmonella overproduce flagellin in vitro . (A) Western blot for flagellin from WT and flgM− S. Typhimuirum cell pellets or supernatant; 1.5×108 cell equivalents were loaded in each lane. (B) Motility of Salmonella measured by using a 3 g/L of agar LB plate grown at 37 C. (C) Bacterial growth in LB broth measured by OD 600. (D) Salmonella induced cell death in thioglycollate elicited peritoneal macrophages measured by LDH release assay. (E) TLR5 activity measured using an NF-κB luciferase reporter CHO cell assay; MTLR5-CHO cells were stimulated with 1×105 heat killed cells (pellet), or 1×105 cell equivalents supernatant from WT or flgM− bacteria. Data are representative of two to three independent experiments.
(TIFF)
Increased in inflammatory cytokines in serum of WT SL1344 infected mice. ELISA measurement of serum cytokine for mice infected with 1000 cfu of SL1344 WT, flgM− Salmonella or PBS (Mock): TNF-α (A), IL-1B (B), IL-6 (C), IL12/IL12 p40 (D). Figures A–D represent data from three independent experiments (WT n = 12; flgM− n = 15; mock n = 4). Statistical analysis with one-way ANOVA using the Kruskal-Wallis test and Dunn’s multiple comparisons test, * = p<0.05. *** = p<0.001.
(TIF)
Increased in inflammatory cytokines in serum of Caspase-1−/− infected mice. C57BL/6 WT or Caspase-1−/− mice were infected with 1000 cfu of flgM− Salmonella. ELISA measurement of serum cytokine for TNF-α (A), IL-1B (B), IL-6 (C), IL-12/IL23 p40 (D). Figures A–D represent data from two independent experiments (WT n = 9; caspase-1−/− n = 8). ELISAs were not performed for all cytokines due to poor serum yield. Mann-Whitney test * = p<0.05. ** = p<0.01.
(TIF)
Decreased virulence of flgM− Salmonella is dependent on caspase-1. In vivo competitive index assays where performed by infecting C57BL/6 WT (n = 5) or caspase-1−/− (n = 5) mice with an inoculum containing 500 cfu WT SL1344 and 500 cfu flgM− Salmonella. CFU for the WT and flgM− bacteria were enumerated, and the log ratios (flgM−/WT) were plotted for cecum (A), liver (B), spleen (C), MLN (D). Log ratio was used to demonstrate increases or decreases of virulence between SL1344 WT and flgM−Salmonella. Mann-Whitney test * = p<0.05. ** = p<0.01.
(TIF)
Ultrastructural localization of flgM − Salmonella in the epithelium. Ceca from C57BL/6 WT (A,C) and Casp1−/− (B,D) mice were analyzed for electron microscopy. Salmonella-like bacteria were detected in the lumina for both mice (arrows, A and B), and in the cytosol of some epithelial cells in Casp1−/− mice (arrowheads, B). Rare bacteria were detected in mechanically disrupted epithelial cells of WT mice (asterix, A) and likely represent artifact. The epithelium was well preserved in the WT mice (microvilli - M, macula densa - MD, and mitochondria - M). Focally, bacteria were seen in intracellular vesicles within cells in the epithelial layer (arrowhead, D), possibly representing Salmonella in leukocyte vacuoles.
(TIF)
Caspase-1 controls intracellular Salmonella . C57BL/6 WT or Caspase-1−/− mice were infected with 1000 cfu of flgM− Salmonella containing a stable GFP expressing plasmid. Frozen cecal tissue was stained using F4/80 antibody in WT (A) and caspase-1−/− (B). The percentage of F4/80+ cells associated with GFP+ bacteria (C) and the number of GFP+ Salmonella associated with each F4/80+ cell (D) were quantified for ten 40X- objective high power fields from WT and caspase-1−/− mice. Mann-Whitney test. *** = p<0.001.
(TIF)
Ultrastructural localization of flgM − Salmonella in the lamina propria and submucosa of WT mice. Within the lamina propria and submucosa of C57BL/6 WT mice, Salmonella-like bacteria were detected outside of cells (arrows, A) and inside of cells (arrowheads, A). Examination at higher magnification revealed that the extracellular bacteria (arrows; B,D,E) were adjacent to degenerating cells and cellular debris. The intracellular bacteria were infrequent, and present within simple vesicles contained by a single lipid bilayer (arrowheads, C).
(TIF)
Ultrastructural localization of flgM − Salmonella in the lamina propria and submucosa of Casp1−/− mice. Within the lamina propria and submucosa of C57BL/6 Casp1−/− mice (A–D), Salmonella-like bacteria were detected predominantly inside of cells (arrowheads), and within complex heterogenous vesicles. Some of the bacteria showed loss of integrity of the cell wall (asterix, D).
(TIF)
TLR5 is dispensable in inflammatory responses against flgM − Salmonella . C57BL/6 WT (n = 14) and TLR5−/− (n = 16) mice infected with 1000 cfu of flgM− Salmonella. ELISA measurement of serum cytokine for TNF (A), IL-1B (B), IL-6 (C), IL-12 p40 (D). Figures A-D are the combined data of three independent experiments.
(TIF)
TLR5 promotes cecal colonization of flgM − Salmonella independent of Salmonella flagellin. Bacterial burden WT C57BL/6 (n = 5) and TLR5−/− (n = 4) mice infected with 1000 cfu flgM/flagellin− Salmonella in the cecum (A), spleen (B), liver (C), MLN (D). Mann-Whitney test * = p<0.05.
(TIF)
Acknowledgments
We thank Kelly Hudkins-Loya and the Charles Alpers lab for sharing their materials and expertise in immunofluorescence, histochemistry and electron microscopy; Stephen Libby and Joyce Karlinsey for sharing their knowledge and expertise in Microbiology; Brad Cookson for Salmonella strains and the GFP plasmid; Alicia Brasfield for assistance with mice. We thank Shizou Akira for the TLR5−/− mice, and Richard Flavell for the Casp1−/− mice.
Funding Statement
Marvin A. Lai was supported in part by Public Health Service, National Research Service Award, T32 GM07270, from the National Institute of General Medical Sciences. The work is supported by U.S. NIH grants 5R01AI084803 and 5R01AI062859 (KDS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Janeway CA Jr (1989) Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol 54 Pt 1: 1–13. [DOI] [PubMed] [Google Scholar]
- 2. Stecher B, Hapfelmeier S, Muller C, Kremer M, Stallmach T, et al. (2004) Flagella and chemotaxis are required for efficient induction of Salmonella enterica serovar Typhimurium colitis in streptomycin-pretreated mice. Infect Immun 72: 4138–4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stecher B, Hardt WD (2008) The role of microbiota in infectious disease. Trends Microbiol 16: 107–114. [DOI] [PubMed] [Google Scholar]
- 4. Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, et al. (2006) Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol 7: 569–575. [DOI] [PubMed] [Google Scholar]
- 5. Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE (2006) Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog 2: e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lightfield KL, Persson J, Brubaker SW, Witte CE, von Moltke J, et al. (2008) Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol 9: 1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith KD, Andersen-Nissen E, Hayashi F, Strobe K, Bergman MA, et al. (2003) Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol 4: 1247–1253. [DOI] [PubMed] [Google Scholar]
- 8. Kofoed EM, Vance RE (2011) Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477: 592–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao Y, Yang J, Shi J, Gong YN, Lu Q, et al. (2011) The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477: 596–600. [DOI] [PubMed] [Google Scholar]
- 10. Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, et al. (2001) The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410: 1099–1103. [DOI] [PubMed] [Google Scholar]
- 11. Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL (2001) Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol 167: 1882–1885. [DOI] [PubMed] [Google Scholar]
- 12. Poyet JL, Srinivasula SM, Tnani M, Razmara M, Fernandes-Alnemri T, et al. (2001) Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J Biol Chem 276: 28309–28313. [DOI] [PubMed] [Google Scholar]
- 13. Broz P, Newton K, Lamkanfi M, Mariathasan S, Dixit VM, et al. (2010) Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J Exp Med 207: 1745–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lara-Tejero M, Sutterwala FS, Ogura Y, Grant EP, Bertin J, et al. (2006) Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J Exp Med 203: 1407–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raupach B, Peuschel SK, Monack DM, Zychlinsky A (2006) Caspase-1-mediated activation of interleukin-1beta (IL-1beta) and IL-18 contributes to innate immune defenses against Salmonella enterica serovar Typhimurium infection. Infect Immun 74: 4922–4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, et al. (2010) Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol 11: 1136–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Steiner TS (2007) How flagellin and toll-like receptor 5 contribute to enteric infection. Infect Immun 75: 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gewirtz AT, Yu Y, Krishna US, Israel DA, Lyons SL, et al. (2004) Helicobacter pylori flagellin evades toll-like receptor 5-mediated innate immunity. J Infect Dis 189: 1914–1920. [DOI] [PubMed] [Google Scholar]
- 19. Ciacci-Woolwine F, Blomfield IC, Richardson SH, Mizel SB (1998) Salmonella flagellin induces tumor necrosis factor alpha in a human promonocytic cell line. Infect Immun 66: 1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kinnebrew MA, Ubeda C, Zenewicz LA, Smith N, Flavell RA, et al. (2010) Bacterial flagellin stimulates Toll-like receptor 5-dependent defense against vancomycin-resistant Enterococcus infection. J Infect Dis 201: 534–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McDermott PF, Ciacci-Woolwine F, Snipes JA, Mizel SB (2000) High-affinity interaction between gram-negative flagellin and a cell surface polypeptide results in human monocyte activation. Infect Immun 68: 5525–5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wyant TL, Tanner MK, Sztein MB (1999) Salmonella typhi flagella are potent inducers of proinflammatory cytokine secretion by human monocytes. Infect Immun 67: 3619–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Feuillet V, Medjane S, Mondor I, Demaria O, Pagni PP, et al. (2006) Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc Natl Acad Sci U S A 103: 12487–12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Uematsu S, Jang MH, Chevrier N, Guo Z, Kumagai Y, et al. (2006) Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol 7: 868–874. [DOI] [PubMed] [Google Scholar]
- 25. Vijay-Kumar M, Aitken JD, Kumar A, Neish AS, Uematsu S, et al. (2008) Toll-like receptor 5-deficient mice have dysregulated intestinal gene expression and nonspecific resistance to Salmonella-induced typhoid-like disease. Infect Immun 76: 1276–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, et al. (2010) Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328: 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aldridge P, Hughes KT (2002) Regulation of flagellar assembly. Curr Opin Microbiol 5: 160–165. [DOI] [PubMed] [Google Scholar]
- 28. Kutsukake K (1994) Excretion of the anti-sigma factor through a flagellar substructure couples flagellar gene expression with flagellar assembly in Salmonella typhimurium. Mol Gen Genet 243: 605–612. [DOI] [PubMed] [Google Scholar]
- 29. Kutsukake K, Iino T (1994) Role of the FliA-FlgM regulatory system on the transcriptional control of the flagellar regulon and flagellar formation in Salmonella typhimurium. J Bacteriol 176: 3598–3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cummings LA, Barrett SL, Wilkerson WD, Fellnerova I, Cookson BT (2005) FliC-specific CD4+ T cell responses are restricted by bacterial regulation of antigen expression. J Immunol 174: 7929–7938. [DOI] [PubMed] [Google Scholar]
- 31. McSorley SJ, Ehst BD, Yu Y, Gewirtz AT (2002) Bacterial flagellin is an effective adjuvant for CD4+ T cells in vivo. J Immunol 169: 3914–3919. [DOI] [PubMed] [Google Scholar]
- 32. Adams P, Fowler R, Kinsella N, Howell G, Farris M, et al. (2001) Proteomic detection of PhoPQ- and acid-mediated repression of Salmonella motility. Proteomics 1: 597–607. [DOI] [PubMed] [Google Scholar]
- 33. Cummings LA, Wilkerson WD, Bergsbaken T, Cookson BT (2006) In vivo, fliC expression by Salmonella enterica serovar Typhimurium is heterogeneous, regulated by ClpX, and anatomically restricted. Mol Microbiol 61: 795–809. [DOI] [PubMed] [Google Scholar]
- 34. Tomoyasu T, Takaya A, Isogai E, Yamamoto T (2003) Turnover of FlhD and FlhC, master regulator proteins for Salmonella flagellum biogenesis, by the ATP-dependent ClpXP protease. Mol Microbiol 48: 443–452. [DOI] [PubMed] [Google Scholar]
- 35. Schmitt CK, Darnell SC, O’Brien AD (1996) The Salmonella typhimurium flgM gene, which encodes a negative regulator of flagella synthesis and is involved in virulence, is present and functional in other Salmonella species. FEMS Microbiol Lett 135: 281–285. [DOI] [PubMed] [Google Scholar]
- 36. Karlinsey JE (2007) lambda-Red genetic engineering in Salmonella enterica serovar Typhimurium. Methods Enzymol 421: 199–209. [DOI] [PubMed] [Google Scholar]
- 37.Maloy SR, Stewart VJ, Taylor RK (1996) Genetic Analysis of Pathogenic Bacteria. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
- 38. Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, et al. (1995) Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science 267: 2000–2003. [DOI] [PubMed] [Google Scholar]
- 39. Barthel M, Hapfelmeier S, Quintanilla-Martinez L, Kremer M, Rohde M, et al. (2003) Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun 71: 2839–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hudkins KL, Pichaiwong W, Wietecha T, Kowalewska J, Banas MC, et al. (2010) BTBR Ob/Ob mutant mice model progressive diabetic nephropathy. J Am Soc Nephrol 21: 1533–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alpers CE, Hudkins KL, Pritzl P, Johnson RJ (1991) Mechanisms of clearance of immune complexes from peritubular capillaries in the rat. Am J Pathol 139: 855–867. [PMC free article] [PubMed] [Google Scholar]
- 42. Schmitt CK, Darnell SC, O’Brien AD (1996) The attenuated phenotype of a Salmonella typhimurium flgM mutant is related to expression of FliC flagellin. J Bacteriol 178: 2911–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schmitt CK, Darnell SC, Tesh VL, Stocker BA, O’Brien AD (1994) Mutation of flgM attenuates virulence of Salmonella typhimurium, and mutation of fliA represses the attenuated phenotype. J Bacteriol 176: 368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Baumler AJ, Kusters JG, Stojiljkovic I, Heffron F (1994) Salmonella typhimurium loci involved in survival within macrophages. Infect Immun 62: 1623–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hoek JB, Pastorino JG (2002) Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol 27: 63–68. [DOI] [PubMed] [Google Scholar]
- 46. Chassaing B, Srinivasan G, Delgado MA, Young AN, Gewirtz AT, et al. (2012) Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS One 7: e44328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stecher B, Barthel M, Schlumberger MC, Haberli L, Rabsch W, et al. (2008) Motility allows S. Typhimurium to benefit from the mucosal defence. Cell Microbiol 10: 1166–1180. [DOI] [PubMed] [Google Scholar]
- 48. Stelter C, Kappeli R, Konig C, Krah A, Hardt WD, et al. (2011) Salmonella-induced mucosal lectin RegIIIbeta kills competing gut microbiota. PLoS One 6: e20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Koblansky AA, Jankovic D, Oh H, Hieny S, Sungnak W, et al. (2013) Recognition of profilin by Toll-like receptor 12 is critical for host resistance to Toxoplasma gondii. Immunity 38: 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Broz P, Ruby T, Belhocine K, Bouley DM, Kayagaki N, et al. (2012) Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490: 288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gross cecum inflammation was prevalent in all Salmonella infected mice. Representative gross anatomy pictures of C57BL/6 WT mice infected with 1000 cfu WT SL1344 or flgM− Salmonella (A). Gross anatomy pictures of C57BL/6 WT mice infected with 1000 cfu flgM− or flgM−/flagellin− Salmonella (B). Gross anatomy pictures of C57BL/6 WT or caspase-1−/− mice infected with 1000 cfu flgM− Salmonella (C). Gross anatomy pictures of C57BL/6 WT or TLR5−/− mice infected with 1000 cfu flgM− Salmonella (D). The bar represents 1 cm.
(TIF)
Flagellin and caspase-1 contribute to cytokine gene expression in the cecum. Gene expression in the cecum measured by RT-PCR for C57BL/6 mock-infected mice, or mice infected with SL1344 WT or flgM− Salmonella (A). Genes that are significantly induced in flgM− (*) or WT and flgM− (**) Salmonella infected mice compared to mock-infected mice are designated by the asterix below the x-axis (A); genes that are significantly higher in flgM− infected mice are designated by the symbol (#) above the bars (A). Gene expression in the cecum measured by RT-PCR for C57BL/6 WT for flgM vs flgM/flagellin Salmonella infected mice (B). Gene expression in the cecum measured by RT-PCR for C57BL/6 WT or caspase-1−/−mice infected with flgM− Salmonella (C). Gene expression in the cecum measured by RT-PCR for C57BL/6 WT or TLR5−/− mice infected with flgM− Salmonella (D). ns = no statistical significance. Gene expression was normalized to GAPDH, and comparisons were made using the one-way ANOVA and Bonferroni’s multiple comparisons test. Genes with significant differences (P<0.05) are designated by either symbols (* or #) above the bars.
(TIF)
FlgM − Salmonella overproduce flagellin in vitro . (A) Western blot for flagellin from WT and flgM− S. Typhimuirum cell pellets or supernatant; 1.5×108 cell equivalents were loaded in each lane. (B) Motility of Salmonella measured by using a 3 g/L of agar LB plate grown at 37 C. (C) Bacterial growth in LB broth measured by OD 600. (D) Salmonella induced cell death in thioglycollate elicited peritoneal macrophages measured by LDH release assay. (E) TLR5 activity measured using an NF-κB luciferase reporter CHO cell assay; MTLR5-CHO cells were stimulated with 1×105 heat killed cells (pellet), or 1×105 cell equivalents supernatant from WT or flgM− bacteria. Data are representative of two to three independent experiments.
(TIFF)
Increased in inflammatory cytokines in serum of WT SL1344 infected mice. ELISA measurement of serum cytokine for mice infected with 1000 cfu of SL1344 WT, flgM− Salmonella or PBS (Mock): TNF-α (A), IL-1B (B), IL-6 (C), IL12/IL12 p40 (D). Figures A–D represent data from three independent experiments (WT n = 12; flgM− n = 15; mock n = 4). Statistical analysis with one-way ANOVA using the Kruskal-Wallis test and Dunn’s multiple comparisons test, * = p<0.05. *** = p<0.001.
(TIF)
Increased in inflammatory cytokines in serum of Caspase-1−/− infected mice. C57BL/6 WT or Caspase-1−/− mice were infected with 1000 cfu of flgM− Salmonella. ELISA measurement of serum cytokine for TNF-α (A), IL-1B (B), IL-6 (C), IL-12/IL23 p40 (D). Figures A–D represent data from two independent experiments (WT n = 9; caspase-1−/− n = 8). ELISAs were not performed for all cytokines due to poor serum yield. Mann-Whitney test * = p<0.05. ** = p<0.01.
(TIF)
Decreased virulence of flgM− Salmonella is dependent on caspase-1. In vivo competitive index assays where performed by infecting C57BL/6 WT (n = 5) or caspase-1−/− (n = 5) mice with an inoculum containing 500 cfu WT SL1344 and 500 cfu flgM− Salmonella. CFU for the WT and flgM− bacteria were enumerated, and the log ratios (flgM−/WT) were plotted for cecum (A), liver (B), spleen (C), MLN (D). Log ratio was used to demonstrate increases or decreases of virulence between SL1344 WT and flgM−Salmonella. Mann-Whitney test * = p<0.05. ** = p<0.01.
(TIF)
Ultrastructural localization of flgM − Salmonella in the epithelium. Ceca from C57BL/6 WT (A,C) and Casp1−/− (B,D) mice were analyzed for electron microscopy. Salmonella-like bacteria were detected in the lumina for both mice (arrows, A and B), and in the cytosol of some epithelial cells in Casp1−/− mice (arrowheads, B). Rare bacteria were detected in mechanically disrupted epithelial cells of WT mice (asterix, A) and likely represent artifact. The epithelium was well preserved in the WT mice (microvilli - M, macula densa - MD, and mitochondria - M). Focally, bacteria were seen in intracellular vesicles within cells in the epithelial layer (arrowhead, D), possibly representing Salmonella in leukocyte vacuoles.
(TIF)
Caspase-1 controls intracellular Salmonella . C57BL/6 WT or Caspase-1−/− mice were infected with 1000 cfu of flgM− Salmonella containing a stable GFP expressing plasmid. Frozen cecal tissue was stained using F4/80 antibody in WT (A) and caspase-1−/− (B). The percentage of F4/80+ cells associated with GFP+ bacteria (C) and the number of GFP+ Salmonella associated with each F4/80+ cell (D) were quantified for ten 40X- objective high power fields from WT and caspase-1−/− mice. Mann-Whitney test. *** = p<0.001.
(TIF)
Ultrastructural localization of flgM − Salmonella in the lamina propria and submucosa of WT mice. Within the lamina propria and submucosa of C57BL/6 WT mice, Salmonella-like bacteria were detected outside of cells (arrows, A) and inside of cells (arrowheads, A). Examination at higher magnification revealed that the extracellular bacteria (arrows; B,D,E) were adjacent to degenerating cells and cellular debris. The intracellular bacteria were infrequent, and present within simple vesicles contained by a single lipid bilayer (arrowheads, C).
(TIF)
Ultrastructural localization of flgM − Salmonella in the lamina propria and submucosa of Casp1−/− mice. Within the lamina propria and submucosa of C57BL/6 Casp1−/− mice (A–D), Salmonella-like bacteria were detected predominantly inside of cells (arrowheads), and within complex heterogenous vesicles. Some of the bacteria showed loss of integrity of the cell wall (asterix, D).
(TIF)
TLR5 is dispensable in inflammatory responses against flgM − Salmonella . C57BL/6 WT (n = 14) and TLR5−/− (n = 16) mice infected with 1000 cfu of flgM− Salmonella. ELISA measurement of serum cytokine for TNF (A), IL-1B (B), IL-6 (C), IL-12 p40 (D). Figures A-D are the combined data of three independent experiments.
(TIF)
TLR5 promotes cecal colonization of flgM − Salmonella independent of Salmonella flagellin. Bacterial burden WT C57BL/6 (n = 5) and TLR5−/− (n = 4) mice infected with 1000 cfu flgM/flagellin− Salmonella in the cecum (A), spleen (B), liver (C), MLN (D). Mann-Whitney test * = p<0.05.
(TIF)