Abstract
Objective
Knowledge of referral patterns for specialty cancer care is sparse. Information on both need and reasons for referral of high-risk well-differentiated thyroid cancer patients should provide the foundation needed for eliminating obstacles to appropriate patient referrals and for improving patient care.
Methods
We surveyed 370 endocrinologists involved in thyroid cancer management. From information in a clinical vignette, respondents were asked to identify the reasons they would need to refer a high-risk patient to a more specialized facility for care. We performed multivariable analysis controlling for hospital and physician characteristics.
Results
Thirty-two percent of respondents reported never referring thyroid cancer patients to another facility. Of those that would refer a high-risk patient to another facility, the opportunity for a patient to enter a clinical trial was the most common reason reported (44%), followed by high dose radioactive iodine with or without dosimetry (33%), lateral neck dissection (24%) and external beam radiation (15%). In multivariable analysis, endocrinologists with a higher percentage of their practice devoted to thyroid cancer care were significantly less likely to refer patients to another facility (P=0.003).
Conclusion
The majority of endocrinologists treating thyroid cancer patients report referring a high-risk patient to another facility for some or all of their care. Knowledge of the patterns of physician referrals and the likelihood of need for referral are the foundation for understanding discrepancies in referral rates and obstacles in the referral process.
Keywords: thyroid cancer, referral, radioactive iodine, lateral neck dissection, clinical trial
Introduction
Multiple specialists are often involved in the treatment of patients with high-risk well-differentiated thyroid cancer.(1) Although endocrinologists frequently coordinate patient care, for optimum care, patients at high-risk of recurrence may need to see an experienced thyroid surgeon for resection of local disease, including metastases in the lateral neck.(2) They may need to see a nuclear medicine physician for adjuvant radioactive iodine treatment of residual iodine-avid disease and a radiation oncologist for external beam radiation of unresectable disease.(3, 4) In some cases, when the cancer progresses and does not respond to standard care, an oncologist may facilitate clinical trial enrollment.(5)
Variations in the treatments of high-risk patients exist, (6, 7) but it is not known whether these variations are due to uncertainty regarding appropriate management and/or lack of access to specialized care. In order to assess whether access is a concern, it is necessary to first determine what proportion of physicians treating thyroid cancer patients need to refer high-risk patients to more specialized facilities.
We surveyed 370 endocrinologists involved in thyroid cancer management to assess both need for and reasons for referral of a high-risk thyroid cancer patient to another hospital. We hypothesized that, of the physicians treating thyroid cancer, few are at facilities equipped to address all aspects of high-risk thyroid cancer management.
Methods
Data Source and Study Population
As previously described, we surveyed thyroid surgeons affiliated with the American College of Surgeons Commission on Cancer's National Cancer Database (NCDB).(8) These NCDB affiliated surgeons were asked “Please list the names, specialties, and hospital affiliations of the physicians who provide care to your thyroid cancer patients or administer radioactive iodine when needed.” The 903 physicians identified by the surgeons were the subjects for this second survey study.
To encourage survey response, we used the modified Dillman survey method.(9) This consisted of an initial mailing of an introductory letter, the survey instrument, a postage-paid return envelope, and a small financial gift. Three weeks later, a postcard reminder was sent. This was followed by a second identical survey with postage-paid return envelopes mailed to each non-responder. Data were de-identified and analyzed in summary form only. Double-entry method of the data from returned surveys was performed. Exemption was granted for this study by the University of Michigan Institutional Review Board.
Measures
For the purpose of this study, high-risk well-differentiated thyroid cancer was classified as including both extensive local disease and distant thyroid cancer metastases. Included in the survey was a “high-risk” vignette (Figure 1). Before administration, the survey instrument was piloted in a diverse group of physicians.
Figure 1.
Included in the survey was a “high-risk” clinical vignette.
The primary dependent variable was binary: never need to refer patients to another facility versus do need to refer. Independent variables from the survey included: physician practice setting, specialization, number of specialists in the practice, presence of residents, presence of fellows, percentage of practice devoted to thyroid cancer, and number of patient care hours per week. Since respondents could select more than one practice setting, an algorithm previously described by Alderman et al. was used for practice classification.(10) If academic tertiary care center was selected (in addition to when community and private practice were also selected), the practice setting was classified as being academic. When community-based academic affiliate was selected (in addition to when private practice was also selected), the practice setting was classified as being community- based. If only private practice was selected, the practice setting was classified as private practice. A similar approach was used for specialization. If endocrinology was chosen and a second specialty was also selected, for this analysis the respondent was classified as an endocrinologist.
The independent variable hospital case volume was obtained from the NCDB. As previously described, hospitals were divided into quintiles based on thyroid cancer case volume.(6, 8) We excluded hospitals seeing fewer than seven cases a year since in these hospitals it may be difficult to identify a surgeon who operated on a thyroid cancer patient in the past year. This resulted in the following four case volume categories: low (7-11 thyroid cancer cases/year), low-moderate (12-19 thyroid cancer cases/year), moderate (20-34 thyroid cancer cases/year), and high (≥ 35 thyroid cancer cases/year).
Statistical Analyses
Univariate analyses of hospital and physician correlates of referral were performed using chi square tests and student T tests. Multivariable analysis was performed using logistic regression to assess the independent effect of hospital and physician covariates on the need for referral.
All statistical tests were performed using SAS 9.2 (SAS Institute Inc., Cary, North Carolina). Two sided tests were used with P <0.05 considered statistically significant.
Results
Of the 903 surgeon-identified physicians, 50 were ineligible because they were deceased, ill, retired, not treating thyroid cancer patients, or the mailing address was incorrect. The survey response rate was 63% (534/853). Sixty-nine percent of respondents were endocrinologists (370/534); only the endocrinologists were included in this analysis.
As shown in Table 1, in univariate analysis, high hospital case volume, academic practice setting, greater number of specialists in the practice, presence of residents, presence of fellows, a higher percentage of practice devoted to thyroid cancer, and lower number of patient care hours each week were significantly associated with never referring patients to another facility. In multivariable analysis, however, only a higher percentage of the practice devoted to thyroid cancer and community-based academic affiliate practice setting were statistically associated with never needing to refer.
Table 1.
Factors associated with referrals to other facilities for thyroid cancer management (N=370)
| All Respondents No. (%) | Do not need to refer No. (%) | Do need to refer No. (%) | Univariate P value | Multivariable P value | |
|---|---|---|---|---|---|
| Hospital Case Volume | 0.002 | 0.303 | |||
| Low | 32 (9) | 9 (28) | 23 (72) | ||
| Low-Mod | 71 (19) | 18 (25) | 53 (75) | ||
| Moderate | 124 (33) | 28 (23) | 96 (77) | ||
| High | 142 (39) | 62 (43) | 81 (57) | ||
| Practice Setting | <0.001 | ||||
| Private Practice | 226 (62) | 45 (20) | 181 (80) | 0.297 | |
| Community based academic affiliate | 47 (13) | 23 (49) | 24 (51) | 0.039 | |
| Academic tertiary care center | 74 (20) | 45 (61) | 29 (39) | 0.459 | |
| Other | 17 (5) | 3 (18) | 14 (82) | - | |
| No. specialists in practice (Mean + SD) | 4.5 ± 5.4 | 3.0 ± 2.3 | <0.001 | 0.820 | |
| Residents present | <0.001 | 0.435 | |||
| Yes | 147 (40) | 69 (47) | 78 (53) | ||
| No | 219 (60) | 47 (21) | 172 (79) | ||
| Fellows present | <0.001 | 0.115 | |||
| Yes | 79 (22) | 50 (63) | 29 (37) | ||
| No | 287 (78) | 66 (23) | 221 (77) | ||
| Percentage of practice thyroid cancer (Mean + SD) | 24.5 ± 26.7 | 9.8 ± 9.7 | <0.001 | 0.003 | |
| No. of patient care hours each week | 0.001 | 0.982 | |||
| Up to 20 | 27 (7) | 16 (59) | 11 (41) | ||
| 21-30 | 34 (9) | 17 (50) | 17 (50) | ||
| 31-40 | 77 (21) | 22 (29) | 55 (71) | ||
| 41-60 | 179 (49) | 50 (28) | 129 (72) | ||
| >60 | 52 (14) | 12 (23) | 40 (77) |
Demonstrated in Figure 2 are the reasons physicians would refer a high-risk patient to another facility. Thirty-two percent of respondents reported never needing to refer thyroid cancer patients to another facility. In the vignette describing a 30-year-old woman with distant metastases from papillary thyroid cancer and extensive local disease, referral for consideration of a clinical trial was the most common reported reason for referral (44%), followed by the need for high dose radioactive iodine or dosimetry (33%). Close to one quarter of respondents (24%) reported needing to refer for lateral neck dissection and l5% for external beam radiation.
Figure 2.
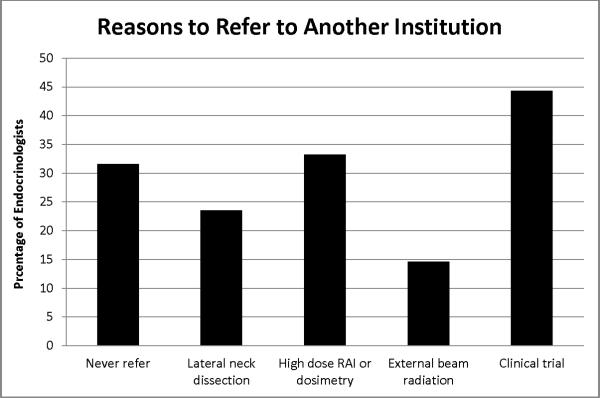
Thirty-two percent of respondents never need to refer thyroid cancer patients to another facility. Of those who would refer a 30 year old woman with distant metastases from papillary thyroid cancer and extensive local disease, request for a clinical trial is the most common reported reason for referral, followed by request for high dose radioactive iodine and/or dosimetry.
Discussion
We found that the majority of endocrinologists surveyed need to refer high-risk thyroid cancer patients to another facility for some or all of their care. The availability of a clinical trial was the most commonly reported reason a physician would need to refer followed by need for high dose radioactive iodine and/or dosimetry.
Before this study, it was known that in the United States there has been an increase in the number of referrals made to specialists(11) and that the reasons physicians choose to refer to a particular physician differ if the referring doctor is a primary care physician versus a specialist.(12) However, the reasons for referral among thyroid cancer specialists were unknown.
Hospitals vary by capabilities and expertise, and this variability can lead to the transfer of patient care to a more specialized provider.(13) There also is some evidence that, for some cancers, patient outcome differs according to physician expertise and case volume.(14, 15) Specifically, surgical expertise correlates with patient outcomes,(16) In some cases, there is a clear role for more extensive surgery, including lateral neck dissection.(2, 17) Whether dosimetry-guided radioactive iodine or fixed dose radioactive iodine is the optimal management for patients with high-risk thyroid cancer is debatable, but many do feel that dosimetry-guided radioactive iodine therapy is preferable in patients with radioactive iodine-avid pulmonary metastases.(18-20) Similarly, external beam radiation prescribed by a radiation oncologist is thought to benefit patients with grossly unresectable disease.(3, 21) More recently, for thyroid cancer patients with progressive disease despite standard care, there has been an increased focus on clinical trials using targeted therapies such as multikinase inhibitors.(22, 23) Thus, there are multiple specialists that can be involved in thyroid cancer care with the potential to improve the outcome of a variety of patients with high-risk thyroid cancer. It is still not clear, however, if this potential has been optimally exploited.
Reasons why high-risk thyroid cancer patients may not attain the highest level of care include lack of experience at their affiliated hospital, absence of a referral from their treating physician to a facility with the required capabilities and expertise,(24) and obstacles to the referral including proximity of the destination hospital and insurance compatibility.
Knowledge of the patterns of physician referrals and the likelihood of need for referral are the foundation for understanding discrepancies in referral rates and obstacles in the referral process. Although the management of the majority of low-risk thyroid cancer patients is relatively straightforward and multiple facilities may be adequately prepared to treat, the treatment of high-risk patients involves multiple specialists and an experienced, equipped treatment center. Recognizing the fact that many facilities may not be equipped to treat these patients is the first step to making certain these patients are referred appropriately. In addition, since the percentage of an endocrinologist's practice devoted to thyroid cancer care was found to be significant in multivariable analysis, this feature may be a key question for patients seeking an endocrinologist affiliated with a treatment facility equip to manage high-risk thyroid cancer patients. This study has implications for future studies assessing treatment patterns for high-risk thyroid cancer patients. For example, age disparities in referrals for specialty surgical care for thyroid cancer have been demonstrated,(25) but it is not clear if this is related to patient selection by age to hospitals with less expertise and lower referral rates or if older patients are not being referred from the same hospitals at the same rates as younger patients.
Strengths of this study include the large sample size of endocrinologists and the novel research question. Limitations are similar to those in other surveys and include non-response bias and risk of the physicians’ report not being consistent with his or her referring behavior. In addition, this study does not address obstacles in the referral process, such as distance and insurance coverage. It is also not clear whether not seeking a referral was because of the ability to provide specialized care locally or because of lack of belief in the value of this care. In addition, the one high-risk scenario provided in our instrument may not be generalizable to all high-risk scenarios, especially with regard to the use of external beam radiation, in which need for referral may be greater in different patient samples.
In conclusion, the majority of physicians treating a high-risk well-differentiated thyroid cancer patient refer the patient to another facility for some or all of their care. Information on physician need for referral is the foundation for future studies exploring discrepancies in referral rates and obstacles in the referral process.
Acknowledgements
This study was endorsed by the American Thyroid Association. The authors would like to thank Brittany Gay, Barbara Salem, Ashley Gay, and Kathryn Schuessler for their work in data collection and processing. This study was funded by K07CA154595-02 to Dr. Haymart from the National Institutes of Health, the University of Michigan Comprehensive Cancer Center Idea Award, the Cancer Surveillance and Outcomes Research Team (CanSORT) Pilot of Feasibility Fund, and the Elizabeth Caroline Crosby Fund.
Footnotes
The authors have no conflicts of interest to disclose.
Contributor Information
Megan R. Haymart, Department of Medicine University of Michigan.
Mousumi Banerjee, Department of Biostatistics University of Michigan.
Di Yang, Department of Biostatistics University of Michigan.
Andrew K. Stewart, National Cancer Database The American College of Surgeons Commission on Cancer.
Jennifer J. Griggs, Department of Medicine and Health Management and Policy University of Michigan.
James C. Sisson, Department of Medicine University of Michigan.
Ronald J. Koenig, Department of Medicine University of Michigan.
References
- 1.Haymart MR, Banerjee M, Yang D, Stewart AK, Koenig RJ, Griggs JJ. The role of clinicians in determining radioactive iodine use for low-risk thyroid cancer. Cancer. 2012 Jun 28; doi: 10.1002/cncr.27721. doi: 10.1002/cncr.27721 [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stack BC, Jr., Ferris RL, Goldenberg D, Haymart M, Shaha A, Sheth S, et al. American Thyroid Association consensus review and statement regarding the anatomy, terminology, and rationale for lateral neck dissection in differentiated thyroid cancer. Thyroid. 2012;22(5):501–8. doi: 10.1089/thy.2011.0312. [DOI] [PubMed] [Google Scholar]
- 3.Chow SM, Yau S, Kwan CK, Poon PC, Law SC. Local and regional control in patients with papillary thyroid carcinoma: specific indications of external radiotherapy and radioactive iodine according to T and N categories in AJCC 6th edition. Endocr Relat Cancer. 2006;13(4):1159–72. doi: 10.1677/erc.1.01320. [DOI] [PubMed] [Google Scholar]
- 4.Tuttle RM, Rondeau G, Lee NY. A risk-adapted approach to the use of radioactive iodine and external beam radiation in the treatment of well-differentiated thyroid cancer. Cancer Control. 2011;18(2):89–95. doi: 10.1177/107327481101800203. [DOI] [PubMed] [Google Scholar]
- 5.Schlumberger M, Sherman SI. Approach to the patient with advanced differentiated thyroid cancer. Eur J Endocrinol. 2012;166(1):5–11. doi: 10.1530/EJE-11-0631. [DOI] [PubMed] [Google Scholar]
- 6.Haymart MR, Banerjee M, Stewart AK, Koenig RJ, Birkmeyer JD, Griggs JJ. Use of radioactive iodine for thyroid cancer. JAMA. 2011;306(7):721–8. doi: 10.1001/jama.2011.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bilimoria KY, Bentrem DJ, Linn JG, Freel A, Yeh JJ, Stewart AK, et al. Utilization of total thyroidectomy for papillary thyroid cancer in the United States. Surgery. 2007;142(6):906–13. doi: 10.1016/j.surg.2007.09.002. discussion 13 e1-2. [DOI] [PubMed] [Google Scholar]
- 8.Haymart M, Banerjee M, Yang D, Stewart A, Doherty G, Koenig R, Griggs J. The relationship between extent of thyroid cancer surgery and use of radioactive iodine. Annals of Surgery. 2012 doi: 10.1097/SLA.0b013e31826c8915. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dillman DA, editor. Mail and Internet Surveys: The Tailored Design Method. ed Second Wiley; New York, New York: 2007. [Google Scholar]
- 10.Alderman AK, Hawley ST, Waljee J, Morrow M, Katz SJ. Correlates of referral practices of general surgeons to plastic surgeons for mastectomy reconstruction. Cancer. 2007;109(9):1715–20. doi: 10.1002/cncr.22598. [DOI] [PubMed] [Google Scholar]
- 11.Barnett ML, Song Z, Landon BE. Trends in physician referrals in the United States, 1999-2009. Arch Intern Med. 2012;172(2):163–70. doi: 10.1001/archinternmed.2011.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnett ML, Keating NL, Christakis NA, O'Malley AJ, Landon BE. Reasons for choice of referral physician among primary care and specialist physicians. J Gen Intern Med. 2012;27(5):506–12. doi: 10.1007/s11606-011-1861-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592–8. doi: 10.1097/MLR.0b013e31820fb71b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349(22):2117–27. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 15.Dimick JB, Birkmeyer JD, Upchurch GR., Jr. Measuring surgical quality: what's the role of provider volume? World J Surg. 2005;29(10):1217–21. doi: 10.1007/s00268-005-7989-4. [DOI] [PubMed] [Google Scholar]
- 16.Sosa JA, Bowman HM, Tielsch JM, Powe NR, Gordon TA, Udelsman R. The importance of surgeon experience for clinical and economic outcomes from thyroidectomy. Ann Surg. 1998;228(3):320–30. doi: 10.1097/00000658-199809000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grant CS, Stulak JM, Thompson GB, Richards ML, Reading CC, Hay ID. Risks and adequacy of an optimized surgical approach to the primary surgical management of papillary thyroid carcinoma treated during 1999-2006. World J Surg. 2010;34(6):1239–46. doi: 10.1007/s00268-009-0307-9. [DOI] [PubMed] [Google Scholar]
- 18.Tuttle RM, Leboeuf R, Robbins RJ, Qualey R, Pentlow K, Larson SM, et al. Empiric radioactive iodine dosing regimens frequently exceed maximum tolerated activity levels in elderly patients with thyroid cancer. J Nucl Med. 2006;47(10):1587–91. [PubMed] [Google Scholar]
- 19.Sisson JC, Shulkin BL, Lawson S. Increasing efficacy and safety of treatments of patients with well-differentiated thyroid carcinoma by measuring body retentions of 131I. J Nucl Med. 2003;44(6):898–903. [PubMed] [Google Scholar]
- 20.Dorn R, Kopp J, Vogt H, Heidenreich P, Carroll RG, Gulec SA. Dosimetry-guided radioactive iodine treatment in patients with metastatic differentiated thyroid cancer: largest safe dose using a risk-adapted approach. J Nucl Med. 2003;44(3):451–6. [PubMed] [Google Scholar]
- 21.Brierley JD. Update on external beam radiation therapy in thyroid cancer. J Clin Endocrinol Metab. 2011;96(8):2289–95. doi: 10.1210/jc.2011-1109. [DOI] [PubMed] [Google Scholar]
- 22.Brose MS, Nutting CM, Sherman SI, Shong YK, Smit JW, Reike G, et al. Rationale and design of decision: a double-blind, randomized, placebo-controlled phase III trial evaluating the efficacy and safety of sorafenib in patients with locally advanced or metastatic radioactive iodine (RAI)-refractory, differentiated thyroid cancer. BMC Cancer. 2011;11:349. doi: 10.1186/1471-2407-11-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sherman SI, Wirth LJ, Droz JP, Hofmann M, Bastholt L, Martins RG, et al. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med. 2008;359(1):31–42. doi: 10.1056/NEJMoa075853. [DOI] [PubMed] [Google Scholar]
- 24.Machens A, Hauptmann S, Dralle H. Referral bias in thyroid cancer surgery: direction and magnitude. Eur J Surg Oncol. 2008;34(5):556–62. doi: 10.1016/j.ejso.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Machens A, Dralle H. Age disparities in referrals to specialist surgical care for papillary thyroid cancer. Eur J Surg Oncol. 2009;35(12):1312–7. doi: 10.1016/j.ejso.2009.07.008. [DOI] [PubMed] [Google Scholar]



