Abstract
Histamine (HA) is a key regulator of experimental allergic encephalomyelitis (EAE), the autoimmune model of multiple sclerosis (MS). HA exerts its effects through four known G protein coupled receptors (GPCR): H1, H2, H3, and H4 (H1-4R). Using HR deficient mice, our lab has demonstrated that H1R, H2R, H3R, and H4R play important roles in EAE pathogenesis, either by regulating encephalitogenic T-cell responses, cytokine production by APCs, blood brain barrier permeability, or T regulatory cell activity, respectively. Histidine decarboxylase deficient mice (HDCKO), which lack systemic HA, exhibit more severe EAE and increased Th1 effector cytokine production by splenocytes in response to myelin oligodendrocyte glycoprotein 35-55. In an inverse approach, we tested the effect of depleting systemic canonical HA signaling on susceptibility to EAE by generating mice lacking all four known GPC-HRs (H1-4RKO mice). Here we report that in contrast to HDCKO mice, H1-4RKO mice develop less severe EAE compared to WT animals. Furthermore, splenocytes from immunized H1-4RKO mice produce less Th1/Th17 effector cytokines compared to WT mice. The opposing results seen between HDCKO and H1-4RKO mice suggest that HA may signal independently of H1-4R and supports the existence of an alternative HAergic pathway in regulating EAE resistance. Understanding and exploiting this pathway has the potential to lead to new disease modifying therapies in MS and other autoimmune and allergic diseases.
Introduction
Histamine (HA) [2-(4-imidazole) ethylamine] is an important mediator involved in regulating various physiological processes like neurotransmission, secretion of pituitary hormones, and gastrointestinal and circulatory functions (1). Additionally, HA is a potent mediator of inflammation and regulates innate and adaptive immune responses (2). Histidine decarboxylase (HDC) synthesizes HA through the decarboxylation of histidine, and mast cells and basophils provide the major source of stored HA in the body (3). However, other cellular sources of HA have recently been identified, including dendritic cells (DCs), T cells, neutrophils, and macrophages (4) and induced or nascent HA secretion occurs in conjunction with increased HDC activity in these cell types. HA mediates its effect through binding to four distinct histamine receptors (HRs), namely H1-H4. All four HRs are 7-transmembrane G-protein-coupled receptors (GPCRs). H1R and H2R couples to Gαq/11 and Gs class of G proteins, respectively, whereas H3R and H4R are coupled to Gi/o (1).
HA plays an important role both in the development of allergic inflammation and autoimmune diseases such as multiple sclerosis (MS) and experimental allergic encephalomyelitis (EAE) the principal animal model of MS. HA and HA releasing agents from mast cells have a dramatic effect on the permeability of the blood brain barrier (BBB) (5, 6). The use of first generation H1R antihistamines, which readily cross the BBB, is associated with a decrease in MS risk (7). MS patients given an H1R antagonist remained stable and improved neurologically (6). In addition, microarray analysis on the chronic plaques of MS patients revealed increased levels of H1R transcripts (8). Similarly, in EAE, T cell clones activated against myelin peptides have increased levels of H1R and H2R transcripts, respectively (9). Mast cell granule stabilizers and H1R specific antagonists reduce EAE severity (10, 11) and mice treated with the H2R agonist dimaprit showed reduced clinical severity and pathology (12). In contrast, the absence of HA leads to an elevation in proinflammatory cytokines and increased susceptibility to EAE in HDCKO mice (13). In both MS and EAE, it is well accepted that MHC class II-restricted CD4+ T cells, which are capable of secreting either IFN-γ (Th1) or IL-17 (Th17) (14), are necessary and sufficient to induce neuropathology.
We have extensively studied the role of HRs in the development of EAE using HRKO mice (4, 15-18). H1RKO mice show a significant delay in the development of EAE and have reduced clinical signs compared to their WT counterparts (15). During myelin oligodendrocyte glycoprotein 35-55 (MOG35-55) induced EAE, T cells from H1RKO mice produce significantly less IFN-γ and increased Th2 cytokines (19). H2RKO mice are also less susceptible to EAE with a blunted Th1 cytokine response in in vitro recall assays (16). H3R is an inhibitory auto/hetero receptor expressed presynaptically on neurons. H3RKO mice develop severe acute early phase EAE and supports the existence of a novel H3R mediated CNS component in the neurogenic control of BBB permeability and peripheral T cell responses (17). H4R is predominantly expressed on hematopoietic cells and exhibits diverse functions (20). H4RKO mice showed increased susceptibility to MOG35-55 induced EAE in association with decreased CNS Treg cell activity (18).
Although the majority of MS and EAE studies have focused on the role of HA signaling through the four known GPC-HRs, there is evidence for HA signaling through non-GPCRs, for example GABAAR, which are ligand-gated ion channels named for their ability to bind the inhibitory neurotransmitter γ-aminobutyric acid (GABA) (21-23). Therefore, to test the hypothesis that HA signaling through non-canonical GPC-HR signaling pathways plays a role in allergic inflammation and the immune responses, we generated mice deficient for the four known HRs (H1-4RKO) and studied them for susceptibility to EAE. Here we report that H1-4RKO mice develop less severe EAE and neuropathology compared WT and HDCKO mice. Furthermore, splenocytes from immunized H1-4RKO mice produce significantly less IFN-γ and H1-4RKO Th1 effector cells are less encephalitogenic under adoptive transfer conditions. Therefore, our data supports the possible existence of an alternative HAergic pathway which in the absence of GPC-HRs significantly reduces susceptibility to EAE.
Materials and methods
Animals
B6.129P-Hrh1tm1Wat (H1RKO) (24), B6.129P-Hrh2tm1Wat (H2RKO) (16), B6.129P2-Hrh3tm1Twl (H3RKO) (17) and B6.129P-Hrh4tm1Thr (H4RKO) mice (Lexicon Genetics, Woodlands Park, TX) (25) were maintained at the University of Vermont, Burlington, VT. All the above strains were backcrossed to C57BL/6J background for >10 generations. H1-4RKO mice were generated by intercrossing individual HR knockout mice (H1RKO X H2RKO X H3RKO X H4RKO). The genetic background of H1-4RKO mice was assessed by the DartMouse™ Speed Congenic Core Facility at the Geisel School of Medicine at Dartmouth (Lebanon, NH). DartMouse uses the Illumina, Inc. (San Diego, CA) GoldenGate Genotyping Assay to interrogate 1449 SNPs spread throughout the genome. The raw SNP data were analyzed using DartMouse’s SNaP-Map™ and Map-Synth™ software, allowing for the determination of the SNP allele at each location. The SNP analysis revealed that the H1-4RKO mice are 97% C57BL/6J, with minor carryover (3%) from 129 only at the retained KO loci. HDC Δ6-8/HDCΔ6-8 (HDCKO) mice were obtained from Dr. Paul J Bryce, Northwestern University, Division of Allergy-Immunology, Feinberg School of Medicine, Chicago, IL 60611, USA (26). The experimental procedures used in this study were approved by the Animal Care and Use Committee of the University of Vermont.
Induction and Evaluation of EAE
Mice were immunized for the induction of EAE using either 1× or 2× immunization protocol (27). For the 2× protocol, mice were injected subcutaneously in the posterior right and left flank with an emulsion containing 100 ug of myelin oligodendrocyte glycoprotein 35-55 (MOG35-55) and an equal volume of complete Freund’s adjuvant (CFA) (Sigma Aldrich, St. Louis, MO) having 200 ug of Mycobacterium tuberculosis H37RA (Difco Laboratories, Detroit, MI); one week later all mice received an identical injection of MOG35-55-CFA. For the 1× protocol, mice were immunized with an emulsion containing 200 ug of MOG35-55 and an equal volume of CFA containing 200 ug of Mycobacterium tuberculosis H37RA. On the day of immunization each mouse received 200 ng of pertussis toxin (PTX) (List Biological Laboratories, Campbell, CA) by i.v.
For passively induced disease, donor mice were immunized using 1× immunization protocol, d12 post-immunization single cell suspensions of DLNs prepared. Cells (10×106 cells/ml in 75 ml tissue culture flasks) were stimulated with MOG35-55 (10 μg/ml) and IL-12 (0.5 ng/ml) for 72 h. Before transferring the cells into recipient mice, cell viability was assessed by trypan blue exclusion; cells stained for intracellular cytokines and culture supernatant also screened for IFN-γ, IL-17 and GM-CSF production by ELISA. After 72 h of culture, cells were washed twice at room temperature with PBS and 1×107 cells/200 μl PBS injected i.v. into WT recipient mice. The mice were monitored for the onset of EAE for 30 days.
Mice were ranked scored daily for clinical quantitative trait variables beginning at day 5 after injection as follows: 0, no clinical expression of disease; 1, flaccid tail without hind limb weakness; 2, hind limb weakness; 3, complete hind limb paralysis and floppy tail; 4, hind leg paralysis accompanied by a floppy tail and urinary or fecal incontinence; 5, moribund. Assessments of clinical quantitative trait variables were performed as previously described (27).
Cytokine and Proliferation assays
For ex vivo cytokine assays, mice were immunized using 2× immunization protocol, spleens and DLNs were harvested on d10, and single cell suspensions were prepared (1 × 106 cells/ml) in RPMI 1640 (10% FBS) and restimulated with 50 μg/ml of MOG35-55. Cell culture supernatants were recovered after 72 h and assayed for IFN-γ, IL-4 and IL-17 by ELISA using anti-IFN-γ, anti-IL-4 and anti-IL-17 mAbs and their respective biotinylated mAbs (BD Biosciences-Pharmingen, San Jose, CA). For proliferation assays, 5×105 cells/well in RPMI 1640 were plated on standard 96-well U-bottom tissue culture plates and stimulated with 0, 1, 2, 10 and 50 μg of MOG35-55 for 72 h at 37oC. During the last 18 h of culture, 1 μCi of [3H] thymidine (PerkinElmer) was added. Cells were harvested onto glass fiber filters and thymidine uptake was determined with a liquid scintillation counter.
CNS-infiltrating mononuclear cell isolation
D15 post-immunization, animals were perfused with saline and brains and SCs removed. A single-cell suspension was obtained and passed through a 70 μm strainer. Mononuclear cells were obtained by Percoll gradient (37%/70%) centrifugation and collected from the interphase. Cells were washed and stimulated for 4 h with PMA + ionomycin in the presence of Brefeldin A (Golgi Plug; BD Biosciences). Cells were labeled with LIVE/DEAD UV-Blue dye (Invitrogen) followed by surface staining (CD45 from Invitrogen and CD4, CD8, TCRγΔ, CD11b, and TCRβ from BD Biosciences). Afterward, cells were fixed, permeabilized, and stained for intracellular IL-17A (BD Biosciences) and IFNγ (Invitrogen).
Antibodies and flow cytometric analysis
Single cell suspensions of thymocytes, lymph node cells and splenocytes were prepared and the red blood cells were lysed with ammonium chloride. Total numbers of cells were counted using the Advia 120 hematology analyzer (Bayer/Siemens, Tarrytown, NY). For flow cytometric analysis, the cells were washed twice and incubated for 30 minutes on ice with the desired fluorochrome-conjugated mAbs or isotype control Ig at 0.5 μg/106 cells. For the identification and phenotypic analysis of TR cells (CD4+CD8−TCRβ+Foxp3+), the following surface anti-mouse mAb were used: anti-CD4 (MCD0417, Caltag); anti-CD8, and anti-CD25 (53-6.7, PC61; BD Pharmingen); anti-TCRβ, and anti-Foxp3 staining set (H57-5987 and FJK-16s; eBioscience), according to the manufacturer’s instructions. Viable cells were selected for flow cytometric analysis (LSR II, BD) based on forward and side scatter properties and analysis was performed using FlowJo software (TreeStar Software, Inc).
Histamine assay
HA concentrations were assessed using an EIA-HA kit according to the manufacturer’s instructions (Cayman Chemicals, Ann Arbor, Michigan). Briefly, 50 μl of derivatization buffer was added to 200 μl undiluted supernatants followed by the addition of 20 μl derivatization reagent. The samples, controls, and standards were added in duplicate to the plate, 100 μl of histamine AChE tracer was added to each well, and the plate was incubated at 4°C for 24 h. The wells were washed and 200 μl Ellman’s Reagent was added and incubated for 30 min in the dark at room temperature while shaking. The plate read at 405 nm when the maximum binding control wells reached an absorbance of 0.2-0.8.
Assessment of antibody responses
Blood was collected from H1-4RKO and C57BL/6J mice d30 post-immunization and sera were stored at −20°C until analyzed. MOG35–55-specific IgG Ab was measured by ELISA as previously described (13). Briefly, 96-well microtiter plates were coated overnight at 4°C with 100 μl of MOG35–55 (0.010 mg/ml) diluted in coating buffer (0.1 M NaHCO3 pH 9.5). The plates were blocked with PBS/1% BSA (blocking buffer) for 2 h. Dilutions of mouse sera from C57BL/6J and H1-4RKO were incubated in MOG35-55 coated wells. Antibody binding was tested by the addition of peroxidase-conjugated monoclonal goat anti-mouse IgG (Southern Biotechnology Associates, Birmingham, AL), each at a 1/5000 dilution in blocking buffer. Enzyme substrate was added and plates were read at 450 nm on a microplate reader.
Cell preparation and culture conditions
From the lymph node and spleen, CD4+ T cells were isolated by negative selection (Qiagen, Valencia, CA). In culture, purified CD4+ T cells (1 × 106 cells/ml) were stimulated with anti-CD3 (5 μg/ml) and anti-CD28 (1 μg/ml) mAbs (BD Biosciences-Pharmingen, San Jose, CA). Supernatants were collected at different time points (24, 48 and 72 h) and analyzed for IFN-γ, IL-4 and IL-2 production by ELISA. CD4+ T cells (1 × 106 cells/ml) were polarized towards Th1, Th2, and Th17 effector cells as previously described (15) analyzed for IFN-γ, IL-4 and IL-17 production by ELISA.
Statistics
Statistical analyses as indicated in the figure legends were performed using GraphPad Prism 5 software (GraphPad software Inc).
Results
H1-4RKO mice exhibit increased resistance to EAE compared to HDCKO and WT animals
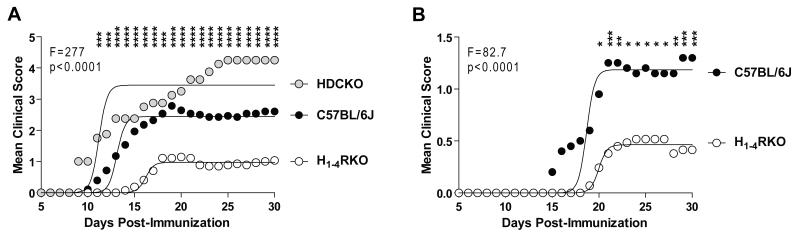
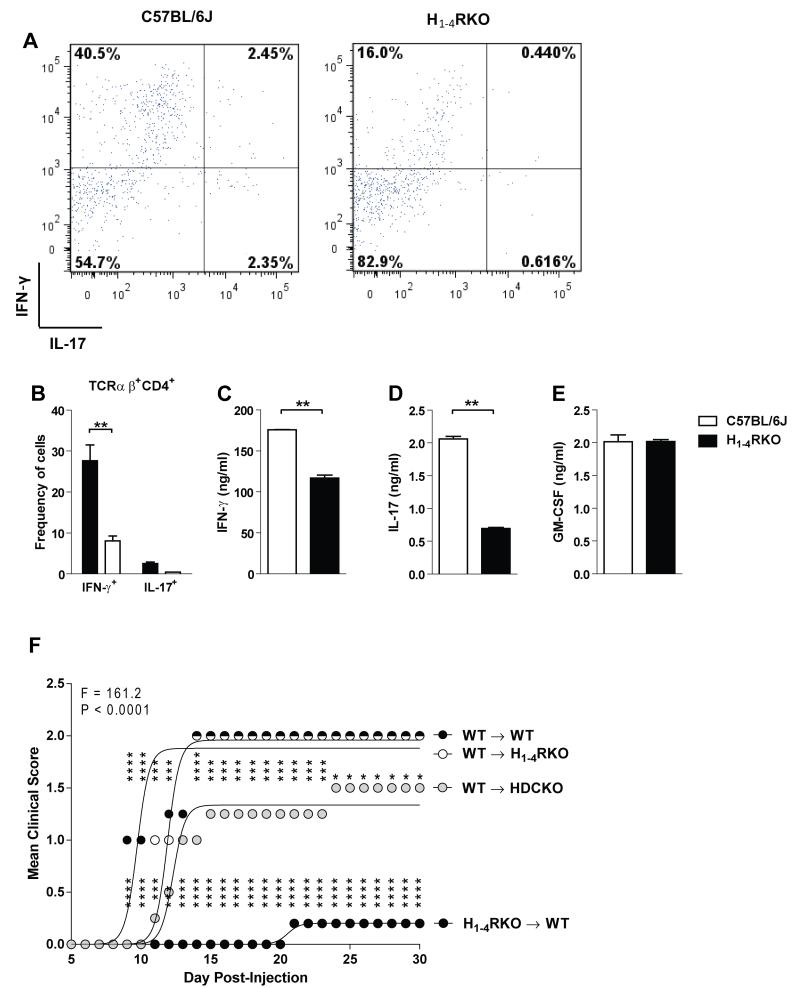
Previously, we demonstrated that mice lacking individual HRs display differential susceptibility to EAE elicited by immunization with a single injection of MOG35-55+CFA+PTX (1× protocol) or two injections of MOG35-55+CFA (2× protocol) (15-18). Additionally, 1× immunized HDCKO mice, which lack HA, exhibit exacerbated EAE (13). In the present study, we assessed susceptibility to MOG35-55-induced EAE in H1-4RKO mice, lacking all four known GPC-HRs, to that of WT and HDCKO mice using the 1× immunization protocol. The severity of the clinical disease courses differed significantly among the strains (F = 277.7; P < 0.0001). Surprisingly, we found that H1-4RKO mice exhibited a significantly less severe clinical disease course than both WT (F = 307.7; P < 0.0001) and HDCKO (F = 485.4; P < 0.0001) mice. The severity of clinical disease in HDCKO mice was significantly greater than WT (F = 74.6; P < 0.0001) and H1-4RKO (F = 485.4; P < 0.0001) mice (Fig. 1A).
Figure 1. H1-4RKO mice exhibit increased resistance to EAE.
(A) WT (n = 28), HDCKO (n = 8) and H1-4RKO (n = 27) mice were immunized using the 1× immunization protocol. (B) WT (n = 20), and H1-4RKO (n = 29) mice were immunized using the 2× immunization protocol. (A and B) The clinical scores following immunization were recorded and the significance of differences between clinical courses was calculated by regression analysis with the best fit curve shown and two-way ANOVA followed by Bonferroni post-hoc multiple comparison test (*, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001).
Analysis of EAE-associated clinical quantitative trait variables revealed that the incidence, cumulative disease score, peak score, number of days affected, mean day of onset, overall severity index, and frequency of lethal disease were significantly lower in H1-4RKO mice compared to both WT and HDCKO mice (Table I). Analysis of EAE susceptibility in H1-4RKO and WT mice using the 2× immunization protocol yielded similar results. H1-4RKO mice exhibit a less severe disease course (F = 82.7; P < 0.0001) (Fig. 1B) and lower incidence, cumulative disease score, peak score, and number of days affected compared to WT mice (Table II). Therefore, in contrast to the increase in EAE susceptibility observed in HDCKO mice, H1-4RKO mice exhibited a dramatic resistance to EAE.
Table I.
Clinical disease traits following immunization of C57BL/6J, H1-4RKO, and HDCKO mice with 1× MOG35–55 + CFA + PTX.
| Strain | Incidencea | CDS | PS | DA | DO | SI | LD |
|---|---|---|---|---|---|---|---|
| B6 | 28/28 (100) | 43.4 ± 3.8 | 3.0 ± 0.2 | 16.8 ± 0.8 | 13.9 ± 0.7 | 2.5 ± 0.2 | 2/28 (7) |
| H1-4RKO | 16/27 (59) | 14.2 ± 2.9 | 1.7 ± 0.3 | 6.3 ± 1.2 | 17.9 ± 1.0 | 2.2 ± 0.1 | 0/16 (0) |
| HDCKO | 8/8 (100) | 68.4± 3.6 | 4.3 ± 0.4 | 22.0 | 9 | 3.1 ± 0.2 | 5/8 (63) |
| Overall | Χ2=17.8, 2 p=0.0001 |
H=36.5 p<0.0001 |
H=19.4 p<0.0001 |
H=42.0 p<0.0001 |
H=28.7 p<0.0001 |
H=10.8 p=0.005 |
Χ2=20.0 p<0.0001 |
| Post-hoc | B6=HDCKO >H1-4RKO |
HDCKO>B6 >H1-4RKO |
HDCKO>B6 >H1-4RKO |
HDCKO>B6 >H1-4RKO |
HDCKO<B6 <H1-4RKO |
HDCKO>B6 =H1-4RKO |
HDCKO>B6 =H1-4RKO |
Animals were considered affected if clinical scores ≥1 were apparent for 2 or more consecutive days (percent affected). Cumulative disease score (CDS), peak score (PS) days affected (DA), day of onset (DO), severity index (SI) and lethal disease (LD). Mean trait values ± SE are shown. The significance of differences for the trait values among the strains was assessed by χ2 analysis (overall incidence) and the Kruskal–Wallis test (H), followed by Dunn’s post hoc multiple comparisons.
Table II.
Clinical disease traits following immunization of C57BL/6J, H1-4RKO with 2× MOG35–55 + CFA.
| Strain | Incidencea | CDS | PS | DA | DO | SI | LD |
|---|---|---|---|---|---|---|---|
| B6 | 16/20 (80) | 14.6 ± 2.9 | 1.7 ± 0.2 | 8.4 ± 1.3 | 19.5 ± 1.0 | 1.7 ± 0.2 | 0/16 |
| H1-4RKO | 6/29 (21) | 5.0 ± 1.9 | 0.6 ± 0.2 | 2.2 ± 0.8 | 20.2 ± 0.6 | 2.2 ± 0.2 | 0/6 |
| p-value | <0.0001 | 0.0007 | 0.0007 | 0.0002 |
Animals were considered affected if clinical scores ±1 were apparent for 2 or more consecutive days (percent affected). Cumulative disease score (CDS), peak score (PS) days affected (DA), day of onset (DO), severity index (SI), and lethal disease (LD). Mean trait values ± SE are shown. Significance of differences in trait values between the strains was assessed using the Fisher’s exact test (incidence) and Mann-Whitney test (clinical disease parameters).
Immune profiling and HA production in H1-4RKO mice
To determine whether the absence of H1-4R inherently influenced immune cell profiles and HA production, we determined the frequency of different cell types in the central and peripheral immune compartments of naïve WT and H1-4RKO mice. There was no significant difference in the total number of cells either in the lymph node or spleen, but H1-4RKO mice had greater numbers of thymocytes compared to WT mice (Fig. S1A). Further analysis revealed a higher frequency of CD4 and CD8 double negative (DN) cells and a lower frequency of CD4 and CD8 double positive (DP) cells in the H1-4RKO mice compared to WT mice (Fig. S1B and S1C). We did not observe any difference in the frequency of single positive (SP) CD4 and CD8 cells between H1-4RKO and WT thymocytes (Fig. S1B and S1C). We also analyzed the frequency of different immune cell subtypes in the lymph node and spleen and found no significant differences (Fig. S1D-F). The only exception being the frequency of splenic B cells, which was decreased in H1-4RKO compared to WT mice (Fig. S1E). Analysis of the WBC differentials in the peripheral blood between H1-4RKO and WT mice revealed no significant differences (Fig. S1G and S1H). Therefore, the absence of the four GPC-HRs neither inherently affects the frequency of T cell subsets nor inflammatory leukocytes in the peripheral immune system, but does exert an influence on total thymic cell numbers and the frequency of DN and DP cells. Lastly, we measured the level of HA in the plasma and found that H1-4RKO mice have significantly higher levels of HA compared to WT mice (Fig. S2).
Impaired differentiation and cytokine production by CD4+ T cells from H1-4RKO mice
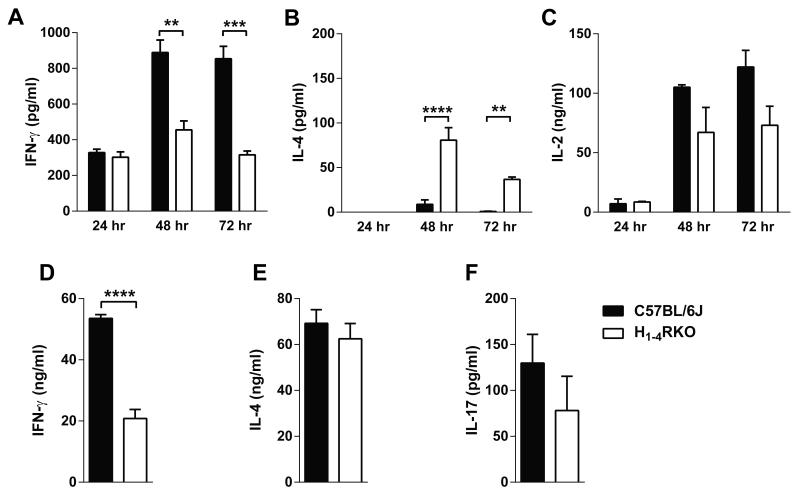
Previous reports indicated that HRs have a role in T cell differentiation and cytokine production (2). To address whether the lack of HRs has an intrinsic effect on the differentiation and/or cytokine production by CD4+ T cells, we stimulated purified CD4+ T cells from the spleen and lymph nodes of naïve WT and H1-4RKO mice with plate bound anti-CD3 and soluble anti-CD28 mAb for 24, 48 and 72 h and screened the culture supernatants for IL-17, IFN-γ, IL-4, and IL-2 production by ELISA. IL-17 was undetectable among the strains. Interestingly, CD4+ T cells from H1-4RKO mice produced less IFN-γ compared to WT CD4+ T cells at 48 and 72 h (Fig. 2A). However, IL-4 production from the stimulated cells was greater in H1-4RKO compared to WT CD4+ T cells (Fig. 2B). We observed no significant difference in the production of IL-2 by these mice (Fig. 2C). These results indicate that CD4+ T cells from H1-4RKO mice upon polyclonal stimulation have an inherent bias towards the Th2 phenotype.
Figure 2. In vitro activated CD4+ T cells from H1-4RKO mice produce decreased IFN-γ.
CD4+ T cells from WT and H1-4RKO mice were activated with anti-CD3 and anti-CD28 mAbs and (A) IFN-γ, (B) IL-4, and (C) IL-2 production at 24, 48, and 72 h post stimulation was determined by ELISA and shown as mean ± standard error of the mean (SEM) of n = 5 per strain. (D, E, and F) CD4+ T cells were activated with anti-CD3 and anti-CD28 mAbs in the presence of Th1, Th2 and, Th17 polarizing cytokines for 4 days. Cells were restimulated with anti-CD3 mAb and the supernatants were collected after 24 h. (D) IFN-γ (E) IL-4, and (F) IL-17 production was analyzed by ELISA and shown as mean ± SEM of n = 5 per strain. In (A, B, and C), significance of differences in cytokine production was determined by two-way ANOVA followed by Bonferroni post-hoc multiple comparison test. In (D, E, and F), significance of differences in cytokine production was determined using the Mann Whitney test (**, p<0.01; ***, p<0.001; ****, p<0.0001).
To address whether the lack of HRs can influence CD4+ T cell differentiation, we purified CD4+ T cells from naïve WT and H1-4RKO mice and in vitro differentiated them into different effector T helper cell subsets. In vitro differentiated Th1 effector cells from H1-4RKO mice produced less IFN-γ compared to Th1 effectors from WT mice (Fig. 2D). We found no difference in the production of IL-4 (Fig. 2E) or IL-17 (Fig. 2F) from Th2 and Th17 effector cells, respectively. Thus, under in vitro nonpolarizing or polarizing conditions, CD4+ T cells from H1-4RKO mice produce less IFN-γ, indicating a deficiency in the Th1 response with a bias towards Th2 under nonpolarizing conditions.
Changes in the Immune Response associated with EAE in H1-4RKO mice
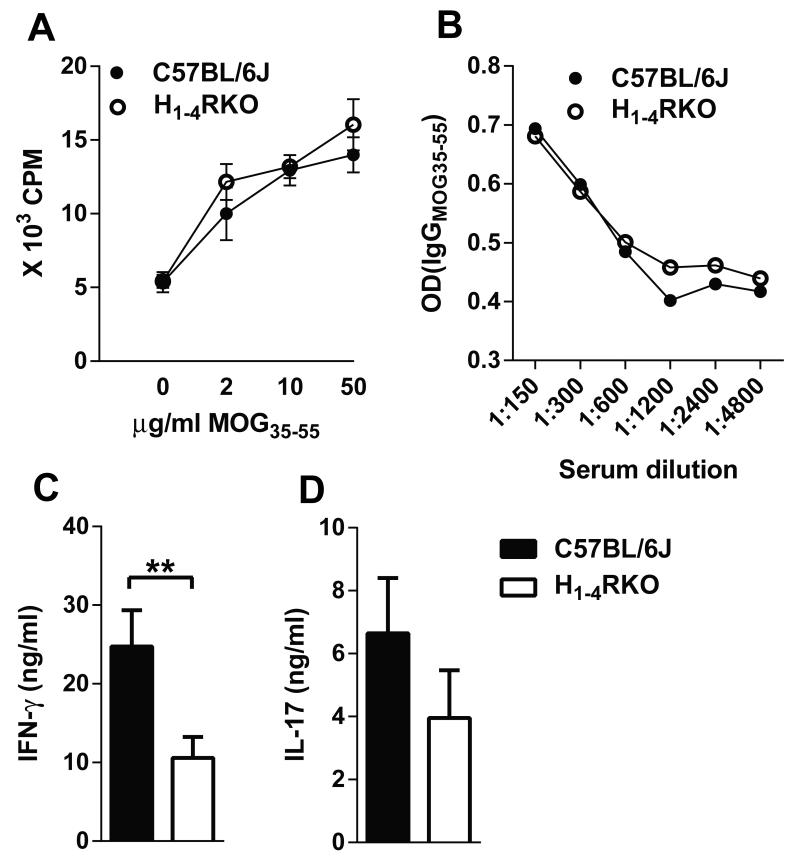
EAE is primarily associated with pathogenic Th1 and Th17 cells.(14) HA and HRs play a role in T cell polarization, proliferation, and cytokine production (2), as well as Ab production by B cells (28). Therefore to elucidate the immune mechanisms associated with differential EAE susceptibility observed in WT and H1-4RKO mice, we compared MOG35-55 specific T cell responses on day 10 (d10) post-immunization. In ex vivo proliferation assays, splenocytes and draining lymph node (DLN) cells from both strains responded equivalently in a dose-dependent fashion to MOG35-55 (Fig. 3A). Splenic and DLN cells from H1-4RKO mice re-stimulated with MOG35-55 produced less IFN-γ (Fig. 3C) and trended towards reduced IL-17 (Fig. 3D) compared to re-stimulated cells from WT mice. We also analyzed IL-4 production by these cells, which is an indicator of an EAE protective Th2 response, but the level was below the limit of detection. Lastly, as HA and HRs can influence Ab production and the frequency of B cells, (24, 28) and H1-4RKO mice have slightly fewer B cells (Fig. S1E), we measured the MOG35-55 specific IgG levels in the sera on d30 1× post-immunization and found no difference in the anti-MOG Ab titers between H1-4RKO and the WT mice (Fig. 3B).
Figure 3. Ex vivo MOG35-55-specific T cell cytokine profiles and serum MOG35-55 specific IgG antibody response in immunized WT and H1-4RKO mice.
(A) MOG35–55-specific T-cell proliferative responses were evaluated by 3H-thymidine incorporation. Mean counts per minute ± standard deviation were calculated from triplicate wells of n = 10 per strain. (B) Serum MOG35–55-specific IgG Ab was measured by ELISA. The significance of differences in proliferation and antibody production were determined by two-way ANOVA followed by Bonferroni post-hoc multiple comparison test. (C) IFN-γ and (D) IL-17 production by MOG35–55 stimulated DLNs and splenocytes from WT and H1-4RKO mice were evaluated by ELISA and shown as mean ± SEM of n = 10 per strain. Significance of differences observed in cytokine production was determined by Mann Whitney test (**, p<0.01).
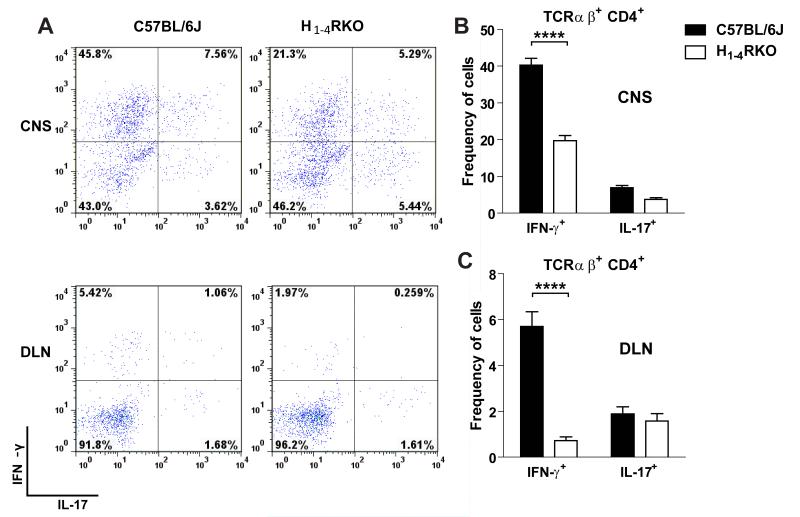
We also evaluated immune responses in the target organ of 1× immunized WT and H1-4RKO mice at d15 post-immunization. Mononuclear cells from the CNS were isolated, stained for cell surface molecules, stimulated with PMA/ionomycin for 4 h in the presence of Brefeldin A, and analyzed by flow cytometry for intracellular cytokines. The total number of infiltrating cells and the number and frequency of CD45+ CNS-infiltrating cells were comparable between WT and H1-4RKO mice (Fig. S3A and S3B). The frequency of TCRαβ+, TCRγΔ+, CD4+, CD8+ T cells, and CD11b+ cells (Fig. S3C-E) was also similar. In contrast, the frequency of IFN-γ producing CD4+ T cells was significantly decreased in H1-4RKO mice compared to WT mice, while that of IL-17 producing cells did not change (Fig. 4A and B). We also evaluated the frequency of IFN-γ and IL-17 producing TCRγΔ+ and CD8+ T cells and found no difference (Fig. S3F and S3G).
Figure 4. Reduced frequency of IFN-γ+ CD4 T cells in the CNS and DLN of immunized H1-4RKO mice.
Mononuclear cells were isolated from the CNS and DLN of 1× immunized mice on d15 post-immunization, stimulated with PMA/ionomycin for 4 hours in the presence of Brefeldin A, stained, and analyzed by flow cytometry. (A) Representative flow cytometric data of the frequency of IFN-γ+ and IL-17+ cells from CNS and DLN, gated on TCRβ+CD4+. (B and C) Percentage of IFN-γ+ and IL-17+ cells from CNS and DLN, gated on TCRβ+CD4+of WT and H1-4RKO mice. Data are shown as mean ± SEM of n = 8 per strain. Significance of differences observed in the percentage of cytokine positive cells were determined by two way ANOVA followed by Bonferroni’s post hoc multiple comparison test (****, p<0.0001).
In addition to the CNS, we also examined the peripheral T cell response d15 post-immunization. Compared to WT mice, no difference was detected in the frequency of TCRγΔ+, TCRαβ+, CD4+, CD8+ T cells, Foxp3+ Treg, and CD11b+ cells in the DLNs of H1-4RKO mice (Fig. S3H-K). There was also no difference in the frequency of IFN-γ or IL-17 producing cells among CD8+ T cells (Fig. S3L). Similar to the CNS, we found that the frequency of IFN-γ producing CD4+ T cells was significantly decreased in H1-4RKO mice compared to WT mice (Fig. 4A and C) with no effect on the frequency of IL-17 producing cells. Taken together, these results suggest that a reduced MOG-specific Th1 response underlies the increased EAE resistance observed in H1-4RKO mice.
T cells from immunized H1-4RKO mice are less encephalitogenic
In the current study, we observed that upon polyclonal stimulation, during in vitro differentiation, and in ex vivo recall assays, Th1 effector cells from H1-4RKO mice produced significantly less IFN-γ. Therefore, to address whether the lack of all four GPC-HRs had any impact on the encephalitogenic potential of T cells, we immunized donor WT and H1-4RKO mice using the 1× immunization protocol and at d12 post-immunization, DLN cells were ex vivo re-stimulated with MOG35-55 and IL-12 for 72 h. Before adoptive transfer, representative cells were stimulated with PMA/ionomycin + Brefeldin A for 4 h, stained for intracellular IFN-γ and IL-17 and supernatants screened for IFN-γ, IL-17, and GM-CSF production. Compared to WT restimulated DLN cells, H1-4RKO cells produced significantly less IFN-γ and IL-17 with no effect on GM-CSF production (Fig. 5A-E). At the end of ex vivo restimulation, an equal number of WT cells were transferred into naïve WT, HDCKO, and H1-4RKO mice. In addition, we transferred restimulated H1-4RKO cells into WT recipients and monitored them for the development of EAE. Interestingly, we found that restimulated cells from WT mice were fully capable of inducing EAE in both WT and H1-4RKO recipients. In contrast, restimulated H1-4RKO cells were unable to induce EAE in WT recipients. Furthermore, we observed that the restimulated WT cells transferred into HDCKO mice also induced EAE but with an intermediary disease course compared to WT recipients (Fig. 5F). These results demonstrate that in the absence of H1-4Rs, T cells are markedly less encephalitogenic.
Figure 5. T cells from H1-4RKO mice are less encephalitogenic.
WT and H1-4RKO mice were immunized using 1× immunization protocol, d12 post-immunization DLN cells were restimulated with MOG35-55 and IL-12 for 72 h. (A) Flow cytometric analysis of WT and H1-4RKO cells stimulated with PMA/ionomycin + Brefeldin A for 4 h and stained intracellularly for IFN-γ and IL-17, gated on CD4 and TCRβ. (B) The percentage of IFN-γ+ and IL-17+CD4+ cells from the DLN of WT and H1-4RKO mice and shown as mean ± SEM of n = 5 per strain. In (B) significance of differences observed in the percentage of cytokine positive cells were determined by two way ANOVA followed by Bonferroni’s post hoc multiple comparison test (**, p<0.01). Cell Culture supernatants were screened for (C) IFN-γ, (D) IL-17, and (E) GM-CSF by ELISA and shown as mean ± SEM of n = 5 per strain. In (C, D, and E) significance of differences in cytokine production was determined using the Mann Whitney test (**, p<0.01). Equal numbers (10 × 106 cells/animal) of restimulated cells were transferred into WT (n = 5/strain) recipients. (F) The clinical scores following immunization were recorded and the significance of differences between clinical courses was calculated by regression analysis with the best fit curve shown and two-way ANOVA followed by Bonferroni post-hoc multiple comparison test (*, p<0.05; ***, p<0.001; ****, p<0.0001).
Blocking GABAAR in H1-4RKO T cells alters functionality in vitro
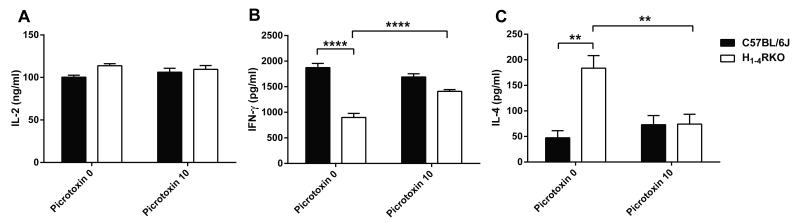
HA mediates its effects by signaling through the four known GPC-HRs. However, it was shown that HA can signal through non-GPCRs, such as GABAAR (21-23). Xenopus oocytes or HEK-293T cells transfected with homomultimeric subunits of GABAAR can form functional HA-gated chloride channels (23). In addition, there is evidence for HA mediated chloride conductance in the mammalian brain, suggesting the existence of HA signaling through non GPC-HRs (22). We hypothesized that in the absence of HRs in H1-4RKO mice HA may similarly signal through GABAAR expressed by CD4+ T cells thereby diminishing their encephalitogenic capacity through altered cytokine production. To address this possibility, we stimulated purified CD4+ T cells from the spleen and lymph nodes of naïve WT and H1-4RKO mice with plate bound anti-CD3 and soluble anti-CD28 mAb for 72 h in presence or absence of the GABAAR antagonist, picrotoxin, and screened the culture supernatants for IL-2, IFN-γ, and IL-4 production by ELISA. We observed no difference in the production of IL-2 (Fig. 6A) between WT and H1-4RKO CD4+ T cells stimulated in the presence or absence of picrotoxin. In the absence of picrotoxin, stimulated CD4+ T cells from H1-4RKO mice produced significantly less IFN-γ (Fig 2A) and more IL-4 (Fig. 2B) compared to WT CD4+ T cells. Blocking GABAAR in H RKO CD4+ 1-4 T cells with picrotoxin significantly increased IFN-γ (Fig. 6B) and decreased IL-4 production (Fig. 6C) supporting the concept that HA can exert its effect through GABAAR expressed in CD4+ T cells and alter cytokine production.
Figure 6. Blocking GABAAR in H1-4RKO T cells alters cytokine production in vitro.
CD4+ T cells from WT and H1-4RKO mice were activated with anti-CD3 and anti-CD28 mAbs in the presence or absence of picrotoxin and (A) IL-2, (B) IFN-γ, and (C) IL-4 production 72 h post stimulation was determined by ELISA and shown as mean ± SEM of n = 5 per strain. In (A, B, and C), significance of differences in cytokine production was determined by two-way ANOVA followed by Bonferroni post-hoc multiple comparison test (**, p<0.01; ****, p<0.0001).
Discussion
Here we have assessed the role of HA in the absence of all known GPC-HRs in EAE susceptibility to test the hypothesis that a non-canonical GPC-HR signaling pathway may influence allergic inflammation and immune responses. The results of our study demonstrate that compared to WT and HDCKO mice, H1-4RKO mice are remarkably resistant to two different protocols of MOG35-55-induced EAE. In contrast, HDCKO mice exhibit increased susceptibility to EAE compared to WT and H1-4RKO mice. The absence of GPC-HRs results in an intrinsic inability of CD4+ T cells to produce IFN-γ and differentiate into Th1 effector T cells. Consequently, decreased susceptibility to EAE in H1-4RKO mice is associated with decreased IFN-γ production by both MOG35-55 specific peripheral and CNS infiltrating CD4+ T cells. Furthermore, in passively induced disease, restimulated WT cells transferred into HDCKO mice also induced EAE, but with a reduced disease course compared to WT recipients, suggesting a role for HA in the effector phase of the disease. In addition, we show that compared to WT effector T cells, H1-4RKO effector T cells, are markedly less encephalitogenic and produce less IFN-γ and IL-17 with no difference in the production of GM-CSF. Our results are in contrast to the observation that GM-CSF is required for the full encephalitogenic potential of T cells (29-31). Our results suggest that under these in vitro polarizing conditions, cells from H1-4RKO mice are less encephalitogenic because of their diminished Th1 and Th17 cytokines. However, it is possible that in vivo within the CNS, infiltrating cells may produce less GM-CSF.
Our results confirm a previous observation showing increased EAE susceptibility in HDCKO mice (13). Since the H1-4RKO mice lack all known GPC-HRs, and are therefore deficient in HA signaling, we anticipated that H1-4RKO mice would be equally susceptible to EAE as HDCKO mice. Rather, these two models of impaired HA signaling have opposing EAE phenotypes. It is known that the prolonged lack of HA affects the level of HR expression, mast cell granule content, and the development of DCs, all of which are important in EAE. Compared to WT mice, HDCKO MOG35-55 specific T cells produce more IFN-γ, TNF-α, and IL-6 (13). Additionally, DCs from HDCKO mice have greater antigen presenting activity than their WT counterparts and tend to more readily polarize T cells toward a Th1 phenotype. Furthermore, HDCKO mice have reduced mast cell numbers with drastically decreased granularity (26).
The contrasting results between H1-4RKO and HDCKO mice suggests a novel HAergic pathway capable of actively regulating resistance to EAE in H1-4RKO mice or alternate ligand binding to canonical GPC-HRs in the absence of HA leads to increased disease severity in HDCKO mice. Support of the latter hypothesis comes from a single unreplicated study suggesting that CCL16 may be a low affinity functional ligand for the H4R capable of eliciting chemotaxis in human and mouse eosinophils (32). Although CCL16 binding to H4R in other immune cells expressing the gene has not been studied, it is theoretically possible that this mechanism leads to the increased lymphocyte chemotaxis, production of proinflammatory cytokines, and EAE susceptibility seen in HDCKO. However, we favor the existence of an alternative HAergic pathway capable of actively regulating increased resistance to EAE in H1-4RKO mice.
We propose that this alternative HAergic pathway may reflect HA acting through either an additional as of yet unidentified high affinity GPC-HR or through less well characterized HA activation of known receptors and/or signaling pathways. Regarding the existence of an unidentified high affinity GPC-HR, it is unlikely that such a receptor has gone undiscovered given the extensive in vitro and in vivo based screens that led to the identification of H3R and H4R (33). Support for later hypothesis comes from the fact that biogenic amines, including HA, can activate ligand gated ion channels (34). Ligand gated ion channels can be either cation channels, which are activated by acetylcholine and serotonin, or anion channels, which are activated by GABA and glycine (35). There is evidence for HA signaling through GABAAR, in that homomultimeric subunits of GABAAR expressed in Xenopus and HEK-293T cells form functional HA-gated chloride channels (23).
Additional evidence for HA activation and signaling through known receptors comes from studies in invertebrates (34, 36-38). Through immunohistochemistry, high levels of HA and HDC were seen in Drosophila photoreceptors (39, 40); however, the functional significance of HA localization in these receptors was not evident until the cloning of two novel HA-gated chloride channels, HisCl-α1 and HisCl-α2 (41). Importantly, the elements that are most important for HA binding and function of HisCl have the greatest mammalian sequence homology with particular subunits of GABAAR and glycine gated chloride channels (42). Additionally, there is evidence in mammalian brain for HA signaling through a non-canonical GPC-HR which is picrotoxin-sensitive and mediated by chloride conductance (22). Taken together these observations support the concept that HA can bind to and signal through GABA and/or glycine receptors. However, unlike the GABAAR β subunits, the glycine receptor β subunits expressed in HEK293 cells do not elicit gating of the channel in response to HA (42).
GABA is a major neurotransmitter in the CNS but is also known to have immunomodulatory activity (43). GABAAR subunits are expressed by immune cells including CD4+ T cells (44) and the production of IL-6 and IL-12 by peritoneal macrophages can be inhibited by exogenous GABA treatment (45). GABA can also modulate CD8+ T cell cytotoxicity and decrease cutaneous delayed-type hypersensitivity reactions (46, 47). Importantly, GABA was shown to reduce myelin basic protein specific T cell proliferative responses (48), and treatment of SJL/J mice with GABAergic agents delays the onset of EAE in association with decreased production of Th1 and Th17 cytokines (49) which is similar to H1-4RKO T cell effector response reported herein. Similarly, low dose GABA treatment of type 1 diabetes prone NOD mice inhibits the development of diabetogenic T cells and suppresses disease progression (44). Furthermore, in the present study, we show that purified CD4+ T cells from H1-4RKO mice stimulated with anti-CD3 + anti-CD28 in the presence of the GABAAR antagonist, picrotoxin, significantly decreased IFN-γ and increased IL-4 production, suggesting, that HA can exert its effect through GABAAR expressed in CD4+ T cells leading to altered cytokine production. Therefore, we anticipate that HA binding to GABA receptors expressed by T cells has the potential to mediate the increased resistance to EAE in H1-4RKO mice. Moreover, we observed that H1-4RKO mice had significantly higher plasma levels of HA compared to WT mice. HRs, especially H2R and H3R, are known to regulate the levels of HA by feedback mechanisms in various systems (50-52). It is likely that higher plasma levels of HA observed in the absence of canonical HRs in H1-4RKO mice may bind to low affinity receptors like GABAAR. Additionally, HA may also act as an endogenous ligand at an unknown allosteric site on the GABAAR subunit potentiating the action of GABA (23).
An additional mechanism whereby HA may mediate disease resistant in H1-4RKO mice is through HA transport. HA transporters are organic cation transporters (OCT) with polyspecificity for transporting molecules across cell membranes. One of these, OCT3 is ubiquitously expressed and HA uptake through OCT3 has been studied in basophils (53). Once inside the cell HA is thought to play a role in cell signaling as a second messenger by binding to cytochrome P450 (P450). P450 is a member of a large and diverse group of heme-containing enzymes involved in the metabolism of xenobiotics, lipids, and hormones (54). In liver microsomes, polyamines, hormones, biogenic amines, and antihistamines can inhibit or displace HA binding to P450 (55). Furthermore, HA binding to P450 has been suggested to modulate its catalytic activity and influence cell growth (56). While the functional significance of HA transport and binding to P450 in the pathogenesis of EAE are unclear, this pathway nevertheless provides for an additional mechanism whereby HA may influence T cells responses and actively suppress EAE.
In summary we have studied the function of endogenous HA on EAE susceptibility in H1-4RKO and HDCKO mice both of which are deficient in HA signaling. Rather than being equivalent in EAE susceptibility and immune responses as predicted, we found H1-4RKO mice to be significantly less susceptible to MOG35-55 induced EAE while HDCKO mice were highly susceptible. Taken together, our findings strongly support the concept that HA acting through mechanisms independent of the four known GPC-HRs mediates increased resistance to EAE. Clearly, delineating the mechanism(s) whereby this alternative inhibitory pathway(s) leads to immune deviation and EAE resistance has the potential to lead to the development of new disease modifying therapies for both allergic and autoimmune diseases.
Supplementary Material
Acknowledgements
The first author, Naresha Saligrama, is the recipient of the Young Investigator Award (second prize) presented by the European Histamine Research Society. We thank Dr. Dimitry N Kremenstov and the members of the Teuscher lab for helpful discussions. We also thank Dr. Robin L Thurmond and Dr. Timothy W Lovenberg, Johnson and Johnson Pharmaceutical Research & Development, LLC, San Diego, CA, USA for providing us with H3RKO and H4RKO mice.
This work was supported by National Institute of Health Grants NS061014, AI041747, NS060901, NS036526, and NS069628 (to C. T).
Abbreviations used in this article
- SC
Spinal cord
- MS
Multiple sclerosis
- EAE
Experimental allergic encephalomyelitis
- HA
Histamine
- HDC
Histidine decarboxylase
- DC
Dendritic cell
- HR
Histamine receptor
- BBB
Blood brain barrier
- GPCR
G-protein-coupled receptor
- MOG35-55
Myelin oligodendrocyte glycoprotein 35-55
- GABA
γ-aminobutyric acid
- DLN
Draining lymph node
- PMA
Phorbol 12-myristate 13-acetate
- WT
Wild-type
- OCT
organic cation transporters
Footnotes
Disclosures The authors declare no financial or commercial conflict of interest.
References
- 1.Parsons ME, Ganellin CR. Histamine and its receptors. Br J Pharmacol. 2006;147(Suppl 1):S127–135. doi: 10.1038/sj.bjp.0706440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akdis CA, Simons FE. Histamine receptors are hot in immunopharmacology. Eur J Pharmacol. 2006;533:69–76. doi: 10.1016/j.ejphar.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 3.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 4.Saligrama N, Noubade R, Case LK, Del Rio R, Teuscher C. Combinatorial roles for histamine H(1) -H(2) and H(3) -H(4) receptors in autoimmune inflammatory disease of the central nervous system. Eur J Immunol. 2012;42:1536–1546. doi: 10.1002/eji.201141859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Behi M, Dubucquoi S, Lefranc D, Zephir H, De Seze J, Vermersch P, Prin L. New insights into cell responses involved in experimental autoimmune encephalomyelitis and multiple sclerosis. Immunol Lett. 2005;96:11–26. doi: 10.1016/j.imlet.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Logothetis L, Mylonas IA, Baloyannis S, Pashalidou M, Orologas A, Zafeiropoulos A, Kosta V, Theoharides TC. A pilot, open label, clinical trial using hydroxyzine in multiple sclerosis. Int J Immunopathol Pharmacol. 2005;18:771–778. doi: 10.1177/039463200501800421. [DOI] [PubMed] [Google Scholar]
- 7.Alonso A, Jick SS, Hernan MA. Allergy, histamine 1 receptor blockers, and the risk of multiple sclerosis. Neurology. 2006;66:572–575. doi: 10.1212/01.wnl.0000198507.13597.45. [DOI] [PubMed] [Google Scholar]
- 8.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 9.Pedotti R, De Voss JJ, Steinman L, Galli SJ. Involvement of both ‘allergic’ and ‘autoimmune’ mechanisms in EAE, MS and other autoimmune diseases. Trends Immunol. 2003;24:479–484. doi: 10.1016/s1471-4906(03)00233-3. [DOI] [PubMed] [Google Scholar]
- 10.Bebo BF, Jr., Yong T, Orr EL, Linthicum DS. Hypothesis: a possible role for mast cells and their inflammatory mediators in the pathogenesis of autoimmune encephalomyelitis. J Neurosci Res. 1996;45:340–348. doi: 10.1002/(SICI)1097-4547(19960815)45:4<340::AID-JNR3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Gregory GD, Bickford A, Robbie-Ryan M, Tanzola M, Brown MA. MASTering the immune response: mast cells in autoimmunity. Novartis Found Symp. 2005;271:215–225. discussion 225-231. [PubMed] [Google Scholar]
- 12.Emerson MR, Orentas DM, Lynch SG, LeVine SM. Activation of histamine H2 receptors ameliorates experimental allergic encephalomyelitis. Neuroreport. 2002;13:1407–1410. doi: 10.1097/00001756-200208070-00012. [DOI] [PubMed] [Google Scholar]
- 13.Musio S, Gallo B, Scabeni S, Lapilla M, Poliani PL, Matarese G, Ohtsu H, Galli SJ, Mantegazza R, Steinman L, Pedotti R. A key regulatory role for histamine in experimental autoimmune encephalomyelitis: disease exacerbation in histidine decarboxylase-deficient mice. J Immunol. 2006;176:17–26. doi: 10.4049/jimmunol.176.1.17. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KH. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2010;162:1–11. doi: 10.1111/j.1365-2249.2010.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noubade R, Milligan G, Zachary JF, Blankenhorn EP, del Rio R, Rincon M, Teuscher C. Histamine receptor H1 is required for TCR-mediated p38 MAPK activation and optimal IFN-gamma production in mice. J Clin Invest. 2007;117:3507–3518. doi: 10.1172/JCI32792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teuscher C, Poynter ME, Offner H, Zamora A, Watanabe T, Fillmore PD, Zachary JF, Blankenhorn EP. Attenuation of Th1 effector cell responses and susceptibility to experimental allergic encephalomyelitis in histamine H2 receptor knockout mice is due to dysregulation of cytokine production by antigen-presenting cells. Am J Pathol. 2004;164:883–892. doi: 10.1016/S0002-9440(10)63176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teuscher C, Subramanian M, Noubade R, Gao JF, Offner H, Zachary JF, Blankenhorn EP. Central histamine H3 receptor signaling negatively regulates susceptibility to autoimmune inflammatory disease of the CNS. Proc Natl Acad Sci U S A. 2007;104:10146–10151. doi: 10.1073/pnas.0702291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.del Rio R, Noubade R, Saligrama N, Wall EH, Krementsov DN, Poynter ME, Zachary JF, Thurmond RL, Teuscher C. Histamine H4 receptor optimizes T regulatory cell frequency and facilitates anti-inflammatory responses within the central nervous system. J Immunol. 2012;188:541–547. doi: 10.4049/jimmunol.1101498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma RZ, Gao J, Meeker ND, Fillmore PD, Tung KS, Watanabe T, Zachary JF, Offner H, Blankenhorn EP, Teuscher C. Identification of Bphs, an autoimmune disease locus, as histamine receptor H1. Science. 2002;297:620–623. doi: 10.1126/science.1072810. [DOI] [PubMed] [Google Scholar]
- 20.Zampeli E, Tiligada E. The role of histamine H4 receptor in immune and inflammatory disorders. Br J Pharmacol. 2009;157:24–33. doi: 10.1111/j.1476-5381.2009.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ringstad N, Abe N, Horvitz HR. Ligand-gated chloride channels are receptors for biogenic amines in C. elegans. Science. 2009;325:96–100. doi: 10.1126/science.1169243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatton GI, Yang QZ. Ionotropic histamine receptors and H2 receptors modulate supraoptic oxytocin neuronal excitability and dye coupling. J Neurosci. 2001;21:2974–2982. doi: 10.1523/JNEUROSCI.21-09-02974.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saras A, Gisselmann G, Vogt-Eisele AK, Erlkamp KS, Kletke O, Pusch H, Hatt H. Histamine action on vertebrate GABAA receptors: direct channel gating and potentiation of GABA responses. J Biol Chem. 2008;283:10470–10475. doi: 10.1074/jbc.M709993200. [DOI] [PubMed] [Google Scholar]
- 24.Banu Y, Watanabe T. Augmentation of antigen receptor-mediated responses by histamine H1 receptor signaling. J Exp Med. 1999;189:673–682. doi: 10.1084/jem.189.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hofstra CL, Desai PJ, Thurmond RL, Fung-Leung WP. Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. J Pharmacol Exp Ther. 2003;305:1212–1221. doi: 10.1124/jpet.102.046581. [DOI] [PubMed] [Google Scholar]
- 26.Ohtsu H, Tanaka S, Terui T, Hori Y, Makabe-Kobayashi Y, Pejler G, Tchougounova E, Hellman L, Gertsenstein M, Hirasawa N, Sakurai E, Buzas E, Kovacs P, Csaba G, Kittel A, Okada M, Hara M, Mar L, Numayama-Tsuruta K, Ishigaki-Suzuki S, Ohuchi K, Ichikawa A, Falus A, Watanabe T, Nagy A. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Lett. 2001;502:53–56. doi: 10.1016/s0014-5793(01)02663-1. [DOI] [PubMed] [Google Scholar]
- 27.Teuscher C, Noubade R, Spach K, McElvany B, Bunn JY, Fillmore PD, Zachary JF, Blankenhorn EP. Evidence that the Y chromosome influences autoimmune disease in male and female mice. Proc Natl Acad Sci U S A. 2006;103:8024–8029. doi: 10.1073/pnas.0600536103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jutel M, Watanabe T, Klunker S, Akdis M, Thomet OA, Malolepszy J, Zak-Nejmark T, Koga R, Kobayashi T, Blaser K, Akdis CA. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature. 2001;413:420–425. doi: 10.1038/35096564. [DOI] [PubMed] [Google Scholar]
- 29.Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 30.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kroenke MA, Chensue SW, Segal BM. EAE mediated by a non-IFN-gamma/non-IL-17 pathway. Eur J Immunol. 2010;40:2340–2348. doi: 10.1002/eji.201040489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakayama T, Kato Y, Hieshima K, Nagakubo D, Kunori Y, Fujisawa T, Yoshie O. Liver-expressed chemokine/CC chemokine ligand 16 attracts eosinophils by interacting with histamine H4 receptor. J Immunol. 2004;173:2078–2083. doi: 10.4049/jimmunol.173.3.2078. [DOI] [PubMed] [Google Scholar]
- 33.Liu C, Ma X, Jiang X, Wilson SJ, Hofstra CL, Blevitt J, Pyati J, Li X, Chai W, Carruthers N, Lovenberg TW. Cloning and pharmacological characterization of a fourth histamine receptor (H(4)) expressed in bone marrow. Mol Pharmacol. 2001;59:420–426. doi: 10.1124/mol.59.3.420. [DOI] [PubMed] [Google Scholar]
- 34.Hardie RC. A histamine-activated chloride channel involved in neurotransmission at a photoreceptor synapse. Nature. 1989;339:704–706. doi: 10.1038/339704a0. [DOI] [PubMed] [Google Scholar]
- 35.Karlin A, Akabas MH. Toward a structural basis for the function of nicotinic acetylcholine receptors and their cousins. Neuron. 1995;15:1231–1244. doi: 10.1016/0896-6273(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 36.McCaman RE, Weinreich D. Histaminergic synaptic transmission in the cerebral ganglion of Aplysia. J Neurophysiol. 1985;53:1016–1037. doi: 10.1152/jn.1985.53.4.1016. [DOI] [PubMed] [Google Scholar]
- 37.Stuart AE. From fruit flies to barnacles, histamine is the neurotransmitter of arthropod photoreceptors. Neuron. 1999;22:431–433. doi: 10.1016/s0896-6273(00)80699-6. [DOI] [PubMed] [Google Scholar]
- 38.Claiborne BJ, Selverston AI. Histamine as a neurotransmitter in the stomatogastric nervous system of the spiny lobster. J Neurosci. 1984;4:708–721. doi: 10.1523/JNEUROSCI.04-03-00708.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarthy PV. Histamine: a neurotransmitter candidate for Drosophila photoreceptors. J Neurochem. 1991;57:1757–1768. doi: 10.1111/j.1471-4159.1991.tb06378.x. [DOI] [PubMed] [Google Scholar]
- 40.Pollack I, Hofbauer A. Histamine-like immunoreactivity in the visual system and brain of Drosophila melanogaster. Cell Tissue Res. 1991;266:391–398. doi: 10.1007/BF00318195. [DOI] [PubMed] [Google Scholar]
- 41.Zheng Y, Hirschberg B, Yuan J, Wang AP, Hunt DC, Ludmerer SW, Schmatz DM, Cully DF. Identification of two novel Drosophila melanogaster histamine-gated chloride channel subunits expressed in the eye. J Biol Chem. 2002;277:2000–2005. doi: 10.1074/jbc.M107635200. [DOI] [PubMed] [Google Scholar]
- 42.Fleck MW, Thomson JL, Hough LB. Histamine-gated ion channels in mammals? Biochem Pharmacol. 2012;83:1127–1135. doi: 10.1016/j.bcp.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 43.Wong CG, Bottiglieri T, Snead OC., 3rd GABA, gamma-hydroxybutyric acid, and neurological disease. Ann Neurol. 2003;54(Suppl 6):S3–12. doi: 10.1002/ana.10696. [DOI] [PubMed] [Google Scholar]
- 44.Tian J, Lu Y, Zhang H, Chau CH, Dang HN, Kaufman DL. Gamma-aminobutyric acid inhibits T cell autoimmunity and the development of inflammatory responses in a mouse type 1 diabetes model. J Immunol. 2004;173:5298–5304. doi: 10.4049/jimmunol.173.8.5298. [DOI] [PubMed] [Google Scholar]
- 45.Reyes-Garcia MG, Hernandez-Hernandez F, Hernandez-Tellez B, Garcia-Tamayo F. GABA (A) receptor subunits RNA expression in mice peritoneal macrophages modulate their IL-6/IL-12 production. J Neuroimmunol. 2007;188:64–68. doi: 10.1016/j.jneuroim.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 46.Bergeret M, Khrestchatisky M, Tremblay E, Bernard A, Gregoire A, Chany C. GABA modulates cytotoxicity of immunocompetent cells expressing GABAA receptor subunits. Biomed Pharmacother. 1998;52:214–219. doi: 10.1016/S0753-3322(98)80019-X. [DOI] [PubMed] [Google Scholar]
- 47.Tian J, Chau C, Hales TG, Kaufman DL. GABA(A) receptors mediate inhibition of T cell responses. J Neuroimmunol. 1999;96:21–28. doi: 10.1016/s0165-5728(98)00264-1. [DOI] [PubMed] [Google Scholar]
- 48.Bjurstom H, Wang J, Ericsson I, Bengtsson M, Liu Y, Kumar-Mendu S, Issazadeh-Navikas S, Birnir B. GABA, a natural immunomodulator of T lymphocytes. J Neuroimmunol. 2008;205:44–50. doi: 10.1016/j.jneuroim.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 49.Bhat R, Axtell R, Mitra A, Miranda M, Lock C, Tsien RW, Steinman L. Inhibitory role for GABA in autoimmune inflammation. Proc Natl Acad Sci U S A. 2010;107:2580–2585. doi: 10.1073/pnas.0915139107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hakanson R, Larsson LI, Liedberg G, Sundler F. Evidence for H2-receptor-mediated feed-back regulation of histamine release from endocrine cells in the rat stomach. J Physiol. 1978;276:151–157. doi: 10.1113/jphysiol.1978.sp012224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hakanson R, Larsson LI, Liedberg G, Rehfeld JF, Sundler F. Suppression of rat stomach histidine decarboxylase activity by histamine: H2-receptor-mediated feed-back. J Physiol. 1977;269:643–667. doi: 10.1113/jphysiol.1977.sp011920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soldani G, Garbarg M, Intorre L, Bertini S, Rouleau A, Schwartz JC. Modulation of pentagastrin-induced histamine release by histamine H3 receptors in the dog. Scand J Gastroenterol. 1996;31:631–638. doi: 10.3109/00365529609009141. [DOI] [PubMed] [Google Scholar]
- 53.Schneider E, Machavoine F, Pleau JM, Bertron AF, Thurmond RL, Ohtsu H, Watanabe T, Schinkel AH, Dy M. Organic cation transporter 3 modulates murine basophil functions by controlling intracellular histamine levels. J Exp Med. 2005;202:387–393. doi: 10.1084/jem.20050195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hrycay EG, Bandiera SM. The monooxygenase, peroxidase, and peroxygenase properties of cytochrome P450. Arch Biochem Biophys. 2012;522:71–89. doi: 10.1016/j.abb.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 55.Brandes LJ, Queen GM, LaBella FS. Displacement of histamine from liver cells and cell components by ligands for cytochromes P450. J Cell Biochem. 2002;85:820–824. doi: 10.1002/jcb.10177. [DOI] [PubMed] [Google Scholar]
- 56.LaBella FS, Brandes LJ. Interaction of histamine and other bioamines with cytochromes P450: implications for cell growth modulation and chemopotentiation by drugs. Semin Cancer Biol. 2000;10:47–53. doi: 10.1006/scbi.2000.0307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.