Abstract
Over time we have come to appreciate that the complex regulation of Rho GTPases involves additional mechanisms beyond the activating role of RhoGEFs, the inactivating function of RhoGAPs and the sequestering activity of RhoGDIs. One class of regulatory mechanisms includes direct modifications of Rho proteins such as isoprenylation, phosphorylation and SUMOylation. Rho GTPases can also regulate each other by means of crosstalk signaling, which is again mostly mediated by GEFs, GAPs and GDIs. More complex mutual regulation ensues when and where two or more Rho proteins activate a common molecular target, i.e., share a common effector. We have recently unraveled a reciprocal mechanism wherein spatiotemporal dynamics of Rac1 activity during migration of Dictyostelium cells is apparently regulated by antagonizing interactions of Rac1-GTP with two distinct effectors. By monitoring specific fluorescent probes, activated Rac1 is simultaneously present at the leading edge, where it participates in Scar/WAVE-mediated actin polymerization, and at the trailing edge, where it induces formation of a DGAP1/cortexillin actin-bundling complex. Strikingly, in addition to their opposed localization, the two populations of activated Rac1 also display opposite kinetics of recruitment to the plasma membrane upon stimulation by chemoattractants. These findings with respect to Rac1 in Dictyostelium suggest a novel principle for regulation of Rho GTPase activity that might also play a role in other cell types and for other Rho family members.
Keywords: Rac1, DGAP1, IQGAP, cortexillin, SCAR/WAVE, cell polarization, actin cytoskeleton, Rho GTPases, Dictyostelium
Eukaryotic cells must establish, maintain and alter their polarity in order to efficiently migrate and orient themselves in response to external clues. Slowly moving cells such as fibroblasts and endothelial cells in culture steer predominantly by gradually changing the direction of extension of their leading edge, and it takes tens of minutes for a cell’s path to deflect significantly.1-3 Rapidly moving cells such as neutrophils and Dictyostelium amoebas, on the other hand, can completely reverse direction of movement within a minute or less.4-6 Moreover, the manner in which such fast-moving cells maneuver is often quite different from their slower counterparts. These cells can turn by shutting down protrusion of the current pseudopodium and initiate extension of a new one at another position.6 As an extreme example of this behavior, a Dictyostelium cell can convert its leading edge into its trailing end, and vice versa, within only 40 seconds (Fig. 1).
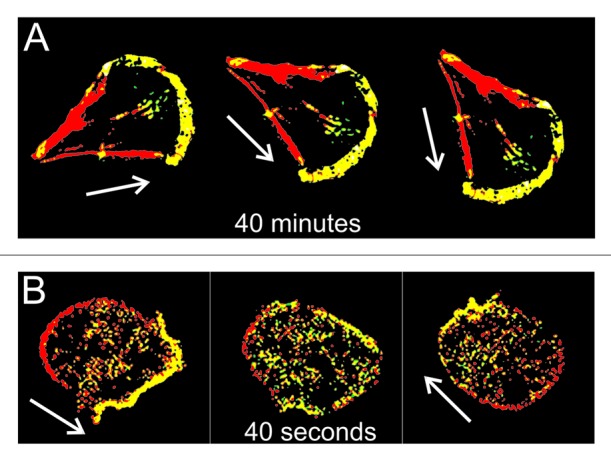
Figure 1.Dictyostelium amoebas accomplish the fastest re-polarization among eukaryotic cells. Whereas, for instance, fibroblasts typically need tens of minutes to sidetrack significantly (A), a Dictyostelium cell can turn “frontside back” within less than a minute (B). Cytoskeletal front and back domains are shown in yellow and red, respectively. Note that posterized pictures of cells are not to scale: fibroblasts are on average 5 times larger than amoebas. White arrows indicate direction of cell movement.
The small GTPases of the Rho family represent a group of signaling proteins that are responsible for the regulation and coordination of numerous cellular activities driven by the actin cytoskeleton.7 Early on it was recognized that prominent members of the Rho family have the capacity to trigger formation of major structures composed of filamentous actin, including stress fibers, lamellipodia and filopodia.7,8 Since then, Rho GTPases have been shown to be essential transducers in several receptor-mediated signaling pathways, and their downstream effectors, which influence remodeling of the actin cytoskeleton, have been identified.9,10 For a comprehensive overview of Rho GTPases-mediated signaling pathways we refer the reader to a number of excellent reviews.7-13 Herein we present a few selected examples of Rho effectors in mammalian cells that either bind directly to actin, or constitute an intermediate layer in signaling cascades that regulate cellular functions driven by the actin cytoskeleton.
Rho GTPases are key molecular switches regulating formation of cellular protrusions.14 Coordinated polymerization and depolymerization of actin are both important for continuous treadmilling of the crosslinked F-actin network in a lamellipodium.15,16 Rac and Cdc42 regulate F-actin polymerization in lamellipodia by activating the Arp2/3 complex, a process mediated through Scar/WAVE and WASP scaffolding protein complexes, respectively.17 Similarly, indirect inactivation of the actin-depolymerizing protein cofilin is effected by Rac through the PAK/Lim-kinase pathway.18 Cdc42 binds to and activates Diaphanous-related formins (Drfs), which are responsible for polymerization of linear actin filaments in filopodia and lamellipodia.19,20 IQGAP proteins constitute another class of Rac and Cdc42 effectors that bind directly to F-actin, but their multiple functional roles are not yet fully understood.21 Formation of actin-myosin II filaments has been suggested to determine the rigidity of the lateral and posterior cellular cortex, and to contribute to retraction of the tail during cell migration. Despite evidence for its interaction with Drfs,22 the main role of Rho appears to be the regulation of actin-myosin II assembly. RhoA activates ROCK and thereby induces phosphorylation of myosin light chain (MLC), which promotes the assembly of actin-myosin II filaments.23
Besides relaying intracellular and extracellular signals to the actin cytoskeleton, Rho GTPases can also regulate each other by crosstalk signaling. Three modes by which these proteins can indirectly interact have been proposed: (1) mutual regulation of activity mediated largely by GEFs or GAPs; (2) regulation of expression and stability mediated by RhoGDI proteins; and (3) regulation of the same downstream pathway through a common effector.24 An example of another signaling strategy is encountered during vulval development in Caenorhabditis elegans, where the small GTPase Ras switches effectors in a temporal sequence, from Raf to RalGEF, to promote divergent and mutually antagonistic cell fates.25 In this commentary we propose a related mechanism in Dictyostelium discoideum, wherein the activity of Rac1 is spatially regulated by antagonizing interactions of Rac1-GTP with two distinct effectors.
Spatially coordinated activation of different Rho GTPases is critical for polarization of migrating mammalian cells. According to the classical view, protrusion of a lamellipodium driven by actin polymerization is induced by activated Rac and Cdc42 at the front of a moving cell, whereas activated Rho regulates myosin-based retraction and detachment from the substratum at the rear.26 A model has been proposed for the spatial segregation of these signals into antagonistic anterior and posterior signaling cascades organized by Rac/Cdc42 and Rho, respectively, which negatively regulate each other.26 Mutual negative regulation of Rho and Rac/Cdc42, mediated by respective GEF proteins, has also been postulated in theoretical models.27,28 Simulations based on these models result in a stable spatial distribution of Rho proteins, which resembles their experimentally determined polarized localization. Exclusive segregation of Rac and Rho into anterior and posterior domains has been challenged experimentally by the use of a FRET-based biosensor for Rac activity in neutrophils, where contrary to expectations, activated Rac was detected in the retracting tails of migrating cells.29 Consistently, Rac1 was shown to promote RhoA-stimulated, myosin II-driven retraction of the trailing cell end.30,31 On the other hand, it has been suggested that Rho activity is also present at the tip region of the lamellipodium in mouse cultured embryonic fibroblasts.32 Quasi-periodical protrusions of the leading edge segments have been associated with RhoA activity, and a pacemaker cycle has been postulated that additionally involves protein kinase A and RhoGDI.33
Correlated fluctuations of lamellipodial segments in large cells occur on a spatial scale of ten microns and a temporal scale of a minute.32,33 Shape fluctuations of Dictyostelium cells typically occur on similar spatial and temporal scales during random motility.6 Therefore, it appears reasonable that, analogous to the situation in mammalian cells, spatiotemporal dynamics of Rho GTPase activity also correlates with, and possibly controls, dynamic cell morphology in Dictyostelium. In order to test this hypothesis, it is necessary to monitor dynamics of activated Rho GTPases in living amoebas. The Dictyostelium genome, however, lacks canonical Rho and Cdc42 family members but instead encodes 18 different Rac proteins.34 We therefore decided to use fluorescent probes that interact with activated forms of three Rac1 isoforms in Dictyostelium (Rac1A, Rac1B and Rac1C), which share an identical effector domain with human Rac1. We used two such probes: a GTPase-binding domain from rat Pak1 kinase fused to a yellow fluorescent protein (GBD-YFP), and a Rac1-GTP-binding protein DGAP1 fused to a red fluorescent protein (mRFP-DGAP1). Interestingly, we found that the two Rac1 effectors were enriched at the opposite regions in the cortex of randomly migrating Dictyostelium cells.35
It is perhaps not that surprising that the two Rac1 effectors localize to different cortical regions in migrating cells. Activated Rac1 proteins interact with numerous proteins implicated in the regulation of actin dynamics and at least a half dozen have been detected in Dictyostelium so far: multiple IQGAP-related proteins, e.g., DGAP136 and GAPA,37 Scar/WAVE,38 formins,39 various PAK-related kinases40 and WASP.41 It is remarkable, however, that the zones of GBD and DGAP1 localization are mutually exclusive, and that the dynamics of their local appearance and disappearance are exactly opposite to each other (Fig. 2A). Whereas such intricate spatiotemporal dynamics can be monitored in non-stimulated, randomly migrating cells, a simplified, uniform response along the cell membrane is induced in chemoattractant pulse experiments. When cAMP or folic acid are applied to competent Dictyostelium cells, the GBD probe is recruited to the plasma membrane (Fig. 2B). At the same time and with highly similar dynamics, the DGAP1 probe is released from the membrane into the cytoplasm (Fig. 2B). These responses are transient and last approximately 30 seconds, a time interval which is comparable to the persistence time of a leading pseudopodium during random migration in Dictyostelium.35
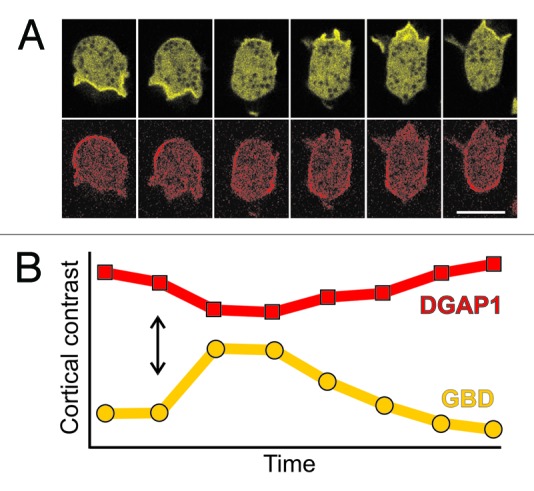
Figure 2. Time scales of spontaneous and induced redistributions of GBD and DGAP1 probes are comparable. (A) A sequence showing spontaneous re-polarization of a randomly migrating cell labeled with GBD-YFP (yellow) and mRFP-DGAP1 (red). Interval between frames, 10 sec; scale bar, 10 μm. (B) Response of the cortical localization of the two probes, GBD-YFP (yellow) and mRFP-DGAP1 (red), to a uniform pulse of chemoattractant. Fifty micromolars of folic acid has been applied to the cell sample at the time-point indicated by the double arrow. The cortical contrast represents the ratio between the average fluorescence intensity of a probe in the cell cortex vs. its average intensity in the cytoplasm. Interval between measurement points: 3.25 sec.
When actin polymerization does not occur, either during spontaneous non-motile intervals or when it is inhibited by polymerization-blocking agents, the cell cortex remains labeled only with the DGAP1 probe.35 Thus, it appears that the major fraction of active Rac1 is bound in a quaternary cortical complex that encompasses DGAP1, Rac1-GTP and the heterodimeric actin-bundling proteins cortexillin I and II.42 In order to initiate local protrusion of a pseudopodium, Arp2/3-mediated nucleation of branched actin filaments has to be triggered. This process is known to be activated by the pentameric Scar/WAVE complex, which resides in its passive, autoinhibited conformation until inhibition is released by binding to active Rac1and acidic phospholipids.43 Our results show that a local release of DGAP1 from the plasma membrane, suggesting disassembly of the quaternary complex, occurs concomitantly with recruitment of the GBD probe to the membrane, which indicates the presence of free Rac1-GTP. These results therefore suggest that the release of active Rac1 from DGAP1/cortexillin, which makes it available for binding to Scar/WAVE, is a prerequisite for initiation of Arp2/3-mediated actin assembly.
Interestingly, the scheme in which Rac1 interacts with two separate effectors at the front and at the back, which negatively regulate each other, sheds new light on results obtained more than a decade ago in a study using DGAP1-null and DGAP1-overexpressing cells.36 DGAP1-overexpressing cells were found to have a decreased F-actin to G-actin ratio, generate fewer actin-based protrusions, and migrate at a slower speed when compared with control.36 These findings are consistent with the notion that more Rac1 is bound to excessive DGAP1 protein in these cells and therefore not available to promote polymerization of actin and associated motility. On the other hand, DGAP1-null cells have the opposite phenotype: they contain more F-actin, make more protrusions, and move significantly faster.36 It therefore appears that in the absence of DGAP1 more free Rac1 is available to drive actin-based motility. These mutant studies thus suggest that an appropriate balance of Rac1 effectors strongly affects the regulation of actin-based motility in Dictyostelium cells.
Actin treadmilling in the lamellipodium is a process characterized by the rapid turnover of its structural and regulatory elements. It has been estimated that, in Dictyostelium, individual actin filaments can grow at a rate of over 1000 subunits per second.44 Consequently, regulatory molecules that control different phases of this process, including Rac1-activated formins and the Scar/WAVE complex, have to be turned on and off at a relatively high rate, probably by binding and releasing Rac1-GTP. It is therefore tempting to speculate that individual activated Rac1 molecules are recycled at a high rate at the leading edge and hence repeatedly detected by the GBD probe. The DGAP1/cortexillin complex has a strong influence on the viscoelastic properties of the cell cortex, harbors multiple actin-binding sites and participates in bundling of actin filaments at the lateral sides and the back of a cell.42,45-47 This complex probably has a comparably slow disassembly rate and therefore effectively acts as a buffer for activated Rac1 in these cortical sites.48 Locally induced disassembly of the DGAP1/cortexillin complex accompanied by the release of Rac1, analogous to its induced release in the chemoattractant pulse experiments, may serve as a trigger for initiation of a new pseudopodium. This process would soften the cortical actin layer by disrupting the crosslinked network of actin filaments beneath the plasma membrane, and simultaneously trigger formation of actin filaments orientated approximately perpendicular to the membrane, which is induced by the Rac1-Scar/WAVE-Arp2/3 pathway.49
Based on recent experimental observations and modeling, it has become increasingly evident that the amoeboid motility of Dictyostelium and similar cells is controlled by an excitable network that encompasses components of the actin cytoskeleton and associated signaling pathways.50 The basic conceptual element of the proposed scheme consists of a double feedback loop between signaling molecules that act as activators and inhibitors of cell motility (Fig. 3A). Despite the fact that the molecular identity of the primary activator and inhibitor and their interactions have not been fully established in Dictyostelium, it has been assumed that the final “readout” of the networks’ activity is the local polymerization of actin.50 Furthermore, it has become increasingly clear that not only one, but multiple feedback loops are involved in this excitable regulatory mechanism, involving global and local, as well as activating and inhibitory interactions.50 One such loop regulates the balance between important signaling phospholipids, phosphatidylinositol [4,5] bis-phosphate (PIP2) and phosphatidylinositol [3,4,5] tris-phosphate (PIP3), that are interconverted by PI3K kinase and PTEN phosphatase.51 The zones labeled with PIP3 and PTEN probes are mutually exclusive and together they cover the entire surface of the plasma membrane in Dictyostelium cells.51 It has been proposed that PIP3 and PTEN constitute an autonomous oscillatory system based on their mutual cross-inhibition.52 This model belongs to a class of mass-conserved reaction-diffusion models where negative regulation of a component in a reaction system can be traced back to its limited supply.53
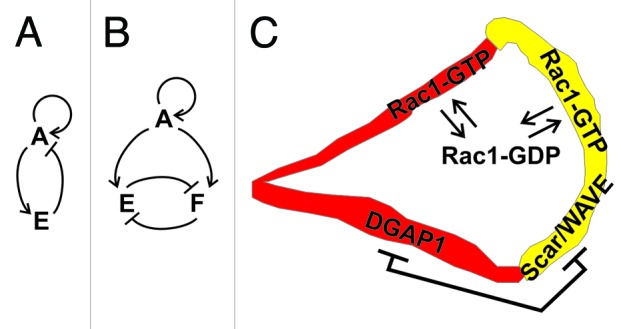
Figure 3. A dual role model for Rac1 in migrating Dictyostelium cells. (A) The basic circuit of an excitable network: activator A activates an effector E, which in turn inhibits A. Auto-activating activity of A is also usually incorporated in the model. (B) A modified version of the model wherein Rac1-GTP plays the role of an activator A that activates two effectors: the DGAP1/cortexillin complex E and the Scar/Wave complex F, which in turn mutually inhibit each other. (C) A schematic representation of regulation and dynamics of Rac1 in a polarized cell. Exchange between the activated form in the membrane and inactivated form in the cytoplasm is governed by the GEF-GAP-GDI-mediated mechanism in both anterior and posterior compartments. Additionally, the two major Rac1 effectors, DGAP1/cortexillin and Scar/WAVE, compete for a common pool of active Rac1 and thereby effectively act as mutual inhibitors.
We propose that the regulation of spatiotemporal dynamics of Rac1 activity is governed by a similar mechanism (Fig. 3B). However, instead of two enzymes that conversely influence the local concentration of PIP3, the central role is assumed by a pair of effectors, DGAP1/cortexillin and Scar/WAVE complex, that compete for a common pool of active Rac1 (Fig. 3C). Under conditions where the total amount of active Rac1 in a cell is limited, and can be adjusted e.g., by the basic GEF-GAP-GDI regulatory mechanism, the mutual inhibition of the two effectors is automatically warranted. This condition is fulfilled in mammalian cells where less than 10% of the major cellular Rho GTPases, including Rac1, are located at the membrane in their GTP-bound state, while the majority is sequestered in a GDI-bound form in the cytoplasm.54,55 It remains to be seen whether other interactions between the two effector complexes in Dictyostelium contribute to the overall feedback network.
In conclusion, we have obtained evidence through the use of specific fluorescently labeled probes that activated Rac1 GTPases are involved in regulation of actin cytoskeleton dynamics both at the front and the back of migrating Dictyostelium cells. Due to its dual role, Rac1 might be one of the key signaling proteins that regulate the sustained morphological oscillations typical for randomly migrating Dictyostelium cells.6 Based on these findings we hypothesize that Rac1 effectors can downregulate each other by competing for a limited pool of active Rac1. It is therefore tempting to speculate that similar mechanisms may play a general role in mutual regulation of signaling pathways downstream of Rho GTPases in various actin-based processes.
Acknowledgments
We thank Dr. Marija-Mary Sopta for critical reading of the manuscript. This work was supported by the Unity through Knowledge Fund grant UKF 1A 10/07 and Ministry of Science, Education and Sport of the Republic of Croatia grant 098-0982913-2858 to I.W., and by grants DAAD-D/07/00065 and DFG FA 330/6-1 to J.F.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/smallgtpases/article/23476
References
- Filić V, Marinović M, Faix J, Weber I. A dual role for Rac1 GTPases in the regulation of cell motility. J Cell Sci. 2012;125:387–98. doi: 10.1242/jcs.089680. [DOI] [PubMed] [Google Scholar]
References
- 1.Ware MF, Wells A, Lauffenburger DA. Epidermal growth factor alters fibroblast migration speed and directional persistence reciprocally and in a matrix-dependent manner. J Cell Sci. 1998;111:2423–32. doi: 10.1242/jcs.111.16.2423. [DOI] [PubMed] [Google Scholar]
- 2.Stokes CL, Lauffenburger DA, Williams SK. Migration of individual microvessel endothelial cells: stochastic model and parameter measurement. J Cell Sci. 1991;99:419–30. doi: 10.1242/jcs.99.2.419. [DOI] [PubMed] [Google Scholar]
- 3.Dunn GA, Brown AF. A unified approach to analysing cell motility. J Cell Sci Suppl. 1987;8:81–102. doi: 10.1242/jcs.1987.supplement_8.5. [DOI] [PubMed] [Google Scholar]
- 4.Friedl P, Borgmann S, Bröcker E-B. Amoeboid leukocyte crawling through extracellular matrix: lessons from the Dictyostelium paradigm of cell movement. J Leukoc Biol. 2001;70:491–509. [PubMed] [Google Scholar]
- 5.Devreotes PN, Zigmond SH. Chemotaxis in eukaryotic cells: a focus on leukocytes and Dictyostelium. Annu Rev Cell Biol. 1988;4:649–86. doi: 10.1146/annurev.cb.04.110188.003245. [DOI] [PubMed] [Google Scholar]
- 6.Weber I. Is there a pilot in a pseudopod? Eur J Cell Biol. 2006;85:915–24. doi: 10.1016/j.ejcb.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–69. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 8.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–14. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 9.Buchsbaum RJ. Rho activation at a glance. J Cell Sci. 2007;120:1149–52. doi: 10.1242/jcs.03428. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz M. Rho signalling at a glance. J Cell Sci. 2004;117:5457–8. doi: 10.1242/jcs.01582. [DOI] [PubMed] [Google Scholar]
- 11.Charest PG, Firtel RA. Big roles for small GTPases in the control of directed cell movement. Biochem J. 2007;401:377–90. doi: 10.1042/BJ20061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 13.Vega FM, Ridley AJ. Rho GTPases in cancer cell biology. FEBS Lett. 2008;582:2093–101. doi: 10.1016/j.febslet.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 14.Ladwein M, Rottner K. On the Rho’d: the regulation of membrane protrusions by Rho-GTPases. FEBS Lett. 2008;582:2066–74. doi: 10.1016/j.febslet.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 15.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–65. doi: 10.1016/S0092-8674(03)00120-X. [DOI] [PubMed] [Google Scholar]
- 16.Le Clainche C, Carlier MF. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol Rev. 2008;88:489–513. doi: 10.1152/physrev.00021.2007. [DOI] [PubMed] [Google Scholar]
- 17.Derivery E, Gautreau A. Generation of branched actin networks: assembly and regulation of the N-WASP and WAVE molecular machines. Bioessays. 2010;32:119–31. doi: 10.1002/bies.200900123. [DOI] [PubMed] [Google Scholar]
- 18.Delorme V, Machacek M, DerMardirossian C, Anderson KL, Wittmann T, Hanein D, et al. Cofilin activity downstream of Pak1 regulates cell protrusion efficiency by organizing lamellipodium and lamella actin networks. Dev Cell. 2007;13:646–62. doi: 10.1016/j.devcel.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faix J, Rottner K. The making of filopodia. Curr Opin Cell Biol. 2006;18:18–25. doi: 10.1016/j.ceb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Block J, Breitsprecher D, Kühn S, Winterhoff M, Kage F, Geffers R, et al. FMNL2 drives actin-based protrusion and migration downstream of Cdc42. Curr Biol. 2012;22:1005–12. doi: 10.1016/j.cub.2012.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brandt DT, Grosse R. Get to grips: steering local actin dynamics with IQGAPs. EMBO Rep. 2007;8:1019–23. doi: 10.1038/sj.embor.7401089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krebs A, Rothkegel M, Klar M, Jockusch BM. Characterization of functional domains of mDia1, a link between the small GTPase Rho and the actin cytoskeleton. J Cell Sci. 2001;114:3663–72. doi: 10.1242/jcs.114.20.3663. [DOI] [PubMed] [Google Scholar]
- 23.Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 2010;67:545–54. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guilluy C, Garcia-Mata R, Burridge K. Rho protein crosstalk: another social network? Trends Cell Biol. 2011;21:718–26. doi: 10.1016/j.tcb.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zand TP, Reiner DJ, Der CJ. Ras effector switching promotes divergent cell fates in C. elegans vulval patterning. Dev Cell. 2011;20:84–96. doi: 10.1016/j.devcel.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu J, Wang F, Van Keymeulen A, Herzmark P, Straight A, Kelly K, et al. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201–14. doi: 10.1016/S0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- 27.Jilkine A, Marée AF, Edelstein-Keshet L. Mathematical model for spatial segregation of the Rho-family GTPases based on inhibitory crosstalk. Bull Math Biol. 2007;69:1943–78. doi: 10.1007/s11538-007-9200-6. [DOI] [PubMed] [Google Scholar]
- 28.Narang A. Spontaneous polarization in eukaryotic gradient sensing: a mathematical model based on mutual inhibition of frontness and backness pathways. J Theor Biol. 2006;240:538–53. doi: 10.1016/j.jtbi.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 29.Gardiner EM, Pestonjamasp KN, Bohl BP, Chamberlain C, Hahn KM, Bokoch GM. Spatial and temporal analysis of Rac activation during live neutrophil chemotaxis. Curr Biol. 2002;12:2029–34. doi: 10.1016/S0960-9822(02)01334-9. [DOI] [PubMed] [Google Scholar]
- 30.Sun CX, Downey GP, Zhu F, Koh ALY, Thang H, Glogauer M. Rac1 is the small GTPase responsible for regulating the neutrophil chemotaxis compass. Blood. 2004;104:3758–65. doi: 10.1182/blood-2004-03-0781. [DOI] [PubMed] [Google Scholar]
- 31.Pestonjamasp KN, Forster C, Sun C, Gardiner EM, Bohl B, Weiner O, et al. Rac1 links leading edge and uropod events through Rho and myosin activation during chemotaxis. Blood. 2006;108:2814–20. doi: 10.1182/blood-2006-01-010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, et al. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009;461:99–103. doi: 10.1038/nature08242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tkachenko E, Sabouri-Ghomi M, Pertz O, Kim C, Gutierrez E, Machacek M, et al. Protein kinase A governs a RhoA-RhoGDI protrusion-retraction pacemaker in migrating cells. Nat Cell Biol. 2011;13:660–7. doi: 10.1038/ncb2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vlahou G, Rivero F. Rho GTPase signaling in Dictyostelium discoideum: insights from the genome. Eur J Cell Biol. 2006;85:947–59. doi: 10.1016/j.ejcb.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Filić V, Marinović M, Faix J, Weber I. A dual role for Rac1 GTPases in the regulation of cell motility. J Cell Sci. 2012;125:387–98. doi: 10.1242/jcs.089680. [DOI] [PubMed] [Google Scholar]
- 36.Faix J, Clougherty C, Konzok A, Mintert U, Murphy J, Albrecht R, et al. The IQGAP-related protein DGAP1 interacts with Rac and is involved in the modulation of the F-actin cytoskeleton and control of cell motility. J Cell Sci. 1998;111:3059–71. doi: 10.1242/jcs.111.20.3059. [DOI] [PubMed] [Google Scholar]
- 37.Adachi H, Takahashi Y, Hasebe T, Shirouzu M, Yokoyama S, Sutoh K. Dictyostelium IQGAP-related protein specifically involved in the completion of cytokinesis. J Cell Biol. 1997;137:891–8. doi: 10.1083/jcb.137.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bear JE, Rawls JF, Saxe CL., 3rd SCAR, a WASP-related protein, isolated as a suppressor of receptor defects in late Dictyostelium development. J Cell Biol. 1998;142:1325–35. doi: 10.1083/jcb.142.5.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schirenbeck A, Bretschneider T, Arasada R, Schleicher M, Faix J. The Diaphanous-related formin dDia2 is required for the formation and maintenance of filopodia. Nat Cell Biol. 2005;7:619–25. doi: 10.1038/ncb1266. [DOI] [PubMed] [Google Scholar]
- 40.Müller-Taubenberger A, Bretschneider T, Faix J, Konzok A, Simmeth E, Weber I. Differential localization of the Dictyostelium kinase DPAKa during cytokinesis and cell migration. J Muscle Res Cell Motil. 2002;23:751–63. doi: 10.1023/A:1024475628061. [DOI] [PubMed] [Google Scholar]
- 41.Myers SA, Han JW, Lee Y, Firtel RA, Chung CY. A Dictyostelium homologue of WASP is required for polarized F-actin assembly during chemotaxis. Mol Biol Cell. 2005;16:2191–206. doi: 10.1091/mbc.E04-09-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faix J, Weber I, Mintert U, Köhler J, Lottspeich F, Marriott G. Recruitment of cortexillin into the cleavage furrow is controlled by Rac1 and IQGAP-related proteins. EMBO J. 2001;20:3705–15. doi: 10.1093/emboj/20.14.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Z, Borek D, Padrick SB, Gomez TS, Metlagel Z, Ismail AM, et al. Structure and control of the actin regulatory WAVE complex. Nature. 2010;468:533–8. doi: 10.1038/nature09623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diez S, Gerisch G, Anderson K, Müller-Taubenberger A, Bretschneider T. Subsecond reorganization of the actin network in cell motility and chemotaxis. Proc Natl Acad Sci U S A. 2005;102:7601–6. doi: 10.1073/pnas.0408546102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simson R, Wallraff E, Faix J, Niewöhner J, Gerisch G, Sackmann E. Membrane bending modulus and adhesion energy of wild-type and mutant cells of Dictyostelium lacking talin or cortexillins. Biophys J. 1998;74:514–22. doi: 10.1016/S0006-3495(98)77808-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stock A, Steinmetz MO, Janmey PA, Aebi U, Gerisch G, Kammerer RA, et al. Domain analysis of cortexillin I: actin-bundling, PIP(2)-binding and the rescue of cytokinesis. EMBO J. 1999;18:5274–84. doi: 10.1093/emboj/18.19.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weber I, Niewöhner J, Faix J. Cytoskeletal protein mutations and cell motility in Dictyostelium. Biochem Soc Symp. 1999;65:245–65. [PubMed] [Google Scholar]
- 48.Ren Y, Effler JC, Norstrom M, Luo T, Firtel RA, Iglesias PA, et al. Mechanosensing through cooperative interactions between myosin II and the actin crosslinker cortexillin I. Curr Biol. 2009;19:1421–8. doi: 10.1016/j.cub.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Medalia O, Weber I, Frangakis AS, Nicastro D, Gerisch G, Baumeister W. Macromolecular architecture in eukaryotic cells visualized by cryoelectron tomography. Science. 2002;298:1209–13. doi: 10.1126/science.1076184. [DOI] [PubMed] [Google Scholar]
- 50.Iglesias PA, Devreotes PN. Biased excitable networks: how cells direct motion in response to gradients. Curr Opin Cell Biol. 2012;24:245–53. doi: 10.1016/j.ceb.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arai Y, Shibata T, Matsuoka S, Sato MJ, Yanagida T, Ueda M. Self-organization of the phosphatidylinositol lipids signaling system for random cell migration. Proc Natl Acad Sci U S A. 2010;107:12399–404. doi: 10.1073/pnas.0908278107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shibata T, Nishikawa M, Matsuoka S, Ueda M. Modeling the self-organized phosphatidylinositol lipid signaling system in chemotactic cells using quantitative image analysis. J Cell Sci. 2012;125:5138–50. doi: 10.1242/jcs.108373. [DOI] [PubMed] [Google Scholar]
- 53.Otsuji M, Ishihara S, Co C, Kaibuchi K, Mochizuki A, Kuroda S. A mass conserved reaction-diffusion system captures properties of cell polarity. PLoS Comput Biol. 2007;3:e108. doi: 10.1371/journal.pcbi.0030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garcia-Mata R, Boulter E, Burridge K. The ‘invisible hand’: regulation of RHO GTPases by RHOGDIs. Nat Rev Mol Cell Biol. 2011;12:493–504. doi: 10.1038/nrm3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boulter E, Garcia-Mata R, Guilluy C, Dubash A, Rossi G, Brennwald PJ, et al. Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1. Nat Cell Biol. 2010;12:477–83. doi: 10.1038/ncb2049. [DOI] [PMC free article] [PubMed] [Google Scholar]


