Abstract
Nitrate is an important nutrient required for plant growth. It also acts as a signal regulating plant development. Nitrate is actively taken up and transported by nitrate transporters (NRT), which form a large family with many members and distinct functions. In contrast to Arabidopsis and rice there is little information about the NRT family in woody plants such as Populus. In this study, a comprehensive analysis of the Populus NRT family was performed. Sixty-eight PtNRT1/PTR, 6 PtNRT2, and 5 PtNRT3 genes were identified in the P. trichocarpa genome. Phylogenetic analysis confirmed that the genes of the NRT family are divided into three clades: NRT1/PTR with four subclades, NRT2, and NRT3. Topological analysis indicated that all members of PtNRT1/PTR and PtNRT2 have 8 to 12 trans-membrane domains, whereas the PtNRT3 proteins have no or up to two trans-membrane domains. Four PtNRT3 members were predicted as secreted proteins. Microarray analyses revealed tissue-specific expression patterns of PtNRT genes with distinct clusters of NRTs for roots, for the elongation zone of the apical stem segment and the developing xylem and a further cluster for leaves, bark and wood. A comparison of different poplar species (P. trichocarpa, P. tremula, P. euphratica, P. fremontii x P. angustifolia, and P. x canescens) showed that the tissue-specific patterns of the NRT genes varied to some extent with species. Bioinformatic analysis of putative cis-regulatory elements in the promoter regions of PtNRT family retrieved motifs suggesting the regulation of the NRT genes by N metabolism, by energy and carbon metabolism, and by phytohormones and stress. Multivariate analysis suggested that the combination and abundance of motifs in distinct promoters may lead to tissue-specificity. Our genome wide analysis of the PtNRT genes provides a valuable basis for functional analysis towards understanding the role of nitrate transporters for tree growth.
Introduction
Nitrate (NO3 −) is a major N source for higher plants [1]. A large proportion of the NO3 − acquired by plants from soil is actively transported through NO3 − transporters (NRT) [2]. To cope with low (<1 mM) or high (>1 mM) NO3 − concentrations in soil plant roots have developed high-affinity and low-affinity nitrate uptake systems [3]. Physiological studies revealed that each system is composed of constitutive and inducible components [4]. The plant nitrate transporters form a large family composed of NRT1/PTR (nitrate/peptide transporters), NRT2, and NRT3 members [3]. The members of the NRT family play multifunctional roles in nitrate uptake and transport throughout the plant body [1], [5]. Tissue-specific expression of NRT family genes has been observed in several species, e.g., Arabidopsis, rice, and cucumber [5]–[7].
The first nitrate transporter (CHL1 or NRT1.1) was identified in a chlorate resistant mutant of Arabidopsis [8]. It takes part in both low-affinity and high-affinity nitrate transport, and also functions as a nitrate sensor to activate the expression of nitrate related genes in plants [9]. Other members of NRT1/PTR are low-affinity nitrate or peptide transporters (PTR). For example, AtNRT1.2 is a constitutive low-affinity nitrate transporter expressed mainly in root hairs and the epidermis of Arabidopsis [10]; further three NRT1 members (NRT1.5, NRT1.8 and NRT1.9) are involved in regulating root to shoot long-distance nitrate translocation [1]; and AtNRT1.4 is expressed in petioles and mid-rips with functions for the nitrate allocation to leaves [3], [11].
Peptide transporters (PTR) are also important members of the NRT1/PTR family [12] because they transport di- and tripeptides as well as many other molecules. In Arabidopsis, tissue-specific expression was shown for AtPTR2 in green siliques, roots, and young seedlings. AtPTR1 and AtPTR4 are expressed in vascular tissues throughout the plant suggesting a role in long-distance peptide transport [13], [14]. AtPTR6 is expressed in senescing leaves and pollen [13]. AtPTR5 is also highly expressed in pollen [15].
Unlike the large subfamily of NRT1/PTR, which consists of 53 members in Arabidopsis, only seven NRT2 genes were found in Arabidopsis. Single, double, and triple knockout mutants of NRT2.1, NRT2.2, NRT2.4, and NRT2.7 in Arabidopsis all have nitrate-related phenotypes and have, therefore, been invoked in nitrate transport [16]–[18]. Most of the AtNRT2 genes are chiefly expressed in the roots, with the exception of AtNRT2.7, whose expression was higher in shoots than in roots [19]. Several recent papers document that the full function of NRT2 proteins is dependent on another family named NRT3 (or NAR2) forming together a two component nitrate uptake system [20]–[23]. The NRT3 family has two members in Arabidopsis [24].
Up to now, the molecular features and the regulation of nitrate transporters have been studied mostly in Arabidopsis thaliana. With the help of Arabidopsis sequences, the whole NRT family has been identified in rice [12] and Lotus japonicus [25]. Plett et al. (2010) included eight NRT1, five NRT2, and four NRT3 genes of P. trichocarpa when comparing NRT genes of dicots and monocots [20], but a comprehensive analysis of the NRT family in trees is still lacking. Because poplars are an important feedstock for sustainable bio-energy production [26], the molecular basis of growth stimulation by nitrogen supply is receiving increasing attention [27]. The family of ammonium transporters has been characterized in Populus [28]. However, nitrate is the major form of nitrogen uptake for poplars [29] and therefore, more information on poplars nitrate transporters is important. Recently, the expression of a number of genes involved in nitrogen metabolism including two NRT1, two NRT2, and three NRT3 genes were compared in two poplar species with contrasting biomass production and revealed distinct patterns in both roots and leaves [30]. However, systematic analyses of the tissue-specific expression patterns of NRT genes have not yet been conducted in poplar.
We present here the first complete analysis of the NRT family in poplar, including the identification of putative NRT genes based on their sequence similarity to Arabidopsis genes, their phylogenetic relationships and their expression profiles in different tissues. We furthermore included the prediction of cis-regulatory elements (CRE) in the 5′- UTR of all putative NRT1/PTR, NRT2, and NRT3 members. CREs play essential roles in regulating gene expression [31], [32]. Previous studies reported several putative NO3 − response motifs in the NiR and NIA promoters of Arabidopsis [33]–[35]. A 150 bp cis-acting element in AtNRT2.1 promoter was first identified as a NO3 – specific regulatory region [36]. We used multivariate analysis to identify links between CRE patterns and tissue-specific expression as a first step to understand the regulatory mechanisms of NRT genes in poplars. Our results obtained with P. trichocarpa and P. tremula provide a valuable basis for further studies to gain insights into the functions of the NRTs in poplar.
Results
Identification of NRT Family Genes
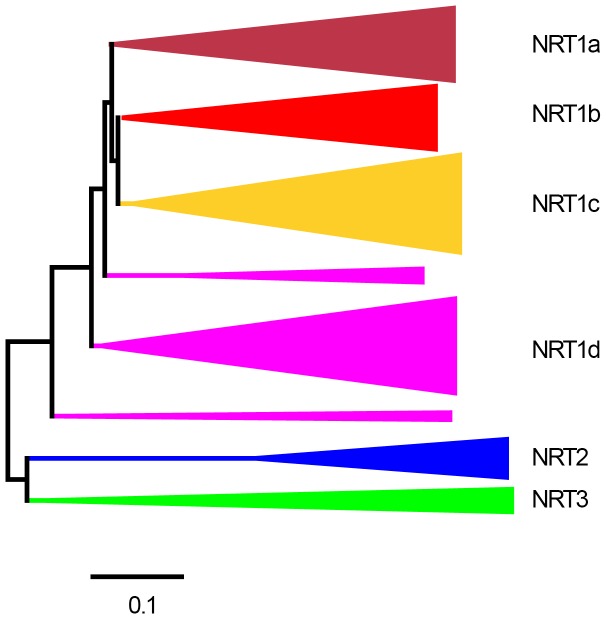
To explore the entire NRT family in poplar, we searched the genome of P. trichocarpa for PtNRT genes based on the full NRT family in Arabidopsis. We retrieved 68 PtNRT1/PTR, 6 PtNRT2, and 5 PtNRT3 genes in poplar (Table S1). We aligned the amino acid sequences of NRT genes of P. trichocarpa and A. thaliana. The resulting phylogenetic tree defined three main clades with NRT1/PTR, NRT2, and NRT3 (Figure 1). The NRT1/PTR family formed four subclades, which were named PtNRT1a, PtNRT1b, PtNRT1c and PtNRT1d (Figure 2). Consistent with Plett et al. (2010) the names of NRTs in poplar were assigned by their sequence similarities with Arabidopsis NRT genes [20]. However, as 36 of the 53 NRT1/PTR members in Arabidopsis have not yet any specific names, we assigned working names to the genes for the purpose of our study (Figure 2, Table S1).
Figure 1. Overview on the phylogeny of the NRT families in P. trichocarpa and A. thaliana.
Detailed information of each clade is presented in figure 2. ClustalX2 was used to perform the alignment of the amino acids and to calculate the final tree with the neighbor-joining method and a bootstrap value of n = 1,000. The tree was displayed using MEGA 5.
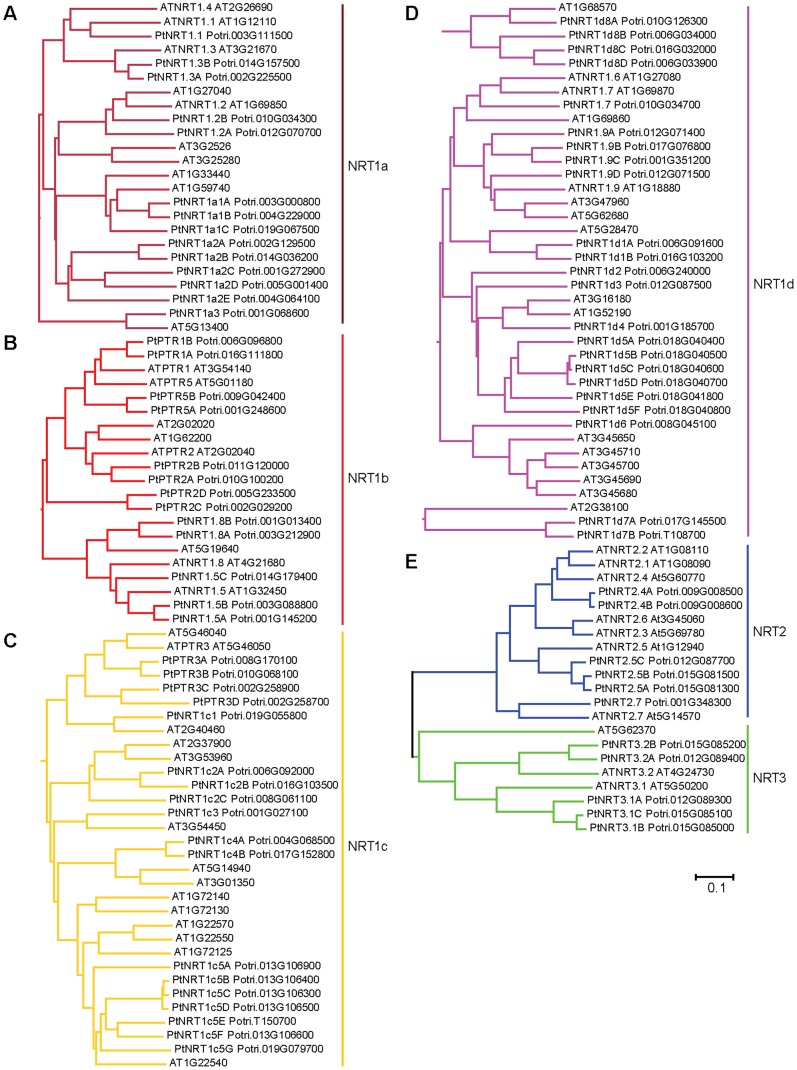
Figure 2. Expanded view of the phylogenetic relationships of the genes in the NRT families of P. trichocarpa and A. thaliana.
A. subclade NRT1a. B. subclade NRT1b. C. subclade NRT1c. D. subclade NRT1d. E. clade NRT2 and NRT3. A–D represent together clade NRT1/PTR. ClustalX2 was used for the alignment of the amino acids and to calculate the final tree with the neighbor-joining method and a bootstrap value of n = 1,000. The tree was displayed using MEGA 5.
The lengths of the 53 PtNRT1/PTR genes range from 1623 bp to 2037 bp with 3 to 6 exons (Table S1). The genes are distributed across 17 of the 19 poplar chromosomes with exception of chromosome 7 and chromosome 15 (Table S1). The lengths of the six PtNRT2 genes range from 1068 bp to 1593 bp with 2 to 3 exons (Table S1). The PtNRT2 genes are localized on four chromosomes: PtNRT2.4A/B on chromosome 9, PtNRT2.5A/B on chromosome 15, PtNRT2.5C on chromosome 12 and PtNRT2.7 on chromosome 1 (Table S1). The five PtNRT3 genes have lengths of 618 bp to 1107 bp with 2 to 3 exons and are located on chromosome 12 and chromosome 15 (Table S1).
The known Arabidopsis NRT1 proteins are distributed in the subclades PtNRT1a, PtNRT1b, and PtNRT1d (Figure 2). The orthologs of NRT1.1, NRT1.2, NRT1.3, and NRT1.4 in Arabidopsis and Populus are present in subclade NRT1a (Figure 2A). The orthologs of NRT1.5 and NRT1.8 and peptide transporters (PTR1, PTR2, PTR4, PTR5, and PTR6) in Arabidopsis and Populus are in subclade NRT1b (Figure 2B). The orthologs of two newly identified Arabidopsis glucosinolate transporters (GTR1 and GTR2) [37] together with NRT1.6, NRT1.7, and NRT1.9 in Arabidopsis and Populus are found in subclade NRT1d (Figure 2D). With the exception of the PTR3 genes, the functions encoded by genes in subclade NRT1c are unknown and names have not yet been assigned to them (Figure 2C). In order to distinguish the unknown poplar NRT1/PTR genes, they were named as PtNRT1+ their subclade assignment and then consecutively numbered (Figure 2, Table S1).
Duplication and multiplication were found in the PtNRT family compared with Arabidopsis. We found twelve duplicated gene pairs in PtNRT1/PTR present on duplicated chromosomes of P. trichocarpa [38] (Figure 2, Table S1). Especially, in subclade NRT1b, 10 out of 13 Populus genes are belonging to those paired PtNRT genes. We found some multiplicated PtNRT genes in subclade PtNRT1a, PtNRT1c, and PtNRT1d. For instance, eight PtNRT genes are the orthologs of only two Arabidopsis AtNRT1s (At1G33440 and At1G97940) in subclade PtNRT1a. Six Populus PtNRT genes are orthologs of At1G22540 in subclade PtNRT1c. In subclade PtNRT1d, six Populus PtNRT1 genes are similar to At1G52190 and At3G16180. No multiplication was found in clades NRT2 and NRT3. Members of AtNRT3 are duplicated or triplicated in Populus. By contrast, some branches of the phylogenetic tree in clade NRT1/PTR and NRT2 contained more Arabidopsis genes than Populus genes. For instance, AtNRT1.1 and AtNRT1.4 are orthologs of one Populus gene named PtNRT1.1 (Figure 2A). Both AtNRT1.6 and AtNRT1.7 are orthologs of PtNRT1.7 (Figure 2D). Another clear deletion event was found in clade NRT2. One branch of clade NRT2 has two Populus genes (PtNRT2.4A/B) but five Arabidopsis genes including AtNRT2.1, AtNRT2.2, AtNRT2.3, AtNRT2.4, and AtNRT2.6 (Figure 2E).
In order to investigate further properties of the PtNRT proteins, we predicted the hydrophobicity by the TMHMM program (http://www.cbs.dtu.dk/services/TMHMM/). The analysis suggests that all members of PtNRT1/PTR and PtNRT2 have 8 to 12 trans-membrane domains (TMDs) (Table S1). In contrast, PtNRT3.1A/B/C were predicted to have only one to two TMDs, and no TMDs were found in PtNRT3.2A/B. The prediction of signal peptides showed that 12 NRT genes encoded putatively secreted proteins (Table S1). Four of those are belonging to the PtNRT3 (PtNRT3.1A/B/C and PtNRT3.2A) family. The other eight putatively secreted proteins are belonging to the PtNRT1/PTR cluster (Table S1). The prediction of signal peptides was also performed for Arabidopsis. We found that AtNRT3.1 and At2g38100, which is the closest ortholog to PtNRT1d7A, are also putatively secreted proteins (Table S1). Overall, these analyses reveal that the structures of the PtNRT3 proteins are distinctly different from those of the PtNRT1/PTR and PtNRT2 genes.
Differential Expression of NRT Genes in Poplar
In order to explore the expression patterns of poplar NRT genes we compiled microarrays from public databases and own analyses for six tissues including the elongation segment of the stem top (T), the developing xylem (DX), wood (W), bark (B), leaves (L), and roots (R) (Table S2). The data base encompassed five poplar species. Significant signal intensities were detected for 57 PtNRT genes on the microarrays (Table S1). Eleven genes have no probesets and eleven genes were not expressed on the microarrays (Table S1). For three of the latter group, quantitative real time polymerase chain reaction (qRT-PCR) data are available (see below under “tissue-specific expression”) suggesting that the expression of these silent genes was too low for detection by microarray analyses. This may also be true for the remaining eight genes or they may be expressed only under specific conditions.
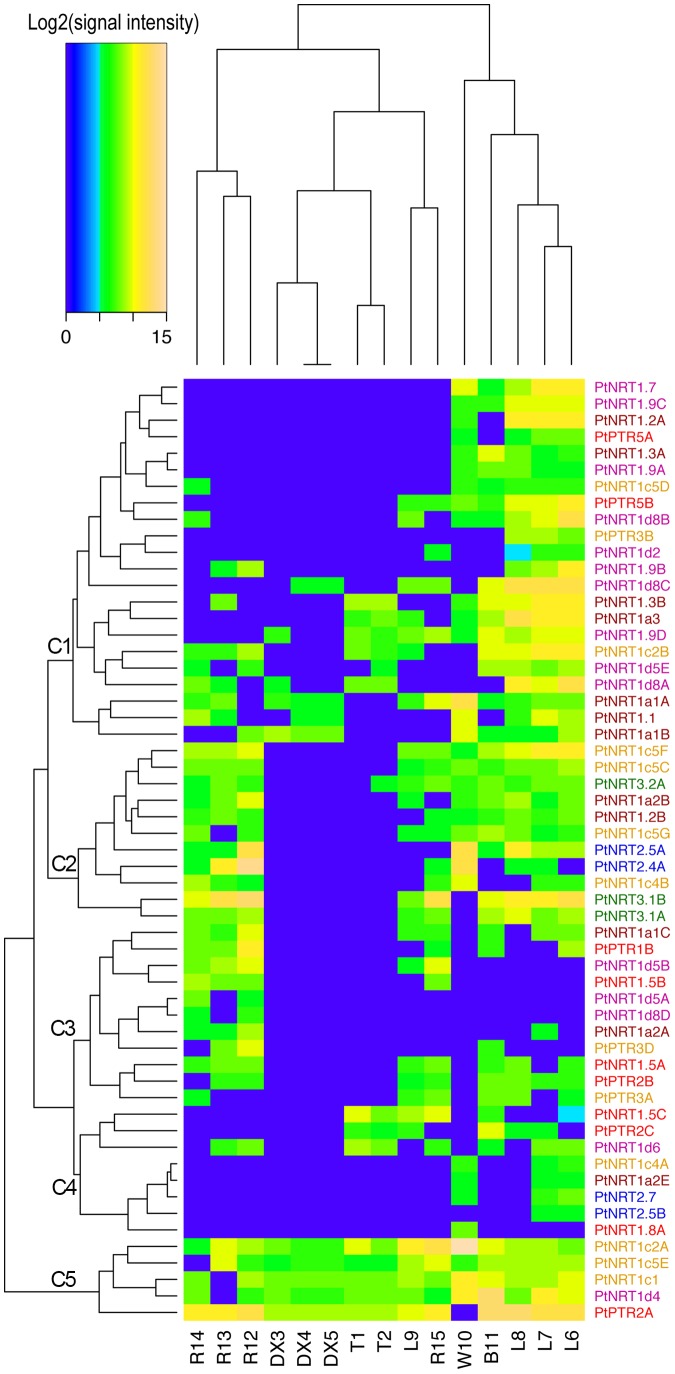
Hierarchical clustering of the available dataset revealed clear differential expression of PtNRT genes in different poplar tissues (Figure 3). The tissues were separated into three clusters: The NRT expression patterns in roots from P. x canescens, P. euphratica and P. tremula formed one cluster, that of the developing xylem and tip together with roots and leaves of axenically grown P. trichocarpa formed another cluster and the third cluster encompassed leaves of P. fremontii x angustifolia and wood, and bark of P. trichocarpa. Based on the expression patterns in the six tissues analyzed, the PtNRT genes were grouped into C1 to C5 (Figure 3). All 22 genes in C1 were expressed in leaves, and 12 of those were also expressed in bark and wood. Genes in C2 were expressed in the roots, leaves, bark, and wood, but not in the developing xylem and tip. All genes in C3 were expressed in roots, and some of those had expression in other tissues. Genes in C5 were expressed in all six tissues. We found that most of the PtNRT genes were expressed in leaves, bark, and wood, whereas a lower number of PtNRT genes were expressed in roots (Figure 3). Very few genes were found in the developing xylem and the elongation zone, all belonging to the PtNRT1/PTR group (Figure 3).
Figure 3. Expression profiles of NRT family genes in Populus across different tissues.
The heatmap represents the hierarchical clustering of average log2(signal intensity) of Populus NRT genes in various tissues. T, stem top; DX, developing xylem; L, leaves; W, wood; B, bark; R, roots. The numbers refer to different experiments compiled in Table S2. The color of the gene name represents the clade in the phylogenetic tree (Figure 1). The Affymetrix microarray data were obtained from ArrayExpression database (http://www.ebi.ac.uk/arrayexpress) and their accession number are shown in Table S2.
Comparing the Tissue-specific Expression of NRT Genes in P. tremula
We selected 21 poplar NRT genes (13 NRT1, 5 NRT2, and 3 NRT3), whose othologs had previously been functionally characterized in Arabidopsis [1] for qRT-PCR in P. tremula. Eight tissues of P. tremula were used including fine roots (FR), coarse roots (CR), developing xylem (DX), wood (W), bark (B), stem top (T), leaves (L), and petioles (P).
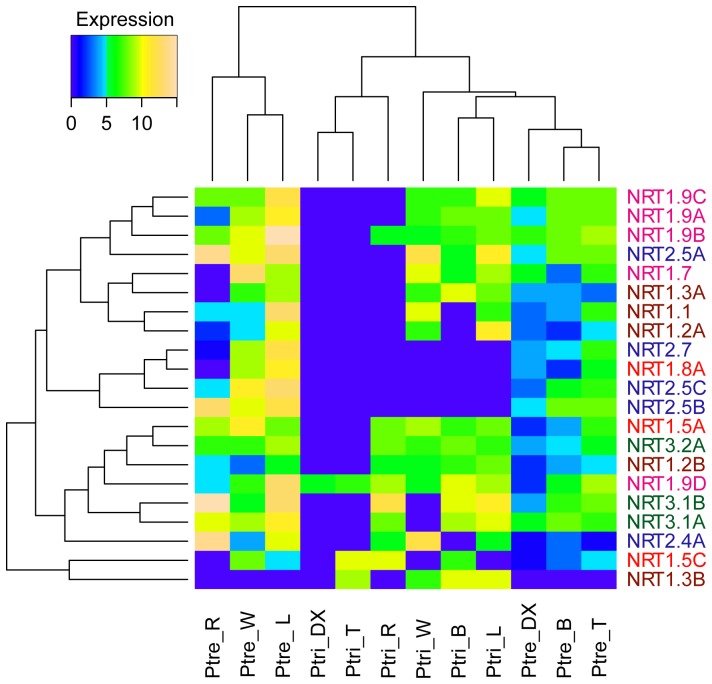
A heatmap was generated to compare the expression of NRT genes of P. tremula (PtreNRT) with those of P. trichocarpa (PtNRT). Hierarchical clustering revealed a clear separation of the two poplar species (Figure 4). Higher signal intensities were observed in P. tremula analyzed by qRT-PCR than in P. trichocarpa analyzed by microarrays. Tissue-specific expression of the selected PtreNRT genes was observed (Figure 4). Leaves, wood, coarse roots, and petioles formed one cluster. Most of the tested PtreNRT genes were highly expressed in those tissues. Developing xylem, bark, and stem tip formed another cluster, which contained fewer expressed PtreNRT genes (Figure 4). We found that bark exhibited diverging expression of NRT genes in two poplar species (Figure 4). In P. trichocarpa NRT genes in bark clustered with leaves, but in P. tremula with developing xylem and stem top. In summary, the results of the qRT-PCR confirmed a clear separation of NRT gene expression in different tissues and pointed to species related differences.
Figure 4. Comparison of tissue-specific expression patterns of NRT genes in P. trichocarpa (Ptri) and P. tremula (Ptre).
The heatmap represents the hierarchical clustering of average log2(relative expression) of NRT genes in stem top (T), bark (B), developing xylem (DX), leaves (L), wood (W), and roots (R). The color of the gene name represents the clade in the phylogenetic tree (Figure 1).
Cis - regulatory Elements of Nitrate Transporters in P. trichocarpa
To investigate whether the differential regulation of the P. trichocarpa NRT genes might be related to differences in regulatory motifs, the 1 kb UTRs of all PtNRT1, PtNRT2, and PtNRT3 genes were analyzed using the PLACE signal scan program. This analysis resulted in 142 different motifs, many of which are related to development, stress, or phytohormones (Table S3). A cluster analysis divided the predicted CREs into 14 groups named as MC1 to MC14 (Figure S1). MC1 contained 9 motifs present in the promoter regions of all NRT genes, namely CAATBOX1, ARR1AT, WRKY71OS, ROOTMOTIFTAPOX1, CACTFTPPCA1, DOFCOREZM, GATABOX, GT1CONSENSUS, and GTGANTG10 (Table S3). These motifs were also the most abundant motifs with more than 11 copies per gene and per motif.
The cluster analysis furthermore showed that the PtNRT genes were divided into nine groups from GC1 to GC9, based on the enrichment of CREs (Figure S1). We found that PtNRT2 genes were all clustered in GC6, and the PtNRT3 genes in GC8. The PtNRT1/PTR clustered in 8 different groups based on their motif patterns. This analysis highlights clear differences of the CRE patterns in the promoters of the PtNRT1/PTR, PtNRT2, and PtNRT3 families, respectively.
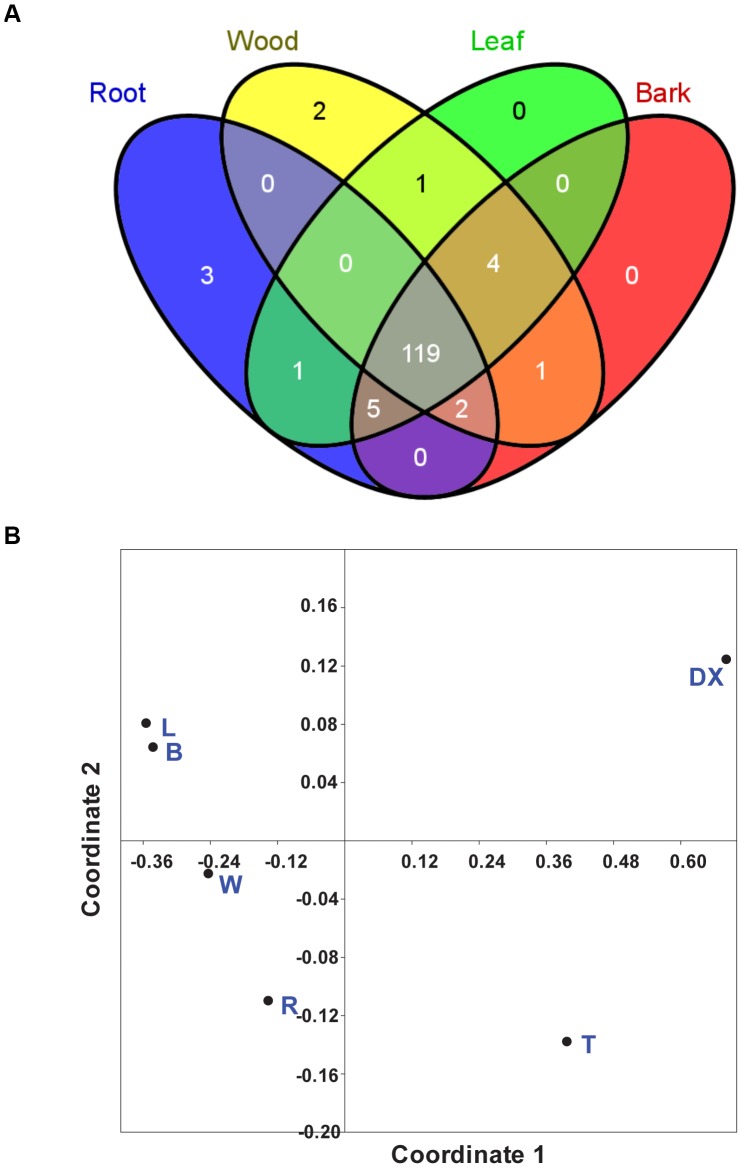
In order to explore potential relationships between the motif patterns and the tissue-specific expression of PtNRT genes, we compared the presence and absence of known motifs across the analyzed tissues (Figure 5A). Most of the motifs (119/136 in P. trichocarpa) were present in the four main tissues leaves, bark, wood and roots (Figure 5A). Roots contained three (ACGTTBOX, ACGTOSGLUB1, E2F1OSPCNA) and wood contained two unique motifs (LRENPCABE, ABREATCONSENSUS), whereas no unique motifs occurred in leaves, bark, developing xylem or tip. Therefore, tissue-specificity of the observed NRT expression patterns cannot be related to the presence or absence of known CREs. Multivariate analysis of motif abundance in the different tissues identified similar patterns for bark and leaves, and distinct patterns for all other tissues (Figure 5B). These results suggest that different regulatory mechanisms may act in specific tissue controlling PtNRT gene expression.
Figure 5. cis-regulatory elements of NRT genes in P. trichocarpa.
A. Overlapping CREs of NRT genes in leaves, bark, wood, and roots. The diagram shows the number of CREs in PtNRT genes expressed in specific tissues. The Venn diagram was drawn using VENNY (http://bioinfogp.cnb.csic.es/tools/venny/). B. Nonmetric multidimensional scaling (NMDS) ordination of CREs abundance and tissue specific expression of PtNRT genes. T, stem top; DX, developing xylem; L, leaves; W, wood; B, bark; R, roots. The data matrix used for the NMDS analyses is shown in Table S3.
Discussion
The Poplar Genes Families NRT1/PTR and NRT3 but not NRT2 are Expanded Compared with Arabidopsis
Here, we present a comprehensive analysis of NRT related genes in poplar. We retrieved a total of 79 sequences with high similarities to the 62 known Arabidopsis genes. In a preceding analysis Plett et al. (2010) included 8 PtNRT1, 5 PtNRT2 and 4 PtNRT3 genes for in silico analysis of NRT genes of dicots and monocots [20]. The present identification of PtNRT family was based on newly characterized NRT genes of Arabidopsis (AtNRT1.9, AtGTP1, and AtGTP2) in addition to those already available before 2010 [37], [39] and relied on an improved annotation of the poplar genome (Populus trichocarpa v3, DOE-JGI, http://www.phytozome.net/poplar). Similar to other species like Arabidopsis, rice and Lotus, a high number of NRT1/PTR genes and a comparatively low number of NRT2 and NRT3 genes were retrieved. In comparison with NRT1/PTR in Arabidopsis (53 genes) and Lotus (37 genes), in both rice (80 genes) and poplar (65 genes) the NRT1/PTR family was expanded [12], [25].
The P. trichocarpa genome is duplicated [38] and therefore, contains more protein-coding genes than Arabidopsis, ranging on average from 1.4 to 1.6 putative Populus genes for each Arabidopsis gene [38]. Whole-genome duplication raises the rate of gene gains and losses [40]. Many studies presented evidence for gene duplication in P. trichocarpa such as the analysis of the complete glutamine synthetase family [41]. A high proportion of the members of the oligopeptide transporter family in P. trichocarpa might be derived from tandem duplication [42]. Our phylogeny based on full-length NRT amino acid sequences revealed that gene expansion and loss occurred also in the NRT family of P. trichocarpa compared with Arabidopsis. We found thirteen duplicated PtNRT1/PTR two NRT3 gene pairs in P. trichocarpa compared with Arabidopsis. For example, three closely related orthologs of Arabidopsis AtNRT2.5 were identified in Populus. Three branches in clade NRT1/PTR had one to two Arabidopsis AtNRT genes but seven to eight Populus PtNRT genes. These results suggest that duplications and multiplications have contributed to the expansion of the PtNRT gene family in Populus.
However, a loss of NRT genes was also observed in NRT1/PTR and NRT2 of P. trichocarpa. For example, AtNRT1.1 and AtNRT1.4 are the orthologs of one poplar gene (PtNRT1.1). AtNRT2.1, AtNRT2.2, and AtNRT2.4 in Arabidopsis have complementary functions in high affinity nitrate transport [43]. The phylogeny showed that AtNRT2.1, AtNRT2.2, AtNRT2.4, together with AtNRT2.3 and AtNRT2.6 are the orthologs of only two poplar proteins (PtNRT2.4A/B). The functional significance of these results will have to be addressed in future studies. Our current analysis provides a valuable basis for a systematic approach to evaluate nitrate uptake and translocation in poplar.
In Arabidopsis, topology predicted that both NRT1s and NRT2s contain 12 putative TMDs with a large hydrophilic loop between TM6 and TM7 [12]. Similarly, we found that the predicted poplar NRT1 and NRT2 proteins have 8 to 12 TMS. In contrast to the NRT1 and NRT2 members of Arabidopsis and P. trichocarpa, the NRT3 proteins in Arabidopsis and P. trichocarpa have either no or few (up to two) TMDs. In addition, PtNRT3.1A/B/C, PtNRT3.2A, and AtNRT3.1A were predicted as secreted proteins. Currently, experimental evidence for NRT3s as secreted protein is missing. However, NRT3 proteins are soluble proteins that have been shown to form a two component complex with NRT2 proteins for high affinity nitrate uptake [5], [21]–[23], [44].
RT-PCR analysis in Arabidopsis revealed that 51 of the 53 NRT1/PTR genes are expressed, whereas two genes could be pseudogenes [12]. To examine whether the identified PtNRT genes in poplar are expressed, we studied the transcript abundance of 79 PtNRT genes using microarray experiments representing six tissues in Populus. The results indicate that 57 PtNRT genes were expressed, 11 PtNRT genes exhibited no signal, and 11 PtNRT genes had no probesets on the arrays. However, 8 of the silent PtNRT genes in P. trichocarpa were expressed in other poplar species, which indicates that some PtNRT genes might have species-specific expression in Populus or may be pseudogenes For example, PtNRT3.2B was silent on the microarrays and we have not been able to amplify this gene by qRT-PCR. Therefore, PtNRT3.2B could be a pseudo gene. Among the selected PtNRT genes, PtNRT1.8B, PtNRT2.4B, PtNRT2.5C, and PtNRT3.1C do not have probesets on the microarrays. One possible reason might be their high sequence similarities with other family members. Our PCR amplification of PtNRT1.8B and PtNRT2.5C showed positive results, and suggested that PtNRT1.8B and PtNRT2.5C are expressed genes. In addition, a study in P. popularis and P. alba × P. glandulosa showed the expression of PtNRT2.4B and PtNRT3.1C [30]. Therefore, all members of PtNRT2 and PtNRT3 except PtNRT3.1B are expressed genes. Eighteen PtNRT1/PTR were not detected as expressed genes. They could be pseudogenes or expressed in other conditions.
Tissue-specific Expression of NRT Genes in Poplar
The members of the NRT1/PTR, NRT2, and NRT3 family play different roles in nitrate uptake and transport throughout the plant body [1], [5]. Our data show that tissue specific expression of NRT genes as documented for Arabidopsis and rice [5]–[7] is also true for poplar. The distinct expression patterns of the PtNRT1/PTR, PtNRT2, and PtNRT3 genes suggest diverse biological functions in specific tissues. The tip and the developing xylem contained a much lower number of expressed NRTs than the other tissues and the expressed NRTs were subsets of wood, leaf, and bark. Unique tissue-specific NRTs were PtNRT1.2A, PtNRT2.7, PtNRT1a2E, and PtNRT1c4A in leaves and wood, PtPTR3B and PtNRT2.5B in leaves, PtNRT1.8A in wood, and PtNRT1.5B, PtNRT1d5A and PtNRT1d5B in roots. Three of these genes have Arabidopsis orthologs with known functions: AtNRT1.2 is a low affinity nitrate transporter that has recently been shown to be also an abscisic acid transporter [45]. AtNRT2.5 is a high affinity nitrate transporter important for growth regulation, which is mainly expressed in leaves of Arabidopsis [46], and AtNRT1.5 is involved in xylem loading of nitrate in roots [47]. NRT1.8 has functions in nitrate removal from the xylem sap in Arabidopsis [21], and we found that PtNRT1.8A was specifically expressed in wood of P. trichocarpa.
The nitrate transporter/receptor AtNRT1.1 ( = nitrate tranceptor CHL1) was the first identified NRT in Arabidopsis and is currently the most extensively studied gene in this family [48]. AtNRT1.1 is expressed widely in roots and shoots with highest expression in the epidermis of the tips of the primary roots; it is also expressed in the endodermis of older roots and weakly expressed in mature parts of roots [49]. It has multiple functions as a nitrate transporter and sensor in roots [9]. It is furthermore expressed in guard cells and functions in stomatal opening [50]. It can also transport auxin, which may explain its participation in the regulation of lateral root growth [51], [52]. In P. trichocarpa its expression was found in leaves and wood, but not yet in roots. This finding was unexpected because a sensor is anticipated to be constitutively present, particularly in roots the primary uptake sites for nitrate. However, if the sensor is relatively stable, its expression can be low. It is also possible that NRT1.1 when expressed in the root tip, where the major NO3 − flux occurs [53] was diluted in extracts of the whole poplar root system and therefore not detected. Indeed, Li et al. (2012) reported low NRT1.1 expression in roots of two different poplar species, which increased in response to N fertilization [30]. Notably roots contained the highly expressed ortholog PtNRT1.2B to the low affinity nitrate/abscisic acid transporter of Arabidopsis, while leaves contained homolog of this gene (PtNRT1.2A). These findings may point to links between the regulation of nitrate uptake and drought stress, which are also corroborated by the CRE analysis (see below).
To transport NO3 − between roots and the aerial parts, NO3 − has to be loaded to the vascular tissues [1]. Many xylem and phloem located NRT genes are belonging to the NRT1/PTR family [1], [54]. For instance, in Arabidopsis, AtNRT1.5, AtNRT1.7, AtNRT1.8 and AtNRT1.9 are expressed in transport tissues and function in long-distance transport or remobilization of NO3 − [1], [54]. In line with these studies, we identified the expression of their poplar orthologs in roots, bark, wood, and leaves. In Arabidopsis, the members of the AtNRT1/PTR family transport not only nitrate but also auxin, carboxylates, di- and tripeptides, and even glucosinolates, and it is assumed that other substrates might be identified in the future [12], [13], [37]. For poplar, we still have no experimental evidence for their natural substrates.
In contrast to the NRT1/PTR family, none of the PtNRT2 and PtNRT3–related transcripts was detected in the developing xylem and the stem tip by microarray analysis. In Arabidopsis, rice, and peach the expression levels of NRT2 genes are generally higher in roots than in shoots [5], [7], [24], [55], [56]. One exception is AtNRT2.7 that exhibits a strong leaf-specific expression and participates in balancing the leaf nitrate content [57]. This notion may also be true for poplar, where PtNRT2.7 exhibited high expression levels in leaves.
In Arabidopsis, AtNRT2.4 participates in phloem nitrate transport [16]. In poplar bark, we did not detect the expression of PtNRT2.4A, but that of PtNRT2.5A. The finding that PtNRT2.5A clustered together with PtNRT3.1A/B may suggest that these proteins are required together for high affinity transporter of nitrate in the bark. Poplar PtNRT2.4 was abundant in roots, wood including the stem tip, and leaves, pointing to participation in long distance transport from the belowground to the above ground tissues.
Interestingly, with the exception of PtNRT1.9D, which is an ortholog to an Arabidopsis nitrate transporters in the phloem [39], none of the NRT genes with similarity to known Arabidopsis nitrate transporters was present in all analyzed P. trichocarpa tissues. NRT1/PTR genes constitutively expressed in all tissues were members of subclade NRT1c and subclade NRT1d, whose Arabidopsis orthologs were classified as members of the major facilitator superfamily involved in oligopeptide transport. However, distinct functions of these NRT1 members are still unclear. A further constitutive PtNRT1c1 (subclade NRT1c) has an Arabidopsis ortholog which can transport di- and tripeptides but not NO3 − in oocytes assays [58]. Therefore, most genes found across all P. trichocarpa tissues do not appear to be specific for NO3 − transport.
Cis-regulatory Elements Related to Specific Expression of PtNRTs in Tissues
CREs play essential roles in regulating gene expression pattern [32]. Here, nine of a total of 142 CRE motifs were found in the promoter regions of all PtNRT genes (Cluster MC1). Two of these motifs (GATABOX, DOFCOREZM) are involved in N metabolic pathways [33], [59], [60]. For instance, in spinach, GATA motifs exist in the promoter region of the nitrite reductase gene that is differentially regulated by ammonium [33]. Transgenic Arabidopsis plants overexpressing Dof1 showed better growth under low-nitrogen conditions [61]. The CAAT motif of CAATBOX1 in cluster MC1 is a general motif for binding of transcription factor in eucaryotes (identified e.g. in the promoter of the legumin) [62]. The GT1CONSENSUS motif is present in the promoter of many light-regulated genes such as the rubisco small subunit [63]. CACTFTPPCA1 is important for the regulation of phosphoenol pyruvate carboxylase [64], a central enzyme for anapleurotic reactions, which are required when metabolites are withdrawn from the citrate cycle for amino acid biosynthesis, e.g. that of glutamate [65]. The ubiquitous presence of these motifs suggests that the PtNRT genes underlie a tight regulation by N as well as plant energy and carbon metabolism. Further motifs in MC1 are related to organ specificity (ROOTMOTIFTAPOX1 [66]) and hormone regulation (ARR1AT, WRKY71OS) [67], [68]. ARR1AT is a binding element [69], [70] for ARR1, which is a cytokinin response transcriptional activator [67], [68]. WRKY71OS, a binding element for WRKY71, encodes a transcriptional repressor of gibberillic acid signaling [71] and is induced by cold, salt and dehydration stress in Musa spp. [72]. Because gibberillic acid and cytokinin are both plant growth hormones our findings place PtNRT genes into a growth regulatory net.
Many of the other motifs, which did not occur in all NRT promoters, still may indicate functional redundancy with the common regulatory elements because we detected a large number of motifs for light (12), sugar (8), hormone (16) and nitrogen regulation (14). Among the hormone-related motifs not only growth, but also abscisic acid-related motifs were identified, which points to drought stress regulation of some NRTs. This notion is also supported by a considerable number of motifs (11) for Myb transcription factors also known to play roles in drought stress [73]–[76].
In addition to ROOTMOTIFTAPOX1 we found further CREs related to tissue-specific expression such as NODCON1G/NODCON2G [77], POLLEN1LELAT52 [78], and TAAAGSTKST1 [79]. However, we could not find a direct relationship between the enrichment of these motifs and the tissue-specific expression of their corresponding PtNRT genes. A possible reason for this lack of correlation could be that CREs are not working independently of each other. For example, Ye et al. (2012) found two cis-regulatory elements (GSE1 and GSE2) important for the expression of genes in green tissues in rice [80]. GSE1 and GSE2 harbored three cis-elements, namely ROOTMOTIFTAPOX1, NODCON2GM, and DOFCOREZM, which appeared to be required together for tissue-specific expression. Our analysis of motif occurrence in the analyzed tissues revealed strong overlap (119/138 = 86%) in leaves, bark, wood and roots. Only two motifs were specific to wood and three to roots, and none to the other tissues analyzed. The specific motifs were very rare, each occurring only in one NRT gene and showed no correlation with distinct tissues. This observation precludes a role in tissue-specificity. Our NMDS analysis suggests that the combination and abundance of motifs in distinct promoters may lead to tissue-specificity because most tissues were clearly separated. Only for leaves and bark our analysis predicts similar regulation.
In conclusion, the results of this study display the identification and an expression analysis of the whole NRT gene family in Populus. The prediction of cis-regulatory elements suggests various possible regulatory mechanisms of PtNRT genes, in particular with regard to phytohormones. These data provide a valuable basis for further studies to gain insights into the functions of nitrate transporters in poplar.
Materials and Methods
Sterile Growth of P. trichocarpa
P. trichocarpa were multiplied by micropropagation and grown under axenic conditions in with autoclaved sand-filled (Ø 0.71–1.25 mm Melo, Göttingen, Germany) Petri dishes supplied with Woody Plant Medium [81]. The plants were grown for five weeks in an acclimatized room (26°C, 60% relative air humidity, day/night length of 16/8 h with a photosynthetic active radiation (PAR) of 150 µmol photons m−2s−1). Then the plants were separated into roots and shoots, shock-frozen in liquid nitrogen and stored at −80°C. For transcript profiling, materials of 15 plants per experiment were pooled, ground in a ball mill (Retsch, Haan, Germany) and used for RNA extraction with a modified method after Chang et al. (1993) [82]. Three independent biological samples were obtained from three experiments. Total RNA was purified with the RNeasy mini kit (Qiagen, Valencia, CA). RNA integrity was assessed on an Agilent 2.100 Bioanalyzer (Agilent, Santa Clara, CA) and analysed on the GeneChip® Poplar Genome Array (Affymetrix, Santa Clara, CA) at the Microarray Facility Tübingen. Raw and normalized data are available at the ArrayExpress database under MEXP-3909 and MEXP-3910 (further information: Table S2).
Plantlets of P. tremula for the Transcript Analysis of PtreNRT Genes
Tissue cultured plantlets of P. tremula were grown on MS medium [83] in an air-conditioned room (22°C air temperature, 30% humidity, and day/night length of 16/8 h, light intensity of 63–70 µmol m−2 s−1) for three weeks. Afterwards, the plantlets were transferred to Long-Ashton hydroponic solution (0.02 mM KNO3, 0.90 mM Ca(NO3)2•4H2O, 0.30 mM MgSO4•7H2O, 0.60 mM KH2PO4, 0.04 mM K2HPO4, 0.01 mM H3BO3, 0.002 mM MnSO4•H2O, 0.007 mM Na2MoO4•2H2O, 0.00001 mM CoSO4•7H2O, 0.0002 mM ZnSO4•7H2O, 0.0002 mM CuSO4•H2O, EDTA-Fe, pH 5.5) and grown in the same room for three weeks. Then the P. tremula plants were transferred to compost soil (Kompostwerk GmbH, Niederorla, Germany) and kept in a greenhouse (22°C air temperature, 30% humidity, day/night length of 16/8 h, light intensity of 70–100 µmol m−2 s−1) for eight weeks from July to September.
For RNA extraction, the plants were harvested. The top (2 cm upper stem segment (T)) was frozen after removal of young leaves. The remaining stem was separated into bark (B), developing xylem (DX), and wood (W). The developing xylem was collected by scraping the exposed surface of wood. Leaf discs (L) were cut out from the fully expanded leaves with a cork borer (diameter 1 cm). Petioles (P) were separated from the leaves. Roots were divided into fine roots (FR) and coarse roots (CR). Each of the harvested tissues from 20 plants were pooled and split to two replicates for RNA extraction. All harvested samples were shock frozen in liquid nitrogen and stored at –80°C.
Identification and Phylogenetic Analysis of NRTs in Poplar
The sequences of NRTs in Arabidopsis thaliana were obtained from TAIR (http://www.arabidopsis.org/index.jsp). Arabidopsis 58 NRTs [12] were used to search against the proteome database in HMMER (http://hmmer.janelia.org/). Nitrate transporter was used as key word to search against poplar genome database (http://www.phytozome.net/) and National Center for Biotechnology Information (NCBI) gene databases (http://www.ncbi.nlm.nih.gov/). After removing the redundant, the remaining sequences were further used for the phylogenetic analysis.
Multiple amino acid alignments of NRTs were generated using Clustal X2 (http://www.clustal.org/) (Larkin et al. 2007). The phylogenetic trees were constructed using the neighbor-joining method with the bootstrapped option n = 1000 in MEGA 5 (http://www.megasoftware.net/) [84]. Based on the results of the phylogenetic analysis, the P. trichocarpa NRT1s were named as PtNRT1+clade name.
Trans-membrane domains were predicted by TMHMM v2. (http://www.cbs.dtu.dk/services/TMHMM/).
Cis-regulatory Elements of NRT Genes in Poplar and Arabidopsis
The 1 kb upstream of all NRT genes in Arabidopsis thaliana and P. trichocarpa were obtained from ENSEMBL plant (http://plants.ensembl.org/index.html) and phytozome (http://www.phytozome.net/), respectively. The cis-regulatory elements were scanned in PLACE [85] and Cistome (http://bar.utoronto.ca/cistome/cgi-bin/BAR_Cistome.cgi) with the following settings: Ze cutoff = 3.0 (0< z <10), functional depth cutoff for PSSMs = 0.35 (0.0< d <1.0), and the proportion of genes the motif should be found in = 0 to discover all the motifs in each family genes (0.0<p<1.0). Both strands were searched.
For CRE analysis only NRTs found in P. trichocarpa were usable. Overlapping CREs were documented by Venn diagrams [86]. To generate a tissue-CRE matrix, the total number of elements per motif was counted for the NRTs expressed in each tissue. The matrix was analyzed by nonmetric multidimensional scaling (NMDS) in the open access software PAST version 2.17c (http://folk.uio.no/ohammer/past/) using the Bray Curtis index as similarity measure [87].
Transcript Analysis using Affymetrix Genechips
The published datasets of Affymetrix genechip analyses were downloaded from Arrayexpress (http://www.ebi.ac.uk/arrayexpress) (Table S2). All Affymetrix based CEL files were transformed to signal intensity values using the affy package from R software (http://www.bioconductor.org). Expression heatmaps were generated in R using package gplots (http://cran.r-project.org/web/packages/gplots/index.html).
Primer Design for the Quantitative RT-PCR
Based on functionally characterized NRT genes in Arabidopsis (9 NRT1, 7 NRT2 and 2 NRT3), their homologs in the poplar (15 NRT1, 6 NRT2 and 5 NRT3) were used for the qRT-PCR analysis. To develop specific primers of poplar NRT genes multi-alignment analysis for the coding sequence was conducted by the software ClustalX2 and web-server MultiAli (http://multalin.toulouse.inra.fr/multalin/). Exon and intron information within the coding sequence was obtained from website phytozome (http://www.phytozome.net/) and Gene Structure Display Server (http://gsds.cbi.pku.edu.cn/). To improve the quality of primers and prevent missmatch and DNA contamination, the specific primers were obtained based on the condition that the selected sequences are on the non-conserved regions. Exon-exon junction primers or exon-exon primers with intron inside were developed. Finally, 21 specific primer pairs for qRT-PCR were designed with a predicted melting temperature (Tm) of 60±2°C, primer length of 18–24 nucleotides, product size of 100–250 base pairs and a GC content of 40%–60% using web server Primer3 from SimGene.com (http://simgene.com/Primer3). The details are shown in Table S4.
For eight NRT genes exon-exon junction primers were designed. For seven NRT genes exon - exon primers with an intron inside of the amplified product were designed. For six NRT genes only exon specific primers were found. All the primer pairs were tested in the plant leaves of P. trichocarpa and P. tremula. In order to distinguish the NRT transcript in two poplar species we named the NRT in P. trichocarpa as PtNRT and the NRT in P. tremula as PtreNRT in our study. Because of the high similarity and properties between the coding sequence of following gene pairs: NRT1.5A and NRT1.5B (92%); NRT2.4A and NRT2.4B (97%); and NRT3.1B and NRT3.1C (97%), the primers were designed on the common sequences of NRT1.5A and NRT1.5B, NRT2.4 A and NRT2.4B, and NRT3.1B and NRT3.1C, and named as NRT1.5A, NRT2.4A, and NRT3.1B, respectively.
RNA Isolation, cDNA Synthesis and Quantitative Real Time PCR
Total RNA of the samples was extracted using the cetyltrimethyl ammonium bromide (CTAB) method [82]. The RNA integrity was checked by 2% agar gel electrophoresis. DNA contamination was removed by DNAase using the TURBO DNA-free Kit according to the manual instructions (Applied Biosystems, Darmstadt, Germany). The extracted RNA was subjected to PCR with ACT1 (POPTR_0001s31700) primers (Table S4) to check if DNA was completely removed. The PCR amplified solution was run on a 2% agarose gel to make sure no product show on the gel. Then, first-strand cDNA synthesis was carried out with approximately 1 µg RNA using a First Strand cDNA Synthesis Kit (MBI-Fermentas, St.Leon-Rot, Germany). To evaluate the quality 0.2 µl cDNA was subjected to PCR using primer pairs of ACT1 and run on a 2% agarose gel. The prepared cDNA was used for the qRT-PCR analysis. PCR amplification was run for 35 cycles with denaturation at 94°C for 30 seconds, annealing at 60°C for 30 seconds, and extension at 72°C for 30 seconds in a LightCycler® 480 Detection system (Roche Diagnostics, Mannheim, Germany) using SYBR Green Master kit (Roche Diagnostics, Mannheim, Germany). To calculate the relative expression of the gene in each sample, the 2−ΔΔCT method was used [88].
Supporting Information
Cluster analysis of cis -regulatory elements of PtNRT genes. Color key represents the copy numbers of CREs in 1 kb promoter regions of PtNRT genes. The color of the gene name represents the clade in the phylogenetic tree (Figure 1). The analysis resulted in nine clusters of PtNRT genes (GC1–GC9) and 14 clusters of CRE motifs (MC1–MC14).
(TIF)
Identification of NRT1/PTR1 , NRT2 , and NRT3 genes in P. trichocarpa (Populus trichocarpa v3. genome ).
(XLSX)
Microarrays used for the transcript analysis of Populus NRT genes in leaves, bark, wood, developing xylem, stem top in the elongation zone, and roots.
(XLSX)
Mean expression level and motif abundances of PtNRT family genes.
(XLSX)
Specific primers for NRT genes and housekeeping genes in Populus .
(XLSX)
Acknowledgments
We thank C. Kettner for providing tissue-cultured poplar plants.
Funding Statement
PhD scholarships were provided by the German Academic Exchange Service (DAAD) to HB and DE and by the Niedersächsisches Exzellenzcluster “Function Biodiversity Research” to KV. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wang YY, Hsu PK, Tsay YF (2012) Uptake, allocation and signaling of nitrate. Trends Plant Sci 17: 458–467 doi: 10.1016/j.tplants.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 2. Gojon A, Krouk G, Perrine WF, Laugier E (2011) Nitrate transceptor(s) in plants. J Exp Bot 62: 2299–2308 doi: 10.1093/jxb/erq419 [DOI] [PubMed] [Google Scholar]
- 3. Dechorgnat J, Nguyen CT, Armengaud P, Jossier M, Diatloff E, et al. (2010) From the soil to the seeds: the long journey of nitrate in plants. J Exp Bot 62: 1349–1359 doi: 10.1093/jxb/erq409 [DOI] [PubMed] [Google Scholar]
- 4. Crawford NM, Glass ADM (1998) Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci 3: 389–395 doi: 10.1016/S1360-1385(98)01311-9 [Google Scholar]
- 5. Feng H, Yan M, Fan X, Li B, Shen Q, et al. (2011) Spatial expression and regulation of rice high-affinity nitrate transporters by nitrogen and carbon status. J Exp Bot. 62: 2319–2332 doi: 10.1093/jxb/erq403 [DOI] [PubMed] [Google Scholar]
- 6. Migocka M, Warzybok A, Kłobus G (2012) The genomic organization and transcriptional pattern of genes encoding nitrate transporters 1 (NRT1) in cucumber. Plant and Soil 364: 254–260 doi: 10.1007/s11104-012-1345-x [Google Scholar]
- 7. Okamoto M, Vidmar JJ, Glass ADM (2003) Regulation of NRT1 and NRT2 gene families of Arabidopsis thaliana: responses to nitrate provision. Plant Cell Physiol 44: 304–317 doi: 10.1093/pcp/pcg036 [DOI] [PubMed] [Google Scholar]
- 8. Tsay YF, Schroeder JI, Feldmann KA, Crawford NM (1993) The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell 72: 705–713 doi: 10.1016/0092-8674(93)90399-B [DOI] [PubMed] [Google Scholar]
- 9. Ho CH, Lin SH, Hu HC, Tsay YF (2009) CHL1 functions as a nitrate sensor in plants. Cell 138: 1184–1194 doi: 10.1016/j.cell.2009.07.004 [DOI] [PubMed] [Google Scholar]
- 10. Huang NC, Liu KH, Lo HJ, Tsay YF (1999) Cloning and functional characterization of an Arabidopsis nitrate transporter gene that encodes a constitutive component of low-affinity uptake. Plant Cell 11: 1381–1392 doi: 10.1105/tpc.11.8.1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chiu CC, Lin CS, Hsia AP, Su RC, Lin HL, et al. (2004) Mutation of a nitrate transporter, AtNRT1:4, results in a reduced petiole nitrate content and altered leaf development. Plant Cell Physiol 45: 1139–1148 doi: 10.1093/pcp/pch143 [DOI] [PubMed] [Google Scholar]
- 12. Tsay YF, Chiu CC, Tsai CB, Ho CH, Hsu PK (2007) Nitrate transporters and peptide transporters. FEBS Lett 581: 2290–2300 doi: 10.1016/j.febslet.2007.04.047 [DOI] [PubMed] [Google Scholar]
- 13. Weichert A, Brinkmann C, Komarova N, Dietrich D, Thor K, et al. (2012) AtPTR4 and AtPTR6 are differentially expressed, tonoplast-localized members of the peptide transporter/nitrate transporter 1 (PTR/NRT1) family. Planta 235: 311–323 doi: 10.1007/s00425-011-1508-7 [DOI] [PubMed] [Google Scholar]
- 14. Dietrich D, Hammes U, Thor K, Suter-Grotemeyer M, Flückiger R, et al. (2004) AtPTR1, a plasma membrane peptide transporter expressed during seed germination and in vascular tissue of Arabidopsis . Plant J 40: 488–499 doi: 10.1111/j.1365-313X.2004.02224.x [DOI] [PubMed] [Google Scholar]
- 15. Komarova NY, Thor K, Gubler A, Meier S, Dietrich D, et al. (2008) AtPTR1 and AtPTR5 transport dipeptides in planta. Plant Physiol 148: 856–869 doi: 10.1104/pp.108.123844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kiba T, Feria-Bourrellier A-B, Lafouge F, Lezhneva L, Boutet-Mercey S, et al. (2012) The Arabidopsis nitrate transporter NRT2.4 plays a double role in roots and shoots of nitrogen-starved plants. Plant Cell 24: 245–258 doi: 10.1105/tpc.111.092221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chopin F, Orsel M, Dorbe MF, Chardon F, Truong HN, et al. (2007) The Arabidopsis ATNRT2.7 nitrate transporter controls nitrate content in seeds. Plant Cell 19: 1590–1602 doi: 10.1105/tpc.107.050542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Little DY, Rao H, Oliva S, Daniel-Vedele F, Krapp A, et al. (2005) The putative high-affinity nitrate transporter NRT2.1 represses lateral root initiation in response to nutritional cues. Proc Natl Acad Sci USA 102: 13693–13698 doi: 10.1073/pnas.0504219102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang R, Okamoto M, Xing X, Crawford NM (2003) Microarray analysis of the nitrate response in Arabidopsis roots and shoots reveals over 1,000 rapidly responding genes and new linkages to glucose, trehalose-6-phosphate, iron, and sulfate metabolism. Plant Physiol 132: 556–567 doi: 10.1104/pp.103.021253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Plett D, Toubia J, Garnett T, Tester M, Kaiser BN, et al. (2010) Dichotomy in the NRT gene families of dicots and grass species. PLoS One 5: e15289 doi: 10.1371/journal.pone.0015289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li JY, Fu YL, Pike SM, Bao J, Tian W, et al. (2010) The Arabidopsis nitrate transporter NRT1.8 functions in nitrate removal from the xylem sap and mediates cadmium tolerance. Plant Cell 22: 1633–1646 doi: 10.1105/tpc.110.075242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yong Z, Kotur Z, Glass ADM (2010) Characterization of an intact two-component high-affinity nitrate transporter from Arabidopsis roots. Plant J 63: 739–748 doi: 10.1111/j.1365-313X.2010.04278.x [DOI] [PubMed] [Google Scholar]
- 23. Kotur Z, Mackenzie N, Ramesh S, Tyerman SD, Kaiser BN, et al. (2012) Nitrate transport capacity of the Arabidopsis thaliana NRT2 family members and their interactions with AtNAR2.1. New Phytol 194: 724–731 doi: 10.1111/j.1469-8137.2012.04094.x [DOI] [PubMed] [Google Scholar]
- 24. Orsel M, Chopin F, Leleu O, Smith SJ, Krapp A, et al. (2006) Characterization of a two-component high-affinity nitrate uptake system in Arabidopsis. Physiology and protein-protein interaction. Plant Physiol 142: 1304–1317 doi: 10.1104/pp.106.085209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Criscuolo G, Valkov VT, Parlati A, Alves LM, Chiurazzi M (2012) Molecular characterization of the Lotus japonicus NRT1(PTR) and NRT2 families. Plant Cell Environ 35: 1567–1581 doi: 10.1111/j.1365-3040.2012.02510.x [DOI] [PubMed] [Google Scholar]
- 26.Polle A, Janz D, Teichmann T, Lipka V (2013) Poplar genetic engineering: promoting desirable wood characteristics and pest resistance. Appl Microbiol Biotechnol. doi: 10.1007/s00253-013-4940-8 [DOI] [PubMed]
- 27. Plavcova L, Hacke UG, Almeida-Rodriguez AM, Li E, Douglas CJ (2013) Gene expression patterns underlying changes in xylem structure and function in response to increased nitrogen availability in hybrid poplar. Plant Cell Environ 36: 186–199 doi: 10.1111/j.1365-3040.2012.02566.x [DOI] [PubMed] [Google Scholar]
- 28. Couturier J, Montanini B, Martin F, Brun A, Blaudez D, et al. (2007) The expanded family of ammonium transporters in the perennial poplar plant. New Phytol 174: 137–150 doi: 10.1111/j.1469-8137.2007.01992.x [DOI] [PubMed] [Google Scholar]
- 29. Rennenberg H, Wildhagen H, Ehlting B (2010) Nitrogen nutrition of poplar trees. Plant Biol 12: 275–291 doi: 10.1111/j.1438-8677.2009.00309.x [DOI] [PubMed] [Google Scholar]
- 30. Li H, Li M, Luo J, Cao X, Qu L, et al. (2012) N-fertilization has different effects on the growth, carbon and nitrogen physiology, and wood properties of slow- and fast-growing Populus species. J Exp Bot 63: 6173–6185 doi: 10.1093/jxb/ers271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wittkopp PJ, Kalay G (2012) cis-regulatory elements: molecular mechanisms and evolutionary processes underlying divergence. Nat Rev Genet 13: 59–69 doi: 10.1038/nrg3095 [DOI] [PubMed] [Google Scholar]
- 32.Griffiths AJF (2008) An introduction to genetic analysis. New York: W.H. Freeman.
- 33. Rastogi R, Bate N, Sivasankar S, Rothstein S (1997) Footprinting of the spinach nitrite reductase gene promoter reveals the preservation of nitrate regulatory elements between fungi and higher plants. Plant Mol Biol 34: 465–476 10.1023/A:1005842812321. [DOI] [PubMed] [Google Scholar]
- 34. Hwang SY, Lindroth RL (1997) Clonal variation in foliar chemistry of aspen: effects on gypsy moths and forest tent caterpillars. Oecologia 111: 99–108 doi: 10.1007/s004420050213 [DOI] [PubMed] [Google Scholar]
- 35. Konishi M, Yanagisawa S (2010) Identification of a nitrate-responsive cis-element in the Arabidopsis NIR1 promoter defines the presence of multiple cis-regulatory elements for nitrogen response. Plant J 63: 269–282 doi: 10.1111/j.1365-313X.2010.04239.x [DOI] [PubMed] [Google Scholar]
- 36. Girin T, Lejay L, Wirth J, Widiez T, Palenchar PM, et al. (2007) Identification of a 150 bp cis-acting element of the AtNRT2.1 promoter involved in the regulation of gene expression by the N and C status of the plant. Plant Cell Environ 30: 1366–1380 doi: 10.1111/j.1365-3040.2007.01712.x [DOI] [PubMed] [Google Scholar]
- 37. Nour-Eldin HH, Andersen TG, Burow M, Madsen SR, Jorgensen ME, et al. (2012) NRT/PTR transporters are essential for translocation of glucosinolate defence compounds to seeds. Nature 488: 531–534 doi: 10.1038/nature11285 [DOI] [PubMed] [Google Scholar]
- 38. Tuskan GA, DiFazio S, Jansson S, Bohlmann J, Grigoriev I, et al. (2006) The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 313: 1596–1604 doi: 10.1126/science.1128691 [DOI] [PubMed] [Google Scholar]
- 39. Wang YY, Tsay YF (2011) Arabidopsis nitrate transporter NRT1.9 is important in phloem nitrate transport. Plant Cell 23: 1945–1957 doi: 10.1105/tpc.111.083618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guo YL (2013) Gene family evolution in green plants with emphasis on the origination and evolution of Arabidopsis thaliana genes. Plant J 73: 941–951 doi: 10.1111/tpj.12089 [DOI] [PubMed] [Google Scholar]
- 41. Castro-Rodriguez V, Garcia-Gutierrez A, Canales J, Avila C, Kirby E, et al. (2011) The glutamine synthetase gene family in Populus . BMC Plant Biol 11: 119 doi: 10.1186/1471-2229-11-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cao J, Huang J, Yang Y, Hu X (2011) Analyses of the oligopeptide transporter gene family in poplar and grape. BMC Genomics 12: 465 doi: 10.1186/1471-2164-12-465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li W, Wang Y, Okamoto M, Crawford NM, Siddiqi MY, et al. (2007) Dissection of the AtNRT2.1:AtNRT2.2 inducible high-affinity nitrate transporter gene cluster. Plant Physiol 143: 425–433 doi: 10.1104/pp.106.091223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shinji I, Yuka ITO, Yuki S, Yuka F, Misa T, et al. (2009) Two-component high-affinity nitrate transport system in barley: Membrane localization, protein expression in roots and a direct protein-protein interaction. Plant Biotechnology 26: 197–205 doi: 10.5511/plantbiotechnology.26.197 [Google Scholar]
- 45. Kanno Y, Hanada A, Chiba Y, Ichikawa T, Nakazawa M, et al. (2012) Identification of an abscisic acid transporter by functional screening using the receptor complex as a sensor. Proc Natl Acad Sci USA 109: 9653–9658 doi: 10.1073/pnas.1203567109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kechid M, Desbrosses G, Rokhsi W, Varoquaux F, Djekoun A, et al. (2013) The NRT2.5 and NRT2.6 genes are involved in growth promotion of Arabidopsis by the plant growth-promoting rhizobacterium (PGPR) strain Phyllobacterium brassicacearum STM196. New Phytol 198: 514–524 doi: 10.1111/nph.12158 [DOI] [PubMed] [Google Scholar]
- 47. Lin SH, Kuo HF, Canivenc G, Lin CS, Lepetit M, et al. (2008) Mutation of the Arabidopsis NRT1.5 nitrate transporter causes defective root-to-shoot nitrate transport. Plant Cell 20: 2514–2528 doi: 10.1105/tpc.108.060244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Alvarez JM, Vidal EA, Gutiérrez RA (2012) Integration of local and systemic signaling pathways for plant N responses. Curr Opin Plant Biol 15: 185–191 doi: 10.1016/j.pbi.2012.03.009 [DOI] [PubMed] [Google Scholar]
- 49. Nazoa P, Vidmar JJ, Tranbarger TJ, Mouline K, Damiani I, et al. (2003) Regulation of the nitrate transporter gene AtNRT2.1 in Arabidopsis thaliana : responses to nitrate, amino acids and developmental stage. Plant Mol Biol 52: 689–703 doi: 10.1023/A:1024899808018 [DOI] [PubMed] [Google Scholar]
- 50. Guo FQ, Young J, Crawford NM (2003) The nitrate transporter AtNRT1.1 (CHL1) functions in stomatal opening and contributes to drought susceptibility in Arabidopsis . Plant Cell 15: 107–117 doi: 10.1105/tpc.006312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Krouk G, Lacombe B, Bielach A, Perrine-Walker F, Malinska K, et al. (2010) Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev Cell 18: 927–937 doi: 10.1016/j.devcel.2010.05.008 [DOI] [PubMed] [Google Scholar]
- 52. Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, et al. (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 doi: 10.1016/S0092-8674(03)00924-3 [DOI] [PubMed] [Google Scholar]
- 53. Luo ZB, He J, Li H, Luo J, Ma C, et al. (2013) A transcriptomic network underlies microstructural and physiological responses to cadmium in Populus × canescens . Plant Physiol 162: 424–439 doi: 10.1104/pp.113.215681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fan SC, Lin CS, Hsu PK, Lin SH, Tsay YF (2009) The Arabidopsis nitrate transporter NRT1.7, expressed in phloem, is responsible for source-to-sink remobilization of nitrate. Plant Cell 21: 2750–2761 doi: 10.1105/tpc.109.067603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nakamura Y, Umemiya Y, Masuda K, Inoue H, Fukumoto M (2007) Molecular cloning and expression analysis of cDNAs encoding a putative Nrt2 nitrate transporter from peach. Tree Physiol 27: 503–510 doi: 10.1093/treephys/27.4.503 [DOI] [PubMed] [Google Scholar]
- 56. Katayama H, Mori M, Kawamura Y, Tanaka T, Mori M, et al. (2009) Production and characterization of transgenic rice plants carrying a high-affinity nitrate transporter gene (OsNRT2.1). Breeding Science 59: 237 doi: 10.1270/jsbbs.59.237 [Google Scholar]
- 57. Orsel M, Krapp A, Daniel-Vedele F (2002) Analysis of the NRT2 nitrate transporter family in Arabidopsis. Structure and gene expression. Plant Physiol 129: 886–896 doi: 10.1104/pp.005280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Komarova NY, Meier S, Meier A, Grotemeyer MS, Rentsch D (2012) Determinants for Arabidopsis peptide transporter targeting to the tonoplast or plasma membrane. Traffic 13: 1090–1105 doi: 10.1111/j.1600-0854.2012.01370.x [DOI] [PubMed] [Google Scholar]
- 59. Marzluf GA (1997) Genetic regulation of nitrogen metabolism in the fungi. Microbiol Mol Biol Rev 61: 17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yanagisawa S, Akiyama A, Kisaka H, Uchimiya H, Miwa T (2004) Metabolic engineering with Dof1 transcription factor in plants: Improved nitrogen assimilation and growth under low-nitrogen conditions. Proc Natl Acad Sci USA 101: 7833–7838 doi: 10.1073/pnas.0402267101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yanagisawa S (2004) Dof domain proteins: plant-specific transcription factors associated with diverse phenomena unique to plants. Plant Cell Physiol 45: 386–391 doi: 10.1093/pcp/pch055 [DOI] [PubMed] [Google Scholar]
- 62. Shirsat A, Wilford N, Croy R, Boulter D (1989) Sequences responsible for the tissue specific promoter activity of a pea legumin gene in tobacco. Mol Gen Genet 215: 326–331 doi: 10.1007/BF00339737 [DOI] [PubMed] [Google Scholar]
- 63. Villain P, Mache R, Zhou D-X (1996) The Mechanism of GT Element-mediated Cell Type-specific Transcriptional Control. J Biol Chem 271: 32593–32598 doi: 10.1074/jbc.271.51.32593 [DOI] [PubMed] [Google Scholar]
- 64. Gowik U, Burscheidt J, Akyildiz M, Schlue U, Koczor M, et al. (2004) cis-regulatory elements for mesophyll-specific gene expression in the C4 plant flaveria trinervia, the promoter of the C4 phosphoenolpyruvate carboxylase gene. Plant Cell 16: 1077–1090 doi: 10.1105/tpc.019729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stitt M, Zeeman SC (2012) Starch turnover: pathways, regulation and role in growth. Curr Opin Plant Biol 15: 282–292 doi: 10.1016/j.pbi.2012.03.016 [DOI] [PubMed] [Google Scholar]
- 66. Elmayan T, Tepfer M (1995) Evaluation in tobacco of the organ specificity and strength of the rolD promoter, domain A of the 35S promoter and the 35S2 promoter. Transgenic Res 4: 388–396 doi: 10.1007/BF01973757 [DOI] [PubMed] [Google Scholar]
- 67. Sakai H, Aoyama T, Oka A (2000) Arabidopsis ARR1 and ARR2 response regulators operate as transcriptional activators. Plant J 24: 703–711 doi: 10.1111/j.1365-313X.2000.00909.x [DOI] [PubMed] [Google Scholar]
- 68. Taniguchi M, Sasaki N, Tsuge T, Aoyama T, Oka A (2007) ARR1 directly activates cytokinin response genes that encode proteins with diverse regulatory functions. Plant Cell Physiol 48: 263–277 doi: 10.1093/pcp/pcl063 [DOI] [PubMed] [Google Scholar]
- 69. Oka A, Sakai H, Iwakoshi S (2002) His-Asp phosphorelay signal transduction in higher plants: receptors and response regulators for cytokinin signaling in Arabidopsis thaliana . Genes Genet Syst 77: 383–391 doi: 10.1266/ggs.77.383 [DOI] [PubMed] [Google Scholar]
- 70. Hatorangan M, Sentausa E, Wijaya G (2009) In silico identification of cis-regulatory elements of phosphate transporter genes in rice (Oryza sativa L.). J Crop Sci Biotechnol 12: 25–30 doi: 10.1007/s12892-008-0054-8 [Google Scholar]
- 71. Zhang ZL, Xie Z, Zou X, Casaretto J, Ho TD, et al. (2004) A rice WRKY gene encodes a transcriptional repressor of the gibberellin signaling pathway in aleurone cells. Plant Physiol 134: 1500–1513 doi: 10.1104/pp.103.034967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shekhawat US, Ganapathi T, Srinivas L (2011) Cloning and characterization of a novel stress-responsive WRKY transcription factor gene (MusaWRKY71) from Musa spp. cv. Karibale Monthan (ABB group) using transformed banana cells. Mol Biol Rep. 38: 4023–4035 doi: 10.1007/s11033-010-0521-4 [DOI] [PubMed] [Google Scholar]
- 73. Urao T, Yamaguchi-Shinozaki K, Urao S, Shinozaki K (1993) An Arabidopsis myb homolog is induced by dehydration stress and its gene product binds to the conserved MYB recognition sequence. Plant Cell 5: 1529–1539 doi: 10.1105/tpc.5.11.1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Abe H, Yamaguchi-Shinozaki K, Urao T, Iwasaki T, Hosokawa D, et al. (1997) Role of Arabidopsis MYC and MYB homologs in drought- and abscisic acid-regulated gene expression. Plant Cell 9: 1859–1868 doi: 10.1105/tpc.9.10.1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nakashima K, Yamaguchi-Shinozaki K (2006) Regulons involved in osmotic stress-responsive and cold stress-responsive gene expression in plants. Physiologia Plantarum 126: 62–71 doi: 10.1111/j.1399-3054.2005.00592.x [Google Scholar]
- 76. Abe H, Urao T, Ito T, Seki M, Shinozaki K, et al. (2003) Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 15: 63–78 doi: 10.1105/tpc.006130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Campos-Soriano L, Gomez-Ariza J, Bonfante P, San Segundo B (2011) A rice calcium-dependent protein kinase is expressed in cortical root cells during the presymbiotic phase of the arbuscular mycorrhizal symbiosis. BMC Plant Biol 11: 90 doi: 10.1186/1471-2229-11-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bate N, Twell D (1998) Functional architecture of a late pollen promoter: pollen-specific transcription is developmentally regulated by multiple stage-specific and co-dependent activator elements. Plant Mol Biol 37: 859–869 doi: 10.1023/A:1006095023050 [DOI] [PubMed] [Google Scholar]
- 79. Plesch G, Ehrhardt T, Mueller-Roeber B (2001) Involvement of TAAAG elements suggests a role for Dof transcription factors in guard cell-specific gene expression. Plant J 28: 455–464 doi: 10.1046/j.1365-313X.2001.01166.x [DOI] [PubMed] [Google Scholar]
- 80. Ye R, Zhou F, Lin Y (2012) Two novel positive cis-regulatory elements involved in green tissue-specific promoter activity in rice (Oryza sativa L ssp.). Plant Cell Rep 31: 1159–1172 doi: 10.1007/s00299-012-1238-8 [DOI] [PubMed] [Google Scholar]
- 81. Lloyd G, McCown B (1980) Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Proc Plant Prop Soc 30: 421–427. [Google Scholar]
- 82. Chang S, Puryear J, Cairney J (1993) A simple and efficient method for isolating RNA from pine trees. Plant Molecular Biology Reporter 11: 113–116 doi: 10.1007/BF02670468 [Google Scholar]
- 83. Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum 15: 473–497 doi: 10.1111/j.1399-3054.1962.tb08052.x [Google Scholar]
- 84. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731–2739 doi: 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database. Nucleic Acids Res 27: 297–300 doi: 10.1093/nar/27.1.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oliveros J (2007) VENNY. An interactive tool for comparing lists with Venn diagrams. Venny website: http://bioinfogp.cnb.csic.es/tools/venny/. Accessed 2012 April.
- 87.Hammer Ø, Harper DAT (2001) PAST: Paleontological statistics software package for education and data analysis. Available: http://palaeo-electronica.org/2001_1/past/issue1_01.htm. Accessed 2013 May 19.
- 88. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 25: 402–408 doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cluster analysis of cis -regulatory elements of PtNRT genes. Color key represents the copy numbers of CREs in 1 kb promoter regions of PtNRT genes. The color of the gene name represents the clade in the phylogenetic tree (Figure 1). The analysis resulted in nine clusters of PtNRT genes (GC1–GC9) and 14 clusters of CRE motifs (MC1–MC14).
(TIF)
Identification of NRT1/PTR1 , NRT2 , and NRT3 genes in P. trichocarpa (Populus trichocarpa v3. genome ).
(XLSX)
Microarrays used for the transcript analysis of Populus NRT genes in leaves, bark, wood, developing xylem, stem top in the elongation zone, and roots.
(XLSX)
Mean expression level and motif abundances of PtNRT family genes.
(XLSX)
Specific primers for NRT genes and housekeeping genes in Populus .
(XLSX)