The risk of a seizure in temporal association with a TMS session in a patient with epilepsy is less than 2%, both for single and paired-pulse TMS (Schrader et al., 2004) and for repetitive TMS (rTMS) (Bae et al., 2007). With rare exceptions (Dhuna et al., 1991), TMS-associated seizures in epilepsy patients have been clinically similar to the patients’ typical spontaneous seizures, leaving uncertainty as to whether they are causally related to stimulation. Here we describe a patient with an epileptic brain malformation who, while undergoing paired-pulse TMS over a cortical region distant from his seizure focus, had a spontaneous seizure of his usual semiology, with demonstrated ictal EEG onset from his expected epileptogenic focus.
The patient was a 22-year-old man with gray matter heterotopia and drug-resistant symptomatic focal (localization-related) epilepsy that began at age 8. His typical seizures were characterized by tonic stiffening and distortion of the left face, lasting up to 31/2 min and occurring every 2–6 weeks, with no precipitating factors identified. He had been taking sodium valproate 2500 mg (serum level 158 μg/mL) and zonisamide 200 mg (serum level 12.2 μg/mL) each day for more than 6 months. His neurological examination showed a long latency of speech and avoidance of eye contact; other findings were normal. Brain MRI demonstrated two areas of nodular heterotopia along the right lateral ventricle. Scalp EEG recordings demonstrated ictal onset in the right frontal lobe (most evident at the F4 electrode in a 10–20 recording system); interictal epileptiform activity was seen in the right frontal and temporal regions.
He was enrolled in a TMS–EEG study approved by our institutional review board for the exploration of cortical physiology in developmental brain malformations, after informed consent was obtained. He had never previously received TMS. Stimulation was delivered with a neuronavigated system (Nexstim Ltd., Helsinki, Finland) and a figure-of-eight coil (external diameter: 70 mm each wing). EEG was recorded continuously using 60 scalp electrodes distributed according to the 10–10 international system (sampling rate: 1450 Hz; offline fast Fourier transform filtering: 1–50 Hz; reference to a forehead electrode).
Initially, the patient received 91 single biphasic pulses with intensity between 30% and 58% of maximum stimulator output (MSO) over the right primary motor cortex (M1); the target muscle was the left first dorsal interosseus. The resting motor threshold (RMT) was identified as 46% MSO. Then, 35 single biphasic pulses (55% MSO–120% RMT) were delivered over a programmed “control” target at the posterior end of the right inferior frontal gyrus, situated about 10 cm away from the presumed seizure focus and identified based on lack of aberrant brain connectivity in this region, as determined by diffusion tensor imaging and resting-state functional connectivity MRI.
Subsequently, 38 monophasic pulses (intensity 63–84% MSO) were given over M1 to establish the intensity needed to obtain motor evoked potentials of about 1 mV. The following monophasic pulses were then delivered over the programmed target: (a) 22 single pulses (76% MSO); (b) 22 paired pulses with an interstimulus interval (ISI) of 3 ms (50% and 76% MSO); (c) 22 paired pulses (ISI 12 ms, 50% and 76% MSO); (d) 6 paired pulses (ISI 100 ms, 76% MSO). All stimulations were separated by at least 5 s; in total, 286 pulses were delivered over approximately 45 min, with on average a 3.5 min break between each condition.
During paradigm (d), a clinical seizure was observed. The patient’s head showed tonic version toward the left side, and he was unresponsive to his father. There was no forced gaze deviation or extremity movement or stiffening noted. After about 90 s, he recovered awareness but was amnesic for the entire event. Neurological examination within 5 min of the event revealed no new abnormalities. The patient’s father reported that this event was identical to his typical seizures in severity, duration, and characteristics; the most recent prior seizure had occurred about 4 weeks earlier. The patient had no subsequent problems after the session, and his next seizure occurred 38 days later, spontaneously at home.
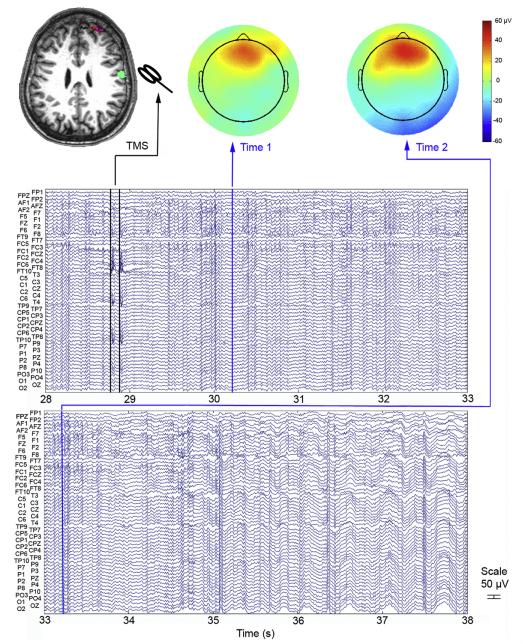
Continuous scalp EEG demonstrated that about 1.5 s after the third paired-pulse stimulation in paradigm (d), rhythmic fast waveforms suggestive of ictal epileptiform activity became visible from the right and midline anterior frontal region, the presumed site of the patient’s seizure focus based on prior clinical evaluation and neurodiagnostic results (Fig. 1). Within about 4 s after ictal onset, epileptiform activity had spread more broadly across many electrodes. As the clinical seizure was not evident immediately, 3 subsequent paired-pulse stimulations were delivered after ictal EEG onset, with no apparent effect on epileptiform activity.
Fig. 1.
Ictal EEG recording of a TMS-associated seizure arising from a focus independent of the stimulation site. This 10-s excerpt of continuous scalp EEG was recorded during TMS over a right posterior frontal cortical target (green area on MRI), when a complex partial seizure occurred. Ictal onset was demonstrated from the right/midline anterior frontal region (rhythmic fast activity between 30 and 31 s, particularly at F2 and F6 electrodes; red area on topographical maps), precisely where the expected seizure focus had been localized based on clinical and neuroimaging characteristics (red area on MRI) and well anterior to the site of stimulation. Vertical blue lines indicate the timepoints at which the two topographical maps are plotted; vertical black lines indicate artifact related to paired-pulse TMS.
While there have been several seizures captured on continuous EEG during TMS (Dhuna et al., 1991), in our case ictal onset was documented to be not from the site of stimulation, but rather from the patient’s expected seizure focus as determined from prior neurodiagnostic studies. Taking a number of factors into account, we believe this seizure was most likely not causally related to the stimulation. First, this focus was consistent with the patient’s semiology (with tonic head version and absence of clonic movements suggestive of contralateral anterior frontal ictal activity) and was located about 10 cm distant from the site of TMS, a separation great enough to insure that the focus was not stimulated directly as a consequence of the coil discharge. Because TMS can have effects on remote regions that are anatomically or functionally connected to the stimulated area, we cannot exclude the possibility that stimulation might have indirectly activated the epileptogenic zone or lowered the seizure threshold. However, the clinical seizure was in all respects identical to the patient’s typical spontaneous events, and occurred within an interval consistent with his usual seizure frequency. Admittedly, since the patient was naïve to TMS, it is possible that anxiety or increased arousal due to the procedure might have contributed to a lowering of the seizure threshold.
Although limited to a single case, our report suggests the need for caution in interpreting the relationship between in-session seizures during TMS and the brain stimulation itself, and highlights the valuable contribution that continuous EEG recording can make in TMS studies of cortical physiology in patients with known epilepsy. In particular, EEG may allow for early detection of epileptiform activity when TMS is applied well outside the motor cortex, as in the current case. Our findings emphasize the importance of a careful clinical and epileptologic assessment before TMS. For patients at risk of seizures, continuous monitoring and the presence of a physician with expertise in the acute management of seizures is recommended (Rossi et al., 2009).
Acknowledgements
Dr. Vernet was supported by the Fyssen Foundation (France). Dr. Yoo was supported by a National Research Foundation of Korea Grant funded by the Korean Government MEST, Basic Research Promotion Fund (NRF-013-2010-1-E00018). Dr. Pascual-Leone serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Allied Mind, Neosync, and Novavision, and holds intellectual property on the real-time integration of transcranial magnetic stimulation (TMS) with electroencephalography (EEG) and magnetic resonance imaging (MRI). Dr. Chang was supported by the National Institutes of Health (K23 NS049159, R01 NS073601), the Epilepsy Foundation, and the Milton Fund of Harvard University.
References
- Bae EH, Schrader LM, Machii K, Alonso-Alonso M, Riviello JJ, Jr, Pascual-Leone A, et al. Safety and tolerability of repetitive transcranial magnetic stimulation in patients with epilepsy: a review of the literature. Epilepsy Behav. 2007;10:521–8. doi: 10.1016/j.yebeh.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Dhuna A, Gates J, Pascual-Leone A. Transcranial magnetic stimulation in patients with epilepsy. Neurology. 1991;41:1067–71. doi: 10.1212/wnl.41.7.1067. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–39. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader LM, Stern JM, Koski L, Nuwer MR, Engel J., Jr Seizure incidence during single- and paired-pulse transcranial magnetic stimulation (TMS) in individuals with epilepsy. Clin Neurophysiol. 2004;115:2728–37. doi: 10.1016/j.clinph.2004.06.018. [DOI] [PubMed] [Google Scholar]