Abstract
Heparin has been widely used as an anticoagulant for more than 80 years. However, there is now considerable evidence that heparin also possesses anti-inflammatory activity, both experimentally and clinically. Importantly in many instances, the anti-inflammatory actions of heparin are independent of anticoagulant activity raising the possibility of developing novel drugs based on heparin that retain the anti-inflammatory activity. Heparin exhibits anti-inflammatory activities via a variety of mechanisms including neutralization of cationic mediators, inhibition of adhesion molecules, and the inhibition of heparanase, all involved in leukocyte recruitment into tissues. It is anticipated that furthering our understanding of the anti-inflammatory actions of heparin will lead to the development of novel anti-inflammatory drugs for a variety of clinical indications.
1. Introduction
Heparin has been used for over eighty years as an anticoagulant. Despite its widespread use, the exact mechanism for the anticoagulant activity of heparin was not elucidated until the 1960s and the specific polysaccharide sequence within the heparin molecule required for this interaction was not defined until nearly twenty years later [1]. The inherent nature of heparin being a polydisperse heterogeneous molecule continues to make this a complex material to work with. In addition to the well described anticoagulant effect of heparin, a range of polysaccharides, some derived from heparin, and some from related structures, have been found to interact with a wide variety of biological pathways and systems, raising the possibility that such drugs may have wider therapeutic uses than inhibiting coagulation. These other activities of heparin and related drugs are less well understood than anticoagulant activity, but such drugs are now under investigation for a wide range of clinical indications, particularly for the treatment of inflammatory diseases.
Heparin is a polysaccharide, and heparin has several unusual characteristics. Firstly, it is polydisperse in nature; that is, it does not possess a defined single structure in the manner of a simple low-molecular-weight drug such as aspirin. Rather, heparin contains a range of saccharide chains of variable lengths and structural diversity and will typically have an average molecular weight of 14 to 18 kDa, but can contain polysaccharides from 10 to over 100 monosaccharide units [1]. The second feature is that heparin is a highly sulphated molecule, and due to this property has a very high negative charge which allows it to bind to a very wide array of positively charged biological materials (see [2] for a review). Heparin belongs to the glycosaminoglycan (GAG) family of polysaccharides which are characterised by alternating hexuronic acid and hexosamine disaccharides as the backbone structure (see Figure 1), although there are a number of other molecules that fall into the GAG family (see Table 1).
Figure 1.
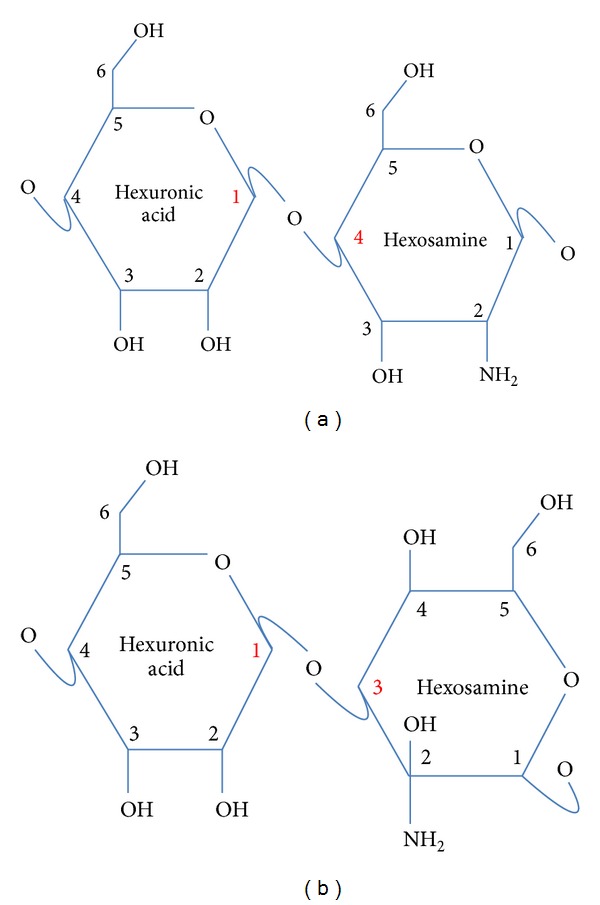
Generic disaccharide backbone structure of glycosaminoglycans with (a) 1–4 linkage, common to heparin and heparan sulphate and (b) 1–3 linkage, common to chondroitin and dermatan sulphates. Numbers on the rings relate to the carbon position, hydroxyl, and amine groups can be sulphated and alternatively orientated.
Table 1.
Examples of glycosaminoglycans (GAGs) and their main backbone components.
| Table of GAGs | Hexuronic acid | Hexosamine | Glycosidic linkage | Average sulphate per disaccharide |
|---|---|---|---|---|
| Hyaluronic acid | GlcUA | βGlcNAc | 1–3, 1–4 | 0 |
| Chondroitin sulphate | GlcUA or GlcUA(2S) | GalNAc or GalNAc(4S) or GalNAc(6S) or GalNAc(4S,6S) | 1–3, 1–4 | 0–2 |
| Heparin | GlcUA or IdoUA(2S) | GlcNAc or GlcNS or GlcNAc(6S) or GlcNS(6S) | 1–4 | 2-3 |
| Heparan sulphate | GlcUA or IdoUA or IdoUA(2S) | GlcNAc or GlcNS or GlcNAc(6S) or GlcNS(6S) | 1–4 | 0–2 |
| Dermatan sulphate | GlcUA or IdoUA or IdoUA(2S) | GalNAc or GalNAc(4S) or GalNAc(6S) or GalNAc(4S,6S) | 1–3, 1–4 | 0–2 |
Hexuronic acid—GlcUA: β-D-glucuronic acid; GlcUA(2S): 2-O-sulfo-β-D-glucuronic acid; IdoUA(2S): 2-O-sulfo-α-L-iduronic acid; IdoUA: α-L-iduronic acid.
Hexosamine—βGlcNAc: β-D-N-acetylglucosamine; GlcNAc: α-D-N-acetylglucosamine; GalNAc: β-D-N-acetylgalactosamine; GalNAc(4S): β-D-N-acetylgalactosamine-4-O-sulfate; GalNAc(6S): β-D-N-acetylgalactosamine-6-O-sulfate; GalNAc(4S,6S): β-D-N-acetylgalactosamine-4-O, 6-O-sulfate; GlcNS: α-D-N-sulfoglucosamine; GlcNS(6S): α-D-N-sulfoglucosamine-6-O-sulfate.
Unfractioned heparin and low-molecular-weight heparins (formed from fractionation or degradation of heparin by different chemical methods, e.g., tinzaparin—(by enzymatic digestion, dalteparin—by nitrous acid depolymerisation, enoxaparin—alkaline β-elimination, and parnaparin—oxidative depolymerisation)) are widely used as anticoagulants in a range of different clinical indications. However, in mammals, heparin is uniquely found in mast cells, which reside within mucosal and connective tissues suggesting that physiologically heparin may be involved in the regulation of inflammatory responses. Indeed, I suggested in 1991 that endogenous heparin may well serve as an endogenous regulator of the inflammatory response in much the same way as various mechanisms have been shown to homeostatically regulate the actions of neurotransmitters and hormones [3]. Mast cells contain an array of inflammatory mediators packed in to their granules which are released on stimulation, and heparin has been found packed in conjunction with a range of cationic molecules, for example, chymase and tryptase. Due to the high negative charge of heparin, a widely held view has been that heparin serves as a packing agent, allowing the containment and storage of large quantities of these various positivity charged mediators in very close proximity [4]. However, it is now recognized that many different proteins involved in the inflammatory cascade have heparin-binding domains in their structure allowing them to recognise and bind heparin, and in many cases heparin is able to inhibit the action of these proteins (see [2]). Thus, endogenous heparin may have an important role in helping control the localised inflammatory response, rather than the coagulation system and indeed heparin has been shown to be released by mast cells [5, 6] and circulating heparin like material has been identified in patients with allergy [7], presumably due to the repeated mast cell degranulation that occurs in such patients.
In addition to endogenous heparin being an anti-inflammatory agent, there are now many experimental and clinical studies demonstrating positive anti-inflammatory activities of heparin (see below), suggesting that such activities could be exploited for therapeutic use. However, the use of heparin itself as an anti-inflammatory drug is currently limited by the anticoagulant activity of the molecule, notwithstanding the observations that when heparin is applied by alternative topical routes of administration, for example, inhalation, and even at higher than conventional anticoagulant doses, no significant anticoagulant effects have been noted systemically [8, 9]. However, the observations, by a number of laboratories, that the anti-inflammatory actions of heparin are independent of its anticoagulant activity [10–13] have spurred the field into investigating novel molecules that mimic the anti-inflammatory activities of heparin, whilst lacking anticoagulant activity and these will be discussed in further detail below.
2. Nonanticoagulant Effects of Heparin
2.1. Effects on Inflammatory Mediators
Heparin can inhibit the activation of a range of inflammatory cells [14–25], an effect that is due in part to the binding and neutralisation of inflammatory mediators and enzymes released during an inflammatory response (reviewed by [2]) that would otherwise go on to activate such cells. Likewise, certain enzymes and cytotoxic mediators released from these cells, involved in propagation of the inflammatory response and subsequent tissue damage and remodelling, have also been shown to be inhibited by heparin, including elastase [26, 27], cathepsin G [26], eosinophil peroxidase [28], eosinophil cationic protein [29], major basic protein [30], certain cytokines (reviewed by [31]), and chemokines (reviewed in [32]).
Many growth factors, including basic fibroblast growth factor [33] and transforming growth factor-beta [34, 35], both of which are involved in the regulation of smooth muscle proliferation, (a feature of the tissue remodelling seen in diseases including asthma, atherosclerosis, and coronary stenosis), are bound by heparin. A long established property of heparin is that of inhibition of vascular smooth muscle cell proliferation [36], an effect which is known to be independent of the anticoagulant actions of heparin [6], and which extends to airway smooth muscle [35, 37, 38].
Heparin is also known to inhibit the degranulation of isolated human mast cells in response to a variety of stimuli, and hence inhibit the release of histamine [20, 39]. This effect is considered to be due to inhibition of inositol 1,4,5-triphosphate- (IP3-) dependent calcium release by heparin. The action of IP3 on the endoplasmic reticulum is potently and competitively blocked by heparin applied to permeabilised mast cells in vitro [40]. IgE-mediated degranulation of mast cells in vitro was found to be inhibited by two fractions of heparin, one which lacked anticoagulant activity and was actually the more potent preparation in this respect, evidence that this effect also does not depend upon the anticoagulant effects of heparin [16]. The cytotoxic effects of TNF-α-activated eosinophils on endothelial cells are also markedly inhibited by heparin [24], as is the homotypic aggregation and chemotaxis of eosinophils in response to complement factor C5a, another inflammatory mediator bound by heparin [25, 41]. Furthermore, unfractionated heparin inhibits lipopolysaccharide-induced activation of endothelial cells via inhibition of p38 MAPK and NF-KB [42].
Heparin has been shown to bind to the surface of neutrophils [43] and can inhibit their degranulation [18, 21], homotypic aggregation [17, 18, 44, 45], the production of superoxide anions, the activity of lysosomal enzymes [17], and the ability of neutrophils to activate platelets [17, 19], again in a manner that is not dependent upon anticoagulant activity. Furthermore, heparin is able to inhibit neutrophil activation in response to thrombin-stimulated platelet products, in addition to inhibiting thrombin-induced platelet aggregation [22], and at high concentrations, platelet α-granule secretion is inhibited [23].
2.2. Effects on Cellular Adhesion
An important component of the inflammatory response is the adherence of inflammatory cells to the vascular endothelium and their subsequent diapedesis into tissues. This is now a well-characterized process, and heparin has been shown to inhibit each of the different stages involved in inflammatory cell recruitment into tissues (reviewed in [2]). Thus, heparin has been shown to inhibit leucocyte-endothelial adhesion, both in vitro (reviewed in [2, 17, 46, 47]) and in vivo [13, 48–53], as well as to limit the ultimate accumulation of cells in inflamed tissues, in response to both allergic [11, 54–56] and nonallergic [13, 48, 50, 57, 58] stimuli.
Heparin is known to bind directly to several adhesion molecules expressed during inflammation and the structural requirements for these interactions are becoming increasingly well characterised (e.g., reviewed by [59]). On leucocytes, L-selectin, a molecule involved in early adhesive interactions between inflammatory cells and the vessel wall, is bound by heparin [60], and endothelial heparan sulphate is able to act as an endothelial ligand for this molecule during cell rolling [61]. The β2-integrin adhesion molecule mac-1 (macrophage-1; CD11b/CD18), important for the firm adhesion of leucocytes to endothelium, is also bound by heparin [62, 63] to an extent that surface immobilised heparin is able to support mac-1-dependent neutrophil adhesion under flow conditions in vitro [62]. Therefore, soluble heparin may inhibit mac-1-dependent interactions between leucocytes and the endothelium; the effects of heparin on leucocyte adhesion in vivo have been found to be dependent on such an interaction with mac-1 [51]. On endothelial cells, heparin binds to P-selectin [64], a selectin adhesion molecule involved in the early sequestration of neutrophils during inflammation. Indeed, the antimetastatic effects of heparin can be ascribed, at least in part, to inhibition of P- and L-selectin function [65, 66]. The selectins are a family of glycoprotein adhesion molecules comprising an epidermal growth factor (EGF) like moiety, repeating sequences mimicking those found on complement binding proteins and an NH2-terminal lectin domain. It is via the lectin domain that these molecules Ca2+-dependently bind to carbohydrate structures on the surfaces of interacting cells. Selectins are concerned predominantly with the rolling stages of adhesion, without which firm adhesion and transmigration cannot proceed [67]. However, despite structural congruencies between the selectins, it has been demonstrated that heparin is unable to bind to E-selectin [60]. This difference is known to rely upon two specific amino-acid residues in the EGF-like domain of the selectins, in that if these residues are altered, E-selectin can be made to bind heparin, and the ability of P-selectin to bind heparin diminished [68]. This differential effect may possess physiological significance with respect to the role of endogenous heparin and, possibly, heparan sulphate in the inflammatory process. Indeed, a similar selectivity of binding can be observed amongst key members of the immunoglobulin superfamily adhesion proteins. Heparin has been shown to bind PECAM-1 (platelet endothelial cell adhesion molecule-1 [69]), an IgSF-adhesion molecule thought to be involved in leucocyte transmigration due to its location at intercellular junctions on the endothelium. The homotypic aggregation of PECAM-1-transfected fibroblasts was found to be inhibited by heparin in a manner dependent upon interaction with the second immunoglobulin domain [70]. Similarly, heparin is able to bind directly to neuronal cell adhesion molecule (NCAM), through a heparin-binding region located on the second immunoglobulin domain [71], as well as through a further heparin-binding region on the first immunoglobulin domain [72]; such interactions with heparan sulphate are important for the physiological functioning of this protein in neuronal development [73]. However, the IgSF-adhesion molecules intercellular adhesion molecule- (ICAM-) 1 and ICAM-2, expressed on vascular endothelium and ligands for leucocyte β2-integrins, do not appear to be bound by heparin. However, given that heparin can affect the functioning of ICAM-1 indirectly, by binding of mac-1, it is plausible that the cell trafficking associated with physiological immune surveillance, facilitated by, for example, interactions between lymphocyte function-related antigen (LFA-1; CD11a/CD18) and ICAM-1/2 may be spared while those associated with excessive cell recruitment during inflammation may be inhibited.
2.3. Inhibition of Heparanase
The ubiquitous distribution of heparan sulphate proteoglycans (HSPGs) in mammalian systems provides a clear indication of the physiological importance of these molecules, which are thought to contribute to growth and development, are key structural components of extracellular matrices, and are involved in the localisation and bioactivity of a wide array of mediators, including enzymes, growth factors, cytokines, and chemokines (reviewed by [74, 75]). The endo-β glucuronidase heparanase (HPSE1) is responsible for the site-selective cleavage of heparan sulphate chains, thus regulating the activity of the wide range of proteins that is functionally dependent upon HSPG. HPSE1 has now been sequenced and cloned [76–79] and HPSE1 exists as a 50 kDa and 8 kDa heterodimer processed from a single, inactive 65 kDa proenzyme ([76] reviewed by [80]). The catalytic sites on the enzyme have also now been characterized [81].
HPSE1 activity has been demonstrated in spleen, lymph nodes, leucocytes and platelets, as well as in endothelial and smooth muscle cells. Moreover, the well-accepted role of HPSE1 in cancer (reviewed by [82]) is underscored by the fact that in human tumours, mRNA for HPSE1 is markedly increased with respect to corresponding normal tissues and that HPSE1 activity in tumour cells has been found to correlate positively with metastatic potential [83]. There are many similarities between leucocyte diapedesis and tumour cell metastasis, and given the evidence that in HPSE1 is involved the latter, it is perhaps not surprising that this enzyme has also been reported as a potential target for novel anti-inflammatory drugs [82].
The potential importance of heparanase activity is illustrated by the fact that in tissue sections from inflammatory bowel disease patients, when compared to healthy tissues, areas of extensive GAG disruption are visible on vascular endothelium and basement membrane, which correlate with localised areas of inflammation [84] and increased levels of GAG degradation products have been found in the urine of patients with asthma, which is thought to reflect the breakdown of extracellular matrices as a result of the inflammatory processes in the airway [85].
It is, therefore, of interest that heparin has long been known to be an inhibitor of HPSE1 activity [86], and it is also well established that heparan-degrading enzymes are released by certain leucocytes during the process of diapedesis [87, 88]. Indeed, when heparin is used at low doses in lymphocyte-driven inflammatory processes such as allergic encephalomyelitis [89, 90], delayed-type hypersensitivity (DTH) [10] and graft-versus-host reactions [91, 92], leucocyte infiltration into tissues is markedly inhibited, and it has been suggested that this effect is via inhibition by heparin of HPSE1. However, recent data with selective inhibitors of HPSE1 have not confirmed anti-inflammatory activity with such drugs in nonallergic inflammatory models, perhaps questioning a central role for this enzyme in leucocyte infiltration (D. Spina, Personal Communication). It has further been demonstrated that vascular endothelial cells also secrete heparanase and that exposure of endothelial cells to proinflammatory cytokines upregulates this secretion [93, 94], further suggesting an important role for this enzyme in inflammation. Moreover, the development of DTH reactions has been found to correlate with endothelial heparanase expression in mice [94].
2.4. Effects on Acute Inflammatory Responses
In animal studies, pretreatment with heparin has been shown to inhibit eosinophil infiltration into the inflamed lung [54, 55, 95] and skin [57], neutrophil accumulation in the inflamed peritoneal cavity [13, 50], independently of anticoagulant activity [11, 13], and to inhibit vascular permeability induced by certain autacoids [96, 97] or the bacterial formyl peptide [97]. Additionally, platelet-activating factor-induced bronchial hyperresponsiveness was inhibited by heparin administration in rabbits [54] and similar effects have been reported in an allergic sheep model, whereby inhaled heparin was found to inhibit the acute airway responses to inhaled allergen [15], an effect that was shared by very low-molecular-weight and nonanticoagulant heparins [16], and in guinea pigs, whereby the protective effect of heparin against bronchial hyperresponsiveness to methacholine was suggested to be due to preservation of nitric oxide signalling in the airway [98].
Heparin has been found, in a number of preclinical models, to protect against ischaemia-reperfusion injury. Thus, in a hamster dorsal skin chamber model, leucocyte-endothelial adhesion induced by ischaemia-reperfusion is inhibited by heparin pretreatment [99], as is cardiac muscle damage [100]. Furthermore, administration of heparin subsequent to transient focal cerebral ischaemia in rats was found to reduce the degree of brain injury by inhibiting reperfusion-induced leucocyte accumulation [58]. Heparin has also recently been suggested as a plausible agent for limitation of the delayed neurological injury that follows subarachnoid haemorrhage [101], by virtue of its broad anti-inflammatory effects. Clearly, the potential to promote further haemorrhage in this setting is a legitimate concern, but it has been suggested that systemic, subanticoagulant doses of heparin may be sufficient to elicit beneficial effects in this condition [101], and, moreover, intracisternal administration of heparin has been found to be protective following experimental subarachnoid haemorrhage in rats [102]. However, given that the anti-inflammatory properties of heparin appear largely to be separable from its effects on coagulation, nonanticoagulant heparin-like molecules may provide a safer approach to treating this important clinical problem in the future.
It has long been appreciated that the anti-inflammatory effects of heparin observed preclinically have also been extended into a number of clinical settings. Heparin has potential use in human inflammatory disease and was first assessed for this purpose in the 1960s, in small, subjectively assessed trials [103, 104]. More recently, in controlled studies, heparin has shown potential in the management of clinical asthma [8, 14, 105, 106] and chronic obstructive pulmonary disease (COPD) [107, 108]. In patients with allergic rhinitis, topical heparin has been observed to reduce eosinophil recruitment into the nose [56] following allergen exposure and to be of value in the treatment of inflammatory bowel disease ([109–111]; reviewed by [112]), although meta-analyses of these trials have concluded that there is currently insufficient evidence to support the use of heparin for the treatment of active ulcerative colitis [113, 114].
Importantly, however, in none of these clinical studies was heparin treatment found to elicit significant haemorrhagic side effects, either when administered systemically or locally. Indeed, in a study performed specifically to address the effects of inhaled heparin on coagulation parameters [9], it was found that almost 40% of a single inhaled dose of heparin is detectable in the lung 24 h later, with no significant effects on blood coagulation. However, given that the anticoagulant actions of heparin appear not to be necessary for the majority of beneficial effects seen in models of inflammation, it seems likely that novel drugs which retain the anti-inflammatory effects of the parent heparin molecule, without the anticoagulant effects will be useful in the management of inflammatory diseases that have been found to respond positively to the administration of heparin or low-molecular-weight heparin; for example, selectively 2,3-O-desulphated heparin, which is currently in clinical trials for COPD, is one such approach [12].
3. Effects of Heparin in Cancer
Due to the common use of heparins for the prophylaxis of venous thromboembolism (VTE) in cancer patients, a sizeable body of evidence exists to suggest that heparin confers benefit in the treatment of cancer that is additional to the direct effects of this drug on blood coagulation (see [115–122]). Analysis of trials of heparin treatment in cancer patients indicates an improved rate of survival [117] and meta-analyses performed specifically to assess the effects of heparin and LMW heparin treatment on survival in cancer patients have indicated positive effects [123, 124].
As discussed previously, the accumulation of metastatic tumour cells into tissues, like leucocytes, is dependent upon adhesion to the vascular endothelium and subsequent diapedesis and many similarities exist between the processes utilised by inflammatory cells and tumour cells in this respect (reviewed by [125]), including a dependency on platelet activation [126, 127]. However, the involvement of anticoagulant mechanisms in these effects is less clear than is perhaps the case for many of the anti-inflammatory properties of heparin. Heparin has been demonstrated repeatedly to reduce metastasis of carcinoma cells in animal models (e.g., [80, 128–131]). However, with respect to the contribution of the anticoagulant effects of the drug, it has been suggested that the basis of the antimetastatic effects of unfractionated heparin lies in the inhibition of fibrin deposition around tumour cells, a factor considered to protect the cells from immune attack [128]. Nonetheless, many studies have found that fractions of heparin with much reduced anticoagulant activity, or none at all, also inhibit metastasis (e.g., [129, 131]). Specific mechanisms thought to be involved in this effect include inhibition of heparanase activity [116], selectin function [126] and the tissue factor pathway [132]. Tissue factor can promote angiogenesis and metastasis via mechanisms both related and unrelated to plasma coagulation [118]. It has been suggested that the effects of heparin and related molecules in models of tumour growth and metastasis rely, at least in part, on the promotion of tissue factor pathway inhibitor (TFPI) release from endothelial cells [132]. Regarding selectin function, clinically relevant levels of LMW heparin, with respect to anticoagulation, have been shown to inhibit experimental metastasis in a manner that correlates with the ability to inhibit P- and L-selectin function; the pentasaccharide fondaparinux, which lacks this ability, was found to be without effect in the same assays, at levels normalised for anticoagulant activity [65], suggesting that it is not the anticoagulant effects per se of heparin that contribute most significantly to effects on tumour cell metastasis. Moreover, mice deficient in both P- and L-selectin were found to be protected against experimental metastasis and, importantly, in these mice, treatment with heparin conferred no further protection [66], in contrast to the marked effects seen in wild-type animals in a range of studies. Protective effects of heparin in cancer models extend beyond the inhibition of metastasis to include those on tumour growth and angiogenesis. Heparin has long been known to be antiangiogenic and its inhibitory effects on heparanase are again well established to be involved in this effect (reviewed by [133]). Growth-factor-induced endothelial cell proliferation is inhibited by unfractionated and LMW heparins [134–136]. Whilst standard LMW heparins in this respect were found to be more potent than unfractionated heparin [135, 136], ultralow-molecular-weight species, including the anticoagulant pentasaccharide fondaparinux, were without effect [136]. Moreover, antiangiogenic and antimetastatic effects may further be mediated through interference with the chemokine system, which is known to be involved in these phenomena (reviewed by [137]).
Therefore, it is likely that heparins inhibit angiogenesis and metastasis via an array of mechanisms, including but by no means limited to heparanase inhibition.
4. Effects of Heparin on Wound Healing and Tissue Repair
Administration of heparin by inhalation has been found to be a viable option for the management of smoke inhalation injury in survivors of fire (reviewed by [138, 139]), which reduces the acute lung injury that contributes significantly to morbidity and mortality in these patients, both alone and in combination with N-acetylcysteine [140].
Indeed, there are a number of reports of heparin being used, topically or systemically, to treat burns, although there are a lack of effectively controlled studies in this area for clear conclusions to be drawn as to the efficacy of this approach (reviewed by [141]). However, isolated case reports continue to emerge, suggesting that heparin is able to promote tissue repair and inhibit inflammation in burns patients (e.g., [142]). Whilst controlled, randomised studies are required to assess the potential utility of heparin in this setting, the demonstrated anti-inflammatory effects in other experimental systems do indicate the potential for a useful effect. Furthermore, in animal models, application of heparin-binding epidermal growth factor-like growth factor (HB-EGF), which is known to be upregulated both in human burn tissue and during healing of experimental burn tissue [143], has been shown to promote healing of partial-thickness burn injuries specifically through potentiation of the expression of transforming growth factor-α, another member of the EGF family of growth factors involved in wound repair [144], and to promote healing of ileal tissue following experimental reanastomosis surgery [145]. It has recently been reported that tissue localisation of HB-EGF, through binding to HSPG, mediates the function of the growth factor as a juxtacrine inhibitor of cell proliferation and that disruption of this binding allows the released HB-EGF to function as an autocrine mitogen [146]. Therefore, it is possible that soluble heparin at the site of injury could act competitively to release this growth factor from heparin sulphate binding sites and promote its participation in tissue repair. Moreover, topically applied heparin has been found to promote effective tissue repair in rabbit trachea, in a model of tissue healing following airway surgery [147], further suggesting that the immunomodulatory effects of heparin may be useful in the specific situation of tissue repair following localised injury.
5. Other Conditions Benefiting from Heparin Treatment
Heparin [148] and the related molecule, pentosan polysulphate, have been shown to have beneficial activity in the treatment of interstitial cystitis and indeed the latter drug has been approved for such use in a number of countries. Another interesting area is the potential use of heparin(s) to treat and prevent protracted labour. Some clinicians had noted that when pregnant women were administered low-molecular-weight heparins (LMWHs) for the prevention of thrombosis, there was a shorter induction time to labour [149–151]. It has been suggested that this effect may relate to inhibition of IL-8. Recent phase 2 clinical studies conducted with tafoxiparin sponsored by Dilafor have confirmed that this LMWH is effective in reducing the incidence of extended labour (http://www.dilafor.com/).
Another possible use of heparin in the treatment of cystic fibrosis was presented [152] and early clinical studies have reported that heparin administered by inhalation provides clinical benefit in patients with COPD (http://www.vectura.com/) which may result from the ability of heparin to act as a mucolytic agent [153] and/or via its well documented effect on neutrophil activations (see above), a major inflammatory cell infiltrating the lung of patients with cystic fibrosis.
6. New Approaches to Treatment
There has long been interest in developing heparin-based anticoagulants that do not require parenteral administration and with respect to, for example, the management of chronic inflammatory diseases, the need for convenient and acceptable methods of drug delivery is arguably an even greater issue. In some circumstances, such as inflammatory diseases of the lung, local administration of heparin by inhalation is an option, but where systemic effects are required, an efficient and predictable drug absorption profile becomes necessary. Absorption of unmodified, unfractionated, and LMW heparins has been reported following oral administration in rats [154–156], and in rats and humans when administered with the absorption-enhancing delivery agent sodium N-[8(2-hydroxybenzoyl)amino]caprylate (SNAC) [157–160]. Similarly, augmentation of heparin absorption via the pulmonary [161–167] and nasal [168–170] routes has been described, when the drug is coadministered with delivery systems including polyethyleneimines, cyclodextrins, alkylmaltosides, alkanoylsucroses, poly-L-arginine, and within PEGylated nanocarriers. Moreover, heparin has been administered successfully in validation studies of needle-free injection devices [171–173], designed to reduce the pain and inconvenience associated with the regular administration of substances such as insulin, presenting a possible alternative to conventional subcutaneous injection of heparins. In all of these studies, measurement of coagulation parameters was used to assess the efficacy of heparin delivery. However, the fact that a robust and well-characterised effect of heparin can be measured, following the administration of standard heparins by nonstandard routes, is promising with respect to the potential delivery of heparin species for nonanticoagulant uses.
A number of new approaches are being investigated to exploit these non-anticoagulant actions of heparin. Heparin and LMWHs are being investigated in a range of diseases (see Table 2) and molecules such as the nonanticoagulant o-desulphated heparin [12]) are in clinical development (Table 2). In addition, a number of polysaccharides of different length and sulphation patterns have been described in the literature [16, 21, 174–178] which are undergoing preclinical and clinical investigation for a range of diseases.
Table 2.
Some examples of clinical trials where heparin is used as a treatment beyond anticoagulant activity (taken from the US National Institute of Health online database http://www.clinicaltrials.gov/ and EU Clinical Trials Register http://www.clinicaltrialsregister.eu/ in Sept., 2012).
| Condition | Intervention | Purpose | Phase | Trial identifier(s) |
|---|---|---|---|---|
| Infertility | Heparin, nadroparin | Heparin increases outcome in poor responders undergoing in vitro fertilisation | Phases 2 and 3 |
NCT01315093
2011-003080-30 |
|
| ||||
| Haemodialysis | Heparin | Evaluates if topically applied heparin to placebo on suitability of constructed primary arteriovenous fistulas | Phase 2 |
NCT01382888
2011-000455-16 |
|
| ||||
| Inhalation burns, smoke inhalation injury | Heparin | Efficacy of nebulised heparin on lung injury score in inhalation burn injury over normal care | Phase 2 | NTC01454869 |
|
| ||||
| Lung cancer | Tinzaparin/enoxaparin | LMWH can inhibit tumour growth and metastasis and enhance survival of patients | Phase 3 |
NCT00475098, NCT00771563, and 2007-007696 |
|
| ||||
| Inflammation | Enoxaparin | Treatment of inflammation in intraocular lens implantation and chronic glomerulonephritis | Phase 4 Phase 3 |
NCT00986076
2005-002989-11 |
|
| ||||
| Adenocarcinoma of the colon | Tinzaparin | LMWH reduction of metastases and recurrence in patients as seen in animal models | Phase 3 | NCT01455831 |
|
| ||||
| Breast, colorectal, Lung, prostate, and venoocclusive cancers | Dalteparin, nadroparin | Assesses if LMWH beyond inhibition of thrombosis improves quality of life over standard treatment | Phases 2 and 3 | NCT00003674, 2005-005336-27 |
|
| ||||
| Vulvodynia | Enoxaparin | LMWH may reduce pain in women with vulvodynia | Phase 2 | NCT00874484 |
|
| ||||
| Ulcerative colitis | Deligoparin | uLMWH may help to reduce inflammation in ulcerative colitis | Phases 2 and 3 | NCT00033943 |
|
| ||||
| Diabetic foot ulcers | Dalteparin | Treatment on chronic foot ulcers due to peripheral arterial occlusive disease in diabetics | Phases 2 and 3 | NCT00765063 |
|
| ||||
| Ovarian cancer | Dalteparin | Assesses the disease response with LMWH over standard therapy | Phase 2 | NCT00239980 |
|
| ||||
| Metastatic Pancreatic cancer | O-Desulphated heparin (ODSH) | Determines if ODSH is efficacious in patients receiving normal therapy | Phase 2 | NCT01461915 |
|
| ||||
| Pregnancy complications | Enoxaparin | Evaluate the efficacy of LMWH on pregnancy outcome in women with previous pregnancy complications | Phase 3 | 2006-004205-26 |
|
| ||||
| Burns | Heparin | Assess analgesic effect of heparin in topical and parenterally treatment | Phase 2 | 2007-004304-12 |
|
| ||||
| Cystic fibrosis | Heparin | Nebulised heparin on easing cystic fibrosis | Phase 2 | 2007-006276-11 |
|
| ||||
| Pulmonary conditions | Heparin, desulfated heparin | Improves lung function in obstructive pulmonary conditions | Phase 2 | 2006-006378-32, 2010-024168-16 |
|
| ||||
| Labour | Nonanticoagulant LMWH | Reducing prolonged labour | Phase 2 | 2006-005839-20 |
|
| ||||
| Microalbuminuria | Sulodexide (LMWH and dermatan sulphate) | Treats microalbuminuria in type 2 diabetes | Phase 3 | 2005-003158-91 |
Additionally, a number of GAG analogues that bind cytokines are under development as novel anti-inflammatory drugs [48, 179]. There is also considerable interest in novel polysaccharides from marine sources such as fucoidans, as anti-inflammatory drugs (see [180]).
Acknowledgments
The author would like to acknowledge the help of John Hogwood for the preparation of the table and figures and of Rebecca Lever in preparing this paper.
References
- 1.Mulloy B, Gray E, Barrowcliffe TW. Characterization of unfractionated heparin: comparison of materials from the last 50 years. Thrombosis and Haemostasis. 2000;84(6):1052–1056. [PubMed] [Google Scholar]
- 2.Lever R, Page CP. Non-anticoagulant effects of heparin: an overview. In: Lever R, Mulloy B, Page CP, editors. Heparin—A Century of Progress. Vol. 207. Berlin, Germany: Springer; 2012. pp. 281–305. (Handbook of Experimental Pharmacology). [DOI] [PubMed] [Google Scholar]
- 3.Page CP. One explanation of the asthma paradox: inhibition of natural anti-inflammatory mechanism by β2-agonists. Lancet. 1991;337(8743):717–720. doi: 10.1016/0140-6736(91)90289-2. [DOI] [PubMed] [Google Scholar]
- 4.Humphries DE, Wong GW, Friend DS, et al. Heparin is essential for the storage of specific granule proteases in mast cells. Nature. 1999;400(6746):769–772. doi: 10.1038/23481. [DOI] [PubMed] [Google Scholar]
- 5.Green WF, Konnaris K, Woolcock AJ. Effect of salbutamol, fenoterol, and sodium cromoglycate on the release of heparin from sensitized human lung fragments challenged with Dermatophagoides pteronyssinus allergen. American Journal of Respiratory Cell and Molecular Biology. 1993;8(5):518–521. doi: 10.1165/ajrcmb/8.5.518. [DOI] [PubMed] [Google Scholar]
- 6.Guyton JR, Rosenberg RD, Clowes AW, Karnovsky MJ. Inhibition of rat arterial smooth muscle cell proliferation by heparin. in vivo studies with anticoagulant and nonanticoagulant heparin. Circulation Research. 1980;46(5):625–634. doi: 10.1161/01.res.46.5.625. [DOI] [PubMed] [Google Scholar]
- 7.Lasser EC, Simon RA, Lyon SG, Hamblin AE, Stein R. Heparin-like anticoagulants in asthma. Allergy. 1987;42(8):619–625. doi: 10.1111/j.1398-9995.1987.tb00393.x. [DOI] [PubMed] [Google Scholar]
- 8.Diamant Z, Timmers MC, Van Der Veen H, Page CP, Van Der Meer FJ, Sterk PJ. Effect of inhaled heparin on allergen-induced early and late asthmatic responses in patients with atopic asthma. American Journal of Respiratory and Critical Care Medicine. 1996;153(6 I):1790–1795. doi: 10.1164/ajrccm.153.6.8665036. [DOI] [PubMed] [Google Scholar]
- 9.Bendstrup KE, Chambers CB, Jensen JI, Newhouse MT. Lung deposition and clearance of inhaled 99mTc-heparin in healthy volunteers. American Journal of Respiratory and Critical Care Medicine. 1999;160(5 I):1653–1658. doi: 10.1164/ajrccm.160.5.9809123. [DOI] [PubMed] [Google Scholar]
- 10.Sy MS, Schneeberger E, McCluskey R, Greene MI, Rosenberg RD, Benacerraf B. Inhibition of delayed-type hypersensitivity by heparin depleted of anticoagulant activity. Cellular Immunology. 1983;82(1):23–32. doi: 10.1016/0008-8749(83)90137-5. [DOI] [PubMed] [Google Scholar]
- 11.Seeds EAM, Page CP. Heparin inhibits allergen-induced eosinophil infiltration into guinea-pig lung via a mechanism unrelated to its anticoagulant activity. Pulmonary Pharmacology & Therapeutics. 2001;14(2):111–119. doi: 10.1006/pupt.2000.0277. [DOI] [PubMed] [Google Scholar]
- 12.Fryer A, Huang YC, Rao G, et al. Selective O-desulfation produces nonanticoagulant heparin that retains pharmacological activity in the lung. Journal of Pharmacology and Experimental Therapeutics. 1997;282(1):208–219. [PubMed] [Google Scholar]
- 13.Lever R, Smailbegovic A, Page CP. Locally available heparin modulates inflammatory cell recruitment in a manner independent of anticoagulant activity. European Journal of Pharmacology. 2010;630(1-3):137–144. doi: 10.1016/j.ejphar.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed T, Garrigo J, Danta I. Preventing bronchoconstriction in exercise-induced asthma with inhaled heparin. The New England Journal of Medicine. 1993;329(2):90–95. doi: 10.1056/NEJM199307083290204. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed T, Syriste T, Mendelssohn R, et al. Heparin prevents antigen-induced airway hyperresponsiveness: interference with IP3-mediated mast cell degranulation? Journal of Applied Physiology. 1994;76(2):893–901. doi: 10.1152/jappl.1994.76.2.893. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed T, Campo C, Abraham MK, et al. Inhibition of antigen-induced acute bronchoconstriction, airway hyperresponsiveness, and mast cell degranulation by a nonanticoagulant heparin: comparison with a low molecular weight heparin. American Journal of Respiratory and Critical Care Medicine. 1997;155(6):1848–1855. doi: 10.1164/ajrccm.155.6.9196085. [DOI] [PubMed] [Google Scholar]
- 17.Bazzoni G, Nuñez AB, Mascellani G, Bianchini P, Dejana E, Del Maschio A. Effect of heparin, dermatan sulfate, and related oligo-derivatives on human polymorphonuclear leukocyte functions. Journal of Laboratory and Clinical Medicine. 1993;121(2):268–275. [PubMed] [Google Scholar]
- 18.Brown RA, Lever R, Jones NA, Page CP. Effects of heparin and related molecules upon neutrophil aggregation and elastase release in vitro . British Journal of Pharmacology. 2003;139(4):845–853. doi: 10.1038/sj.bjp.0705291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evangelista V, Piccardoni P, Maugeri N, De Gaetano G, Cerletti C. Inhibition by heparin of platelet activation induced by neutrophil-derived cathepsin G. European Journal of Pharmacology. 1992;216(3):401–405. doi: 10.1016/0014-2999(92)90437-9. [DOI] [PubMed] [Google Scholar]
- 20.Inase N, Schreck RE, Lazarus SC. Heparin inhibits histamine release from canine mast cells. American Journal of Physiology. 1993;264(4):L387–L390. doi: 10.1152/ajplung.1993.264.4.L387. [DOI] [PubMed] [Google Scholar]
- 21.Lever R, Lo WT, Faraidoun M, et al. Size-fractionated heparins have differential effects on human neutrophil function in vitro . British Journal of Pharmacology. 2007;151(6):837–843. doi: 10.1038/sj.bjp.0707298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piccardoni P, Evangelista V, Piccoli A, De Gaetano G, Walz A, Cerletti C. Thrombin-activated human platelets release two NAP-2 variants that stimulate polymorphonuclear leukocytes. Thrombosis and Haemostasis. 1996;76(5):780–785. [PubMed] [Google Scholar]
- 23.Rohrer MJ, Kestin AS, Ellis PA, et al. High-dose heparin suppresses platelet alpha granule secretion. Journal of Vascular Surgery. 1992;15(6):1000–1009. [PubMed] [Google Scholar]
- 24.Slungaard A, Vercellotti GM, Walker G, Nelson RD, Jacob HS. Tumor necrosis factor α/cachectin stimulates eosinophil oxidant production and toxicity towards human endothelium. Journal of Experimental Medicine. 1990;171(6):2025–2041. doi: 10.1084/jem.171.6.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teixeira MM, Rossi AG, Hellewell PG. Adhesion mechanisms involved in C5a-induced eosinophil homotypic aggregation. Journal of Leukocyte Biology. 1996;59(3):389–396. doi: 10.1002/jlb.59.3.389. [DOI] [PubMed] [Google Scholar]
- 26.Redini F, Tixier JM, Petitou M, Choay J, Robert L, Hornebeck W. Inhibition of leucocyte elastase by heparin and its derivatives. Biochemical Journal. 1988;252(2):515–519. doi: 10.1042/bj2520515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh RL, Dillon TJ, Scicchitano R, McLennan G. Heparin and heparan sulphate are inhibitors of human leucocyte elastase. Clinical Science. 1991;81(3):341–346. doi: 10.1042/cs0810341. [DOI] [PubMed] [Google Scholar]
- 28.Pégorier S, Wagner LA, Gleich GJ, Pretolani M. Eosinophil-derived cationic proteins activate the synthesis of remodeling factors by airway epithelial cells. The Journal of Immunology. 2006;177:4861–4869. doi: 10.4049/jimmunol.177.7.4861. [DOI] [PubMed] [Google Scholar]
- 29.Fredens K, Dahl R, Venge P. In vitro studies of the interaction between heparin and eosinophil cationic protein. Allergy. 1991;46(1):27–29. doi: 10.1111/j.1398-9995.1991.tb00538.x. [DOI] [PubMed] [Google Scholar]
- 30.Swaminathan GJ, Myszka DG, Katsamba PS, Ohnuki LE, Gleich GJ, Acharya KR. Eosinophil-granule major basic protein, a C-type lectin, binds heparin. Biochemistry. 2005;44(43):14152–14158. doi: 10.1021/bi051112b. [DOI] [PubMed] [Google Scholar]
- 31.Muramatsu T, Muramatsu H. Glycosaminoglycan-binding cytokines as tumor markers. Proteomics. 2008;8(16):3350–3359. doi: 10.1002/pmic.200800042. [DOI] [PubMed] [Google Scholar]
- 32.Shute J. Glycosaminoglycan and chemokine/growth factor interactions. In: Lever R, Mulloy B, Page CP, editors. Heparin—A Century of Progress. Vol. 207. Heidelberg, Germany: Springer; 2011. pp. 307–324. (Handbook of Experimental Pharmacology). [Google Scholar]
- 33.Bono F, Rigon P, Lamarche I, Savi P, Salel V, Herbert JM. Heparin inhibits the binding of basic fibroblast growth factor to cultured human aortic smooth-muscle cells. Biochemical Journal. 1997;326(part 3):661–668. doi: 10.1042/bj3260661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCaffrey TA, Falcone DJ, Brayton CF, Agarwal LA, Welt FG, Weksler BB. Transforming growth factor-b activity is potentiated by heparin via dislocation of the transforming growth factor-b/a2-macroglobulin inactive complex. The Journal of Cell Biology. 1989;109(1):441–448. doi: 10.1083/jcb.109.1.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okona-Mensah KB, Shittu E, Page C, Costello J, Kilfeather SA. Inhibition of serum and transforming growth factor beta (TGF-β1)-induced DNA synthesis in confluent airway smooth muscle by heparin. British Journal of Pharmacology. 1998;125(4):599–606. doi: 10.1038/sj.bjp.0702046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clowes AW, Karnowsky MJ. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977;265(5595):625–626. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- 37.Kanabar V, Hirst SJ, O’Connor BJ, Page CP. Some structural determinants of the antiproliferative effect of heparin-like molecules on human airway smooth muscle. British Journal of Pharmacology. 2005;146(3):370–377. doi: 10.1038/sj.bjp.0706333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kilfeather SA, Tagoe S, Perez AC, Okona-Mensa K, Matin R, Page CP. Inhibition of serum-induced proliferation of bovine tracheal smooth muscle cells in culture by heparin and related glycosaminoglycans. British Journal of Pharmacology. 1995;114(7):1442–1446. doi: 10.1111/j.1476-5381.1995.tb13367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dragstedt CA, Wells JA, Rocha E, Silva M. Inhibitory effect of heparin upon histamine release by trypsin, antigen, and protease. Proceedings of the Society for Experimental Biology and Medicine. 1942;51:191–192. [Google Scholar]
- 40.Ghosh TK, Eis PS, Mullaney JM, Ebert CL, Gill DL. Competitive, reversible, and potent antagonism of inositol 1,4,5-trisphosphate-activated calcium release by heparin. Journal of Biological Chemistry. 1988;263(23):11075–11079. [PubMed] [Google Scholar]
- 41.Matzner Y, Marx G, Drexler R, Eldor A. The Inhibitory effect of heparin and related glycosaminoglycans on neutrophil chemotaxis. Thrombosis and Haemostasis. 1984;52:134–137. [PubMed] [Google Scholar]
- 42.Li S, Zheng Z, Li X, Xiochun M. Unfractionated heparin inhibits lipopolysaccharide-induced response through blocking p38 MAPK and NF-KB activation on endothgelial cells. Cytokine. 2012;60(1):114–121. doi: 10.1016/j.cyto.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 43.Leculier C, Benzerara O, Couprie N, et al. Specific binding between human neutrophils and heparin. British Journal of Haematology. 1992;81(1):81–85. doi: 10.1111/j.1365-2141.1992.tb08176.x. [DOI] [PubMed] [Google Scholar]
- 44.Freischlag JA, Colburn MD, Quiñones-Baldrich WJ, Moore WS. Heparin, urokinase, and ancrod alter neutrophil function. Journal of Vascular Surgery. 1992;16(4):565–574. doi: 10.1067/mva.1992.39288. [DOI] [PubMed] [Google Scholar]
- 45.Pasini FL, Pasqui AL, Ceccatelli L. Heparin inhibition of polymorphonuclear leukocyte activation in vitro. A possible pharmacological approach to granulocyte-mediated vascular damage. Thrombosis Research. 1984;35(5):527–537. doi: 10.1016/0049-3848(84)90284-6. [DOI] [PubMed] [Google Scholar]
- 46.Silvestro L, Viano I, Macario M, et al. Effects of heparin and its desulfated derivatives on leukocyte-endothelial adhesion. Seminars in Thrombosis and Hemostasis. 1994;20(3):254–258. doi: 10.1055/s-2007-1001910. [DOI] [PubMed] [Google Scholar]
- 47.Smailbegovic A, Lever R, Page CP. The effects of heparin on the adhesion of human peripheral blood mononuclear cells to human stimulated umbilical vein endothelial cells. British Journal of Pharmacology. 2001;134(4):827–836. doi: 10.1038/sj.bjp.0704321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson Z, Kosco-Vilbois MH, Herren S, et al. Interference with heparin binding and oligomerization creates a novel anti-inflammatory strategy targeting the chemokine system. Journal of Immunology. 2004;173(9):5776–5785. doi: 10.4049/jimmunol.173.9.5776. [DOI] [PubMed] [Google Scholar]
- 49.Ley K, Cerrito M, Arfors KE. Sulfated polysaccharides inhibit leukocyte rolling in rabbit mesentery venules. American Journal of Physiology. 1991;260(5):H1667–H1673. doi: 10.1152/ajpheart.1991.260.5.H1667. [DOI] [PubMed] [Google Scholar]
- 50.Nelson RM, Cecconi O, Roberts WG, Aruffo A, Linhardt RJ, Bevilacqua MP. Heparin oligosaccharides bind L- and P-selectin and inhibit acute inflammation. Blood. 1993;82(11):3253–3258. [PubMed] [Google Scholar]
- 51.Salas A, Sans M, Soriano A, et al. Heparin attenuates TNF-alpha induced inflammatory response through a CD11b dependent mechanism. Gut. 2000;47(1):88–96. doi: 10.1136/gut.47.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tangelder GJ, Arfors KE. Inhibition of leukocyte rolling in venules by protamine and sulfated polysaccharides. Blood. 1991;77(7):1565–1571. [PubMed] [Google Scholar]
- 53.Xie X, Thorlacius H, Raud J, Hedqvist P, Lindbom L. Inhibitory effect of locally administered heparin on leukocyte rolling and chemoattractant-induced firm adhesion in rat mesenteric venules in vivo. British Journal of Pharmacology. 1997;122(5):906–910. doi: 10.1038/sj.bjp.0701454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sasaki M, Herd CM, Page CP. Effect of heparin and low-molecular weight heparinoid on PAF-induced airway responses in neonatally immunized rabbits. British Journal of Pharmacology. 1993;110(1):107–112. doi: 10.1111/j.1476-5381.1993.tb13778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seeds EAM, Horne AP, Tyrrell DJ, Page CP. The effect of inhaled heparin and related glycosaminoglycans on allergen-induced eosinophil infiltration in guinea-pigs. Pulmonary Pharmacology. 1995;8(2-3):97–105. doi: 10.1006/pulp.1995.1012. [DOI] [PubMed] [Google Scholar]
- 56.Vancheri C, Mastruzzo C, Armato F, et al. Intranasal heparin reduces eosinophil recruitment after nasal allergen challenge in patients with allergic rhinitis. Journal of Allergy and Clinical Immunology. 2001;108(5):703–708. doi: 10.1067/mai.2001.118785. [DOI] [PubMed] [Google Scholar]
- 57.Teixeira MM, Hellewell PG. Suppression by intradermal administration of heparin of eosinophil accumulation but not oedema formation in inflammatory reactions in guinea-pig skin. British Journal of Pharmacology. 1993;110(4):1496–1500. doi: 10.1111/j.1476-5381.1993.tb13991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yanaka K, Nose T, Hindman BJ. Heparin ameliorates brain injury by inhibiting leukocyte accumulation. Stroke. 1996;27(11):2146–2147. [PubMed] [Google Scholar]
- 59.Fritzsche J, Alban S, Ludwig RJ, et al. The influence of various structural parameters of semisynthetic sulfated polysaccharides on the P-selectin inhibitory capacity. Biochemical Pharmacology. 2006;72(4):474–485. doi: 10.1016/j.bcp.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 60.Koenig A, Norgard-Sumnicht K, Linhardt R, Varki A. Differential interactions of heparin and heparan sulfate glycosaminoglycans with the selectins: implications for the use of unfractionated and low molecular weight heparins as therapeutic agents. Journal of Clinical Investigation. 1998;101(4):877–889. doi: 10.1172/JCI1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giuffrè L, Cordey AS, Monai N, Tardy Y, Schapira M, Spertini O. Monocyte adhesion to activated aortic endothelium: role of L-selectin and heparan sulfate proteoglycans. Journal of Cell Biology. 1997;136(4):945–956. doi: 10.1083/jcb.136.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diamond MS, Alon R, Parkos CA, Quinn MT, Springer TA. Heparin is an adhesive ligand for the leukocyte integrin Mac-1 (CD11b/CD18) Journal of Cell Biology. 1995;130(6):1473–1482. doi: 10.1083/jcb.130.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peter K, Schwarz M, Conradt C, et al. Heparin inhibits ligand binding to the leukocyte integrin Mac-1 (CD11b/CD18) Circulation. 1999;100(14):1533–1539. doi: 10.1161/01.cir.100.14.1533. [DOI] [PubMed] [Google Scholar]
- 64.Skinner MP, Lucas CM, Burns GF, Chesterman CN, Berndt MC. GMP-140 binding to neutrophils is inhibited by sulfated glycans. Journal of Biological Chemistry. 1991;266(9):5371–5374. [PubMed] [Google Scholar]
- 65.Stevenson JL, Choi SH, Varki A. Differential metastasis inhibition by clinically relevant levels of heparins—correlation with selectin inhibition, not antithrombotic activity. Clinical Cancer Research. 2005;11(19):7003–7011. doi: 10.1158/1078-0432.CCR-05-1131. [DOI] [PubMed] [Google Scholar]
- 66.Stevenson JL, Varki A, Borsig L. Heparin attenuates metastasis mainly due to inhibition of P- and L-selectin, but non-anticoagulant heparins can have additional effects. Thrombosis Research. 2007;120(2):S107–S111. doi: 10.1016/S0049-3848(07)70138-X. [DOI] [PubMed] [Google Scholar]
- 67.Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65(5):859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 68.Revelle BM, Scott D, Beck PJ. Single amino acid residues in the E-and P-selectin epidermal growth factor domains can determine carbohydrate binding specificity. The Journal of Biological Chemistry. 1996;271:16160–16170. doi: 10.1074/jbc.271.27.16160. [DOI] [PubMed] [Google Scholar]
- 69.Watt SM, Williamson J, Genevier H, et al. The heparin binding PECAM-1 adhesion molecule is expressed by CD34+ hematopoietic precursor cells with early myeloid and B-lymphoid cell phenotypes. Blood. 1993;82(9):2649–2663. [PubMed] [Google Scholar]
- 70.DeLisser HM, Yan HC, Newman PJ, Muller WA, Buck CA, Albelda SM. Platelet/endothelial cell adhesion molecule-1 (CD31)-mediated cellular aggregation involves cell surface glycosaminoglycans. Journal of Biological Chemistry. 1993;268(21):16037–16046. [PubMed] [Google Scholar]
- 71.Cole GJ, Loewy A, Glaser L. Neuronal cell-cell adhesion depends on interactions of N-CAM with heparin-like molecules. Nature. 1986;320(6061):445–447. doi: 10.1038/320445a0. [DOI] [PubMed] [Google Scholar]
- 72.Kiselyov VV, Berezin V, Maar TE, et al. The first immunoglobulin-like neural cell adhesion molecule (NCAM) domain is involved in double-reciprocal interaction with the second immunoglobulin-like NCAM domain and in heparin binding. Journal of Biological Chemistry. 1997;272(15):10125–10134. doi: 10.1074/jbc.272.15.10125. [DOI] [PubMed] [Google Scholar]
- 73.Kallapur SG, Akeson RA. The neural cell adhesion molecule (NCAM) heparin binding domain binds to cell surface heparan sulfate proteoglycans. Journal of Neuroscience Research. 1992;33(4):538–548. doi: 10.1002/jnr.490330406. [DOI] [PubMed] [Google Scholar]
- 74.Powell AK, Yates EA, Fernig DG, Turnbull JE. Interactions of heparin/heparan sulfate with proteins: appraisal of structural factors and experimental approaches. Glycobiology. 2004;14(4):17R–30R. doi: 10.1093/glycob/cwh051. [DOI] [PubMed] [Google Scholar]
- 75.Turnbull J, Powell A, Guimond S. Heparan sulfate: decoding a dynamic multifunctional cell regulator. Trends in Cell Biology. 2001;11(2):75–82. doi: 10.1016/s0962-8924(00)01897-3. [DOI] [PubMed] [Google Scholar]
- 76.Fairbanks MB, Mildner AM, Leone JW, et al. Processing of the human heparanase precursor and evidence that the active enzyme is a heterodimer. Journal of Biological Chemistry. 1999;274(42):29587–29590. doi: 10.1074/jbc.274.42.29587. [DOI] [PubMed] [Google Scholar]
- 77.Hulett MD, Freeman C, Hamdorf BJ, Baker RT, Harris MJ, Parish CR. Cloning of mammalian heparanase, an important enzyme in tumor invasion and metastasis. Nature Medicine. 1999;5(7):803–809. doi: 10.1038/10525. [DOI] [PubMed] [Google Scholar]
- 78.Kussie PH, Hulmes JD, Ludwig DL, et al. Cloning and functional expression of a human heparanase gene. Biochemical and Biophysical Research Communications. 1999;261(1):183–187. doi: 10.1006/bbrc.1999.0962. [DOI] [PubMed] [Google Scholar]
- 79.Vlodavsky I, Friedmann Y, Elkin M, et al. Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis. Nature Medicine. 1999;5(7):793–802. doi: 10.1038/10518. [DOI] [PubMed] [Google Scholar]
- 80.Parish CR, Freeman C, Hulett MD. Heparanase: a key enzyme involved in cell invasion. Biochimica et Biophysica Acta. 2001;1471(3):M99–M108. doi: 10.1016/s0304-419x(01)00017-8. [DOI] [PubMed] [Google Scholar]
- 81.Hulett MD, Hornby JR, Ohms SJ, et al. Identification of active-site residues of the pro-metastatic endoglycosidase heparanase. Biochemistry. 2000;39(51):15659–15667. doi: 10.1021/bi002080p. [DOI] [PubMed] [Google Scholar]
- 82.McKenzie EA. Heparanase: a target for drug discovery in cancer and inflammation. British Journal of Pharmacology. 2007;151(1):1–14. doi: 10.1038/sj.bjp.0707182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McKenzie E, Tyson K, Stamps A, et al. Cloning and expression profiling of Hpa2, a novel mammalian heparanase family member. Biochemical and Biophysical Research Communications. 2000;276(3):1170–1177. doi: 10.1006/bbrc.2000.3586. [DOI] [PubMed] [Google Scholar]
- 84.Murch SH, MacDonald TT, Walker-Smith JA, Levin M, Lionetti P, Klein NJ. Disruption of sulphated glycosaminoglycans in intestinal inflammation. Lancet. 1993;341(8847):711–714. doi: 10.1016/0140-6736(93)90485-y. [DOI] [PubMed] [Google Scholar]
- 85.Shute JK, Parmar J, Holgate ST, Howarth PH. Urinary glycosaminoglycan levels are increased in acute severe asthma—a role for eosinophil-derived gelatinase B? International Archives of Allergy and Immunology. 1997;113(1-3):366–367. doi: 10.1159/000237604. [DOI] [PubMed] [Google Scholar]
- 86.Bar-Ner M, Eldor A, Wasserman L, et al. Inhibition of heparanase-mediated degradation of extracellular matrix heparan sulfate by non-anticoagulant heparin species. Blood. 1987;70(2):551–557. [PubMed] [Google Scholar]
- 87.Lider O, Mekori YA, Miller T, et al. Inhibition of T lymphocyte heparanase by heparin prevents T cell migration and T cell-mediated immunity. European Journal of Immunology. 1990;20(3):493–499. doi: 10.1002/eji.1830200306. [DOI] [PubMed] [Google Scholar]
- 88.Matzner Y, Vlodavsky I, Bar-Ner M, Ishai-Michaeli R, Tauber AI. Subcellular localization of heparanase in human neutrophils. Journal of Leukocyte Biology. 1992;51(6):519–524. doi: 10.1002/jlb.51.6.519. [DOI] [PubMed] [Google Scholar]
- 89.Lider O, Baharav E, Mekori YA, et al. Suppression of experimental autoimmune diseases and prolongation of allograft survival by treatment of animals with low doses of heparins. Journal of Clinical Investigation. 1989;83(3):752–756. doi: 10.1172/JCI113953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Willenborg DO, Parish CR. Inhibition of allergic encephalomyelitis in rats by treatment with sulfated polysaccharides. Journal of Immunology. 1988;140(10):3401– 3405. [PubMed] [Google Scholar]
- 91.Gorski A, Lao M, Gradowska L, Nowaczyk M, Wasik M, Lagodzinski Z. New strategies of heparin treatment used to prolong allograft survival. Transplantation Proceedings. 1991;23(4):2251–2252. [PubMed] [Google Scholar]
- 92.Naparstek E, Slavin S, Weiss L, et al. Low-dose heparin inhibits acute graft versus host disease in mice. Bone Marrow Transplantation. 1993;12(3):185–189. [PubMed] [Google Scholar]
- 93.Chen G, Wang D, Vikramadithyan R, et al. Inflammatory cytokines and fatty acids regulate endothelial cell heparanase expression. Biochemistry. 2004;43(17):4971–4977. doi: 10.1021/bi0356552. [DOI] [PubMed] [Google Scholar]
- 94.Edovitsky E, Lerner I, Zcharia E, Peretz T, Vlodavsky I, Elkin M. Role of endothelial heparanase in delayed-type hypersensitivity. Blood. 2006;107(9):3609–3616. doi: 10.1182/blood-2005-08-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Seeds EAM, Hanss J, Page CP. The effect of heparin and related proteoglycans on allergen and PAF-induced eosinophil infiltration. Journal of Lipid Mediators. 1993;7(3):269–278. [PubMed] [Google Scholar]
- 96.Carr J. The anti-inflammatory action of heparin: heparin as an antagonist to histamine, bradykinin and prostaglandin E1 . Thrombosis Research. 1979;16(3-4):507–516. doi: 10.1016/0049-3848(79)90097-5. [DOI] [PubMed] [Google Scholar]
- 97.Jones H, Paul W, Page CP. The effects of heparin and related molecules on vascular permeability and neutrophil accumulation in rabbit skin. British Journal of Pharmacology. 2002;135(2):469–479. doi: 10.1038/sj.bjp.0704505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maarsingh H, De Boer J, Kauffman HF, Zaagsma J, Meurs H. Heparin normalizes allergen-induced nitric oxide deficiency and airway hyperresponsiveness. British Journal of Pharmacology. 2004;142(8):1293–1299. doi: 10.1038/sj.bjp.0705848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Becker M, Menger MD, Lehr HA. Heparin-released superoxide dismutase inhibits postischemic leukocyte adhesion to venular endothelium. American Journal of Physiology. 1994;267(3):H925–H930. doi: 10.1152/ajpheart.1994.267.3.H925. [DOI] [PubMed] [Google Scholar]
- 100.Kilgore KS, Tanhehco EJ, Naylor KB, Lucchesi BR. Ex vivo reversal of heparin-mediated cardioprotection by heparinase after ischemia and reperfusion. Journal of Pharmacology and Experimental Therapeutics. 1999;290(3):1041–1047. [PubMed] [Google Scholar]
- 101.Simard JM, Schreibman D, Aldrich EF, et al. Unfractionated heparin: multitargeted therapy for delayed neurological deficits induced by subarachnoid hemorrhage. Neurocritical Care. 2010;13(3):439–449. doi: 10.1007/s12028-010-9435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tekkök IH, Tekkök S, Ozcan OE, Erbengi T, Erbengi A. Preventive effect of intracisternal heparin for proliferative angiopathy after experimental subarachnoid haemorrhage in rats. Acta Neurochirurgica. 1994;127(1-2):112–117. doi: 10.1007/BF01808557. [DOI] [PubMed] [Google Scholar]
- 103.Dolowitz DA, Dougherty TF. The use of heparin as an anti-inflammatory agent. LaryngoScope. 1960;70:873–874. [PubMed] [Google Scholar]
- 104.Dolowitz DA, Dougherty TF. The use of heparin in the control of allergies. Annals of Allergy. 1965;23:309–313. [PubMed] [Google Scholar]
- 105.Antczak M, Kuna P. Heparin inhibits allergen induced airway response in asthmatics. Results of a double blind placebo-controlled, crossover study. Journal of Allergy and Clinical Immunology. 1995;95:p. 386. [Google Scholar]
- 106.Bowler SD, Smith SM, Laverombe PS. Heparin inhibits the immediate response to antigen in the skin and lungs of allergic subjects. The American Review of Respiratory Disease. 1993;147(1):160–163. doi: 10.1164/ajrccm/147.1.160. [DOI] [PubMed] [Google Scholar]
- 107.Venge P, Pedersen B, Håkansson L, Hällgren R, Lindblad G, Dahl R. Subcutaneous administration of hyaluronan reduces the number of infectious exacerbations in patients with chronic bronchitis. American Journal of Respiratory and Critical Care Medicine. 1996;153(1):312–316. doi: 10.1164/ajrccm.153.1.8542136. [DOI] [PubMed] [Google Scholar]
- 108.Brown RA, Allegra L, Matera MG, Page CP, Cazzola M. Additional clinical benefit of enoxaparin in COPD patients receiving salmeterol and fluticasone propionate in combination. Pulmonary Pharmacology and Therapeutics. 2006;19(6):419–424. doi: 10.1016/j.pupt.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 109.Evans RC, Wong VS, Morris AI, Rhodes JM. Treatment of corticosteroid-resistant ulcerative colitis with heparin—a report of 16 cases. Alimentary Pharmacology & Therapeutics. 1997;11(6):1037–1040. doi: 10.1046/j.1365-2036.1997.00252.x. [DOI] [PubMed] [Google Scholar]
- 110.Gaffney PR, O’Leary JJ, Doyle CT, et al. Response to heparin in patients with ulcerative colitis. Lancet. 1991;337(8735):238–239. doi: 10.1016/0140-6736(91)92201-c. [DOI] [PubMed] [Google Scholar]
- 111.Gaffney PR, Doyle CT, Gaffney A, Hogan J, Hayes DP, Annis P. Paradoxical response to heparin in 10 patients with ulcerative colitis. American Journal of Gastroenterology. 1995;90(2):220–223. [PubMed] [Google Scholar]
- 112.Michell NP, Lalor P, Langman MJ. Heparin therapy for ulcerative colitis? Effects and mechanisms. European Journal of Gastroenterology & Hepatology. 2001;13:449–456. doi: 10.1097/00042737-200104000-00026. [DOI] [PubMed] [Google Scholar]
- 113.Chande N, McDonald JW, Macdonald JK. Unfractionated or low-molecular weight heparin for induction of remission in ulcerative colitis. Cochrane Database of Systematic Reviews. 2008;(2) doi: 10.1002/14651858.CD006774.pub2.CD006774 [DOI] [PubMed] [Google Scholar]
- 114.Shen J, Ran ZH, Tong JL, Xiao SD. Meta-analysis: the utility and safety of heparin in the treatment of active ulcerative colitis. Alimentary Pharmacology & Therapeutics. 2007;26(5):653–663. doi: 10.1111/j.1365-2036.2007.03418.x. [DOI] [PubMed] [Google Scholar]
- 115.Borsig L. Antimetastatic activities of heparins and modified heparins. Experimental evidence. Thrombosis Research. 2010;125:S66–71. doi: 10.1016/S0049-3848(10)70017-7. [DOI] [PubMed] [Google Scholar]
- 116.Engelberg H. Actions of heparin that may affect the malignant process. Cancer. 1999;85:257–272. doi: 10.1002/(sici)1097-0142(19990115)85:2<257::aid-cncr1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 117.Hettiarachchi RJK, Smorenburg SM, Ginsberg J, Levine M, Prins MH, Buller HR. Do heparins do more than just treat thrombosis? The influence of heparins on cancer spread. Thrombosis and Haemostasis. 1999;82(2):947–952. [PubMed] [Google Scholar]
- 118.Mousa SA. Heparin and low-molecular weight heparins in thrombosis and beyond. Methods in Molecular Biology. 2010;663:109–132. doi: 10.1007/978-1-60761-803-4_3. [DOI] [PubMed] [Google Scholar]
- 119.Niers TMH, Klerk CPW, DiNisio M, et al. Mechanisms of heparin induced anti-cancer activity in experimental cancer models. Critical Reviews in Oncology/Hematology. 2007;61(3):195–207. doi: 10.1016/j.critrevonc.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 120.Smorenburg SM, Van Noorden CJF. The complex effects of heparins on cancer progression and metastasis in experimental studies. Pharmacological Reviews. 2001;53(1):93–105. [PubMed] [Google Scholar]
- 121.Zacharski LR, Ornstein DL. Heparin and cancer. Thrombosis and Haemostasis. 1988;80(1):10–23. [PubMed] [Google Scholar]
- 122.Zacharski LR, Ornstein DL, Mamourian AC. Low-molecular-weight heparin and cancer. Seminars in Thrombosis and Hemostasis. 2000;26(3):69–77. doi: 10.1055/s-2000-9499. [DOI] [PubMed] [Google Scholar]
- 123.Akl EA, van Doormaal FF, Barba M, et al. Parenteral anticoagulation for prolonging survival in patients with cancer who have no other indication for anticoagulation. Cochrane Database of Systematic Reviews. 2007;(3) doi: 10.1002/14651858.CD006652.CD006652 [DOI] [PubMed] [Google Scholar]
- 124.Kuderer NM, Khorana AA, Lyman GH, Francis CW. A meta-analysis and systematic review of the efficacy and safety of anticoagulants as cancer treatment: impact on survival and bleeding complications. Cancer. 2007;110(5):1149–1161. doi: 10.1002/cncr.22892. [DOI] [PubMed] [Google Scholar]
- 125.Vlodavsky I, Friedmann Y. Molecular properties and involvement of heparanase in cancer metastasis and angiogenesis. Journal of Clinical Investigation. 2001;108(3):341–347. doi: 10.1172/JCI13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Borsig L, Wong R, Feramisco J, Nadeau DR, Varki NM, Varki A. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(6):3352–3357. doi: 10.1073/pnas.061615598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pitchford SC, Yano H, Lever R, et al. Platelets are essential for leukocyte recruitment in allergic inflammation. Journal of Allergy and Clinical Immunology. 2003;112(1):109–118. doi: 10.1067/mai.2003.1514. [DOI] [PubMed] [Google Scholar]
- 128.Alonso DF, Bertolesi GE, Farias EF, Eijan AM, Joffe EBD, De Cidre LL. Antimetastatic effects associated with anticoagulant properties of heparin and chemically modified heparin species in a mouse mammary tumor model. Oncology Reports. 1996;3(1):219–222. doi: 10.3892/or.3.1.219. [DOI] [PubMed] [Google Scholar]
- 129.Mousa SA, Linhardt R, Francis JL, Amirkhosravi A. Anti-metastatic effect of a non-anticoagulant low-molecular-weight heparin versus the standard low-molecular-weight heparin, enoxaparin. Thrombosis and Haemostasis. 2006;96(6):816–821. doi: 10.1160/th06-05-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nakajima M, Irimura T, Nicolson GL. Heparanases and tumor metastasis. Journal of Cellular Biochemistry. 1988;36(2):157–167. doi: 10.1002/jcb.240360207. [DOI] [PubMed] [Google Scholar]
- 131.Sciumbata T, Caretto P, Pirovano P, et al. Treatment with modified heparins inhibits experimental metastasis formation and leads, in some animals, to long-term survival. Invasion and Metastasis. 1996;16(3):132–143. [PubMed] [Google Scholar]
- 132.Amirkhosravi A, Meyer T, Amaya M, et al. The role of tissue factor pathway inhibitor in tumor growth and metastasis. Seminars in Thrombosis and Hemostasis. 2007;33(7):643–652. doi: 10.1055/s-2007-991531. [DOI] [PubMed] [Google Scholar]
- 133.Vlodavsky I, Eldor A, Haimovitz-Friedman A, et al. Expression of heparanase by platelets and circulating cells of the immune system: possible involvement in diapedesis and extravasation. Invasion and Metastasis. 1992;12(2):112–127. [PubMed] [Google Scholar]
- 134.Takahashi H, Ebihara S, Okazaki T, Asada M, Sasaki H, Yamaya M. A comparison of the effects of unfractionated heparin, dalteparin and danaparoid on vascular endothelial growth factor-induced tumour angiogenesis and heparanase activity. British Journal of Pharmacology. 2005;146(3):333–343. doi: 10.1038/sj.bjp.0706344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Khorana AA, Sahni A, Altland OD, Francis CW. Heparin inhibition of endothelial cell proliferation and organization is dependent on molecular weight. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23:2110–2115. doi: 10.1161/01.ATV.0000090671.56682.D7. [DOI] [PubMed] [Google Scholar]
- 136.Marchetti M, Vignoli A, Russo L, et al. Endothelial capillary tube formation and cell proliferation induced by tumor cells are affected by low molecular weight heparins and unfractionated heparin. Thrombosis Research. 2008;121(5):637–645. doi: 10.1016/j.thromres.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 137.Mehrad B, Keane MP, Strieter RM. Chemokines as mediators of angiogenesis. Thrombosis and Haemostasis. 2007;97(5):755–762. [PMC free article] [PubMed] [Google Scholar]
- 138.Cancio LC. Airway management and smoke inhalation injury in the burn patient. Clinics in Plastic Surgery. 2009;36(4):555–567. doi: 10.1016/j.cps.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 139.Toon MH, Maybauer MO, Greenwood JE, Maybauer DM, Fraser JF. Management of acute smoke inhalation injury. Critical Care and Resuscitation. 2010;12(1):53–61. [PubMed] [Google Scholar]
- 140.Miller AC, Rivero A, Ziad S, Smith DJ, Elamin EM. Influence of nebulized unfractionated heparin and N-acetylcysteine in acute lung injury after smoke inhalation injury. Journal of Burn Care and Research. 2009;30(2):249–256. doi: 10.1097/BCR.0b013e318198a268. [DOI] [PubMed] [Google Scholar]
- 141.Oremus M, Hanson MD, Whitlock R, et al. A systematic review of heparin to treat burn injury. Journal of Burn Care and Research. 2007;28(6):794–804. doi: 10.1097/BCR.0b013e3181599b9b. [DOI] [PubMed] [Google Scholar]
- 142.Chacon JMF, De Andrea MLM, Blanes L, Ferreira LM. Effects of topical application of 10,000 IU heparin on patients with perineal dermatitis and second-degree burns treated in a public pediatric hospital. Journal of Tissue Viability. 2010;19(4):150–158. doi: 10.1016/j.jtv.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 143.Cribbs RK, Harding PA, Luquette MH, Besner GE. Endogenous production of heparin-binding EGF-like growth factor during murine partial-thickness burn wound healing. Journal of Burn Care and Rehabilitation. 2002;23(2):116–125. doi: 10.1097/00004630-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 144.Cribbs RK, Luquette MH, Besner GE. Acceleration of partial-thickness burn wound healing with topical application of heparin-binding EGF-like growth factor (HB-EGF) Journal of Burn Care and Rehabilitation. 1998;19(2):95–101. doi: 10.1097/00004630-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 145.Radulescu A, Zhang HY, Chen CL, et al. Heparin-binding egf-like growth factor promotes intestinal anastomotic healing. Journal of Surgical Research. 2010;171(2):540–550. doi: 10.1016/j.jss.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Prince RN, Schreiter ER, Zou P, et al. The heparin-binding domain of HB-EGF mediates localization to sites of cell-cell contact and prevents HB-EGF proteolytic release. Journal of Cell Science. 2010;123(13):2308–2318. doi: 10.1242/jcs.058321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sen S, Meteoglu I, Ogurlu M, Sen S, Derinceoz OO, Barutca S. Topical heparin: a promising agent for the prevention of tracheal stenosis in airway surgery. Journal of Surgical Research. 2009;157(1):e23–e29. doi: 10.1016/j.jss.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 148.Lilly JD, Parsons CL. Bladder surface glycosaminoglycans is a human epithelial permeability barrier. Surgery, Gynecology & Obstetrics. 1990;171(6):493–496. [PubMed] [Google Scholar]
- 149.Hellgren M, Andersson E, Bystrom B, et al. Dalteparin shortens human labour. Journal of Thrombosis and Haemostasis. 2007;5(Supplement 2) [Google Scholar]
- 150.Osman I, Young A, Ledingham MA, et al. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Molecular Human Reproduction. 2003;9(1):41–45. doi: 10.1093/molehr/gag001. [DOI] [PubMed] [Google Scholar]
- 151.Ekman-Ordeberg G, Hellgren M, Kerud A, et al. Low molecular weight heparin stimulates myometrial contractility and cervical remodeling in vitro . Acta Obstetricia et Gynecologica Scandinavica. 2009;88(9):984–989. doi: 10.1080/00016340903176818. [DOI] [PubMed] [Google Scholar]
- 152.Serisier DJ, Shute JK, Hockey PM, Higgins B, Conway J, Carroll MP. Inhaled heparin in cystic fibrosis. European Respiratory Journal. 2006;27(2):354–358. doi: 10.1183/09031936.06.00069005. [DOI] [PubMed] [Google Scholar]
- 153.King M, Rubin BK. Pharmacological approaches to discovery and development of new mucolytic agents. Advanced Drug Delivery Reviews. 2002;54(11):1475–1490. doi: 10.1016/s0169-409x(02)00156-4. [DOI] [PubMed] [Google Scholar]
- 154.Hiebert LM. Oral heparins. Clinical Laboratory. 2002;48:111–116. [PubMed] [Google Scholar]
- 155.Hiebert LM, Ping T, Wice SM. Enhanced antithrombotic effects of unfractionated heparin in rats after repeated oral doses and its relationship to endothelial heparin concentration. British Journal of Pharmacology. 2008;153(6):1177–1184. doi: 10.1038/bjp.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pinel C, Wice SM, Hiebert LM. Orally administered heparins prevent arterial thrombosis in a rat model. Thrombosis and Haemostasis. 2004;91(5):919–926. doi: 10.1160/TH03-08-0527. [DOI] [PubMed] [Google Scholar]
- 157.Baughman RA, Kapoor SC, Agarwal RK, Kisicki J, Catella-Lawson F, FitzGerald GA. Oral delivery of anticoagulant doses of heparin. A randomized, double- blind, controlled study in humans. Circulation. 1998;98(16):1610–1615. doi: 10.1161/01.cir.98.16.1610. [DOI] [PubMed] [Google Scholar]
- 158.Berkowitz SD, Marder VJ, Kosutic G, Baughman RA. Oral heparin administration with a novel drug delivery agent (SNAC) in healthy volunteers and patients undergoing elective total hip arthroplasty. Journal of Thrombosis and Haemostasis. 2003;1(9):1914–1919. doi: 10.1046/j.1538-7836.2003.00340.x. [DOI] [PubMed] [Google Scholar]
- 159.Gonze MD, Salartash K, Sternbergh WC, Baughman RA, Leone-Bay A, Money SR. Orally administered unfractionated heparin with carrier agent is therapeutic for deep venous thrombosis. Circulation. 2000;101(22):2658–2661. doi: 10.1161/01.cir.101.22.2658. [DOI] [PubMed] [Google Scholar]
- 160.Pineo GF, Hull RD, Marder VJ. Orally active heparin and low-molecular-weight heparin. Current Opinion in Pulmonary Medicine. 2001;7(5):344–348. doi: 10.1097/00063198-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 161.Bai S, Ahsan F. Synthesis and evaluation of pegylated dendrimeric nanocarrier for pulmonary delivery of low molecular weight heparin. Pharmaceutical Research. 2009;26(3):539–548. doi: 10.1007/s11095-008-9769-y. [DOI] [PubMed] [Google Scholar]
- 162.Bai S, Ahsan F. Inhalable liposomes of low molecular weight heparin for the treatment of venous thromboembolism. Journal of Pharmaceutical Sciences. 2010;99(11):4554–4564. doi: 10.1002/jps.22160. [DOI] [PubMed] [Google Scholar]
- 163.Bai S, Gupta V, Ahsan F. Inhalable lactose-based dry powder formulations of low molecular weight heparin. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2010;23(2):97–104. doi: 10.1089/jamp.2009.0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Qi Y, Zhao G, Liu D, et al. Delivery of therapeutic levels of heparin and low-molecular-weight heparin through a pulmonary route. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(26):9867–9872. doi: 10.1073/pnas.0402891101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rawat A, Yang T, Hussain A, Ahsan F. Complexation of a poly-L-arginine with low molecular weight heparin enhances pulmonary absorption of the drug. Pharmaceutical Research. 2008;25(4):936–948. doi: 10.1007/s11095-007-9442-x. [DOI] [PubMed] [Google Scholar]
- 166.Yang T, Mustafa F, Ahsan F. Alkanoylsucroses in nasal delivery of low molecular weight heparins: in vivo absorption and reversibility studies in rats. Journal of Pharmacy and Pharmacology. 2004;56(1):53–60. doi: 10.1211/0022357022377. [DOI] [PubMed] [Google Scholar]
- 167.Yang T, Mustafa F, Bai S, Ahsan F. Pulmonary delivery of low molecular weight heparins. Pharmaceutical Research. 2004;21(11):2009–2016. doi: 10.1023/b:pham.0000048191.69098.d6. [DOI] [PubMed] [Google Scholar]
- 168.Mustafa F, Yang T, Khan MA, Ahsan F. Chain length-dependent effects of alkylmaltosides on nasal absorption of enoxaparin. Journal of Pharmaceutical Sciences. 2004;93(3):675–683. doi: 10.1002/jps.10579. [DOI] [PubMed] [Google Scholar]
- 169.Yang T, Hussain A, Paulson J, Abbruscato TJ, Ahsan F. Cyclodextrins in nasal delivery of low-molecular-weight heparins: in vivo and in vitro studies. Pharmaceutical Research. 2004;21(7):1127–1136. doi: 10.1023/b:pham.0000032998.84488.7a. [DOI] [PubMed] [Google Scholar]
- 170.Yang T, Hussain A, Bai S, Khalil IA, Harashima H, Ahsan F. Positively charged polyethylenimines enhance nasal absorption of the negatively charged drug, low molecular weight heparin. Journal of Controlled Release. 2006;115(3):289–297. doi: 10.1016/j.jconrel.2006.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Baer CL, Bennett WM, Folwick DA, Erickson RS. Effectiveness of a jet injection system in administering morphine and heparin to healthy adults. American Journal of Critical Care. 1996;5:42–48. [PubMed] [Google Scholar]
- 172.Wagner S, Dues G, Sawitzky D, Frey P, Christ B. Assessment of the biological performance of the needle-free injector INJEX using the isolated porcine forelimb. British Journal of Dermatology. 2004;150(3):455–461. doi: 10.1111/j.1365-2133.2004.05853.x. [DOI] [PubMed] [Google Scholar]
- 173.Hollingsworth SJ, Hoque K, Linnard D, Corry DG, Barker SG. Delivery of low molecular weight heparin for prophylaxis against deep vein thrombosis using a novel, needle-less injection device (J-Tip) Annals of the Royal College of Surgeons of England. 2000;82(6):428–431. [PMC free article] [PubMed] [Google Scholar]
- 174.Casu B, Vlodavsky I, Sanderson RD. Non-anticoagulant heparins and inhibition of cancer. Pathophysiology of Haemostasis and Thrombosis. 2008;36(3-4):195–203. doi: 10.1159/000175157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mousa SA. Heparin, low molecular weight heparin, and derivatives in thrombosis, angiogenesis, and inflammation: emerging links. Seminars in Thrombosis and Hemostasis. 2007;33(5):524–533. doi: 10.1055/s-2007-982084. [DOI] [PubMed] [Google Scholar]
- 176.Baba M, Snoeck R, Pauwels R, De Clercq E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrobial Agents and Chemotherapy. 1988;32(11):1742–1745. doi: 10.1128/aac.32.11.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sezer AD, Hatipoğlu F, Cevher E, Oğurtan Z, Baş AL, Akbuğa J. Chitosan film containing fucoidan as a wound dressing for dermal burn healing: preparation and in vitro/in vivo evaluation. AAPS PharmSciTech. 2007;8(2, article no. 39) doi: 10.1208/pt0802039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ahmed T, Smith G, Vlahov I, Abraham WM. Inhibition of allergic airway responses by heparin derived oligosaccharides: identification of a tetrasaccharide sequence. Respiratory Research. 2012;13(article 6) doi: 10.1186/1465-9921-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Severin IC, Soares A, Hantson J, et al. Glycosaminoglycan analogues as a novel anti-inflammatory strategy. Frontiers in Immunology. 2012;293:1–12. doi: 10.3389/fimmu.2012.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Colliec-Jouault S, Bavington C, Delbarre-Lodrat C. Heparin-like entities from marine organisms. In: Lever R, Mulloy B, Page CP, editors. Heparin—A Century of Progress. Vol. 207. Berlin, Germany: Springer; 2012. pp. 423–429. (Handbook of Experimetnal Pharmacology). [DOI] [PubMed] [Google Scholar]


