A new study provides insight into aging and age-related diseases by linking telomere dysfunction to a decline in mitochondrial number and function.
Common observation, bolstered by a wealth of epidemiologic evidence, demonstrates that aging is accompanied by a profound increase in the incidence of cardiovascular disease. Understanding the mechanisms that underlie the aging process might, therefore, provide clues and even potential therapeutic targets for cardiovascular disease, as well as a host of other age-related pathologies. Unfortunately, even though there has been substantial progress in our understanding of the aging process, no single theory of why we age has emerged. Early studies analyzing what led to the senescence of cultured human cells focused on the telomere, the specialized region on the end of each chromosome. Each cell division is known to result in the shortening of overall telomere length. After multiple cell divisions, proliferating cells finally erode the telomere down to a critical length. At this point, the cell responds by invoking a DNA damage response not unlike what happens when DNA is damaged by exogenous radiation or chemicals. This damage response culminates in the activation of the tumor suppressor, p53. Once activated, p53 can, in turn, promote growth arrest, cell death, or perhaps most importantly for aging, cellular senescence. While our knowledge of telomere biology was unfolding, parallel studies were arguing that aging was not, in fact, caused by any alterations at the ends of chromosomes or, for that matter, any nuclear event at all. Rather, these studies implicated a decline in mitochondrial function or a rise in reactive oxygen species as the critical factor for why we age.1 Now, a new study by Ronald DePinho and colleagues suggests that both sides of what might be called this ageold debate might have been right all along. This new study demonstrates that telomeres, through p53, act to regulate overall metabolic function.2 In making such a connection, the mitochondria and the nucleus appeared to have gotten a lot closer and the basis for why we age has became a lot clearer.
The model used to connect telomeres with mitochondrial function involved a mouse with impaired telomere maintenance caused by a targeted deletion of the enzyme telomerase. These Tert−/− animals have been shown previously to develop severely short telomeres when backcrossed for four or more generations (known as G4 or generation 4 mice). Interestingly, the G4 Tert−/− animals developed pathologies not only in highly proliferative tissues, as might be expected, but also in predominantly postmitotic organs, such as the heart. To understand how nonproliferating tissues, such as the heart, might be affected by the absence of telomerase, DePinho and colleagues analyzed the transcriptome of wild-type and G4 mice. Strikingly, pathway analysis revealed that the absence of telomerase led to significant alterations in those genes regulating oxidative phosphorylation, mitochondrial function, antioxidant defense and metabolism. The authors then realized that a family of transcriptional regulators, peroxisome proliferator-activated receptor gamma coactivator 1 alpha and beta (PGC-1α and PGC-1β) had been implicated as an important regulator of all these processes. This suggested that telomere shortening might somehow repress the expression of PGC-1α and PGC-1β. The author subsequently confirmed this hypothesis and then proceeded to show that telomere dysfunction results in reduced mitochondrial number and function in the heart, as well as other tissues. Interestingly, reinstitution of telomerase could, at least partially, correct the mitochondrial dysfunction.
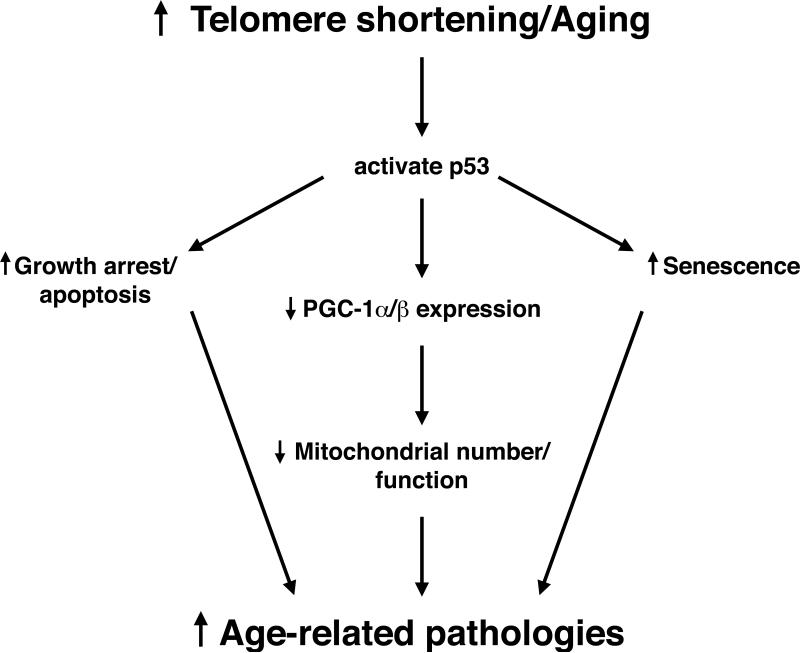
The authors next sought to understand how telomere shortening represses the expression of the PGC-1 family of coactivators. Here, they took advantage of previous observations that in their mouse model, p53 was activated as a consequence of telomere attrition.3 Furthermore, past studies had shown that animal models exhibiting constitutively activated p53 are often characterized by a phenotype consistent with accelerated aging.4,5 Using both genetic and biochemical approaches, the authors demonstrated that telomere dysfunction activated p53, and once activated, p53 was an important and physiologic repressor of PGC-1α and PGC-1β expression. This led to a model wherein telomere shortening and, by extension, aging leads to the activation of a p53-dependent pathway that culminates in altered mitochondrial function (see Figure).
Figure.
A connection between telomere shortening in the nucleus and mitochondrial dysfunction. Key to this connection is the activation of the tumor suppressor, p53, that acts as a transcriptional repressor of the PGC-1 family of transcriptional coactivators. PGC-1α/β are, in turn, important regulators of mitochondrial biogenesis. See text for additional details.
What are the implications of these results for human aging and for the treatment of age-related pathologies? Interestingly, recent analysis has demonstrated that in humans, conditions associated with a genetic impairment of telomere maintenance results in a constellation of symptoms, including inherited bone marrow failure, pulmonary fibrosis, and hepatic cirrhosis.6 Although these families do not appear to spontaneously develop cardiovascular disease, there are a number of other studies linking shortened telomeres to higher susceptibility for cardiovascular disease.7 It will, therefore, be of interest to see whether in any of the above situations, shortening of telomeres results in measurable alterations in mitochondrial number or function. Follow-up studies may also be able to tease out the relative importance of p53 as a repressor of PGC-1α and PGC-1β expression vs. its other well-described role in mediating growth arrest, apoptosis, or senescence. It is conceivable that different organs and tissues will differ considerably in the relative importance of the various p53-regulated pathways. In essence, aging may not be a uniform process in the body, and different tissues may age at different rates and through different mechanisms. Therapeutically, one implication of this new study is that augmenting PGC-1α and PGC-1β expression might be able to reverse some of the age-dependent decline in tissue or organ function. Although this possibility is intriguing, it is important to note that too much PGC-1α and PGC-1β expression can have adverse effects, as well.8 Further analysis will hopefully address these and related questions. At least for now, the description of a molecular connection between DNA maintenance and mitochondrial function represents an important first step. Furthermore, these results strengthen the intriguing notion that the molecular basis for why we develop conditions, such as heart failure or atherosclerosis, may be buried within the secrets of how and why we age.
Acknowledgment
I am grateful to I. Rovira for help with this manuscript.
Sources of Funding
Supported by funds from the Ellison Medical Foundation and the NIH Intramural program.
Footnotes
Telomere Dysfunction Induces Metabolic and Mitochondrial Compromise Sahin et al Nature. 2011;470:359--365
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–-495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, Maser RS, Tonon G, Foerster F, Xiong R, Wang YA, Shukla SA, Jaskelioff M, Martin ES, Heffernan TP, Protopopov A, Ivanova E, Mahoney JE, Kost-Alimova M, Perry SR, Bronson R, Liao R, Mulligan R, Shirihai OS, Chin L, DePinho RA. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–-365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chin L, Artandi SE, Shen Q, Tam A, Lee SL, Gottlieb GJ, Greider CW, DePinho RA. P53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell. 1999;97:527–-538. doi: 10.1016/s0092-8674(00)80762-x. [DOI] [PubMed] [Google Scholar]
- 4.Maier B, Gluba W, Bernier B, Turner T, Mohammad K, Guise T, Sutherland A, Thorner M, Scrable H. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 2004;18:306–-319. doi: 10.1101/gad.1162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C, Hee Park S, Thompson T, Karsenty G, Bradley A, Donehower LA. P53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–-53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 6.Young NS. Telomere biology and telomere diseases: implications for practice and research. Hematology Am Soc Hematol Educ Program. 2010;2010:30–-35. doi: 10.1182/asheducation-2010.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Meyer T, Rietzschel ER, De Buyzere ML, Van Criekinge W, Bekaert S. Telomere length and cardiovascular aging: the means to the ends? Ageing Res Rev. 2010 doi: 10.1016/j.arr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–-856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]