Abstract
Renewed awareness of the significant morbidity and mortality that Shigella causes among young children in developing countries combined with technological innovations in vaccinology has led to the development of novel vaccine strategies in the past five years. Along with advancement of classical vaccines in clinical trials and new sophisticated measurements of immunological responses, much new data has been produced lending promise to the potential for production of safe and effective Shigella vaccines. Herein we review the recent progress in Shigella vaccine development within the framework of persistent obstacles.
Introduction
Shigella diarrheal illness remains an important cause of morbidity and mortality globally, particularly among children < 5 years of age in developing countries. In 1999, it was estimated that Shigella was causing annually ~ 113 million episodes and 0.6 million deaths1. As a pathogen that invades and destroys intestinal mucosa, Shigella is less amenable to the salutory effects of oral rehydration compared to enterotoxigenic pathogens that cause dehydrating diarrhea. Antibiotics are the standard of care for shigellosis but therapeutic options are limited by the widespread prevalence of resistant strains, as in Asia where resistance to ciprofloxacin has become common2, 3. Resistance has increased to the three second line choices, reaching moderate levels (30–50%) for pivmecillinam and azithromycin, and has appeared to third generation cephalosporins mediated by extended spectrum β-lactamases2, 4–6. As therapeutic options narrow, the need for a safe and effective Shigella vaccine becomes more pressing. Herein we review advances in Shigella vaccine development during the past 5 years, highlighting new vaccine technologies.
Current taxonomy and epidemiology of Shigella
Shigella is an antigenically diverse pathogen whose taxonomy undergoes periodic modifications. The current official taxonomy encompasses four species (or Groups) and 49 serotypes and subtypes that include S. dysenteriae (Group A types), S. flexneri (Group B, 13 types and subtypes), S. boydii (Group C, 20 types) and S. sonnei (Group D, 1 type). There are also more than a dozen putative new type or subtype strains that are being considered for possible official classification. Among these, arguably, the most important are S. flexneri provisional serotype variants Y394 and 88–893 that are likely to be designated S. flexneri 7a and 7b, respectively7.
Since the ingestion of minute inocula (10 organisms) can lead to shigellosis, Shigella disseminates easily in settings where there is overcrowding, limited access to water, compromise of personal hygiene and inadequate sanitation. S. flexneri serotypes are the major agents of endemic shigellosis among children in developing countries, while S. sonnei is the predominant serotype associated with Shigella diarrheal illness in sub-populations in industrialized country settings where Shigella infections persist and in transitional countries; S. sonnei is also an important agent of travelers’ diarrhea8. S. boydii serotypes are uncommonly associated with diarrheal illness. The Global Enteric Multicenter Study (GEMS) of moderate and severe diarrhea (MSD) among children < 60 months of age in 7 developing countries in sub-Saharan Africa and South Asia determined the serotypes of Shigella isolates9, 10. Among > 1,100 isolates of Shigella associated with cases of MSD, 66% were S. flexneri and 24% were S. sonnei; S. boydii and S. dysenteriae serotypes collectively accounted for only 10% of the isolates. Four serotypes, S. flexneri 2a, S. flexneri 3a, S. flexneri 6 and S. sonnei, comprised 65% of all the isolates.
S. dysenteriae type 1 (the “Shiga bacillus”), the only serotype that elaborates Shiga toxin, can cause severe clinical disease with complications, including hemolytic uremic syndrome and historically has led to devastating epidemics and pandemics with high case fatality in all age groups. Shiga dysentery typically appears in developing countries experiencing upheaval of civil society or natural disaster11. Although Shiga disease has virtually disappeared within the past decade, it can reappear at any time.
For vaccine developers preparing a broad-based Shigella vaccine based on serotypes, the GEMS and other large survey data suggest that a quadravalent vaccine containing strains or antigens from S. sonnei and S. flexneri 2a, 3a, and 6, would directly cover ~65% of current circulating strains; with cross protection based on shared S. flexneri group antigens, such a quadravalent vaccine could cover >85% of currently circulating Shigella strains12, 13. Many argue for including S. dysenteriae 1 coverage in a serotype-based vaccine, in the expectation that pandemic Shiga dysentery will return and a vaccine could constitute an important public health tool.
Genomics
Genomic and proteomic technologies have elucidated complete Shigella genomes and protein profiles14–18, providing information that impacts vaccine development. The revelations made have encouraged vaccine development strategies based on the identification of proteins conserved among Shigella and related E. coli enteropathogens (vide infra). Moreover, the genome analyses that document the phylogenetic relatedness of Shigella and E. coli are prompting a possible reclassification of Shigella as a member of the E. coli species. Such a taxonomic revision will have to include input from clinicians and epidemiologists to minimize confusion in the clinical and disease control arenas.
Pathogenesis and clinical features of Shigella infection
All serotypes follow a similar pathogenesis which involves translocation through ileal and colonic M cells, macrophage uptake basolateral invasion of epithelial cells, and dissemination within the mucosa (reviewed in19, 20). After an incubation period of 1 to 4 days, shigellosis usually begins with systemic symptoms, including fever, headache, malaise, anorexia, and occasional vomiting. Watery diarrhea typically precedes dysentery21 and may be the only clinical manifestation of mild infection22. Watery diarrhea arises from the action of enterotoxins in the jejunum, whereas bloody diarrhea results from invasion of the colonic epithelium. Frank dysentery manifests as frequent scanty stools of containing blood and mucus, accompanied by lower abdominal cramps and rectal tenesmus. Patients with severe infection may pass more than 20 dysenteric stools daily. Shigellosis in otherwise healthy individuals is generally self-limited and resolves within 5 to 7 days, without sequelae. However, extraintestinal complications may occur23 including generalized convulsions and encephalopathy. Hemolytic uremic syndrome (HUS) can accompany S. dysenteriae 1 infection24. Acute, life-threatening complications are sometimes seen in malnourished children in developing countries. In the United States, Shigella bacteremia has rarely been reported among HIV-infected and other immunocompromised patients25.
Whereas the molecular mechanisms that determine Shigella invasiveness, rupture of the phagocytic vacuole, movement through the host-cell cytoplasm, and modulation of the innate immune response have been intensively studied, less is known about how the organism evokes diarrhea. Nevertheless, secretogenic proteins elaborated by Shigella strains have been identified (Table 1) and serve as targets for attenuating mutations or as new vaccine antigens.
Table 1.
Factors contributing to diarrhea in Shigella
| Gene(s) | Location | Function(s) | Reference | ||
|---|---|---|---|---|---|
| Enterotoxins | |||||
| ShET1 | set1A, set1B | chromosome she PAI S. flexneri 2a and EAEC | Enterotoxin (Ussing chamber) Rabbit ileal loops | 26, 27, 110 | |
| ShET2 | sen, ospD3 | virulence plasmid Shigella, EIEC | Enterotoxin (Ussing chamber) TTSS effector | 47 | |
| SPATES | |||||
| SigA | sigA | Chromosome, she PAI Shigella, EIEC | Enterotoxin (rabblit ileal loop) Fodrin degradation | 34, 40 | |
| Pic | pic | chromosome, she PAI EAEC042, UPEC, S. flexneri 2a | Mucinase, enterotoxicity, immune modulation | 38–40 | |
| SepA | sepA | virulence plasmid Shigella | Rabbit ileal loop inflammation enterotoxicity | 36, 111 | |
Shigella enterotoxins
In 1995 we identified Shigella enterotoxins 1 and 2 (ShET1 and ShET2,) by demonstrating their ability to cause fluid accumulation in rabbit ileal loops (ShET1), and greater potential difference and short circuit current (Isc) in Ussing chambers (both measures of electrolytes and water secretion)26, 27. ShET2 secretion requires the T3SS in S. flexneri 2a. Functional studies of a ShET2 mutant demonstrating a reduction in interleukin-8 (IL-8) secretion following invasion suggests that this toxin might also participate in Shigella-induced inflammation in epithelial cells28. A role for these two toxins in disease was determined in clinical trials where a reduction in reactogenicity in live attenuated S. flexneri 2a vaccine strains containing mutations in these two toxins was demonstrated29. ShET1 and ShET2 continue to serve as targets for attenuating mutations in multiple vaccine candidates30–32.
Serine Protease Autotransporters of Enterobacteriaceae (SPATES)
The most common secretion mechanism across the Gram negative envelope is the autotransporter system, in which the full length species is passed across the inner membrane by virtue of the Sec apparatus, and then employs its own C-terminus to effect translocation across the outer membrane. A large and growing family of SPATEs has been identified, produced almost exclusively by pathogenic E. coli, Shigella and Salmonella strains33. Three SPATES have been identified as potential contributors to the enterotoxic activity of Shigella (Table 1).
SigA is a chromosomally-encoded class I SPATE (cytotoxic to epithelial cells) in S. flexneri 2a that exerts cytopathic effect in HEp-2 cells34, suggesting that it may be a cell-altering toxin with a role in pathogenesis. SigA was demonstrated to be partly responsible for the ability of S. flexneri to stimulate fluid accumulation in ligated rabbit ileal loops34. A fragment of SigA is the basis for one conserved protein vaccine strategy 35.
SepA is a class II non-cytotoxic SPATE with a potential role demonstrated in the rabbit ligated ileal loop assay, in which a mutant in SepA exhibited significantly less inflammation and tissue damage than the wild type parent strain36. We have demonstrated in Ussing chamber studies that SepA exerts enterotoxic activity (unpublished).
Pic is a SPATE encoded on the chromosomes of EAEC 042, urinary pathogenic E. coli and Shigella flexneri 2a37, 38. Functional analyses of the Pic protein implicate this factor in mucinase activity, serum resistance, hemagglutination, enterotoxicity and immune modulation in targeting a broad range of human leukocyte adhesion proteins39. The gene encoding Pic is located as overlapping DNA on the opposite strand as set1AB, encoding ShET138. Thus, live attenuated strains containing mutations in ShET1 also contain mutations in Pic.
Whereas most commensal and diarrheagenic E. coli encode few SPATEs, most Shigella strains harbor one or more SPATE-encoding gene(s)40, suggesting that class I SPATEs are important in Shigella pathogenesis. Moreover, the most common Shigella serotypes (e.g., S. flexneri 2a, 2b) generally carry the greatest number of SPATE-encoding genes. The class II Pic protease is largely found in S. flexneri 2a, which is globally the most important Shigella serotype. As more is learned about the precise roles of the SPATEs, their inclusion in vaccine strategies may be expanded.
Animal Models
No small animal model recapitulates all aspects of Shigella pathogenesis, as seen in humans. Non-human primates (NHP) constitute one useful model, since they exhibit diarrhea and dysentery following oral infection with virulent Shigella strains and have aided efficacy evaluation of vaccine candidates41–43. However, the cost and availability of NHPs, combined with the fact that enormous inocula (>109 CFU) are required to consistently induce shigellosis, are recognized drawbacks. The guinea pig keratoconjunctivitis model described by Sereny, which correlates with the ability of the organism to invade cells, spread through a single cell layer, and induce inflammation44, is helpful for evaluating the safety of live vaccines, as well as for testing the efficacy of many types of vaccines. The mouse lung model is employed for the same purposes, i.e., to reveal the inflammatory potential of live vaccines and its mutants and to demonstrate protection conferred by Shigella vaccines44, 45. A model involving intrarectal inoculation of guinea pigs46, which leads to severe acute rectocolitis and a robust inflammatory response, is also useful in vaccine evaluation.
We have shown that Shigella strains and their extracellular enterotoxins induce ion flux (correlating with a secretory state) in rabbit tissue mounted in Ussing chambers26, 47. Ussing chambers provide a far more quantitative readout than rabbit ileal loops of pathophysiologic effects on mucosal epithelium. We have also used mouse small intestine to study Shigella enterotoxic activity in ex vivo models.
Vaccine Candidates
The lack of an ideal small animal model of Shigella infection represents one hurdle in vaccine development. Despite this obstacle, multiple vaccine strategies have been advanced in the past 5 years, buoyed by increased knowledge of Shigella epidemiology and pathogenesis and of human immune responses to the pathogen, as well as by new vaccinology technologies. These efforts can be categorized into two broad approaches including: 1) serotype based vaccines, or 2) conserved antigen vaccines (Table 2). Serotype-specific strategies extend the demonstration that an initial clinical infection stimulates acquired immunity and serotype-specific protection against shigellosis. This has been well documented in challenge studies in non-human primates48, in adult volunteers experimentally infected with virulent strains49, 50 and in epidemiological studies in endemic regions51.
Table 2.
Shigella Vaccine Candidates
| O-Antigen Directed
| |||
|---|---|---|---|
| Live Attenuated | Gene Mutations | Development Stage | Reference |
|
| |||
| S. flexneri 2a CVD 1204 | guaBA | Phase 1 | 29 |
| S. flexneri 2a CVD 1208S | guaBA, set, sen | Phase 2 | 30 |
| S. dysenteriae 1 CVD 1256 | guaBA, sen, stxA | Preclinical | 112 |
| S. sonnei WRSs1 | virG | Phase 1 | 113 |
| S. sonnei WRSs2, 3 | virG, senA, senB, msbB2 | Preclinical NHP | 32, 67, 114, 115 |
| S. flexneri 2a SC602 | virG, iuc | Phase 1–2 | 63, 69, 70 |
| S. flexneri 2a WRSf2G11, 12, 15 | virG, senA, senB, msbB2 | Preclinical | 31, 68, 116 |
| S. dysenteriae 1WRSd1 | virG, stxAB | Phase 1 | 117, 118 |
|
| |||
| Conjugate | |||
|
| |||
| ChemicalConjugate | S. flexneri 2a LPS-rEPA | Phase 1–3 | 55, 56, 119 |
| S. sonnei LPS-rEPA | Phase 1–3 | 55, 56, 119 | |
| S. dysenteriae 1 LPS-rEPA | Preclinical | 120 | |
|
| |||
| GlycoVaxyn Bioconjugate | S. dysenteriae 1 LPS-exoA | Phase 1 | 59 |
|
| |||
| Synthetic Carbohydrate | |||
|
| |||
| Synthetic Oligosaccharide | O-antigen mimic-tetanus toxoid | Preclinical | 60, 121 |
|
| |||
| Common Protein Directed | |||
|
| |||
| Purified Ipa proteins | S. flexneri 2a IpaB + IpaD | Preclinical | 82 |
|
| |||
| GMMA vesicles | OM and periplasmic proteins | Preclinical | 87 |
|
| |||
| Conserved Proteins IcsP, SigA | Protein fragments | Preclinical | |
|
| |||
| Combined O-Antigen Specific Plus Common Protein | |||
|
| |||
| Invaplex | LPS + Ipa proteins B, C, and D | Phase 1 | 80, 81 |
Serotype-targeted vaccines
Serotype-specific candidates include conjugate vaccines composed of purified Shigella O polysaccharides conjugated to a protein carrier, genetically engineered O polysaccharide protein fusions and live attenuated strains. The most advanced conjugate vaccines, developed by investigators at the National Institute of Child Health and Human Development, include S. flexneri 2a LPS conjugated to recombinant Pseudomonas exoprotein A (rEPA) and S. sonnei LPS conjugated to rEPA. These conjugates were shown to be safe and immunogenic in adults and young children52. Furthermore, the S. sonnei conjugate was efficacious against disease when tested in Israeli soldiers in field trials 53–55. Recently the S. sonnei and S. flexneri 2a conjugate vaccines were tested for efficacy in Israeli children aged 1–4 yrs. There was not enough disease due to S. flexneri to calculate efficacy. The S. sonnei conjugate did not meet the primary aim of the study in providing significant efficacy overall in children <4 years of age. However, when sub-group analyses by age were undertaken it was revealed that 71% efficacy was observed against S. sonnei infection in the 3–4 yr old age group, 35.5% efficacy in 2–3 yr olds but no efficacy was found in children aged 1–2 yrs at the time of vaccination56. Efficacy paralleled the age-related immune responses induced by the vaccine. One interpretation of these data is that the Shigella conjugate vaccine boosted children old enough to have had likely previous exposure but was unable to prime and protect immunologically naïve young children under 2 years of age.
A novel bioconjugate vaccine technology has been advanced by investigators at GlycoVaxyn that utilizes recombinant DNA technology to catalyze the in vivo synthesis of conjugate vaccines. The glycosylation machinery from Campylobacter was cloned in an E. coli production strain to generate a protein carrier glycosylated with the O-antigen specific Shigella LPS, which was then purified as a conjugate vaccine 57, 58. A S. dysenteriae 1 O-antigen-EPA conjugate vaccine was produced in this system and tested in a Phase 1 trial where it was found to be safe and immunogenic following two doses 59.
Investigators at the Institut Pasteur have formulated carbohydrate vaccines encompassing synthetic oligosaccharides mimicking the protective determinants carried by the Shigella O antigen. The synthetic oligosaccharides fused to tetanus toxoid resulted in a functional O-antigen mimic recognized by human serum 60, 61. The use of synthetic technology may allow great flexibility in the production of vaccine antigens.
Live attenuated vaccines represent another strategy based on the serotype specificity of the human protective response and include the advantage of presenting much more of the antigenic repertoire of the bacteria to the host immune system. Live attenuated vaccines have induced protective responses against virulent challenge in volunteer studies and have protected adult and pediatric populations against disease in controlled field trials62–64. While sophisticated genetic techniques allow the introduction of specifically targeted modifications into vaccine strains, achieving the correct balance of safety and immunogenicity has been a formidable challenge65, 66. Two attenuating strategies continue to progress through clinical trials. We have engineered a series of Shigella strains containing mutations in guaBA, encoding critical enzymes for bacterial metabolism, and in the sen and set loci, encoding ShET1 and ShET2. S. flexneri 2a strain CVD 1208S appeared safe and immunogenic in Phase 1 studies13, 30 and has advanced through process development, cGMP manufacture and to Phase 2 clinical studies. The CVD is advancing an overall strategy utilizing five attenuated strains including S. dysenteriae 1, S. sonnei, S. flexneri 2a, S. flexneri 3a and S. flexneri 6 to encompass the most important strains and the type- and group-specific antigens found on all Shigella isolates12, 13.
Investigators at the Walter Reed Army Institute of Research (WRAIR) have developed a series of live attenuated vaccine candidates containing a fundamental mutation in the virG (icsA) gene which is required for cell to cell spread of the bacteria. Additional mutations in some strains include genes encoding ShET1 and ShET2 as well as the msbB gene which is thought to detoxify lipid A of LPS and render the strain less reactogenic31, 32, 67, 68. S. flexneri 2a vaccine SC602, containing mutations in virG and iuc (encoding aerobactin), was previously demonstrated to be immunogenic and protective against challenge in North American volunteers although reactogenic at moderate and high doses 63, 69. This vaccine was subsequently tested in healthy adults and school age children (8-10 yrs) in a Shigella-endemic region of Bangladesh, where single oral doses of 104, 105, or 106 CFU resulted in minimal vaccine shedding, minimal reactogenicity, no transmission and low immune stimulation70.
This study underscores a critical point surrounding the use of orally administered live attenuated strains in Shigella-endemic regions where the nutritional and immune status as well as the microbiota of individuals may well affect vaccine performance71. Other oral live attenuated vaccines including polio, cholera and rotavirus have engendered lower immune responses among vaccinees in developing countries compared to those in industrialized countries72–75. Nonetheless, some of these vaccines have provided protection against severe illness in these populations75, 76 and it is expected that the optimal formulation and vaccination regimen of a live attenuated Shigella vaccine will be equally successful72, 77, 78.
Conserved antigen vaccines
The concept of using an antigen conserved among Shigella strains as an immunogen to provide broad protection is the basis for several new vaccine candidates. The most advanced vaccine that contains components of conserved proteins plus serotype specific O-antigen has been established by investigators at WRAIR. Shigella Invaplex was initially formulated from a bacterial extract composed of invasion plasmid antigen proteins (Ipa) which are highly conserved among all Shigella serotypes, and LPS. In animals, only serotype-specific protection has been demonstrated, mediated by the LPS component79. Invaplex has been shown to be safe and immunogenic following intranasal delivery in volunteers80, 81 Current studies are underway to optimize formulation and delivery.
In related efforts, a vaccine composed of purified IpaB plus IpaD has been demonstrated to confer homologous as well as heterologous protection in a mouse model of Shigella infection when delivered with adjuvant82. Antibodies against invasion plasmid antigens (Ipas) are produced after natural and experimental human infection and are believed to contribute to protection83, 84. A vaccine that could induce protective anti-Ipa responses could provide protection against all Shigella strains expressing these highly conserved antigens.
The use of outer membrane protein preparations as vaccine formulations has also been explored85, 86. A novel protein vesicle technology named Generalized Modules of Membrane Antigens (GMMA) is an industrial, high yielding production process for genetically derived outer membrane particles composed of predicted Shigella outer membrane and periplasmic proteins without LPS87. In preclinical mouse studies, immunization with GMMA provided 65–100% protection against lethal challenge.
Taking advantage of genomic and proteomic data, investigators at the International Vaccine Institute (IVI) have identified two conserved protein candidates including IcsP2, an outer membrane protease which cleaves IcsA from the surface and which is present on all Shigella species and EIEC, and SigA2, a SPATE present on all S. flexneri 2a, S. boydii and S. sonnei. These antigens have demonstrated protection in animal models 35
Immune Responses
The evaluation of vaccine candidates relies on an understanding of which responses are critical for protection. Studies from humans and non-human primates following natural infection and vaccination provide the most relevant data and suggest that a complex series of responses engaging multiple arms of the immune system are involved in immunity to disease caused by Shigella.
Immunity induced by natural infection or vaccination
Humans develop an array of immune responses following Shigella infection, including humoral and cell-mediated immune (CMI) responses. Of particular importance are the high levels of serum IgG and IgA antibodies against Shigella O-antigen, which appear 1–2 weeks following primary exposure. Results from multiple epidemiological51 and seroepidemiological studies 88–90 suggest that O-specific antibodies play a critical role in protection. Antibodies against Ipas are also produced after natural and experimental infection and believed to contribute to protection83, 84, 91.
In addition to systemic immunity, strong mucosal immune responses are induced 63, 92. Gut-derived O-specific IgA antibody secreting cells (ASC) are believed to play a critical role in protection against Shigella. These cells are detected in peripheral blood 7 to 10 days after exposure to the organism or vaccine and are believed to represent a pool of transiently migrating antigen-specific B cells with the capacity to home to mucosal effector sites where they will participate in host defenses by producing local antibodies. IgA O-antigen ASCs represent a measure of oral priming that has been associated with efficacy of live attenuated vaccines63, 93. The number of O-specific IgA ASCs and the levels of O-specific serum IgG are commonly used as primary readouts of immunogenicity in clinical trials of attenuated live and non-living whole cell oral vaccines and serum IgG O antibody for parenteral O polysaccharide-protein conjugate vaccines; moreover, these are generally considered to be predictors of the efficacy of these vaccines. Secretory IgA (sIgA) also appears to have a major role limiting the duration of illness94, 95. However, measurements of sIgA in mucosal secretions (e.g., stool and saliva) can be variable and there is no consensus in the literature showing a clear association with resistance to infection93.
The efficacy of conjugate vaccines has been correlated with high levels of IgG O-antibody56, 96. It has been speculated that the organism may be inactivated by parenterally induced IgG leaked into the intestinal lumen, possibly through complement-mediated lysis in the epithelial cell surface97. It is reasonable to assume that the presence of a critical level of protective IgG antibodies implies the presence of strong underlying T helper immunity, yet there are no reports of T cell measurements in Shigella-conjugate vaccine studies.
Shigella infection has also been shown to induce CMI including upregulation of interferon (IFN)-γ receptor expression and production of pro-inflammatory cytokines, including IFN-γ98, 99. Moreover, an expansion of T cells, particularly CD8+ and T-cell receptor (TCR) γ δ+ T-cell subsets in the gut mucosa100, has been described in the rectal mucosa of shigellosis patients. Of note, increased levels of activated and memory CD4+ and CD8+ T cells and expansion of defined TCR Vβ families have been reported in peripheral blood of patients with shigellosis101, 102. However, a direct association between CMI responses and protection has not been demonstrated and the extent to which these responses contribute to clearing infection and to the pathogenesis of shigellosis remains unknown.
Mucosal immunological priming and effector responses induced by wild type or attenuated Shigella vaccine strains
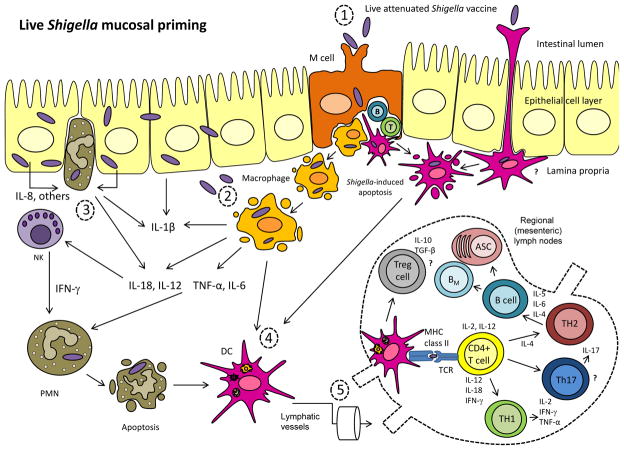
Our current understanding of the processes involved in Shigella pathogenesis offers some insights into how the organism may interact with the immune cells in the gastrointestinal mucosa and trigger immune responses following infection (Figure 1A). Upon re-exposure to the organism, the host displays a plethora of immunological effector mechanisms to resist the infection (Figure 1B). Of particular importance are antibodies and immune cells in the gut (e.g., O-specific ASC and plasma cells, sIgA, memory B cells (BM), Th1 and Th2 T cells) which will provide the first line of defense to prevent the organism from invading the epithelial barrier.
Figure 1.
Figure 1A. Live Shigella mucosal priming. (1) From the intestinal lumen, Shigella crosses the intestinal epithelial barrier through the M cells, possibly by a receptor-mediated uptake 107, and it is endocytosed by macrophages and dendritic cells in the subepithelial region of the M cell pocket. Conceivably, the organism could also be sampled from the lumen by DC residing between epithelial cells through their extended dendrites108. (2) The bacteria escape the phagocytic vacuole and induce apoptosis of the infected cells. As a result, live organisms are released and invade epithelial cells from the basolateral side, spreading from cell to cell throughout the mucosal epithelial layer. (3) Infected epithelial cells in turn secrete IL-8 and other chemotactic factors that will recruit polymorphonuclear (PMN) lymphocytes. Activated macrophages and PMN secrete a cascade of pro-inflammatory molecules (e.g., IL-18, IL-1β, TNF-α, IL-6, IFN-γ) further attracting phagocytic cells, which would ultimately kill and clear the organism. This initial inflammatory response sets the stage for adaptive immunological priming. (4) Apoptotic infected macrophages, neutrophils and other antigenic material released from infected cells may be taken up by DC, allowing for presentation and cross-presentation of bacterial antigens to T cells109. These antigen-loaded DC are transported to adjacent interfollicular T cell zones of mucosal lymphoid follicles or regional lymph nodes where they stimulate naïve T cells. Stimulated T cells in turn proliferate vigorously and differentiate into effector and memory T cells. This process leads to the activation of CD4+ Th2 cells, which support the production of antibodies, and Th1 cells, that will facilitate and expand inflammatory responses. Being an intracellular pathogen, Shigella is also expected to activate cytotoxic CD8+ T cells (CTL) that could eventually kill infected cells (e.g., intraepithelial -IEL- CTL) and secrete IFN-γ and other cytokines to further enhance Th1 CMI. B and T cells primed by mucosal pathogens acquire homing receptor molecules that will allow them to migrate to mucosal effector sites.
Figure 1B. Immunological effector mechanisms. A. Innate immunity. Shigella infection triggers an inflammatory response in the intestinal epithelium with recruitment of PMN, macrophages and NK cells, which will capture and kill the organism. These activated phagocytic cells release pro-inflammatory cytokines, which in turn contribute to the recruitment of B and T cells. B. Adaptive immunity. (1) IgA produced by mucosal ASC are secreted through the epithelial cells. IgG, produced by local IgG ASC or present in circulation can diffuse into the intestinal lumen or be actively transported through the FcRn receptor. Both antibodies could block the organisms abrogating cell attachment. (2) Antibodies could also block the bacteria that have breached the intestinal epithelial barrier preventing further cell invasion. IgG can mediate bacterial killing through opsonophagocytosis or lysis in the presence of complement. (3) Th1 cells could limit bacterial dissemination through induction of IEL with cytotoxic capacity. (4) Th2 cells provide support for production of antibodies and contribute to B cell differentiation and induction of BM cells. B memory cells can reactivate and mount a quick anamnestic response upon antigen exposure. Although the presence of antibodies against the O-polysaccharide and Shigella Ipas appear to be critical for protection, the contribution of other immunological effectors is likely necessary to clear an infection.
Abbreviations: PMN: polymorphonuclear lymphocytes; DC: dendritic cells; CTL: cytotoxic T cells (CTL); BM: B memory cells; FcRn: Fc-γ (IgG) neonatal intestinal receptor; IEL: intraepithelial lymphocytes; NK: natural killer. Black arrows indicate soluble protein mediators (e.g cytokines); blue arrows indicate changes in cell phenotype; grey arrows depict cytokines and molecular mediators that drive cell proliferation and differentiation in the lymph nodes.
Anamnestic humoral immune responses, largely dependent on the presence of B memory (BM) cells, are generally faster and higher in magnitude than primary responses and are crucial for protection from subsequent infections103. We have recently provided direct evidence for the presence of BM cells specific for Shigella antigens in volunteers immunized with a single oral dose of S. flexneri 2a CVD 1204 or CVD 1208 attenuated vaccine candidates104, 105. Using a modified BM ELISPOT assay we found that volunteers developed IgA and IgG BM to S. flexneri 2a LPS and IpaB antigens which were correlated with serum antibody responses. Moreover, we found elevated frequencies of IgA+, but not IgG+, CD19+ CD27+ CD20+ and CD19+ CD27+ CD20+ CD20−/dim B cell subsets expressing the gut homing receptor integrin α4/β7 28 days after oral vaccination, suggesting an increase in the circulating pool of Shigella-specific IgA BM and plasmablasts with gut homing potential in individuals that developed humoral responses to Shigella. These responses are finely regulated, with mucosal DC playing a central role in imprinting mucosal homing receptors on T and B cells and inducing IgA differentiation and regulatory T cells (Treg). A competent immune system in a healthy individual can clear the pathogen within 7–10 days of infection.
These findings are important for the development of effective vaccines since the presence of BM are likely to contribute to the persistence of systemic and mucosal antibodies and the ability to mount an anamnestic response when circulating antibody levels have already declined106. We therefore suggest including measurements of specific BM to relevant antigens in future vaccine studies since they might represent an important immunological correlate of protection and indicate long-term immunity.
Conclusions
Technological advancements including genomic technologies, synthetic carbohydrate chemistry, in vivo conjugation techniques and sophisticated assays to measure immune responses have facilitated the development and evaluation of a new generation of Shigella vaccines. In parallel, the progression of clinical trials assessing classical conjugate and live attenuated vaccines is revealing new information about relevant immune responses associated with protection. Despite these advances, there is still no consensus on what constitute critical immunological correlates of protection; this remains an obstacle impeding the development of safe and efficacious vaccines. This issue is complicated by the lack of an optimal small animal model for studying disease and the effects of vaccines leading to reliance, ultimately, on clinical trials for relevant data. Notwithstanding these difficulties, this is a time for optimism as in recent years there has been a renewed recognition of the excessive morbidity and mortality that Shigella causes among young children in developing countries and a commitment to diminish this burden. This has translated to new funding partners joining the efforts of other traditional supporters in providing resources for Shigella vaccine development. As a consequence, consortia of scientists have been contributing their expertise toward the common goal of developing safe and efficacious Shigella vaccines that might in the future become widely utilized public health tools to diminish the burden of shigellosis in populations most in need.
Review criteria.
We searched for articles focusing on original research on Shigella vaccine development. A PubMed search was performed using the search terms “Shigella” and “vaccines”. All papers identified were English-language full-text papers. We also reviewed the reference lists of identified articles for further relevant papers.
Key Points.
Shigella continues to be a leading cause of morbidity and mortality exerting the greatest burden in children in less industrialized countries.
Recent epidemiological studies (GEMS) confirmed the distribution of multiple serotypes in geographical regions as important causes of infection.
Studies on pathogenesis have revealed new virulence factors which may serve as targets for attenuation in live vaccine strains or as potential vaccine antigens.
Vaccine strategies may be divided into serotype-targeted or conserved protein antigen approaches and there are multiple candidates in various stages of development and evaluation.
New immunological measurements are shedding light on important protective responses.
Acknowledgments
Funding was provided by the following grants: U19 AI090873 (AF, EMB, MFP), U19 AI082655 (CCHI:MBS), U54 AI57168 (MML, EMB), R01 AI059223 (EMB), R01 AI089519 (MFP), grants from the Enteric Vaccine Initiative-Program for Appropriate Technology in Health (MML, MFP, EMB) and grants # 38874 and OPP1033572 from the Bill and Melinda Gates Foundation (MML, KLK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Footnotes
Competing interests
Myron M. Levine is the co-inventor of the patent for the attenuated Shigella flexneri 2a vector vaccine expressing two putative protective antigens of Enterotoxigenic Escherichia coli. However, heretofore no company has licensed this technology, thus there is no commercial “product” in development. The patent exists on paper only.
Reference List
- 1.Kotloff KL, et al. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ. 1999;77:651–666. [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang W, et al. Wide dissemination of multidrug-resistant Shigella isolates in China. J Antimicrob Chemother. 2011;66:2527–2535. doi: 10.1093/jac/dkr341. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh S, et al. Genetic characteristics and changing antimicrobial resistance among Shigella spp. isolated from hospitalized diarrhoeal patients in Kolkata, India. J Med Microbiol. 2011;60:1460–1466. doi: 10.1099/jmm.0.032920-0. [DOI] [PubMed] [Google Scholar]
- 4.Vinh H, et al. A changing picture of shigellosis in southern Vietnam: shifting species dominance, antimicrobial susceptibility and clinical presentation. BMC Infect Dis. 2009;9:204. doi: 10.1186/1471-2334-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khatun F, et al. Changing species distribution and antimicrobial susceptibility pattern of Shigella over a 29-year period (1980–2008) Epidemiol Infect. 2011;139:446–452. doi: 10.1017/S0950268810001093. [DOI] [PubMed] [Google Scholar]
- 6.Taneja N, Mewara A, Kumar A, Verma G, Sharma M. Cephalosporin-resistant Shigella flexneri over 9 years (2001–09) in India. J Antimicrob Chemother. 2012;67:1347–1353. doi: 10.1093/jac/dks061. [DOI] [PubMed] [Google Scholar]
- 7.Foster RA, et al. Structural elucidation of the O-antigen of the Shigella flexneri provisional serotype 88–893: structural and serological similarities with S. flexneri provisional serotype Y394 (1c) Carbohydr Res. 2011;346:872–876. doi: 10.1016/j.carres.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Hyams KC, et al. Diarrheal disease during Operation Desert Shield. N Engl J Med. 1991;325:1423–1428. doi: 10.1056/NEJM199111143252006. [DOI] [PubMed] [Google Scholar]
- 9.Kotloff K, Blackwelder WC, Nasrin D, et al. The Global Enterics Multicenter Study (GEMS) of Diarrheal Disease in Young CHildren in Dveeloping Countries: Epidemiologic and Clinical Methods of the Case-Control Study. Clinical Infectious Diseases. 2012 doi: 10.1093/cid/cis753. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panchalingam S, Antonio M, Hossain A, et al. Diagnostic Microbiology Methods in the GEMS-1 Case/Control Study. Clinical Infectious Diseases. 2012 doi: 10.1093/cid/cis754. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control Prevention. Health Status of displaced persons following Cival War - Burundi Dcemebr 1993 – January 1994. 1994;43:701–703. [PubMed] [Google Scholar]
- 12.Noriega FR, et al. Strategy for cross-protection among Shigella flexneri serotypes. Infect Immun. 1999;67:782–788. doi: 10.1128/iai.67.2.782-788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine MM, Kotloff KL, Barry EM, Pasetti MF, Sztein MB. Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard road. Nat Rev Microbiol. 2007;5:540–553. doi: 10.1038/nrmicro1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin Q, et al. Genome sequence of Shigella flexneri 2a: insights into pathogenicity through comparison with genomes of Escherichia coli K12 and O157. Nucleic Acids Res. 2002;30:4432–4441. doi: 10.1093/nar/gkf566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang F, et al. Genome dynamics and diversity of Shigella species, the etiologic agents of bacillary dysentery. Nucleic Acids Res. 2005;33:6445–6458. doi: 10.1093/nar/gki954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J, Chen L, Yu J, Sun L, Jin Q. ShiBASE: an integrated database for comparative genomics of Shigella. Nucleic Acids Res. 2006;34:D398–D401. doi: 10.1093/nar/gkj033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei J, et al. Complete genome sequence and comparative genomics of Shigella flexneri serotype 2a strain 2457T. Infect Immun. 2003;71:2775–2786. doi: 10.1128/IAI.71.5.2775-2786.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ying TY, et al. Immunoproteomics of membrane proteins of Shigella flexneri 2a 2457T. World J Gastroenterol. 2005;11:6880–6883. doi: 10.3748/wjg.v11.i43.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phalipon A, Sansonetti PJ. Shigella’s ways of manipulating the host intestinal innate and adaptive immune system: a tool box for survival? Immunol Cell Biol. 2007;85:119–129. doi: 10.1038/sj.icb7100025. [DOI] [PubMed] [Google Scholar]
- 20.Schroeder GN, Hilbi H. Molecular pathogenesis of Shigella spp.: controlling host cell signaling, invasion, and death by type III secretion. Clin Microbiol Rev. 2008;21:134–156. doi: 10.1128/CMR.00032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DuPont HL, Hornick RB, Dawkins AT, Snyder MJ, Formal SB. The response of man to virulent Shigella flexneri 2a. J Infect Dis. 1969;119:296–299. doi: 10.1093/infdis/119.3.296. [DOI] [PubMed] [Google Scholar]
- 22.Taylor DN, et al. The role of Shigella spp., enteroinvasive Escherichia coli, and other enteropathogens as causes of childhood dysentery in Thailand. J Infect Dis. 1986;153:1132–1138. doi: 10.1093/infdis/153.6.1132. [DOI] [PubMed] [Google Scholar]
- 23.Ashkenazi S, Dinari G, Zevulunov A, Nitzan M. Convulsions in childhood shigellosis. Clinical and laboratory features in 153 children. Am J Dis Child. 1987;141:208–210. doi: 10.1001/archpedi.1987.04460020098036. [DOI] [PubMed] [Google Scholar]
- 24.Koster F, et al. Hemolytic-uremic syndrome after shigellosis. Relation to endotoxemia and circulating immune complexes. N Engl J Med. 1978;298:927–933. doi: 10.1056/NEJM197804272981702. [DOI] [PubMed] [Google Scholar]
- 25.Nelson MR, Shanson DC, Hawkins DA, Gazzard BG. Salmonella, Campylobacter and Shigella in HIV-seropositive patients. AIDS. 1992;6:1495–1498. doi: 10.1097/00002030-199212000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Fasano A, Noriega FR, Liao FM, Wang W, Levine MM. Effect of shigella enterotoxin 1 (ShET1) on rabbit intestine in vitro and in vivo. Gut. 1997;40:505–511. doi: 10.1136/gut.40.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fasano A, et al. Shigella enterotoxin 1: an enterotoxin of Shigella flexneri 2a active in rabbit small intestine in vivo and in vitro. J Clin Invest. 1995;95:2853–2861. doi: 10.1172/JCI117991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farfan MJ, Toro CS, Barry EM, Nataro JP. Shigella enterotoxin-2 is a type III effector that participates in Shigella-induced interleukin 8 secretion by epithelial cells. FEMS Immunol Med Microbiol. 2011;61:332–339. doi: 10.1111/j.1574-695X.2011.00778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kotloff KL, et al. Deletion in the Shigella enterotoxin genes further attenuates Shigella flexneri 2a bearing guanine auxotrophy in a phase 1 trial of CVD 1204 and CVD 1208. J Infect Dis. 2004;190:1745–1754. doi: 10.1086/424680. [DOI] [PubMed] [Google Scholar]
- 30.Kotloff KL, et al. Safety and Immunogenicity of CVD 1208S, a Live, Oral DeltaguaBA Deltasen Deltaset Shigella flexneri 2a Vaccine Grown on Animal-Free Media. Hum Vaccin. 2007;3 doi: 10.4161/hv.4746. [DOI] [PubMed] [Google Scholar]
- 31.Ranallo RT, et al. Two live attenuated Shigella flexneri 2a strains WRSf2G12 and WRSf2G15: A new combination of gene deletions for 2nd generation live attenuated vaccine candidates. Vaccine. 2012;30:5159–5171. doi: 10.1016/j.vaccine.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Bedford L, et al. Further characterization of Shigella sonnei live vaccine candidates WRSs2 and WRSs3-plasmid composition, invasion assays and Sereny reactions. Gut Microbes. 2011;2:244–251. doi: 10.4161/gmic.2.4.17042. [DOI] [PubMed] [Google Scholar]
- 33.Dutta PR, Cappello R, Navarro-Garcia F, Nataro JP. Functional comparison of serine protease autotransporters of enterobacteriaceae. Infect Immun. 2002;70:7105–7113. doi: 10.1128/IAI.70.12.7105-7113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Hasani K, et al. The sigA gene which is borne on the she pathogenicity island of Shigella flexneri 2a encodes an exported cytopathic protease involved in intestinal fluid accumulation. Infect Immun. 2000;68:2457–2463. doi: 10.1128/iai.68.5.2457-2463.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Czerkinsky c, Kim DW. US2010/0136045. Novel Shigella Protein Antigens and Methods. 2010;12/692:187.
- 36.Benjelloun-Touimi Z, Si TM, Montecucco C, Sansonetti PJ, Parsot C. SepA, the 110 kDa protein secreted by Shigella flexneri: two-domain structure and proteolytic activity. Microbiology. 1998;144 (Pt 7):1815–1822. doi: 10.1099/00221287-144-7-1815. [DOI] [PubMed] [Google Scholar]
- 37.Henderson IR, Czeczulin J, Eslava C, Noriega F, Nataro JP. Characterization of pic, a secreted protease of Shigella flexneri and enteroaggregative Escherichia coli. Infect Immun. 1999;67:5587–5596. doi: 10.1128/iai.67.11.5587-5596.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Behrens M, Sheikh J, Nataro JP. Regulation of the overlapping pic/set locus in Shigella flexneri and enteroaggregative Escherichia coli. Infect Immun. 2002;70:2915–2925. doi: 10.1128/IAI.70.6.2915-2925.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruiz-Perez F, et al. Serine protease autotransporters from Shigella flexneri and pathogenic Escherichia coli target a broad range of leukocyte glycoproteins. Proc Natl Acad Sci U S A. 2011;108:12881–12886. doi: 10.1073/pnas.1101006108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boisen N, Ruiz-Perez F, Scheutz F, Krogfelt KA, Nataro JP. Short report: high prevalence of serine protease autotransporter cytotoxins among strains of enteroaggregative Escherichia coli. Am J Trop Med Hyg. 2009;80:294–301. [PMC free article] [PubMed] [Google Scholar]
- 41.Formal SB, et al. Protection of monkeys against experimental shigellosis with a living attenuated oral polyvalent dysentery vaccine. J Bacteriol. 1966;92:17–22. doi: 10.1128/jb.92.1.17-22.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Formal SB, LaBrec EH, Palmer A, Falkow S. Protection of Monkeys Against Experimental Shigellosis with Attenuated Vaccines. J Bacteriol. 1965;90:63–68. doi: 10.1128/jb.90.1.63-68.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shipley ST, et al. A challenge model for Shigella dysenteriae 1 in cynomolgus monkeys (Macaca fascicularis) Comp Med. 2010;60:54–61. [PMC free article] [PubMed] [Google Scholar]
- 44.Sereny B. Experimental shigella keratoconjunctivitis; a preliminary report. Acta Microbiol Acad Sci Hung. 1955;2:293–296. [PubMed] [Google Scholar]
- 45.Mallett CP, VanDeVerg L, Collins HH, Hale TL. Evaluation of Shigella vaccine safety and efficacy in an intranasally challenged mouse model. Vaccine. 1993;11:190–196. doi: 10.1016/0264-410x(93)90016-q. [DOI] [PubMed] [Google Scholar]
- 46.Shim DH, et al. New animal model of shigellosis in the Guinea pig: its usefulness for protective efficacy studies. J Immunol. 2007;178:2476–2482. doi: 10.4049/jimmunol.178.4.2476. [DOI] [PubMed] [Google Scholar]
- 47.Nataro JP, et al. Identification and cloning of a novel plasmid-encoded enterotoxin of enteroinvasive Escherichia coli and Shigella strains. Infect Immun. 1995;63:4721–4728. doi: 10.1128/iai.63.12.4721-4728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Formal SB, et al. Effect of prior infection with virulent Shigella flexneri 2a on the resistance of monkeys to subsequent infection with Shigella sonnei. J Infect Dis. 1991;164:533–537. doi: 10.1093/infdis/164.3.533. [DOI] [PubMed] [Google Scholar]
- 49.Herrington DA, et al. Studies in volunteers to evaluate candidate Shigella vaccines: further experience with a bivalent Salmonella typhi-Shigella sonnei vaccine and protection conferred by previous Shigella sonnei disease. Vaccine. 1990;8:353–357. doi: 10.1016/0264-410x(90)90094-3. [DOI] [PubMed] [Google Scholar]
- 50.Kotloff KL, et al. A modified Shigella volunteer challenge model in which the inoculum is administered with bicarbonate buffer: clinical experience and implications for Shigella infectivity. Vaccine. 1995;13:1488–1494. doi: 10.1016/0264-410x(95)00102-7. [DOI] [PubMed] [Google Scholar]
- 51.Ferreccio C, et al. Epidemiologic patterns of acute diarrhea and endemic Shigella infections in children in a poor periurban setting in Santiago, Chile. Am J Epidemiol. 1991;134:614–627. doi: 10.1093/oxfordjournals.aje.a116134. [DOI] [PubMed] [Google Scholar]
- 52.Passwell JH, et al. Safety and immunogenicity of Shigella sonnei-CRM9 and Shigella flexneri type 2a-rEPAsucc conjugate vaccines in one- to four-year-old children. Pediatr Infect Dis J. 2003;22:701–706. doi: 10.1097/01.inf.0000078156.03697.a5. [DOI] [PubMed] [Google Scholar]
- 53.Robbins JB, Chu C, Schneerson R. Hypothesis for vaccine development: protective immunity to enteric diseases caused by nontyphoidal salmonellae and shigellae may be conferred by serum IgG antibodies to the O-specific polysaccharide of their lipopolysaccharides. Clin Infect Dis. 1992;15:346–361. doi: 10.1093/clinids/15.2.346. [DOI] [PubMed] [Google Scholar]
- 54.Taylor DN, et al. Synthesis, characterization, and clinical evaluation of conjugate vaccines composed of the O-specific polysaccharides of Shigella dysenteriae type 1, Shigella flexneri type 2a, and Shigella sonnei (Plesiomonas shigelloides) bound to bacterial toxoids. Infect Immun. 1993;61:3678–3687. doi: 10.1128/iai.61.9.3678-3687.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen D, et al. Safety and immunogenicity of investigational Shigella conjugate vaccines in Israeli volunteers. Infect Immun. 1996;64:4074–4077. doi: 10.1128/iai.64.10.4074-4077.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Passwell JH, et al. Age-related efficacy of Shigella O-specific polysaccharide conjugates in 1-4-year-old Israeli children. Vaccine. 2010;28:2231–2235. doi: 10.1016/j.vaccine.2009.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ihssen J, et al. Production of glycoprotein vaccines in Escherichia coli. Microb Cell Fact. 2010;9:61. doi: 10.1186/1475-2859-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feldman MF, et al. Engineering N-linked protein glycosylation with diverse O antigen lipopolysaccharide structures in Escherichia coli. Proc Natl Acad Sci U S A. 2005;102:3016–3021. doi: 10.1073/pnas.0500044102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dro P, Sinclair M. GlycoVaxyn Phase I Clinical Study Shows Positive Data with Shigella dysenteriae Vaccine Candidate. 2010 http://www.glycovaxyn.com/content/news/releases/10%2010%2008.pdf. [ http://www.glycovaxyn.com/content/news/releases/10%2010%2008.pdf]
- 60.Phalipon A, et al. A synthetic carbohydrate-protein conjugate vaccine candidate against Shigella flexneri 2a infection. J Immunol. 2009;182:2241–2247. doi: 10.4049/jimmunol.0803141. [DOI] [PubMed] [Google Scholar]
- 61.Kubler-Kielb J, Vinogradov E, Chu C, Schneerson R. O-Acetylation in the O-specific polysaccharide isolated from Shigella flexneri serotype 2a. Carbohydr Res. 2007;342:643–647. doi: 10.1016/j.carres.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Porter CK, Thura N, Ranallo RT, Riddle MS. The Shigella human challenge model. Epidemiol Infect. 2012:1–10. doi: 10.1017/S0950268812001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coster TS, et al. Vaccination against shigellosis with attenuated Shigella flexneri 2a strain SC602. Infect Immun. 1999;67:3437–3443. doi: 10.1128/iai.67.7.3437-3443.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mel DM, Terzin AL, Vuksic L. Studies on vaccination against bacillary dysentery. 3. Effective oral immunization against Shigella flexneri 2a in a field trial. Bull World Health Organ. 1965;32:647–655. [PMC free article] [PubMed] [Google Scholar]
- 65.Ranallo RT, Barnoy S, Thakkar S, Urick T, Venkatesan MM. Developing live Shigella vaccines using lambda Red recombineering. FEMS Immunol Med Microbiol. 2006;47:462–469. doi: 10.1111/j.1574-695X.2006.00118.x. [DOI] [PubMed] [Google Scholar]
- 66.Venkatesan MM, Ranallo RT. Live-attenuated Shigella vaccines. Expert Rev Vaccines. 2006;5:669–686. doi: 10.1586/14760584.5.5.669. [DOI] [PubMed] [Google Scholar]
- 67.Barnoy S, et al. Shigella sonnei vaccine candidates WRSs2 and WRSs3 are as immunogenic as WRSS1, a clinically tested vaccine candidate, in a primate model of infection. Vaccine. 2011;29:6371–6378. doi: 10.1016/j.vaccine.2011.04.115. [DOI] [PubMed] [Google Scholar]
- 68.Ranallo RT, et al. Virulence, inflammatory potential, and adaptive immunity induced by Shigella flexneri msbB mutants. Infect Immun. 2010;78:400–412. doi: 10.1128/IAI.00533-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Katz DE, et al. Two studies evaluating the safety and immunogenicity of a live, attenuated Shigella flexneri 2a vaccine (SC602) and excretion of vaccine organisms in North American volunteers. Infect Immun. 2004;72:923–930. doi: 10.1128/IAI.72.2.923-930.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rahman KM, et al. Safety, dose, immunogenicity, and transmissibility of an oral live attenuated Shigella flexneri 2a vaccine candidate (SC602) among healthy adults and school children in Matlab, Bangladesh. Vaccine. 2011;29:1347–1354. doi: 10.1016/j.vaccine.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 71.Gordon JI, Dewey KG, Mills DA, Medzhitov RM. The human gut microbiota and undernutrition. Sci Transl Med. 2012;4:137ps12. doi: 10.1126/scitranslmed.3004347. [DOI] [PubMed] [Google Scholar]
- 72.Levine MM. Immunogenicity and efficacy of oral vaccines in developing countries: lessons from a live cholera vaccine. BMC Biol. 2010;8:129. doi: 10.1186/1741-7007-8-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hanlon P, et al. Trial of an attenuated bovine rotavirus vaccine (RIT 4237) in Gambian infants. Lancet. 1987;1:1342–1345. doi: 10.1016/s0140-6736(87)90649-0. [DOI] [PubMed] [Google Scholar]
- 74.John TJ, Jayabal P. Oral polio vaccination of children in the tropics. I. The poor seroconversion rates and the absence of viral interference. Am J Epidemiol. 1972;96:263–269. doi: 10.1093/oxfordjournals.aje.a121457. [DOI] [PubMed] [Google Scholar]
- 75.Armah GE, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:606–614. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 76.Balraj V, John TJ, Thomas M, Mukundan S. Efficacy of oral poliovirus vaccine in rural communities of North Arcot District, India. Int J Epidemiol. 1990;19:711–714. doi: 10.1093/ije/19.3.711. [DOI] [PubMed] [Google Scholar]
- 77.Madhi SA, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362:289–298. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 78.Su-Arehawaratana P, et al. Safety and immunogenicity of different immunization regimens of CVD 103-HgR live oral cholera vaccine in soldiers and civilians in Thailand. J Infect Dis. 1992;165:1042–1048. doi: 10.1093/infdis/165.6.1042. [DOI] [PubMed] [Google Scholar]
- 79.Turbyfill KR, Kaminski RW, Oaks EV. Immunogenicity and efficacy of highly purified invasin complex vaccine from Shigella flexneri 2a. Vaccine. 2008;26:1353–1364. doi: 10.1016/j.vaccine.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 80.Riddle MS, et al. Safety and immunogenicity of an intranasal Shigella flexneri 2a Invaplex 50 vaccine. Vaccine. 2011;29:7009–7019. doi: 10.1016/j.vaccine.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 81.Tribble D, et al. Safety and immunogenicity of a Shigella flexneri 2a Invaplex 50 intranasal vaccine in adult volunteers. Vaccine. 2010;28:6076–6085. doi: 10.1016/j.vaccine.2010.06.086. [DOI] [PubMed] [Google Scholar]
- 82.Martinez-Becerra FJ, et al. Broadly protective Shigella vaccine based on type III secretion apparatus proteins. Infect Immun. 2012;80:1222–1231. doi: 10.1128/IAI.06174-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li A, Zhao CR, Ekwall E, Lindberg AA. Serum IgG antibody responses to Shigella invasion plasmid-coded antigens detected by immunoblot. Scand J Infect Dis. 1994;26:435–445. doi: 10.3109/00365549409008617. [DOI] [PubMed] [Google Scholar]
- 84.Oaks EV, Hale TL, Formal SB. Serum immune response to Shigella protein antigens in rhesus monkeys and humans infected with Shigella spp. Infect Immun. 1986;53:57–63. doi: 10.1128/iai.53.1.57-63.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Adamus G, Mulczyk M, Witkowska D, Romanowska E. Protection against keratoconjunctivitis shigellosa induced by immunization with outer membrane proteins of Shigella spp. Infect Immun. 1980;30:321–324. doi: 10.1128/iai.30.2.321-324.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pore D, Mahata N, Pal A, Chakrabarti MK. Outer membrane protein A (OmpA) of Shigella flexneri 2a, induces protective immune response in a mouse model. PLoS One. 2011;6:e22663. doi: 10.1371/journal.pone.0022663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Berlanda SF, et al. High yield production process for Shigella outer membrane particles. PLoS One. 2012;7:e35616. doi: 10.1371/journal.pone.0035616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cohen D, Green MS, Block C, Rouach T, Ofek I. Serum antibodies to lipopolysaccharide and natural immunity to shigellosis in an Israeli military population. J Infect Dis. 1988;157:1068–1071. doi: 10.1093/infdis/157.5.1068. [DOI] [PubMed] [Google Scholar]
- 89.Cohen D, Green MS, Block C, Slepon R, Ofek I. Prospective study of the association between serum antibodies to lipopolysaccharide O antigen and the attack rate of shigellosis. J Clin Microbiol. 1991;29:386–389. doi: 10.1128/jcm.29.2.386-389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Van De Verg LL, Herrington DA, Boslego J, Lindberg AA, Levine MM. Age-specific prevalence of serum antibodies to the invasion plasmid and lipopolysaccharide antigens of Shigella species in Chilean and North American populations. J Infect Dis. 1992;166:158–161. doi: 10.1093/infdis/166.1.158. [DOI] [PubMed] [Google Scholar]
- 91.Oberhelman RA, et al. Prospective study of systemic and mucosal immune responses in dysenteric patients to specific Shigella invasion plasmid antigens and lipopolysaccharides. Infect Immun. 1991;59:2341–2350. doi: 10.1128/iai.59.7.2341-2350.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kotloff KL, et al. Safety, immunogenicity, and transmissibility in humans of CVD 1203, a live oral Shigella flexneri 2a vaccine candidate attenuated by deletions in aroA and virG. Infect Immun. 1996;64:4542–4548. doi: 10.1128/iai.64.11.4542-4548.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kotloff KL, et al. Evaluation of the safety, immunogenicity, and efficacy in healthy adults of four doses of live oral hybrid Escherichia coli-Shigella flexneri 2a vaccine strain EcSf2a-2. Vaccine. 1995;13:495–502. doi: 10.1016/0264-410x(94)00011-b. [DOI] [PubMed] [Google Scholar]
- 94.Hayani KC, et al. Concentration of milk secretory immunoglobulin A against Shigella virulence plasmid-associated antigens as a predictor of symptom status in Shigella-infected breast-fed infants. J Pediatr. 1992;121:852–856. doi: 10.1016/s0022-3476(05)80327-0. [DOI] [PubMed] [Google Scholar]
- 95.Cleary TG, Winsor DK, Reich D, Ruiz-Palacios G, Calva JJ. Human milk immunoglobulin A antibodies to Shigella virulence determinants. Infect Immun. 1989;57:1675–1679. doi: 10.1128/iai.57.6.1675-1679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cohen D, et al. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet. 1997;349:155–159. doi: 10.1016/S0140-6736(96)06255-1. [DOI] [PubMed] [Google Scholar]
- 97.Robbins JB, Chu C, Schneerson R. Hypothesis for vaccine development: protective immunity to enteric diseases caused by nontyphoidal salmonellae and shigellae may be conferred by serum IgG antibodies to the O-specific polysaccharide of their lipopolysaccharides. Clin Infect Dis. 1992;15:346–361. doi: 10.1093/clinids/15.2.346. [DOI] [PubMed] [Google Scholar]
- 98.Raqib R, et al. Persistence of local cytokine production in shigellosis in acute and convalescent stages. Infect Immun. 1995;63:289–296. doi: 10.1128/iai.63.1.289-296.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Raqib R, et al. Delayed and reduced adaptive humoral immune responses in children with shigellosis compared with in adults. Scand J Immunol. 2002;55:414–423. doi: 10.1046/j.1365-3083.2002.01079.x. [DOI] [PubMed] [Google Scholar]
- 100.Islam D, Christensson B. Disease-dependent changes in T-cell populations in patients with shigellosis. APMIS. 2000;108:251–260. doi: 10.1034/j.1600-0463.2000.d01-52.x. [DOI] [PubMed] [Google Scholar]
- 101.Islam D, Bardhan PK, Lindberg AA, Christensson B. Shigella infection induces cellular activation of T and B cells and distinct species-related changes in peripheral blood lymphocyte subsets during the course of the disease. Infect Immun. 1995;63:2941–2949. doi: 10.1128/iai.63.8.2941-2949.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Islam D, Wretlind B, Lindberg AA, Christensson B. Changes in the peripheral blood T-Cell receptor V beta repertoire in vivo and in vitro during shigellosis. Infect Immun. 1996;64:1391–1399. doi: 10.1128/iai.64.4.1391-1399.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sanz I, Wei C, Lee FE, Anolik J. Phenotypic and functional heterogeneity of human memory B cells. Semin Immunol. 2008;20:67–82. doi: 10.1016/j.smim.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Simon JK, et al. Antigen-specific B memory cell responses to lipopolysaccharide (LPS) and invasion plasmid antigen (Ipa) B elicited in volunteers vaccinated with live-attenuated Shigella flexneri 2a vaccine candidates. Vaccine. 2009;27:565–572. doi: 10.1016/j.vaccine.2008.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Simon JK, et al. Antigen-specific IgA B memory cell responses to Shigella antigens elicited in volunteers immunized with live attenuated Shigella flexneri 2a oral vaccine candidates. Clin Immunol. 2011;139:185–192. doi: 10.1016/j.clim.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Traggiai E, Puzone R, Lanzavecchia A. Antigen dependent and independent mechanisms that sustain serum antibody levels. Vaccine. 2003;21 (Suppl 2):S35–S37. doi: 10.1016/s0264-410x(03)00198-1. [DOI] [PubMed] [Google Scholar]
- 107.Tyrer PC, Ruth FA, Kyd JM, Otczyk DC, Cripps AW. Receptor mediated targeting of M-cells. Vaccine. 2007;25:3204–3209. doi: 10.1016/j.vaccine.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 108.Ng SC, Kamm MA, Stagg AJ, Knight SC. Intestinal dendritic cells: their role in bacterial recognition, lymphocyte homing, and intestinal inflammation. Inflamm Bowel Dis. 2010;16:1787–1807. doi: 10.1002/ibd.21247. [DOI] [PubMed] [Google Scholar]
- 109.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12:557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 110.Noriega FR, Liao FM, Formal SB, Fasano A, Levine MM. Prevalence of Shigella enterotoxin 1 among Shigella clinical isolates of diverse serotypes. J Infect Dis. 1995;172:1408–1410. doi: 10.1093/infdis/172.5.1408. [DOI] [PubMed] [Google Scholar]
- 111.Benjelloun-Touimi Z, Sansonetti PJ, Parsot C. SepA, the major extracellular protein of Shigella flexneri: autonomous secretion and involvement in tissue invasion. Mol Microbiol. 1995;17:123–135. doi: 10.1111/j.1365-2958.1995.mmi_17010123.x. [DOI] [PubMed] [Google Scholar]
- 112.Wu T, Grassel C, Levine MM, Barry EM. Live attenuated Shigella dysenteriae type 1 vaccine strains overexpressing shiga toxin B subunit. Infect Immun. 2011;79:4912–4922. doi: 10.1128/IAI.05814-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kotloff KL, et al. Phase I evaluation of delta virG Shigella sonnei live, attenuated, oral vaccine strain WRSS1 in healthy adults. Infect Immun. 2002;70:2016–2021. doi: 10.1128/IAI.70.4.2016-2021.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Barnoy S, et al. Characterization of WRSs2 and WRSs3, new second-generation virG(icsA)-based Shigella sonnei vaccine candidates with the potential for reduced reactogenicity. Vaccine. 2010;28:1642–1654. doi: 10.1016/j.vaccine.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Collins TA, et al. Safety and colonization of two novel VirG(IcsA)-based live Shigella sonnei vaccine strains in rhesus macaques (Macaca mulatta) Comp Med. 2008;58:88–94. [PMC free article] [PubMed] [Google Scholar]
- 116.Ranallo RT, Thakkar S, Chen Q, Venkatesan MM. Immunogenicity and characterization of WRSF2G11: a second generation live attenuated Shigella flexneri 2a vaccine strain. Vaccine. 2007;25:2269–2278. doi: 10.1016/j.vaccine.2006.11.067. [DOI] [PubMed] [Google Scholar]
- 117.McKenzie R, et al. Safety and immunogenicity of WRSd1, a live attenuated Shigella dysenteriae type 1 vaccine candidate. Vaccine. 2008;26:3291–3296. doi: 10.1016/j.vaccine.2008.03.079. [DOI] [PubMed] [Google Scholar]
- 118.Venkatesan MM, et al. Construction, characterization, and animal testing of WRSd1, a Shigella dysenteriae 1 vaccine. Infect Immun. 2002;70:2950–2958. doi: 10.1128/IAI.70.6.2950-2958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cohen D, et al. Clinical trials of Shigella vaccines in Israel. Adv Exp Med Biol. 1996;397:159–167. doi: 10.1007/978-1-4899-1382-1_21. [DOI] [PubMed] [Google Scholar]
- 120.Chu CY, et al. Preparation, characterization, and immunogenicity of conjugates composed of the O-specific polysaccharide of Shigella dysenteriae type 1 (Shiga’s bacillus) bound to tetanus toxoid. Infect Immun. 1991;59:4450–4458. doi: 10.1128/iai.59.12.4450-4458.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Theillet FX, et al. Effects of backbone substitutions on the conformational behavior of Shigella flexneri O-antigens: implications for vaccine strategy. Glycobiology. 2011;21:109–121. doi: 10.1093/glycob/cwq136. [DOI] [PubMed] [Google Scholar]