Abstract
Skeletal loading and unloading has a pronounced impact on bone remodeling, a process also regulated by insulin-like growth factor 1 (IGF-1) signaling. Skeletal unloading leads to resistance to the anabolic effect of IGF-1, while reloading after unloading restores responsiveness to IGF-1. However, a direct study of the importance of IGF-1 signaling in the skeletal response to mechanical loading remains to be tested. In this study, we assessed the skeletal response of osteoblast-specific Igf-1 receptor deficient (Igf-1r−/−) mice to unloading and reloading. The mice were hindlimb unloaded for 14 days and then reloaded for 16 days. Igf-1r−/− mice displayed smaller cortical bone and diminished periosteal and endosteal bone formation at baseline. Periosteal and endosteal bone formation decreased with unloading in Igf-1r+/+ mice. However, the recovery of periosteal bone formation with reloading was completely inhibited in Igf-1r−/− mice, although reloading-induced endosteal bone formation was not hampered. These changes in bone formation resulted in the abolishment of the expected increase in total cross-sectional area with reloading in Igf-1r−/− mice compared to the control mice. These results suggest that the Igf-1r in mature osteoblasts has a critical role in periosteal bone formation in the skeletal response to mechanical loading.
Keywords: Insulin-like growth factor 1 receptor, osteoblasts, unloading, reloading, periosteal bone formation
Introduction
Bone is a dynamic tissue that is responsive and sensitive to mechanical stimuli [1]. Regions undergoing increased mechanical loading augment bone mass [2]. Bone loss results from skeletal unloading during long-term spaceflight and prolonged bed rest caused by fractures, spinal injuries, and strokes [3, 4]. Astronauts dramatically lose bone mass in the hip at 1.5%/month on long-duration missions on the International Space Station [5]. Recovery of bone mineral density in astronauts after completing missions is much slower than the bone loss during spaceflight [6–8]. Skeletal unloading leads to decreased bone mass due to reduced bone formation and increased bone resorption [3, 9]. Despite identification of the cell types and signaling molecules responsible for skeletal unloading and loading [10, 11], the underlying mechanisms by which bone senses and responds to mechanical stimuli have not been fully elucidated.
Insulin-like growth factor-1 (IGF-1) is critical for bone homeostasis and required to achieve normal bone growth and bone mass [12–14]. IGF-1 and the IGF-1 receptor (IGF-1R) are expressed in osteoblasts [15, 16]. In vivo and in vitro studies have revealed that the most pronounced role of IGF-1 in osteoblasts is to stimulate osteoblast function and bone formation. Targeted over-expression of Igf-1 in mature osteoblasts leads to increased bone formation [17]. Mice carrying Igf-1 receptor (Igf-1r) deletion in mature osteoblasts exhibit decreases in cortical thickness as well as bone formation [18, 19]. These lines of evidence demonstrate that the IGF-1/IGF-1R system in mature osteoblasts is indispensable for maintaining normal bone mass and bone formation.
Osteoblasts and osteocytes stimulated by mechanical loading produce IGF-1 [20, 21]. Skeletal unloading leads to resistance to the anabolic effect of IGF-1 on bone, while bone responsiveness to IGF-1 is restored with reloading following unloading [22, 23]. However, a direct study of the importance of IGF-1 signaling in the skeletal response to mechanical loading remains to be tested. In this study, we assessed the skeletal response of osteoblast-specific Igf-1r deficient mice to unloading and reloading.
Materials and Methods
Mice
Animal protocols were approved by the Institutional Animal Care and Use Committee at the Veterans Administration Medical Center, San Francisco. Homozygous conditional mice in which exon 3 of the Igf-1r gene was flanked by loxP sites [18] were bred with heterozygous mice in which cre recombinase was expressed under the control of the human osteocalcin promoter (gift from Dr. Thomas Clemens) [24] to generate osteoblast-specific Igf-1r deficient mice. The mice were maintained under pathogen-free conditions and fed ad libitum. We hindlimb-unloaded 12-week-old mice for 14 days, as described previously [25–27], and then reloaded them for 16 days.
Histomorphometry
Tibiae were fixed, dehydrated and embedded in methyl methacrylate. Ten-μm cross-sectional undecalcified sections from a region immediately proximal to the tibio-fibular junction at the tibial diaphysis were cut to assess bone formation measurements in cortical bone. Mosaic-tiled images were converted to a single image by the Axio Vision software (Carl Zeiss). Data were collected using the Bioquant Osteo software (Bioquant Image Analysis) and reported according to standard bone histomorphometry nomenclature [28]. In details, to obtain cortical bone formation measurements, alizarin complexone (30mg/kg, Sigma-Aldrich) and calcein (15 mg/kg, Sigma-Aldrich) were peritoneally given to mice 14 days and 2 days before euthanasia, respectively. The fluorochromes are incorporated into bone matrix and become fluorescent labels on bone surfaces only where and when bone formation occurs before being excreted by the kidney. Bone formation rate/bone surface (BFR/BS) (μm3/μm2/day) is calculated as the multiplication of mineralizing surface (MS)/BS and mineral apposition rate (MAR). MS is bone surface actively mineralized by osteoblasts at a particular time when fluorescent labels are administered and calculated as the total extent of double labeled bone surfaces plus the half of single labeled bone surfaces. It correlates with the extent of bone surface covered with osteoblasts. MAR is the distance between two labels divided by the time (12 days in this study) between two labeling periods. MAR correlates with the activity of osteoblasts [19].
Micro–computed tomography (μCT)
Tibiae were scanned under anesthesia using a SCANO VivaCT 40 (Scano Medical AG) to obtain longitudinal 3D in vivo μCT imaging. A region of interest (ROI) for cortical bone analysis consisted of 0.42 mm proximal to the tibio-fibular junction at the tibial diaphysis. Data were reported according to standard μCT nomenclature [29]. Total cross-sectional area (Tt.Ar) is defined as total cross-sectional area inside the periosteal envelope in cortical bone.
Immunohistochemistry
Femurs were cleaned of adherent tissue, fixed overnight at 4 C in 4% paraformaldehyde in PBS, rinsed in PBS, dehydrated through an ethanol series, cleared in xylene, embedded in paraffin, and cut into 5-μm sections. Deparaffinized and rehydrated sections were incubated with 3% hydrogen peroxide in methanol to block endogenous peroxidase and with protein blocker (Abcam) to block the nonspecific binding of antibodies. Then, the sections were reacted with rabbit IGF-IR antibody (1:200) (Aviva Systems Biology) at 4 C overnight. After washing with PBS, the sections were incubated with biotinylated goat anti-rabbit IgG (Abcam), then streptavidin peroxidase (Abcam), and visualized by 3,3′-diaminobenzidine.
Statistical analysis
Data are expressed as mean ± SE and analyzed by an unpaired Student’s t-test, except using a paired Student’s t-test for longitudinal measurements of μCT analysis.
Results
Deletion of the Igf-1r in mature osteoblasts and osteocytes in Igf-1r−/− mice
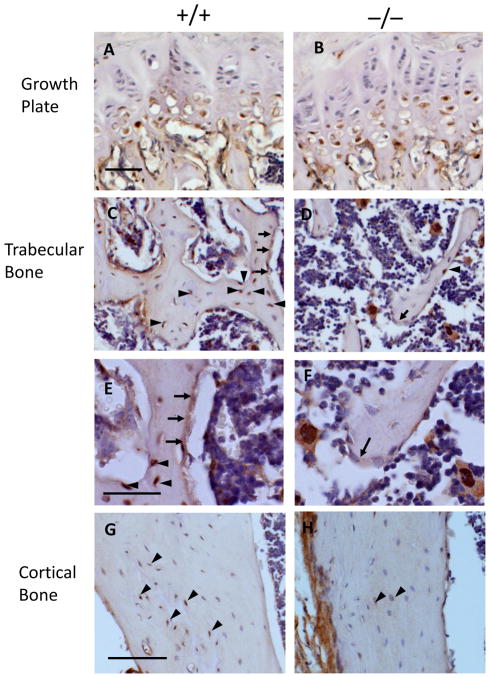
We evaluated mice in which the insulin-like growth factor type-1 receptor was deleted with osteocalcin-driven cre recombinase (Igf-1r−/−) in order to determine the role of the IGF-1R in mature osteoblasts in the skeletal response to unloading and reloading. Before examining the skeletal response of Igf-1r−/− mice to unloading and reloading, we assessed the expression of Igf-1r in the growth plate, trabecular bone and cortical bone by immunohistochemistry (Fig 1). In Igf-1r−/− mice, very little expression of IGF-1R was identified in mature osteoblasts in the secondary spongiosa of trabecular bone as well as in osteocytes in trabecular and cortical bone (Fig. 1D, 1F, 1H), although IGF-1R was expressed in mature osteoblasts and osteocytes in Igf-1r+/+ mice (Fig. 1C, 1E, 1G). IGF-1R expression in prehypertrophic and hypertrophic chondrocytes in the growth plate was comparable between Igf-1r+/+ and Igf-1r−/− mice (Fig. 1A, 1B). These results demonstrate the specific deletion of the Igf-1r in mature osteoblasts and osteocytes in Igf-1r−/− mice.
Fig 1. Expression of IGF-1R in Igf-1r−/− mice.
Immunohistochemistry staining showed that in the growth plate (A & B), IGF-1R (brown) was expressed in the prehypertrophic and hypertrophic chondrocytes in the Igf-1r+/+ (A) mice and the Igf-1r−/− (B) mice; in the trabecular bone of the secondary spongiosa, IGF-1R is expressed in the osteoblasts lining the bone surfaces (arrows) and osteocytes embedded in the bone matrix (arrowheads) in the Igf-1r+/+ mice (C), but very little expression of IGF-1R was identified in the osteoblasts (arrows) and osteocytes (arrow heads) in the Igf-1r−/− mice (D). High magnification pictures of Igf-1r+/+ (E) and Igf-1r−/− (F) mice are shown below. In the cortical bones, IGF-1R is expressed in the osteocytes (brown, arrow heads) in the Igf-1r+/+ mice (G), but very few osteocytes (arrowheads) express IGF-1R in the Igf-1r−/− mice (H). 20 X in A–D, G & H; 40 X in E & F. Bars = 50 μm.
Periosteal and endosteal bone formation in cortical bone is impaired in Igf-1r−/− mice
We assessed Igf-1r−/− mice at baseline by μCT and bone histomorphometry, before determining the role of the IGF-1R in mature osteoblasts in the skeletal response to unloading and reloading. μCT analysis revealed that total cross-sectional area (Tt.Ar) in cortical bone was decreased in Igf-1r−/− male mice compared to that in Igf-1r+/+ littermates (Fig. 2A). Histomorphometry demonstrated that the bone formation rate (BFR/BS) was reduced in both periosteal and endosteal surfaces of cortical bone in Igf-1r−/− mice (Fig. 2B). The reduced BFR/BS was due to a decrease in MAR and MS/BS on both periosteal and endosteal bone surfaces (data not shown). These results indicate that the IGF-1R in mature osteoblasts has a significant role in bone formation in cortical bone.
Fig 2. Periosteal and endosteal bone formation in cortical bone is impaired in Igf-1r−/− mice.
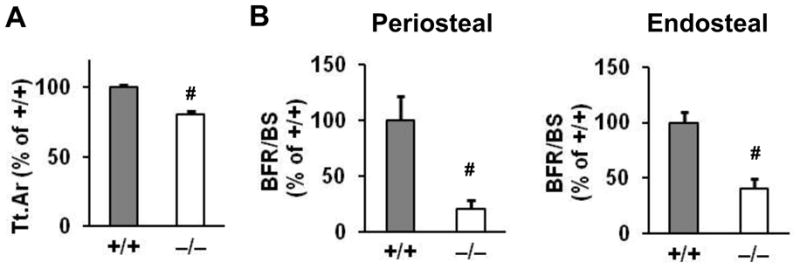
(A) μCT analysis revealed that total cross-sectional area (Tt.Ar) of cortical bone was decreased in Igf-1r−/− male mice. n = 10 in the two groups. (B) Bone histomorphometry showed that bone formation rate (BFR/BS) was decreased in both periosteal and endosteal surfaces of cortical bone in Igf-1r−/− mice. n = 6 in Igf-1r+/+ mice; n = 7 in Igf-1r−/− mice. Data are percent of the results of Igf-1r+/+ mice and shown as mean ± SE. #p < 0.01.
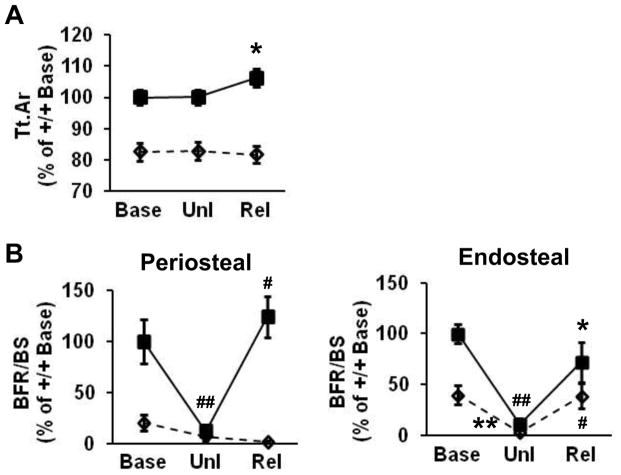
Periosteal bone formation is diminished in Igf-1r−/− mice in the response to skeletal unloading and reloading
We hindlimb-unloaded mice and then reloaded them in order to determine whether the IGF-1R in mature osteoblasts is associated with the skeletal response to unloading and/or mechanical loading. Cortical bone was longitudinally estimated by μCT at baseline and after unloading and reloading. Tt.Ar increased with reloading in Igf-1r+/+ male mice compared to that after unloading in the same group (Fig. 3A). However, the response of Tt.Ar to reloading was diminished in Igf-1r−/− mice, indicating that periosteal bone expansion by reloading is inhibited in Igf-1r−/− mice. Histomorphometry showed that BFR/BS on periosteal surfaces of cortical bone declined with unloading in Igf-1r+/+ mice and then increased with reloading (Fig. 3B.), along with a reduction and an increase in both MAR and MS/BS, respectively (data not shown). In contrast, this response to reloading was completely hindered in Igf-1r−/− mice. On the other hand, BFR/BS on endosteal surfaces of cortical bone fell with unloading and then increased with reloading in both Igf-1r−/− and Igf-1r+/+ mice (Fig. 3B). A change of MAR and MS/BS with unloading and reloading on endosteal bone surfaces was in concordance with the changes of BFR/BS with unloading and reloading, respectively, in both Igf-1r−/− and Igf-1r+/+ mice (data not shown). These results indicate that the IGF-1R in mature osteoblasts plays a crucial role in reloading-induced periosteal but not endosteal bone formation.
Fig 3. Periosteal bone formation is diminished in Igf-1r−/− mice in the response to skeletal unloading and reloading.
(A) Total cross-sectional area (Tt.Ar) increased with reloading (Rel) in Igf-1r+/+ mice (solid squares with solid lines) compared to that after unloading (Unl) in the same group. Note that the response in the Tt.Ar induced by reloading was inhibited in Igf-1r−/− mice (open diamonds with dashed lines). Base, baseline; n = 9 in the two groups. (B) The increased bone formation rate (BFR/BS) induced by reloading (Rel) seen in Igf-1r+/+ mice was completely blocked on periosteal surfaces, but not on endosteal surfaces, of cortical bone in Igf-1r−/− mice. n = 6 in Igf-1r+/+ mice at baseline, n = 8 in Igf-1r+/+ mice with unloading, n = 9 in Igf-1r+/+ mice with reloading, n = 9 in all the group of Igf-1r−/− mice. Solid lines, Igf-1r+/+ mice; dashed, Igf-1r−/− mice. Data are percent of the results of Igf-1r+/+ mice at baseline and shown as mean ± SE. #,*, Unl vs. Rel in the same genotype; ##, Base vs. Unl in Igf-1r+/+mice; **, Base vs. Unl in Igf-1r−/−mice; #,##,**p < 0.01, *p < 0.05.
Discussion
We provide evidence that IGF-1R in mature osteoblasts is required for periosteal bone expansion and formation induced by reloading using osteoblast specific Igf-1r deficient mice that were hindlimb unloaded and then reloaded. Bone histomorphometry revealed that the augmentation in BFR on periosteal surfaces of cortical bone induced by reloading was completely blocked in Igf-1r−/− mice (Fig. 3B). μCT analysis showed that a reloading-induced increase in Tt.Ar in the cortical diaphysis was also completely blunted in Igf-1r−/− mice (Fig. 3A). Several studies have shown that mechanical loading enhances Igf-1 expression in osteoblasts and osteocytes [20, 21]. However, a direct study determining whether the skeletal response to mechanical loading is mediated by IGF-1 signaling has not been previously demonstrated. We provide the evidence that the IGF-1 signaling in mature osteoblasts has a critical role in periosteal surfaces of cortical bone in response to reloading following unloading. In addition to our previous study [23], this study suggests that enhancing IGF-1 signaling in mature osteoblasts in an astronaut after returning to the earth could promote periosteal bone formation and expansion in cortical bone and mitigate the risk of fracture in the astronaut after completing their long-term spaceflight missions.
The response of endosteal bone formation to reloading was comparable between Igf-1r−/− and Igf-1r+/+ mice (Figs. 3B). The Igf-1r is deleted only in the mature osteoblasts in our mouse model. Thus, the response to reloading in endosteal bone likely occurs in the less mature osteoblasts which express the IGF-1R normally, while the response to loading in periosteal bone requires the IGF-1R in mature osteoblasts. The BFR/BS in endosteal bone was much higher than that in periosteal bone at baseline in both Igf-1r−/− and Igf-1r+/+ mice (data not shown), suggesting that less mature osteoblasts have a dominant role in the high turnover sites in the skeletal response to reloading. The deletion of the Igf-1r in less mature osteoblasts will be useful to test whether the IGF-1R is crucial for reloading-induced endosteal bone formation.
BFR/BS on periosteal bone surfaces declined with unloading in Igf-1r+/+ mice and then increased with reloading (Fig. 3B), along with a reduction and an increase in both MAR and MS/BS, respectively. In contrast, this response of BFR/BS (Fig. 3B), MAR and MS/BS to reloading was completely inhibited in Igf-1r−/− mice, implying that IGF-1R in mature osteoblasts could affect the number of active osteoblasts and the activity osteoblasts of at baseline and in the skeletal response to mechanical load [28].
In conclusion, IGF-1 signaling in mature osteoblasts plays a critical role in periosteal bone formation in the skeletal response to reloading, suggesting that IGF-1 signaling is necessary for accelerating periosteal bone formation following the return to normal gravity and that approaches to enhance and maintain IGF-1 signaling in mature osteoblasts may be beneficial after long-term space missions and prolonged bed rest caused by medical conditions.
Highlights.
We tested the response of osteoblast-specific Igf-1r−/− mice to unloading/reloading.
Increased periosteal bone formation with reloading is blocked in Igf-1r−/− mice.
Increased cortical bone expansion with reloading is inhibited in Igf-1r−/− mice.
Igf-1r in mature osteoblasts mediates reloading-induced periosteal bone formation.
Acknowledgments
Funding sources:
National Space Biomedical Research Institute through NASA NCC 9-58 (TK), NIH RO1 AR055924 (DDB)
Veterans Affairs Research Enhancement Award Program (DDB)
Veterans Affairs Merit Review Program (DDB)
We thank Dr. Thomas L Clemens for providing transgenic mice and the SF-VAMC Bone Imaging Core for μCT analysis. This work was supported by the National Space Biomedical Research Institute through NASA NCC 9-58 (TK), NIH RO1 AR055924 (DDB), the Veterans Affairs Research Enhancement Award Program (DDB) and the Veterans Affairs Merit Review Program (DDB).
Abbreviations
- igf-1r
insulin-like growth factor 1 receptor
- Base
baseline
- Unl
unloading
- Rel
reloading
Footnotes
Conflict of interest:
All authors have no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng. 2006;8:455–498. doi: 10.1146/annurev.bioeng.8.061505.095721. [DOI] [PubMed] [Google Scholar]
- 2.Robling AG, Hinant FM, Burr DB, Turner CH. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J Bone Miner Res. 2002;17:1545–1554. doi: 10.1359/jbmr.2002.17.8.1545. [DOI] [PubMed] [Google Scholar]
- 3.LeBlanc AD, Spector ER, Evans HJ, Sibonga JD. Skeletal responses to space flight and the bed rest analog: a review. J Musculoskelet Neuronal Interact. 2007;7:33–47. [PubMed] [Google Scholar]
- 4.Sievanen H. Immobilization and bone structure in humans. Arch Biochem Biophys. 2010;503:146–152. doi: 10.1016/j.abb.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19:1006–1012. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- 6.Lang TF, Leblanc AD, Evans HJ, Lu Y. Adaptation of the proximal femur to skeletal reloading after long-duration spaceflight. J Bone Miner Res. 2006;21:1224–1230. doi: 10.1359/jbmr.060509. [DOI] [PubMed] [Google Scholar]
- 7.Rittweger J, Felsenberg D. Recovery of muscle atrophy and bone loss from 90 days bed rest: results from a one-year follow-up. Bone. 2009;44:214–224. doi: 10.1016/j.bone.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 8.Allen MR, Hogan HA, Bloomfield SA. Differential bone and muscle recovery following hindlimb unloading in skeletally mature male rats. J Musculoskelet Neuronal Interact. 2006;6:217–225. [PubMed] [Google Scholar]
- 9.Bikle DD, Sakata T, Halloran BP. The impact of skeletal unloading on bone formation. Gravit Space Biol Bull. 2003:45–54. [PubMed]
- 10.Ozcivici E, Luu YK, Adler B, Qin YX, Rubin J, Judex S, Rubin CT. Mechanical signals as anabolic agents in bone. Nat Rev Rheumatol. 2010;6:50–59. doi: 10.1038/nrrheum.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobs CR, Temiyasathit S, Castillo AB. Osteocyte mechanobiology and pericellular mechanics. Annu Rev Biomed Eng. 2010;12:369–400. doi: 10.1146/annurev-bioeng-070909-105302. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Nishida S, Sakata T, Elalieh HZ, Chang W, Halloran BP, Doty SB, Bikle DD. Insulin-like growth factor-I is essential for embryonic bone development. Endocrinology. 2006;147:4753–4761. doi: 10.1210/en.2006-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yakar S, Courtland HW, Clemmons D. IGF-1 and bone: New discoveries from mouse models. J Bone Miner Res. 2010;25:2543–2552. doi: 10.1002/jbmr.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev. 2008;29:535–559. doi: 10.1210/er.2007-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang E, Wang J, Chin E, Zhou J, Bondy CA. Cellular patterns of insulin-like growth factor system gene expression in murine chondrogenesis and osteogenesis. Endocrinology. 1995;136:2741–2751. doi: 10.1210/endo.136.6.7750499. [DOI] [PubMed] [Google Scholar]
- 16.Shinar DM, Endo N, Halperin D, Rodan GA, Weinreb M. Differential expression of insulin-like growth factor-I (IGF-I) and IGF-II messenger ribonucleic acid in growing rat bone. Endocrinology. 1993;132:1158–1167. doi: 10.1210/endo.132.3.8440176. [DOI] [PubMed] [Google Scholar]
- 17.Zhao G, Monier-Faugere MC, Langub MC, Geng Z, Nakayama T, Pike JW, Chernausek SD, Rosen CJ, Donahue LR, Malluche HH, Fagin JA, Clemens TL. Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinology. 2000;141:2674–2682. doi: 10.1210/endo.141.7.7585. [DOI] [PubMed] [Google Scholar]
- 18.Zhang M, Xuan S, Bouxsein ML, von Stechow D, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, Clemens TL. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277:44005–44012. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Nishida S, Boudignon BM, Burghardt A, Elalieh HZ, Hamilton MM, Majumdar S, Halloran BP, Clemens TL, Bikle DD. IGF-I receptor is required for the anabolic actions of parathyroid hormone on bone. J Bone Miner Res. 2007;22:1329–1337. doi: 10.1359/jbmr.070517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lean JM, Jagger CJ, Chambers TJ, Chow JW. Increased insulin-like growth factor I mRNA expression in rat osteocytes in response to mechanical stimulation. Am J Physiol. 1995;268:E318–327. doi: 10.1152/ajpendo.1995.268.2.E318. [DOI] [PubMed] [Google Scholar]
- 21.Reijnders CM, Bravenboer N, Tromp AM, Blankenstein MA, Lips P. Effect of mechanical loading on insulin-like growth factor-I gene expression in rat tibia. J Endocrinol. 2007;192:131–140. doi: 10.1677/joe.1.06880. [DOI] [PubMed] [Google Scholar]
- 22.Sakata T, Halloran BP, Elalieh HZ, Munson SJ, Rudner L, Venton L, Ginzinger D, Rosen CJ, Bikle DD. Skeletal unloading induces resistance to insulin-like growth factor I on bone formation. Bone. 2003;32:669–680. doi: 10.1016/s8756-3282(03)00088-7. [DOI] [PubMed] [Google Scholar]
- 23.Boudignon BM, Bikle DD, Kurimoto P, Elalieh H, Nishida S, Wang Y, Burghardt A, Majumdar S, Orwoll BE, Rosen C, Halloran BP. Insulin-like growth factor I stimulates recovery of bone lost after a period of skeletal unloading. J Appl Physiol. 2007;103:125–131. doi: 10.1152/japplphysiol.00111.2007. [DOI] [PubMed] [Google Scholar]
- 24.Dietrich P, Dragatsis I, Xuan S, Zeitlin S, Efstratiadis A. Conditional mutagenesis in mice with heat shock promoter-driven cre transgenes. Mamm Genome. 2000;11:196–205. doi: 10.1007/s003350010037. [DOI] [PubMed] [Google Scholar]
- 25.Globus RK, Bikle DD, Morey-Holton E. Effects of simulated weightlessness on bone mineral metabolism. Endocrinology. 1984;114:2264–2270. doi: 10.1210/endo-114-6-2264. [DOI] [PubMed] [Google Scholar]
- 26.Morey-Holton E, Globus RK, Kaplansky A, Durnova G. The hindlimb unloading rat model: literature overview, technique update and comparison with space flight data. Adv Space Biol Med. 2005;10:7–40. doi: 10.1016/s1569-2574(05)10002-1. [DOI] [PubMed] [Google Scholar]
- 27.Sessions ND, Halloran BP, Bikle DD, Wronski TJ, Cone CM, Morey-Holton E. Bone response to normal weight bearing after a period of skeletal unloading. Am J Physiol. 1989;257:E606–610. doi: 10.1152/ajpendo.1989.257.4.E606. [DOI] [PubMed] [Google Scholar]
- 28.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 29.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]