Abstract
Pulmonary hypertension (PH) that occurs after left-heart failure (LHF), classified as Group 2 PH, involves progressive pulmonary vascular remodeling induced by smooth muscle cell (SMC) proliferation. However, mechanisms involved in the activation of SMCs remain unknown. The objective of this study was to determine the involvement of peroxynitrite and phosphatase-and-tensin homolog on chromosome 10 (PTEN) in vascular SMC proliferation and remodeling in the LHF-induced PH (LHF-PH). LHF was induced by permanent ligation of left anterior descending coronary artery in rats for 4 weeks. MRI, ultrasound, and hemodynamic measurements were performed to confirm LHF and PH. Histopathology, western blot, and real-time polymerase chain reaction analyses were used to identify key molecular signatures. Therapeutic intervention was demonstrated using an antiproliferative compound, HO-3867. LHF-PH was confirmed by significant elevation of mean pulmonary artery pressure (mean pulmonary artery pressure/mm Hg: 35.9±1.8 versus 14.8±2.0, control; P<0.001) and vascular remodeling. HO-3867 treatment decreased mean pulmonary artery pressure to 22.6±0.8 mm Hg (P<0.001). Substantially higher levels of peroxynitrite and significant loss of PTEN expression were observed in the lungs of LHF rats when compared with control. In vitro studies using human pulmonary artery SMCs implicated peroxynitrite-mediated downregulation of PTEN expression as a key mechanism of SMC proliferation. The results further established that HO-3867 attenuated LHF-PH by decreasing oxidative stress and increasing PTEN expression in the lung. In conclusion, peroxynitrite and peroxynitrite-mediated PTEN inactivation seem to be key mediators of lung microvascular remodeling associated with PH secondary to LHF.
Keywords: HO-3867, left-heart failure, peroxynitrite, phosphatase-and-tensin homolog on chromosome 10, pulmonary hypertension
Pulmonary hypertension (PH) that occurs after left-heart failure (LHF) is a debilitating disease that is associated with significant morbidity and mortality.1,2 LHF-PH originates from a sustained increase of left-atrial pressure (pulmonary venous hypertension) because of left-ventricular (LV) dysfunction resulting in chronic increase of pulmonary arterial pressure, which promotes remodeling of the arterial wall, including abnormalities of elastic fibers, intimal fibrosis, and medial hypertrophy. These changes, which are largely progressive and irreversible, lead to vascular stiffness and decrease in pulmonary vasodilatory responsiveness to therapies.1 In addition, the fixed PH with pulmonary vascular resistance >3 Wood units is linked with a higher posttransplantation mortality and hence considered a contraindication for heart transplantation.3 Of the total number of deaths after heart transplantation, ≈20% are attributed to fatal right-ventricular failure.3 Because PH is usually diagnosed at a late stage, a proper understanding of the underlying pathobiology of the disease progression is necessary for designing suitable interventional approaches at an early stage before irreversible pathological changes occur in the pulmonary vascular bed. Currently available treatment options, such as inhaled NO, nitrates, calcium-channel blockers, prostanoids, endothelin-receptor antagonists, statins, PPARγ activators, and phosphodiesterase inhibitors, despite their beneficial effects on PH, have adverse systemic effects.4,5
A phosphatase-and-tensin homolog on chromosome 10 (PTEN), is a modulator of PI3K activity associated with PH-mediated vascular remodeling.6,7 PTEN is a dual-function phosphatase capable of dephosphorylating both lipids and proteins and it negatively regulates PI3K pathway by dephosphorylating phosphatidyl-inositol-triphosphate and pAkt, and hence regulates apoptosis and cell-proliferation pathways. Downregulation of PTEN is linked to smooth muscle cell (SMC) proliferation and vascular remodeling in animal models of arterial restenosis,6–8 PH,6 and lung fibrosis.8,9 Recently, we have reported a significant reduction in PTEN levels in the lung tissues of rats with PH induced by monocrotaline or exposure to hypoxia.7 Reduction in PTEN inversely correlated with pAkt suggesting the involvement of PTEN-mediated modulation of PI3K pathway in the progression of PH. A link between PH and PTEN has been reported in a patient diagnosed with a mutation in PTEN gene (Cowden syndrome).10 This case report speculated that the patient’s possible disposition to developing PH was secondary to the loss of PTEN activity and function. However, the precise role and involvement of PTEN in PH-associated vascular alterations in the general population, especially in PH secondary to LHF has not been determined.
Peroxynitrite, a potent oxidant formed by the reaction of superoxide with NO, has been shown to stimulate vascular SMC proliferation at low concentrations (<2 µmol/L) through a mechanism involving peroxynitrite-mediated activation of ERK and PKC.11,12 Using immunohistochemistry to localize nitrotyrosine, Bowers et al13 showed ubiquitous presence of peroxynitrite in the lung tissue of patients with severe PH. This clinical observation along with the in vitro results of Agbani et al12 on peroxynitrite-induced proliferation of SMCs suggests a possible link between peroxynitrite (oxidant) and the pathogenesis of PH. Despite numerous reports implicating the involvement of reactive oxygen species (ROS) in the pathogenesis of myocardial infarction and heart failure, little is known about the involvement of ROS in the progression of PH associated with heart failure. Hence, we sought to determine whether LHF-PH is mediated by reactive oxidants, leading to a PTEN-dependent vascular SMC proliferation in the lung. We hypothesized that peroxynitrite is a key mediator of SMC proliferation via PTEN signaling. We used an in vivo rat model of LHF-PH induced by permanent ligation of the left anterior descending (LAD) coronary artery. Human pulmonary artery SMCs (PASMC) were used to study the mechanism by which peroxynitrite modulates PTEN and induces cell proliferation. Furthermore, we also tested a novel therapeutic intervention using a unique compound HO-3867, with both antiproliferative and antioxidant properties and known to upregulate PTEN.14,15 The results showed the involvement of reactive oxidants including peroxynitrite and PTEN in the development of LHF-PH and demonstrated a potential therapeutic approach for the management of PH associated with LHF.
Materials and Methods
A more detailed Materials and Methods section is provided in the online-only Data Supplement.
Reagents
HO-3867 was synthesized as reported.16
Experimental Animals
LHF was induced in male Sprague-Dawley rats (body weight: 225–250 g; Harlan Laboratories, South Easton, MA) by permanent ligation of LAD coronary artery. All procedures were performed with the approval of the Institutional Animal Care and Use Committee of The Ohio State University and conformed to the Guide for Care and Use of Laboratory Animals (National Institutes of Health publication 8th edition, 2011).
Treatment With HO-3867
HO-3867 was administered in the diet (100 ppm; Harlan-Teklad Laboratory Animal Diets, South Easton, MA), beginning day 1 after LAD coronary artery ligation, and continued for the entire treatment period of 4 weeks. The 100-ppm dose of HO-3867 was based on our earlier studies that used oral administration of this compound in mice.17,18
In Vitro Studies
Human PASMCs were obtained from Lonza (Walkersville, CA).
Statistical Analysis
Data were expressed as mean±SD for all groups. Statistical analyses were performed using unpaired Student t test for comparing 2 groups or 1-way ANOVA with Fisher LSD post hoc test for comparing multiple groups. Differences between groups were considered significant at P<0.05.
Results
LHF Promotes Pulmonary Vascular Remodeling and Downregulation of PTEN in the Lung
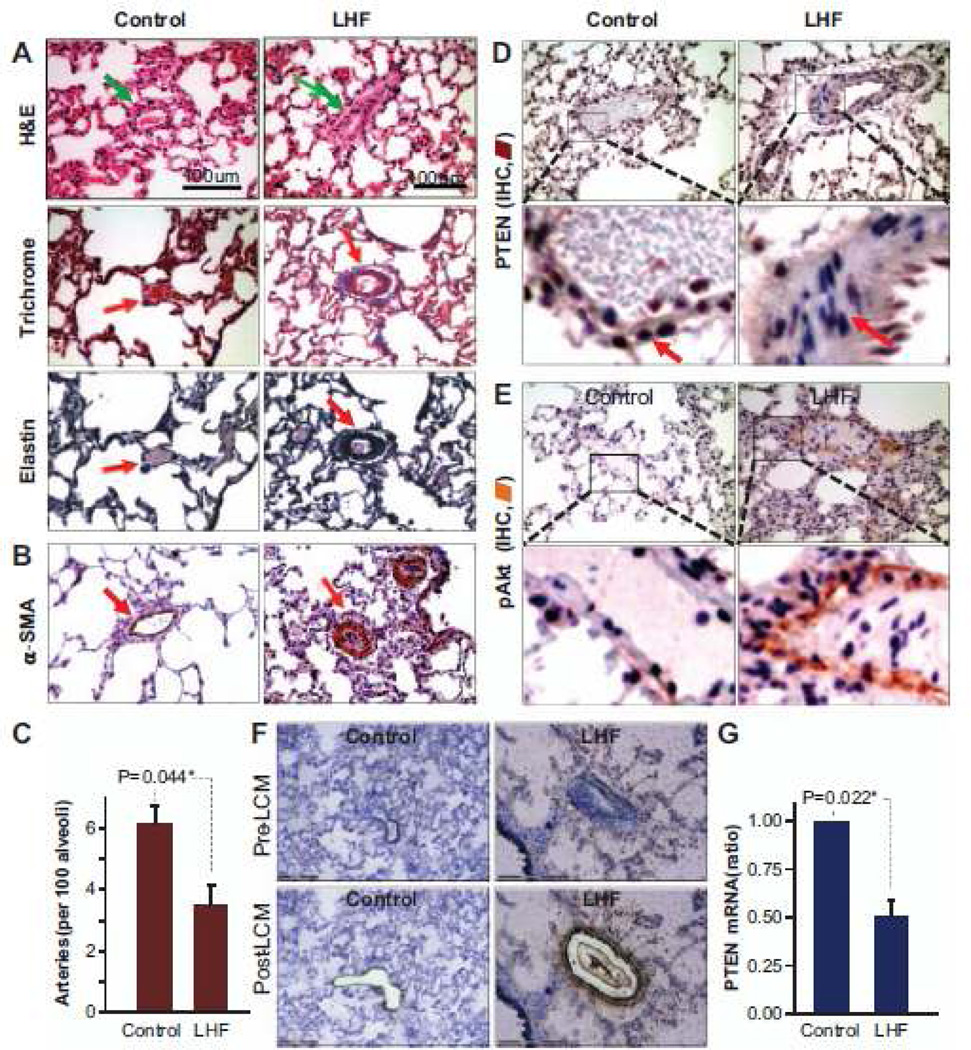
LHF was induced in rats by permanent occlusion of LAD coronary artery for 4 weeks. Cardiac dysfunction and development of PH were confirmed by MRI, echocardiography, and hemodynamic measurements (Figure S1 in the online-only Data Supplement). Histopathology and immunohistochemistry were performed to confirm pulmonary vascular remodeling and to identify the involvement of PTEN and other key downstream proteins in the development of PH secondary to LHF. H&E, Masson trichrome, and elastin staining showed the hallmarks of PH-mediated vascular remodeling including medial thickening, luminal narrowing, perivascular fibrosis, muscularization of the peripheral small arteries, and vascular smooth muscle hyperplasia in the tunica media (Figure 1A). Staining for α-smooth muscle actin, which is a specific marker for vascular SMCs, indicated capillary vessel-wall (medial) thickening (Figure 1B). There was a significant reduction in the peripheral distribution of pulmonary small arteries in the lungs of LHF rats (Figure 1C). Immunohistochemical staining of lung tissues identified extensive loss of PTEN and abundance of pAkt in the pericytes of the tunica intima in the LHF group (Figure 1D and 1E). Real-time polymerase chain reaction analysis of lung vascular cells isolated using laser-capture microdissection (Figure 1F) showed a decrease in the expression of PTEN mRNA in the lungs of LHF group (Figure 1G). Overall, histopathology confirmed the development of vascular remodeling and downregulation of PTEN in the lungs of rats with LHF.
Figure 1.
Left-heart failure leads to pulmonary vascular remodeling and loss of phosphatase-and-tensin homolog on chromosome 10 (PTEN). Histopathology, immunohistochemical analysis, and laser-capture microdissection (LCM) were performed on lung tissues collected from rats 4 weeks after left anterior descending coronary artery ligation. A, Representative images of H&E, Masson trichrome and elastin staining of lung sections show extensive muscularization of the capillary vessel walls in the left-heart failure (LHF) group indicative of vascular remodeling. B, α-smooth muscle actin (α-SMA)-staining indicates capillary vessel-wall thickening in the lung. C, Blood-vessel count shows a decrease in the capillaries in the lungs of LHF rats when compared with control group (mean±SD; n=3 lungs, with 3 slides/lung). D, Representative immunohistochemical staining images (×20 and zoomed view) of the lung show decreased PTEN (D) and increased pAkt expression (E) in the vessel walls of the LHF group. F, Images show the contour of vascular tissue region extracted by LCM for analysis. G, Real-time PCR analysis of the samples collected by LCM show a significant decrease in PTEN expression in the LHF group when compared with control group (mean±SD; n=3 lungs). *P<0.05. These results demonstrate the substantial remodeling of the pulmonary vasculature and loss of PTEN expression after left-heart failure. IHC indicates.
Peroxynitrite Induces Cell Proliferation and Downregulation of PTEN in PASMCs
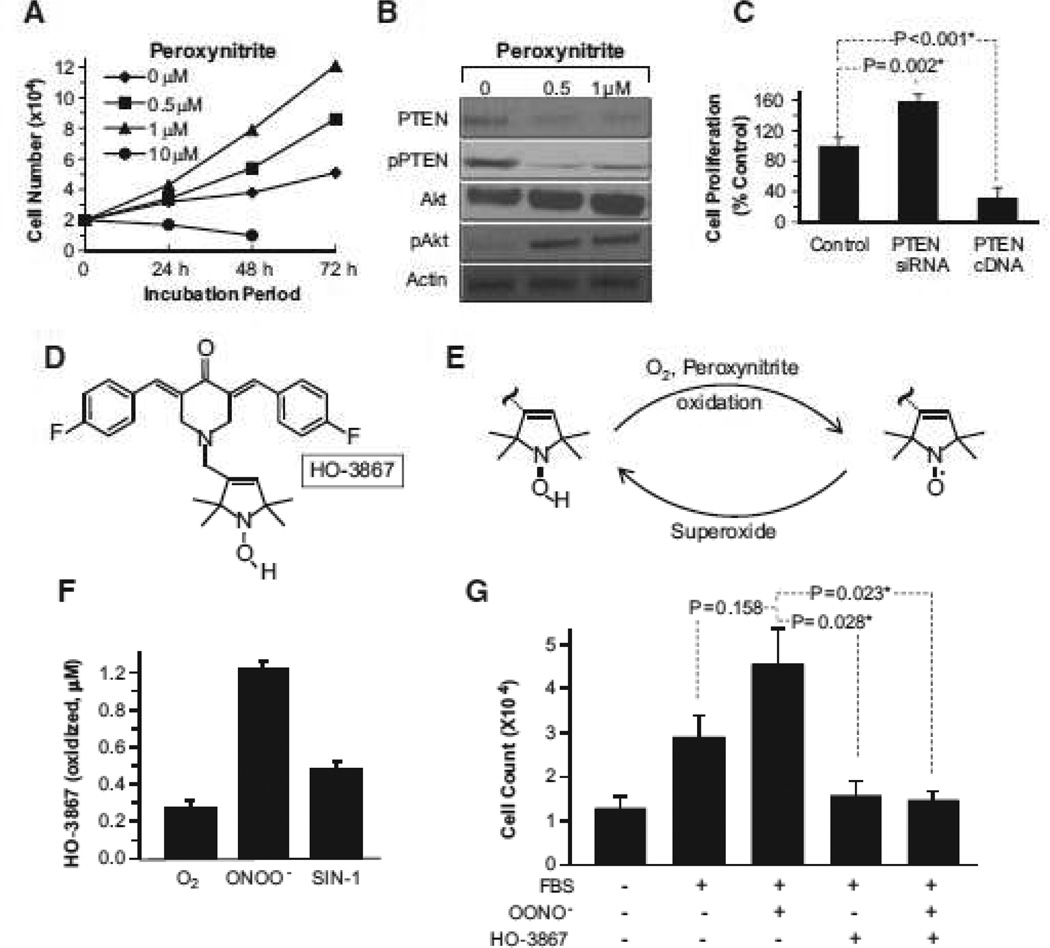
Because PH is associated with abundant production of superoxide in the lung parenchyma,17–19 as well as increased SMC proliferation, we hypothesized that peroxynitrite production in the lungs of LHF-PH modulates the molecular pathways associated with PASMC proliferation. PASMCs were treated with a single, bolus dose of 0.5 µmol/L or 1 µmol/L peroxynitrite. At 48 and 72 hours there was an increase in PASMC proliferation and decreased expression of PTEN and pPTEN and an increase in Akt (total) and pAkt (Ser473; Figure 2A and 2B). The involvement of PTEN in PASMC proliferation was confirmed using PTEN small interfering RNA and PTEN cDNA transfection studies (Figure 2C). We determined the effect of HO-3867 (Figure 2D), a potent antioxidant and PTEN-stabilizing agent,15 on the peroxynitrite-induced proliferation of PASMCs. In cells, HO-3867 undergoes a 1-electron reversible redox conversion to its nitroxide form in the presence of oxidants, such as superoxide and peroxynitrite (Figure 2E). HO-3867 scavenges peroxynitrite and becomes oxidized to nitroxide (Figure 2F), which is readily detectable by electron paramagnetic resonance spectroscopy. The nitroxide metabolite, in turn, is capable of scavenging superoxide anion radicals (SOD-mimetic).14 Addition of HO-3867 to PASMCs in the presence of fetal bovine serum, significantly inhibited peroxynitrite-mediated proliferation (Figure 2G). These results established that low levels of peroxynitrite promote PASMC proliferation, via downregulation of PTEN. Furthermore, HO-3867 is capable of scavenging peroxynitrite, and thereby inhibits the peroxynitrite-induced cell proliferation.
Figure 2.
Effect of peroxynitrite, phosphatase-and-tensin homolog on chromosome 10 (PTEN), and HO-3867 on human pulmonary artery smooth muscle cell (PASMC) proliferation. In vitro studies were conducted using human PASMCs. A, Effect of peroxynitrite on the proliferation of PASMCs. The data show peroxynitrite induces proliferation at lower concentrations (0.5 and 1.0 µmol/L) and seems to be cytotoxic at higher (10 µmol/L) concentration. B, Western blot images show a decrease in the expression of PTEN and pPTEN with a concomitant increase in Akt and pAkt in PASMCs exposed to peroxynitrite. C, Cell-proliferation assay (mean±SD; n=3) of PASMCs transfected with PTEN small interfering RNA (siRNA) or PTEN cDNA indicates the involvement of PTEN in proliferation *P<0.05. D, Molecular structure of HO-3867. E, Redox conversion between the hydroxylamine and nitroxide forms of HO-3867 induced by oxidants. F, Peroxynitrite-induced oxidation of HO-3867 to nitroxide using a single, bolus dose of preformed peroxynitrite solution (100 µmol/L) or SIN-1 (100 µmol/L), a peroxynitrite-releasing agent. Data represent mean±SD (n=4). G, HO-3867 inhibits peroxynitrite-induced proliferation of PASMCs stimulated by fetal bovine serum (FBS; mean±SD; n=3; *P<0.05). The results show that HO-3867 is capable of scavenging peroxynitrite and inhibiting peroxynitrite-induced smooth muscle cell proliferation.
HO-3867 Attenuates the Progression of LHF-PH
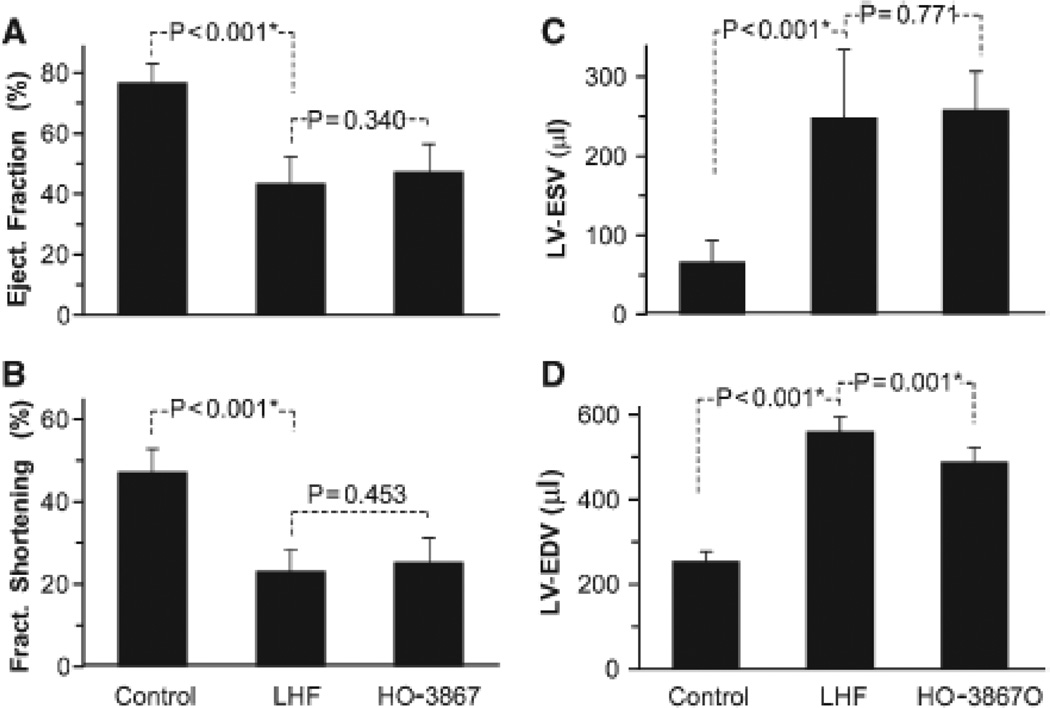
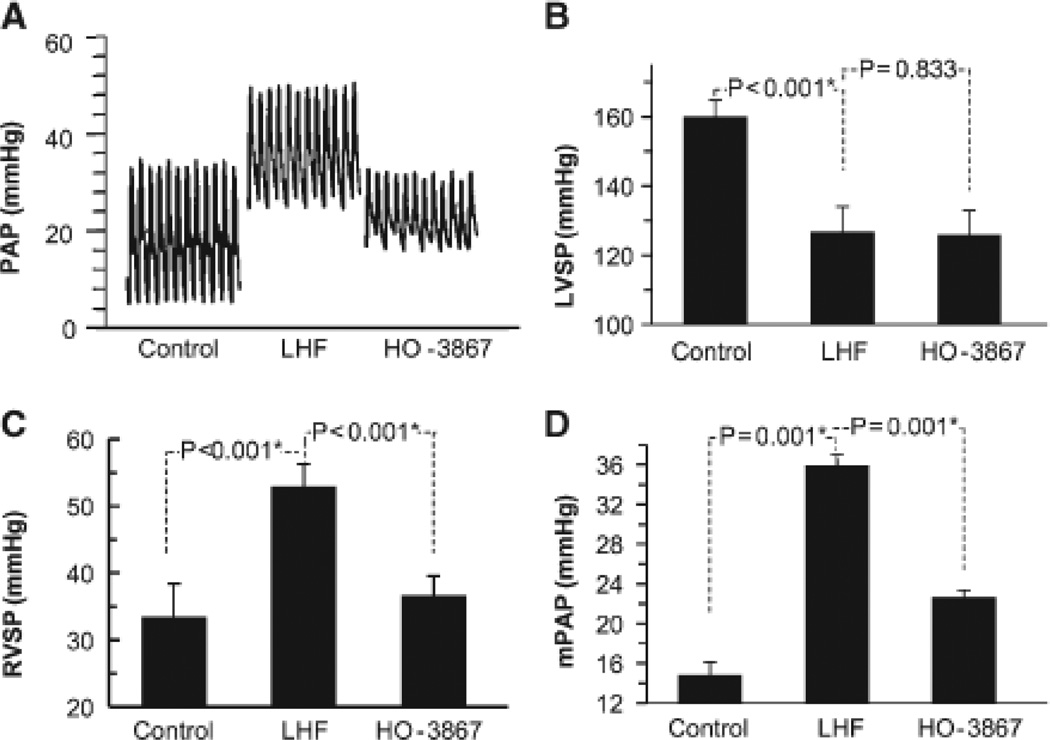
The effect of HO-3867 on LHF-PH was studied on separate groups (cf, Figure S1) of rats subjected to LAD artery occlusion. Wet-weight measurements showed an increase of lung, heart, and LV mass on LAD occlusion for 4 weeks (Figure S2). HO-3867 treatment significantly blunted the increase in lung weight, but had no effect on heart or LV weight. Echocardiography showed that HO-3867 treatment did not have any significant effect on the LV dysfunction observed in the LHF group (Figure 3). However, hemodynamic measurements showed a significant attenuation of right-ventricular systolic pressure and mean pulmonary artery pressure elevation induced by LHF (Figure 4). HO-3867 did not have any effect on LV systolic pressure. Taken together, the functional and hemodynamic data indicated that although HO-3867 had no effect on LV function it attenuated the progression of PH secondary to LHF.
Figure 3.
Effect of HO-3867 on cardiac function. Left-heart failure (LHF) was induced in rats by permanent ligation of left anterior descending coronary artery for 4 weeks. HO-3867 was administered in the feed throughout this period. Echocardiography measurements were performed at the end of 4 weeks postligation. A, Echocardiography results of left-ventricular (LV) ejection fraction, (B) LV fractional shortening, (C) LV end-systolic volume (LV-ESV), and (D) LV end-diastolic volume (LV-EDV; mean±SD; 8 animals/group). *P<0.05. The results show no significant beneficial effect of HO-3867 on left-heart failure.
Figure 4.
HO-3867 attenuates left-heart failure (LHF)-induced increase in pulmonary artery pressure. LHF was induced in rats by permanent ligation of left anterior descending coronary artery for 4 weeks. HO-3867 was administered in the feed throughout the period. Hemodynamic (pressure) measurements were performed at the end of 4 weeks postligation. A, Representative tracings of the pulmonary artery pressure (PAP). B, Left-ventricular systolic pressure (LVSP). C, Right-ventricular systolic pressure (RVSP). D, Mean pulmonary arterial pressure (mPAP). Data represent mean±SD; n=6 animals/group. *P<0.05. The results show HO-3867 significantly attenuates the increase in mPAP observed in the LHF group.
LHF Generates Superoxide and Peroxynitrite in the Lung
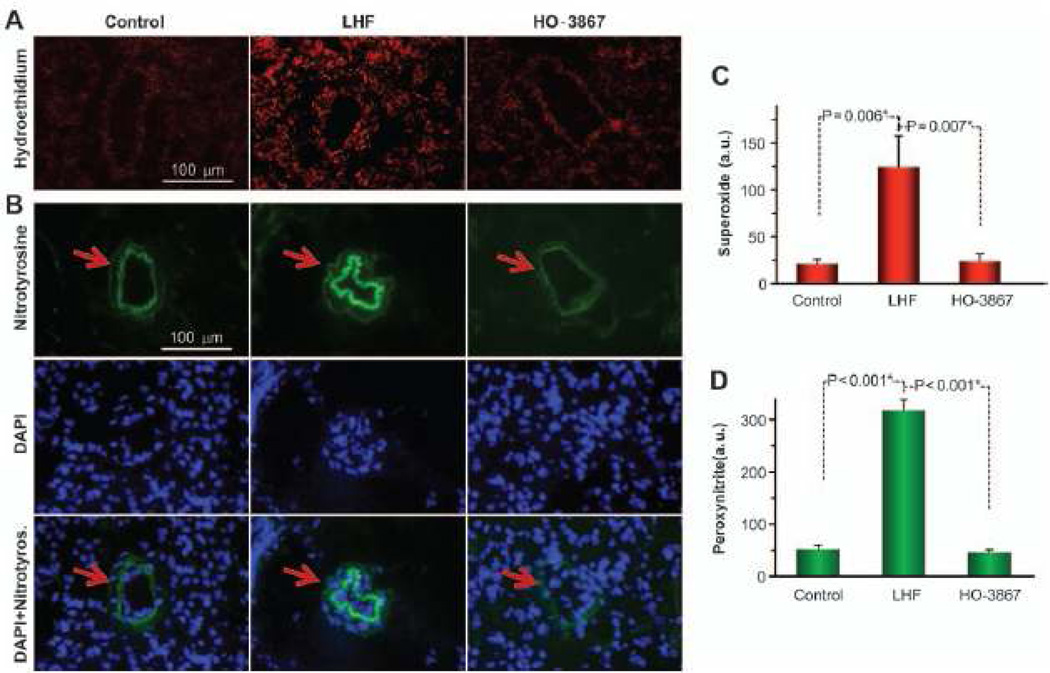
DHE and nitrotyrosine-immunostaining images of lung tissue sections showed an increase in superoxide and peroxynitrite production in the LHF group when compared with the control (Figure 5). The intensity data showed a significant increase in the levels of both superoxide and peroxynitrite in LHF group when compared with baseline (control) lungs. HO-3867 almost completely abolished the elevation in superoxide and peroxynitrite levels in lung tissues caused by LHF, further establishing the role of HO-3867 as an antioxidant.
Figure 5.
Left-heart failure (LHF) induces oxidant production in the lung. LHF was induced in rats by permanent ligation of the left anterior descending coronary artery. HO-3867 was administered in the feed throughout the period. Lung tissues, collected at the end of 4 weeks of ligation, were stained for the determination of oxidant (superoxide) and nitrotyrosine (as surrogate of peroxynitrite). A, Representative images of dihydroethidium staining show a higher level of superoxide production in the lungs of LHF group when compared with the control and HO-3867 groups. B, Representative immunostaining images of nitrotyrosine along with nuclear staining by DAPI show the peroxynitrite levels in the different groups. C, Quantitative analysis of dihydroethidium staining shows a significant increase in superoxide production in the LHF group which is largely attenuated by HO-3867 treatment (mean±SD; n=3 rats with 3 slides/rat). D, Quantitative analysis of the nitrotyrosine images show an increased level of peroxynitrite in the LHF group that is significantly attenuated on HO-3867 (mean±SD; n=3 rats with 3 slides/rat). *P<0.05. The results indicate a substantial production of oxidants in the lungs of the LHF group.
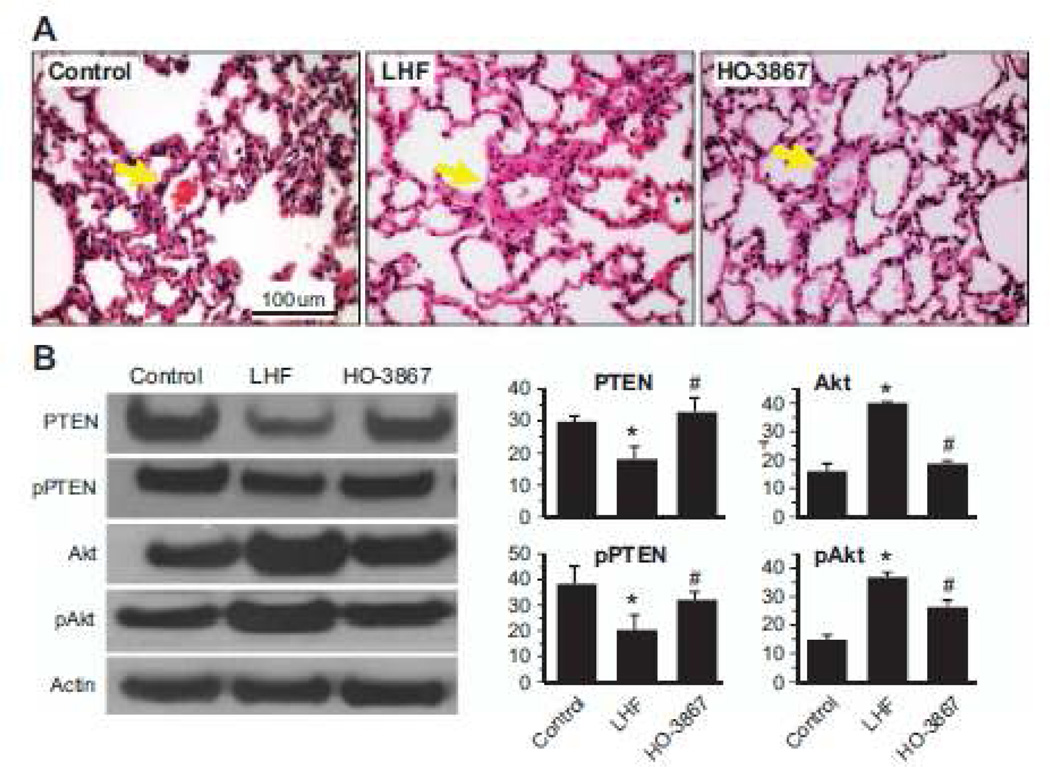
HO-3867 Inhibits Pulmonary Vascular Remodeling by Restoration of PTEN Activity
We next determined the effect of HO-3867 on pulmonary vascular remodeling. H&E staining of HO-3867-treated lungs showed a marked decrease in medial thickening and luminal narrowing that was seen in the untreated LHF lungs (Figure 6A). The results suggested the efficacy of HO-3867 in attenuating the pulmonary vascular remodeling. Western blot analysis of excised whole lung tissues showed a significant decrease in the activity of PTEN in the lungs of LHF rats when compared with control. HO-3867 treatment almost completely restored both the total and active PTEN levels (Figure 6B). It should be noted that the ratio of pPTEN/PTEN or pAkt/Akt did not change (data not shown) on treatment suggesting that both the unphosphorylated and phosphorylated forms are similarly affected. The results showed that loss of PTEN and upregulation of Akt in and treatment with HO-3867 significantly restored PTEN levels and caused downregulation of Akt.
Figure 6.
HO-3867 inhibits vascular remodeling by restoration of phosphatase-and-tensin homolog on chromosome 10 (PTEN) activity in left-heart failure (LHF)-mediated pulmonary hypertension (PH). A, Representative images of H&E staining of lung sections show extensive muscularization of the capillary vessel walls in the LHF group indicative of vascular remodeling in comparison with the control group. The images also show a decrease in vascular remodeling on treatment with HO-3867. The results demonstrate the ability of HO-3867 in decreasing the luminal narrowing and vascular smooth muscle cell hyperplasia associated with LHF-PH. B, Western blot assays were performed on lung tissues collected after 4 weeks of left anterior descending artery ligation. Representative Western blot images and densitometric analysis of total and phosphorylated form of PTEN and Akt, normalized with respect to loading control (Actin), are shown. The data represent mean±SD; n=5; *P<0.05 versus Control; #P<0.05 versus LHF. The results show a significant downregulation of antiproliferative signaling molecule pPTEN and upregulation of prosurvival protein pAkt in the LHF group which is mitigated by HO-3867.
Discussion
PH, regardless of its cause, is a debilitating disease with no effective therapies. The results of the current study provided 2 significant and important conclusions: (1) peroxynitrite and peroxynitrite-mediated PTEN dysregulation seems to be key mediators of pulmonary vascular remodeling; and (2) HO-3867, a curcumin analog with novel antioxidant/antiproliferative properties, is effective in targeting the peroxynitrite/PTEN pathway for the treatment of PH and associated vascular alterations.
PTEN has been previously implicated in the negative regulation of SMC proliferation involved with vascular remodeling.15,19–21 PTEN was significantly downregulated in the lung tissues of rats administered with monocrotaline or exposed to chronic hypoxia.7 In the current study, immunohistochemical staining showed loss of PTEN expression in the lungs of LHF-PH rats. Laser-capture microdissection and real-time polymerase chain reaction results established that the PH-mediated alterations in PTEN were particularly localized to the vascular SMCs of the small- and medium-sized arteries in the lung. Our results are in agreement with a recent study by Nisbet et al22 which showed a significant reduction in the expression of PTEN in the lungs of mice with PH induced by exposure to hypoxia. The study further showed that treatment of mice with rosiglitazone, an activator of PPARγ, could largely restore PTEN levels and attenuate PH suggesting the involvement of PTEN in the progression of vascular remodeling. As a corroborative evidence for the role of PTEN, immunohistochemical staining and protein data from our experiments further showed that Akt, a downstream proliferative signaling protein of the PTEN antagonist PI3K, was markedly activated in the lungs of LHF-PH rats. This relation between PTEN and Akt is similar to that observed in our previous studies on the monocrotaline and hypoxia models of PH.7
Oxidative stress, characterized by increased production of ROS, has been implicated as a primary contributor to the pathogenesis of PH.23 Production of ROS has been reported in the lung biopsy samples taken from patients with severe PH13 and in several rodent models of PH induced by monocrotaline24–26 or hypoxia.22,27–29 The causal link between oxidative stress and progression of PH has been established by many studies, which have shown that antioxidant treatment prevented the induction of PH.25 The current study clearly demonstrated the generation of oxidants in the lungs of heart failure rats. The involvement of ROS is also evident from the fact that LHF rats fed with HO-3867, a scavenger of superoxide and peroxynitrite, showed marked decrease in the oxidant levels in the lung. Nisbet et al,22 using a mouse model of chronic hypoxia showed the generation of superoxide radicals via NADPH oxidase pathway. They further showed that activation of PPARγ by rosiglitazone blunted superoxide radical generation by inhibiting the chronic hypoxia-stimulated Nox4 expression in pulmonary vascular endothelial cells. Taken together, our results and that of others indicate that antioxidant therapy, targeting either the inhibition or scavenging of ROS in the lung vasculature could be beneficial for the treatment of PH.
Our in vitro results indicate that low doses of peroxynitrite induces proliferation of human PASMCs. Agbani et al30 have demonstrated that bovine PASMCs stimulated intracellular peroxynitrite formation on exposure to acute hypoxia. They have further shown that peroxynitrite at concentrations <2 µmol/L was capable of stimulating cell proliferation in pulmonary artery endothelial and SMCs.11,12 Our western blot data indicate that peroxynitrite, at low concentrations, downregulates the expression and activity of PTEN and that PTEN negatively regulates cell proliferation. These studies provide a direct link between peroxynitrite and SMC proliferation involving the activation of PTEN. Although our results and that of Delgado-Esteban et al31 indicate a correlation between peroxynitrite and PTEN activity, the mechanism by which PTEN protein or its activity is degraded by peroxynitrite is yet to be determined. However, it is reasonable to assume that a mechanism similar to that reported32 for hydrogen peroxide-induced reversible inactivation of PTEN could be responsible and operative in the peroxynitrite-PTEN pathway.
HO-3867 is a synthetic analog of curcumin, the principal component of the popular Indian spice turmeric (the rhizome of Curcuma Longa). HO-3867 was designed, synthesized, and characterized in our laboratories.16 It is a dual-acting molecule with targeted antiproliferative and antioxidant capabilities.14 Its antiproliferative activity has been evaluated using a number of cancer cell lines,14 murine xenograft tumors,17,33,34 serum-stimulated SMCs,15 and balloon injury-mediated carotid artery restenosis in rats.15 The compound, usually administered as a dietary supplement, is readily bio-absorbed and retained in tissues for 24 hours.35 HO-3867 is capable of scavenging superoxide radicals (SOD-mimetic), peroxyl radicals,14 and peroxynitrite (this work). Under normoxic conditions, such as those occurring in normal (healthy) tissues, HO-3867 functions as an antioxidant and protects cells from ROS-mediated oxidative damage. Most importantly, data from our laboratory have indicated that HO-3867 is nontoxic to healthy tissues, including heart.18 In the current study, we observed that HO-3867 treatment did not have any grossly discernible effect on heart/LV weight or function. This observation is in contrast to the study of Morimoto et al36 that showed curcumin to have a significant effect on improving cardiac function in rats 7 weeks after MI. The observed difference on their cardioprotective effect could be due to the structural modification or differences in dosing and post-MI duration. However, HO-3867 significantly attenuated the elevation of pulmonary arterial pressure, blunted oxidant levels in lung, and restored key signaling proteins involved in the control of vascular remodeling. The causative role of superoxide in inducing hypoxia-mediated PH has been previously determined by administering antioxidants or overexpressing superoxide-scavenging enzymes such as extracellular superoxide dismutase.28,29
A recent study by Chen et al,37 using a mouse model of PH induced by transverse aortic constriction, showed that lung fibrosis, leukocyte infiltration, and vascular remodeling, but not lung edema, were mainly responsible for heart failure-induced PH. This report, as well as those of other groups using different models of heart failure,38,39 highlighted the importance of targeting pathways involved in lung remodeling for the treatment of PH secondary to heart failure. In our study, we did not observe any significant increase in the ratio of lung wet-weight to dry-weight (data not shown) suggesting that lung edema is not a causative factor in the pathogenesis of PH. The substantial increase in vascular wall-thickening and remodeling observed in the LHF group and their attenuation by HO-3867 in our study seem to suggest that HO-3867 treatment targets the mechanisms related to vascular remodeling. Based on the existing knowledge and results of this study, we proposed a mechanistic pathway involved in the progression of PH secondary to LHF.
To our knowledge, this is the first report of the involvement of oxidative stress in the lung in the development and progression of PH secondary to heart failure. We have identified peroxynitrite as a potential inducer of SMC proliferation by downregulation of PTEN and hence may play a causative role in vascular remodeling associated with the progression of PH. This unique pathway is shown to be a potential target for HO-3867, which seems to play multiple roles in attenuating oxidative stress and inhibiting vascular remodeling in LHF-PH. Although the exact mechanism of the effect of HO-3867 on PH is yet to be elucidated, our data indicate the importance of blunting ROS generation in the lung to inhibit this progressive disorder. Nevertheless, the current study has identified a potential therapeutic approach targeting multiple mechanisms in a preclinical model of PH associated with LHF.
Perspectives
Despite numerous medical and surgical therapeutic options for the management of heart failure, treatments for PH that occurs secondary to heart failure are limited. The primary PH, such as pulmonary arterial hypertension, has been the focus of a large number of investigations leading to drug discovery and clinical trials, the secondary PH due to heart failure has received little attention. At present, there is limited rationale or hope that the same therapeutic options will be effective for treating heart failure patients with PH. Our study clearly implicates the involvement of oxidative stress in the progression of PH secondary to LHF. We show the generation of peroxynitrite, a potent oxidant, in the lungs of heart failure rats. We further demonstrate downregulation of PTEN in the pulmonary arteries resulting in vascular remodeling and vasoconstriction. The findings will enable us in developing targeted therapies for the management of PH. We have also identified a unique multifunctional compound, HO-3867, which attenuates the progression of PH by targeting the antioxidant and antiproliferative pathways. Our future work will explore the specific mechanism of action of HO-3867 in preventing the development of PH and also study the effect of HO-3867 in improving the survival of rats with preexisting PH in comparison with drugs currently used in the management of PH.
Supplementary Material
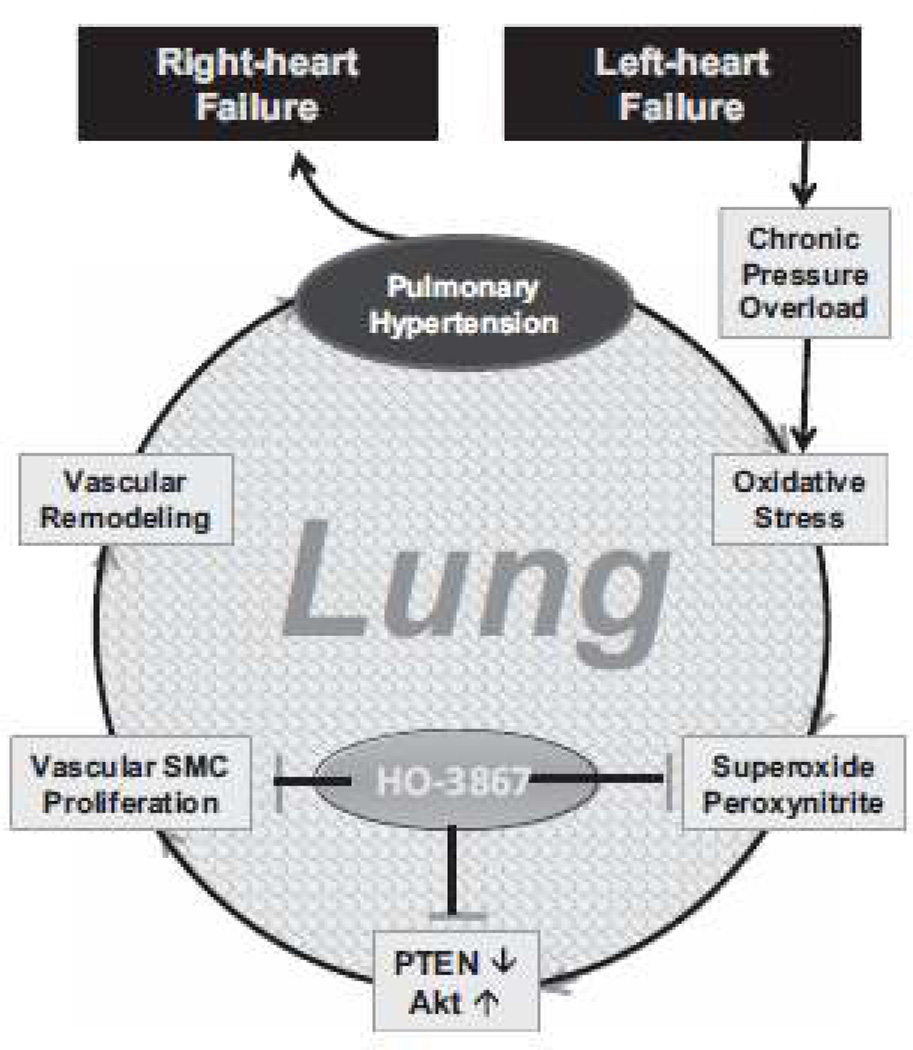
Figure 7.
Illustration of the mechanisms involved in the pathogenesis and progression of pulmonary hypertension (PH) secondary to left-heart failure. Chronic elevation of pulmonary artery pressure, due to after-load pressure from failing left-ventricle, results in oxidative stress and production of reactive oxidants in the lung microvasculature. The oxidants then trigger a cascade of molecular events involving phosphatase-and-tensin homolog on chromosome 10 (PTEN) and leading to smooth muscle cell (SMC) proliferation, vascular remodeling, vasoconstriction, and PH progression, eventually causing RV failure. HO-3867 inhibits the proliferation of LHF-PH at multiple levels (1) scavenging of reactive oxidants, (2) restoring PTEN activity, (3) inhibiting SMC proliferation.
Novelty and Significance.
What Is New?
The study establishes, for the first time, that oxidative stress, particularly peroxynitrite, mediates vascular remodeling by downregulation of PTEN in pulmonary hypertension associated with left-heart failure.
We further report that, a novel compound, HO-3867, with multifunctional activity including antioxidant and antiproliferative properties attenuates the progression of PH secondary to left-heart failure.
What Is Relevant?
PH is a debilitating disease and involves the interactions of numerous molecular pathways in the development and progression of the disease.
PH is associated with significant mortality and morbidity as the elevated pulmonary arterial pressure further complicates the management of congestive heart failure.
There is no known therapy to treat PH.
Summary
We conclude that peroxynitrite-mediated downregulation of PTEN induces lung microvascular remodeling in PH secondary to LHF and HO-3867 limits the progression of PH.
Acknowledgments
The authors thank Uksha Saini, Lakshmi Kuppusamy, and Brian Rivera for their technical assistance with and in vitro cell studies and in vivo drug-treatment.
Sources of Funding
This work was supported by funding from National Institutes of Health grants EB006153 and HL095066 (to P. Kuppusamy) and Hungarian National Research fund OTKA K81123 (to K. Hideg).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
The online-only Data Supplement is available with this article at http://hyper.ahajournals.org/lookup/suppl/doi:10.1161/HYPERTENSIONAHA.111.00514/-/DC1.
References
- 1.Guazzi M, Arena R. Pulmonary hypertension with left-sided heart disease. Nat Rev Cardiol. 2010;7:648–659. doi: 10.1038/nrcardio.2010.144. [DOI] [PubMed] [Google Scholar]
- 2.Guglin M, Khan H. Pulmonary hypertension in heart failure. J Card Fail. 2010;16:461–474. doi: 10.1016/j.cardfail.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Stobierska-Dzierzek B, Awad H, Michler RE. The evolving management of acute right-sided heart failure in cardiac transplant recipients. J Am Coll Cardiol. 2001;38:923–931. doi: 10.1016/s0735-1097(01)01486-3. [DOI] [PubMed] [Google Scholar]
- 4.Umar S, Steendijk P, Ypey DL, Atsma DE, van der Wall EE, Schalij MJ, van der Laarse A. Novel approaches to treat experimental pulmonary arterial hypertension: a review. J Biomed Biotechnol. 2010;2010:702836. doi: 10.1155/2010/702836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryerson CJ, Nayar S, Swiston JR, Sin DD. Pharmacotherapy in pulmonary arterial hypertension: a systematic review and meta-analysis. Respir Res. 2010;11:12. doi: 10.1186/1465-9921-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goncharova EA, Ammit AJ, Irani C, Carroll RG, Eszterhas AJ, Panettieri RA, Krymskaya VP. PI3K is required for proliferation and migration of human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2002;283:L354–L363. doi: 10.1152/ajplung.00010.2002. [DOI] [PubMed] [Google Scholar]
- 7.Ravi Y, Selvendiran K, Meduru S, Citro L, Naidu S, Khan M, Rivera BK, Sai-Sudhakar CB, Kuppusamy P. Dysregulation of PTEN in cardiopulmonary vascular remodeling induced by pulmonary hypertension. Cell Biochem Biophys. 2011 doi: 10.1007/s12013-011-9332-z. (EPub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fagone E, Conte E, Gili E, Fruciano M, Pistorio MP, Lo Furno D, Giuffrida R, Crimi N, Vancheri C. Resveratrol inhibits transforming growth factor-β-induced proliferation and differentiation of ex vivo human lung fibroblasts into myofibroblasts through ERK/Akt inhibition and PTEN restoration. Exp Lung Res. 2011;37:162–174. doi: 10.3109/01902148.2010.524722. [DOI] [PubMed] [Google Scholar]
- 9.White ES, Atrasz RG, Hu B, Phan SH, Stambolic V, Mak TW, Hogaboam CM, Flaherty KR, Martinez FJ, Kontos CD, Toews GB. Negative regulation of myofibroblast differentiation by PTEN (Phosphatase and Tensin Homolog Deleted on chromosome 10) Am J Respir Crit Care Med. 2006;173:112–121. doi: 10.1164/rccm.200507-1058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Natali D, Girerd B, Montani D, Soubrier F, Simonneau G, Humbert M, Sitbon O. Pulmonary arterial hypertension in a patient with Cowden syndrome and anorexigen exposure. Chest. 2011;140:1066–1068. doi: 10.1378/chest.10-2588. [DOI] [PubMed] [Google Scholar]
- 11.Agbani E, Coats P, Wadsworth RM. Threshold of peroxynitrite cytotoxicity in bovine pulmonary artery endothelial and smooth muscle cells. Toxicol In Vitro. 2011;25:1680–1686. doi: 10.1016/j.tiv.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Agbani EO, Coats P, Mills A, Wadsworth RM. Peroxynitrite stimulates pulmonary artery endothelial and smooth muscle cell proliferation: involvement of ERK and PKC. Pulm Pharmacol Ther. 2011;24:100–109. doi: 10.1016/j.pupt.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Bowers R, Cool C, Murphy RC, Tuder RM, Hopken MW, Flores SC, Voelkel NF. Oxidative stress in severe pulmonary hypertension. Am J Respir Crit Care Med. 2004;169:764–769. doi: 10.1164/rccm.200301-147OC. [DOI] [PubMed] [Google Scholar]
- 14.Selvendiran K, Ahmed S, Dayton A, Kuppusamy ML, Tazi M, Bratasz A, Tong L, Rivera BK, Kálai T, Hideg K, Kuppusamy P. Safe and targeted anticancer efficacy of a novel class of antioxidant-conjugated difluorodiarylidenyl piperidones: differential cytotoxicity in healthy and cancer cells. Free Radic Biol Med. 2010;48:1228–1235. doi: 10.1016/j.freeradbiomed.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selvendiran K, Kuppusamy ML, Bratasz A, Tong L, Rivera BK, Rink C, Sen CK, Kálai T, Hideg K, Kuppusamy P. Inhibition of vascular smooth-muscle cell proliferation and arterial restenosis by HO-3867, a novel synthetic curcuminoid, through up-regulation of PTEN expression. J Pharmacol Exp Ther. 2009;329:959–966. doi: 10.1124/jpet.108.150367. [DOI] [PubMed] [Google Scholar]
- 16.Kálai T, Kuppusamy ML, Balog M, Selvendiran K, Rivera BK, Kuppusamy P, Hideg K. Synthesis of N-substituted 3,5-bis(arylidene)-4-piperidones with high antitumor and antioxidant activity. J Med Chem. 2011;54:5414–5421. doi: 10.1021/jm200353f. [DOI] [PubMed] [Google Scholar]
- 17.Selvendiran K, Tong L, Bratasz A, Kuppusamy ML, Ahmed S, Ravi Y, Trigg NJ, Rivera BK, Kálai T, Hideg K, Kuppusamy P. Anticancer efficacy of a difluorodiarylidenyl piperidone (HO-3867) in human ovarian cancer cells and tumor xenografts. Mol Cancer Ther. 2010;9:1169–1179. doi: 10.1158/1535-7163.MCT-09-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dayton A, Selvendiran K, Meduru S, Khan M, Kuppusamy ML, Naidu S, Kálai T, Hideg K, Kuppusamy P. Amelioration of doxorubicin-induced cardiotoxicity by an anticancer-antioxidant dual-function compound, HO-3867. J Pharmacol Exp Ther. 2011;339:350–357. doi: 10.1124/jpet.111.183681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furgeson SB, Simpson PA, Park I, Vanputten V, Horita H, Kontos CD, Nemenoff RA, Weiser-Evans MC. Inactivation of the tumour suppressor, PTEN, in smooth muscle promotes a pro-inflammatory phenotype and enhances neointima formation. Cardiovasc Res. 2010;86:274–282. doi: 10.1093/cvr/cvp425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nemenoff RA, Simpson PA, Furgeson SB, Kaplan-Albuquerque N, Crossno J, Garl PJ, Cooper J, Weiser-Evans MC. Targeted deletion of PTEN in smooth muscle cells results in vascular remodeling and recruitment of progenitor cells through induction of stromal cell-derived factor-1alpha. Circ Res. 2008;102:1036–1045. doi: 10.1161/CIRCRESAHA.107.169896. [DOI] [PubMed] [Google Scholar]
- 21.Huang J, Kontos CD. Inhibition of vascular smooth muscle cell proliferation, migration, and survival by the tumor suppressor protein PTEN. Arterioscler Thromb Vasc Biol. 2002;22:745–751. doi: 10.1161/01.atv.0000016358.05294.8d. [DOI] [PubMed] [Google Scholar]
- 22.Nisbet RE, Bland JM, Kleinhenz DJ, Mitchell PO, Walp ER, Sutliff RL, Hart CM. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. Am J Respir Cell Mol Biol. 2010;42:482–490. doi: 10.1165/rcmb.2008-0132OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabima DM, Frizzell S, Gladwin MT. Reactive oxygen and nitrogen species in pulmonary hypertension. Free Radic Biol Med. 2012;52:1970–1986. doi: 10.1016/j.freeradbiomed.2012.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Csiszar A, Labinskyy N, Olson S, Pinto JT, Gupte S, Wu JM, Hu F, Ballabh P, Podlutsky A, Losonczy G, de Cabo R, Mathew R, Wolin MS, Ungvari Z. Resveratrol prevents monocrotaline-induced pulmonary hypertension in rats. Hypertension. 2009;54:668–675. doi: 10.1161/HYPERTENSIONAHA.109.133397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamezaki F, Tasaki H, Yamashita K, Tsutsui M, Koide S, Nakata S, Tanimoto A, Okazaki M, Sasaguri Y, Adachi T, Otsuji Y. Gene transfer of extracellular superoxide dismutase ameliorates pulmonary hypertension in rats. Am J Respir Crit Care Med. 2008;177:219–226. doi: 10.1164/rccm.200702-264OC. [DOI] [PubMed] [Google Scholar]
- 26.Xu D, Guo H, Xu X, Lu Z, Fassett J, Hu X, Xu Y, Tang Q, Hu D, Somani A, Geurts AM, Ostertag E, Bache RJ, Weir EK, Chen Y. Exacerbated pulmonary arterial hypertension and right ventricular hypertrophy in animals with loss of function of extracellular superoxide dismutase. Hypertension. 2011;58:303–309. doi: 10.1161/HYPERTENSIONAHA.110.166819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoshikawa Y, Ono S, Suzuki S, Tanita T, Chida M, Song C, Noda M, Tabata T, Voelkel NF, Fujimura S. Generation of oxidative stress contributes to the development of pulmonary hypertension induced by hypoxia. J Appl Physiol. 2001;90:1299–1306. doi: 10.1152/jappl.2001.90.4.1299. [DOI] [PubMed] [Google Scholar]
- 28.Hartney T, Birari R, Venkataraman S, Villegas L, Martinez M, Black SM, Stenmark KR, Nozik-Grayck E. Xanthine oxidase-derived ROS upregulate Egr-1 via ERK1/2 in PA smooth muscle cells; model to test impact of extracellular ROS in chronic hypoxia. PLoS ONE. 2011;6:e27531. doi: 10.1371/journal.pone.0027531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nozik-Grayck E, Suliman HB, Majka S, Albietz J, Van Rheen Z, Roush K, Stenmark KR. Lung EC-SOD overexpression attenuates hypoxic induction of Egr-1 and chronic hypoxic pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol. 2008;295:L422–L430. doi: 10.1152/ajplung.90293.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agbani EO, Coats P, Wadsworth RM. Acute hypoxia stimulates intracellular peroxynitrite formation associated with pulmonary artery smooth muscle cell proliferation. J Cardiovasc Pharmacol. 2011;57:584–588. doi: 10.1097/FJC.0b013e3182135e1b. [DOI] [PubMed] [Google Scholar]
- 31.Delgado-Esteban M, Martin-Zanca D, Andres-Martin L, Almeida A, Bolaños JP. Inhibition of PTEN by peroxynitrite activates the phosphoinositide-3-kinase/Akt neuroprotective signaling pathway. J Neurochem. 2007;102:194–205. doi: 10.1111/j.1471-4159.2007.04450.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 33.Selvendiran K, Ahmed S, Dayton A, Ravi Y, Kuppusamy ML, Bratasz A, Rivera BK, Kálai T, Hideg K, Kuppusamy P. HO-3867, a synthetic compound, inhibits the migration and invasion of ovarian carcinoma cells through downregulation of fatty acid synthase and focal adhesion kinase. Mol Cancer Res. 2010;8:1188–1197. doi: 10.1158/1541-7786.MCR-10-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selvendiran K, Ahmed S, Dayton A, Kuppusamy ML, Rivera BK, Kálai T, Hideg K, Kuppusamy P. HO-3867, a curcumin analog, sensitizes cisplatin-resistant ovarian carcinoma, leading to therapeutic synergy through STAT3 inhibition. Cancer Biol Ther. 2011;12:837–845. doi: 10.4161/cbt.12.9.17713. [DOI] [PubMed] [Google Scholar]
- 35.Dayton A, Selvendiran K, Kuppusamy ML, Rivera BK, Meduru S, Kálai T, Hideg K, Kuppusamy P. Cellular uptake, retention and bioabsorption of HO-3867, a fluorinated curcumin analog with potential antitumor properties. Cancer Biol Ther. 2010;10:1027–1032. doi: 10.4161/cbt.10.10.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morimoto T, Sunagawa Y, Kawamura T, Takaya T, Wada H, Nagasawa A, Komeda M, Fujita M, Shimatsu A, Kita T, Hasegawa K. The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats. J Clin Invest. 2008;118:868–878. doi: 10.1172/JCI33160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Guo H, Xu D, Xu X, Wang H, Hu X, Lu Z, Kwak D, Xu Y, Gunther R, Huo Y, Weir EK. Left ventricular failure produces profound lung remodeling and pulmonary hypertension in mice: heart failure causes severe lung disease. Hypertension. 2012;59:1170–1178. doi: 10.1161/HYPERTENSIONAHA.111.186072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jasmin JF, Calderone A, Leung TK, Villeneuve L, Dupuis J. Lung structural remodeling and pulmonary hypertension after myocardial infarction: complete reversal with irbesartan. Cardiovasc Res. 2003;58:621–631. doi: 10.1016/s0008-6363(03)00290-6. [DOI] [PubMed] [Google Scholar]
- 39.Redout EM, van der Toorn A, Zuidwijk MJ, van de Kolk CW, van Echteld CJ, Musters RJ, van Hardeveld C, Paulus WJ, Simonides WS. Antioxidant treatment attenuates pulmonary arterial hypertension-induced heart failure. Am J Physiol Heart Circ Physiol. 2010;298:H1038–H1047. doi: 10.1152/ajpheart.00097.2009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.