ABSTRACT
Fewer than 500 Amur tigers (Panthera tigris altaica) remain in the wild. Due to low numbers and their solitary and reclusive nature, tiger sightings across their range in the Russian Far East and China are rare; sightings of sick tigers are rarer still. Serious neurologic disease observed in several wild tigers since 2001 suggested disease emergence in this endangered species. To investigate this possibility, histology, immunohistochemistry (IHC), in situ hybridization (ISH), and reverse transcription-PCR (RT-PCR) were performed on tissues from 5 affected tigers that died or were destroyed in 2001, 2004, or 2010. Our results reveal canine distemper virus (CDV) infection as the cause of neurologic disease in two tigers and definitively establish infection in a third. Nonsuppurative encephalitis with demyelination, eosinophilic nuclear viral inclusions, and positive immunolabeling for CDV by IHC and ISH were present in the two tigers with available brain tissue. CDV phosphoprotein (P) and hemagglutinin (H) gene products were obtained from brains of these two tigers by RT-PCR, and a short fragment of CDV P gene sequence was detected in lymph node tissue of a third tiger. Phylogenetically, Amur tiger CDV groups with an Arctic-like strain in Baikal seals (Phoca siberica). Our results, which include mapping the location of positive tigers and recognition of a cluster of cases in 2010, coupled with a lack of reported CDV antibodies in Amur tigers prior to 2000 suggest wide geographic distribution of CDV across the tiger range and recent emergence of CDV as a significant infectious disease threat to endangered Amur tigers in the Russian Far East.
IMPORTANCE
Recognition of disease emergence in wildlife is a rare occurrence. Here, for the first time, we identify and characterize a canine distemper virus (CDV), the second most common cause of infectious disease death in domestic dogs and a viral disease of global importance in common and endangered carnivores, as the etiology of neurologic disease and fatal encephalitis in wild, endangered Amur tigers. We establish that in 2010 CDV directly or indirectly killed ~1% of Amur tigers. Location of positive cases over an expansive geographic area suggests that CDV is widely distributed across the tiger range. Interspecies interactions are increasing as human populations grow and expand into wildlife habitats. Identifying animal reservoirs for CDV and identifying the CDV strains that are transmissible to and among wildlife species, including Amur tigers and sympatric critically endangered Amur leopards (Panthera pardus orientalis), is essential for guiding conservation and mitigation efforts.
Observation
Since the beginning of the 20th century, tiger populations have collapsed from an estimated 100,000 tigers among 9 subspecies to fewer than 3,500 individuals (1, 2), and all extant subspecies are currently listed as endangered or critically endangered by the International Union for the Conservation of Nature (http://www.iucn.org/). Poaching, decimation of the tiger prey base, and habitat fragmentation have all contributed to tiger decline (1, 3, 4). For the Amur tiger (Panthera tigris altaica) subspecies, 400 to 500 animals remain in the wild and represent one of the most endangered cat populations on the planet (2). Usually reclusive and seldom observed, adult Amur tigers have been seen uncharacteristically entering villages and wandering onto roadways in the Russian Far East (RFE) since 2001. Even more unusual is abnormal neurologic behavior in the tigers. These observations, along with the diagnosis of morbillivirus infection in a wild Amur tiger in 2004 (histology and immunohistochemistry [IHC] findings were previously described [5]), a cluster of wild tigers with abnormal neurologic behavior in 2010, and a localized rapid and unexpected decline of tigers in the Sikhote-Alin biosphere reserve (SABR) in the RFE since 2009 (6), led to concerns about an infectious disease and in particular canine distemper virus (CDV) as an emerging threat to Amur tigers. Our aim was to identify the cause of neurologic disease and death in endangered Amur tigers in the RFE, establish etiologic linkages between cases over a wide geographic and temporal range, and understand the significance of the disease in tiger conservation.
Tissues collected during necropsy procedures from five adult, free-ranging tigers that died naturally or were destroyed in the RFE in 2001, 2004, or 2010 were available for histopathology, IHC staining, in situ hybridization (ISH), and reverse transcription-PCR (RT-PCR) testing. Brain tissue, critical in assessing CDV infection, was available from two tigers (Pt2004 and Pt2010-3); lung, a primary site of CDV replication, was available from all tigers.
Histologic processing of formalin-fixed tissues was performed using routine methods. Five to eighteen of twenty-two different tissue types (adipose tissue, adrenal gland, artery, brain, heart, kidney, large intestine, liver, lung, lymph node, ovary, pancreas, peripheral nerve, salivary gland, skeletal muscle, spleen, stomach, small intestine, testis, tongue, trachea, or urinary bladder) were available from each animal for histologic review. Bright-field microscopy was performed using a Leica DM2500 microscope (Leica Microsystems Wetzlar GmbH, Wetzlar, Germany).
IHC for canine distemper virus antigen was performed using a primary monoclonal IgG1 anti-CDV surface envelope antibody as described previously and included positive and negative controls (5). Bright-field microscopy was performed as described above.
For ISH, probes to a 600-bp nucleotide region of the phosphoprotein (P) gene of canine distemper virus were designed by Panomics (Affymetrix, Inc., Santa Clara, CA). This region corresponds to nucleotides 1926 to 2526 of the CDV genome (GenBank accession no. AF378705). ISH using Fast Red staining was performed using the Panomics QuantiGene View RNA kit for formalin-fixed paraffin-embedded sections according to the manufacturer’s protocol (product QV0050, QuantiGene ViewRNA FFPE; Affymetrix, Inc., Santa Clara, CA,) and as described previously (7). Sections were counterstained with hematoxylin. Duplicate sections were run without the probe as a negative control. Bright-field microscopy was performed as described above.
For canine distemper virus RT-PCR, RNA was extracted using a standard protocol for xylene deparaffinization of formalin-fixed, paraffin-embedded tissue (a total of 50 µm [10 sections, 5 µm each] of tissue; RNeasy FFPE kit [Qiagen Inc., Valencia CA]). Primer sets were designed from the following regions of the CDV genome: P gene, MorbF, 2132 to 2151; MorbR, 2560 to 2541; CDVF4, 2206 to 2228; and CDVR3, 2319 to 2297; and hemagglutinin (H) gene, CDVH2-F, 8593 to 8619; CDVH2-R, 8842 to 8821; CDVH3-R, 8883 to 8864; CDVH4-R, 8868 to 8850; CDV-HF, 8521 to 8541; and CDV-HR, 8836 to 8815. Nucleotide positions were based on CDV strain A75/17 (GenBank accession no. AF164967). Primers were purchased from Life Technologies (Norwalk, CT). CDV-positive raccoon and fox brain were used as control tissues. No-template negative controls were included in all assays. One-step RT-PCR amplification of CDV P or H gene regions was performed using a standard protocol (Qiagen Inc., Valencia CA). RT-PCR reactions were carried out using an initial 50°C RT step for 30 min, using an annealing temperature of 45°C for 45 s, and 40 cycles. PCR products of correct molecular weight were purified using the ExoSAP-IT reagent (Affymetrix, Santa Clara, CA) or the Qiagen MinElute gel extraction kit and directly sequenced in the forward and reverse directions using an ABI 3730x DNA analyzer for capillary electrophoresis and fluorescent dye terminator detection (Genewiz Inc., South Plainfield, NJ). Sequences were trimmed, aligned, and subjected to analysis using the BLASTn and BLASTx search tools to determine the identities of the viral sequences (GenBank, National Center for Biotechnology Information). The nucleotide sequences of the H gene were translated and aligned using the Geneious alignment tool (Geneious Pro 5.1.7 software; Biomatters Ltd., Auckland, New Zealand) and analyzed for amino acid polymorphisms at positions 530 and 549 in the SLAM receptor binding region of the H gene.
To determine the phylogenetic relationships of tiger CDVs to each other and to other CDV viruses and morbilliviruses, nucleotide sequences for the P and H genes from the tigers and representative CDV strains were aligned (GenBank, National Center for Biotechnology Information; http://www.ncbi.nlm.nih.gov) (Geneious Pro 5.1.7 software; Biomatters Ltd., Auckland, New Zealand). Pairwise identities were obtained by PAUP analysis to create a running P-distance pairwise comparison matrix (PAUP plugin in Geneious Pro). Bayesian analysis was performed using MrBayes 3.1 plugin in Geneious Pro using gamma-distributed rate variation and an HKY85 substitution model (8). The first 25% of a 1,100,000 chain length was discarded as burn-in, and 4 heated chains were run with a subsampling frequency of 200. Rinderpest virus (accession no. AF132934) was used as an outgroup. Trees were finalized and labeled (FigTree v1.3.1 software [Andrew Rambaut, Institute of Evolutionary Biology, University of Edinburgh, 2006 to 2009; http://tree.bio.ed.ac.uk/]). Posterior probability values were calculated.
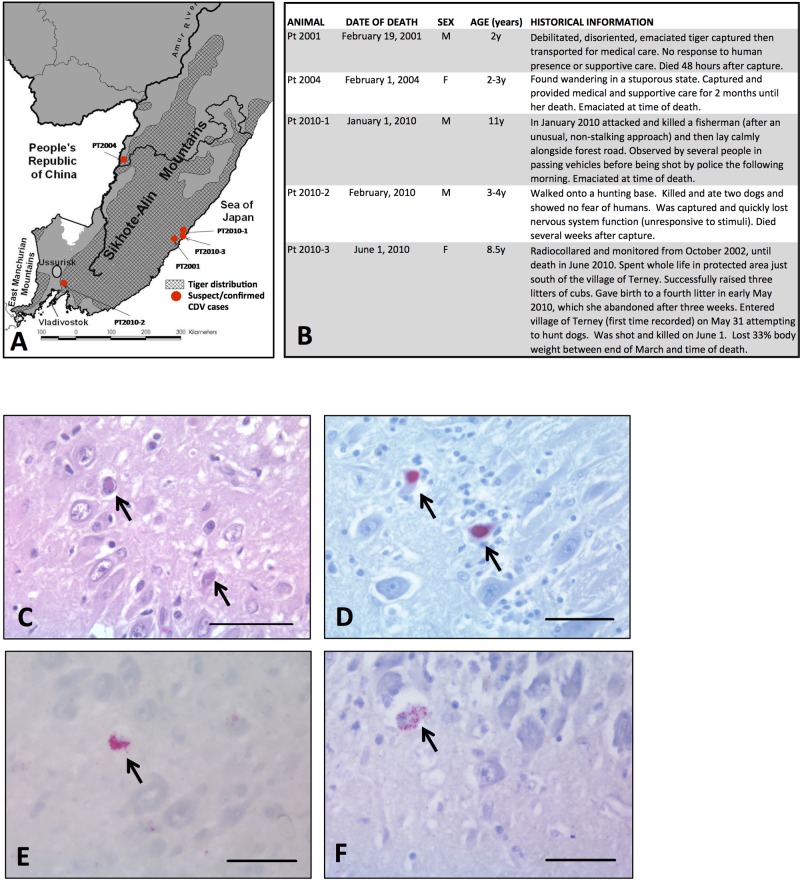
Between January and June 2010, three adult free-ranging Amur tigers (Panthera tigris altaica) (Pt2010-1, Pt2010-2, and Pt2010-3) entered villages in the RFE (Fig. 1A and B). Each was killed (Pt2010-1 and Pt2010-3) or died naturally (Pt-2010-2) after exhibiting abnormal neurologic behavior (disorientation, lack of response to stimulation, and/or nonaggressive fearlessness). Prior to 2010, two other free-ranging Amur tigers (Pt2001 and Pt2004) were captured and died after exhibiting similar neurologic behavior (Fig. 1A and B). Four of the five tigers were emaciated or showed extreme weight loss at the time of death (Fig. 1B).
FIG 1 .
Geographical distribution (A) and historical information (B) for tigers in the Russian Far East that died or were killed due to abnormal neurologic behavior in 2001, 2004, or 2010. (C) Tiger Pt2010-3: hematoxylin-and-eosin-stained section of brain with neuronal intranuclear eosinophilic viral inclusions (arrow). (D) Tiger Pt2010-3: positive immunohistochemical staining of neurons with monoclonal IgG primary antibody to CDV viral envelope protein antigen (arrows) (fast-red staining). (E and F) Positive in situ hybridization (fast red) of probes to CDV P gene sequence in CDV-infected neurons in tiger Pt2004 (E) and tiger Pt2010-3 (F). Bar = 50 µm in all images.
Brain tissue was available from Pt2010-3 and Pt2004 (histology and IHC for the latter were previously reported [5], and tissue was reprocessed and reviewed for this article). Histologic lesions in the brains were identical and consisted of nonsuppurative viral encephalitis with severe demyelination. Brightly eosinophilic neuronal and glial cell nuclear viral inclusions and positive immunohistochemical staining in these cell types for an envelope component of CDV were also seen (Fig. 1C and D, respectively). The findings were severe and were sufficient to result in the clinically observed neurologic behavior in both cases and natural death in Pt2004 (5). Mild or moderate lymphoid depletion was seen in lymph nodes of Pt2010-1 and Pt2001, respectively, and moderate lymphoid depletion was seen in spleens from Pt-2001, Pt2004, and Pt2010-1. Intralesional viral RNA was confirmed in both tiger brains using ISH to a 600-bp segment of the CDV P gene (Fig. 1E and F). Viral inclusions, IHC staining, or ISH consistent with CDV infection were not seen in nonneural tissues, including lung or lymphoid tissues, from any of the tigers (data not shown). Concurrent, transmissible infectious disease was not seen.
Extracted RNA from select formalin-fixed paraffin-embedded (FFPE) tissues was analyzed by RT-PCR for morbillivirus and CDV phosphoprotein P and H genes using multiple primer sets. Positive results were obtained in 3 of 5 tigers: Pt2004 (histologic and IHC description previously reported [5]), Pt2010-2, and Pt-2010-3. CDV P gene products ranging in size from 114 bp to 430 bp and a 291-bp H gene product were recovered from the brains of both Pt2004 and Pt2010-3. In Pt2010-2, lymph node tissue was positive for a 114-bp fragment of the CDV P gene and was H gene negative. Possible reasons for failure to recover H gene sequence from this tiger include RNA degradation due to autolysis and/or cross-linking due to formalin fixation and/or prolonged formalin fixation prior to RT-PCR. In addition to the possibility of true negatives, these complications could have prevented identification of positive cases among the remaining two tigers (Pt2001 and Pt2010-1) or additional tissues in positive cases. Not having access to brain, the optimal target tissue in these neurologic tigers, may also explain the failure to identify additional positive tigers.
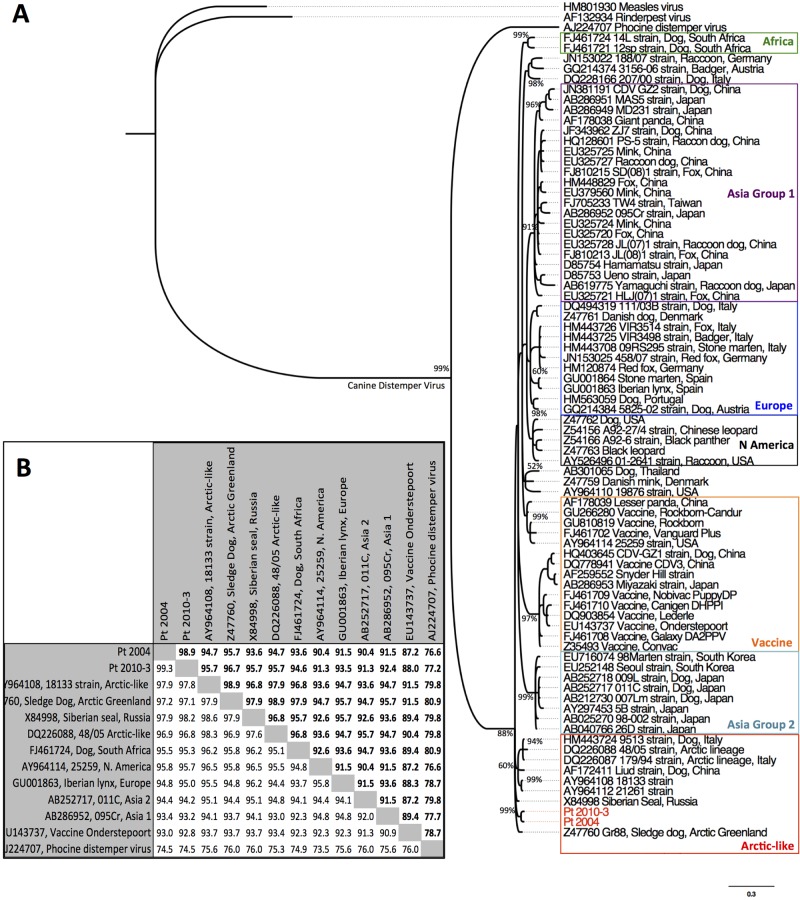
Gene product sequences from Pt2004, Pt2010-2, and Pt2010-3 were aligned with sequences of representative morbilliviruses, CDV sequences, and each other. Alignments of morbilliviruses and CDV strains were distributed as expected within viral clades and geographic distribution groups for Asia, Africa, Europe, and North America. H gene segments from Pt2004 and Pt2010-3 were 99.3% identical to each other (Fig. 2B). Phylogenetic analysis segregated tiger H and P gene sequences within the Arctic-like strains (Fig. 2A, H gene phylogeny; P gene phylogeny not shown). BLASTn and PAUP distance matrix analysis showed tiger CDV H gene segments having closest identity (97.9%) to Arctic-like CDV strain 18133 (9) and a Baikal seal (Phoca siberica) strain (10) (Fig. 2B). Our results indicate that tiger CDV is an Arctic-like strain similar to those from Greenland (11), China (12), Russia (10), and the United States (9).
FIG 2 .
(A) Bayesian phylogenetic tree of the H gene nucleotide alignment from tigers Pt2004 and Pt2010-3 and representative CDV sequences obtained from GenBank. Sequences were aligned using Geneious Pro software. Bayesian posterior probabilities of branching demonstrate the robustness of the individual groups. (B) Distance matrix analysis of CDV H gene sequences. Pairwise identities of nucleotide and amino acid sequences (boldface) between different strains of CDV were obtained through GenBank and generated from a pairwise distance matrix calculated using PAUP software.
The critical amino acid residues G530 and Y549 in the SLAM receptor binding domain of the CDV hemagglutinin protein have been shown to determine host cell tropism in vitro. G → N, R, or D or Y → H substitution at the 530 or 549 residue, respectively, is proposed to be associated with CDV transmission from domestic dogs (Canis lupus familiaris) and disease emergence in novel host species (13). Both tiger sequences lacked the Y549 → H substitution but contained an N residue at position G530. Because the G530 → N substitution is a consistent finding in Arctic-like strains in general, including those in dogs and wildlife (12–14), we cannot attribute a recent substitution event at this residue to disease emergence in tigers. If this amino acid is under positive selective pressure, the change may have occurred through a dog-to-wildlife transmission prior to 1988, when Arctic-like strains, which include the G530 → N mutation, were first detected in Baikal seals and sled dogs in Greenland (14). Subsequent reintroduction of virus with this substitution into the domestic dog population may explain why the substitution is a predominant synapomorphy in the Arctic-like lineage. Another interesting finding was three unique amino acid changes (V538 → I, T548 → M, and D570 → N) in the tiger H gene sequence that have not been observed previously in Arctic-like strains. These findings suggest that the tiger Arctic-like CDV is distinct; however, additional information about Arctic-like strains is needed to be confident in this conclusion.
CDV is the second most common cause of infectious disease death in domestic dogs and is a significant viral disease of global importance in common and endangered wild carnivores (15). It is a multihost pathogen, and interactions with and disease transmission from abundant wildlife reservoir species, such as raccoon dogs (Nyctereutes procyonoides) or domestic dogs, are likely to be as important, if not more important, for disease transmission and population effect than infection among tigers alone due to low tiger numbers and population density (16). In the RFE, little appears to be known about the distribution and strains of CDV that are circulating in domestic dogs and wildlife. However, our identification of positive tiger CDV cases separated by 200 km to 300 km suggests wide distribution for the Arctic-like CDV strain that infects and kills Amur tigers.
Low rates of vaccination and CDV infection are present in domestic dogs in Russia, and direct transmission of CDV from infected, unvaccinated dogs to tigers is a significant concern, since Amur tigers are known to encounter and kill domestic dogs (17). In one survey, only 16% of village dogs were vaccinated against CDV and 58% of unvaccinated dogs were seropositive for antibodies to the virus, indicating high endemic exposure (18). In the same report, 15% of wild tigers (n = 40) sampled between 2000 and 2004 were seropositive for CDV antibodies, with no seropositive tigers detected prior to 2000 (n = 27) (18); both Pt2004 and Pt2010-3 were seropositive for antibodies to CDV (1:256; virus neutralization [VN] ≥ 1:4 positive threshold value) two (5) and three (data not shown) months, respectively, prior to their deaths.
CDV is a preventable infectious disease, and vaccination strategies, all of which have limitations and significant challenges in a wildlife setting (19, 20), are likely to be considered for protecting endangered Amur tigers. Because dogs are a known CDV reservoir, one strategy is to vaccinate domestic dogs to decrease transmission risk to susceptible wildlife. This strategy was initiated in the Serengeti ecosystem in 2003 in response to several significant CDV mortality events in lions (Panthera leo) (21). The success of this strategy to date is unclear, since at least one CDV outbreak has occurred since initiation of the vaccination program (21). A second strategy is direct wildlife vaccination, which because of small numbers of animals, limited range, and known high disease-associated mortality is a critical component in conservation programs for the endangered black-footed ferret (Mustela nigripes) (22) and critically endangered Santa Catalina Island fox (Urocyon littoralis catalinae) (23). Vaccination with recombinant vectored vaccines has been safely used and is the recommendation for captive tigers in Association of Zoos and Aquariums (AZA)-accredited zoos (http://www.aazv.org/displaycommon.cfm?an=1&subarticlenbr=273) and for nondomestic canid and other wildlife species (modified-live vaccines can induce disease and should not be used). Recombinant vectored vaccines may provide an option in wild tiger vaccination strategies, which in addition to safety must also consider efficacy, practicality, limitations, cost, and unintended consequences of vaccination (including increased disease susceptibility to CDV or other pathogens) in target or nontarget species (19, 20).
Infectious disease as the cause of population decline or (less commonly) extinction in free-ranging wildlife is a recognized threat to species survival; however, our ability to identify these events and their significance as they are occur and in time to mitigate their effects is rare (24). The exact timing of CDV emergence in the RFE Amur tiger population is speculative. The absence of positive serology prior to 2000 (18), lack of documented observations of neurologically ill tigers by scientists (5, 6, 18) or people living in tiger range (personal communication, Igor Gregorivich) prior to 2001, and a cluster of cases in 2010 suggest CDV emergence after 2000 (whether earlier individual cases or previous waves of tiger CDV infection and mortality occurred but were undetected prior to 2000 remains to be rigorously investigated). Additionally, in 2010 alone, CDV infection directly or indirectly killed approximately 1.0% of wild Amur tigers (2 adults and 3 abandoned cubs). These deaths reflect the immediate, direct effects of CDV infection and more than likely underestimate actual CDV-related deaths. In addition and at the population level, the long-term impact of losing reproductively active animals, especially females like Pt2010-3, will exceed the direct effects of individual animal infection alone through lost productivity of both the dam and her offspring (25).
Our study is the first to confirm and genetically characterize a CDV that is killing wild, endangered Amur tigers in the RFE. Our results indicate that tiger CDV is an Arctic-like strain similar to CDV in Baikal seals in Russia and domestic dogs. Our report illustrates the importance of long-term wildlife monitoring and health surveillance in identifying emerging threats in endangered species. It also shows how through these efforts we are afforded an opportunity to develop and implement mitigation activities, including identification of CDV reservoir species and consideration and assessment of vaccination strategies, to reduce disease risk in Amur tigers and sympatric critically endangered Amur leopards (Panthera pardus orientalis).
Nucleotide sequence accession numbers.
Tiger CDV P and H gene sequences were deposited in GenBank (accession numbers KC579363 [Pt2004; H gene], KC579361 [Pt2004; P gene], and KC579362 [Pt2010-3; H gene]). Accession numbers for tiger-derived sequences and all other sequences are presented in the figures.
ACKNOWLEDGMENTS
Funding was generously provided by the Dunemere Foundation.
We thank Melissa Miller and Judy St. Leger for the positive-control CDV tissues and John Goodrich, Kathy Quigley, Charles Leathers, Alfred Ngbokoli, Daniel Friedman, Damien Joly, Enkhtuvshin Shiilegdamba, Kate Jenks, and Jamie Phillips for materials, advice, and logistical support. Special thanks go to Carol Oddoux, our Russian colleagues and the field staff that participated on the necropsy teams, and Séamus Maclennan and Martin Gilbert for reviewing the manuscript.
Footnotes
Citation Seimon TA, Miquelle DG, Chang TY, Newton AL, Korotkova I, Ivanchuk G, Lyubchenko E, Tupikov A, Slabe E, McAloose D. 2013. Canine distemper virus: an emerging disease in wild endangered Amur tigers (Panthera tigris altaica). mBio 4(4):e00410-13. doi:10.1128/mBio.00410-13.
REFERENCES
- 1. Dinerstein E, Loucks C, Wikramanayake E, Ginsberg J, Sanderson E, Seidensticker J, Forrest J, Bryja G, Heydlauff A, Klenzendorf S, Leim-Gruber P, Mills J, O’Brien TG, Shrestha M, Simons R, Songer M. 2007. The fate of wild tigers. BioScience 57:508–514 [Google Scholar]
- 2. Henry P, Miquelle D, Sugimoto T, McCullough DR, Caccone A, Russello MA. 2009. In situ population structure and ex situ representation of the endangered Amur tiger. Mol. Ecol. 18:3173–3184 [DOI] [PubMed] [Google Scholar]
- 3. Kinnaird MG, Sanderson EW, O’Brien TG, Wibisono HT, Woolmer G. 2003. Deforestation trends in a tropical landscape and implications for endangered large mammals. Conserv. Biol. 17:245–257 [Google Scholar]
- 4. Smith JL, Tunhikorn S, Tanhan S, Simcharoen S, Kanchanasaka B. 1999. Metapopulation structure of tigers in Thailand, p 166–175 In Seidensticker J, Christie S, Jackson P, Riding the tiger: tiger conservation in human-dominated landscapes. Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 5. Quigley KS, Evermann JF, Leathers CW, Armstrong DL, Goodrich J, Duncan NM, Miquelle DG. 2010. Morbillivirus infection in a wild Siberian tiger in the Russian Far East. J. Wildl. Dis. 46:1252–1256 [DOI] [PubMed] [Google Scholar]
- 6. Smirnov EN, Miquelle DG, Zaumyslova OY. 2012. Population dynamics of the Amur tiger in Sikhote-Alin Zapovednik: 1966–2011, p 159–178 Sikhote-Alin Biosphere Region: condition of ecosystems and their components: a volume of scientific work for the 75th anniversary of the Sikhote-Alin Reserve. DalNauka, Vladivostok, Russia. (In Russian.) [Google Scholar]
- 7. Seimon TA, McAloose D, Raphael B, Honkavuori KS, Chang T, Hirschberg DL, Lipkin WI. 2012. A novel herpesvirus in 3 species of pheasants: mountain peacock pheasant (Polyplectron inopinatum), Malayan peacock pheasant (Polyplectron malacense), and Congo peafowl (Afropavo congensis). Vet. Pathol. 49:482–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huelsenbeck JP, Ronquist F. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755 [DOI] [PubMed] [Google Scholar]
- 9. Pardo ID, Johnson GC, Kleiboeker SB. 2005. Phylogenetic characterization of canine distemper viruses detected in naturally infected dogs in North America. J. Clin. Microbiol. 43:5009–5017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mamaev LV, Denikina NN, Belikov SI, Volchkov VE, Visser IK, Fleming M, Kai C, Harder TC, Liess B, Osterhaus AD, Barrett T. 1995. Characterisation of morbilliviruses isolated from Lake Baikal seals (Phoca sibirica). Vet. Microbiol. 44:251–259 [DOI] [PubMed] [Google Scholar]
- 11. Bohm J, Blixenkrone-Møller M, Lund E. 1989. A serious outbreak of canine distemper among sled-dogs in northern Greenland. Arctic Med. Res. 48:195–203 [PubMed] [Google Scholar]
- 12. Martella V, Cirone F, Elia G, Lorusso E, Decaro N, Campolo M, Desario C, Lucente MS, Bellacicco AL, Blixenkrone-Møller M, Carmichael LE, Buonavoglia C. 2006. Heterogeneity within the hemagglutinin genes of canine distemper virus (CDV) strains detected in Italy. Vet. Microbiol. 116:301–309 [DOI] [PubMed] [Google Scholar]
- 13. McCarthy AJ, Shaw MA, Goodman SJ. 2007. Pathogen evolution and disease emergence in carnivores. Proc. Biol. Sci. 274:3165–3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bolt G, Jensen TD, Gottschalck E, Arctander P, Appel MJ, Buckland R, Blixenkrone-Møller M. 1997. Genetic diversity of the attachment (H) protein gene of current field isolates of canine distemper virus. J. Gen. Virol. 78:367–372 [DOI] [PubMed] [Google Scholar]
- 15. Deem SL, Spelman LH, Yates RA, Montali RJ. 2000. Canine distemper in terrestrial carnivores: a review. J. Zoo Wildl. Med. 31:441–451 [DOI] [PubMed] [Google Scholar]
- 16. Craft ME, Hawthorne PL, Packer C, Dobson AP. 2008. Dynamics of a multihost pathogen in a carnivore community. J. Anim. Ecol. 77:1257–1264 [DOI] [PubMed] [Google Scholar]
- 17. Goodrich JM, Seryodkin IV, Miquelle DG, Beriznuk SI. 2011. Conflicts between Amur (Siberian) tigers and humans in the Russian Far East. Biol. Conserv. 144:584–592 [Google Scholar]
- 18. Goodrich JM, Quigley KS, Lewis JC, Astafiev AA, Slabi EV, Miquelle DG, Smirnov EN, Kerley LL, Armstrong DL, Quigley HB, Hornocker MG. 2012. Serosurvey of free-ranging Amur tigers in the Russian Far East. J. Wildl. Dis. 48:186–189 [DOI] [PubMed] [Google Scholar]
- 19. Chauvenet AL, Durant SM, Hilborn R, Pettorelli N. 2011. Unintended consequences of conservation actions: managing disease in complex ecosystems. PLoS ONE 6:e28671. 10.1371/journal.pone.0028671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Munson L, Terio KA, Ryser-Degiorgis MP, Lane EP, Courchamp F. 2010. Wild felid diseases: conservation implications and management strategies, p 237–259 In MacDonald DW, Loveridge AJ, Biology and conservation of wild felids. Oxford University Press, Oxford, United Kingdom [Google Scholar]
- 21. Craft ME. 2010. Ecology of infectious diseases in Serengeti lions, p 263–282 In MacDonald DW, Loveridge AJ (ed), Biology and conservation of wild felids. Oxford University Press, Oxford, United Kingdom [Google Scholar]
- 22. Holmes BE. 2008. A review of black-footed ferret reintroduction in northwest Colorado, 2001–2006, p 43 Technical Note 426 U.S. Department of the Interior, Bureau of Land Management, White River Field Office, Meeker, CO [Google Scholar]
- 23. Timm SF, Munson L, Summers BA, Terio KA, Dubovi EJ, Rupprecht CE, Kapil S, Garcelon DK. 2009. A suspected canine distemper epidemic as the cause of a catastrophic decline in Santa Catalina Island foxes (Urocyon littoralis catalinae). J. Wildl. Dis. 45:333–343 [DOI] [PubMed] [Google Scholar]
- 24. MacPhee RD, Greenwood AD. 2013. Infectious disease, endangerment, and extinction. Int. J. Evol. Biol. 2013:571939 http://dx.doi.org/10.1155/2013/571939 [DOI] [PMC free article] [PubMed]
- 25. Kerley LL, Goodrich JM, Miquelle DG, Smirnov EN, Quigley HB, Hornocker MG. 2003. Reproductive parameters of wild female Amur (Siberian) tigers (Panthera tigris altaica). J. Mammal. 84:288–298 [Google Scholar]