ABSTRACT
Infective endocarditis and kidney infections are serious complications of Staphylococcus aureus sepsis. We investigated the role of superantigens (SAgs) in the development of lethal sepsis, infective endocarditis, and kidney infections. SAgs cause toxic shock syndrome, but it is unclear if SAgs contribute to infective endocarditis and kidney infections secondary to sepsis. We show in the methicillin-resistant S. aureus strain MW2 that lethal sepsis, infective endocarditis, and kidney infections in rabbits are critically dependent on high-level SAgs. In contrast, the isogenic strain lacking staphylococcal enterotoxin C (SEC), the major SAg in this strain, is attenuated in virulence, while complementation restores disease production. SAgs’ role in infective endocarditis appears to be both superantigenicity and direct endothelial cell stimulation. Maintenance of elevated blood pressure by fluid therapy significantly protects from infective endocarditis, possibly through preventing bacterial accumulation on valves and increased SAg elimination. These data should facilitate better methods to manage these serious illnesses.
IMPORTANCE
The Centers for Disease Control and Prevention reported in 2007 that Staphylococcus aureus is the most significant cause of serious infectious diseases in the United States (R. M. Klevens, M. A. Morrison, J. Nadle, S. Petit, K. Gershman, et al., JAMA 298:1763–1771, 2007). Among these infections are sepsis, infective endocarditis, and acute kidney injury. Infective endocarditis occurs in 30 to 60% of patients with S. aureus bacteremia and carries a mortality rate of 40 to 50%. Over the past decades, infective endocarditis outcomes have not improved, and infection rates are steadily increasing (D. H. Bor, S. Woolhandler, R. Nardin, J. Brusch, D. U. Himmelstein, PLoS One 8:e60033, 2013). There is little understanding of the S. aureus virulence factors that are key for infective endocarditis development and kidney abscess formation. We demonstrate that superantigens are critical in the causation of all three infections. We show that their association results from both superantigenicity and direct toxic effects on endothelial cells, the latter likely contributing to delayed endothelium healing. Our studies contribute significantly to understanding the development of these illnesses and are expected to lead to development of important therapies to treat such illnesses.
Introduction
Staphylococcus aureus is the second leading cause of bacteremia and the leading cause of infective endocarditis (IE) (1–4). Medical advances, such as intravascular and prosthetic devices and surgical procedures, and an increasing population with underlying conditions, such as diabetes mellitus, liver disease, renal hemodialysis, and immunosuppression, have contributed to the surge of S. aureus infections in health care settings and in the community (4, 5).
S. aureus bacteremia results often from skin infections, infected catheters, surgical wounds, pneumonia, or intravenous drug use and carries a mortality rate of 20 to 40% (6, 7). S. aureus’ ability to cause metastatic infections, such as IE and deep tissue abscesses, contributes to the high fatality rate associated with S. aureus bacteremia (8). IE, which accounts for up to one-third of the complications of S. aureus bacteremia, is an infection of the heart endothelium, predominantly valves, that results in the formation of large vegetative lesions (1, 4). Vegetations are a meshwork of host factors, including fibrin and platelets, and bacterial aggregates (9). IE is associated with a high risk for congestive heart failure and systemic embolization resulting in strokes, metastatic abscesses, persistent bacteremia, and toxic shock syndrome (TSS), all of which can lead to death (5, 10).
Various S. aureus surface virulence factors are associated with the pathogenesis of IE, particularly those involved in survival in the bloodstream (i.e., SOK, a surface factor promoting resistance to oxidative/neutrophil killing) and tissue adherence/colonization (i.e., coagulases Coa and von Willebrand factor binding protein [vWbp] and clumping factor ClfA) (11, 12). However, evidence suggests that superantigens (SAgs), secreted virulence factors involved in host immune evasion, also contribute to IE. In a rabbit model, infection with SAg-deficient strains produces minimal vegetations, while ectopic expression of TSS toxin 1 (TSST-1) in these strains results in large vegetations with high bacterial counts (13). A recent study observed that neutralization of the SAg staphylococcal enterotoxin C (SEC) with a soluble, high-affinity T cell receptor β-chain protected rabbits against lethal sepsis and dramatically reduced vegetation size in rabbits infected with MW2 (an SEC-producing strain) (14). Furthermore, an international study of S. aureus strains from definite IE patients demonstrated a high prevalence of SAg genes encoding TSST-1, SEC, SEG (staphylococcal enterotoxin), and SEI (staphylococcal enterotoxin-like I) among IE isolates compared to prevalence among isolates from soft tissue infections (15).
We investigated the association of SAgs with IE and disease sequelae in the sensitive rabbit model of IE and sepsis. We used the community-associated, methicillin-resistant (MRSA) MW2 strain as a representative of S. aureus isolates with high capacity to cause infective endocarditis, and we provide evidence that high-level SAgs, as exemplified by SEC in MW2, are critical virulence factors in lethal sepsis, IE, and kidney injury. Use of fluid replacement to promote SAg elimination by kidney filtration and to offset SAg-induced hypotension decreased both IE vegetation size and bacterial growth within the vegetations. We demonstrate immune cell recruitment to vegetative lesions and direct SEC induction of interleukin 8 (IL-8) in primary human aortic endothelial cells (HAECs). IL-8 production was found to be dependent on signaling via G-protein-coupled receptors (GPCR), metalloproteinases (MMPs), and vascular endothelial growth factor receptors (VEGFR).
RESULTS
Lethality resulting from S. aureus MW2 bacteremia is due to SEC.
Persistent S. aureus bacteremia is linked to high mortality rates in humans, where lethality results from cardiotoxicity and/or sepsis (5). Recently, Coa, ClfA, and vWbp were described as essential for lethality in a murine model of staphylococcal sepsis (12). However, murine models do not account for the action of SAgs, as mice on a per kilogram basis are >109 more resistant to SAgs than humans, while rabbits resemble humans in susceptibility to SAgs (16). MW2 encodes multiple SAgs, including SEC and SEl-X (14). SEC is produced at 80 to 100 µg/ml in liquid culture, while the other SAgs are produced at 0.0001 to 0.03 µg/ml. MW2 and its derivatives lacking sec or selx were tested in the rabbit model of IE and sepsis. An selx deletion was also chosen given its recent association with necrotizing pneumonia in the MRSA strain LAC (17). Of note, SEl-X is produced at much higher levels in LAC than in MW2.
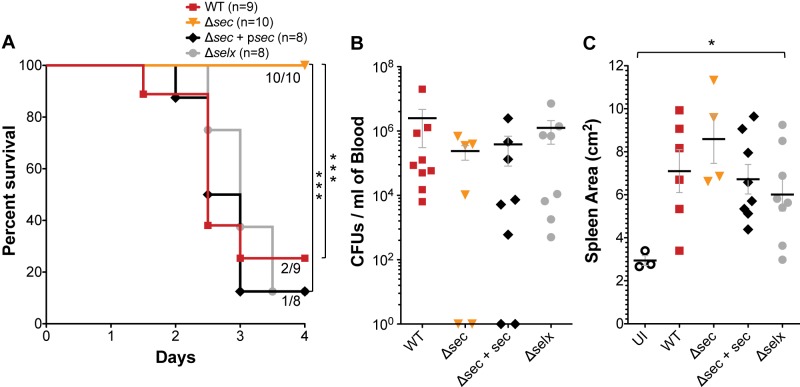
Bacteria were injected intravenously at 2 × 107 to 3 × 107 CFU/rabbit after mechanical damage of the aortic valves, and infection was allowed to proceed for a maximum of 4 days. In this time period, MW2 caused lethal sepsis in 80% of rabbits, while the isogenic sec deletion (MW2Δsec) strain failed to cause comparable lethality (90% survived to day 4) (Fig. 1A). Deletion of selx did not affect MW2 lethality, demonstrating that SEC is the dominant virulence SAg in this strain. Complementation of the MW2Δsec strain restored lethality to wild-type levels (Fig. 1A). Furthermore, survival of rabbits infected with the MW2Δsec strain occurred even as bacterial blood counts were similar to those of the wild type, and rabbits exhibited similar degrees of splenomegaly due to bacteremia (Fig. 1B and C). Thus, bacteremia alone does not account for the lethal outcomes in S. aureus infection, indicating the action of a SAg is required.
FIG 1 .
Deletion of sec in S. aureus strain MW2 protects rabbits against lethality in the IE and sepsis model despite high-load bacteremia. (A) Percent survival of rabbits infected intravenously with 2 × 107 to 3 × 107 CFU of wild-type MW2, the MW2Δsec strain, the MW2Δsec strain with psec (ectopic expression), or the MW2Δselx strain after mechanical damage of the aortic valves. ***, P = 0.0009 (MW2 versus MW2Δsec) and P = 0.0002 (MW2 versus MW2Δsec + psec), log-rank, Mantel-Cox test. (B) Bacterial counts per milliliter of blood recovered from the rabbits postmortem. (C) Enlargement of the spleen resulting from S. aureus bacteremia. UI, uninfected. *, P = 0.0385, one-way ANOVA and nonparametric Kruskal-Wallis test. (B and C) Horizontal lines and error bars represent mean values ± standard errors of the means (SEM). P values of ≤0.05 are considered statistically significant. No P value means no statistical significance.
SEC is critical for infective endocarditis.
IE, a life-threatening and difficult-to-treat complication of S. aureus bacteremia, particularly affects individuals with damaged hearts (5). In the rabbit model, vegetative lesions develop on aortic valve cusps upon seeding of the organism at sites of mechanical damage. MW2 formed large vegetations with an off-white cauliflower-like appearance (Fig. 2A). Vegetations were frequently found on multiple valve leaflets and often associated with blood clots. Some vegetations destroyed aortic valve leaflets and extended into adjacent tissue, while others extended onto the surface of the aortic artery (Fig. 2A). All rabbits infected with MW2 (9/9) developed vegetations; most (6/9 vegetations) weighed 92 to 125 mg and contained 1 × 108 to 70 × 108 CFU. Rabbits that succumbed before day 2 had smaller vegetations (28 to 46 mg), with 2 × 107 to 30 × 107 CFU (Fig. 3B and C). Of the 10 rabbits infected with the MW2Δsec strain, 7 had no vegetations, and 3 had small vegetations weighing 6, 10, and 35 mg; one vegetation was sterile, and the other two had 1 × 103 and 5 × 108 CFU, respectively. Complementation of the MW2Δsec strain restored its ability to cause IE, whereas deletion of selx had no effect on vegetation formation or recovered bacteria (Fig. 2B and C). These results highlight the critical role of high-level SAgs in the pathogenesis of IE.
FIG 2 .
Deletion of sec in S. aureus strain MW2 protects rabbits against IE. (A) Representative images of vegetative lesions on aortic valve cusps caused by wild-type MW2; (B) total weight of vegetations dissected from aortic valves after intravenous inoculation with 2 × 107 to 3 × 107 CFU of wild-type MW2, the MW2Δsec strain, the MW2Δsec strain with psec (ectopic expression), or the MW2Δselx strain; (C) bacterial counts recovered from aortic valve vegetations shown in panel B. ***, P = 0.0002; **, P = 0.0011; one-way ANOVA and nonparametric Kruskal-Wallis test. Horizontal lines and error bars represent mean values ± SEM. P values of ≤0.05 are considered statistically significant.
FIG 3 .
Deletion of sec in S. aureus strain MW2 protects rabbits against development of acute renal infarction and abscess formation and partially against lung necrosis and hemorrhage in the infective endocarditis and sepsis model. (A) Representative images of kidneys harvested from infected rabbits demonstrating the severity of kidney injury by SEC-producing S. aureus strains. (B) Quantification of renal injury and abscess formation plotted as the percentage of the kidney surface area with visible kidney injury in rabbits infected intravenously with 2 × 107 to 3 × 107 CFU of wild-type MW2, the MW2Δsec strain, the MW2Δsec strain with psec (ectopic expression), or the MW2Δselx strain after mechanical damage of the aortic valves. *, P = 0.025, nonparametric (one-way ANOVA) Kruskal-Wallis test. Horizontal lines and error bars represent mean values ± SEM. P values of ≤0.05 are considered statistically significant. (C) Representative images of lungs harvested from infected rabbits. Gross examination shows an improvement in the pathology of the lungs in rabbits infected with the sec deletion strain.
SEC contributes to kidney injury.
To assess the contribution of SAgs to S. aureus hematogenous spread and establishment of metastatic infections, rabbits were examined for the presence of kidney and lung injury. Gross examination of the kidneys from MW2-infected rabbits revealed multiple areas of tissue ischemia and renal infarction (Fig. 3A). The majority contained abscesses that covered up to 35% of the surfaces (Fig. 3B). Although rabbits that did not live past day 2 had no or small abscesses, at least one kidney presented signs of massive renal infarction, suggesting that tissue ischemia developed due to hypoperfusion. In sharp contrast, the majority of the rabbits (7/10) infected with the MW2Δsec strain had normal kidneys, no ischemic tissue, and no abscesses. Two rabbits had tissue ischemia and minute abscesses (Fig. 3B). Kidneys from rabbits infected with the MW2Δsec complemented strain or the MW2Δselx strain exhibited pathology similar to that of kidneys from rabbits infected with MW2 (Fig. 3A and C).
Lungs from MW2-infected rabbits showed phenotypes that included diffuse or hemorrhagic consolidation, hemorrhagic pleural effusion, and tissue necrosis. No abscesses were observed. sec deletion protected rabbits only against tissue necrosis (Fig. 3C).
Characterization of vegetative lesions.
We hypothesized that SAgs contribute to IE by aiding in initiation and/or growth of vegetations. SAgs could accomplish these by (i) superantigenicity (induction of massive cytokine production and hypotension) and/or (ii) direct effects on aortic endothelial cells (induction of localized cytotoxicity and persistent inflammation). To address these possibilities, we first performed histological analysis of vegetative lesions and investigated immune cell infiltration.
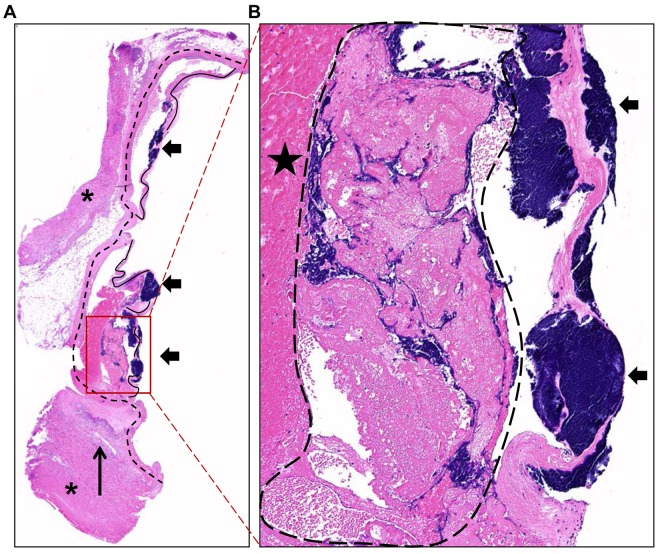
Staphylococcal vegetations had the classic appearance of fibrinous endocarditis lesions, with extensive bacterial colonies detected within the lesions (Fig. 4A). These lesions were composed of fibrin aggregates with pockets of platelets, erythrocytes, and bacterial cocci embedded within the aggregates. However, bacteria macroclusters were found focused on the valve leaflets (Fig. 4B). These S. aureus clusters were also present in nascent vegetations, prior to the formation of the large vegetation meshwork composed of host factors and bacteria within the valve cusps, indicating that settling and growth of the organism onto damaged valves preceded septic fibrinous aggregate development (see Fig. S1 in the supplemental material). Furthermore, recruitment of inflammatory cells was observed in the endocardium adjacent to the vegetative lesions, including small foci of necropurulent inflammation in the nearby heart muscle (Fig. 4A; see also Fig. S1 and S2 in the supplemental material).
FIG 4 .
Hematoxylin and eosin-stained section of vegetative lesions on aortic valves characteristic of staphylococcal endocarditis. (A) A 2× composite of a vegetative lesion containing heart muscle (asterisks), valves (solid lines), and aortic artery (dashed lines) with vegetations (block arrows) primarily centered on the valves. Foci of necropurulent inflammation were detected in the nearby adjacent heart muscle (long arrow). (B) A ×10 magnification of the vegetative lesion shown in panel A highlights the presence of extensive bacterial colonies within the valve leaflets (block arrows). Vegetations are composed of aggregates of fibrin (star) with pockets of platelets, erythrocytes, and large masses of bacterial cocci consistent with S. aureus (dashed outline) in addition to bacterial macroclusters centered on the valves.
Fluid replacement inhibits vegetation formation and decreases bacterial burden.
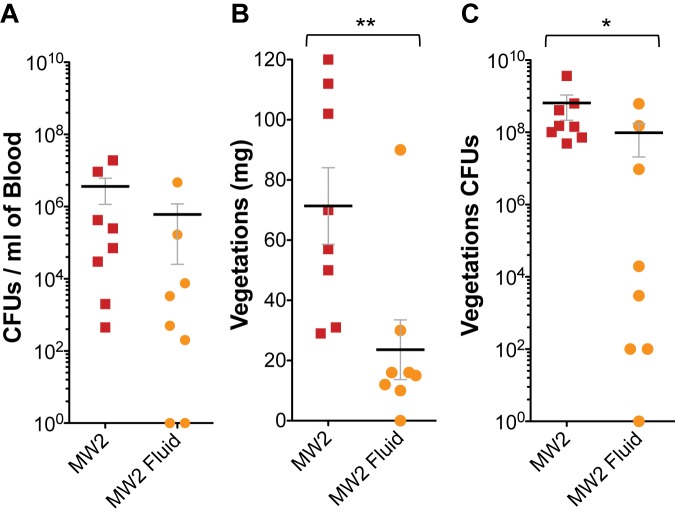
S. aureus IE in humans frequently results from hematogenous spread from a primary infection site, such as intravascular catheters, surgical wounds, pneumonia, or skin and soft tissue infections (5). SAgs produced at those sites affect the vasculature causing capillary leak and hypotension (18, 19). Hypotension may favor S. aureus’ ability to settle onto damaged heart valves (as observed histologically) and cause IE. To test this, rabbits infected with MW2 were treated with 25-ml volumes of sterile 0.9% saline subcutaneously (twice daily) to offset some of the fluid loss that occurs during the course of the infection and maintain blood tension. Fluid-treated rabbits were compared to identical numbers of untreated infected rabbits at days 2 and 3 postinfection. Rabbits treated with fluid had significantly smaller heart valve vegetations (0 to 30 mg in 7/8 rabbits versus 29 to 112 mg) and significantly fewer bacterial counts in the vegetations than untreated rabbits, even though similar numbers of bacteria were recovered from the blood of all animals, treated or untreated (Fig. 5). The observation that fluid administration reduces vegetation formation in IE suggests that fluid may increase vascular circulation, counteracting the effects of SAgs, reducing S. aureus’ capacity to colonize damaged heart valves under flow conditions, and at the same time eliminating SAgs and proinflammatory mediators through kidney filtration.
FIG 5 .
Fluid replacement therapy inhibits vegetation formation and decreases bacterial burden. For fluid replacement, 25-ml volumes of sterile 0.9% saline solution were injected subcutaneously twice daily. (A) Bacterial counts per milliliter of blood recovered from the rabbits postmortem; (B) total weight of vegetations dissected from aortic valves after intravenous inoculation with 2 × 107 to 3 × 107 CFU of wild-type MW2; (C) bacterial counts recovered from aortic valve vegetations shown in panel B. **, P = 0.0074; *, P = 0.0312; nonparametric Mann-Whitney test. Horizontal lines and error bars represent mean values ± SEM. P values of ≤0.05 are considered statistically significant.
SEC induces IL-8 in aortic endothelial cells dependent on GPCR, metalloendopeptidase, and VEGFR signaling.
The second possible mechanism of SAg action in IE is direct targeting of the aortic endothelium, inducing localized production of proinflammatory mediators, such as IL-8, which recruit polymorphonuclear cells (PMNs) and induce tissue toxicity. To test this, primary human aortic endothelial cells (HAECs) were incubated with purified SEC, and IL-8 was measured in culture supernates. We found that HAECs secrete IL-8 upon SEC stimulation in a concentration-dependent manner (Fig. 6A). High concentrations of SEC induced HAEC cytotoxicity, which explains the decreased levels of IL-8 in supernates at high doses (see Fig. S3 in the supplemental material). Since the epithelial cell response to TSST-1 is dependent on metalloendopeptidases and epidermal growth factor receptors (EGFRs) (20), we hypothesized that similar signaling mechanisms may occur in HAECs to promote localized inflammation and progression of vegetative lesions. HAECs were thus treated with specific inhibitors prior to SEC induction. The inhibitors used were TAPI-1 (pan-ADAM and matrix metalloproteinase [MMP] inhibitor), gallein (Gβγ inhibitor), and axitinib (VEGFR inhibitor). Secretion of IL-8 was decreased in the presence of each inhibitor in a concentration-dependent manner (Fig. 6B to D). These results indicate that metalloendopeptidase-mediated transactivation of VEGFR is necessary for SEC induction of IL-8 in HAECs, but this mechanism is dependent on signaling via an unknown G-protein-coupled receptor (GPCR).
FIG 6 .
SEC induces IL-8 production in primary human aortic endothelial cells (HAECs), and inhibitors of various IL-8 transcription activating pathways in epithelial/endothelial cells alter the SEC-induced IL-8 production by primary HAECs. (A) IL-8 detected in culture supernates after 6 h of incubation of primary HAECs with increasing concentrations of SEC (**, P = 0.005). Panels B to D show IL-8 detected in culture supernates after 30 min of treatment with inhibitors and 6 h of incubation with 10 µg/ml of SEC. (B) TAPI-1 (pan-ADAM and matrix metalloproteinase inhibitor; *, P = 0.016); (C) gallein (Gβγ inhibitor; *, P = 0.011); (D) axitinib (vascular endothelial growth factor receptor inhibitor; **, P = 0.008). P values were determined with the one-way ANOVA and nonparametric Kruskal-Wallis test. P values of ≤0.05 are considered statistically significant.
DISCUSSION
Sepsis and IE are serious infections of the blood and heart valves. S. aureus strains that are exceptional at causing IE in rabbits produce TSST-1, SEB, and/or SEC (21). Recently, a select group of staphylococcal SAg genes were shown to be highly prevalent in S. aureus strains from a multinational collection of IE isolates (15). However, these results were largely dismissed, with SAgs deemed as biomarkers for strains with IE potential. Here, we used MRSA MW2 to address the contribution of SAgs directly to the development of IE. MW2 produces SEC at high levels and is extraordinary in its ability to produce large septic vegetations in the sensitive rabbit model. Through gene deletion and complementation, we provide definitive evidence for the requirement of SEC in IE. This effect is not due to decreased survival of the MW2Δsec strain in the bloodstream, as bacterial counts recovered from the blood of rabbits were similar to those of the wild type. Therefore, SEC action is required for vegetation formation on heart valves. Furthermore, we have previously shown that SAg-deficient strains, when expressing TSST-1 ectopically, have increased capacities to generate septic vegetations compared to TSST-1-negative isogenic strains (13). These results highlight the critical role of SAgs in IE and explain the high prevalence of these genes in strains from IE patients.
The S. aureus virulence factors SOK, Coa, ClfA, and vWbp contribute to IE, probably by increasing survival in the bloodstream or on damaged tissue (11, 12). SAgs are known for causing immune system dysregulation and TSS (22). Hence, their role in vegetation formation is more elusive. We hypothesized that SAgs could support IE by at least two nonmutually exclusive mechanisms: (i) hypotension and immune system dysregulation (superantigenicity), which allows the organism to settle onto damage tissues, evade immune responses, and persist, and (ii) localized cytotoxicity and persistent inflammation, preventing healing of the damaged site and promoting accumulation of host factors. We provide evidence that supports both mechanisms.
First, our studies show that fluid replacement inhibits vegetation formation. In 1991, P. K. Lee et al. demonstrated that fluid replacement protected rabbits from the lethal effects of TSST-1, a hallmark publication that established the association between SAg-induced vascular leakage and toxic shock syndrome (19). IE develops in susceptible individuals with S. aureus bacteremia, frequently arising from extracardial infection sites (5). SAgs produced at these sites can lead to hypotension and, even if mild or inapparent, could favor settling of the organism onto the damaged heart valves and cause a buildup of SAg systemically. The success in reducing vegetation size and bacterial burden in infected rabbits treated with fluid highlights the importance of early fluid replacement to avoid SAg-induced changes that increase difficulty in IE management. In agreement with our studies, D. M. Mattis et al. demonstrated in the IE and sepsis model that rabbits infected with MW2 and treated intravenously with soluble, high-affinity Vβ-T cell receptor chains specific for SEC, as a neutralizing agent, had dramatically reduced vegetations and bacterial counts compared to those of untreated rabbits (14). Altogether, the data suggest that interfering with the cardiovascular effects of SAgs, vascular leakage and hypotension, protects against development of IE.
Second, histological analysis of aortic vegetative lesions showed large clusters of bacteria colonies growing seemingly uncontested on the valve leaflets and regions of necropurulent inflammation. These observations indicate a role for localized inflammation and endothelial cell toxicity in the etiology of IE. We found that purified SEC induces production of the chemokine IL-8 in primary HAECs. IL-8 production is dependent on signaling via GPCRs and metalloendopeptidase-mediated transactivation of VEGFRs. A similar mechanism has been previously described for TSST-1 induction of IL-8 in human vaginal epithelial cells (20). We propose that SAg effects on vascular endothelia cause localized inflammation and barrier dysfunction, allowing SAgs access to the endocardium while recruiting immune cells, perpetuating the production of proinflammatory cytokines, tissue toxicity, and immune evasion.
Of great importance, S. aureus IE results in systemic embolization of cardiac vegetations in approximately half the cases, which is significantly higher than IE caused by other bacteria (4). One in three patients with IE develop kidney injury and renal failure (23, 24). However, little is known about the pathogenesis of kidney injury during IE and toxic shock syndrome. In our model, kidney abscesses are observed only in rabbits infected with SEC+ strains, and as mentioned previously, the organisms are present in the bloodstream at wild-type levels. Therefore, the presence of S. aureus in the circulation, even at high levels, is not the major determinant of renal injury, IE, or shock; SAgs and their role in inflammation, toxicity, or immune dysregulation are required. In patients with septic shock, acute renal failure occurs at a rate of 1 in 5, and the principal mechanisms include ischemia or hypoperfusion, immunologically mediated glomerulonephritis, and acute renal arterial obstruction (25–27). Our studies suggest that kidney injury and abscesses are likely to be the effect of embolization of cardiac vegetations. However, other mechanisms, such as tissue ischemia, may play a role in early death (<48 h), since these rabbits had small vegetations and presented signs of massive renal infarction with small or no kidney abscesses. Further studies will be required to determine the exact mechanism of kidney injury in our model and whether intervention strategies can be develop to prevent renal injury in IE patients.
In conclusion, we provide definitive evidence for the critical role of SAgs in the etiology of IE and lethal sepsis as tested in the highly sensitive rabbit model. We demonstrate that both superantigenicity and direct stimulation of cardiac endothelial cells account for the SAg involvement. If patients with IE succumb, they do so because of heart failure, embolic strokes, and sepsis and shock associated with metastatic abscesses, all effects that can be secondary to SAg contributions to vegetation formation. An additional critical finding in our studies is the key role SAgs have in development of kidney abscesses. With all this knowledge, it becomes possible that patients may be treated with therapeutic agents that neutralize SAgs, limit disease progression, and thereby increase survival.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Community-associated, methicillin-resistant USA400 strain MW2 was originally obtained in the Upper Midwest from a young patient who succumbed to necrotizing pneumonia. Lyophilized stocks of low passage number are maintained in the Schlievert laboratory. MW2 encodes the SAgs SEC, SEA, SE-like H, SE-like K, SE-like L, SE-like Q, and SE-like X. However, SAgs are variably produced during growth in liquid culture, where SEC is produced at approximately 100,000 ng/ml and the rest are produced at very low levels (0.075 to 30 ng/ml). For endocarditis experiments, strains were grown overnight in Todd-Hewitt (TH) broth (Becton Dickinson, Sparks, MD) at 37°C, diluted, and washed in phosphate-buffered saline (PBS) before infection.
Construction of S. aureus MW2 sec or selx in-frame deletions and complemented strains.
The MW2 sec deletion plasmid was constructed by PCR amplifying the sequences upstream and downstream of sec with primer sets EcoRI-UPsecF (5′ ATCCTAGAATTCAGGCACAGCAATGTGTTCA 3′)/f1-UPsecR (5′ GCTAGCACGCGTCTCCTTCATCCAACATTCCC 3′) and f2-DNsecF (5′ ACGCGTGCTAGCGAGTGAAGATAGAAGTCCACCTTACA 3′)/AvaI-DNsecR (5′ ATCCTACCCGGGGGCAAGCATCAAACAGTTACAAC 3′), respectively. The selx deletion plasmid was constructed with upstream primers EcoRI-UPselx-F (5′ ATCCTAGAATTCCGAGTCGATAGGGCCACCAG 3′)/f1-UPselx-R (5′ GCTAGCACGCGTGACGTGCTCATGAATTAAATTCATC 3′) and downstream primers f2-DNselx-F (5′ ACGCGTGCTAGCGTTAGGTATCTAAAGGTGCCTAAC 3′)/AvaI-DNselx-R (5′ ATCCTACCCGGGCCATTCTAGTAGACACCTCAGTCG 3′). The fragments were spliced together by overlapping amplification with the primer pair EcoRI-UPsecF/AvaI-DNsecR or EcoRI-UPselx-F/AvaI-DNselx-R, digested with EcoRI and AvaI, and inserted into pJB38 (28). The resulting plasmids, pJB38-Δselx (pWSP1) or pJB38-Δsec (pWSP4), were electroporated into RN4220 and moved into MW2 by transduction with bacteriophage ϕ11. The gene deletions were introduced by allelic exchange and confirmed by PCR with the primer sets EXTsecF (5′ AACCTGAACCTACTGTTGTTAA 3′)/EXTsecR (5′ CTCTCGTACTATATATGGTGGTG 3′) for sec or EXTselxF (5′ GATGTAATGTATGCGTCG 3′)/EXTselxR (5′ CTTTGACTATAACTTCGGAGTTG 3′) for selx. Plasmid loss in selected clones was confirmed by streaking on TH plates containing chloramphenicol (10 µg/ml). The gene deletions were further confirmed by Western blotting. For complementation of the MW2Δsec strain, sec was expressed from the pCE104 plasmid under the control of the tstH promoter (pP tstOE vector) (29). SEC overexpression by the MW2Δsec strain grown in dialyzable beef heart medium was comparable to that of MW2, as determined by quantitative Western blotting (116 µg/ml for MW2 versus 90 µg/ml for the MW2Δsec strain with psec).
Rabbit model of IE and lethal sepsis.
The combined IE and sepsis model was performed as previously described (22). Briefly, young adult New Zealand white rabbits weighing 2 to 3 kg were obtained from Bakkom Rabbitry (Red Wing, MN) and anesthetized with ketamine and xylazine. The left carotid artery of each was exposed and a hard plastic catheter inserted until the aortic valve was reached (pulsation of the catheter ensured proper placement). The catheter was left in place for 2 h to induce mechanical damage to the valve and then removed, and the neck incision was sutured. Rabbits were injected through the marginal ear veins with 2 × 107 to 3 × 107 bacteria resuspended in PBS. Rabbits were monitored four times a day for survival for up to 4 days. Rabbits infected with the sec-complemented strain were injected subcutaneously with 200 µl of erythromycin (5 mg/ml) twice daily. For fluid replacement, rabbits received 25-ml volumes of sterile 0.9% NaCl subcutaneously in the nape of their necks twice a day. All rabbits receiving fluid were matched to rabbits without fluid at 2 and 3 days postinfection. At the end of experiments, surviving rabbits were euthanized with Euthasol. At necropsy, rabbits were assessed for overall health: kidney, lung, and liver injury and gastrointestinal tract abnormalities (loose stools and diarrhea). Kidneys, lung, heart, and spleen were removed for gross examination. Hearts were examined for the presence of aortic vegetations; if present, vegetations were carefully dissected, weighed, homogenized, and plated on sheep blood plates to determine bacterial counts within the vegetations. Kidneys were examined for the presence of tissue ischemia, infarcts, and abscesses. Lungs were examined for the presence of hemorrhagic consolidation and pleural effusion, tissue necrosis, and abscesses. Spleen size was used as an internal control for the level of bacteremia in each rabbit. Venous blood was recovered and homogenized, and bacterial counts were quantified on sheep blood plates. Two of the rabbits that were infected with wild-type MW2 were used for histopathology, and therefore vegetation weight and bacterial counts could not be determined. All animal experiments were performed according to established guidelines and a protocol approved by the University of Iowa Institutional Animal Care and Use Committee (protocol 1106138).
Cell culture, inhibitors, and cytokine and cytotoxicity assays.
Primary human aortic endothelial cells (HAECs) were purchased from Invitrogen (C-006-5C). Cells were maintained in tissue culture-treated flasks or plates at 37° C in 7% CO2 in medium 200 (Invitrogen, M-200-500) supplemented with endothelial growth kit (ATCC; PCS-100-041), 25 IU/ml penicillin, 25 µg/ml streptomycin, 40 µg/ml gentamicin, and 2.5 µg/ml amphotericin B (Gibco). This is referred to as complete medium. Medium 200 without any supplements or antibiotics is referred to as minimal medium. HAECs were seeded at 50,000 cells/well in 96-well plates in complete medium. At 24 h, complete medium was removed and replaced with minimal medium. At 48 h, medium was removed and minimal medium containing SEC, α-toxin, or SEC and α-toxin or medium alone was added to the cells (toxins were added at various concentrations depending on the experiment). For inhibition experiments, TAPI-1 (Enzo Life Sciences; BML-PI134-0001), gallein (Tocris; 3090), or axitinib (Tocris; 4350) were added 30 min prior to SEC treatment. Supernatant fluids were removed 6 h after treatment, and secreted IL-8 was measured via enzyme-linked immunosorbent assay (ELISA) (R&D Systems; DY208) according to the manufacturer’s instructions. The cells were assayed for viability using the MTS assay (Promega; G3580).
Statistical analyses.
Statistical significance in survival experiments was determined using the log-rank, Mantel-Cox test (GraphPad Prism Software). Significance across means was carried out using one-way analysis of variance (ANOVA), Kruskal-Wallis test, or Mann-Whitney test (GraphPad Prism Software).
SUPPLEMENTAL MATERIAL
Hematoxylin and eosin-stained section of a nascent aortic vegetative lesion. (A) A ×10 magnification of a nascent vegetative lesion containing heart muscle (asterisks), valves (solid lines), aortic artery (dashed lines) with vegetations (block arrows) primarily centered on the valves, and foci of necropurulent inflammation detected in the nearby adjacent heart muscle (long arrow). (B) Zoomed image of a nascent vegetation highlighting the S. aureus macroclusters on the valves and association with aortic endothelium in the absence of large fibrinous aggregates of large vegetations (dashed outline). Download
Hematoxylin and eosin-stained section of vegetative lesions on aortic valves characteristic of staphylococcal endocarditis. (A) A 2× composite of a vegetative lesion containing heart muscle (asterisks), valves (solid lines), and aortic artery (dashed lines) with vegetations (block arrows) primarily centered on the valves. Foci of necropurulent inflammation were detected in the nearby adjacent heart muscle (long arrow). (B) A ×20 magnification of the necropurulent inflammation shown in panel A highlighting the presence of extensive neutrophil recruitment and tissue damage, with degenerate neutrophils (neutrophils that have degranulated) at the edge of the endocardium and aortic endothelium. Download
Cytotoxicity measured after 6 h of incubation of primary human aortic endothelial cells (HAECs) with decreasing concentration of SEC. *, P = 0.0296 for SEC; *, P = 0.0129 for α-toxin. P values were determined with the one-way ANOVA and nonparametric Kruskal-Wallis test. ns, not statistically significant. Download
ACKNOWLEDGMENTS
This research was supported by NIH grants AI74283 (P.M.S.), AI57153 (P.M.S.), AI83211 (A.R.H.), and AI73366 (M.L.P.). P.M.S. is a member of the Great Lakes Regional Center of Excellence in biodefense and Emerging Infectious Diseases. W.S.-P. was supported by NIH training grant T32AI007511.
Footnotes
Citation Salgado-Pabón W, Breshears L, Spaulding AR, Merriman JA, Stach CS, Horswill AR, Peterson ML, Schlievert PM. 2013. Superantigens are critical for Staphylococcus aureus infective endocarditis, sepsis, and acute kidney injury. mBio 4(4):e00494-13. doi:10.1128/mBio.00494-13.
REFERENCES
- 1. Murdoch DR, Corey GR, Hoen B, Miró JM, Fowler VG, Bayer AS, Karchmer AW, Olaison L, Pappas PA, Moreillon P, Chambers ST, Chu VH, Falcó V, Holland DJ, Jones P, Klein JL, Raymond NJ, Read KM, Tripodi MF, Utili R, Wang A, Woods CW, Cabell CH, International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators 2009. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch. Intern. Med. 169:463–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shorr AF, Tabak YP, Killian AD, Gupta V, Liu LZ, Kollef MH. 2006. Health care-associated bloodstream infection: A distinct entity? Insights from large U.S. database. Crit. Care Med 34:2588–2595 [DOI] [PubMed] [Google Scholar]
- 3. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309–317. 10.1086/421946 [DOI] [PubMed] [Google Scholar]
- 4. Fowler VG, Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, Corey GR, Spelman D, Bradley SF, Barsic B, Pappas PA, Anstrom KJ, Wray D, Fortes CQ, Anguera I, Athan E, Jones P, van der Meer JT, Elliott TS, Levine DP, Bayer AS, ICE Investigators 2005. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 293:3012–3021 [DOI] [PubMed] [Google Scholar]
- 5. Naber CK. 2009. Staphylococcus aureus bacteremia: epidemiology, pathophysiology, and management strategies. Clin. Infect. Dis. 48(Suppl 4):S231–S237 [DOI] [PubMed] [Google Scholar]
- 6. Fowler VG, Jr, Olsen MK, Corey GR, Woods CW, Cabell CH, Reller LB, Cheng AC, Dudley T, Oddone EZ. 2003. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch. Intern. Med. 163:2066–2072 [DOI] [PubMed] [Google Scholar]
- 7. Park KH, Lee YM, Hong HL, Kim T, Park HJ, Park SY, Moon SM, Chong YP, Kim SH, Lee SO, Choi SH, Jeong JY, Kim MN, Woo JH, Kim YS. 2012. Persistent catheter-related Staphylococcus aureus bacteremia after catheter removal and initiation of antimicrobial therapy. PLoS One 7:e46389. 10.1371/journal.pone.0046389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lautenschlager S, Herzog C, Zimmerli W. 1993. Course and outcome of bacteremia due to Staphylococcus aureus: evaluation of different clinical case definitions. Clin. Infect. Dis. 16:567–573 [DOI] [PubMed] [Google Scholar]
- 9. Thiene G, Basso C. 2006. Pathology and pathogenesis of infective endocarditis in native heart valves. Cardiovasc. Pathol. 15:256–263 [DOI] [PubMed] [Google Scholar]
- 10. Vos FJ, Kullberg BJ, Sturm PD, Krabbe PF, van Dijk AP, Wanten GJ, Oyen WJ, Bleeker-Rovers CP. 2012. Metastatic infectious disease and clinical outcome in Staphylococcus aureus and Streptococcus species bacteremia. Medicine 91:86–94 [DOI] [PubMed] [Google Scholar]
- 11. Malachowa N, Kohler PL, Schlievert PM, Chuang ON, Dunny GM, Kobayashi SD, Miedzobrodzki J, Bohach GA, Seo KS. 2011. Characterization of a Staphylococcus aureus surface virulence factor that promotes resistance to oxidative killing and infectious endocarditis. Infect. Immun. 79:342–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McAdow M, Kim HK, Dedent AC, Hendrickx AP, Schneewind O, Missiakas DM. 2011. Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLOS Pathog. 7:e1002307. 10.1371/journal.ppat.1002307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pragman AA, Yarwood JM, Tripp TJ, Schlievert PM. 2004. Characterization of virulence factor regulation by SrrAB, a two-component system in Staphylococcus aureus. J. Bacteriol. 186:2430–2438. 10.1128/JB.186.8.2430-2438.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mattis DM, Spaulding AR, Chuang-Smith ON, Sundberg EJ, Schlievert PM, Kranz DM. 2013. Engineering a soluble high-affinity receptor domain that neutralizes staphylococcal enterotoxin C in rabbit models of disease. Protein Eng. Des. Sel. 26:133–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nienaber JJ, Sharma Kuinkel BK, Clarke-Pearson M, Lamlertthon S, Park L, Rude TH, Barriere S, Woods CW, Chu VH, Marín M, Bukovski S, Garcia P, Corey GR, Korman T, Doco-Lecompte T, Murdoch DR, Reller LB, Fowler VG, International Collaboration on Endocarditis-Microbiology Investigators 2011. Methicillin-susceptible Staphylococcus aureus endocarditis isolates are associated with clonal complex 30 genotype and a distinct repertoire of enterotoxins and adhesins. J. Infect. Dis. 204:704–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schlievert PM. 2009. Cytolysins, superantigens, and pneumonia due to community-associated methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 200:676–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wilson GJ, Seo KS, Cartwright RA, Connelley T, Chuang-Smith ON, Merriman JA, Guinane CM, Park JY, Bohach GA, Schlievert PM, Morrison WI, Fitzgerald JR. 2011. A novel core genome-encoded superantigen contributes to lethality of community-associated MRSA necrotizing pneumonia. PLOS Pathog. 7:e1002271. 10.1371/journal.ppat.1002271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee PK, Vercellotti GM, Deringer JR, Schlievert PM. 1991. Effects of staphylococcal toxic shock syndrome toxin 1 on aortic endothelial cells. J. Infect. Dis. 164:711–719 [DOI] [PubMed] [Google Scholar]
- 19. Lee PK, Deringer JR, Kreiswirth BN, Novick RP, Schlievert PM. 1991. Fluid replacement protection of rabbits challenged subcutaneous with toxic shock syndrome toxins. Infect. Immun. 59:879–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Breshears LM, Schlievert PM, Peterson ML. 2012. A disintegrin and metalloproteinase 17 (ADAM17) and epidermal growth factor receptor (EGFR) signaling drive the epithelial response to Staphylococcus aureus toxic shock syndrome toxin-1 (TSST-1). J. Biol. Chem. 287:32578–32587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spaulding AR, Satterwhite EA, Lin YC, Chuang-Smith ON, Frank KL, Merriman JA, Schaefers MM, Yarwood JM, Peterson ML, Schlievert PM. 2012. Comparison of Staphylococcus aureus strains for ability to cause infective endocarditis and lethal sepsis in rabbits. Front. Cell. Infect. Microbiol. 2:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McCormick JK, Yarwood JM, Schlievert PM. 2001. Toxic shock syndrome and bacterial superantigens: an update. Annu. Rev. Microbiol. 55:77–104 [DOI] [PubMed] [Google Scholar]
- 23. Majumdar A, Chowdhary S, Ferreira MA, Hammond LA, Howie AJ, Lipkin GW, Littler WA. 2000. Renal pathological findings in infective endocarditis. Nephrol. Dial. Transplant. 15:1782–1787 [DOI] [PubMed] [Google Scholar]
- 24. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Kidney Acute, Injury Network 2007. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit. Care 11:R31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Russell JA, Singer J, Bernard GR, Wheeler A, Fulkerson W, Hudson L, Schein R, Summer W, Wright P, Walley KR. 2000. Changing pattern of organ dysfunction in early human sepsis is related to mortality. Crit. Care Med. 28:3405–3411 [DOI] [PubMed] [Google Scholar]
- 26. Lucas S. 2007. The autopsy pathology of sepsis-related death. Diagn. Histopathol. 13:375–388 [Google Scholar]
- 27. Doi K, Leelahavanichkul A, Yuen PS, Star RA. 2009. Animal models of sepsis and sepsis-induced kidney injury. J. Clin. Invest. 119:2868–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bose JL, Fey PD, Bayles KW. 2013. Genetic tools to enhance the study of gene function and regulation in Staphylococcus aureus. Appl. Environ. Microbiol. 79:2218–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schlievert PM, Jablonski LM, Bonach GA. 2000. Pyrogenic toxin superantigen site specificity in toxic shock syndrome and food poisoning in animals. Infect. Immun. 68:3630–3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hematoxylin and eosin-stained section of a nascent aortic vegetative lesion. (A) A ×10 magnification of a nascent vegetative lesion containing heart muscle (asterisks), valves (solid lines), aortic artery (dashed lines) with vegetations (block arrows) primarily centered on the valves, and foci of necropurulent inflammation detected in the nearby adjacent heart muscle (long arrow). (B) Zoomed image of a nascent vegetation highlighting the S. aureus macroclusters on the valves and association with aortic endothelium in the absence of large fibrinous aggregates of large vegetations (dashed outline). Download
Hematoxylin and eosin-stained section of vegetative lesions on aortic valves characteristic of staphylococcal endocarditis. (A) A 2× composite of a vegetative lesion containing heart muscle (asterisks), valves (solid lines), and aortic artery (dashed lines) with vegetations (block arrows) primarily centered on the valves. Foci of necropurulent inflammation were detected in the nearby adjacent heart muscle (long arrow). (B) A ×20 magnification of the necropurulent inflammation shown in panel A highlighting the presence of extensive neutrophil recruitment and tissue damage, with degenerate neutrophils (neutrophils that have degranulated) at the edge of the endocardium and aortic endothelium. Download
Cytotoxicity measured after 6 h of incubation of primary human aortic endothelial cells (HAECs) with decreasing concentration of SEC. *, P = 0.0296 for SEC; *, P = 0.0129 for α-toxin. P values were determined with the one-way ANOVA and nonparametric Kruskal-Wallis test. ns, not statistically significant. Download