Abstract
Background
Posttraumatic stress disorder (PTSD) is characterized as a disorder of exaggerated defensive physiological arousal. The novel aim of the present research was to investigate within PTSD a potential dose-response relationship between past trauma recurrence and current comorbidity and intensity of physiological reactions to imagery of trauma and other aversive scenarios.
Methods
A community sample of principal PTSD (n = 49; 22 single-trauma exposed, 27 multiple-trauma exposed) and control (n = 76; 46 never-trauma exposed, 30 trauma exposed) participants imagined threatening and neutral events while acoustic startle probes were presented and the eye-blink response (orbicularis occuli) was recorded. Changes in heart rate, skin conductance level, and facial expressivity were also indexed.
Results
Overall, PTSD patients exceeded control participants in startle reflex, autonomic responding, and facial expressivity during idiographic trauma imagery and, though less pronounced, showed heightened reactivity to standard anger, panic, and physical danger imagery. Concerning subgroups, control participants with and without trauma exposure showed isomorphic patterns. Within PTSD, only the single-trauma patients evinced robust startle and autonomic responses, exceeding both control participants and multiple-trauma PTSD. Despite greater reported arousal, the multiple-trauma relative to single-trauma PTSD group showed blunted defensive reactivity associated with more chronic and severe PTSD, greater mood and anxiety disorder comorbidity, and more pervasive dimensional dysphoria (e.g., depression, trait anxiety).
Conclusions
Whereas PTSD patients generally show marked physiological arousal during aversive imagery, concordant with self-reported distress, the most symptomatic patients with histories of severe, cumulative traumatization show discordant physiological hyporeactivity, perhaps attributable to sustained high stress and an egregious, persistent negative affectivity that ultimately compromises defensive responding.
Keywords: Anger, anxiety, anxiety sensitivity, chronicity, comorbidity, cumulative trauma, depression, diagnostic subtypes, emotional reactivity, facial expressivity, heart rate, mental imagery, multiple trauma, narrative imagery, psychophysiology, PTSD, skin conductance, startle, trauma, trauma duration, trauma recurrence
This research explores a potential dose-response relationship between trauma recurrence and intensity of physiological reactions to trauma memories in principal posttraumatic stress disorder (PTSD). Epidemiological work has revealed that exposure to multiple compared with single traumatic events more strongly predisposes the development of PTSD (1). Cumulative exposure is associated with more severe (2–4) and chronic posttraumatic stress (5,6), more generalized symptoms (e.g., nonspecific anxiety, anger) (7–9), increased morbidity rates (e.g., depression, panic, substance abuse) (9,10), and poorer socio-occupational functioning (2,6,11). In effect, multiple compared with single traumatic exposure more perniciously sensitizes individuals to subsequent stress (1), prolonging pathological emotional processing across numerous symptom domains.
Script-driven emotional imagery is a valuable tool in studies of PTSD, permitting presentation of idiographic trauma challenges. Findings that physiological arousal to fear imagery parallels anticipatory reactions to threatening events corroborates its ecological validity (12), similarly mobilizing the autonomic nervous system (e.g., heart rate, skin conductance), communicating threat through facial musculature (e.g., corrugator “frown” muscle), and prompting somatic reflexive action (e.g., startle potentiation) (13,14). Animals confronting survival threat show similar reactions, mediated by the brain’s defense circuit (centered on the amygdala) (15,16) and neuroimaging studies suggest that a comparable circuit (17–19) underlies human fear.
Autonomic and somatic hyperarousal during trauma-related imagery are hallmark symptoms of PTSD (20) demonstrated in many trauma populations (combat [21–23], childhood sexual abuse [24], breast cancer [25], war zone nursing [26], heterogeneous civilian events [27]). Exaggerated reactivity is not consistent, however, across all physiological measures. Although heart rate and skin conductance responses are often both recorded, frequently only a single autonomic measure shows increases during trauma-related imagery (21,24,27–29). Equivalent reactivity between control and PTSD groups has been observed in autonomic or facial muscle measures (30) and diminished rather than heightened fear potentiation and heart rate responses in PTSD (31–34). Surveying a cumulative sample of 96 patients across multiple studies, Pitman et al. (25) estimated that 30% to 40% of PTSD participants are physiologically nonresponsive during trauma-related processing.
In a series of imagery investigations, Cuthbert et al. (34), Cook et al. (35), McNeil et al. (36), Weerts and Lang (37), and Lang et al. (12,38,39) have explored evoked defensive arousal differences across the spectrum of anxiety diagnoses: specific and social phobia patients demonstrated the greatest autonomic and startle responses. Paradoxically, patients with more pervasive and diffuse anxiety symptomatology—panic disorder with agoraphobia, generalized anxiety disorder (GAD)—showed less robust fear potentiation (despite reports of intense fear). This reflex blunting was consistently more pronounced across and within respective diagnoses, coincident with increased clinician-rated severity, poorer prognosis, greater comorbidity (depression and anxiety), elevated questionnaire-based indexes of negative affectivity, and lengthier disorder chronicity (40,41), suggesting that defensive engagement during imagery might be compromised by long-term stress and accompanying dysphoria.
In the current study, it was expected that similar to many studies (21,23), PTSD patients as a whole would demonstrate heightened defense circuit activation relative to control participants when confronting trauma-related imagery (i.e., potentiating startle and enhancing skin conductance, heart rate, and facial muscle action [corrugator]). Furthermore, patients and control participants were expected to react similarly during neutral scenarios and threatening contexts for which defensive mobilization is normal and adaptive (e.g., facing an attacking animal). Standard anger and panic attack scenarios were also assessed in expectation that these symptom-relevant, but nontrauma-related, scenarios would prompt more reactivity in patients than control participants (30,42), as PTSD patients often report anger during aversive imagery (21,23,28) and anger (43) and panic attacks (44,45) are prominent posttraumatic symptoms.
Regarding trauma extent in PTSD, single-trauma PTSD patients were expected to show robust physiological responses during aversive imagery similar to phobic disorders (36–41). However, multiple-trauma PTSD patients—likely more severe with higher depression and anxiety comorbidity—would demonstrate blunted physiology as found in other anxiety spectrum disorders characterized by pervasive anxiety and prominent depression. Finally, control participants with a trauma history were not expected to differ in responsiveness from nonexposed control participants (46).
Methods and Materials
Participants
Participants (81% Caucasian) were assessed at the University of Florida Fear and Anxiety Disorders Clinic: 49 treatment-seeking adults with principal diagnoses of PTSD (66% female) and 76 healthy community control participants (71% female).
Diagnostic Classification
Diagnostic groups were established using the Anxiety Disorder Interview Schedule for DSM-IV (ADIS-IV) (47), a semi-structured interview for assessing current anxiety, mood, substance use, and somatoform disorders and for screening psychosis and major medical disease.
For multiple Axis I disorders, diagnostic primacy was determined by clinician-rated severity (ranging from 0, No features present, to 5, Diagnosis present; severe) reflecting both distress and interference. control participants denied current or lifetime diagnoses of psychiatric illness.
For trauma subtype assignment, patients with a lifetime history of one criterion A event (reported during ADIS administration and meeting both A1 and A2 criteria) were classified single-trauma (n = 22), whereas those with two or more were classified multiple-trauma (n = 27; Table 1). Multiple-trauma patients reported a minimum of three different types of high magnitude traumas (e.g., interpersonal/assaultive violence). Types of exposure in addition to the index trauma are listed in Table 2. control participants were simply classified as exposed (n = 30) or nonexposed (n = 46) to at least one trauma. All trauma-positive participants endorsed direct exposure.
Table 1.
Proportion of Control and PTSD Groups by Index Trauma
| Trauma type | Trauma-Exposed Control (n = 30) | Single-Trauma PTSD (n = 22) | Multiple-Trauma PTSD (n = 27) |
|---|---|---|---|
| Childhood | |||
| Accident/Injury | .10 | 0 | 0 |
| Natural Disaster | .03 | 0 | 0 |
| Physical &/Sexual Abuse | .03 | 0 | .33 |
| Sexual Assaulta | .03 | .09 | .11 |
| Witnessed Injury/Death | .17 | 0 | 0 |
| Adulthood | |||
| Accident/Injury | .13 | .45 | .11 |
| Combat Exposure | .03 | 0 | .07 |
| Domestic Violence/Stalking | 0 | 0 | .07 |
| Natural Disaster | .07 | .05 | 0 |
| Sexual &/Physical Assault | .14 | .23 | .26 |
| Witnessed Injury/Death | .27 | .18 | .04 |
PTSD, posttraumatic stress disorder.
Includes childhood stranger and extra/intrafamilial single incident sexual assaults.
Table 2.
Proportion of Multiple-Trauma PTSD Group Endorsing the Following Traumatic Events in Addition to Index Trauma
| Trauma Type | Multiple-Trauma PTSD (n = 27) |
|---|---|
| Childhood | |
| Accident/Injury | .04 |
| Natural Disaster | 0 |
| Physical &/Sexual Abuse | .26 |
| Sexual Assaulta | .33 |
| Witnessed Injury/Death | .18 |
| Adulthood | |
| Accident/Injury | .11 |
| Combat Exposure | 0 |
| Domestic Violence/Stalking | .22 |
| Natural Disaster | .04 |
| Sexual &/Physical Assault | .15 |
| Witnessed Injury/Death | .18 |
PTSD, posttraumatic stress disorder.
Includes childhood stranger and extra/intrafamilial single incident sexual assaults.
Procedure
The University of Florida Institutional Review Board (IRB-01) approved the study and participants provided informed consent before assessment. Participants completed questionnaires and interview in the morning; psychophysiological assessment and clinical debriefing followed in the afternoon.
Experimental Stimuli
Twenty-four narrative imagery texts were used (48). Analyses focused on two idiographic, “personal” threat narratives representing each patient’s primary clinical fear1 or for control participants their “worst fear” experiences (Table S1 in Supplement 1). Standard scenes included two anger (witnessing a dog intentionally harmed, having parking spot taken), two panic attack (in busy checkout line, while driving), four survival threat (physical attack by animal/human), and two neutral (watching documentary, reading magazine) events. Filler scripts were low arousal or engaging pleasant scenes to impede development of an overall unpleasant arousal context. Scripts were ~20 words designed to quickly reveal affect and reflect active participation. A woman recorded the scenes using minimal prosody for presentation over earphones (Telephonics TDH-49, Telephonics Corporation, Huntington, New York).
Imagery Assessment
Seated in a quiet, dimly lit room with electrodes placed, participants were instructed to listen to the auditory scripts with eyes closed, vividly imagining the events described, as if actively involved. Throughout the recording session, soft tones cued participants to relax, breathe slowly, and silently repeat the word “one” to stabilize between-trial physiological activity (49). Imagery scripts were interspersed every 36 seconds in the tone series with content pseudorandomized so that no more than two stimuli of the same hedonic valence (pleasant, neutral, unpleasant) or content category (e.g., survival threat) were presented consecutively. The script series was repeated in a counterbalanced order.
Trials consisted of a 1-second baseline, the 6-second auditory script, and 12 seconds of imagery. Startle probes (50 msec 95 dB[A] white noise, instantaneous rise-time) were presented at 4 to 5.5 seconds or 10 to 11.5 seconds postscript onset, or both, and on 25% of intertrial intervals, at 22 to 23.5 seconds postimagery offset.
Following imagery assessment, participants rated each scene for experienced pleasure and emotional arousal (50).
Experimental Control and Data Collection
A PC-compatible computer running VPM software (51) controlled stimulus presentation and data acquisition. Bioamplifiers recorded electromyograph (EMG) potentials at left orbicularis occuli and corrugator supercilii, skin conductance level (SCL), and electrocardiogram (ECG) as reported (41).
Data Reduction and Analysis
Univariate analyses of variance (ANOVAs) and Tukey honestly significant difference tests for planned comparisons determined group differences in demographic and questionnaire data.
Using VPM software, EMG, SCL (normalized [log(SCL+1)]), and ECG R-R intervals (converted to beats-per-minute) were reduced into half-second bins. Responses were determined by subtracting amplitude during the 1 second before script presentation from averages during the 12-second imagery period.
Startle blinks from orbicularis oculi EMG represented the magnitude difference between onset and peak muscle potential (52), standardized within subject in relation to the mean and standard deviation of intertrial probe responses (34).
Using SPSS (SPSS Inc., Chicago, Illinois), omnibus repeated measures ANOVAs were performed separately for each physiological measure, with diagnostic status (control subject, patient) as the between-subjects factor and imagery content as the within-subjects factor. Startle and autonomic reactivity during imagery have been shown to strongly covary with rated emotional arousal (34–36); thus, contents were entered according to the linear increase in arousal reported by the patients (i.e., neutral, anger, panic, survival threat, idiographic/personal threat). Significant overall group effects were followed up with between-group tests by contents to specify which imagery scenarios evoked different sensitivities in patients and control participants, facilitating comparisons with preceding imagery studies of PTSD that utilized different contents (21–28). Within-group comparisons explicated interactions. Analyses were repeated for exposure subtypes (i.e., no exposure/trauma-exposed control subject; single-trauma/multiple-trauma PTSD). Guided by prior investigations focused on idiographic threat-related imagery (20,22,27), group comparisons on that content were tested irrespective of omnibus results. Wilks’ lambda addressed sphericity issues (53).
Results
PTSD Versus Control Groups
Affective Judgments
Both groups rated personal threat images most and neutral scenes least unpleasant, F(4,116) = 145.26, p <.001. Patients rated panic and personal threat scenes more unpleasant than control participants, ps <.05. Furthermore, control participants rated personal threat, anger, and survival threat scenes equivalently, all ns; patients rated personal threat as more aversive than all other contents, all ps <.001; content × diagnosis interaction, F(4,116) = 3.77, p <.01.
Emotional arousal also varied with content (Table 3), content F(4,116) = 119.98, p <.001; content × diagnosis F(4,116) = 3.55, p <.01. control participants rated personal threat scenes most arousing followed by survival threat, anger, panic, and neutral scenes. Patients showed the same extremes, but anger, panic, and survival threat did not differ. Additionally, patients endorsed higher arousal than control participants for panic, anger,2 and neutral scenes.
Table 3.
Mean Responses and Standard Deviations to Imagery Scenes by Control and PTSD Groups
| Response Modality/Imagery Scene | Control | PTSD | Group Effect |
|---|---|---|---|
| Pleasure (1–9) | |||
| Neutral | 6.93 (1.53) | 6.41 (1.74) | F(1,121) = 3.04, ns |
| Anger | 2.79 (1.22)a | 2.59 (1.14)a | F(1,121) = .90, ns |
| Panic attack | 4.00 (1.08)a | 3.49 (1.49)a | F(1,121) = 4.82, p < .05 |
| Survival threat | 2.68 (.99)a | 2.59 (1.36)a | F(1,121) = .20, ns |
| Personal/idiographic threat | 2.65 (1.41)a | 1.62 (1.11)a | F(1,119) = 17.99, p < .001 |
| Arousal (1–9) | |||
| Neutral | 2.31 (1.54) | 3.10 (1.78) | F(1,121) = 6.81, p < .05 |
| Anger | 6.15 (1.78)a | 6.73 (1.71)a | F(1,121) = 3.22, p < .05b |
| Panic attack | 5.37 (1.90)a | 6.88 (1.44)a | F(1,121) = 21.80, p < .001 |
| Survival threat | 6.69 (1.53)a | 6.99 (1.70)a | F(1,121) = 1.00, ns |
| Personal/idiographic threat | 7.69 (1.67)a | 8.12 (1.66)a | F(1,119) = 1.93, ns |
| Startle Reflex (t score) | |||
| Neutral | 49.76 (5.01) | 52.16 (10.78) | F(1,107) = 2.50, ns |
| Anger | 51.16 (6.08) | 56.34 (15.56)a | F(1,107) = 5.54, p < .05 |
| Panic attack | 51.51 (7.20)a | 55.30 (9.53)a | F(1,107) = 5.53, p < .05 |
| Survival threat | 54.57 (8.22)a | 57.42 (13.80)a | F(1,107) = 1.83, ns |
| Personal/idiographic threat | 53.58 (7.33)a | 59.37 (24.86)a | F(1,107) = 3.24, p < .05b |
| SCL Δ (log [μS + 1]) | |||
| Neutral | −.006 (.036) | −.013 (.061) | F(1,118) = 4.87, p < .05 |
| Anger | .0005 (.035) | .012 (.048) | F(1,119) = 2.52, ns |
| Panic attack | .0009 (.042) | .011 (.067) | F(1,117) = .98, ns |
| Survival threat | .004 (.022)a | .031 (.089)a | F(1,118) = 6.30, p < .05 |
| Personal/idiographic threat | .045 (.086)a | .063 (.145)a | F(1,118) = .79, ns |
| Heart Rate Δ (bpm) | |||
| Neutral | −.46 (2.03) | −.95 (2.04) | F(1,120) = 1.68, ns |
| Anger | −.27 (1.43) | −.68 (1.37) | F(1,120) = 2.49, ns |
| Panic attack | −.22 (1.50) | .20 (1.62)a | F(1,121) = 2.40, ns |
| Survival threat | .04 (1.00)a | –.29 (1.12)a | F(1,121) = 3.02, ns |
| Personal/idiographic threat | 1.00 (1.00)a | 2.02 (3.07)a | F(1,120) = 4.56, p < .05 |
| Corrugator EMG Δ (μV) | |||
| Neutral | −.07 (.79) | .18 (1.71) | F(1,122) = 1.19, ns |
| Anger | 1.17 (2.77)a | .80 (1.67)a | F(1,122) = .72, ns |
| Panic attack | .49 (1.40)a | .60 (1.24)a | F(1,122) = .19, ns |
| Survival threat | .96 (1.98)a | 1.34 (2.10)a | F(1,122) = 1.05, ns |
| Personal/idiographic threat | 1.17 (2.60)a | 2.66 (5.15)a | F(1,122) = 4.52, p < .05 |
Note: Pleasure rated on SAM (50): 1 = completely unhappy, 9 = completely happy; Arousal rated on SAM: 1 = completely relaxed, 9 = completely aroused.
Δ, change; bpm, residual beats per minute after removal of baseline effects; EMG, electromyographic; μS, microsiemen; μV, microvolt; PTSD, posttraumatic stress disorder; SAM, Self-Assessment Manikin; SCL, skin conductance level.
Within-group comparison to neutral significant at p < .05.
One-tailed test.
Baseline Physiology
No group differences emerged for blink magnitude to intertrial startle probes or for SCL, corrugator, or orbicularis activity in the 1-second baseline before script onset (Table S2 in Supplement 1). Consistent with preceding studies (54), patients exceeded control participants in heart rate, F(1,121) = 25.24, p < .001.3
Startle Reflex Potentiation
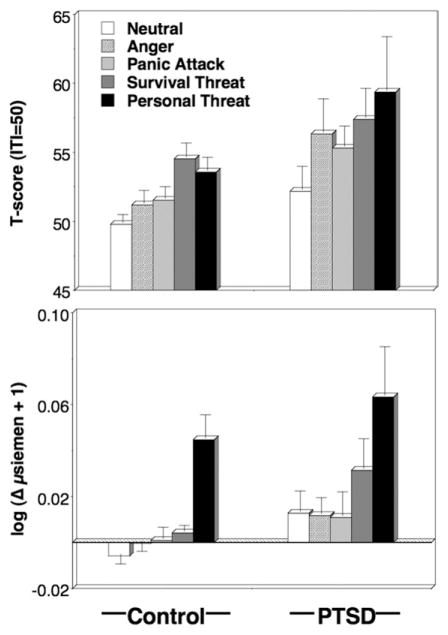
Blink magnitude (Figure 1, Table 3) was larger during unpleasant compared with neutral imagery, content F(4,104) = 7.85, p < .001, all unpleasant-neutral comparisons, p < .01; patients were generally more reactive than control participants, diagnosis F(1,107) = 5.14, p < .05, content × diagnosis F(4,104) = 1.00, ns, exceeding control participants in reflexes elicited during all unpleasant contents except survival threat.
Figure 1.
Mean startle reflex responses (standardized to the distribution of responses during intertrial intervals; top panel) and skin conductance level change (bottom panel) during neutral, anger, panic, survival threat, and personal threat imagery in control and PTSD groups. Error bars refer to standard error of the mean. PTSD, posttraumatic stress disorder.
Autonomic and Facial Responses
control participants and patients showed similar patterns of sympathetic reactivity across contents, content F(4,114) = 6.03, p < .001, content × diagnosis F(4,114) = .96, ns, with increased skin conductance during survival and personal threat relative to neutral imagining, ps < .01. An overall difference was suggested, diagnosis F(1,117) = 3.90, p < .05, attributable to larger increases for patients during survival threat and unexpectedly, although consistent with arousal ratings, for neutral as well (Figure 1, Table 3).
Paralleling startle and SCL findings, heart rate change was modulated by imagery scene, content F(4,116) =10.46, p < .001, with significant increases above neutral for panic, survival, and personal threat imagery, ps < .05. Contents varied similarly between groups, content × diagnosis F(4,116) = 2.03, ns, except that control participants showed a significant linear increase from neutral, with personal threat most extreme, followed by survival threat; conversely, patients showed their second largest response to imagery of panic attacks—a response discordant from their arousal ratings, content × diagnosis (linear contrast), F(1,116) = 4.23, p < .05. As predicted, acceleration in patients surpassed control participants during personal threat imagery (Table 3).
Both patients and control participants demonstrated increased corrugator tension during unpleasant relative to neutral imagining (Figure 2), content F(4,119) = 8.87, p < .001. However, reactivity to specific contents differed within diagnosis, content × diagnosis F(4,119) = 2.51, p < .05: control participants augmented similarly to anger, survival, and personal threat imagery and more modestly, but reliably, for panic scenarios, comparisons with neutral ps < .01. In contrast, patients showed by far the most robust contraction to personal threat imagery, exceeding all other contents, ps < .05. Furthermore, the survival threat increase was secondary, surpassing reactivity to panic and anger and the minimal response to neutral scenarios. Notably, patients’ corrugator response to personal threat reliably surmounted that for control participants (Table 3).4
Figure 2.
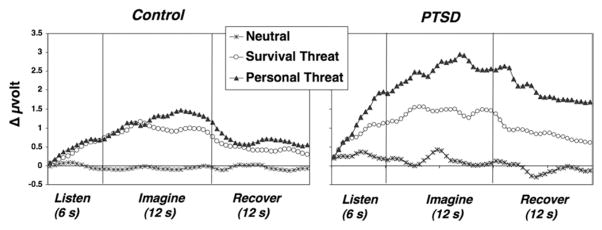
Corrugator electromyography change in half-second averages during neutral, survival, and personal threat script perception; imagery; and recovery for control (left panel) and PTSD (right panel) groups. Throughout all epochs, PTSD patients were reliably more reactive than control subjects to personal threat. PTSD, posttraumatic stress disorder.
Trauma Subtypes
Evaluative Ratings
Multiple-trauma patients rated imagery contents overall more aversive than both control groups, diagnosis F(3,117) = 5.88, p < .01, control subgroups versus multiple-trauma comparisons, ps < .01. Single-trauma patients were intermediate (subgroup comparisons ns). Specifically for personal threat, both PTSD subgroups exceeded control participants, content × diagnosis F(12,301.91) = 1.79, p < .05, control versus patient comparisons, ps < .05. Multiple-trauma patients also surpassed control participants in overall arousal, diagnosis F(3,117) = 4.75, p < .01, multiple-trauma versus trauma-exposed control participants, p < .01, versus nontrauma, p < .05, with single-trauma patients again intermediate.
Defensive Physiology
The exaggerated startle potentiation characteristic of the overall PTSD group was clearly driven by the strong responding of single-trauma patients,5 group F(3,105) = 5.33, p < .01, exceeding both the multiple-trauma and control groups, ps < .01 (Figure 3). Between-group content tests were significant except neutral and survival threat. Single-trauma patients showed augmented responding relative to control and multiple-trauma groups during anger and personal threat, ps < .01, and compared with control participants during panic imagery, p < .05. For the multiple-trauma group, responses to personal threat did not differ from neutral imagery and were also reliably less than for both panic and survival threat imagery, post hoc: ps < .05. Startle response differences between neutral and unpleasant imagery were also analyzed for the single-trauma patients. These results further underscored the defensive hyper-responsivity of the single-trauma group: fear potentiation was greater for the single-trauma than control and multiple-trauma groups for both anger, diagnosis F(3,105) = 3.15, p < .05, and personal threat imagery, diagnosis F(3,105) = 4.31, p < .01, all between-groups, ps < .05.
Figure 3.
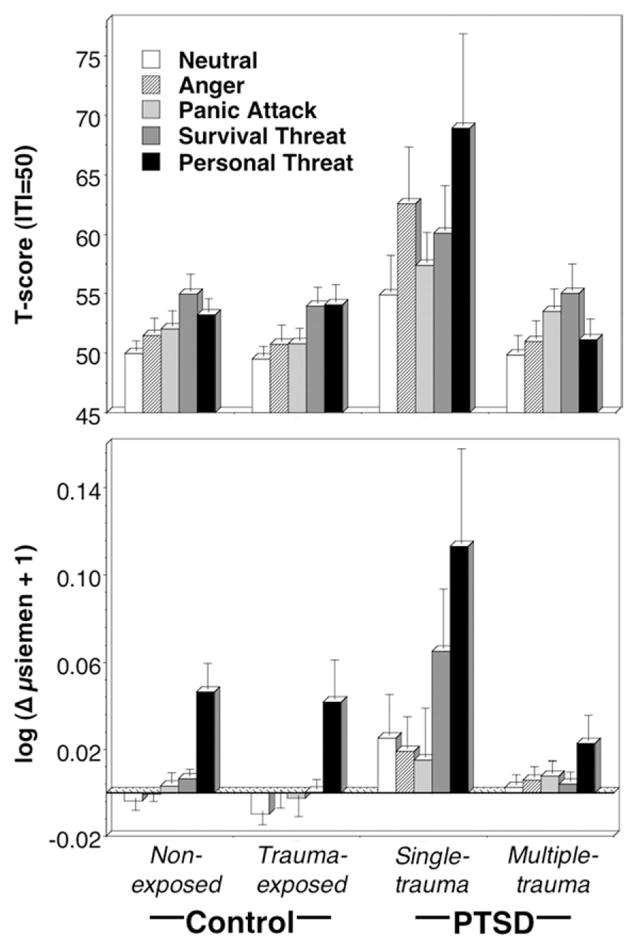
Mean startle reflex responses (top panel) standardized to the distribution of responses during intertrial intervals and skin conductance level change (bottom panel) during neutral, anger, panic, survival threat, and personal threat imagery for nonexposed and trauma-exposed control groups and single- and multiple-trauma PTSD groups. Error bars refer to standard error of the mean. PTSD, posttraumatic stress disorder.
A strikingly similar pattern emerged in skin conductance (Figure 3): single-trauma patients showed heightened sympathetic activation relative to the multiple-trauma and both control groups, ps < .05: content F(4,112) = 6.33, p < .001, diagnosis F(3,115) =4.42, p < .01, content × diagnosis F (12,296.62) = .25, ns. For survival threat, the single-trauma group evinced larger conductance increases than the multiple-trauma and both control groups, ps < .01, diagnosis F(3,116) = 7.13, p < .001, and during personal threat imagery reliably exceeded the multiple-trauma group, p < .05, with the same tendency relative to control participants, ps < .05,2 diagnosis F(3,116) = 2.90, p < .05. This same pattern of increased responding in the single-trauma group was weakly evident during neutral imagery, p < .06, no such trend was found for multiple-trauma patients.
Heart rate changes during imagery were generally similar over contents in the four subgroups. However, planned comparisons for personal threat imagery revealed greater acceleration in the single-trauma group (M =2.66, SD =3.75) than for both nonexposed (M =1.05, SD =2.28) and trauma-exposed (M = .93, SD =2.04) control participants, ps < .05.2
In contrast to startle and autonomic indexes, marked corrugator reactions during survival and personal threat imagery were equivalent for both single- and multiple-trauma groups, significantly greater than for control participants.6
Trauma Characteristics
Relative to the single-trauma group, the multiple-trauma group indicated significantly more prevalent intentional trauma (single: 40.9%, multiple index: 92.6%, multiple additional: 74.1%), childhood trauma (single: 9.1%, multiple index: 44.4%, multiple additional: 51.9%), and specifically, childhood sexual and/or physical abuse (single: 0%, multiple index: 33.3%, multiple additional: 26%). Rate of index trauma occurring to self (vs. witnessing) was similarly high in both patient groups (single: 81.8%, multiple: 96.3%).7 Overall, these characteristics suggest not only cumulative but also more severe trauma exposure in the multiple-trauma group. In post hoc analyses to explore the attenuated reactivity of the multiple-trauma group, these variables exerted neither a main effect nor interaction on any measure of defensive physiology.
Trauma Duration and PTSD Chronicity
Traumatic events persisted over a lengthier period for multiple-trauma patients (M = 18.14 years, SD = 11.87), with the initial event occurring at approximately 11 years (SD = 9.24) and the most recent event at 29 years (SD = 10.0); traumatic exposure in single-trauma PTSD occurred at approximately 32 years of age (SD = 13.35). Post-traumatic stress disorder onset for single-trauma patients was approximately 9 years later than for multiple-trauma patients (Table 4). Because age at evaluation did not differ, PTSD chronicity was significantly longer in the multiple-trauma than single-trauma patients (Figure 4).
Table 4.
Demographic, Interview, and Questionnaire Responses (Means and Standard Deviations) for Control and PTSD Exposure Subtypes
| Measure | Nonexposed Control | Trauma-Exposed Control | Single-Trauma PTSD | Multiple-Trauma PTSD | Group Effect |
|---|---|---|---|---|---|
| Questionnaire Measures | |||||
| ASI total | 8.41 (6.40)c,d | 10.05 (7.45)c,d | 28.0 (15.74)a,b,d | 43.17 (9.88)a,b,c | F(3,113) = 89.46, p < .001 |
| STAXI-Trait | 13.89 (3.47)c,d | 15.23 (4.52)c,d | 20.63 (7.41)a,b | 19.46 (6.22)a,b | F(3,117) = 11.53, p < .001 |
| STAXI-State | 10.26 (.74)c,d | 10.53 (1.74)c,d | 15.55 (7.28)a,b | 16.38 (7.64)a,b | F(3,119) =79.89, p < .001 |
| STAI-Trait | 30.87 (8.51)c,d | 30.69 (8.82)c,d | 53.67 (15.92)a,b,d | 61.87 (8.76)a,b,c | F(3,116) = 73.91, p < .001 |
| STAI-State | 28.59 (7.17)c,d | 27.87 (7.86)c,d | 54.55 (16.48)a,b | 59.37 (10.77)a,b | F(3,119) = 79.89, p < .001 |
| BDI total | 3.00 (3.85)c,d | 4.30 (5.42)c,d | 23.29 (11.45)a,b,d | 28.82 (8.38)a,b,c | F(3,120) = 10.89, p < .001 |
| QMI total | 87.83 (31.91) | 75.50 (24.62) | 97.95 (38.74) | 87.96 (33.07) | F(3,117) = 2.07, ns |
| Interview Measures | |||||
| Re-experiencing symptoms (0–40) | 25.86 (8.03) | 27.69 (8.15) | F(1,47) = .62, ns | ||
| Strategic avoidance symptoms (0–16) | 10.20 (4.57)d | 12.11 (3.77)c | F(1,47) = 2.60, p < .05e | ||
| Emotional numbing symptoms (0–24) | 12.50 (6.44)d | 17.76 (5.37)c | F(1,47) = 9.72, p < .01 | ||
| Hyperarousal symptoms (0–40) | 24.32 (5.90)d | 27.67 (6.14)c | F(1,47) = 3.12, p < .05e | ||
| Age of disorder onset (years) | 32.09 (12.81)d | 23.74 (13.15)c | F(1,47) = 5.00, p < .05 | ||
| PTSD chronicity (years) | 5.05 (10.31)d | 17.22 (14.25)c | F(1,47) = 11.24, p < .01 | ||
| PTSD severity (0–5) | 3.77 (.81)d | 4.44 (.58)c | F(1,47) = 11.41, p < .01 | ||
| Prognosis (1–4) | 1.86 (.77)d | 3.04 (.90)c | F(1,47) = 23.38, p < .001 | ||
| Comorbid Axis I disorders (count) | 1.23 (1.48)d | 2.52 (1.42)c | F(1,47) = 9.83, p < .01 | ||
| Comorbid anxiety disorder (%) | 31.8d | 74.1c | X2(1) =8.75, p < .01 | ||
| Comorbid depressive disorder (%) | 51.5d | 85.2c | X2(1) = 5.58, p < .05 | ||
| Demographics | |||||
| Age (years) | 31.00 (11.08)d | 33.00 (12.48) | 37.14 (13.60) | 40.96 (11.14)a | F(3,121) =4.48, p < .01 |
| Gender (% female) | 67.39 | 63.33 | 68.18 | 74.07 | X2(3) = .77, ns |
| Race (% Caucasian) | 80.44 | 90.00 | 72.73 | 77.78 | X2(3) = 2.72, ns |
| College graduate (%) | 63.04d | 56.67d | 40.91a | 22.22a,b | X2(3) = 12.36, p < .001 |
Note. PTSD symptoms clusters = sum of severity ratings (9-point scale ranging from 0, None, to 8, Very severe) endorsed for each DSM-IV PTSD criterion on ADIS-IV (47) according to clusters (57). Age of onset = patient-reported onset of PTSD diagnosis. Chronicity of PTSD = years from patient-reported onset of diagnosis to assessment. PTSD Severity = clinician-rated severity (6-point scale ranging from 0, No features present, to 5, Diagnosis present; severe) reflecting both distress and interference. Prognosis = clinician-rated estimate of treatment prognosis (4-point scale ranging from 1, Excellent, to 4, Poor).
ADIS-IV, Anxiety Disorder Interview Schedule for DSM-IV; ASI, Anxiety Sensitivity Index (82); BDI, Beck Depression Inventory (85); HSD, honestly significant difference; PTSD, posttraumatic stress disorder; QMI Total, Questionnaire on Mental Imagery (86); STAXI-State, State scale of State Trait Anger Expression Inventory (83); STAXI-Trait, Trait scale STAXI (83); STAI-Trait, Trait scale of State Trait Anxiety Inventory (84); STAI-State, State scale of STAI (84).
Post hoc between-group comparison to nonexposed control is significant at p < .05 (results of Tukey HSD pairwise comparisons).
Post hoc between-group comparison to trauma-exposed control group is significant at p < .05 (results of Tukey HSD pairwise comparisons).
Post hoc between-group comparison to single-trauma PTSD group is significant at p < .05 (results of Tukey HSD pairwise comparisons).
Post hoc between-group comparison to multiple-trauma PTSD group is significant at p < .05 (results of Tukey HSD pairwise comparisons).
One-tailed test based on directional hypothesis that multiple-trauma PTSD group would exceed single-trauma PTSD group.
Figure 4.
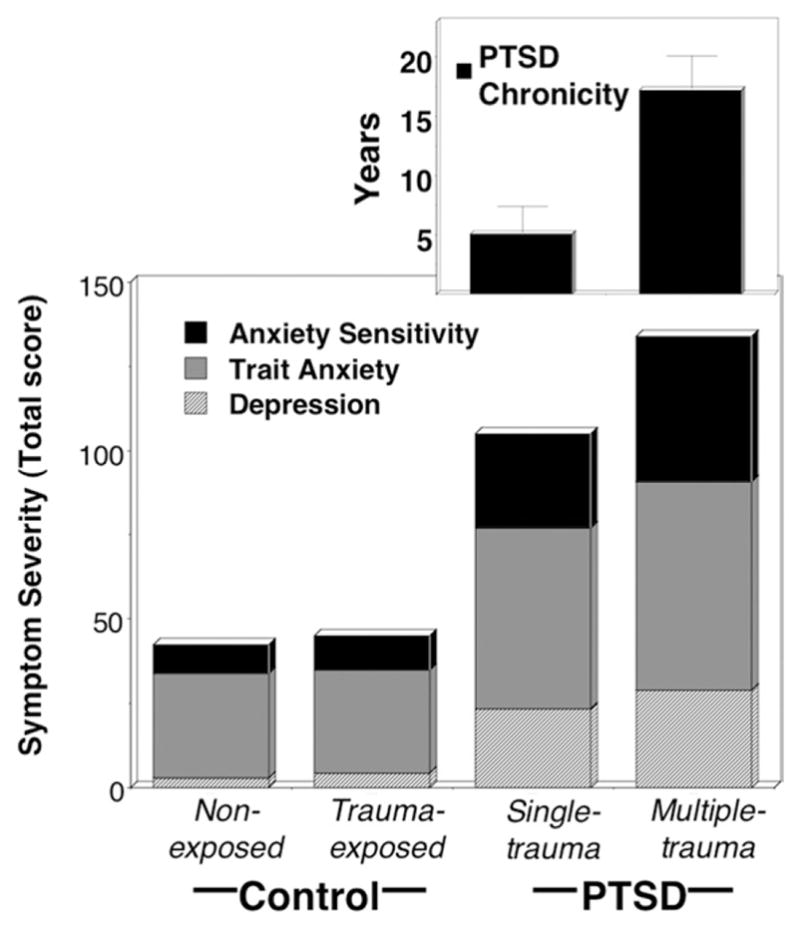
To illustrate the differences in total negative affectivity between groups, mean stacked symptom severity scores on the STAI (trait), BDI, and ASI for nonexposed and trauma-exposed control groups and single-trauma and multiple-trauma PTSD groups are illustrated in the bottom panel. In the top panel, mean duration of PTSD (i.e., disorder chronicity) in years for single-trauma and multiple-trauma PTSD groups are plotted, showing that broad negative affectivity and chronicity demonstrate concurrent increases. ASI, Anxiety Sensitivity Index; BDI, Beck Depression Inventory; PTSD, post-traumatic stress disorder; STAI, State Trait Anxiety Inventory.
Symptomatology
A highly consistent pattern of elevated distress and functional impairment was observed in the multiple-trauma compared with single-trauma patients but with the single-trauma group still far exceeding both control groups (Table 4). Specifically, questionnaire measures of anxiety sensitivity, non-specific trait anxiety, and depression were lowest for the two control groups, increased in severity for the single-trauma group, and were highest for the multiple-trauma patients (Figure 4).8 The multiple-trauma patients consistently surpassed the single-trauma patients in frequency of comorbid anxiety and depression, total number of Axis I disorders, and clinician-conferred ratings of PTSD severity and poorer treatment prognosis. In analysis of PTSD symptom clusters as delineated by Suvak et al. (57), single-trauma and multiple-trauma groups indicated commensurate severity of re-experiencing, while the multiple-trauma exceeded the single-trauma group in extent of emotional numbing and hyperarousal and showed the same trend for strategic avoidance. Ability to generate vivid mental imagery was equivalent across subgroups.
Discussion
Defensive Physiology and PTSD
As in many prior studies of idiographic trauma imagery, the total cohort of PTSD patients evinced more pronounced heart rate acceleration than control participants (21) and, concordant with more extreme aversiveness ratings, greater facial expressions of displeasure (34,58). Patients also surpassed control participants in startle reflex potentiation during idiographic threat-related imagery, consistent with enhanced limbic (particularly amygdala) and paralimbic activation shown in parallel neuroimaging research (59–61). Although mean skin conductance change loomed larger in PTSD patients than control participants, the groups did not differ significantly (as in a subset of earlier studies [23,27,30,34]). Both patient and control groups rated their “worst” threatening scenes most arousing and correspondingly evinced their most palpable electrodermal increases when imagining these scenarios.
Posttraumatic stress disorder patients also surpassed control participants in responses to standard unpleasant imagery, rating anger and panic scenes more aversive and arousing and showing greater startle potentiation. Imagery of animal and human survival threats prompted elevated skin conductance in PTSD, similar to reactions reported during imagery of standard exposure to combat (21,22), missile attack (62), and nursing war zone casualties (26). Overall, PTSD patients showed defensive hyper-reactivity foremost to trauma-related imagery, as well as a broader sensitivity to aversive cues as found subsequent to shock-threat conditioning (63).
Defensive Physiology and Trauma Recurrence
control participants, whether or not they had experienced prior traumatic events, showed similar, reliable physiological increases and subjective distress during aversive imagery. However, the impression prompted by the overall comparison of patients and control participants—that PTSD patients reliably exceed control participants in defensive reactivity—was qualified by the subgroup analyses. The hyperreactivity of the PTSD group was carried by less than half the total cohort of patients, specifically those with posttraumatic stress consequent to a single, discrete trauma. Single-trauma PTSD patients showed more robust startle and skin conductance responses than both control participants and multiple-trauma PTSD patients. The latter group, in fact, showed no startle potentiation or conductance increases during personal threat that differed from those amid neutral imagery. Furthermore, single-trauma patients clearly exceeded both control groups in heart rate increases during personal threat; the lesser reaction of the multiple-trauma group did not surpass control participants.
Evaluative Reports and Facial Expressivity
Both single- and multiple-trauma PTSD groups reported that unpleasant imagery prompted greater experienced negative emotion than did control participants, with the highest ratings for multiple-trauma patients, emphasizing a dramatic discordance from this group’s impaired defensive activation in autonomic and reflex potentiation. Curiously, corrugator frown muscle action was not strongly discordant with ratings, as it increased significantly during unpleasant imagery in both patient subgroups, suggesting that facial expression and evaluative reports are coordinate communication channels under instrumental control.
Physiological Blunting in PTSD
Current findings for multiple-trauma PTSD of obtunded startle during aversive imagery seem paradoxical. However, Cuthbert et al. (34) also observed this phenomenon in PTSD during imagery in their smaller sample. Similarly, a reduced electrodermal response has been reported (64) during threat of shock in PTSD relative to control participants. The reason for this attenuation in multiple-trauma PTSD is unclear. Although dissociation is a viable candidate process, multiple investigators have shown no impact of high dissociative tendencies on the defensive physiology of PTSD during imagery (33,65,66) and the profile of multiple-trauma PTSD here revealed heightened negative facial expressivity during aversive imagery rather than complete defensive suppression (67). Additionally, lack of heart rate increase during aversive imagery has been associated with verbal reports of dissociation in PTSD (68,69); however, here, multiple-trauma patients showed significant heart rate increases relative to neutral processing that, although less pronounced than for single-trauma patients, were equivalent to control participants responding to their worst fear. Considering that multiple-trauma patients endorsed more extreme aversiveness and arousal ratings and equivalent ability to generate vivid imagery to the other groups, the data do not strongly support a dissociation/disengagement interpretation.
Concerning neural mediation of reduced reactivity, several neuroimaging studies found no heightened amygdala activation during trauma imagery in PTSD (70–73) and one study even observed amygdala deactivation (74) relative to control participants. Startle potentiation and skin conductance increases to emotionally salient cues have been described as downstream effects of amygdala activation (15,16,75,76). The present findings of their coincident attenuation in chronic PTSD patients with cumulative trauma histories suggest deficient amygdalar recruitment during internally generated trauma recall that may extend to nontrauma-related contents nevertheless pertinent to the long-term posttraumatic presentation (i.e., anger, panic, physical danger).
Trauma Recurrence, Chronicity, and Comorbidity
The multiple-trauma patients sustained more, higher magnitude traumatic events that began at an earlier age and posttraumatic stress persisted, on average, over three times longer than the single-trauma patients (i.e., 17 years and 5 years, respectively). Recurrent compared with single traumatization was also associated with more severe PTSD that, importantly, was concomitant with more extreme and broader anxious and depressive comorbidity, as quantified by questionnaires (i.e., trait anxiety and anger, cognitive and somatic symptoms of depression), and prevalence of additional anxiety and mood diagnoses.
When compared with prior studies of trauma imagery (23,25,27,28,62,77), the multiple-trauma PTSD sample was particularly extreme in comorbidity of anxiety and depression. For example, the veterans in the Orr et al. sample (23) who showed increased skin conductance and heart rate acceleration to idiographic trauma imagery were most similar in trait anxiety (State-Trait Anxiety Inventory; trait M = 48.4) and depression (Beck Depression Inventory = 19.4) to the present highly reactive, single-trauma group. Furthermore, 74% of the multiple-trauma group met criteria for a comorbid anxiety disorder and 85% surpassed the threshold for comorbid depression, far exceeding the prevalence in the single group (anxiety disorder: 32%; depressive disorder 52%). Importantly, previous PTSD samples that demonstrated exaggerated defensive reactivity were generally characterized by depression comorbidity at (21,22) or below (27) the level of the single-trauma group—far below the multiple-trauma group.
The Anxiety Spectrum
The current findings suggest that for PTSD patients attenuated defensive reactivity is associated with broad distress, severe and recurrent trauma exposure, and lengthier disorder chronicity. This blunting phenomenon has been observed not only in other broadly symptomatic anxiety disorders (i.e., panic disorder with agoraphobia and GAD [34,38–40]) but also within fear diagnoses (41). For example, in social phobia, the most severe patients (generalized social phobia with comorbid depression) endorsed the most pronounced negative affectivity and enduring dysfunction but showed the least physiological reactivity during aversive imagery.
High Stress and Defensive Responses
Animal data suggest that variations in stressor intensity, duration, and recurrence can result in dampened defensive responses. For example, using a conditioning paradigm, Davis and Astrachan (78) observed a nonmonotonic relationship between fear-potentiated startle and shock intensity: rats exposed to light cues paired with intermediate levels of shock evinced the greatest conditioned potentiation; rats exposed to low shock intensities demonstrated modest augmentation; and those exposed to the highest shock intensity demonstrated no discernible increase in startle magnitude. Chalmers et al. (79) similarly found an absence of conditioned fear potentiation among rats exposed to highly intense, prolonged, and inescapable shock. More specific to stressor chronicity, animals exposed to brief (i.e., 10 days) and/or less severe “resident/intruder” stress demonstrated hypervigilance and hyperarousal, whereas those exposed to longer duration stress (20 to 30 days) developed more generalized anxiety and depressive-like symptoms—including passivity, limited movement, and reduced communication and consumption behaviors—that persisted even in the absence of the aggressor (80,81).
Conclusion
Single- and multiple-trauma exposures yield identifiably different psychophysiological profiles, obscured when PTSD is considered, irrespective of trauma recurrence. Posttraumatic stress disorder secondary to a discrete trauma is characterized by heightened defensive reactivity during aversive imagery, whereas PTSD after higher magnitude, multiple traumas is marked by higher anxious and depressive comorbidity and a blunted reflex reaction. These findings suggest that trauma accumulation and the associated context may prompt sustained traumatic stress, ultimately impairing defensive physiological reflexes and broadening symptom severity. In summary, patients’ verbal reports were consistent with the diagnostic criteria implicating exaggerated hyperarousal in PTSD. However, objective physiological measures revealed that defensive responding did not uniformly increase with PTSD severity. In fact, the most extreme constellation of psychopathology was characterized by a compromised defense response to aversive imagery.
Supplementary Material
Acknowledgments
This work was supported in part by a National Institute of Mental Health Grant (P50 MH 72850) to the Center for the Study of Emotion and Attention, University of Florida, Gainesville, Florida, and a National Research Service Award Research Fellowship (F31 MH069048) to the first author.
Special thanks to the following individuals for this assistance in data collection: Cyd C. Strauss, Eleni Dimoulas, Denise M. Sloan, Greg Perlman, and Bethany Wangelin. Special thanks to Danny Kaloupek and Andreas Keil for their critiques of manuscript drafts.
Footnotes
Personal scenes were based upon descriptions of prior experiences. For patients with PTSD, both personal scenes described fearful and threatening aspects of their index trauma. Among the control participants, 60.5% of participants described a traumatic or potentially traumatic event (e.g., physical assault, motor vehicle accident, witnessing violence, home invasion) for at least one of their two personal scenes, whereas others described intense nontraumatic, fearful events (e.g., giving a speech, receiving injections, undergoing surgery, panic attacks, exposure to snakes/insects).
One-tailed test based on directional hypothesis that patients would exceed control participants.
Analyses for heart rate change were calculated on residuals secondary to removing the trial-specific baseline (1-second average before script onset) effects via linear regression.
In follow-up analyses of baseline and imagery physiology, gender exerted neither a main effect nor interaction in any measure.
No reliable differences emerged between patient and control subtypes in blink magnitude to intertrial startle probes or for SCL, corrugator, or orbicularis EMG activity in the 1-second baseline before script onset. Single-trauma (M = 74.94, SD = 13.03) and multiple-trauma (M = 75.90, SD = 12.65) patients showed similarly rapid baseline heart rate, both demonstrating higher rates than control participants (nonexposed, M =65.14, SD =10.81; trauma-exposed, M =65.05, SD =9.06), group, F(3,119) = 8.31, p < .001.
Twenty-seven of the 49 patients indicated current use of psychotropic medication. Most frequently, these were selective serotonin reuptake inhibitors (34.7%) and/or benzodiazepines (25.5%). The effects of these and less frequently endorsed compounds (e.g., norepinephrine and dopamine reuptake inhibitors, 8.2%; serotonin norepinephrine reuptake inhibitors, 6.1%) were assessed by comparing resting and imagery reactivity among the medicated and nonmedicated patients both for patients as a whole and within subtypes. Considering either general psychotropic usage or more specific classes of drugs, no reliable effects emerged, perhaps due to the relatively small proportion of the sample on any single medication. These null medication findings are consistent with prior physiological investigations in PTSD (23,33,55,56). Reported usage of prescription and over-the-counter physical health medications for promoting physical health, as well as recreational substance use were also collected but low frequencies of endorsement precluded statistical analysis.
The prevalence rates for trauma-exposed control participants were intentional trauma (46.7%), childhood trauma (36.7%), childhood sexual and/or physical abuse (.03%), and trauma occurring to self (versus witnessing) (43.3%).
Age did not differ among trauma-exposed control participants and PTSD patients, although the nonexposed control participants were somewhat younger than the multiple-trauma PTSD group (Table 3). Both PTSD subgroups were similar in age (21,22,24) or younger (23) than prior PTSD samples that showed robust defensive engagement during aversive imagery.
All authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
References
- 1.Breslau N, Chilcoat HD, Kessler RC, Davis GC. Previous exposure to trauma and PTSD effects of subsequent trauma: Results from the Detroit Area Survey of Trauma. Am J Psychiatry. 1999;156:902–907. doi: 10.1176/ajp.156.6.902. [DOI] [PubMed] [Google Scholar]
- 2.Dohrenwend BP, Turner JB, Turse NA, Adams BG, Koenen KC, Marshall R. The psychological risks of Vietnam for U.S. veterans: A revisit with new data and methods. Science. 2006;313:979–982. doi: 10.1126/science.1128944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Follette VM, Polusny MA, Bechtle AE, Naugle AE. Cumulative trauma: The impact of child sexual abuse, adult sexual assault, and spouse abuse. J Trauma Stress. 1996;9:25–35. doi: 10.1007/BF02116831. [DOI] [PubMed] [Google Scholar]
- 4.Green BL, Goodman LA, Krupnick JL, Corcoran CB, Petty RM, Stockton P, Stern NM. Outcomes of single versus multiple trauma exposure in a screening sample. J Trauma Stress. 2000;13:271–286. doi: 10.1023/A:1007758711939. [DOI] [PubMed] [Google Scholar]
- 5.Clancy CP, Graybeal A, Tompson WP, Badgett KS, Feldman ME, Calhoun PS, et al. Lifetime trauma exposure in veterans with military-related posttraumatic stress disorder: Association with current symptomatology. J Clin Psychiatry. 2006;67:1346–1353. doi: 10.4088/jcp.v67n0904. [DOI] [PubMed] [Google Scholar]
- 6.Kulka RA, Schlenger WE, Fairbank JA, Hough RL, Jordan BK, Marmar CR, et al. Trauma and the Vietnam War Generation: Report of Findings from the National Vietnam Veterans Readjustment Study. New York: Brunner/Mazel; 1990. [Google Scholar]
- 7.Briere J, Kaltman S, Green BL. Accumulated childhood trauma and symptom complexity. J Trauma Stress. 2008;21:223–226. doi: 10.1002/jts.20317. [DOI] [PubMed] [Google Scholar]
- 8.Mollica RF, McInnes K, Poole C, Tor S. Dose-effect relationships of trauma to symptoms of depression and post-traumatic stress disorder among Cambodian survivors of mass violence. Br J Psychiatry. 1998;173:482–488. doi: 10.1192/bjp.173.6.482. [DOI] [PubMed] [Google Scholar]
- 9.Lu W, Mueser KT, Rosenberg SD, Jankowski MK. Correlates of adverse childhood experiences among adults with severe mood disorders. Psychiatr Serv. 2008;59:1018–1026. doi: 10.1176/ps.2008.59.9.1018. [DOI] [PubMed] [Google Scholar]
- 10.McCauley J, Kern DE, Kolodner K, Dill L, Schroeder AF, DeChant HK, et al. Clinical characteristics of women with a history of childhood abuse: Unhealed wounds. JAMA. 1997;277:1362–1368. [PubMed] [Google Scholar]
- 11.Cloitre M, Cohen LR, Edelman RE, Han H. Posttraumatic stress disorder and extent of trauma exposure as correlates of medical problems and perceived health among women with childhood abuse. Women Health. 2001;34:1–17. doi: 10.1300/J013v34n03_01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang PJ, Levin DN, Miller GA, Kozak MJ. Fear behavior, fear imagery, and the psychophysiology of emotion: The problem of affective response integration. J Abnorm Psychol. 1983;92:276–306. doi: 10.1037//0021-843x.92.3.276. [DOI] [PubMed] [Google Scholar]
- 13.Lang PJ, Kozak MJ, Miller GA, Levin DN, McLean A., Jr Emotional imagery: Conceptual structure and pattern of somato-visceral response. Psychophysiology. 1980;17:179–192. doi: 10.1111/j.1469-8986.1980.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 14.Vrana SR, Lang PJ. Fear imagery and the startle-probe reflex. J Abnorm Psychol. 1990;99:189–197. doi: 10.1037//0021-843x.99.2.189. [DOI] [PubMed] [Google Scholar]
- 15.Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: A tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- 16.Lang PJ, Davis M. Emotion, motivation, and the brain: Reflex foundations in animal and human research. Prog Brain Res. 2006;156:3–29. doi: 10.1016/S0079-6123(06)56001-7. [DOI] [PubMed] [Google Scholar]
- 17.Sabatinelli D, Bradley MM, Fitzsimmons JR, Lang PJ. Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage. 2005;24:1265–1270. doi: 10.1016/j.neuroimage.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 19.Costa VD, Bradley MM, Lang PJ. Emotional imagery activates reward circuits. Psychophysiology. 2009;46:S63. [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 21.Pitman RK, Orr SP, Forgue DF, de Jong JB, Claiborn JM. Psychophysiologic assessment of posttraumatic stress disorder imagery in Vietnam combat veterans. Arch Gen Psychiatry. 1987;44:970–975. doi: 10.1001/archpsyc.1987.01800230050009. [DOI] [PubMed] [Google Scholar]
- 22.Keane TM, Kolb LC, Kaloupek DG, Orr SP, Blanchard EB, Thomas RG, et al. Utility of psychophysiological measurement in the diagnosis of posttraumatic stress disorder: Results from a Department of Veterans Affairs Cooperative Study. J Consult Clin Psychol. 1998;66:914–923. doi: 10.1037//0022-006x.66.6.914. [DOI] [PubMed] [Google Scholar]
- 23.Orr SP, Pitman RK, Lasko NB, Herz LR. Psychophysiological assessment of posttraumatic stress disorder imagery in World War II and Korean combat veterans. J Abnorm Psychol. 1993;102:152–159. doi: 10.1037//0021-843x.102.1.152. [DOI] [PubMed] [Google Scholar]
- 24.Orr SP, Lasko NB, Metzger LJ, Berry NJ, Ahern CE, Pitman RK. Psychophysiologic assessment of women with posttraumatic stress disorder resulting from childhood sexual abuse. J Consult Clin Psychol. 1998;66:906–913. doi: 10.1037//0022-006x.66.6.906. [DOI] [PubMed] [Google Scholar]
- 25.Pitman RK, Lanes DM, Williston SK, Guillaume JL, Metzger LJ, Gehr GM, Orr SP. Psychophysiologic assessment of posttraumatic stress disorder in breast cancer patients. Psychosomatics. 2001;42:133–140. doi: 10.1176/appi.psy.42.2.133. [DOI] [PubMed] [Google Scholar]
- 26.Carson MA, Paulus LA, Lasko NB, Metzger LJ, Wolfe J, Orr SP, Pitman RK. Psychophysiologic assessment of posttraumatic stress disorder in Vietnam nurse veterans who witnessed injury or death. J Consult Clin Psychol. 2000;68:890–897. [PubMed] [Google Scholar]
- 27.Shalev AY, Orr SP, Pitman RK. Psychophysiologic assessment of traumatic imagery in Israeli civilian patients with posttraumatic stress disorder. Am J Psychiatry. 1993;150:620–624. doi: 10.1176/ajp.150.4.620. [DOI] [PubMed] [Google Scholar]
- 28.Pitman RK, Orr SP, Forgue DF, Altman B, de Jong JB, Herz LR. Psychophysiologic responses to combat imagery of Vietnam veterans with posttraumatic stress disorder versus other anxiety disorders. J Abnorm Psychol. 1990;99:49–54. doi: 10.1037//0021-843x.99.1.49. [DOI] [PubMed] [Google Scholar]
- 29.McDonagh-Coyle A, McHugo GJ, Friedman MJ, Schnurr PP, Zayfert C, Descamps M. Psychophysiological reactivity in female sexual abuse survivors. J Trauma Stress. 2001;14:667–683. doi: 10.1023/A:1013081803429. [DOI] [PubMed] [Google Scholar]
- 30.Davis JM, Adams HE, Uddo M, Vasterling JJ, Sutker PB. Physiological arousal and attention in veterans with posttraumatic stress disorder. J Psychopathol Behav Assess. 1996;18:1–20. [Google Scholar]
- 31.Cohen H, Kotler M, Matar MA, Kaplan Z, Loewenthal U, Miodownik H, Cassuto Y. Analysis of heart rate variability in posttraumatic stress disorder patients in response to a trauma-related reminder. Biol Psychiatry. 1998;44:1054–1059. doi: 10.1016/s0006-3223(97)00475-7. [DOI] [PubMed] [Google Scholar]
- 32.Cohen H, Benjamin J, Geva AB, Matar MA, Kaplan Z, Kotler M. Autonomic dysregulation in panic disorder and in post-traumatic stress disorder: Application of power spectrum analysis of heart rate variability at rest and in response to recollection of trauma or panic attacks. Psychiatry Res. 2000;96:1–13. doi: 10.1016/s0165-1781(00)00195-5. [DOI] [PubMed] [Google Scholar]
- 33.Halligan SL, Michael T, Wilhelm FH, Clark DM, Ehlers A. Reduced heart rate responding to trauma reliving in trauma survivors with PTSD: Correlates and consequences. J Trauma Stress. 2006;19:721–734. doi: 10.1002/jts.20167. [DOI] [PubMed] [Google Scholar]
- 34.Cuthbert BN, Lang PJ, Strauss C, Drobes D, Patrick CJ, Bradley MM. The psychophysiology of anxiety disorder: Fear memory imagery. Psychophysiology. 2003;40:407–422. doi: 10.1111/1469-8986.00043. [DOI] [PubMed] [Google Scholar]
- 35.Cook EW, 3rd, Melamed BG, Cuthbert BN, McNeil DW, Lang PJ. Emotional imagery and the differential diagnosis of anxiety. J Consult Clin Psychol. 1988;56:734–740. doi: 10.1037//0022-006x.56.5.734. [DOI] [PubMed] [Google Scholar]
- 36.McNeil DW, Vrana SR, Melamed BG, Cuthbert BN, Lang PJ. Emotional imagery in simple and social phobia: Fear versus anxiety. J Abnorm Psychol. 1993;102:212–225. doi: 10.1037//0021-843x.102.2.212. [DOI] [PubMed] [Google Scholar]
- 37.Weerts TC, Lang PJ. Psychophysiology of fear imagery: Differences between focal phobia and social performance anxiety. J Consult Clin Psychol. 1978;46:1157–1159. doi: 10.1037//0022-006x.46.5.1157. [DOI] [PubMed] [Google Scholar]
- 38.Lang PJ, McTeague LM, Cuthbert BN. Fearful imagery and the anxiety disorder spectrum. In: Rothbaum BO, editor. Pathological Anxiety: Emotional Processing in Etiology and Treatment. New York: Guilford Publications; 2005. pp. 56–77. [Google Scholar]
- 39.Lang PJ, McTeague LM, Cuthbert BN. Fear, anxiety, depression, and the anxiety disorder spectrum: A psychophysiological analysis. In: Treat T, Baker T, editors. Psychological Clinical Science: Recent Advances in Theory and Practice. Integrative Perspectives in Honor of Richard M. McFall. Mahwah, NJ: Lawrence Erlbaum Associates; 2007. pp. 167–195. [Google Scholar]
- 40.Lang PJ, McTeague LM. The anxiety disorder spectrum: Fear imagery, physiological reactivity, and differential diagnosis. Anxiety Stress Coping. 2009;22:5–25. doi: 10.1080/10615800802478247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McTeague LM, Lang PJ, Laplante MC, Cuthbert BN, Strauss CC, Bradley MM. Fearful imagery in social phobia: Generalization, comorbidity, and physiological reactivity. Biol Psychiatry. 2009;65:374–382. doi: 10.1016/j.biopsych.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beckham JC, Vrana SR, Barefoot JC, Feldman ME, Fairbank J, Moore SD. Magnitude and duration of cardiovascular responses to anger in Vietnam veterans with and without posttraumatic stress disorder. J Consult Clin Psychol. 2002;70:228–234. doi: 10.1037//0022-006x.70.1.228. [DOI] [PubMed] [Google Scholar]
- 43.Chemtob CM, Novaco RW, Hamada RS, Gross DM, Smith G. Anger regulation deficits in combat-related posttraumatic stress disorder. J Trauma Stress. 1997;10:17–36. doi: 10.1023/a:1024852228908. [DOI] [PubMed] [Google Scholar]
- 44.Falsetti SA, Resnick HS. Frequency and severity of panic attack symptoms in a treatment seeking sample of trauma victims. J Trauma Stress. 1997;10:683–689. doi: 10.1023/a:1024810206381. [DOI] [PubMed] [Google Scholar]
- 45.Nixon RD, Resick PA, Griffin MG. Panic following trauma: The etiology of acute posttraumatic arousal. J Anxiety Disord. 2004;18:193–210. doi: 10.1016/S0887-6185(02)00290-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blanchard EB, Hickling EJ, Buckley TC, Taylor AE, Vollmer A, Loos WR. Psychophysiology of posttraumatic stress disorder related to motor vehicle accidents: Replication and extension. J Consult Clin Psychol. 1996;64:742–751. doi: 10.1037//0022-006x.64.4.742. [DOI] [PubMed] [Google Scholar]
- 47.Brown TA, Barlow DH, DiNardo PA, Barlow DH. The Anxiety Disorder Interview Schedule for DSM-IV: Adult Version. New York: Oxford University Press; 1994. [Google Scholar]
- 48.Bradley MM, Lang PJ. Affective Norms for English Text (ANET): Affective Ratings of Text and Instruction Manual. Technical Report. D-1. Gainesville, FL: University of Florida; 2007. [Google Scholar]
- 49.Benson H. The Relaxation Response. New York: Morrow; 1975. [Google Scholar]
- 50.Bradley MM, Lang PJ. Measuring emotion: The Self-Assessment Manikin and the Semantic Differential. J Behav Ther Exp Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 51.Cook EW., 3rd . VPM Reference Manual. Birmingham, AL: University of Alabama; 2000. [Google Scholar]
- 52.Globisch J, Hamm A, Schneider R, Vaitl D. A computer program for scoring reflex eyeblink and electrodermal responses written in Pascal. Psychophysiology. 1993;39:S30. [Google Scholar]
- 53.Vasey MW, Thayer JF. The continuing problem of false positives in repeated measures ANOVA in psychophysiology: A multivariate solution. Psychophysiology. 1987;24:479–486. doi: 10.1111/j.1469-8986.1987.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 54.Buckley TC, Kaloupek DG. A meta-analytic examination of basal cardiovascular activity in posttraumatic stress disorder. Psychosom Med. 2001;63:585–594. doi: 10.1097/00006842-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 55.Peri T, Ben-Shakhar G, Orr SP, Shalev AY. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biol Psychiatry. 2000;47:512–519. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- 56.Shalev AY, Rogel-Fuchs Y. Auditory startle reflex in post-traumatic stress disorder patients treated with clonazepam. Isr J Psychiatry Relat Sci. 1992;29:1–6. [PubMed] [Google Scholar]
- 57.Suvak M, Maguen S, Litz BT, Silver RC, Holman EA. Indirect exposure to the September 11 terrorist attacks: Does symptom structure resemble PTSD? J Trauma Stress. 2008;21:30–39. doi: 10.1002/jts.20289. [DOI] [PubMed] [Google Scholar]
- 58.Pole N. The psychophysiology of posttraumatic stress disorder: A meta-analysis. Psychol Bull. 2007;133:725–746. doi: 10.1037/0033-2909.133.5.725. [DOI] [PubMed] [Google Scholar]
- 59.Rauch SL, van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, et al. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch Gen Psychiatry. 1996;53:380–387. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- 60.Piefke M, Pestinger M, Arin T, Kohl B, Kastrau F, Schnitker R, et al. The neurofunctional mechanisms of traumatic and non-traumatic memory in patients with acute PTSD following accident trauma. Neurocase. 2007;13:342–357. doi: 10.1080/13554790701851494. [DOI] [PubMed] [Google Scholar]
- 61.Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- 62.Shalev AY, Peri T, Gelpin E, Orr SP, Pitman RK. Psychophysiologic assessment of mental imagery of stressful events in Israeli civilian post-traumatic stress disorder patients. Compr Psychiatry. 1997;38:269–273. doi: 10.1016/s0010-440x(97)90059-6. [DOI] [PubMed] [Google Scholar]
- 63.Grillon C, Morgan CA., 3rd Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. J Abnorm Psychol. 1999;108:134–142. doi: 10.1037//0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- 64.Blechert J, Michael T, Grossman P, Lajtman M, Wilhelm FH. Autonomic and respiratory characteristics of posttraumatic stress disorder and panic disorder. Psychosom Med. 2007;69:935–943. doi: 10.1097/PSY.0b013e31815a8f6b. [DOI] [PubMed] [Google Scholar]
- 65.Kaufman ML, Kimble MO, Kaloupek DG, McTeague LM, Bachrach P, Forti AM, Keane TM. Peritraumatic dissociation and physiological response to trauma-relevant stimuli in Vietnam combat veterans with posttraumatic stress disorder. J Nerv Ment Dis. 2002;190:167–174. doi: 10.1097/00005053-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 66.Nixon RD, Bryant RA, Moulds ML, Felmingham KL, Mastrodomenico JA. Physiological arousal and dissociation in acute trauma victims during trauma narratives. J Trauma Stress. 2005;18:107–113. doi: 10.1002/jts.20019. [DOI] [PubMed] [Google Scholar]
- 67.Griffin MG, Resick PA. Mechanic MB. Objective assessment of peritraumatic dissociation: Psychophysiological indicators. Am J Psychiatry. 1997;54:1081–1088. doi: 10.1176/ajp.154.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hopper JW, Frewen PA, van der Kolk BA, Lanius RA. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: Symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J Trauma Stress. 2007;20:713–725. doi: 10.1002/jts.20284. [DOI] [PubMed] [Google Scholar]
- 69.Lanius RA, Williamson PC, Boksman K, Densmore M, Gupta M, Neufeld RW, et al. Brain activation during script-driven imagery induced dissociative responses in PTSD: A functional magnetic resonance imaging investigation. Biol Psychiatry. 2002;52:305–311. doi: 10.1016/s0006-3223(02)01367-7. [DOI] [PubMed] [Google Scholar]
- 70.Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: A positron emission tomography study. Biol Psychiatry. 1999;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry. 2005;57:832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 72.Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, et al. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. Am J Psychiatry. 1999;156:575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- 73.Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, et al. Neural correlates of traumatic memories in posttraumatic stress disorder: A functional MRI investigation. Am J Psychiatry. 2001;158:1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- 74.Phan KL, Britton JC, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Arch Gen Psychiatry. 2006;63:184–192. doi: 10.1001/archpsyc.63.2.184. [DOI] [PubMed] [Google Scholar]
- 75.Laine CM, Spitler KM, Mosher CP, Gothard KM. Behavioral triggers of skin conductance responses and their neural correlates in the primate amygdala. J Neurophysiol. 2009;101:1749–1754. doi: 10.1152/jn.91110.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sequeira H, Roy JC. Cortical and hypothalamo-limbic control of electrodermal responses. In: Roy JC, Boucsein W, Fowles DC, Gruzelier JH, editors. Progress on Electrodermal Research. New York: Plenum; 1993. pp. 93–114. [Google Scholar]
- 77.Orr SP, Claiborn JM, Altman B, Forgue DF, de Jong JB, Pitman RK, Herz LR. Psychometric profile of posttraumatic stress disorder, anxious, and healthy Vietnam veterans: Correlations with psychophysiologic responses. J Consult Clin Psychol. 1990;58:329–335. doi: 10.1037//0022-006x.58.3.329. [DOI] [PubMed] [Google Scholar]
- 78.Davis M, Astrachan DI. Conditioned fear and startle magnitude: Effects of different footshock or backshock intensities used in training. J Exp Psychol Anim Behav Process. 1978;4:95–103. doi: 10.1037//0097-7403.4.2.95. [DOI] [PubMed] [Google Scholar]
- 79.Chalmers DV, Hohf JC, Levine S. The effects of prior shock stimulation on the behavioral and physiological responses to intense acoustic stimuli. Physiol Behav. 1974;12:711–718. doi: 10.1016/0031-9384(74)90004-3. [DOI] [PubMed] [Google Scholar]
- 80.Avgustinovich DF, Kovalenko IL, Kudryavtseva NN. A model of anxious depression: Persistence of behavioral pathology. Neurosci Behav Physiol. 2005;35:917–924. doi: 10.1007/s11055-005-0146-6. [DOI] [PubMed] [Google Scholar]
- 81.Rygula R, Abumaria N, Flügge G, Fuchs E, Rüther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: Impact of chronic social stress. Behav Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 82.Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav Res Ther. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- 83.Spielberger CD. Manual for the State-Trait Anger Expression Inventory. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- 84.Spielberger CD, Gorsuch RL, Lushene PR, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (STAI) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 85.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory. 2. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 86.Sheehan PW. A shortened form of Betts’ questionnaire upon mental imagery. J Clin Psychol. 1967;223:380–389. doi: 10.1002/1097-4679(196707)23:3<386::aid-jclp2270230328>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.