Abstract
BACKGROUND
Poor medication adherence is a significant problem in hypertensive African Americans. Although motivational interviewing (MINT) is effective for adoption and maintenance of health behaviors in patients with chronic diseases, its effect on medication adherence remains untested in this population.
METHODS
This randomized controlled trial tested the effect of a practice-based MINT counseling versus usual care (UC) on medication adherence and blood pressure (BP) in 190 hypertensive African Americans (88% women; mean age 54 years). Patients were recruited from two community-based primary care practices in New York City. The primary outcome was adherence measured by electronic pill monitors; the secondary outcome was within-patient change in office BP from baseline to 12 months.
RESULTS
Baseline adherence was similar in both groups (56.2% and 56.6% for MINT and UC respectively, p = 0.94). Based on intent-to-treat analysis using mixed effects regression, a significant time X group interaction with model-predicted post-treatment adherence rates of 43% and 57% were found in the UC and MINT groups, respectively (p = 0.027), with a between-group difference of 14% (95% CI, −0.2% to −27%). The between-group difference in systolic and diastolic BP was −6.1 mm Hg (p = .065) and −1.4 mm Hg (p = .465), respectively, in favor of the MINT group.
CONCLUSIONS
A practice-based MINT counseling led to steady maintenance of medication adherence over time, compared to significant decline in adherence for UC patients. This effect was associated with a clinically meaningful net reduction in systolic BP in favor of the MINT group.
Keywords: Motivational Interviewing, Medication Adherence, African American, Hypertension
INTRODUCTION
African Americans have higher prevalence of hypertension and poorer hypertension-related outcomes than whites.1-3 Poor medication adherence may explain the poor BP control in African Americans.4, 5 Behavioral counseling strategies are effective in improving medication adherence chronic diseases patients,6, 7 with little data in hypertensive African Americans. Successful interventions include those that are emotionally supportive; involve patients in their care; address patients’ beliefs about medications; and enhance patients’ confidence in their ability to overcome barriers to adherence.8-13 Motivational interviewing (MINT), a counseling approach that has gained increased popularity in primary care practices,14 encompasses several of these characteristics. MINT is defined as directive, patient-centered counseling designed to motivate patients for change by helping them recognize and resolve the discrepancy between their behavior, personal goals and values.15 In patients with chronic diseases, MINT is effective in facilitating the adoption and maintenance of recommended health behaviors including weight loss, smoking cessation and dietary habits.16-20 Despite its proven efficacy, the effect of MINT on medication adherence remains untested in hypertensive African Americans in primary care settings.
In this randomized controlled trial, we tested the effect of a practice-based MINT versus usual care (UC) on medication adherence and blood pressure (BP), among hypertensive African Americans. We hypothesized a greater effect of MINT on medication adherence compared to UC at 12 months; and that this effect would be associated with a significant reduction in clinic BP.
METHODS
Setting and Patients
As described elsewhere, 21 patients were recruited from two community-based primary care practices affiliated with New York Presbyterian Hospital Ambulatory Care Network. Eligible patients were identified via electronic medical records (EMR) and asked to participate during routine office visits by trained research assistants (RA). Eligibility criteria included self-identification as African American or black; age ≥ 18 years; diagnosis of hypertension; taking at least one antihypertensive medication; uncontrolled BP on two successive office visits prior to screening (BP ≥140/90 mmHg or ≥130/80 mmHg for those with kidney disease or diabetes);22 and fluency in English. Patients who agreed to participate provided written informed consent. Cornell and Columbia University Institutional Review Boards approved the study.
Baseline assessment and follow-up visits
At baseline, trained RAs assessed patients’ demographics and clinical history. Patients’ EMR were reviewed for clinic BP, and history of comorbidity was documented using Charlson comorbidity index.23 Each patient was provided and taught how to use an electronic pill cap equipped with the Medication Events Monitoring System (MEMS) (Aprex Corporation, Fremont, California, USA). Patients were instructed to bring their MEMS to all study visits. Follow-up assessments were conducted every 3 months, during which patients’ adherence data were downloaded from their MEMS pill caps and their clinic BP readings were retrieved from their EMR. Final assessment was conducted at 12 months. All patients were reimbursed $25 after each study visit.
Randomization
After baseline assessment, patients were randomly assigned to either UC or MINT group by the study statistician, using sealed envelopes. Separate randomization schedules were developed from a computerized random-number generator, balanced at set intervals, using permutated blocks, to assure equal numbers in each group. Due to the nature of the behavioral intervention, neither the patients nor the RAs were blinded to the intervention. However, the clinic staff that recorded the BP data were blinded to patient assignment. It is important to note that medication adherence data were downloaded automatically into the computer from the MEMS caps through a reader. Thus, both the research assistants and patients could not affect MEMS adherence outcome.
Intervention
Details of the intervention are described elsewhere.21 Briefly, patients in the MINT group received usual care plus behavioral counseling about medication adherence using MINT techniques. Each patient received a 30-40 minute MINT counseling session at 3, 6, 9 and 12 months. All sessions were conducted by trained RAs using a structured MINT counseling script.21 All sessions were audio-taped and fidelity of the RAs to MINT techniques was assessed regularly by a trained MINT rater, who provided feedback to the RAs based on the taped recordings.24 Because the use of MEMS pill caps is associated with increased adherence, 25 the intervention was delivered 3 months post-randomization in order to allow for habituation to the use of MEMS, and assessment of patients’ baseline medication adherence level.
Patients in the UC group did not receive MINT counseling, but completed all assessments at the same time intervals as the MINT group.
Outcomes and Measurements
The primary outcome was medication adherence between 10-12 months assessed with MEMS pill caps, the accepted “gold standard” for adherence assessment.26 Patients were required to monitor adherence to one antihypertensive medication taken once daily. For each patient, there is a value from 0-k, where k is the number of times MEMS recorded an opening for each day the patient was in the study. Patients were assigned missing values for the days their MEMS were not used (i.e., “drug holidays”). The values for each day were converted to a binary record, 0 = MEMS not opened, while 1 = MEMS opened once/day. This metric (known as taking adherence), was the proportion of days in which the patient took his or her medication as prescribed (once daily in this case).27
The secondary outcome was within-patient change in systolic and diastolic BP from baseline to 12 months. Because of the desire to mimic real-world primary care practice, we did not influence the BP measurement protocol used by the individual practices. All BP data were extracted from patients’ EMR log of BP taken during patient's routine office visits. Thus, BP measurements were carried out by nurses or certified medical assistants with mercury sphygmomanometers.21 For this purpose, patients were required to be seated with their arms bare and at least one BP reading was taken for each patient (as indicated in the electronic medical records).
Statistical Analysis
The sample size was determined by a power analysis using a moderate change in adherence rates as the effect size, power of .80 and significance level of α=0.05. This analysis suggested a sample size of 86 patients per group, but 190 patients were randomized (95 per group). The primary outcome was MEMS adherence between 10-12 months. However, MEMS data were categorized as missing for those patients who dropped out of the study and those who did not return their MEMS caps. To conform to intent-to-treat principles, mixed effects regression models were used to test the time X group interaction for the primary analysis. For this purpose, all available data for all patients was used to estimate and test the intervention effect. However, the description of the results and follow-up contrasts focused on the group differences between 10-12 months. Additionally, we carried out extensive evaluation of the missing value patterns to determine if data were missing at random (MAR) before testing the intervention effects. For the 30 patients (16%) for which there were no MEMS data due to damage of the MEMS pill caps, we evaluated the extent to which these patients differed from other patients on all available data and undertook sensitivity analyses to assess the possible effects of the missing data on the study results.
RESULTS
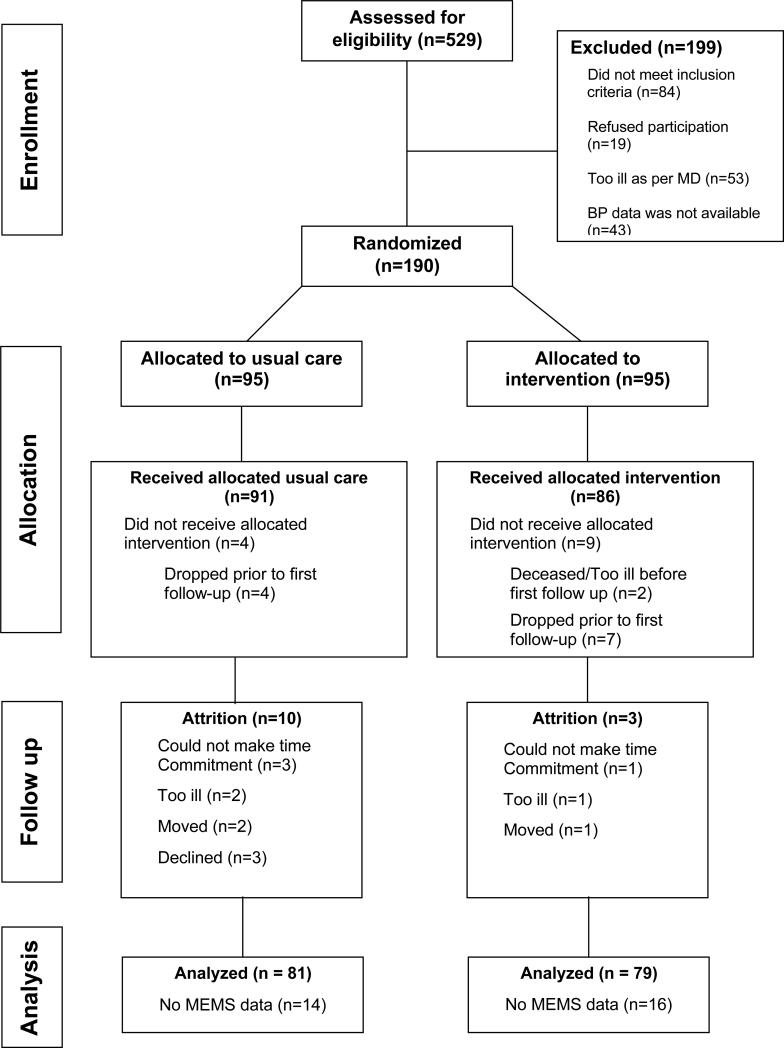
We screened 529 patients, of whom 330 were eligible, and we enrolled 190 into the trial (Figure 1). Their mean age was 54 years; 88% were female; 17% were married; 77% had high school or college education; with over half unemployed. The baseline mean systolic BP was 144 mmHg (SD=19.2), with a mean diastolic BP of 86.6 mmHg (SD=11.4). Forty-five percent had a Charlson comorbidity score ≥ 3 with one-third reporting diabetes; 8% had heart failure and 4% had kidney disease. As shown in Table 1, there were no significant differences between both groups at baseline, and their adherence rates were also similar (56.2% ± 35.5% for MINT versus 56.6% ± 34.1% for UC, p = 0.948).
Figure 1.
Consort Flow Diagram.
Table 1.
Comparison of Baseline Characteristics by Randomization Group
| Characteristic | Value, n (%) | ||
|---|---|---|---|
| Intervention (n = 95) | Control (n = 95) | p-value | |
| Mean Age +/− SD | 53.45 (11.35) | 54.04 (12.81) | .29 |
| Female (%) | 84 (50) | 84 (50) | .59 |
| Marital Status (%) | |||
| Single | 49 (58) | 35 (42) | |
| Married | 13 (39) | 20 (61) | .12 |
| Separated | 26 (45) | 32 (55) | |
| Widower | 7 (47) | 8 (53) | |
| Education (%) | |||
| Elementary | 21 (48) | 23 (52) | |
| High school/GED | 41 (48) | 44 (52) | .49 |
| Some college | 33 (54) | 28 (46) | |
| Employment Status (%) | |||
| Employed full time | 15 (52) | 14 (48) | |
| Employed part time | 8 (62) | 5 (38) | |
| Retired | 12 (60) | 8 (40) | .29 |
| Not working | 60 (45) | 68 (55) | |
| Type of insurance (%) | |||
| HMO | 11 (85) | 2 (15) | |
| Medicare | 11 (50) | 11 (50) | .06 |
| Medicaid | 65 (46) | 75 (54) | |
| Self | 8 (53) | 7 (47) | |
| Annual income (%) | |||
| Unknown | 14 (48) | 15 (52) | |
| ≤$20,000 | 58 (48) | 64 (52) | .33 |
| >$20,000 | 23 (59) | 16 (41) | |
| CHF (%) | 6 (43) | 8 (57) | .42 |
| Stroke (%) | 11 (44) | 14 (56) | .37 |
| Diabetes (%) | 31 (51) | 30 (49) | .44 |
| Kidney disease (%) | 4 (57) | 3 (43) | .48 |
| Charlson Comorbidity Score (%) | |||
| 0 | 12 (35) | 22 (65) | .43 |
| 1-2 | 34 (55) | 28 (45) | |
| ≥3 | 36 (51) | 35 (49) | |
| Mean DBP (+/− SD) | 145.79 (19.76) | 143.11 (18.36) | .33 |
| Mean SBP (+/− SD) | 86.22 (11.67) | 87.09 (12.49) | .59 |
| Antihypertensive Medications (%) | |||
| ACE-Inhibitor | 43 (45) | 32 (34) | 0.47 |
| Beta Blocker | 29 (31) | 28 (30) | 0.76 |
| Calcium Channel Blocker | 27 (28) | 28 (30) | 0.76 |
| Diuretic | 32 (34) | 39 (41) | 0.64 |
| Combination | 20 (21) | 21 (22) | 0.76 |
p-value indicates differences in values between intervention and control groups
HMO: Healthcare Maintenance Organization SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure
Medication Adherence: Completer Analysis
A total of 190 MEMS pill caps were distributed to patients. Of these, 160 caps were returned (84%), while 30 patients (16%) had no usable MEMS data due to damage. Of the 160 patients with MEMS data, 111 (70%) had complete MEMS data. Among these 111 patients, the MINT group had a higher adherence rate compared to UC (60% vs. 47% respectively, p = .054) with a between-group difference of 13% (95% CI, −0.2% to 27%).
Medication Adherence: Intent to Treat Analysis
The 160 patients with MEMS data contributed a total of 520 observations to the analyses. If all 160 patients had complete data, there would have been 640 (four assessment periods X 160 patients) MEMS observations. Thus, the available data represents 520/640 = 81% of the possible MEMS data. The primary analysis was mixed effects regression using all 520 observations. A critical assumption for using mixed effects regression with incomplete data is that the data were MAR. Based on Little's MCAR test we could not conclude that the data were MCAR (chi-square = 26.7, df = 15, p = .031). Thus, we created a variable indicating whether or not a patient had missing data, and correlated this variable with adherence rates at the four assessment periods. The correlations between missingness and these adherence rates were small and non-significant. Most importantly, the correlation with the final post-treatment adherence rates at 10-12 months was −.015, p=.872, suggesting that the data can be treated as MAR.
Overall, there was a significant reduction in adherence throughout the study period that corresponded to a reduction in adherence rate of 4% per quarter, and the test of this overall downward linear trend was significant (t with 127.5 df = −2.77, p = .006). To test whether these trends differ between the MINT and UC groups, models containing a time (quarterly trend) X intervention interaction were fitted. The parameter for the critical time X intervention interaction of .0456 was statistically significant (t with 121.4 df =2.24, p=.027). To interpret this interaction, the parameter estimate from the mixed effects regression model was used to predict the pre-intervention and post-intervention adherence rates for both groups. Using mixed regression analysis, a significant time X group interaction with model-predicted post-intervention adherence rates of 43% and 57% was found for the UC and MINT groups, respectively (p=.027), see Table 2.
Table 2.
Outcome Measures based on the Mixed Regression Intent to Treat Analyses
| UC (N = 79) | MINT (N = 81) | |||
|---|---|---|---|---|
| Medication Adherence | Pre | Post | Pre | Post |
| Predicted Medication Adherence Scores (%) | 55.2% | 42.9% | 55.4% | 56.9% |
| Change | −12. 3% | +.5% | ||
| UC (N = 95) | MINT (N = 95) | |||
|---|---|---|---|---|
| Blood Pressure | Pre | Post | ||
| Predicted SBP (mm Hg) | 141.9 | 136.8 | 144.2 | 133.0 |
| Change (mm Hg) | −5.1 | −11.2 | ||
| Predicted DBP (mmHg) | 86.3 | 82.82 | 86.0 | 81.1 |
| Change (mm Hg) | −3.48 | −4.92 | ||
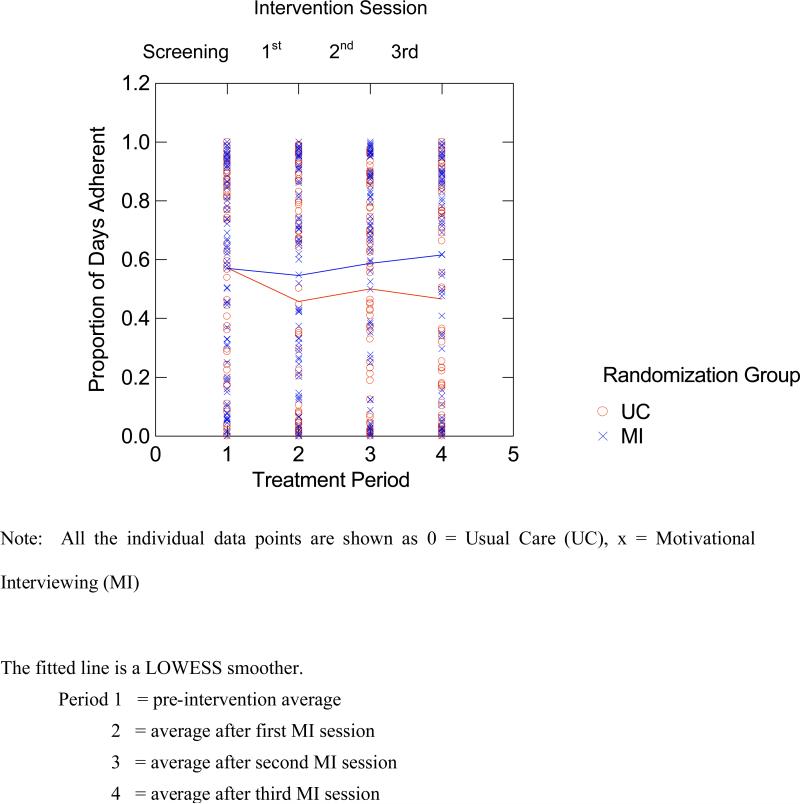
In order to describe the observed differences in the adherence pattern across time for both groups, we fitted a LOWESS smoother to the data obtained (Figure 2). Then we evaluated the pre-post differences in adherence separately for the UC and MINT groups in follow-up contrasts, using mixed regression but with time (assessment period) specified as a four-level factor. In this analysis there was a significant drop between the baseline versus post-intervention adherence rate for the UC group (−12.9%, t=−2.89 with 159 df, p=.004) and a slight increase between the baseline versus post-intervention adherence rate for the MINT group (1.1%, t = .24 with 159 df, p=.810). This analysis indicates that the primary effect of MINT was to prevent the decline in medication adherence observed in the UC group. Based on the parameter estimates from the mixed regression analysis there was a significant overall drop in adherence of about 4% per quarter, but this effect was off-set with an additional gain of about 0.5% per quarter for the MINT group. Thus, there was a significant decrease in adherence for the UC group from baseline to 12 months and an overall non-significant increase in adherence for the MINT group; this difference is reflected in the significant group X contrast interaction.
Figure 2.
Results of LOWESS Smoother Curve Testing the Time X Treatment Interaction
Sensitivity analyses of patients without MEMS Data
As stated previously, 30 patients had no useable MEMS data. These 30 patients were evenly split between the MINT and UC groups (N=14 and 16, respectively) and there was no difference between the 160 patients who had MEMS data and the 30 who had none on any of the baseline measures. Nonetheless, the extent to which the results of the primary analyses would change under various scenarios of hypothetical data for these 30 patients was evaluated. Under the most extreme case where the adherence rates for these 30 patients is the opposite of those found in the 160 patients with data (that is the 14 UC subjects had higher adherence rates than the 16 MINT subjects), the significant effect was lost. However, under less extreme possible data patterns, we found a smaller but significant effect in favor of the MINT group. For example, we assumed that for this group of patients there was no treatment effect and we assigned all 30 patients the overall average adherence rate at each of the four assessment periods and then repeated the mixed regression analyses with these patients included. We tested the obtained parameter estimate for the time X treatment interaction from this analysis against the standard error and degrees of freedom from the analysis with 160 patients (to avoid taking advantage of the reduced variability in the data that results from assigning the mean to 30 patients). The results of this analysis indicated a smaller, but still statistically significant, group X time intervention effect in favor of the MINT group (t with 121.4 df = 1.78, p=.039, one-tailed).
Blood Pressure
Mixed regression analysis was used to compare the effects of MINT versus UC on systolic and diastolic BP. Parameter estimates from the mixed regression analysis indicated a significant overall drop in systolic BP of 5.1 mmHg across 12 months for both groups (t with 145.1, df = −2.26, p=.026) and the MINT group showed an additional drop (time X treatment interaction) of 6.1 mmHg (t with150.5 df = −1.86, p = .065; Table 2). For diastolic BP, there was also a significant overall drop of 3.5 mmHg across the 12 months (t with 151.2, df = −2.61, p=.01), but the MINT group did not show an additional drop (time X treatment interaction, t with 156.7, df = −.73, p=.465; Table 2).
COMMENTS
In this practice-based trial, MINT counseling led to steady maintenance of medication adherence over 12 months, compared to a significant decline noted in the UC group. Although not statistically significant, this effect was associated with a clinically meaningful net reduction in systolic BP in favor of the MINT group. To our knowledge, this is the only randomized trial that tested the long-term effect of practice-based MINT on medication adherence and BP control in hypertensive African Americans. We are not aware of any practice-based behavioral intervention targeted at medication adherence in this patient population. Another major strength of our study is the long duration of medication adherence assessment with the objective MEMS. Previous adherence intervention trials suffered from the lack of objective measures of adherence, short-term duration of the outcomes assessed, and low minority participation.6, 7, 9, 28
Motivational interviewing is an increasingly popular patient-centered approach to behavioral management of chronic diseases in primary-care settings.29, 30 One mechanism through which MINT exerts its positive effects on health behaviors is enhancement of self-motivation,14, 31 which increases patients’ readiness to change and confidence in their ability to overcome barriers necessary to achieve a desired outcome. Although results from recent reviews indicate the positive effect of MINT on psychological, physiological, and lifestyle-change outcomes in patients with chronic diseases, 29, 30 only one pilot study assessed the effect of MINT on self-reported adherence to medications in HIV patients.32 In that study, patients randomized to the intervention group received 3 MINT sessions delivered by nurses whereas the control group patients received UC. Although not significant, at two months, patients in the MINT group reported higher self-reported adherence scores and fewer missed doses. While this study provides preliminary evidence for the role of MINT in improving medication adherence, the lack of an objective measure of adherence and short duration limits the interpretation of the true effect of MINT on medication adherence. In our study, we assessed medication adherence for a much longer duration (12 months) with an objective and accepted “gold standard” for adherence assessment.
Although MINT was not used, similar effects of behavioral counseling approach on adherence in patients with other chronic diseases have been reported.33-38 However, majority of these studies had short duration of adherence monitoring (typically 3 to 6 months) and were not practice-based.34-38 In one of the few MEMS studies that extended beyond a 6-month monitoring period, Weber et al. also reported steady maintenance of MEMS adherence in 60 HIV positive patients randomized to monthly cognitive behavioral therapy compared to usual care for a 12- month period.33 While there was no significant worsening of adherence in the intervention arm, the usual care arm showed significant reduction of 8.7% per year in MEMS adherence (p=.006).33
While not statistically significant, the net reduction in systolic BP of 5 mm hg over 12 months in favor of the MINT group achieved in our study was clinically meaningful. Woollard et al. reported a similar finding in an 18-week trial that tested the effect of MINT versus usual care among 166 hypertensive patients followed in a general practice in Australia.39 In that study, patients randomized to six face-to-face, monthly MINT sessions, including one low-intensity, face-to-face session and five telephone MINT sessions delivered by trained nurses, showed net significant decreases in systolic BP at 18 weeks (−4 and −2 mmHg respectively, p< .05), compared with UC. In traditional antihypertensive drug trials, the magnitude of systolic BP reduction noted in our trial has been associated with significant cardiovascular risk reduction and mortality.22
We should note two limitations of this study. First, majority of patients were low-income women, which limits generalizability of our findings to the broader African-American population. Second, the greater reduction seen in SBP for the MINT group could be explained by greater intensity in medication adjustment. But we did not collect this data and as such cannot make any statement about this claim. Despite these limitations, our findings suggests that MINT counseling delivered every 3 months in a practice-based setting, led to steady maintenance of medication adherence in hypertensive African Americans compared to UC. This effect was associated with a clinically meaningful reduction in systolic BP. These findings set the stage for future studies to assess the cost-effectiveness of this approach for maintenance of adherence to prescribed antihypertensive medications in this high-risk population. Future studies should explore the integration of MINT into standard practice for this patient population, especially given its widespread use for self-management behaviors in patients with chronic diseases.
ACKNOWLEDGEMENTS
The study was supported by grant R01 HL69408 from NHLBI, NIH, Bethesda, MD, USA. The funding agency played no role in the design, conduct, or reporting of the study, or in the decision to submit this manuscript for publication.
Footnotes
The authors have no financial disclosures to report.
DISCLOSURE
All authors declare there are no competing or financial relationships that may lead to a conflict of interest.
REFERENCES
- 1.CDC/NCHS NHANES III [1988--94] 2000 Feb;49(5) 2000. [Google Scholar]
- 2.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 3.Wong MD, Shapiro MF, Boscardin WJ, Ettner SL. Contribution of major diseases to disparities in mortality. N Engl J Med. 2002 Nov 14;347:1585–1592. doi: 10.1056/NEJMsa012979. [DOI] [PubMed] [Google Scholar]
- 4.Bosworth HB, Dudley T, Olsen MK, Voils CI, Powers B, Goldstein MK, Oddone EZ. Racial differences in blood pressure control: potential explanatory factors. Am J Med. 2006;119:70, e79–15. doi: 10.1016/j.amjmed.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Hyre AD, Krousel-Wood MA, Muntner P, Kawasaki L, DeSalvo KB. Prevalence and predictors of poor antihypertensive medication adherence in an urban health clinic setting. J Clin Hypertens (Greenwich) 2007;9:179–186. doi: 10.1111/j.1524-6175.2007.06372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haynes RB, Montague P, Oliver T, McKibbon KA, Brouwers MC, Kanani R. Interventions for helping patients to follow prescriptions for medications. Cochrane Database Syst Rev. 2000:2. doi: 10.1002/14651858.CD000011. [DOI] [PubMed] [Google Scholar]
- 7.Schroeder K, Fahey T, Ebrahim S. Interventions for improving adherence to treatment in patients with high blood pressure in ambulatory settings. Cochrane Database Syst Rev. 2004:CD004804. doi: 10.1002/14651858.CD004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller NH, Hill M, Kottke T, Ockene IS. The multilevel compliance challenge: recommendations for a call to action. A statement for healthcare professionals. Circulation. 1997;95:1085–1090. doi: 10.1161/01.cir.95.4.1085. [DOI] [PubMed] [Google Scholar]
- 9.Morrison A WA, Berger M. Interventions to improve antihypertensive drug adherence: a quantitative review of trials. Formulary. 2000;35:234–255. [Google Scholar]
- 10.Prochaska JO, Crimi P, Lapsanski D, Martel L, Reid P. Self-change processes, self-efficacy and self-concept in relapse and maintenance of cessation of smoking. Psychol Rep. 1982;51:983–990. doi: 10.2466/pr0.1982.51.3.983. [DOI] [PubMed] [Google Scholar]
- 11.Rogers CR. Carl Rogers on the development of the person-centered approach. Person centered review. 1986;1:257–259. [Google Scholar]
- 12.Roter DL, Hall JA, Merisca R, Nordstrom B, Cretin D, Svarstad B. Effectiveness of interventions to improve patient compliance: a meta- analysis. Med Care. 1998;36:1138–1161. doi: 10.1097/00005650-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Stewart MA. Effective physician-patient communication and health outcomes: A review. Canadian Medical Association Journal. 1996;152:1423. [PMC free article] [PubMed] [Google Scholar]
- 14.Emmons KM, Rollnick S. Motivational interviewing in health care settings. Opportunities and limitations. Am J Prev Med. 2001 Jan;20:68–74. doi: 10.1016/s0749-3797(00)00254-3. [DOI] [PubMed] [Google Scholar]
- 15.Rollnick S, Miller W. What is Motivational Interviewing? Behavioral and Cognitive Psychotherapy. 1995;23:325–334. doi: 10.1017/S1352465809005128. [DOI] [PubMed] [Google Scholar]
- 16.Dilorio C, Resnicow K, McDonnell M, Soet J, McCarty F, Yeager K. Using motivational interviewing to promote adherence to antiretroviral medications: A pilot study. Journal of the Association of Nurses in AIDS Care. 2003;14:52–62. doi: 10.1177/1055329002250996. [DOI] [PubMed] [Google Scholar]
- 17.Resnicow K, Jackson A, Wang T, De AK, McCarty F, Dudley WN, Baranowski T. A motivational interviewing intervention to increase fruit and vegetable intake through Black churches: results of the Eat for Life trial. Am J Public Health. 2001;91:1686–1693. doi: 10.2105/ajph.91.10.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55:305–312. [PMC free article] [PubMed] [Google Scholar]
- 19.Smith DE, Heckemeyer CM, Kratt PP, Mason DA. Motivational interviewing to improve adherence to a behavioral weight- control program for older obese women with NIDDM. A pilot study. Diabetes Care. 1997;20:52–54. doi: 10.2337/diacare.20.1.52. [DOI] [PubMed] [Google Scholar]
- 20.West DS, DiLillo V, Bursac Z, Gore SA, Greene PG. Motivational interviewing improves weight loss in women with type 2 diabetes. Diabetes Care. 2007;30:1081–1087. doi: 10.2337/dc06-1966. [DOI] [PubMed] [Google Scholar]
- 21.Ogedegbe GO, Schoenthaler A, Richardson T, Lewis L, Belue R, Espinosa E, Spencer J, Allegrante JP, Charlson ME. An RCT of the effect of motivational interviewing on medication adherence in hypertensive African Americans: Rationale and design. Contemporary Clinical Trials. 2007;28:169–181. doi: 10.1016/j.cct.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, Ogedegbe G, Orwig D, Ernst D, Czajkowski S. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol. 2004;23:443–451. doi: 10.1037/0278-6133.23.5.443. [DOI] [PubMed] [Google Scholar]
- 25.Burnier M. Medication adherence and persistence as the cornerstone of effective antihypertensive therapy. Am J Hypertens. 2006;19:1190–1196. doi: 10.1016/j.amjhyper.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Cramer JA. Microelectronic systems for monitoring and enhancing patient compliance with medication regimens. Drugs. 1995;49:321–327. doi: 10.2165/00003495-199549030-00001. [DOI] [PubMed] [Google Scholar]
- 27.Murray MD, Young J, Hoke S, Tu W, Weiner M, Morrow D, Stroupe KT, Wu J, Clark D, Smith F, Gradus-Pizlo I, Weinberger M, Brater DC. Pharmacist intervention to improve medication adherence in heart failure: a randomized trial. Ann Intern Med. 2007;146:714–725. doi: 10.7326/0003-4819-146-10-200705150-00005. [DOI] [PubMed] [Google Scholar]
- 28.McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. Jama. 2002;288:2868–2879. doi: 10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]
- 29.Britt E, Hudson SM, Blampied NM. Motivational interviewing in health settings: a review. Patient Educ Couns. 2004;53:147–155. doi: 10.1016/S0738-3991(03)00141-1. [DOI] [PubMed] [Google Scholar]
- 30.Knight KM, McGowan L, Dickens C, Bundy C. A systematic review of motivational interviewing in physical health care settings. Br J Health Psychol. 2006;11:319–332. doi: 10.1348/135910705X52516. [DOI] [PubMed] [Google Scholar]
- 31.Resnicow K, DiIorio C, Soet JE, Ernst D, Borrelli B, Hecht J. Motivational interviewing in health promotion: it sounds like something is changing. Health Psychol. 2002;21:444–451. [PubMed] [Google Scholar]
- 32.DiIorio C, Resnicow K, McDonnell M, Soet J, McCarty F, Yeager K. Using motivational interviewing to promote adherence to antiretroviral medications: a pilot study. J Assoc Nurses AIDS Care. 2003;14:52–62. doi: 10.1177/1055329002250996. [DOI] [PubMed] [Google Scholar]
- 33.Weber R, Christen L, Christen S, Tschopp S, Znoj H, Schneider C, Schmitt J, Opravil M, Gunthard HF, Ledergerber B. Effect of individual cognitive behaviour intervention on adherence to antiretroviral therapy: prospective randomized trial. Antivir Ther. 2004;9:85–95. [PubMed] [Google Scholar]
- 34.De Geest S, Schafer-Keller P, Denhaerynck K, Thannberger N, Kofer S, Bock A, Surber C, Steiger J. Supporting medication adherence in renal transplantation (SMART): a pilot RCT to improve adherence to immunosuppressive regimens. Clin Transplant. 2006;20:359–368. doi: 10.1111/j.1399-0012.2006.00493.x. [DOI] [PubMed] [Google Scholar]
- 35.Rawlings MK, Thompson MA, Farthing CF, Brown LS, Racine J, Scott RC, Crawford KH, Goodwin SD, Tolson JM, Williams VC, Shaefer MS. Impact of an educational program on efficacy and adherence with a twice-daily lamivudine/zidovudine/abacavir regimen in underrepresented HIV-infected patients. J Acquir Immune Defic Syndr. 2003;34:174–183. doi: 10.1097/00126334-200310010-00007. [DOI] [PubMed] [Google Scholar]
- 36.Simoni JM, Pantalone DW, Plummer MD, Huang B. A randomized controlled trial of a peer support intervention targeting antiretroviral medication adherence and depressive symptomatology in HIV-positive men and women. Health Psychol. 2007;26:488–495. doi: 10.1037/0278-6133.26.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner GJ, Kanouse DE, Golinelli D, Miller LG, Daar ES, Witt MD, Diamond C, Tilles JG, Kemper CA, Larsen R, Goicoechea M, Haubrich RH. Cognitive-behavioral intervention to enhance adherence to antiretroviral therapy: a randomized controlled trial (CCTG 578). Aids. 2006;20:1295–1302. doi: 10.1097/01.aids.0000232238.28415.d2. [DOI] [PubMed] [Google Scholar]
- 38.Holzemer WL, Bakken S, Portillo CJ, Grimes R, Welch J, Wantland D, Mullan JT. Testing a nurse-tailored HIV medication adherence intervention. Nurs Res. 2006;55:189–197. doi: 10.1097/00006199-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Woollard J, Beilin L, Lord T, Puddey I, MacAdam D, Rouse I. A controlled trial of nurse counselling on lifestyle change for hypertensives treated in general practice: preliminary results. Clin Exp Pharmacol Physiol. 1995;22:466–468. doi: 10.1111/j.1440-1681.1995.tb02046.x. [DOI] [PubMed] [Google Scholar]