Abstract
Purpose
Local failure after definitive chemoradiation therapy for unresectable esophageal cancer remains problematic. Little is known about the failure pattern based on modern day radiation treatment volumes. We hypothesized that most local failures would be within the gross tumor volume (GTV), where the bulk of the tumor burden resides.
Methods and Materials
We reviewed treatment volumes for 239 patients who underwent definitive chemoradiation therapy and compared this information with failure patterns on follow-up positron emission (PET). Failures were categorized as within the GTV, the larger clinical target volume (CTV, which encompasses microscopic disease), or the still larger planning target volume (PTV, which encompasses setup variability) or outside the radiation field.
Results
At a median follow-up time of 52.6 months (95% CI: 46.1 – 56.7 months), 119 patients (50%) had experienced local failure, 114 (48%) had distant failure, and 74 (31%) had no evidence of failure. Of all local failures, 107 (90%) were in the GTV, 27 (23%) in the CTV; and 14 (12%) in the PTV. In multivariate analysis, GTV failure was associated with tumor status (T3/T4 vs. T1/T2: OR=6.35, p value =0.002), change in standardized uptake value on PET before and after treatment (decrease >52%: OR=0.368, p value = 0.003) and tumor length (>8 cm: 4.08, p value = 0.009).
Conclusions
Most local failures after definitive chemoradiation for unresectable esophageal cancer occur in the GTV. Future therapeutic strategies should focus on enhancing local control.
Keywords: Esophageal cancer, dose escalation, failure patterns
Localized esophageal carcinomas are highly aggressive and difficult to cure, as they often persist or recur after definitive chemoradiation. Esophageal cancer is the eighth most common causes of cancer, with 482,000 new cases and 407,000 deaths estimated worldwide in 2008, making it the sixth most common cause of death from cancer.1 The prevalence of esophageal cancer, specifically adenocarcinoma, in North America continues to increase in parallel with the growth in prevalence of obesity and gastroesophageal reflux disease leading to Barrett’s metaplasia.2-4 Although surgery continues to be the standard approach for most localized esophageal cancers, cure rates after surgery alone have been poor, with 3- to 5-year survival rates ranging from 6% to 35%.5-7 The current trimodality approach, combining chemotherapy, radiation therapy (RT), and surgery, has significantly improved prognosis,8 with several studies showing improved survival rates.9, 10
However, many patients cannot tolerate surgery or decline it; for such individuals, definitive chemoradiation is the standard approach. The combination of RT and chemotherapy has additive effects in terms of local control and overall survival in this select population.7, 9, 11, 12 However, the optimal dose of radiation for this purpose continues to be a topic of debate, with current recommendation is to use the same dose for preoperative RT as for definitive treatment, i.e., 50.4 Gy.13 Several studies have attempted to evaluate the potential benefit of dose-escalation for esophageal cancer. The largest such study Intergroup 0123 / Radiation Therapy Oncology Group 94-05,14 found that escalating the dose to 64.8 Gy did not improve survival or local-regional control yet may have contributed to increased morbidity. However, that study used two-dimensional conformal RT (2D-CRT) with a sequential boost for dose escalation, with larger margins for both the primary and high-dose volumes than are now considered standard practice. These large treatment fields may have resulted in excessive overall toxicity. Since that study was completed, advances in the technologies associated with tumor imaging and radiation planning and delivery have allowed improved accuracy.
The use of highly conformal intensity-modulated radiation therapy (IMRT) has been demonstrated by several groups to provide additional flexibility in modifying dose distributions and improving normal tissue sparing.15 Compared with 2D-CRT, IMRT results in improved conformality and dose reduced to proximal critical structures. Further, use of a simultaneous integrated radiation boost with the IMRT offers the additional advantage of simultaneously delivering a higher dose to the primary tumor (at 2.2 Gy or 2.3 Gy per fraction) while lower conventional doses are used to treat subclinical disease or electively treated regions (at 1.8 Gy or 2.0 Gy per fraction).16 Nevertheless, despite these advances the doses used to treat esophageal cancer have remained the same. The principle of radiation dose escalation has translated into improved local control and enhanced survival for patients with other solid tumors, and one would anticipate similar benefits for patients with esophageal cancer, however, such studies have not been conducted.17-19 Since the current standard is to use the same RT dose for definitive and for preoperative therapy, we hypothesized that the treatment failure after definitive chemoradiation would occur in most patients within the gross tumor volume (GTV), where the tumor burden is largest.
We sought here to document the sites of failure after standard-dose definitive chemoradiation therapy for esophageal cancer in terms of three commonly used radiation treatment volumes: GTV, the larger clinical target volume (CTV), and the still larger planning target volume (PTV). We further assessed a variety of patient- and disease-related characteristics for their potential utility as risk factors for identifying patterns of failure, reasoning that such factors would be useful in personalizing therapeutic approaches.
MATERIALS AND METHODS
We retrospectively identified 239 patients with unresectable esophageal cancer treated at The University of Texas MD Anderson Cancer Center from January 2002 through January 2009. All patients had been treated with definitive chemoradiation therapy. We assessed failures within the GTV, CTV, and PTV (as defined below) and distant failure on posttreatment PET, CT, or PET/CT scans and compared those scans with the original CT-based radiation treatment plans. The institutional review board of MD Anderson Cancer Center approved this post hoc analysis.
For treatment simulation and planning purposes, all patients had undergone 4-dimensional (4D) computed tomography (CT) scanning to account for respiratory motion as follows. The CT images were acquired first while the patient was free-breathing, and 4D images were acquired immediately thereafter. During the 4D CT image acquisition, patient respiration was monitored with an external respiratory gating system (Real-Time Position Management Respiratory Gating System; Varian Medical Systems, Palo Alto, CA). Each 4D CT image set consisted of 10 CT data sets representing 10 equally divided breathing phases in a complete respiratory cycle. The 4D CT images provided quantitative, time-dependent 3D information about internal organ motion, allowing quantitative description of internal organ motion for both treatment targets and normal organs.
The GTV was contoured on the planning CT scans by the attending radiation oncologist using all available resources, including data from PET/CT fusion scans, endoscopic ultrasonography images, and diagnostic CT images. The GTV was expanded to the CTV by extending the radiation coverage 3 cm superiorly, 3 cm inferiorly, 1 cm laterally, and 3 cm into the gastric mucosa. The PTV was then generated by using a uniform 0.5-cm expansion beyond the borders of the CTV. All organs at risk (e.g., heart, lung and liver) were outlined. All patients were to be treated to a total dose of 50.4 Gy, given in 28 fractions, with concurrent fluorouracil chemotherapy. All radiation treatment was to be delivered as IMRT; treatment plans were generated with the Pinnacle planning system (Phillips Medical Systems, Andover, MA).
The treatment failures were assessed based on serial posttreatment images that included PET scans, CT scans, and images from endoscopic evaluations. Failure location (GTV, CTV, PTV, or outside the radiation field) was identified by fusing current PET scans with the treatment plan CT scan. For simplicity, any failure within the radiation treatment volume was considered local (because these volumes encompassed prophylactic nodal coverage) and any failures outside the radiation treatment volume were considered distant. Patients were considered to have failure if pathologically proven or documented radiographically though serial progression. All failure patterns were included in the analysis regardless of the timing of previously failures.
Continuous variables were summarized by descriptive statistics such as means, standard deviations, medians, and ranges. Categorical variables were tabulated by frequency and percentage. Fisher’s exact test or chi-square test and Wilcoxon’s rank sum test were used to compare patient characteristics between patients with and without failure. Logistic regression models were fit for multivariate analyses to evaluate associations between GTV failure and any of the clinical factors with a p value less than 0.15 in the univariate analysis. The Kaplan-Meier product-limit method and log rank test were applied to estimate survival probabilities and compare survival, respectively. Univariate and multivariate Cox proportional hazards models were fit to evaluate potential associations between overall survival or progression-free survival with clinical factors. The backward selection procedure was used for model selections. SAS 9.2 (SAS Institute Inc., Cary, NC 27513) and S-Plus 8.0 (TIBCO software Inc., Palo Alto, CA 94304) statistical software were used for statistical data analysis.
RESULTS
Patient Characteristics
Table 1 shows the baseline characteristics of the 239 patients included in this retrospective analysis. The median patient age was 66 years (range, 30-90 years); 206 (86%) were male and 33 (14%) were female. Most patients had T3 tumors (81%), N1 disease (67%), M0 disease (79%), and poorly differentiated tumors (54%). The most common tumor histology was adenocarcinoma (76%), and most tumors (79%) were located in the lower thoracic region. Median tumor length was 5 cm (range, 1-17 cm), and the median baseline standardized uptake value (SUV) on PET was 11.7 (range, 0-51). Treatment characteristics are listed in Table 2. Nearly all patients (99%) received concurrent chemotherapy, and 46% received induction chemotherapy as well. The median radiation dose was 50.4 Gy (range, 32.4-66 Gy).
Table 1.
Patient characteristics
| Characteristic | Value or No. of Patients (%) |
|---|---|
| Sex | |
| Female | 33 (14%) |
| Male | 206 (86%) |
| Age, years | |
| median (range) | 66 (30-90) |
| Tumor category | |
| T1 | 7 (3%) |
| T2 | 24 (10%) |
| T3 | 193 (81%) |
| T4 | 13 (5%) |
| TX | 2 (1%) |
| Lymph node category | |
| N0 | 77 (32%) |
| N1 | 160 (67%) |
| NX | 2 (1%) |
| Metastasis category | |
| M0 | 189 (79%) |
| M1a | 28 (12%) |
| M1b | 22 (9%) |
| Tumor location | |
| Cervical | 3 (1%) |
| Upper thoracic | 18 (7%) |
| Mid-thoracic | 29 (12%) |
| Lower thoracic | 189 (79%) |
| Tumor histology | |
| Adenocarcinoma | 181 (76%) |
| Squamous cell | 57 (24%) |
| Neuroendocrine | 1 (0.4%) |
| Tumor grade | |
| G1 (well differentiated) | 2 (1%) |
| G2 (moderately differentiated) | 107 (45%) |
| G3 (poorly differentiated) | 129 (54%) |
| GX (undetermined) | 1 (0.4%) |
| Tumor length, cm | |
| median (range) | 5 (1-17) |
Table 2.
Treatment Characteristics and Outcomes
| Characteristic | All Patients (n=239) |
Patients with T1/T2 Tumors (n=31) |
Patients with T3/T4 Tumors (n=208) |
|---|---|---|---|
| Induction chemotherapy | |||
| Yes | 111 (46%) | ||
| No | 128 (54%) | ||
| Concurrent chemotherapy | |||
| Yes | 237 (99%) | ||
| No | 2 (1%) | ||
| Radiation dose, Gy | |||
| median (range) | 50.4 (32.4-66) | ||
| Initial Max. SUV | |||
| median (range) | 11.7 (0-51) | ||
| Post treatment Max. SUV | |||
| median (range) | 4.4 (0-19.9) | ||
| Outcomes | |||
| Local failure | 119 (50%) | 7 (23%) | 112 (54%) |
| GTV failure | 107 (90%) | 6 (86%) | 101 (90%) |
| CTV failure | 27 (23%) | 3 (43%) | 24 (21%) |
| PTV failure | 14 (12%) | 0 (0%) | 14 (12%) |
| Distant failure | 114 (48%) | 6 (19%) | 108 (52%) |
| No evidence of disease | 74 (31%) | 22 (71%) | 52 (25%) |
Abbreviations: SUV, standardized uptake value; GTV, gross tumor volume; CTV, clinical target volume; PTV, planning target volume.
Patterns of Locoregional Failure
Patients were followed until death or from 10.9 months to 88.4 months (median: 44.4 months) in those alive at last evaluation. At the time of last follow-up contact in December 2010, 119 patients (50%) had had local recurrence; 116 (48%) had had distant failure, defined as outside the radiation field; and 74 patients (31%) had no evidence of disease at last follow-up. Representative scans illustrating local failure within the GTV, CTV, and PTV are shown in Figure 1. The numbers of failures in each volume are illustrated in Figure 2. Of the 119 patients who had local failure, 107 (90%) had failure in the GTV, 27 (23%) in the CTV, and 14 (12%) in the PTV. Eighty-six patients (72%) had failure only in the GTV, but others had failure in more than one volume: 14 (12%) in the GTV and CTV, 1 (1%) in the GTV and PTV, and 6 (5%) in the GTV, CTV, and PTV. Salvage surgery was performed on 25% (30/116) of the patient with local failure. Tumor stage was strongly associated with local control; 71% of patients with T1/T2 disease had local disease control versus only 25% of patients with T3/T4 disease (P =0.0009)(Table 2). Tumor length was strongly associated with local control; 60% of patients with tumor ≤ 8cm disease had local disease control versus only 32 % of patients with > 8 cm in length (P =0.005). We found no difference in failure patterns based on histology.
Figure 1.

Demonstration of various failure patterns based on the original treatment planning coronal CT scans (on the left) illustrating the treated radiation volume, with matched post treatment PET scans (on the right) demonstrating recurrence patterns, which are visible as bright spots on the fusion PET/CT scans. 1A) Failure in the gross tumor volume (GTV) in the center of the treatment field. 1B) Failure in the clinical target volume (CTV), GTV in green, CTV in yellow and PTV in teal. 1C) Failure within the planning target volume (PTV). 1D) Failure outside the PTV.
Figure 2.
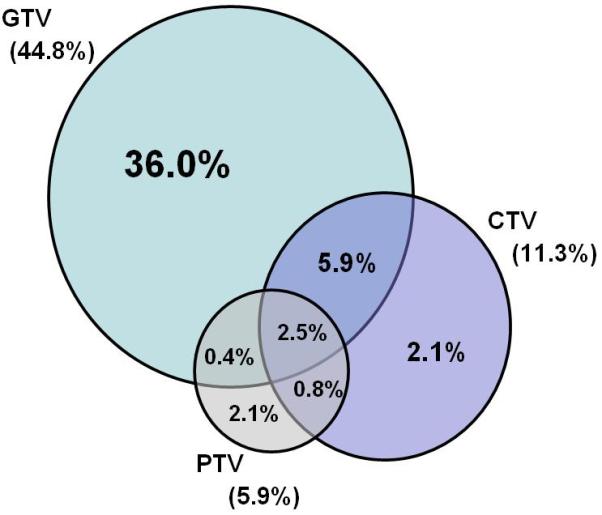

Patterns of local failures based on the original radiation treatment volumes, 90% within the gross tumor volume (GTV), 23% in the clinical target volume (CTV), and 12% in the planning target volume (PTV).
Overall Survival and Progression-Free Survival
At a median follow-up interval of 52.6 months, the median progression-free survival (PFS) time was 14.7 months (95% CI: 12.3-16.5 months). PFS times were better for women (median, 26.2 months) than for men (13.2 months) (P=0.011). PFS was associated with baseline T category, being 27% at 2 years for patients with T3/T4 versus 70% for patients with T1/T2 tumors (P<0.0001). Under multivariate analysis, PFS times continued to be worse for men (hazard ratio [HR] 2.23, 95% CI 1.32-3.77, P=0.0027) and those with N1 (HR 2.40, 95% CI 1.35-4.29, P=0.003) and M1a disease (HR 1.80, 95% CI 1.26-2.57, P=0.0012). In addition, having a post treatment SUV > 4.3 also predicted for worse PFS (HR 1.62, 95% CI 1.18-2.21, P=0.0026) Induction chemotherapy was not associated with PFS (13.8 months with vs. 15.0 months without, P=0.667).
The median overall survival (OS) time for the entire cohort was 27.3 months (95% CI: 22.7 – 33.7 months). OS was associated with baseline T status (P≤ 0.0001), N status (P=0.001), and tumor grade (P≤ 0.0001), and marginally associated with sex (P=0.078, with women seemingly having longer OS than men). Median survival times based on the site of first failure were 27.8 months for local, 21.7 months for distant and 52.5 months for no failure. Having a failure within the GTV (as opposed to all other failure patterns plus patients without failure) influenced OS as well; the median OS time for patients with GTV failure was 23.3 months (95% CI = 20.00 – 31.32) versus 31.6 months for those with no GTV failure (95% CI = 24.31–not reached) (P=0.0009). The 2-year and 3-year OS rates were also significantly different for those with and without GTV failure: at 2 years, OS rates were 49.1% (95% CI = 40.3–59.8%) with GTV failure versus 58.7% (95% CI 50.9%-67.7%) without (P=0.0009); at 3 years, OS rates were 34.4% (95% CI = 26.2%–45.2%) for those with GTV failure versus 48.6% (95% CI 40.6%-58.1%) for those without (P=0.0009) (Fig. 3).
Figure 3.
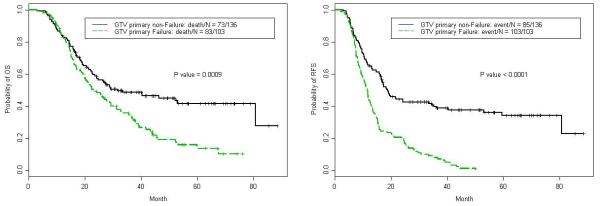
Kaplan-Meier estimates of overall survival according to failure within the gross tumor volume after definitive chemoradiation therapy for unresectable esophageal cancer compared to all other patients without GTV failure.
Predictors of GTV Primary Failure
To identify which clinical factors, if any, may be associated with failure in the GTV, we used Fisher’s exact tests and Wilcoxon rank sum test to compare categorical variables and continuous variables for patients with and without GTV failure. On univariate analysis, GTV failure was associated with being male (P=0.0185), having M1b disease (P=0.013), having a tumor > 8 cm long (P=0.005), having a posttreatment SUV > 4.3 (P=0.011), and having a pretreatment-to-posttreatment change in SUV of ≤ 52% (P=0.015). Tumor status correlated strongly with GTV failure: 16% of patients with T1 or T2 tumors (4 of 24) had GTV failures compared with 47% of those with T3 or T4 tumors (98 of 208) (P=0.0009). Overall locoregional-disease–free survival rates were also different depending on tumor status (77.4% [24 of 31] for T1/T2 vs. 46.2% [96 of 208] for T3/T4, P = 0.001). Interestingly, the rate of failure within the GTV was found to be higher among patients receiving induction chemotherapy (50% vs. 36% for those who did not receive induction chemotherapy, P=0.032). Younger age may also have been associated with higher risk of GTV failure (P=0.056) (Table 3).
Table 3.
Fisher’s exact test or chi-square test to determine the association of GTV failure and covariates
| Failure in Gross Tumor Volume | |||
|---|---|---|---|
| Variable | No | Yes | P Value |
| Sex | |||
| Female | 25 (76%) | 8 (24%) | 0.0185 |
| Male | 111 (54%) | 95 (46%) | |
| Age, y | |||
| ≤ 65 | 57 (50%) | 56 (50%) | 0.0561 |
| > 65 | 79 (63%) | 47 (37%) | |
| Tumor category | |||
| T1/T2 | 26 (84%) | 5 (16%) | 0.0009 |
| T3/T4 | 110 (53%) | 98 (47%) | |
| Lymph node category | |||
| N0 | 44 (57%) | 33 (43%) | 0.6686 |
| N1 | 90 (56%) | 70 (44%) | |
| NX | 2 (100%) | 0 (0%) | |
| Metastasis category | |||
| M0 | 113 (60%) | 76 (40%) | 0.013 |
| M1a | 17 (61%) | 11 (39%) | |
| M1b | 6 (27%) | 16 (73%) | |
| Tumor location | |||
| Cervical | 2 (67%) | 1 (33%) | 0.9873 |
| Upper thoracic | 10 (56%) | 8 (44%) | |
| Mid-thoracic | 16 (55%) | 13 (45%) | |
| Lower thoracic | 108 (57%) | 81 (43%) | |
| Tumor histology | |||
| Adenocarcinoma | 101 (56%) | 80 (44%) | 0.3954 |
| Squamous cell | 35 (61%) | 22 (39%) | |
| Neuroendocrine | 0 (0%) | 1 (100%) | |
| Tumor grade | |||
| G1/G2 | 63 (58%) | 46 (42%) | 0.7982 |
| G3/GX | 73 (56%) | 57 (44%) | |
| Tumor length | |||
| ≤ 8 cm | 122 (60%) | 81 (40%) | 0.0051 |
| > 8 cm | 9 (32%) | 19 (68%) | |
| Induction chemotherapy | |||
| No | 81 (63%) | 47 (37%) | 0.0325 |
| Yes | 55 (49%) | 56 (50%) | |
| Status | |||
| Alive | 63 (76%) | 20 (24%) | <0.0001 |
| Dead | 73 (47%) | 83 (53%) | |
| CTV failure | |||
| No | 126 (59%) | 86 (41%) | 0.0269 |
| Yes | 10 (37%) | 17 (63%) | |
| PTV failure | |||
| No | 129 (57%) | 96 (43%) | 0.5908 |
| Yes | 7 (50%) | 7 (50%) | |
| Distant failure | |||
| No | 76 (61%) | 49 (39%) | 0.2028 |
| Yes | 60 (53%) | 54 (47%) | |
| Pretreatment iSUV | |||
| ≤ 6 (median) | 29 (64%) | 16 (36%) | 0.1943 |
| > 6 (median) | 89 (54%) | 77 (46%) | |
| Post treatment SUV | |||
| ≤ 4.3 (median) | 69 (65%) | 37 (35%) | 0.0105 |
| > 4.3 (median) | 50 (48%) | 55 (52%) | |
| SUV change | |||
| ≤ 52% | 30 (42%) | 41 (58%) | 0.0147 |
| > 52% | 68 (61%) | 44 (39%) | |
Abbreviations: GTV, gross tumor volume; CTV, clinical target volume; PTV, planning target volume; iSUV, standardized uptake value.
In multivariate analysis, having a T3 or T4 tumor compared with a T1 or T2 tumor was associated with higher risk of GTV failure (odds ratio [OR] 6.35, 95% CI 1.92-20.95, P=0.002). Risk of GTV failure was also associated with extent of change in SUV before versus after chemoradiation, with a decrease of > 52% conferring a lower risk of GTV failure (OR 0.37, 95% CI 0.19-0.72, P=0.003). Tumor length was also a predictor of GTV failure on multivariate analysis, both as a categorical value (> 8 cm) (OR 4.08, 95% CI 1.42-11.69, P=0.009) (Table 4). Tumor length as a continuous variable in the multivariate analysis also suggested that bigger tumor was associated with a higher risk to develop GTV failure (OR 1.23, 95% CI 1.06-1.41, P=0.005).
Table 4.
Multivariate logistic regression analysis for GTV failure
| Variable | Odds Ratio (95% CI) |
P Value |
|---|---|---|
| Tumor category | ||
| T1/T2 | ||
| T3/T4 | 6.345 (1.922-20.947) | 0.002 |
| iSUV decrease | ||
| ≤ 52% | ||
| > 52% | 0.368 (0.188-0.718) | 0.003 |
| Tumor length (categorical) | ||
| ≤ 8 cm | ||
| > 8 cm | 4.078 (1.422-11.690) | 0.009 |
Abbreviation: CI, confidence interval.
DISCUSSION
With current treatment, the outcome for patients with unresectable esophageal cancer is poor 20. Here we demonstrate that localized EC treated with definitive chemoradiation , the treatment failure is often in the GTV. We also identified several pretreatment risk factors as being associated with risk of GTV failure, including having a T3 or T4 tumor, a tumor > 8 cm long, and perhaps age < 65 years. These factors may be helpful in identifying patients who could benefit from alternate strategies. Given the advances that have taken place in target identification and treatment delivery, we propose that it is time to revisit the concept of dose escalation in a personalized approach to therapy that is based on these risk factors.21-23
Our findings suggest that stratifying patients based on risk factors could be useful in terms of identifying those patients at highest risk of local failure. Tumor status at diagnosis was one such finding, with T1 or T2 tumors having a local control rate of 77% local control versus only 46% for T3 or T4 tumors, suggesting that many earlier stage tumors can be treated effectively with definitive chemoradiation. Nevertheless, we found that patients with T1/T2 disease were still more likely to experience local rather than distant failure, suggesting that some patients with early-stage disease may benefit from intensification of local therapy. Because nonsurgical treatment of more advanced T3/T4 disease continues to be associated with high failure rates, most such patients with unresectable disease might benefit from escalation of local therapy as well. This is supported by previous studies demonstrating that for solid tumors, a minimum of 65 Gy to 70 Gy would be needed for tumor control.24 While chemotherapy can help to some degree, it does not seem to be able to change the fact that this dose (50.4 Gy in 28 fractions) is inadequate to achieve a high probability for local control.
Therapeutic intensification can be done in one of two ways: by escalating the radiation dose and by adding radiation-sensitizing agents such as biological-based therapeutics. With regard to the first approach, several recent reports have shown the utility of dose escalation for esophageal cancer, especially given the new treatment modalities and techniques now available.22 In the previously noted dose escalation trial RTOG 94-05, dose escalation was thought to be ineffective and highly toxic; most of the patients in that study could not receive even a 50.4 Gy dose owing to toxicity.20
We recently showed that a simultaneous integrated boost IMRT technique could increase the dose to the primary gross tumor by 28% 22 while simultaneously achieving substantial reductions in cardiac and pulmonary doses secondary to improved treatment planning techniques. Proton-beam therapy has also been shown to effectively increase the dose to the primary tumor while reducing toxicity to proximal critical structures compared with 3-D conformal and IMRT techniques23, 25-27.
Another potential means of overcoming chemoradiation resistance of esophageal cancer is the use of rational targeted therapeutics that increases the therapeutic ratio of radiation by sensitizing tumor tissues. Inhibition of key kinases in oncologic signaling pathways such as PI3K/AKT can lead to effective radiosensitization, which may translate into improved local control. The encouraging findings seen from the combination of Cetuximab with radiation therapy for head and neck cancers have led to the adoption of similar approaches for esophageal cancer.28 Currently the RTOG is investigating the addition of Cetuximab to chemoradiation therapy for esophageal cancer.29, 30 Other biologic targets being investigated for this purpose include inhibitors of Hedgehog,31 BCL-2, VEGF, mTOR, and HSP-90.32 The hope is that such treatments will address resistant cancer cell populations that do not respond to chemoradiation therapy.
Although dose escalation has been shown to improve local control and survival in patients with other types of solid tumors,33 caution is warranted in applying this concept to esophageal cancer. Given the proximity of the esophagus to the heart and lung, great care must be taken to ensure that improvements in local control are not achieved at the cost of greater morbidity. High-dose radiation therapy could, for example, substantially increase the risk of esophageal stricture and or perforation, a potentially life-threatening complication.34 Moreover, dose escalation should probably not be used for tumors with extensive gastric penetration, as the tolerance of the gastric mucosa to radiation is even lower than that of the esophagus.35, 36 Finally, even though we found that the GTV was the most common site of initial failure, our patients also experienced high rates of systemic failure; thus efforts to increase local control may not necessarily translate into improved survival unless systemic therapies also improve. Thus far, trials to evaluate intensification of chemotherapy through the addition of induction chemotherapy have shown a mixed response37, 38. Perhaps the utility of this approach will not be fully realized until the high local failure rate is addressed.
Among the limitations of this study were its retrospective nature, with the associated biases, and the fact that techniques for disease staging improved considerably over the 7-year time span of the study. Moreover, although the focus of our analysis was on local failure, systemic failure was also all too common in our patient population; thus efforts to improve overall survival may have to await improvements in systemic therapies for control of distant as well as local disease. Some of the strengths of our analysis include the large number of patients with a relatively rare form of esophageal cancer, the consistency of the treatment dose and technique used over the period of study, and the relatively long follow-up time.
In summary, we found that local control after definitive chemoradiation therapy for esophageal cancer remains a problem and that most local failures occur within the GTV. It is warranted to explore potential ways of improving local control including IMRT, hadron therapy, and the use of more effective dose fractionation strategies. It seems appropriate to evaluate patient-based risk factors such as tumor status, tumor length, and other biological correlates that seem to predict local versus systemic relapses.
ACKNOWLEDGEMENTS
* This paper is dedicated to the memory of a dear patient Ms. Margaret Hollinshead Ley, who taught us the importance of living life to the fullest and reminds us that behind every data point lies a human being and a family which also suffers from their diagnosis.
Research supported in part by Dallas, Park, Sultan, Smith, and Cantu Family funds; the Reivercreek and Schecter Private foundations; Kevin and Frazier funds; the Multidisciplinary Research Program Grant from The University of Texas MD Anderson Cancer Center; and National Cancer Institute grants CA142072, CA127672, and CA129906
Footnotes
Financial disclosures: The authors declare no potential conflicts of interest.
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Brown LM, Swanson CA, Gridley G, et al. Adenocarcinoma of the esophagus: role of obesity and diet. J Natl Cancer Inst. 1995;87(2):104–9. doi: 10.1093/jnci/87.2.104. [DOI] [PubMed] [Google Scholar]
- 3.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100(16):1184–7. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97(2):142–6. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 5.Kelsen DP, Winter KA, Gunderson LL, et al. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol. 2007;25(24):3719–25. doi: 10.1200/JCO.2006.10.4760. [DOI] [PubMed] [Google Scholar]
- 6.Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med. 1997;337(3):161–7. doi: 10.1056/NEJM199707173370304. [DOI] [PubMed] [Google Scholar]
- 7.Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TP. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335(7):462–7. doi: 10.1056/NEJM199608153350702. [DOI] [PubMed] [Google Scholar]
- 8.Kleinberg L, Forastiere AA. Chemoradiation in the management of esophageal cancer. J Clin Oncol. 2007;25(26):4110–7. doi: 10.1200/JCO.2007.12.0881. [DOI] [PubMed] [Google Scholar]
- 9.Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26(7):1086–92. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaast A, et al. Effect of preoperative concurrent chemoradiotherapy on survival of patients with resectable esophageal or esophagogastric junction cancer: Results from a multicenter randomized phase III study. J Clin Oncol. 2010;28(15s) [Google Scholar]
- 11.Forastiere AA. Treatment of locoregional esophageal cancer. Semin Oncol. 1992;19(4 Suppl 11):57–63. [PubMed] [Google Scholar]
- 12.Herscher LL, Cook JA, Pacelli R, Pass HI, Russo A, Mitchell JB. Principles of chemoradiation: theoretical and practical considerations. Oncology (Williston Park) 1999;13(10 Suppl 5):11–22. [PubMed] [Google Scholar]
- 13.Ajani M Jaffer, Berkaii-Saab M Tanios, Bentrem M Daivd. NCCN Clinical Pratcie Guidlines in Oncology. al e. Available from URL: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 14.Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20(5):1167–74. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 15.Marks LB, Ma J. Challenges in the clinical application of advanced technologies to reduce radiation-associated normal tissue injury. Int J Radiat Oncol Biol Phys. 2007;69(1):4–12. doi: 10.1016/j.ijrobp.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Orlandi E, Palazzi M, Pignoli E, Fallai C, Giostra A, Olmi P. Radiobiological basis and clinical results of the simultaneous integrated boost (SIB) in intensity modulated radiotherapy (IMRT) for head and neck cancer: A review. Critical reviews in oncology/hematology. 2010;73(2):111–25. doi: 10.1016/j.critrevonc.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53(5):1097–105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 18.Martel MK, Ten Haken RK, Hazuka MB, et al. Estimation of tumor control probability model parameters from 3-D dose distributions of non-small cell lung cancer patients. Lung Cancer. 1999;24(1):31–7. doi: 10.1016/s0169-5002(99)00019-7. [DOI] [PubMed] [Google Scholar]
- 19.Kong FM, Ten Haken RK, Schipper MJ, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys. 2005;63(2):324–33. doi: 10.1016/j.ijrobp.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Minsky BD, Pajak TF, Ginsberg RJ. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–74. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 21.Zhu XR, Sahoo N, Zhang X, et al. Intensity modulated proton therapy treatment planning using single-field optimization: the impact of monitor unit constraints on plan quality. Medical Physics. 2010;37(1210) doi: 10.1118/1.3314073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welsh J, Palmer MB, Ajani JA, et al. Esophageal Cancer Dose Escalation using a Simultaneous Integrated Boost Technique. International journal of radiation oncology, biology, physics. 2010 doi: 10.1016/j.ijrobp.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welsh J, Riley B, Palmera M, et al. Intensity Modulated Proton Therapy Allows Dose Escalation and Normal-Tissue Sparing in Locally Advanced Distal Esophageal Tumors. International Journal of Radiation Oncology*Biology*Physics. 2010;78(3) doi: 10.1016/j.ijrobp.2010.07.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shukovsky LJ, Fletcher GH. Time-dose and tumor volume relationships in the irradiation of squamous cell carcinoma of the tonsillar fossa. Radiology. 1973;107(3):621–6. doi: 10.1148/107.3.621. [DOI] [PubMed] [Google Scholar]
- 25.Chang JY, Komaki R, Lu C, et al. Phase 2 study of high-dose proton therapy with concurrent chemotherapy for unresectable stage III nonsmall cell lung cancer. Cancer. 2011 doi: 10.1002/cncr.26080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang JY, Komaki R, Wen HY, et al. Toxicity and Patterns of Failure of Adaptive/Ablative Proton Therapy for Early-Stage, Medically Inoperable Non-Small Cell Lung Cancer. International journal of radiation oncology, biology, physics. 2011 doi: 10.1016/j.ijrobp.2010.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu SW, Li JM, Chang JY, et al. A treatment planning comparison between proton beam therapy and intensity-modulated x-ray therapy for recurrent nasopharyngeal carcinoma. Journal of X-ray science and technology. 2010;18(4):443–50. doi: 10.3233/XST-2010-0265. [DOI] [PubMed] [Google Scholar]
- 28.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus Cetuximab for Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2006;354(6):567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 29.Suntharalingam M Mohan. A Phase III Trial Evaluating the Addition of Cetuximab to Paclitaxel, Cisplatin, and Radiation for Patients with Esophageal Cancer Who Are Treated Without Surgery. Available from URL: http://www.rtog.org/members/protocols/0436/summary_changes.html.
- 30.Pinto C, Di Fabio F, Barone C, et al. Phase II study of cetuximab in combination with cisplatin and docetaxel in patients with untreated advanced gastric or gastro-oesophageal junction adenocarcinoma (DOCETUX study) Br J Cancer. 2009;101(8):1261–8. doi: 10.1038/sj.bjc.6605319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei L, Xu Z. Cross-signaling among phosphinositide-3 kinase, mitogen-activated protein kinase and sonic hedgehog pathways exists in esophageal cancer. Int J Cancer. doi: 10.1002/ijc.25673. [DOI] [PubMed] [Google Scholar]
- 32.Lee W, Patel JH, Lockhart AC. Novel targets in esophageal and gastric cancer: beyond antiangiogenesis. Expert Opin Investig Drugs. 2009;18(9):1351–64. doi: 10.1517/13543780903179286. [DOI] [PubMed] [Google Scholar]
- 33.Pollack MD Alan, Zagars Gunar K., Starkschall George. Prosate cancer radation dose resposne: Results of the M.D Anderson Phase II randomized trial. Int. J. Radiation Oncology Biol. Phys. 2002;53(5):1097–105. doi: 10.1016/s0360-3016(02)02829-8. PH.D. MD. PD. [DOI] [PubMed] [Google Scholar]
- 34.Marks LB, Zeng J, Light K, Kahn D, Zhou S. 116: Radiation-Induced Esophageal Stricture Following Therapy for Lung Cancer: Its Clinical Course and Analysis Comparing Stricture Length With Isodose Levels. International Journal of Radiation Oncology*Biology*Physics. 2006;66(3, Supplement 1):S66–S67. [Google Scholar]
- 35.van der Geld YG, Senan S, van Sörnsen de Koste JR, Verbakel WFAR, Slotman BJ, Lagerwaard FJ. A Four-Dimensional CT-Based Evaluation of Techniques for Gastric Irradiation. International Journal of Radiation Oncology*Biology*Physics. 2007;69(3):903–09. doi: 10.1016/j.ijrobp.2007.06.062. [DOI] [PubMed] [Google Scholar]
- 36.Caudry M, Escarmant P, Maire JP, Demeaux H, Guichard F, Azaloux H. Radiotherapy of gastric cancer with a three field combination: Feasibility, tolerance, and survival. International Journal of Radiation Oncology*Biology*Physics. 1987;13(12):1821–27. doi: 10.1016/0360-3016(87)90347-6. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe M, Nagai Y, Kinoshita K, et al. Induction chemotherapy with docetaxel/cisplatin/5-fluorouracil for patients with node-positive esophageal cancer. Digestion. 2011;83(3):146–52. doi: 10.1159/000321797. [DOI] [PubMed] [Google Scholar]
- 38.McCurdy M, McAleer MF, Wei W, et al. Induction and concurrent taxanes enhance both the pulmonary metabolic radiation response and the radiation pneumonitis response in patients with esophagus cancer. International journal of radiation oncology, biology, physics. 2010;76(3):816–23. doi: 10.1016/j.ijrobp.2009.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]


