Abstract
Phenotypic heterogeneity complicates detection of genomic loci predisposing to type 2 diabetes, potentially obscuring or unmasking specific loci. We conducted ordered subsets linkage analyses (OSA) for diabetes-related quantitative traits (fasting insulin and glucose, HbA1c and 28-year time averaged fasting plasma glucose (tFPG)) from 330 families of the Framingham Offspring Study. We calculated mean body mass index (BMI), waist circumference (WC), and a diabetes ‘age-of-onset score’ for each family. We constructed subsets by adding one family at a time in increasing (lean family to obese) or decreasing (obese to lean) adiposity order or increasing or decreasing propensity to develop diabetes at a younger age, with the OSA LOD reported as the maximum LOD observed in any subset. Permutation p-values tested the hypothesis that phenotypic ordering showed stronger linkage than random ordering. On chromosome 1, ordering by increasing family mean WC increased linkage to tFPG at 256 cM from LOD = 2.4 to 3.5 (permuted p=0.02) and to HbA1c at 180 cM from LOD = 2.0 to 3.3 (p=0.01). On chromosome 19, ordering by decreasing WC increased linkage to fasting insulin at 68 cM from LOD = 2.7 to 4.6 (p=0.002), and ordering by decreasing propensity to develop diabetes at a young age increased linkage to fasting insulin at 73 cM from LOD = 2.7 to 4.0 (p=0.046). We conclude that chromosomes 1 and 19 could harbor adiposity-interacting diabetes susceptibility genes. Such interactions might also influence trait-locus associations and may be useful to consider in diabetes genome-wide association studies.
Keywords: type 2 diabetes mellitus, insulin resistance, genetics, risk factors, longitudinal study, linkage study, genetics, genomics
Introduction
Control of accelerating rates of type 2 diabetes requires an understanding of the gene-environment interactions involved in its pathogenesis. Type 2 diabetes is a genetic disorder, but reproducible evidence on gene-environment interactions conferring susceptibility to its common form is only slowly emerging. Genetic signals may be affected by phenotypic heterogeneity that confound associations of genes with disease expression by obscuring some susceptibility loci, unmasking others, or producing ‘non-genetic’ phenocopies. In particular, three major phenotypic diabetes risk factors: obesity, older age-related onset, and parental diabetes all may act as ‘environmental’ modifiers of disease penetrance. Obesity may lead to diabetes by exacerbating underlying genetically-mediated susceptibility pathways, via pleiotropic locus effects, or through intermediate, non-genetic pathways producing diabetic phenocopies. Older age is a strong diabetes risk factor, but typical older-onset diabetes may represent a different phenotype (or have a different genetic basis) than younger-onset diabetes. Parental diabetes may confer risk for diabetes by transmission of susceptibility genes or by familial health-related behaviors or norms.
Genome-wide linkage analysis has been used in inherited disease gene discovery. Phenotypic stratification is a strategy to limit heterogeneity in linkage studies. Prior evidence suggests that stratification can strengthen LOD scores, increase confidence that linkage peaks contain true diabetes genes, and identify family subsets more likely to be segregating diabetes genes. (1–6) In the Framingham Heart Study (FHS), obesity is common; most cases of diabetes occur at older ages, and about 1 in 7 children have one or more diabetic parents. (7) In earlier work we performed unstratified analyses of linkage to diabetes-related quantitative trait loci (QTL) in pedigrees of the FHS. We identified modest evidence for linkage to glucose-related quantitative traits on chromosomes 1q and 10q as well as elsewhere in the genome. (8) Some of these linkage signals were strengthened by statistical control for body mass index (BMI), others were weakened, and yet others were not altered by BMI adjustment. We also examined unstratified linkage to insulin-related quantitative traits in FHS participants without diabetes but found few substantive linkage peaks. (9) However, in other FHS studies, we found significant interactions between ApoE gene polymorphisms (APOE, chromosome 19q13.2), obesity and insulin resistance, (10) and an interaction by obesity on risk for diabetes and insulin resistance associated with variation in the gene for interleukin 6 (IL6, chromosome 7p21). (11) These observations suggest that phenotypic heterogeneity may be altering some genetic signals, and point to stratification of linkage analyses to improve evidence for linkage and provide evidence of obesity interaction on genomic risk for diabetes.
In this paper we set out to test the hypothesis that accounting for phenotypic heterogeneity would strengthen linkage to diabetes-related traits compared with random-ordered unstratified analyses. We focused specifically on regions with a priori evidence for linkage or obesity interaction in order to reduce type 1 error, specifically, on chromosomes 1q, 7q, 10q, 19q. Our goal was to strengthen evidence for linkage at specific loci and to gain insight into ways in which common diabetes phenotypic variants alter genomic linkage to diabetes-related quantitative traits.
Methods and Procedures
Study Subjects
Subjects were participants in the community-based FHS. (8) FHS subjects include participants in the Original Cohort and Offspring Studies and are primarily of mixed white European descent. The Cohort (parents) includes 5209 subjects (including 1404 couples) examined for cardiovascular disease and its risk factors, including diabetes, biennially since 1949. Children of the Cohort (and the children’s spouses) participate in the Framingham Offspring Study, including 5124 subjects aged 12 to 58 years at enrollment in 1971. Offspring have been examined every 4 years since study onset except with 8 years between exams 1 and 2. The FHS design allows the connection of parents with offspring into a large number of pedigrees. Of these families, the largest 330 pedigrees were selected for genotyping, and in order to evaluate linkage in these pedigrees, the largest were split, resulting in 347 extended families. Of the 2514 FHS participants in these pedigrees, 1865 were genotyped and of these, 464 were from the original FHS Cohort and 1401 were from the Offspring Cohort. In these families, there are 537 nuclear families with 108 (20%) with one Offspring subject, 163 (30%) with two, 154 (29%) with three, 78 (15%) with four, 17 (3%) with five, and 17 (3%) with six or more. In the extended pedigrees there are 1554 sib pairs (of Offspring subjects), 572 avuncular pairs and 738 1st cousin pairs. Among the 1554 sib pairs, 1314 pairs both have FPG measured, and 586 of the 738 cousin pairs both have FPG measured. This study was approved by the Boston University IRB and written informed consent was obtained from each subject.
Clinical Phenotyping
FHS phenotyping methods, including assessment of alcohol, cigarette and medication use, have been reported previously. (12) Diabetes-related quantitative trait data in this analysis come from Offspring exam 5 (1991–1994) or from enrollment from exam 1 through exam 7 (1998–2001), the most recent follow-up exam. Briefly, subjects underwent a standardized medical history and physical exam, provided a fasting blood sample, and (for those without medication-treated diabetes) underwent a 75 gram oral glucose tolerance test. From these we measured fasting and 2hr post-challenge glucose and insulin, hemoglobin A1c (HbA1c,) the 28-year mean FPG level calculated using the mean FPG from ≥2 exams, and homeostasis model-assessed insulin resistance, where HOMA IR = (fasting insulin × fasting glucose)/22.5. (13) Fasting insulin and HOMA-IR levels are validated surrogate measures of insulin resistance. (14; 15) Height, weight, and waist circumference were measured with the subject standing; we measured the waist circumference at the umbilicus and calculated body mass index (BMI) as kg/m2.
We defined diabetes as use of oral hypoglycemic or insulin therapy at the index exam or any prior exam or a FPG ≥7.0 mmol/l the index exam and any one or more prior exams. We established diabetes age-of-onset as the mid-point of the interval between the age at the examination at which diabetes was first recognized and the preceding exam, or by chart review for subjects presenting with diagnosed diabetes at enrollment; 114 subjects were classified with diabetes. Over 98% of diabetes in the FHS is type 2 diabetes. (7) The mean age-of-onset of Offspring diabetes was 58±9 SD years; as of exam 7, 9.3% of diabetic subjects had developed diabetes by age 40 years, 33.0% by age 50, 68.1% by age 60, and 99.7% by age 80. FPG or 75 gm oral glucose tolerance test data are not available for parents, so we defined parental diabetes as hypoglycemic treatment or a casual plasma glucose level ≥200 mg% at any Cohort exam. The mean age-of-onset of parental diabetes was 60±10 SD yr. Among offspring in 330 families, 19.1% had one parent with diabetes (15.6% have paternal diabetes and 13.1% have maternal diabetes) 1.9% had both parents with diabetes, and 23% had unknown parental diabetes status.
Laboratory methods
Laboratory methods for quantitative traits and genotyping have been described previously. (8; 12) Insulin was measured with a total immunoreactive insulin assay (Diagnostic Products Corp, Los Angeles, CA). Coefficients of variation (CV) were <3% for glucose, <2.5% for HbA1c and <10% for insulin. Microsatellite short tandem repeat (STR) markers were assayed by the Marshfield Mammalian Genotyping Service using a set of 401 markers covering the genome at an average density of one marker every 10 cM and with an average heterozygosity of 0.77 (Screening Set v. 9). (http://research.marshfieldclinic.org/genetics/) Map distances were taken from Screening Set v.9 and the Marshfield ‘build your own map’ facility.
Statistical analysis
We used sex-specific linear regression models to adjust individual trait levels for age and age squared, physical activity, alcohol consumption, smoking status, and estrogen usage in women, or in separate models, these and BMI (to further reduce trait variability due to individual BMI). We excluded Offspring subjects treated with exogenous insulin from the analyses of HOMA-IR and fasting insulin. In the regression models, we included all remaining subjects with available data (1,705 men and 1,904 women with FPG; 1,629 and 1,815 with fasting insulin, 1,237 men and 1,408 women with HbA1c, and 2,453 men and 2,610 women with 28-year mean FPG). We performed unstratified multipoint linkage analyses on residuals derived from adjusted levels of exam 5 FPG, fasting insulin, HbA1c, and 28-year mean FPG using the variance component analysis (VCA) model implemented in GENEHUNTER. (16–18) The model makes no assumptions about the mode of transmission and uses all of the data from the pedigree without breaking it up into smaller subsets. The variance-component model partitions the variability of a multivariate normal trait into components for a quantitative trait locus (QTL), the residual polygenic component and the random individual error. Linkage is evaluated by whether the variance component for the QTL is statistically significantly greater than zero. Comparison of likelihoods on a log base 10 scale with the QTL variance set to zero and one with the QTL freely estimated provides a LOD score. Using marker spacings of ~10 cM, for each trait tested in an unordered analysis, a LOD score of 3.0 would be required for genome-wide significance. (19)
Family specific trait levels were derived using individual values to calculate mean and standard deviation (SD) BMI and waist circumference for each family. For diabetes age-of-onset, we extended to pedigrees the survival method developed by Li and Zhong for nuclear families by using normally distributed frailty parameters to calculate the propensity for individuals to develop diabetes by a given age. (20) We obtained Martingale residuals from these models, then averaged the residuals for each family to estimate a family ‘age-of-onset score’, where low or negative values indicate a family with one or more members developing diabetes at a relatively young age, and high positive scores indicate a family where most or all members had reached older age without development of diabetes.
We performed ordered subsets linkage analyses (OSA) to diabetes-related quantitative traits using family-specific LOD scores generated from the initial linkage analysis (18) and implemented in the Order Subsets algorithm in R (www.r-project.org). (21) We ranked families according to the family-specific value of the trait of interest, including mean family BMI, mean family WC, and mean family-specific diabetes ‘age-of-onset score’. Also, we performed a stratified linkage analysis by separating the families into two strata of none vs. one or more parents with diabetes. The OSA analysis proceeded as follows. For a each chromosome, diabetes-related quantitative trait and ordering strategy (for instance, ordering from lean families to obese, or obese to lean), we started with the family-specific LOD score of the family with the initial lowest (or highest) trait level. We added family-specific LOD scores, one family at a time, in trait rank order, until all families were included. After each family was added, we determined the LOD score for the current subset of families, noting the mean family trait value and cM location at that LOD score. We continued the procedure until all families were added, and then report the maximum LOD score observed over all the subsets, and its location, and the family mean and SD BMI, waist circumference, or Martingale residual at which the maximum LOD was obtained. For each ordering strategy we derived permutation p-values from 1000 random family orderings to test the hypothesis that ordering by family phenotype gave stronger linkage than random ordering. For permutation of parental diabetes stratified analyses, we randomly divided the families into two groups with the same sizes as the parental diabetes subsets, calculated the LOD score observed in each group, and defined a P value as the proportion of times the permutation LOD score was greater than or equal to the observed LOD score. Analyses were not adjusted for multiple statistical testing; so we report actual P values for each unordered vs. ordered linkage contrast, and limit our key interpretations to loci with a priori linkage or association evidence in FHS.
Results
Family Characteristics
Among 347 families, the median family mean BMI was 27.1 kg/m2, with interquartile bounds of 25.5 to 28.3 kg./m2 (Figure 1). The median family mean waist circumference was 94.6 cm, with interquartile bounds of 88.5 to 100.0 cm. Among 114 subjects developing diabetes, the mean age-of-onset was 55.3 (SD 11.5) years, and among 1287 remaining free of diabetes, the mean age at last follow-up was 57.7 (SD 10.2). We estimated the propensity to develop diabetes at a young age or to remain free of diabetes at older age by deriving Martingale residuals from a Cox regression model; the distribution of this ‘age-of-onset score’ is shown in Figure 1. Of 347 families, 103 (30%) had one or more parents in the family with diabetes.
Figure 1.

Cumulative distributions of median family mean BMI in 347 families (left-hand panel); median family mean waist circumference; (middle panel) and Martingale residuals, interpreted as a ‘diabetes age-of-onset propensity score’ (right-hand panel).
Linkage Ordered by Family BMI or Waist Circumference
On chromosome 1, ordering subsets by increasing mean BMI or waist circumference from lean to obese families significantly increased linkage to BMI-adjusted HbA1c, 28-yr mean FPG, fasting insulin and HOMA-IR (Table 1 and Figure 2). For instance, in Figure 2, linkage to BMI-adjusted 28-yr mean FPG increased from a LOD of 2.36 in unordered VCA to a LOD of 3.51 (p=0.022 compared with random ordering of a similar number of families). Figure 2 shows that on chromosome 1 linkage at 260.9 cM to 28-yr mean FPG increased among leaner families, with maximum linkage occurring once the mean waist circumference reached 100.2 cm (at 226 families contributing). BMI-adjusted HbA1c showed a similar result on chromosome 1, with the LOD score increasing to over 3.3 by ordering families from lean to obese.
Table 1.
Linkage to Diabetes Traits Ordered by Family Mean BMI or Waist Circumference
| Diabetes-Related Quantitative Trait* | a priori linkage† | Locus | LOD‡ | simulation p-value | No. Families | Mean BMI§ (kg/m2) | SD BMI (kg/m2) | Family Subset Order | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Chr | cM | VCA | OSA | |||||||
|
|
|
|
|
|
|
|||||
| HbA1c (BMI) | trait and locus | 1 | 180.8 | 2.02 | 3.35 | 0.027 | 139 | 28.10 | 4.93 | Mean BMI (Lean to Obese) |
| HbA1c (BMI) | trait and locus | 2 | 173.0 | 2.87 | 4.08 | 0.025 | 186 | 30.37 | 5.25 | Mean BMI (Lean to Obese) |
| HOMA-IR | locus | 3 | 209.4 | 1.49 | 2.58 | 0.009 | 240 | 25.48 | 3.56 | Mean BMI (Obese to Lean) |
| Fasting Insulin (BMI) | trait and locus | 19 | 68.1 | 2.71 | 4.07 | 0.018 | 175 | 26.76 | 3.00 | Mean BMI (Obese to Lean) |
| HOMA-IR (BMI) | trait and locus | 19 | 73.1 | 2.01 | 3.39 | 0.015 | 173 | 26.91 | 4.03 | Mean BMI (Obese to Lean) |
| Fasting Insulin | trait and locus | 19 | 73.1 | 1.89 | 3.00 | 0.016 | 123 | 27.79 | 2.70 | Mean BMI (Obese to Lean) |
| HOMA-IR | trait and locus | 19 | 73.1 | 1.13 | 2.26 | 0.012 | 122 | 27.86 | 5.45 | Mean BMI (Obese to Lean) |
| 28 yr mean fasting plasma glucose (BMI) | trait and locus | 21 | 35.3 | 1.96 | 3.08 | 0.026 | 320 | 31.69 | 1.74 | Mean BMI (Lean to Obese) |
|
| ||||||||||
| Mean WC§ (cm) | SD WC (cm) | |||||||||
|
| ||||||||||
| 28 yr mean fasting plasma glucose (BMI) | trait and locus | 1 | 260.9 | 2.36 | 3.51 | 0.022 | 262 | 100.2 | 10.0 | Mean Waist (Lean to Obese) |
| HbA1c (BMI) | trait and locus | 1 | 180.8 | 2.02 | 3.31 | 0.012 | 175 | 102.3 | 6.6 | Mean Waist (Lean to Obese) |
| HOMA-IR (BMI) | locus | 1 | 226.2 | 1.48 | 2.82 | 0.003 | 35 | 104.0 | 9.3 | Mean Waist (Obese to Lean) |
| Fasting plasma glucose | trait and locus | 3 | 89.9 | 2.28 | 3.37 | 0.048 | 241 | 88.7 | 15.5 | Mean Waist (Obese to Lean) |
| Fasting Insulin (BMI) | trait and locus | 19 | 68.1 | 2.71 | 4.61 | 0.002 | 129 | 96.3 | 10.5 | Mean Waist (Obese to Lean) |
| HOMA-IR (BMI) | trait and locus | 19 | 73.1 | 2.01 | 3.93 | 0.001 | 117 | 97.3 | 11.4 | Mean Waist (Obese to Lean) |
| 28 yr mean fasting plasma glucose (BMI) | trait and locus | 21 | 36.8 | 1.96 | 3.43 | 0.005 | 255 | 99.9 | 16.5 | Mean Waist (Lean to Obese) |
Traits adjusted for age and age2 in sex-specific models; (BMI) signifies additional adjustment for individual body mass index; HbA1c = hemoglobin A1c; HOMA-IR = homeostasis model insulin resistance. LOD scores >3.0 are shown in bold.
a priori linkage signifies substantive linkage observed in prior Framingham studies for both trait and locus (for instance, HbA1c at 180 cM on chromosome 1) or locus only (for instance, diabetes-related linkage on chromosome 1 but not to insulin traits)
LOD score for unordered variance components analysis (VCA) or variance components ordered-subsets analysis (OSA)
Family mean body mass index (BMI) or waist circumference (WC) at which maximum LOD score observed; SD signifies family standard deviation of mean.
Figure 2.
Chromosome 1 ordered-subsets linkage analyses (OSA) of hemoglobin A1c (left-hand panels, HbA1c adjusted for body mass index [BMI], and ordered by increasing family mean BMI); and 28-year mean fasting plasma glucose (right-hand panels, FPG adjusted for BMI and ordered by increasing family mean waist circumference). The top panels show LOD score by the number of families sequentially introduced by ordering, with the family number yielding the maximum LOD score indicated by the vertical dashed line; the middle panels show the mean and standard deviation (SD) of family BMI (left) or waist circumference (right) according to the number of families sequentially introduced by ordering, with the mean (SD) family obesity metric of the family producing the maximum LOD score indicated by the vertical dashed line; and the bottom panels show the LOD score by chromosomal 1 position (in centimorgans, cM) for all families (solid line) and the subset of families producing the maximum OSA LOD score (dashed line).
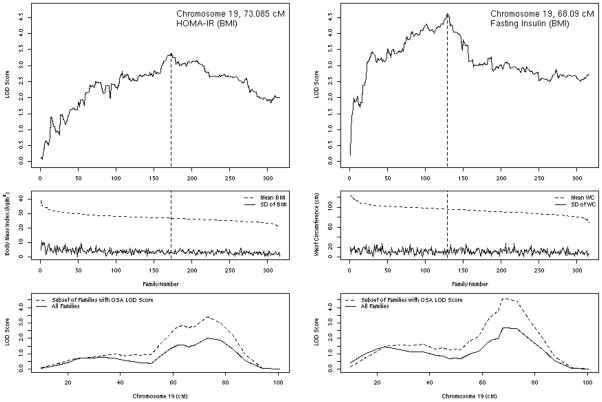
On chromosome 19, ordering subsets by decreasing mean BMI or waist circumference from obese to lean families significantly increased linkage to BMI-adjusted HOMA-IR and fasting insulin (Table 1 and Figure 3). For instance, linkage to BMI-adjusted HOMA-IR increased from a LOD of 2.01 in unordered VCA to a LOD of 3.39 (p=0.015 compared with random ordering of a similar number of families). As can been seen in Figure 3, on chromosome 19 linkage at 73.1 cM to HOMA-IR increased among more obese families, with maximum linkage occurring once the mean BMI had declined to 26.9 kg/m2 (at 173 families contributing). Ordering families by obesity status also significantly increased linkage to glycemic or insulin traits on chromosomes 2, 3, and 21 (Table 1). In most cases for HbA1c and 28-yr mean FPG, ordering from lean to obese families until the BMI or waist circumference reached the obese range (BMI >30 kg/m2 or waist circumference > 100 cm) produced the most dramatic increases in linkage (where LOD > 3.0), while ordering insulin traits from obese to lean produced the most dramatic changes, but here the most obese families contributed the most, with no additional linkage information contributed by families with BMI less than about 26 kg/m2 or waist circumference less than about 96 cm.
Figure 3.
Chromosome 19 ordered-subsets linkage analyses (OSA) of homeostasis model insulin resistance (left-hand panels, HOMA-IR adjusted for body mass index [BMI] and ordered by decreasing family mean BMI); and fasting insulin (right-hand panels, fasting insulin adjusted for BMI and ordered by decreasing family mean waist circumference). The top panels show LOD score by the number of families sequentially introduced by ordering, with the family number yielding the maximum LOD score indicated by the vertical dashed line; the middle panels show the mean and standard deviation (SD) of family BMI (left) or waist circumference (right) according to the number of families sequentially introduced by ordering, with the mean (SD) family obesity metric of the family producing the maximum LOD score indicated by the vertical dashed line; and the bottom panels show the LOD score by chromosomal 19 position (in centimorgans, cM) for all families (solid line) and the subset of families producing the maximum OSA LOD score (dashed line).
We previously reported substantive linkage to 28-yr mean FPG on chromosome 10, but obesity OSA did not alter linkage signals for any diabetes-related quantitative trait at this locus. For instance, on chromosome 10 at 79 cM, the unordered LOD for 28-yr mean FPG was 4.32; ordering by decreasing mean waist circumference increased the LOD to 4.98, but this was not different compared with random ordering of a similar number of families (p=0.26). In addition, on chromosome 7 we found no striking linkage or evidence for improved linkage by obesity OSA. For instance, the strongest evidence was for HbA1c at 160.1 cM on chromosome 7, with ordering by increasing family standard deviation of BMI, the LOD increased from 1.78 in unordered analysis to 2.59 in OSA (p=0.044).
Ordering by Diabetes Age-Of-Onset Score
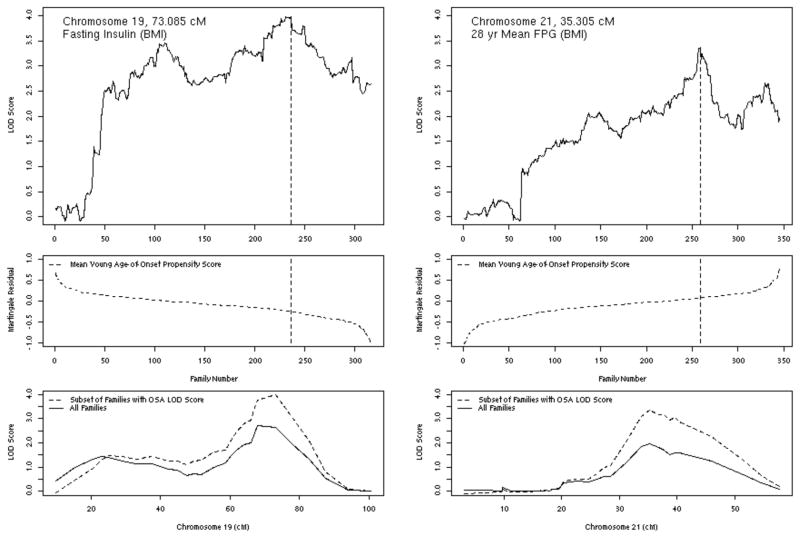
On chromosome 19, ordering subsets by decreasing propensity to develop diabetes at a young age significantly increased linkage to BMI-adjusted fasting insulin (Figure 4). Linkage to BMI-adjusted fasting insulin increased from a LOD of 2.71 in unordered VCA to a LOD of 3.99 (p=0.046 compared with random ordering of a similar number of families). As can been seen in Figure 4, on chromosome 19 linkage at 73.1 cM to fasting insulin increased steadily with falling Martingale residual, indicating that families tending to have older age-of-onset or not to have developed diabetes by older age contributed the most to linkage. On chromosome 21 (Figure 4), linkage to BMI-adjusted 28-year mean FPG at 35.3 cM increased from a LOD of 1.96 in unordered analysis to a LOD of 3.37 (p=0.013) with ordering by increasing Martingale residual, here suggesting that the families with a propensity to develop diabetes at young age contributed the most information for linkage. Also on chromosome 21 at 35.3 cM, ordering linkage to BMI-adjusted HOMA-IR by increasing propensity to develop diabetes at a young age increased linkage from a LOD of 1.40 in unordered analysis to a LOD of 2.46 (p=0.014). Ordering by diabetes age-of-onset propensity did not significantly improve linkage for any quantitative trait on chromosome 10.
Figure 4.
Chromosome 19 (left hand panels) and 21 (right hand panels) ordered-subsets linkage analyses (OSA) of fasting insulin (left-hand panels, fasting insulin adjusted for body mass index [BMI] and ordered by decreasing diabetes age-of-onset propensity score); and 28-year mean fasting plasma glucose (right-hand panels, FPG adjusted for BMI and ordered by increasing diabetes age-of-onset propensity score). The top panels show LOD score by the number of families sequentially introduced by ordering, with the family number yielding the maximum LOD score indicated by the vertical dashed line; the middle panels show the diabetes age-of-onset propensity score according to the number of families sequentially introduced by ordering, with the mean diabetes age-of-onset propensity score of the family producing the maximum LOD score indicated by the vertical dashed line; and the bottom panels show the LOD score by chromosomal position (in centimorgans, cM) for all families (solid line) and the subset of families producing the maximum OSA LOD score (dashed line).
Stratifying by Parental Diabetes
When we stratified families with one or more subjects with diabetes in the parental generation (102 families) vs none, linkage to BMI-adjusted 28-yr mean FPG on chromosome 1 at 231.1 cM increased from a LOD of 2.36 to a LOD of 2.96 (p=0.051 compared with 102 randomly selected families). Stratifying by parental diabetes did not significantly increase any other LOD score to near or greater than 3.0 at any other locus.
Discussion
In this study we have shown that accounting for phenotypic heterogeneity can strengthen linkage to diabetes-related quantitative traits compared with unordered linkage analysis. We focused specifically on regions with a priori evidence for linkage or obesity interaction in the community-based FHS. On chromosome 1, we found that leaner families contributed the most to linkage with glycemic traits with similar phenomena on chromosomes 2 and 21, although these are loci without exceedingly strong prior evidence for linkage. The data suggest that at these glycemia-linked loci, obesity may obscure diabetes-related genetic signals. Similar observations have been made with regard to the protective effect of the Alanine allele at PPARG P12A, which is diminished when obesity is increased. (22; 23) Conversely, on chromosome 19 in the region of APOE, we found that more obese families contributed the most to linkage with insulin traits, with a similar phenomenon on chromosome 3. The data suggest that at these loci, greater adiposity may unmask diabetes-related genetic signals. While chromosomes 1 and 19 may harbor adiposity-interacting diabetes-linked loci, chromosome 10 appears to harbor substantive diabetes-linked loci unaffected by the presence or absence of obesity. Although other studies have suggested that ordering by propensity to develop diabetes at a young age may strengthen linkage, (5; 6) in FHS this approach did not have widespread effects on linkage. Whereas previous work suggested parent-of-origin effects in diabetes transmission in FHS, (7) stratifying by parental diabetes did not have substantive effects on linkage to diabetes at any locus.
Until the recent availability of genome-wide association (GWA) approaches, few proven candidate diabetes susceptibility genes had been identified. (24) Genome-wide linkage to diabetes has been the primary strategy for novel diabetes gene discovery, but with its theoretical limitations has not consistently led to fruitful discovery. (25–27) In FHS, our data replicated linkage seen in other studies within a ~20 cM region on chromosome 1q21-24 (27) and a ~25 cM region on chromosome 10q23-25. (4; 28–30), but linkage peaks were modest and in some cases influenced by covariate adjustment for BMI, leading us to suspect obesity-by-locus interactions on linkage to diabetes-related QTLs. In addition, we previously found that variation at the apoE locus (at about 68.9 cM on 19q13.31-.32) was not associated with insulin resistance unless interaction by obesity was accounted for, with obese but not lean men with the APOE 4 genotype having significantly higher fasting insulin levels than those with the E2 or E3 genotypes. (10; 31) In another study of variation in IL6 (chromsome 7p2), we found a significant interaction between IL-6 genotype and BMI on levels of insulin resistance in men, with obese homozygotes for the minor C allele being most resistant. (11) These observations led us to consider an OSA approach to assess obesity-modified linkage on chromosomes 1, 7, 10, and 19 specifically. We did look for OSA-strengthened linkage at other loci, but consider those findings as hypothesis-generating, as an unbiased genome-wide approach may give false-positive signals from inflated type 1 error due to uncontrolled multiple testing. The results of our analysis suggest that, among the major risk factors for type 2 diabetes, variations in family adiposity have the most substantive effect to modify linkage to diabetes-related quantitative traits.
Obesity is a powerful risk factor for type 2 diabetes, increasing diabetes relative risk by as much as 10-fold. (32–34) Obesity may act through pleiotropic genetic effects of obesity loci, (5; 35–37) or may act as an ‘environmental’ effect on risk of type 2 diabetes by exacerbating insulin resistance and beta-cell dysfunction through secretion of free fatty acids and adipokines. (38–40) Evidence that a fewer than a third of obese subjects are insulin resistant or eventually develop diabetes (41; 42) suggests that obese subjects represent a mixed population of phenocopies and those truly at increased genetic risk. In type 2 diabetes, where multiple susceptibility loci are suspected, different pedigree members may appear to be phenotypically similar but actually have different genetic underpinnings, making inference from allele sharing among those with similar diabetes traits more difficult. The presence of obesity phenocopies dilutes the pool of true genetically at-risk subjects, increasing the probability of type II error in genetic studies. Pleiotropic effects raise the possibility of type I error, where linkage information associated with diabetes could primarily increase risk for obesity and vice versa. (43)
We hypothesized that stratification of pedigrees by obesity status may help to refine the risk phenotype and focus linkage signals for type 2 diabetes-related quantitative traits. We identified families that were on average more or less obese and sequentially introduced families into a linkage model, seeking the subset of families contributing most to linkage and significantly increasing evidence for linkage compared with similarly sized, randomly-ordered sets of families. Several prior studies have shown the promise of the approach, although not all have used permutation to test if ordering produced a better-than-random improvement in evidence for linkage. On chromosome 1, Vionnet et al showed that the maximum LOD score associated with diabetes was 1.2 in the whole sample, but after stratification of pedigrees into normal or obese (BMI < or ≥ 27 kg/m2), the LOD score at this locus increased to 2.5 among lean subjects. (1) Other studies have used ordering to find linkage to regions not seen in FHS. Parker et al assessed linkage to type 2 diabetes, finding on chromosome 18p that the LOD score was 0.66 in the entire sample, but increased progressively with successively more restrictive phenotypic definition such that the LOD score was 4.2 when the most obese 20% of pedigrees (BMI >36 kg/m2) were analyzed. (2) Aulchenko et al also found linkage on chromosome 18p (LOD 2.3). (3) One marker in this region was associated with diabetes (OR 6.7); the association was substantially stronger when the sample was limited to the top 25% of BMI (OR 12.3). In deCODE type 2 diabetes families, Reynisdottir et al found linkage to diabetes on chromosome 5q; in the overall sample, the LOD in this region was 2.9, but increased to 3.6 when the sample was limited to subjects with BMI ≥30 kg/m2). (4) No linkage to diabetes was found this region among subjects with BMI <30 kg/m2. In aggregate these results imply that the next generation of genome-wide association approaches to diabetes gene discovery may benefit from consideration of gene-by-adiposity interactions or phenotypic sub-setting for novel gene discovery.
Recent candidate gene and GWA studies have identified several replicated diabetes risk genes including a variant in FTO (16q12.2), that increases diabetes risk through association with obesity. FTO provides an important example whereby an apparent genetic risk factor for diabetes in fact creates genetic risk for obesity, which then in turn becomes a metabolic environmental mediator of diabetes risk. (43) Interestingly, many of the newly discovered variants increase diabetes risk by causing an impairment in insulin secretion (44; 45); a careful consideration of obesity as a modifier of diabetes genes may uncover novel loci associated with insulin resistance. Thus, obesity-diabetes pleiotropic and gene-environment interactions in diabetes GWA studies is likely to be a fruitful avenue for future research in FHS and other population samples.
Our study has some limitations. FHS is an average-diabetes-risk population sample, with relatively few people at the extreme end of the hyperglycemia phenotype distribution truncating, to some degree, the variance distribution of diabetes-related quantitative traits that may limit our ability to detect linkage to QTLs. Low risk also makes analyses sensitive to addition or subtraction of people with diabetes. In our prior insulin trait linkage study we excluded all subjects with diabetes and found no substantive linkage on chromosome 19, while in the present study, even in unordered analyses, we find substantive linkage in chromosome 19. (9) In the present study we retained all people with diabetes except those treated with exogenous insulin in the analysis of HOMA-IR and fasting insulin, as insulin therapy confounds measurement of plasma insulin as a quantitative trait; otherwise, FHS subjects with diabetes have the highest glucose and insulin levels and so remain the most informative subjects for diabetes-related gene discovery. A limitation of analysis of diabetes age-of-onset is that in FHS relatively few people develop diabetes before about age 55 years. A limitation of analysis of parental diabetes as an environmental risk factor is the relative homogeneity of the race/ethnic/socioeconomic background of the FHS sample, theoretically limiting to some degree the possible range of familial health-related behaviors or norms. This race/ethnic homogeneity also limits the generalizability of FHS genetic findings to other race/ethnic groups. Finally, we do not provide an exhaustive genome-wide inventory of obesity-interacting diabetes susceptibility loci, due to concerns about type 1 error when testing multiple ordered strata at hundreds of microsatellite loci.
In summary, we used an OSA approach to examine three major diabetes risk factors – parental diabetes, offspring obesity, and variable age-of-onset – that are likely to contribute substantial phenotypic heterogeneity in genetic studies of type 2 diabetes. We found that among these, obesity had the most widespread and pronounced effect modifying linkage to diabetes-related quantitative traits in the population-based FHS. We show that obesity can mask, expose, or have little influence on specific genomic loci linked to diabetes-related quantitative traits. Familial obesity stratification significantly strengthened evidence for linkage on several chromosomes, including chromosomes 1, 2, 3, 19 and 21, but had little effect on chromosomes 7 and 10. While some of these loci might be deserving of particular scrutiny using novel, more powerful GWA approaches, the results of our study indicate that gene-environment interaction, and the effect modification imposed by obesity in particular, should be considered in next-generation genome-wide approaches to type 2 diabetes genetic discovery.
Acknowledgments
Supported by R21 DK65732, the National Heart, Lung, and Blood Institute’s Framingham Heart Study (Contract No. N01-HC-25195), and by an American Diabetes Association Career Development Award to Dr. Meigs. Dr. Meigs currently has research grants from GlaxoSmithKline and sanofi-aventis and has received consulting fees from Interleukin Genetics, Kalypsis, and Outcomes.
Footnotes
The authors have no other disclosures to report.
Contributor Information
James B. Meigs, Email: jmeigs@partners.org.
Alisa K. Manning, Email: amanning@bu.edu.
Josée Dupuis, Email: dupuis@bu.edu.
Chunyu Liu, Email: liuc@bu.edu.
Jose C. Florez, Email: jcflorez@partners.org.
L. Adrienne Cupples, Email: adrienne@bu.edu.
References
- 1.Vionnet N, Hani EH, Dupont S, et al. Genomewide search for type 2 diabetes-susceptibility genes in french whites: evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q27-qter and independent replication of a type 2-diabetes locus on chromosome 1q21-q24. Am J Hum Genet. 2000;67:1470–1480. doi: 10.1086/316887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parker A, Meyer J, Lewitzky S, et al. A gene conferring susceptibility to type 2 diabetes in conjunction with obesity is located on chromosome 18p11. Diabetes. 2001;50:675–680. doi: 10.2337/diabetes.50.3.675. [DOI] [PubMed] [Google Scholar]
- 3.Aulchenko YS, Vaessen N, Heutink P, et al. A genome-wide search for genes involved in type 2 diabetes in a recently genetically isolated population from the Netherlands. Diabetes. 2003;52:3001–3004. doi: 10.2337/diabetes.52.12.3001. [DOI] [PubMed] [Google Scholar]
- 4.Reynisdottir I, Thorleifsson G, Benediktsson R, et al. Localization of a susceptibility gene for type 2 diabetes to chromosome 5q34-q35.2. Am J Hum Genet. 2003;73:323–335. doi: 10.1086/377139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanson RL, Ehm MG, Pettitt DJ, et al. An autosomal genomic scan for loci linked to type II diabetes mellitus and body-mass index in Pima Indians. Am J Hum Genet. 1998;63:1130–1138. doi: 10.1086/302061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frayling TM, Wiltshire S, Hitman GA, et al. Young-onset type 2 diabetes families are the major contributors to genetic loci in the Diabetes UK Warren 2 genome scan and identify putative novel loci on chromosomes 8q21, 21q22, and 22q11. Diabetes. 2003;52:1857–1863. doi: 10.2337/diabetes.52.7.1857. [DOI] [PubMed] [Google Scholar]
- 7.Meigs JB, Cupples LA, Wilson PWF. Parental transmission of type 2 diabetes mellitus: the Framingham Offspring Study. Diabetes. 2000;49:2201–2207. doi: 10.2337/diabetes.49.12.2201. [DOI] [PubMed] [Google Scholar]
- 8.Meigs JB, Manning AK, Fox CS, et al. Genome-wide Association with Diabetes-Related Traits in the Framingham Heart Study. BMC Medical Genetics. 2007 doi: 10.1186/1471-2350-8-S1-S16. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panhuysen C, Cupples L, Wilson P, Herbert A, Myers R, Meigs J. A genome scan for loci linked to quantitative insulin traits in persons without diabetes: the Framingham Offspring Study. Diabetologia. 2003;46:579–587. doi: 10.1007/s00125-003-1066-z. [DOI] [PubMed] [Google Scholar]
- 10.Elosua R, Demissie S, Cupples LA, et al. Obesity Modulates the Association among APOE Genotype, Insulin, and Glucose in Men. Obes Res. 2003;11:1502–1508. doi: 10.1038/oby.2003.201. [DOI] [PubMed] [Google Scholar]
- 11.Herbert A, Liu C, Karamohamed S, et al. BMI modifies associations of IL-6 genotypes with insulin resistance: the Framingham Study. Obesity (Silver Spring) 2006;14:1454–1461. doi: 10.1038/oby.2006.165. [DOI] [PubMed] [Google Scholar]
- 12.Meigs JB, Nathan DM, Wilson PWF, Cupples LA, Singer DE. Metabolic risk factors worsen continuously across the spectrum of nondiabetic glucose tolerance: the Framingham Offspring Study. Annals of Internal Medicine. 1998;128:524–533. doi: 10.7326/0003-4819-128-7-199804010-00002. [DOI] [PubMed] [Google Scholar]
- 13.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 14.Anderson RL, Hamman RF, Savage PJ, et al. Exploration of simple insulin sensitivity measures derived from frequently sampled intravenous glucose tolerance (FSIGT) tests. The Insulin Resistance Atherosclerosis Study. American Journal of Epidemiology. 1996;142:724–732. doi: 10.1093/aje/142.7.724. [DOI] [PubMed] [Google Scholar]
- 15.Hanley AJ, Williams K, Gonzalez C, et al. Prediction of type 2 diabetes using simple measures of insulin resistance: combined results from the San Antonio Heart Study, the Mexico City Diabetes Study, and the Insulin Resistance Atherosclerosis Study. Diabetes. 2003;52:463–469. doi: 10.2337/diabetes.52.2.463. [DOI] [PubMed] [Google Scholar]
- 16.Fulker DW, Cherny SS, Cardon LR. Multipoint interval mapping of quantitative trait loci, using sib pairs. Am J Hum Genet. 1995;56:1224–1233. [PMC free article] [PubMed] [Google Scholar]
- 17.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pratt SC, Daly MJ, Kruglyak L. Exact multipoint quantitative-trait linkage analysis in pedigrees by variance components. Am J Hum Genet. 2000;66:1153–1157. doi: 10.1086/302830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang HK, Siegmund D. Mapping quantitative trait loci in oligogenic models. Biostatistics. 2001;2:147–162. doi: 10.1093/biostatistics/2.2.147. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Zhong X. Multivariate survival models induced by genetic frailties, with application to linkage analysis. Biostatistics. 2002;3:57–75. doi: 10.1093/biostatistics/3.1.57. [DOI] [PubMed] [Google Scholar]
- 21.Hauser ER, Watanabe RM, Duren WL, Bass MP, Langefeld CD, Boehnke M. Ordered subset analysis in genetic linkage mapping of complex traits. Genet Epidemiol. 2004;27:53–63. doi: 10.1002/gepi.20000. [DOI] [PubMed] [Google Scholar]
- 22.Florez JC, Jablonski KA, Sun MW, et al. Effects of the type 2 diabetes-associated PPARG P12A polymorphism on progression to diabetes and response to troglitazone. J Clin Endocrinol Metab. 2007;92:1502–1509. doi: 10.1210/jc.2006-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludovico O, Pellegrini F, Di Paola R, et al. Heterogeneous effect of peroxisome proliferator-activated receptor gamma2 Ala12 variant on type 2 diabetes risk. Obesity (Silver Spring) 2007;15:1076–1081. doi: 10.1038/oby.2007.617. [DOI] [PubMed] [Google Scholar]
- 24.Moore AF, Florez JC. Genetic susceptibility to type 2 diabetes and implications for antidiabetic therapy. Annu Rev Med. 2008 doi: 10.1146/annurev.med.59.090706.135315. in press. [DOI] [PubMed] [Google Scholar]
- 25.Florez JC, Hirschhorn J, Altshuler D. The inherited basis of diabetes mellitus: implications for the genetic analysis of complex traits. Annu Rev Genomics Hum Genet. 2003;4:257–291. doi: 10.1146/annurev.genom.4.070802.110436. [DOI] [PubMed] [Google Scholar]
- 26.Risch N, Merikangas K. The future of genetic studies of complex human diseases. Science. 1996;273:1516–1517. doi: 10.1126/science.273.5281.1516. [DOI] [PubMed] [Google Scholar]
- 27.McCarthy MI. Growing evidence for diabetes susceptibility genes from genome scan data. Curr Diab Rep. 2003;3:159–167. doi: 10.1007/s11892-003-0040-y. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh S, Watanabe RM, Valle TT, et al. The Finland-United States Investigation of Non-Insulin-Dependent Diabetes Mellitus Genetics (FUSION) Study I. An Autosomal Genome Scan for Genes That Predispose to Type 2 Diabetes. Am J Hum Genet. 2000;67:1174–1185. [PMC free article] [PubMed] [Google Scholar]
- 29.Wiltshire S, Hattersley AT, Hitman GA, et al. A genomewide scan for loci predisposing to type 2 diabetes in a u.k. population (the diabetes uk warren 2 repository): analysis of 573 pedigrees provides independent replication of a susceptibility locus on chromosome 1q. Am J Hum Genet. 2001;69:553–569. doi: 10.1086/323249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.An P, Hong Y, Weisnagel SJ, et al. Genomic scan of glucose and insulin metabolism phenotypes: the HERITAGE Family Study. Metabolism. 2003;52:246–253. doi: 10.1053/meta.2003.50030. [DOI] [PubMed] [Google Scholar]
- 31.Meigs JB, Ordovas JM, Cupples LA, et al. Apolipoprotein E isoform polymorphisms are not associated with insulin resistance: the Framingham Offspring Study. Diabetes Care. 2000;23:669–674. doi: 10.2337/diacare.23.5.669. [DOI] [PubMed] [Google Scholar]
- 32.Knowler WC, Pettitt DJ, Savage PJ, Bennett PH. Diabetes incidence in Pima Indians: contributions of obesity and parental diabetes. American Journal of Epidemiology. 1981;113:144–156. doi: 10.1093/oxfordjournals.aje.a113079. [DOI] [PubMed] [Google Scholar]
- 33.Wilson PW, McGee DL, Kannel WB. Obesity, very low density lipoproteins, and glucose intolerance over fourteen years: the Framingham Study. American Journal of Epidemiology. 1981;114:697–704. doi: 10.1093/oxfordjournals.aje.a113240. [DOI] [PubMed] [Google Scholar]
- 34.Carey VJ, Walters EE, Colditz GA, et al. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses’ Health Study. American Journal of Epidemiology. 1997;145:614–619. doi: 10.1093/oxfordjournals.aje.a009158. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell BD, Kammerer CM, Mahaney MC, et al. Genetic analysis of the IRS. Pleiotropic effects of genes influencing insulin levels on lipoprotein and obesity measures. Arterioscler Thromb Vasc Biol. 1996;16:281–288. doi: 10.1161/01.atv.16.2.281. [DOI] [PubMed] [Google Scholar]
- 36.Hong Y, Despres JP, Rice T, et al. Evidence of pleiotropic loci for fasting insulin, total fat mass, and abdominal visceral fat in a sedentary population: the HERITAGE family study. Obes Res. 2000;8:151–159. doi: 10.1038/oby.2000.16. [DOI] [PubMed] [Google Scholar]
- 37.Duggirala R, Blangero J, Almasy L, et al. A major locus for fasting insulin concentrations and insulin resistance on chromosome 6q with strong pleiotropic effects on obesity-related phenotypes in nondiabetic Mexican Americans. Am J Hum Genet. 2001;68:1149–1164. doi: 10.1086/320100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- 39.Hotamisligil GS, Spiegelman BM. TNF-alpha: a key component of obesity-diabetes link. Diabetes. 1994;43:1271–1278. doi: 10.2337/diab.43.11.1271. [DOI] [PubMed] [Google Scholar]
- 40.Hotta K, Funahashi T, Bodkin NL, et al. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes. 2001;50:1126–1133. doi: 10.2337/diabetes.50.5.1126. [DOI] [PubMed] [Google Scholar]
- 41.Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G. Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR) J Clin Invest. 1997;100:1166–1173. doi: 10.1172/JCI119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meigs JB, Wilson PW, Fox CS, et al. Body Mass Index, Metabolic Syndrome and Risk of Type 2 Diabetes or Cardiovascular Disease. J Clin Endocrinol Metab. 2006;91:2906–2912. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 43.Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grarup N, Rose CS, Andersson EA, et al. Studies of association of variants near the HHEX, CDKN2A/B and IGF2BP2 genes with type 2 diabetes and impaired insulin release in 10,705 Danish subjects validation and extension of genome-wide association studies. Diabetes. 2007 doi: 10.2337/db07-0856. [DOI] [PubMed] [Google Scholar]
- 45.Pascoe L, Tura A, Patel SK, et al. Common variants of the novel type 2 diabetes genes, CDKAL1 and HHEX/IDE, are associated with decreased pancreatic {beta}-cell function. Diabetes. 2007 doi: 10.2337/db07-0634. [DOI] [PubMed] [Google Scholar]