Abstract
Although significant progress has occurred in the past 20 years regarding our understanding of Alzheimer disease pathogenesis, we have yet to identify disease-modifying therapeutics capable of substantially altering the clinical course of this prevalent neurodegenerative disease. In this short review, we discuss 2 approaches that are currently being tested clinically (γ-secretase inhibition and γ-secretase modulation) and emphasize the significant differences between these 2 therapeutic approaches. We also discuss certain genetic- and biomarker-based translational and clinical trial paradigms that may assist in developing a useful therapeutic agent.
Genetic models have long been used to devise and evaluate rational approaches to the treatment of high-prevalence diseases such as coronary artery disease and stroke. One of the most notable examples was in the pioneering studies of Mabuchi et al1 reporting the highly beneficial effects of a 3-hydroxy-3-methylglutaryl–coenzyme A reductase inhibitor, compactin, in patients with familial hypercholesterolemia. 3-Hydroxy-3-methylglutaryl–coenzyme A reductase is the enzyme that catalyzes the conversion of 3-hydroxy-3-methylglutaryl–coenzyme A to mevalonate, the rate-limiting step in cholesterol synthesis. This historic study involving 7 patients with a mutation in the low-density lipoprotein receptor gene (LDLR) reported the successful lowering of low-density lipoprotein cholesterol levels without changing or even slightly increasing high-density lipoprotein cholesterol levels. That small proof-of-concept clinical pharmacodynamic study eventually paved the way to the subsequent development of a related class of molecules known as the synthetic statins (eg, fluvastatin, atorvastatin, and rosuvastatin), and according to the American Heart Association (http://www.heart.org), statins can safely elicit a selective lowering of low-density lipoprotein cholesterol levels and long-term administration of statins can reduce the incidence of coronary artery disease and stroke by more than 25% to 30%.
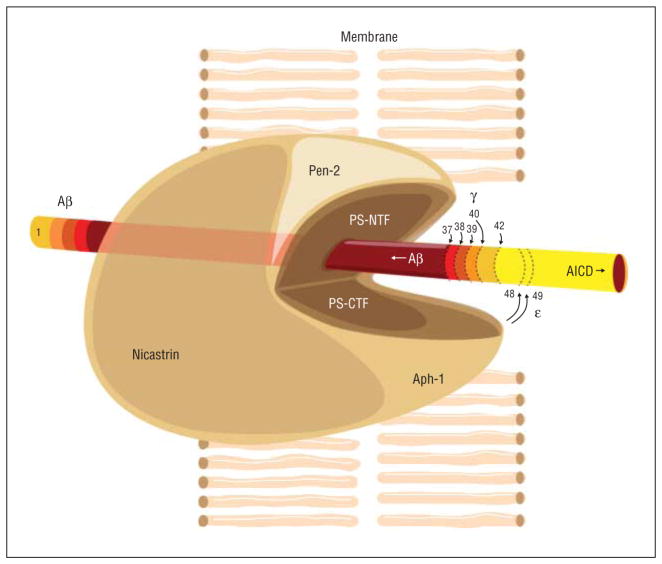
Alzheimer disease (AD) affects approximately 1 in 8 Americans aged 65 years or older. Unfortunately, unlike coronary artery disease and stroke, there are currently no effective disease-modifying or preventive therapies available. However, more than 200 different mutations have been identified in 3 genes associated with an autosomal dominant form of AD, referred to as early-onset familial Alzheimer disease (EOFAD). It is an aggressive form of AD that has served as a template for many of the cellular and transgenic animal models being used to study the pathogenesis of AD and is relevant to the more common late-onset AD.2 The 3 genes causally implicated in EOFAD, which typically affects carriers before age 65 years and sometimes as early as the third or fourth decades, are APP (amyloid precursor protein), PSEN1 (presenilin 1), and PSEN2 (presenilin 2). The presenilin genes encode 2 highly homologous proteins that can independently assemble along with 3 other membrane-associated proteins, namely, nicastrin, anterior pharynx defective 1, and presenilin enhancer 2 (Pen-2), into a large membrane-bound enzymatic complex (Figure 1) that plays a key role in metabolizing a large number of type 1 membrane proteins, including APP.2 The rationale for targeting this enzymatic complex, referred to as γ-secretase, as a therapeutic approach for AD stems from the fact that γ-secretase performs the terminal series of proteolytic cleavages that generate and release extracellularly a number of peptides known collectively as β-amyloid (Aβ).2 Most secreted Aβ peptides range in size from approximately 34 to 42 amino acids (Figure 1). One of these, Aβ42 (42 amino acids long), is the major peptide found in neuritic plaques and diffuse amyloid deposits, neuropathological lesions found in abundance in the brains of patients with AD at autopsy. In EOFAD, these and perhaps others that develop further downstream (eg, neurofibrillary tangles, another major neuropathological hallmark of AD) arise owing to a change in the ratio of cerebral levels of Aβ42 relative to the next longest Aβ peptide, Aβ40 (which is by far the most abundant of all secreted Aβ peptides), increasing the Aβ42/Aβ40 ratio approximately 2-fold.2 Initial therapeutic interventions targeting γ-secretase focused on curbing total Aβ peptide production by directly inhibiting γ-secretase activity through a class of compounds referred to as γ-secretase inhibitors (GSIs).3 The GSIs attenuate the production of all major Aβ peptide variants (eg, Aβ42, Aβ40, Aβ39, Aβ38, and Aβ37) equivalently (Figure 2) and inhibit the generation of an APP intracellular domain and the Notch-1 receptor intracellular domain. Notch is a key substrate of γ-secretase, and the Notch-1 receptor intracellular domain peptide plays an important role in the differentiation of multiple cell types.4 The GSIs also cause accumulation of the γ-secretase substrate C99 (the C-terminal fragment of APP generated by the Aβ-cleaving enzyme BACE 1) as well as C-terminal fragments from a number of other type 1 membrane proteins believed to be degraded by γ-secretase.5 Eli Lilly and Co recently reported (http://www.lilly.com) that in 2 phase 3 clinical trials involving more than 2600 mildly to moderately affected patients with probable AD, the GSI semagacestat failed to slow disease progression and led to worsened cognition and performance of activities of daily living. In addition, semagacestat was associated with an increased risk of skin cancer. The explanation for these disappointing clinical outcomes is currently unknown but may be mechanistically related to inhibiting the γ-secretase enzyme complex and attenuating the processing of 1 or more of its substrates including the C-terminal fragments of APP, Notch-1, and possibly other substrates.5
Figure 1.
γ-Secretase processing of amyloid precursor protein C-terminal fragments (CTFs). A cartoon illustration shows the various subunits of the γ-secretase enzyme complex performing a number of different putative γ-secretase–mediated proteolytic activities. Assembly and maturation of the γ-secretase enzyme begins with the formation of a binary complex between nicastrin and anterior pharynx defective 1 (Aph-1). Presenilin (PS) binds to the nicastrin/Aph-1 complex to form an inactive trimeric complex. The PSN-terminal fragment (NTF) and the PS-CTF collectively harbor the catalytic center of γ-secretase, which requires binding of presenilin enhancer 2 (Pen-2) for the end oproteolysis of PS into the PS-NTF and the PS-CTF, rendering activation of γ-secretase. The active γ-secretase enzyme can cleave the amyloid precursor protein CTF at numerous sites, yielding a host of peptide products (amyloid precursor protein intracellular domain [AICD], β-amyloid (Aβ) 1–42, Aβ1–40, Aβ1–39, Aβ1–38, and Aβ1–37). The relative size of the curved arrows at the γ sites indicates the relative use of each processing site. The ε cleavages (larger curved arrows at amino acids 48 and 49 of the 99–amino acid–long amyloid precursor protein CTF) generate the AICDs, which remain inside the cell (as indicated by the direction of the right arrow) and may actually travel to the nucleus, while the various Aβ peptides are released extracellularly (as indicated by the direction of the left arrow) immediately on cleavage by γ-secretase within the membrane. The different colors correspond to the different proteolytic processing products.
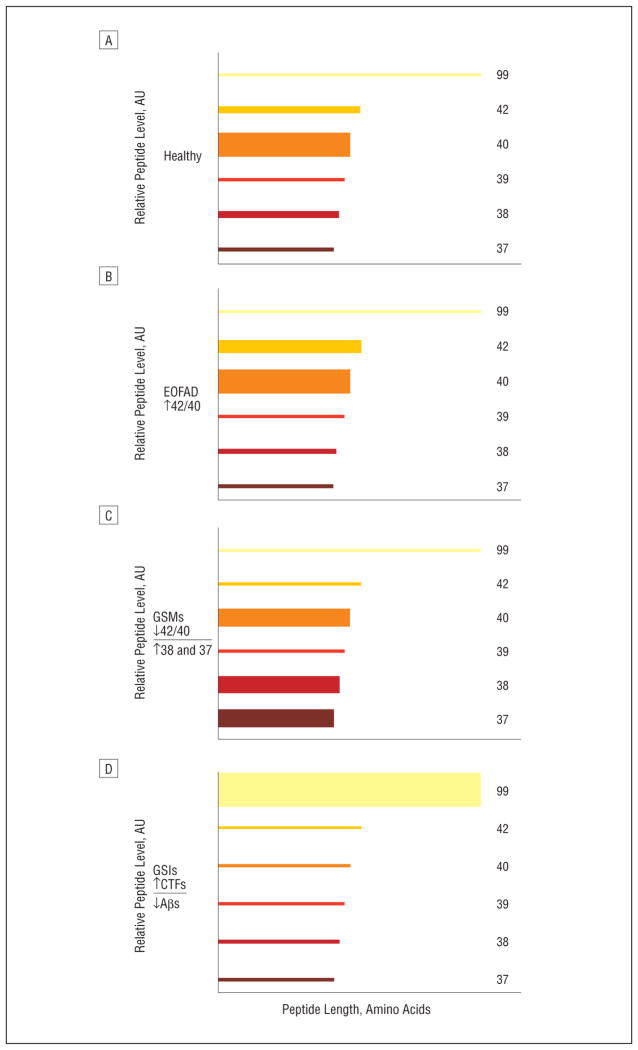
Figure 2.
Semiquantitative estimation of the outcome in healthy individuals (A) and in individuals harboring early-onset familial Alzheimer disease (EOFAD)–linked mutations before (B) and after receiving either a γ-secretase modulator (GSM) (C) or a γ-secretase inhibitor (GSI) (D). The graphs represent shifts in the different forms of β-amyloid (Aβ) that result from processing by normal (wild-type) γ-secretase, mutations in the presenilin component of γ-secretase that result in EOFAD, and the effects of treatment with GSIs or GSMs. The heights (ordinate) and lengths (abscissa) of each Aβ peptide variant correspond to the relative abundance and the number of amino acids, respectively. The GSMs elicit a unique bidirectional biomarker profile with respect to their effects on Aβ peptide variants (decreasing Aβ42 and Aβ40 levels, while increasing Aβ38 and Aβ37 levels). AU indicates arbitrary units; CTFs, C-terminal fragments.
Interestingly, the vast majority of EOFAD-linked mutations do not correlate with an overall increase in γ-secretase activity, nor do they appear to increase total Aβ peptide levels. Instead, most appear to cause a 2-fold increase in the ratio of Aβ42/Aβ40 (Figure 2). This increased Aβ42/Aβ40 ratio is a highly reproducible biochemical phenotype (in vitro and in vivo) for the predominant effect of the more than 200 EOFAD-linked genetic mutations, including a number of mutations in APP (those proximal to the γ-secretase cleavage site in the APP protein), all of those in PSEN2, and most of the large number of mutations in PSEN1.2 Therefore, a therapeutic rationale based on this EOFAD genetics model would entail modulating γ-secretase activity (decreasing the Aβ42/Aβ40 ratio), not inhibiting this important enzyme complex. The feasibility of this more selective EOFAD genetics-based approach was first identified using a series of nonsteroidal anti-inflammatory drug (NSAID)–like compounds, eg, ibuprofen and sulin-dac sulfide.6 However, the NSAID-like compounds lack potency (≥50–100μM in vitro half-maximal inhibitory concentrations for selective lowering of the Aβ42 level in cell-based assays) and later proved to have very poor brain penetrance.6,7 Despite having less than stellar pharmacodynamic and pharmacokinetic properties, a large phase 3 clinical trial involving more than 1600 mildly affected patients with AD was conducted using the NSAID-like compound R-flurbiprofen (Tarenflurbil; sponsored by Myriad Genetics, Inc). Unfortunately, this study failed to show any clinical benefit.7
More recently, a potent series of compounds referred to as the non–NSAID-like or non–carboxylic acid–containing γ-secretase modulators (GSMs), harboring a completely different chemical scaffold from that of NSAIDs, have received considerable attention from pharmaceutical companies, biotechnology concerns, and academic laboratories. Within the last 18 months, more than 60 new patent applications have been published on slight variations of GSM chemical scaffolds highly similar to those originally reported by members of our team.8 This may seem surprising given the recent failures of the GSI semagacestat and Tarenflurbil. However, those 2 compounds had the limitations mentioned earlier. Although GSIs and GSMs target the γ-secretase enzyme complex, they appear to have distinct mechanisms of action as measured by their effects on the levels of the various Aβ peptide variants and C-terminal fragments such as C99.3,8 Unlike the GSIs, some these nonacidic GSMs (a series of diarylaminothiazoles) were found to be capable of potently inhibiting Aβ42 production in vitro (cell-based half-maximal inhibitory concentrations in the low nanomolar range) and in vivo.8 At slightly higher concentrations, they reduce Aβ40 levels only moderately while concomitantly increasing the levels of the shorter Aβ38 and Aβ37 peptide variants (Figure 2).8 Moreover, one of these nonacidic diarylaminothiazole-containing GSMs was shown to markedly reduce Aβ deposition in a transgenic mouse model of AD following long-term daily administration for 29 consecutive weeks and did not show adverse effects that are commonly associated with long-term administration of GSIs in rodents such as goblet cell hyperplasia.8
Potent GSMs that can selectively decrease the production of Aβ42 will need to be rigorously tested during translation to clinical trials. The failure of R-flurbiprofen (Tarenflurbil) in the phase 3 trial highlights a critical first step: the need to demonstrate that a GSM engages its target and selectively lowers the Aβ42 level in the brain in humans. This can be examined by pharmacokinetic and pharmacodynamic studies that analyze cerebrospinal fluid and plasma. Serial sampling of cerebrospinal fluid via an indwelling lumbar catheter may help to define the precise time course and selectivity of inhibition of Aβ42 production and facilitate dose selection. These studies can also analyze other forms of Aβ such as Aβ38, which, based on transgenic mouse studies, may increase in response to a potent GSM.8 The studies can address the question of whether there is a rebound increase in Aβ production when drug levels fall—a problem previously cited with GSIs.9
In planning clinical trials to test a potent GSM in relation to AD, several key issues need to be considered. It is unknown how much the Aβ42 level needs to be lowered to have significant clinical benefits. Although dosing can be bracketed from phase 1 pharmacokinetic and pharmacodynamic studies, it is unclear whether reduction of Aβ42 production by 25% (a moderate effect) or by 50% or higher (a large effect) is necessary to influence amyloid deposition and neuritic plaque dynamics in the brain. Monitoring plasma and cerebrospinal fluid during long-term dosing and the use of biomarkers such as amyloid imaging can provide tools to monitor these parameters. Biomarkers such as structural magnetic resonance imaging can supplement clinical assessment to provide insights into whether sustained lowering of the Aβ42 level (and a concomitant increase in the Aβ38 level) has benefits on brain structure and function. Toxic effects will need to be monitored carefully in view of the experience with semagacestat.
Although clinical trials have typically targeted people with symptomatic AD at a mild to moderate stage of dementia, this may not be the best approach for GSMs. First, the extent of brain amyloid burden does not correlate with the degree of cognitive impairment in AD. It is possible that a cascade of neurodegenerative processes eventually becomes independent of Aβ or amyloid-related toxic effects and leads to progressive dementia in AD; decreasing Aβ42 production may not be successful without simultaneously addressing other potential mechanisms of disease. Second, extensive structural neuronal pathology is already present in the hippocampus and other brain regions in people with mild to moderate AD, which limits the degree of recovery or improvement that may be achieved, even if treatment with GSMs leads to a dramatic reduction in amyloid burden. For these reasons, clinical trials of GSMs are likely to be more promising if conducted in people with very early symptomatic AD (eg, mild cognitive impairment).
With many potent GSMs currently in various stages of preclinical and clinical development, perhaps we should take a lesson from the history of compactin and prioritize limited proof-of-concept trial(s) with well-studied EOFAD kindreds whose disease course, extending from pre-clinical phases to clinical outcomes, has been well mapped. Proof-of-principle studies have been proposed among presymptomatic or mildly affected carriers of an extended Colombian family of more than 5000 members carrying a single PSEN1 genetic mutation who have been thoroughly studied for the past few decades. To meet this need, GSMs must be capable of modulating a γ-secretase enzyme complex harboring EOFAD-linked mutations. Interestingly, unpublished experiments in 2 of our laboratories (R.E.T. and S.L.W.) suggest that these types of aminothiazole-containing GSMs work at least as well if not better against all 5 of the mutant presenilin 1–expressing cell lines tested so far compared with the cell lines expressing wild-type presenilin 1. Studies with carriers of familial AD–linked mutations could serve as important tests of therapeutic regimens targeting γ-secretase and the amyloid cascade hypothesis.
Footnotes
Financial Disclosure: Drs Wagner and Tanzi are shareholders and cofounders of a privately held company (Neurogenetic Pharmaceuticals, Inc) that holds rights to a GSM currently in pre-clinical development.
Author Contributions: Study concept and design: Wagner, Tanzi, and Galasko. Analysis and interpretation of data: Mobley. Drafting of the manuscript: Wagner, Tanzi, and Galasko. Critical revision of the manuscript for important intellectual content: Tanzi and Mobley. Obtained funding: Mobley. Administrative, technical, and material support: Mobley. Study supervision: Mobley.
Additional Contributions: Nora Joelson, BA, created the figures. We thank the Cure Alzheimer’s Fund, the Down Syndrome Research and Treatment Foundation, and the Hillblom Foundation for their past and continued support of the discovery and development of GSMs.
References
- 1.Mabuchi H, Haba T, Tatami R, et al. Effect of an inhibitor of 3-hydroxy-3-methyglutaryl coenzyme A reductase on serum lipoproteins and ubiquinone-10-levels in patients with familial hypercholesterolemia. N Engl J Med. 1981;305 (9):478–482. doi: 10.1056/NEJM198108273050902. [DOI] [PubMed] [Google Scholar]
- 2.Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120(4):545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JJ, Holtz G, Baskin PP, et al. Reductions in β-amyloid concentrations in vivo by the γ-secretase inhibitors BMS-289948 and BMS-299897. Biochem Pharmacol. 2005;69(4):689–698. doi: 10.1016/j.bcp.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 4.De Strooper B, Annaert W, Cupers P, et al. A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398(6727):518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 5.Wakabayashi T, De Strooper B. Presenilins: members of the γ-secretase quartets, but part-time soloists too. Physiology (Bethesda) 2008;23:194–204. doi: 10.1152/physiol.00009.2008. [DOI] [PubMed] [Google Scholar]
- 6.Weggen S, Eriksen JL, Das P, et al. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414(6860):212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- 7.Green RC, Schneider LS, Amato DA, et al. Tarenflurbil Phase 3 Study Group. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA. 2009;302(23):2557–2564. doi: 10.1001/jama.2009.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kounnas MZ, Danks AM, Cheng S, et al. Modulation of γ-secretase reduces β-amyloid deposition in a transgenic mouse model of Alzheimer’s disease. Neuron. 2010;67(5):769–780. doi: 10.1016/j.neuron.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martone RL, Zhou H, Atchison K, et al. Begacestat (GSI-953): a novel, selective thiophene sulfonamide inhibitor of amyloid precursor protein γ-secretase for the treatment of Alzheimer’s disease. J Pharmacol Exp Ther. 2009;331(2):598–608. doi: 10.1124/jpet.109.152975. [DOI] [PubMed] [Google Scholar]