Abstract
Objectives:
A hierarchical generalized linear model (HGLM) was applied to estimate the transmission pattern of scrub typhus from 2001 to 2011 in the Republic of Korea, based on spatial and temporal correlation.
Methods:
Based on the descriptive statistics of scrub typhus incidence from 2001 to 2011 reported to the Korean Centers for Disease Control and Prevention, the spatial and temporal correlations were estimated by HGLM. Incidences according to age, sex, and year were also estimated by the best-fit model out of nine HGLMs. A disease map was drawn to view the annual regional spread of the disease.
Results:
The total number of scrub typhus cases reported from 2001 to 2011 was 51,136: male, 18,628 (36.4%); female, 32,508 (63.6%). The best-fit model selected was a combination of the spatial model (Markov random-field model) and temporal model (first order autoregressive model) of scrub typhus transmission. The peak incidence was 28.80 per 100,000 persons in early October and the peak incidence was 40.17 per 100,000 persons in those aged 63.3 years old by the best-fit HGLM. The disease map showed the spread of disease from the southern central area to a nationwide area, excepting Gangwon-do (province), Gyeongsangbuk-do (province), and Seoul.
Conclusion:
In the transmission of scrub typhus in Korea, there was a correlation to the incidence of adjacent areas, as well as that of the previous year. According to the disease map, we are unlikely to see any decrease in the incidence in the near future, unless ongoing aggressive measures to prevent the exposure to the vector, chigger mites, in rural areas, are put into place.
Keywords: aged, epidemiology, incidence, female, Korea, linear models, Orientia tsutsugamushi, scrub typhus
1. Introduction
Scrub typhus is one of the major febrile diseases occurring in autumn in the Republic of Korea (hereafter, “Korea”). Its causative organism, Orientia tsutsugamushi is transmitted by chigger mites such as Leptotrombidium pallidum and Leptotrombidium scutellare[1]. Chigger mites are carried by wild rodents. Apodemus agrarius, Mus musculus, Microtus fortis, Micromys minutus, Tscherskia triton, and Rattus norvegicus have been known to be serologically positive for O. tsutsugamushi in Korea [2]. Reports of the incidence of scrub typhus in Korea have been increasing [3]. To identify the annual pattern of scrub typhus incidence according to age and sex in Korea from 2001 to 2011, we propose the use of a hierarchical generalized linear model (HGLM), which has been used to identify the annual pattern of malaria in Korea [4]. In this paper, using a variety of combinations of spatial and temporal correlation models, we aim to address the following questions: (1) What is the annual changing pattern of incidence? (2) What is the geographical pattern of incidence? (3) Are there spatial or temporal correlations of the scrub typhus incidence pattern?
The HGLM is applied to understand how the incidence of scrub typhus has spread spatially over consecutive years, which helps in control and prevention of scrub typhus transmission in Korea.
2. Materials and Methods
2.1. Data collection and management
Data were collected by the Korean Centers for Disease Control and Prevention from 2001 to 2011 from medical clinics and hospitals, as well as health centers, based on the “Law on the prevention and control of infectious diseases” in Korea. Scrub typhus is one of the communicable diseases that should be reported to a health center, by law. Age, sex, city or county of resident, and month and year of occurrence, were obtained from the reporting system. Data manipulation was performed in the same way as in our previous work on Plasmodium vivax malaria [4]. Data are grouped according to sex, address, and year. The age of each group is the mean age of infected persons. Also, the month of infection was a mean of the number of months (period of time) each person was reported to be infected. The incidence in each city or county was standardized, based on the number of infections per 100,000 persons in the population. Populations in each city or county were obtained from the website of Statistics Korea, available from: http://kostat.go.kr. In this analysis, we used the same R script as in our previous work and the same dhglmfit() function in the R package dhglm from the Comprehensive R Archive Network (CRAN) [5].
2.2. Statistical model
where, ηitk is the linear predictor, μitk is the conditional expected numbers of cases, Nitk is the population of group (city or county) acquainted from Statistics Korea according to sex and year, ageik is the mean age of infection occurrence between year 2001 and 2011 out of ith city or county, monthi is the mean month of infection occurrence between year 2001 and 2011 out of ith city or county, b(ageik) is the smoothing spline on the mean age of infection occurrence, c(monthi) is the smoothing spline on the mean month of infection occurrence, ui is the random effects for temporal model and vit is the random effects for spatial model.
We considered 3 spatial models for ui and 3 temporal models for vit, which were the same as in our previous work [4]. Spatial Model 1 is the independent model, where the incidence of a region is assumed to be independent of adjacent regions, ui∼i:i:d: N(0; λ1)
Spatial model 2 and 3 assume spatial effects where the incidence of a region is affected through adjacent regions. Spatial model 2 is the intrinsic autoregressive model, ui∼IAR with kernel ∑i∼j(ui − uj)2/λ1. Spatial model 3 is the Markov random-field model, ui∼MRF with var(u)−1=(I − ρN)/λ1
Temporal model 1 is the independent model, the incidence of each year does not depend upon the previous years, vit∼i:i:d: N(0; λ2)
Temporal model 2 and 3 assume temporal effects, where the incidence of each year depends upon the previous year. Temporal model 2 is the random work model, where vit=vt∼RW with kernel ∑t (vt − vt−1)2/λ2
Temporal model 3 is the first order autoregressive model, vit∼AR(1) with kernel ∑i∑t(vt − ρvt−1)2/λ2
The conditional Akaike Information Criterion (AIC) was used to find the best fitting model for scrub typhus incidence in Korea from 2001 to 2011.
2.3. Disease mapping
Disease maps of scrub typhus from 2001 to 2011 in Korea were drawn using MapWizard for Excel [6].
3. Results
3.1. The best-fit model
Out of the nine models, the combination model of Spatial Model 3 and Temporal Model 3 was the best-fit model, with the lowest conditional AIC value (Table 1).
Table 1.
Value of conditional Akaike information criterion of each combination model for the estimation of three spatial and three temporal correlations of scrub typhus in Korea from 2001 to 2011, according to the cases reported to the Korea Centers for Disease Control and Prevention.
| Temporal model
|
||||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Spatial model | 1 | 113,692.1 | 113,236.6 | 109,421.0 |
| 2 | 113,578.8 | 113,116.4 | 109,368.2 | |
| 3 | 113,530.2 | 113,074.7 | 109,253.0 | |
3.2. Temporal distribution of scrub typhus
The total number of scrub typhus cases reported from 2001 to 2011 was 51,136: male, 18,628 (36.4%); female, 32,508 (63.6%). The change in incidence per 100,000 persons, according to year and sex, is shown in Figure 1. There was a dramatic increase of incidence from 2003 to 2005. After that, the incidence has fluctuated. The monthly incidence per 100,000 persons is shown in Figure 2. October and November are the peak months of incidence, 56.94 and 36.21, respectively. The mean month of incidence was mid-October and the peak incidence was in early October, with 28.80 cases per 100,000 persons by the best-fit HGLM (Figure 3).
Figure 1.
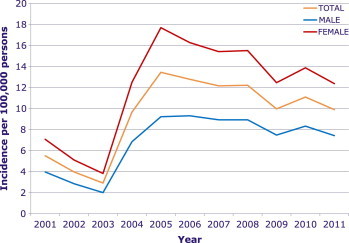
Annual incidence of scrub typhus per 100,000 persons reported to the Korea Centers for Disease Control and Prevention from 2001 to 2011 according to sex.
Figure 2.
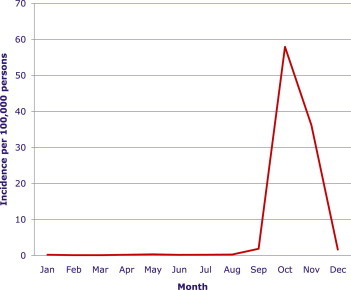
Monthly incidence of scrub typhus per 100,000 persons reported to the Korea Centers for Disease Control and Prevention from 2001 to 2011. The peak incidence can be seen in October, at 57.94.
Figure 3.
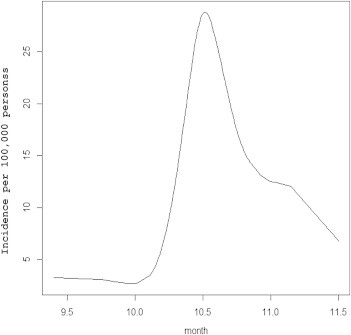
Mean month of incidence of scrub typhus from smoothing spline data reported to the Korea Centers for Disease Control and Prevention 2001–2011. The mean month of incidence was mid-October and the time of peak incidence was in early October (28.80 per 100,000 persons) after estimation by the best-fit hierarchical generalized linear model.
3.3. Spatial distribution of scrub typhus
The disease map of scrub typhus showed the annual pattern of incidence. The core place of transmission of scrub typhus was Jiri-san (a large mountain) in the southern-central area of the Korean Peninsula (Figure 4). The transmission area has since spread nationwide, except to Gangwon-do (province), Gyeongsangbuk-do (province), and Seoul.
Figure 4.
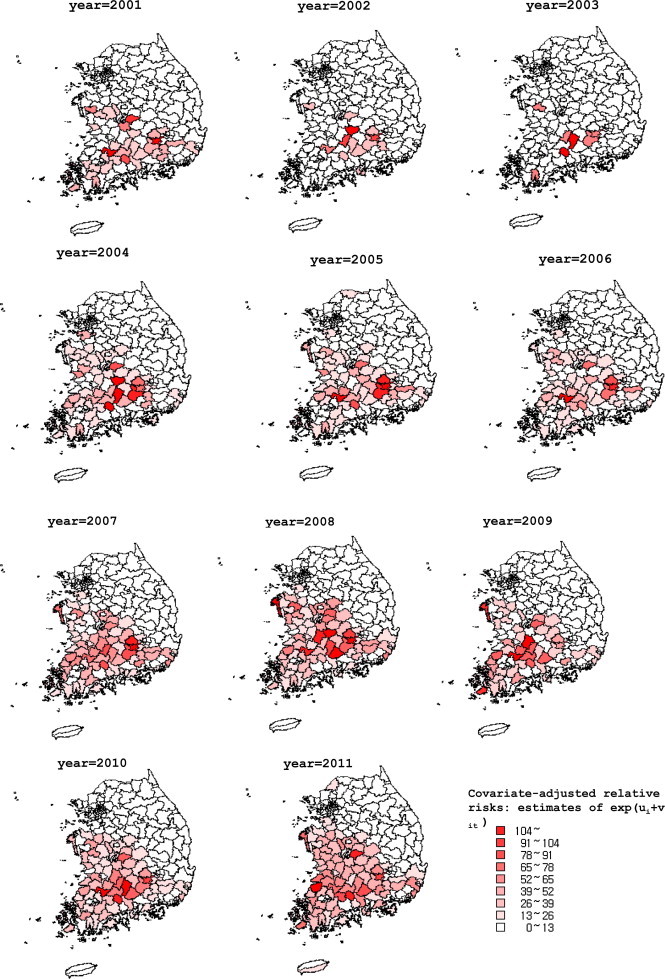
Disease maps of incidence of scrub typhus reported to the Korea Centers for Disease Control and Prevention from 2001 to 2011. The incidence per 100,000 persons is presented after estimation by the best-fit hierarchical generalized linear model.
3.4. Incidence according to age
The 70–74-year-old age group is the most vulnerable to scrub typhus, with an incidence of 442.64 per 100,000 persons (Figure 5). The smoothing spline chart of incidence distribution according to age after HGML estimation is shown in Figure 6. The peak incidence was 40.17 at age 63.3 years old.
Figure 5.
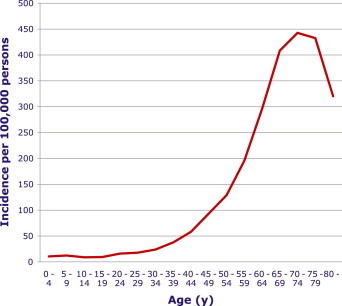
Incidence of scrub typhus reported to the Korea Centers for Disease Control and Prevention from 2001 to 2011 according to age. The peak incidence (442.64 per 100,000 persons) was the 70–74-year-old age group.
Figure 6.
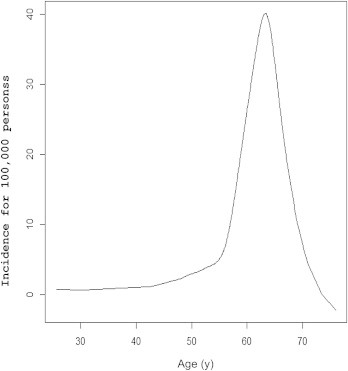
Mean age of incidence of scrub typhus from smoothing spline data reported to the Korea Centers for Disease Control and Prevention from 2001 to 2011. The mean ages of males and females were 57.3 and 60.9 years, respectively. The peak incidence was 40.17 per 100,000 persons at age 63.3 years, after estimation by the best-fit hierarchical generalized linear model.
4. Discussion
Since scrub typhus is a vector (chigger mite) transmitted infectious disease, its incidence can be easily estimated, in that there is a temporal correlation to adjacent areas and a spatial correlation to the previous year. The above results also confirmed this speculation: the combination model of Spatial Model 3 (Markov random field model) and Temporal Model 3 (the first order autoregressive random work model) provided the best fit to scrub typhus transmission in Korea. Notably, this particular combination model was also determined to be the best fit model in a previous study on P. vivax malaria, a mosquito-transmitted infectious disease [4]. Since scrub typhus is transmitted by chigger mites and its larvae mainly reside in small rodents, the spatial correlation can be explained easily: the spread of the vector is influenced by the activity area of the rodents. Chigger mites live on or in soil and eat insect eggs. They maintain a life cycle through four developmental stages: eggs – larvae – pupae – adults. Since chigger mites can survive during the winter period and are believed to lay eggs mainly in the summer in Korea, the survival of chigger mites during winter can influence the incidence of the next year.
Alarmingly, the temporal distribution of scrub typhus has spread rapidly from the southern central mountainous areas in the Korean Peninsula to a nearly nationwide area (Figure 4). Gangwon-do (province) and Gyeongsangbuk-do (province) are still protected by Taebaek-sanmaek and Charyeong-sanmaek (two mountain ranges). Furthermore, Seoul is still far from an endemic area of scrub typhus; however, since the spread rate is rapid and transmission proceeds to northern areas, measures for the prevention of transmission should be stressed. According to the annual pattern of scrub typhus transmission, a decrease in the incidence in the near future seems unlikely. Meanwhile, there may be an increase of incidence of scrub typhus if there is no active preventive program (Figure 1).
The peak incidence season of scrub typhus in Korea is well known to be October through November (Figure 3). This was confirmed by the HGLM estimation (Figure 4). The tissue fluid of mammals is necessary for the larvae of chigger mites to be metamorphosed to pupae. Since chigger mites are believed to lay eggs in the summer in Korea, unlike in tropical areas, autumn is the season during which they suck the blood of mammals. After an incubation period of 1–3 weeks, an infected person may visit a clinic or health center. To Korean physicians, three febrile illnesses during the autumn are well-known infectious diseases: scrub typhus, leptospirosis, and hemorrhagic fever with renal syndrome. Those three infectious diseases can be differentiated easily by serologic testing. It is routine for physicians to order serological testing if scrub typhus is suspected. Also, the communicable disease reporting system is well maintained, so that the incidence of scrub typhus can be estimated accurately.
The higher incidence in aged females (63.6%) is a special issue regarding scrub typhus epidemiology in Korea (Figures 1 and 5). This observation was consistent with the estimation by HGLM (Figure 6). In Fuyang City, Anhui Province, China, out of 104 patients, 49.0 % of scrub typhus patients were female [7]. The annual incidence of scrub typhus in Miyazaki prefecture, Japan, in 2001–2005, showed an equal distribution between males and females, with a male to female ratio of 68:61 out of 129 patients [8]. The greater incidence in aged females than that in males can be explained by the fact that aged females in rural areas work hard outdoors and they are vulnerable to scrub typhus, due to their poor protective measures. In a previous study, wearing a long-sleeved shirt while working, keeping work clothes off the grass, and always using a mat to rest outdoors showed protective associations [9]. Although these preventive measures are well-known to public health experts, it is difficult for them to disseminate to vulnerable age and sex groups. The eradication of the vector, the chigger mite, or the reservoir host, rodents, is also difficult or impossible. The only reasonable approach is personal protection and a reduction in the rodent density near one’s home by appropriate protective knowledge and behavior. The Korea Centers for Disease Control and Prevention has performed a cost–benefit analysis of the economic feasibility of a prevention program. In this program, leaflets, arm warmers, work clothes, and repellents were provided to farmers, along with an education program by a primary health center. Since there was a significant economic value of this program by reducing the incidence of scrub typhus, the ongoing implementation of the program was suggested as an approach to decreasing the incidence [10].
In conclusion, there was a temporal correlation to the previous or following year, as well as a spatial correlation to adjacent regions, in the transmission of scrub typhus in Korea, when nine combination models were tested by HGLM. Notably, the distribution pattern of scrub typhus also showed a rapid spread to adjacent areas, and a decrease in the incidence cannot be expected in the near future, unless an ongoing effective nationwide prevention program is implemented.
Acknowledgments
This work was supported by a research grant of the Korea Centers for Disease Control and Prevention in 2012 (2012E2400100).
References
- 1.Lee SH, Lee YS, Lee IY, et al. Monthly occurrence of vectors and reservoir rodents of scrub typhus in an endemic area of Jeollanamdo, Korea. Korean J Parasitol. 2012 Dec;50(4):327–31. doi: 10.3347/kjp.2012.50.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim HC, Lee IY, Chong ST, et al. Serosurveillance of scrub typhus in small mammals collected from military training sites near the DMZ, Northern Gyeonggi-do, Korea, and analysis of the relative abundance of chiggers from mammals examined. Korean J Parasitol. 2010 Sep;48(3):237–43. doi: 10.3347/kjp.2010.48.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim BN, Gordillo LF, Kim Y. A model for the transmission dynamics of Orientia tsutsugamushi among its natural reservoirs. J Theor Biol. 2010 Sep;266(1):154–61. doi: 10.1016/j.jtbi.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Noh M, Lee Y, Oh S, et al. Spatial and temporal distribution of Plasmodium vivax malaria in Korea estimated with a hierarchical generalized linear model. Osong public Health Res Perspect. 2012 Dec;3(4):192–8. doi: 10.1016/j.phrp.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noh M, Lee Y. dhglm: Double hierarchical generalized linear models R package version 10 [Internet] Vienna (Austria): The R Foundation for Statistical Computing; 2012. [cited 2012 Dec 1]. Available from: http://CRAN.R-project.org/packageZdhglm. [Google Scholar]
- 6.MapWizard for excel, version 1.0. Seoul: TasTech Corp; 2006. [Google Scholar]
- 7.Zhang S, Song H, Liu Y, et al. Scrub typhus in previously unrecognized areas of endemicity in China. Clin Microbiol. 2010 Apr;4:1241–4. doi: 10.1128/JCM.01784-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto S, Ganmyo H, Iwakiri A, et al. Annual incidence of tsutsugamushi disease in Miyazaki prefecture, Japan in 2001–2005. Jpn J Infect Dis. 2006 Dec;59(6):404–5. [PubMed] [Google Scholar]
- 9.Kweon SS, Choi JS, Lim HS, et al. A community-based case-control study of behavioral factors associated with scrub typhus during the autumn epidemic season in South Korea. Am J Trop Med Hyg. 2009 Mar;80(3):442–6. [PubMed] [Google Scholar]
- 10.Kim J, Lee E, Rhee HC. Cost-benefit analysis of the tsutsugamushi disease prevention program in South Korea. Jpn J Infect Dis. 2012;65(3):222–7. doi: 10.7883/yoken.65.222. [DOI] [PubMed] [Google Scholar]


