Abstract
Objective To assess the level of partner relationship satisfaction among mothers of children with different severity of congenital heart defects (CHD) compared with mothers in the cohort. Methods Mothers of children with mild, moderate, or severe CHD (n = 182) and a cohort of mothers of children without CHD (n = 46,782) from the Norwegian Mother and Child Cohort Study were assessed at 5 time points from pregnancy to 36 months postpartum. A 5-item version of the Relationship Satisfaction scale was used, and relevant covariates were explored. Results The trajectories of relationship satisfaction among mothers of children with varying CHD severity did not differ from the trajectories in the cohort. All women in the cohort experienced decreasing relationship satisfaction from 18 months after delivery up to 36 months after delivery. Conclusions Having a child with CHD, regardless of severity, does not appear to exacerbate the decline in relationship satisfaction.
Keywords: chronic illness in children, congenital heart defects, motherhood, partnership, relationship stability
Introduction
Congenital heart defects (CHD) occur in 0.5–1% of all live births (Hoffman & Kaplan, 2002), making CHD the most common congenital malformation. Because of considerable improvements in survival rates (Allen, Gauvreau, Bloom, & Jenkins, 2003; Eskedal et al., 2005), many children with CHD today require long-term intensive care from their caregivers. The increased demand of bringing up a child with severe illness might cause extraordinary stress on the relationship between the parents. As there is little new research available on parents’ relationship satisfaction and stability when they have a child with CHD, more current knowledge is needed.
Severity of CHD varies greatly from minor defects with little functional significance to major life-threatening defects requiring acute and invasive surgical treatment. Having a child with CHD, especially severe CHD, is a stressful life condition for the parents. Fear of losing their child, prolonged hospital visits, medical and surgical interventions, risk of financial burden, feeding problems, and uncertainties about complications and long-term sequelae such as impaired growth, impaired cognitive functioning, and other developmental problems are some of the stressors the parents might encounter (Clemente, Barnes, Shinebourne, & Stein, 2001; Karsdorp, Everaerd, Kindt, & Mulder, 2007; Lawoko, 2007; Saenz, Beebe, & Triplett, 1999). Mothers seem to be especially affected, experiencing physical and psychological stress, guilt, and sadness more frequently than fathers (Knafl, 2000). A recent study by our research group found that mothers of children with the most severe CHD are at risk of experiencing prolonged symptoms of depression and anxiety over time (Solberg et al., 2012). These stressors might generate relationship instability, making the postpartum period a difficult and vulnerable phase for the mothers. On the other hand, poor adaptation does not have to be the ultimate outcome. In accordance with the family stress and coping model by McCubbin, Thompson and McCubbin (1996), families of chronically ill children seek to restore balance and progress to a state of long-term adaptation. This adaptation is a process of reducing discrepancies between expectations and reality and can be conceptualized as a continuum ranging from positive adaptation to negative adaptation. This model takes into account several factors affecting the association between caring for a child with CHD and the parents’ well-being. CHD severity alone cannot predict the maternal response, but other intervening factors such as the families’ prior experience with stress, state and trait anxiety of each family member, social support, socioeconomic situation, problem-solving, and communication skills must also be considered.
In this model relationship, satisfaction can be seen as an outcome of adaptation, but on the other hand, it can as well be a significant predictor of mothers’ ability to cope and adapt with the responsibilities of caring for the child with severe illness (Benson & Gross, 1989). A satisfying relationship and a supportive partner can function as a buffer against distress and hopelessness (Murphy, Christian, Caplin, & Young, 2007). Further, high relationship satisfaction is important for retaining and increasing life satisfaction (Dyrdal, Røysamb, Nes, & Vittersø, 2011; Kersh, Hedvat, Hauser-Cram, & Warfield, 2006). A satisfying relationship is apparently not only beneficial for the couple but also an integral element for children’s growth and development. Wallander, Varni, Babani, Banis, and Wilcox (1989) found that family cohesiveness and cooperation are protective mechanisms for the social competence of children with chronic illness.
Research suggests that having children in general may influence marital happiness in a negative manner (Twenge, Campbell, & Foster, 2003). Some studies report that parents also experience increased satisfaction the first weeks postpartum, referred to as the “baby honeymoon” (Hobbs, 1968; Miller & Sollie, 1980). Although the overall evidence shows that the relationship often suffers when having children, it is not clear whether having a child with CHD poses an even greater risk for the couple’s relationship, as there are few recent studies on this issue. Assuming that more dissatisfied couples tend to divorce, we looked at three relevant studies on divorce and separation in families of children with CHD; the findings in these studies are contradictory.
Joesch and Smith (1997) demonstrated that divorce rates may be related to type and degree of impairment; mothers of children with CHD were at higher risk of divorce or separation compared with mothers of children with milder diseases (such as allergies, migraines, and asthmas) and healthy control subjects. The study included children aged 0–17 years, making it difficult to specify whether the results varied by the age of the child. Unfortunately, the study does not report the CHD diagnosis, making it difficult to know the severity of CHD.
Interestingly, two older studies also showed that there were not more relationship problems and divorce when parents had a child with CHD (Finley, Putherbough, Cook, Netley, & Rowe, 1979; Silbert, Newburger, & Fyler, 1982). Silbert et al. (1982) compared divorce rates in a 5-year period and found that parents of children with CHD were not at higher risk of marriage break-up than parents of children with mild or no CHD. Finley et al. (1979) focused on one single type of heart defect and found that parents of children with tetralogy of Fallot had the same marital stability as parents of children with appendectomy. The study had a small sample size, and the results are difficult to generalize to other heart defects. These two studies were completed ∼30 years ago; the survival rate in children with CHD has increased considerably since then owing to advances in treatment, diagnosis, and health care. Another historical change is that the risk of divorce has increased generally in the population during the past decades and is high in most Western countries (Amato & James, 2010).
As there is limited updated research on the relationship satisfaction of mothers of children with CHD, it is important to consider the literature on relationship satisfaction, marital distress, and marital stability in parents of children with other chronic illnesses and disabilities. Despite contradictory findings, these studies commonly hypothesize that the children’s condition is detrimental to marital satisfaction (Risdal & Singer, 2004). However, in reviews of this literature, there is a growing recognition that having a child with illness or disability does not necessarily pose a threat to the parents’ marital relationship. Benson and Gross (1989) reported in their review that the presence of a child with a congenital handicap or illness had both positive and negative effects on the partner relationship. Another review and a meta-analysis proposed that couples experience more marital dissatisfaction and distress but are not more at risk for divorce than parents of healthy children (Risdal & Singer, 2004; Sabbeth & Leventhal, 1984). These findings demonstrate the importance of considering relationship satisfaction and divorce rates as separate outcomes, as couples can be dissatisfied and distressed and still stay together.
A population sample of mothers from a large pregnancy cohort allowed us to compare trajectories of relationship satisfaction among mothers of children with mild, moderate, and severe CHD with the trajectories of relationship satisfaction in cohort of mothers of children without CHD. Based on previous findings, which suggest that severe CHD constitutes a long-term stressor for the parents, we examined whether variations in relationship satisfaction covaried with the severity of the child’s CHD. Repeated assessments conducted during and after pregnancy allowed the charting of the trajectories of relationship satisfaction. According to the family stress and coping model discussed previously, we hypothesized that the mothers will aim to retain high relationship satisfaction and marital stability over time, and that the first months postpartum will be most challenging period in this adaptation process. To our knowledge, this longitudinal study is the first to examine levels and changes of relationship satisfaction in mothers of children with a wide spectrum of CHD from early pregnancy to 36 months after delivery.
Methods
Participants and Procedures
Prior publications within this CHD cohort have described developmental impairments, emotional and behavioral problems in children with CHD, and anxiety, depression, and well-being among mothers of children with CHD (e.g., Brandlistuen et al., 2010; Dale et al., 2012; Solberg et al., 2012; Stene-Lasen et al., 2011). As described in our previous work, our study had a case–cohort design, linking a nationwide CHD registry administrated at the Department of Pediatrics at Oslo University Hospital to an ongoing prospective epidemiological study (N = 46,964), the Norwegian Mother and Child Cohort Study (MoBa), (www.fhi.no/morogbarn) conducted by the Norwegian Institute of Public Health (Magnus et al., 2006). The MoBa national cohort study presents a broad basis for studying health development, as the sampling frame comprised all pregnant women in Norway. The women received a postal invitation, informational brochure, informed consent form, and the first questionnaire with their appointment for the routine ultrasound examination at gestational week 17–18. Recruitment started in 1999 and ended in 2008. The participation rate was ∼38.5%, and the cohort currently includes 90,725 mothers (Norwegian Institute of Public Health, 2010). The present study had access to data from five assessments: at pregnancy week 17–18 (T1), at pregnancy week 30 (T2), at 6 months after delivery (T3), at 18 months after delivery (T4), and at 36 months after delivery (T5). No exclusion criteria were applied, and response rates among mothers who consented to participate in the study decreased from 95% during pregnancy to 85% at 6 months, 72% at 18 months, and 59% at 36 months after delivery. During the total duration of this study, ∼96% of the mothers reported being in a stable relationship, with 50% being married and 47% co-habiting at T2. In the present study, we included mothers with valid responses to questionnaire items concerning relationship satisfaction. In addition to information from MoBa, we used information on the child’s status at birth from the Medical Birth Registry of Norway (Irgens, 2000). Clinical information on the diagnosis and treatment of children with CHD was available through a nationwide CHD registry administered by the Department of Pediatrics, Pediatric Cardiology Unit, at University Hospital Oslo, Rikshospitalet, Norway, which serves as a national tertiary center for these children (Eskedal et al., 2005). The CHD registry includes information on Norwegian live-born children with significant CHD, including all forms of CHD from mild to severe. All examinations, diagnoses, procedures, and contacts with patients with CHD are entered into the database with assigned dates. This study was based on version 6 (2011) of the quality-assured data files of MoBa. The regional Committee for Medical Research and the Norwegian Data Inspectorate approved the study.
Mothers of infants with CHD in the MoBa cohort were identified by matching the personal identification numbers in the two databases. A case match at 36 months identified 318 mothers of children with (1) mild CHD, (2) moderate CHD, and (3) severe CHD. Children without CHD served as a comparison group (46,782) from the same age cohort. Of 318 mothers of children with CHD identified by the CHD registry, 93 had ceased to participate at 36 months, among which 28% had severe heart defects. A Chi-square test showed no differences between the attrition sample and mothers continuing to participate owing to frequencies of mild, moderate, and severe CHD, χ2 (2, N = 318) = 3.266, p = .19, and a two-tailed two-sample t-test showed no difference in mean scores of relationship satisfaction at 18 months, t(172) = 0.58, p = .56.
Exclusion criteria were syndromes or chromosomal defects (e.g., Down syndrome, Di George, Williams syndrome) (n = 19) and significant comorbid medical conditions (e.g., extracardiac malformations, cancer, intestinal malformation, or severe asthma) (n = 22). Further, two mothers were excluded whose children had an unclear comorbid condition or syndrome, thus arriving at a case group of 182 mothers of children with CHD.
Materials
Classification of Heart Defects
Based on previously accepted methods (Hoffman & Kaplan, 2002), two senior pediatric cardiologists (coauthors H.H. and L.T.E.) classified the cardiac defects according to severity and treatment-related aspects as follows: (1) “Mild CHD” (n = 81): The children with mild CHD were generally asymptomatic and without need of treatment, as the defect usually resolved spontaneously. This group of children need follow-up after the neonatal period and/or additional medical control subjects. Medical follow-up and the CHD diagnosis may have an impact on the parents’ perceptions of the infant’s health; (2) “Moderate CHD” (n = 37): The children could be symptomatic and required treatment and/or follow-up through childhood. No patient was treated more than once, and interventions were medically simple, e.g., balloon dilatation or closure of defects by catheter technique or surgery. The most common heart defects in this group were left-to-right shunts, pulmonary valve stenosis, and noncritical coarctation of the aorta; (3) “Severe CHD” (n = 64): These children had complex heart defects and/ or serious heart defects and were usually clearly symptomatic and sometimes critically ill in the newborn period or early infancy. They all required treatment with surgery, catheter intervention, or both, often repeatedly. The most common heart defect in this group was transposition of the great arteries. Table I shows a diagnosis chart for the different CHD groups.
Table I.
Diagnostic Groups
| Mild CHD | n | Moderate CHD | n | Severe CHD | n |
|---|---|---|---|---|---|
| Small/medium ventricular septal defect | 33 | Ventricular septum defect | 14 | Transposition of the great arteries | 18 |
| Patent ductus arteriosus | 15 | Atrial septal defect | 6 | Fallot (pulmonary atresia with ventricular septal defect) | 7 |
| Atrial septal defect/Patent foramen ovalea | 17 | Coarctation of the aorta | 5 | Coarctation of the aorta with interruption of theaortic arch | 8 |
| Other mild CHDb | 16 | Valvular pulmonary stenosis | 5 | Aortic stenosis | 7 |
| Patent ductus arteriosus | 7 | Pulmonary stenosis | 5 | ||
| Univentricular heart | 4 | ||||
| Large ventricular septal defect | 3 | ||||
| Hypoplastic left heart syndrome | 3 | ||||
| Double outlet right ventricle | 2 | ||||
| Pulmonary atresia with intact ventricular septum | 2 | ||||
| Persistant truncus arteriosus | 2 | ||||
| Atrioventricular septal defect | 2 | ||||
| Total anomalous pulmonary venous drainage | 1 | ||||
| Total | 81 | 37 | 64 |
Note. CHD = congenital heart defect.
aUnclear diagnosis (ASD or PFO) at the time diagnosis was made.
be.g., Mild valvar disease.
Relationship Satisfaction Scale
A five-item version of the Relationship Satisfaction scale (RSS) (Røysamb, Vittersø, & Tambs, 2012) was used to assess maternal relationship satisfaction. Developed for the MoBa study, the scale items refer to mothers’ satisfaction with their relationship with their partner or marital spouse. The scale is based on typical items used in previously well-established scales (Blum & Mehrabian, 1999; Hendrick, 1988), and the same scale has been used in other studies (Dyrdal et al., 2011; Rosand, Slinning, Eberhard-Gran, Roysamb, & Tambs, 2011). The initial validation of the scale was performed on the basis of psychometric study, including a separate population-based Norwegian sample and the pilot sample in the MoBa. The scale showed good psychometric properties, such as high Cronbach’s α, correlates .92 with the Quality of Marriage index (Norton, 1983) and generally exhibits high structural and predictive validity (Røysamb et al., 2012). The five-item version correlates .97 with the full 10-item version (Røysamb et al., 2012). Mothers rated their relationship satisfaction at pregnancy week 18 and 30 and after delivery at 6, 18, and 36 months. The five items, with response categories from 1 = totally agree to 6 = totally disagree, were phrased as follows: “My partner and I have problems in our relationship”; “I am very happy in my relationship”; “My partner is generally understanding”; “I am satisfied with the relationship with my partner”; “We agree about how children should brought up.” Four responses were reversed so that high scores overall reflect high satisfaction. Cronbach’s alphas indicated high reliability for the RSS index, with α = .85, .85, .87, .88, and .90 at T1, T2, T3, T4, and T5, respectively.
Covariates
Gestational age, child birth weight, and maternal age were retrieved from the Medical Birth Registry of Norway (Irgens, 2000). Gestational age in weeks was determined by means of ultrasound examination and operationalized as a continuous variable. Birth weight in grams and maternal age in years was measured as a continuous variable. Maternal education was assessed by six ordinal education levels. The levels were transformed into a continuous variable of the number of years of education that the mothers had completed at T1. Social support was measured at T1 and T5 by the following item: “Do you have anyone other than your husband/partner that you can ask for support in a difficult situation?”; the response categories were dichotomized as “No” and “Yes.” Maternal psychological distress was measured at T1 using the SCL-5, a shortened version of the Hopkins Symptom Checklist shown to correlate strongly with the SCL-25 index (Strand, Dalgard, Tambs, & Rognerud, 2003). SCL-5 has five items, with four response categories from 1 = not bothered to 4 = very bothered; a typical item was “Worrying too much about things.” The index was scored as the mean of the item score, where a mean score <2 is considered within the normal range on the SCL-5. Cronbach’s α was α = .82. Divorce and separation was measured at 36 months after delivery by the following item: “In the last 18 months, have you become divorced or separated or ended your relationship with your partner?”; the response categories were “No” and “Yes.”
Statistical Analyses
Listwise deletion bias of missing values was avoided by using maximum likelihood imputation procedures for missing data (Schafer & Graham, 2002). An expectation maximization algorithm (Dempster, Laird, & Rubin, 1977) was used to impute values for missing scores on the RSS. Missing values were substituted by each scale’s own response parameters from T1, T2, T3, T4, and T5. Missing values in relationship satisfaction were not imputed when all the five items were missing on each scale. The percentages of overall missing values were reduced from 9.3 to 5.4% for T1, from 9.7 to 5.5% for T2, from 9.5 to 4.4% for T3, from 14.5 to 5.6% for T4, and from 8.2 to 3.7% for T5.
To minimize skewness and kurtosis, we computed logarithmic transformation involving reflection before transformation for each RSS item for all measurement times. Then, the scores were re-reflected; therefore, high scores imply high satisfaction. After transformation, the skewness from T1 to T5 were, respectively, −1.53, −1.65, −1.40, −1.36, and −1.14, and the kurtosis were, respectively, 3.53, 4.25, 2.42, 2.16, and 1.47. Standardized regression coefficients and standardized marginal means were computed before creating tables and figures to improve readability. To explore the effect of time and CHD groups on RSS scores, mixed between-within subjects ANCOVAs were carried out (Tabachnick & Fidell, 2007). The within-subjects factor was the time of measurement (T1–T5). The between-subjects factor was CHD group (mild, moderate, severe), including the cohort control subjects. This case–cohort design has the advantage that cases and cohort control subjects are selected from the same population, and we avoid the problem of constructing an appropriate control group. The relative sizes of the groups reflect their proportion of the overall population.
To investigate whether changes in relationship satisfaction were related to the CHD proper and not merely a consequence of confounding conditions, the CHD groups were compared with the cohort on plausible confounding variables using ANOVA and the Bonferroni post hoc test for continuous variables and the chi-square test for categorical variables. The findings were then adjusted for the covariates of birth weight and gestational age, which were all shown to differ significantly between the CHD groups and the cohort group (see Table II). The Pearson correlation between birth weight and gestational age was acceptable, r = .62, and justifies including the variables as separate covariates.
Table II.
Characteristics of CHD Groups Compared with Cohort Control Subjects
| Cohort (n = 46,782) | Mild CHD (n = 81) | Moderate CHD (n = 37) | Severe CHD (n = 64) | χ2/F | Pa | |
|---|---|---|---|---|---|---|
| Mother | ||||||
| Age at birth of child (M ± SD) | 30.3 ± 4.44 | 30.5 ± 4.95 | 30.7 ± 4.76 | 30.3 ± 4.34 | .12 | .950 |
| Parity ≥1 sibling (%) | 53.2 | 49.4 | 54.1 | 53.1 | 4.80 | .923 |
| Education, years (M ± SD) | 14.7 ± 2.27 | 14.2 ± 2.39 | 14.9 ± 2.07 | 14.6 ± 2.47 | 1.57 | .194 |
| Divorced/separated at 36 months postpartum, yes (%) | 3.3 | 2.5 | 2.9 | 3.3 | .15 | .100 |
| Social support gestation week 18, yes (%) | 96.8 | 100.0 | 97.2 | 93.3 | 4.93 | .177 |
| Social support at 36 months postpartum, yes (%) | 96.8 | 97.5 | 100.0 | 91.8 | 6.22 | .101 |
| Psychological distress gestation week 18, score from 1 to 4 (M ± SD) | 1.2 ± .37 | 1.2 ± .31 | 1.2 ± .34 | 1.3 ± .38 | .15 | .928 |
| Psychological distress 6 months postpartum, score from 1 to 4 (M ± SD) | 1.2 ± .33 | 1.2 ± .29 | 1.2 ± .45 | 1.5 ± .47 | 8.82 | <.0001 |
| Relationship satisfaction at T1, raw score from 1 to 6: (M ± SD) | 5.3 ± .69 | 5.2 ± .70 | 5.3 ± .73 | 5.2 ± .84 | .37 | .772 |
| Relationship satisfaction at T2, raw score from 1 to 6: (M ± SD) | 5.3 ± .68 | 5.3 ± .66 | 5.4 ± .52 | 5.2 ± .81 | 1.21 | .304 |
| Relationship satisfaction at T3, raw score from 1 to 6: (M ± SD) | 5.2 ± .77 | 5.3 ± .72 | 5.4 ± .49 | 5.1 ± .81 | 1.88 | .131 |
| Relationship satisfaction at T4, raw score: (M ± SD) | 5.1 ± .82 | 5.1 ± .84 | 5.2 ± .69 | 5.0 ± .84 | .56 | .639 |
| Relationship satisfaction at T5, raw score from 1 to 6: (M ± SD) | 4.4 ± .54 | 4.4 ± .51 | 4.3 ± .50 | 4.3 ± .49 | .54 | .639 |
| Child | ||||||
| Boys/girls (%) | 51.2/48.8 | 44.4/55.6 | 62.2/37.8 | 57.8/42.2 | 4.38 | .223 |
| Gestational week (M ± SD) | 39.4 ± 1.94 | 38.5 ± 3.33b | 39.1 ± 2.52 | 38.7 ± 2.38b | 8.71 | <.0001 |
| Birth weight, g (M ± SD) | 3,575 ± 586 | 3,478 ± 914 | 3,455 ± 716 | 3,328 ± 739b | 4.92 | .002 |
| Operated for CHD (%) | – | – | 51.4 | 92.2 | – | – |
| Catheterized (%) | – | 2.5 | 35.1 | 25.0 | – | – |
Note. Table showing case numbers and within-group percentages; CHD = congenital heart defect; M = mean; SD = standard deviation; T1 = gestation week 18; T2 = gestation week 30; T3 = 6 months after delivery; T4 = 18 months after delivery; T5 = 36 months after delivery
aP-values were calculated using ANOVA for continuous variables and χ2 test for categorical variables.
bDiffers significantly from the cohort.
Cohen’s d was calculated to obtain a measure of effect size for the group differences. Bonferroni-corrected post hoc tests were performed for each assessment period and between groups, with p ≤ 0.05 considered significant. To estimate the sample power, we used Cohen’s f2 for ANOVA and multiple regression (Cohen, 1992). Cohen’s f2 showed that the minimum required sample size to detect a medium to large difference in our analysis with alpha level .05 was at least 54 cases in each group. For a desired power of .80, we had a sufficient number of cases in the mild and severe CHD groups to detect medium-to-large differences and to detect only large differences in the moderate CHD group.
The analyses were computed using PASW software version 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Characteristics of the Study Population
As most children with CHD are born to healthy mothers and the mothers form a random sample from the original cohort, the mothers share similar background variables. Mothers of children in the three CHD groups did not differ from the mothers in the cohort with respect to variables such as parity, education, age at the time of the child’s birth, reported social support at T1 and T5, and psychological distress before birth (Table II). Children with severe CHD had a lower birth weight, and children with mild CHD and severe CHD had a younger gestational age.
Divorce and Separation
There were no differences between the CHD groups and the cohort with respect to divorce and separation (p = .100) (Table II).
Maternal Distress
Mothers of children with severe CHD reported higher levels of distress compared with the cohort control subjects at 6 months postpartum (p < .0001) (Table II).
Effect of CHD Groups and Trajectories of Maternal Relationship Satisfaction
After adjustment for the covariates of child birth weight and gestational age in the analysis, the overall main effect of Groups (mild CHD, moderate CHD, severe CHD, and cohort control subjects) on maternal RSS score was not significant (F [3, 42,320] = .640, p = .589). Comparing the cohort with the mild, moderate, and severe CHD groups, respectively, Cohen’s d for the marginal means showed negligible effects at T1 (d = .03; .06; .04), negligible and small effects at T2 (d = .03; .22; .09), small effects at T3 (d = .20; .25; .25), negligible and small effects at T4 (d = .09; .09; .23), and negligible effects at T5 (d = .06; .11; .09).
Moreover, the overall effect of Time of measurement (T1–T5) on maternal RSS score was significant (F [4, 169,356] = 234.629, p < .0001). Bonferroni-corrected pairwise comparisons for Time revealed that all groups had significantly lower overall RSS scores at T4 and T5 as compared with RSS scores at T1 (p < .0001). Cohen’s d for the overall marginal means for all groups showed a negligible effect from T1 to T2 (d = .09), a small effect from T2 to T3 (d = .15), a small effect from T3 to T4 (d = .26) and a large effect from T4 to T5 (d = 1.09).
There was a non-significant interaction between the effects of Group and Time of measurement (F [12, 169,280] = 1.614, p = .091), indicating that mothers’ RSS scores varied as a function of measurement time.
Cross-Sectional Analysis at 36 Months
To investigate the extent to which maternal distress at 6 months postpartum affected the relationship satisfaction scores at 36 month postpartum, we included maternal distress as an additional covariate. Adjusting for maternal distress, there was no significant difference between the CHD groups and the cohort control subjects on relationship satisfaction scores (F [1, 43,629] = .173, p = .390), and the Cohen’s d for the marginal means showed negligible effects. There was a significant relationship between maternal distress at 6 months postpartum and relationship satisfaction scores at 36 months postpartum (F [1, 43,629] = 2,931.848, p < .0001). Partial Eta square value of .065 and this is a medium effect with Cohen’s d. Adjusting for maternal psychological distress, the mothers of children with mild, moderate, and severe CHD continue to report the same scores as the cohort control subjects at 36 months postpartum.
Table III shows parameter estimates for all CHD groups compared with the cohort in standard deviations. The estimates revealed that mothers of children with CHD had significantly same RSS scores as the cohort at all measurement times.
Table III.
Parameter Estimates Showing Standardized Regression Coefficients for Maternal Relationship Satisfaction Across CHD Severity from Pregnancy Week 18–36 Months Postpartum
| Pregnancy |
Postpartum |
||||
|---|---|---|---|---|---|
| CHD group | Week 18: RSSa b (CI) | Week 30: RSSa b (CI) | 6 months: RSSa b (CI) | 18 months: RSSa b (CI) | 36 months: RSSa b (CI) |
| Mild (n = 81) | −.03 (−.25 to .19) | .04 (−.18 to .25) | .20 (−.04 to .45) | −.06 (−.33 to .21) | −.05 (−.23 to .13) |
| Moderate (n = 37) | .10 (−.23 to .44) | .18 (−.15 to .51) | .25 (−.13 to .63) | .07 (−.35 to .48) | −.10 (−.38 to .17) |
| Severe (n = 64) | −.09 (−.35 to .18) | −.13 (−.39 to .12) | −.26 (−.58 to .06) | −.23 (−.55 to .09) | −.07 (−.29 to .14) |
| Cohort (n = 46,782) | 0b | 0b | 0b | 0b | 0b |
Note. CHD = congenital heart defects; RSS = Relationship Satisfaction Scale; b = regression coefficient; CI = 95% confidence interval.
aAdjusted for relevant covariates.
bParameter is set to zero because it is redundant.
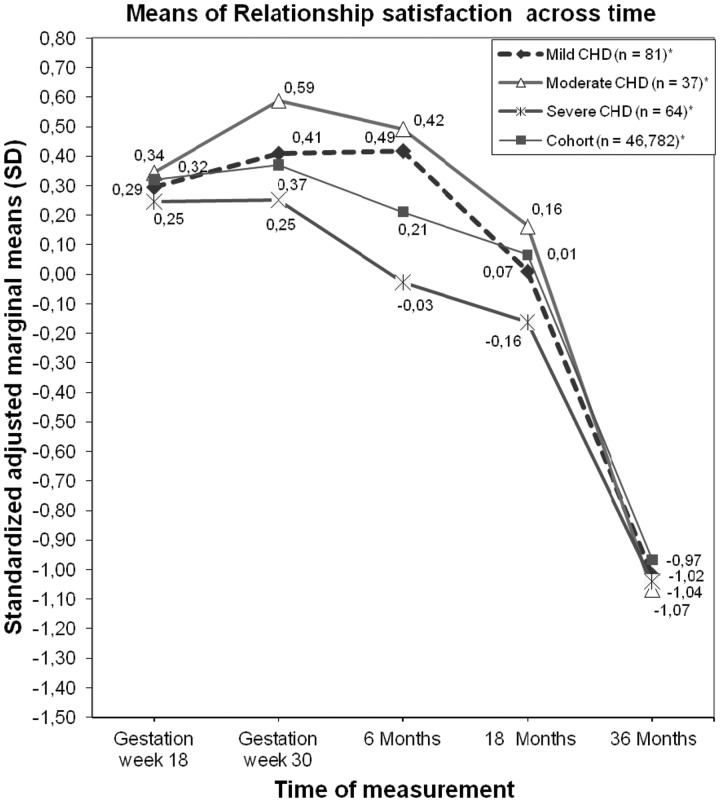
Figure 1 shows standardized adjusted marginal means for overall RSS scores, whereas Table II presents the raw scores with means and standard deviations for the different CHD groups compared with the cohort. Figure 1 shows the results of similar trajectories of RSS, and that the severity of CHD did not have a significant negative effect on RSS.
Figure 1.
Mothers’ standardized adjusted marginal means in fractions of standard deviations for RSS according to the severity of the CHD from pregnancy week 18 to 36 months after delivery. *Standard errors for each group at all measurement times are, respectively, .003, .003, .004, .004, .003 (Cohort); .077, .047, .087, .094, .062 (Mild CHD); .111, .108, .126, .137, .090 (Moderate CHD); .089, .087, .101, .110, .072 (Severe CHD).
Discussion
Because CHD survival is a relatively new phenomenon, little is known about the quality of the relationship between parents caring for CHD survivors. This is the first prospective case–cohort study of differences in relationship satisfaction among mothers of children with CHD from pregnancy to 36 months after pregnancy. Our main finding is that having a child with varying severity of CHD was not associated with reduced relationship satisfaction. This finding must be interpreted on the background that all women in the cohort experienced reduced and decreasing relationship satisfaction, as having a child is by itself associated with decreasing relationship satisfaction over time. This is a well-known finding in previous research on general parenthood, indicating that the first few months comprise a happy period, whereas happiness decreases and returns to pre-event levels or below after a while (Dyrdal et al., 2011; Hobbs, 1968; Miller & Sollie, 1980; Twenge et al., 2003).
There are no recent studies on relationship satisfaction in mothers of children with CHD with which we can compare our results. Studies have shown that having a child with CHD can be experienced as traumatic by the mothers (Lawoko & Soares, 2002, 2006), and a recent study by our research group (Solberg et al., 2012) found that maternal levels of anxiety and depression remain elevated during the 3 years after delivery. Our findings support that there is a significant relationship between maternal distress at 6 months postpartum and relationship satisfaction at 36 months postpartum. However, this distress does not appear to translate into lower relationship satisfaction.
Why did we not find significant lower relationship satisfaction among mothers of children with CHD? In the Resiliency model of adjustment and adaptation of the family by McCubbin et al. (1996), families of chronically ill children seek to restore balance and harmony and promote well-being in response to stress. In their model, a close relationship to the partner becomes the most central enduring and protective social relationship, and maintaining a good relationship can compensate for and help parents to better cope with the negative stressors related to having a child with CHD (Goldbeck & Melches, 2006; McCubbin et al., 1996; Murphy et al., 2007).
We can speculate that having a child with CHD creates in the parents a feeling of stronger mutual obligation to stay together and help each other through a difficult time. On the other hand, a review of the literature on parents of children with congenital handicaps (Benson & Gross, 1989) revealed coherent findings of relationship stability, better communication, and enhanced intimacy (see, e.g., Kazak & Marvin, 1984). Previous research suggested that parents of children with chronic illnesses seek stability; there is a need for normality and certainty, a need for information, and a need for partnership in these families (Fisher, 2001). In a recent study (Dale et al., 2012), we found that maternal levels of general life satisfaction and feelings of joy remained intact from pregnancy to 6 months postpartum, and this study adds that even in highly stressful times, caregivers can also experience the satisfaction and stability provided by a good relationship. In accordance with aforementioned findings, Goldbeck and Melches (2006) demonstrated that the negative impact of a severe disease was buffered if the family had strong intra-family resources. At the same time, this protective function was weakened when families experienced social disadvantage such as single parent status and unemployment.
We cannot rule out the possibility that mothers of children with CHD experienced significant reduction in relationship satisfaction in the first months after birth, when the extent of CHD is known and invasive treatment procedures begin. Owing to small sample size in the moderate CHD group and reduced sample power to reveal small effects, there might be a transient negative effect of severe CHD and positive effect of moderate CHD on relationship satisfaction at 6 months postpartum. However, at 36 months postpartum, this tendency has diminished. Possibly, this particular finding could be a result of a normalization process, where the mothers and the family adapt and return to their “normal” life, at least for a time (Barakat, Alderfer, & Kazak, 2006; Kazak et al., 2006; McCubbin et al., 1996).
Interestingly, mothers of children with CHD did not report a higher percentage of divorce or separation at 36 months postpartum compared with the cohort. This is in accordance with previous findings that divorce did not appear to be more frequent in families of children with CHD than in cohort families (Finley et al., 1979; Silbert et al., 1982).
On the other hand, another study with similar findings on relationship stability among parents of children with disabilities discussed whether this is positive, as having a child with illness may produce a stronger feeling of obligation to stay together (Lundeby & Tøssebro, 2008). It remains to be explored further whether these similarities in divorce rates and relationship satisfaction among mothers of children with CHD and mothers of children without CHD at child age 3 years are sustained as the children get older. Notably, in a previous study (Mauldon, 1992), the strongest association between divorce and having children with a disability was established for older children from age 6 to 9 years. One reason can be that the differences between the child and the child’s peers are more apparent later in the child’s life, e.g., when the child with illness enters school, but we might also wonder whether the long-term stressors could lead to a significant deterioration of the couples’ relationship satisfaction and stability over time. Nonetheless, the similarities between our mothers of children with CHD and mothers of children without CHD are important findings.
Limitations and Strengths
Several limitations of this study need to be addressed. First, the participation rate in MoBa is relatively low (38.5%) and raises a question as to how representative the participants are. A low participation rate might result in selection bias, and the participants have been found to be older and living in more stable relationships than the average Norwegian mother (Nilsen et al., 2009). On one hand, the mothers in this sample are more resourceful than the mothers in the general population; on the other hand, the heart defects were more severe than the severe heart defects treated in centers comparable in size with Oslo University Hospital. As a complement to the severity grading, a mean Aristotele score ranging from 1.5. to 14 can be used for the operated children with CHD (Lacour-Gayet et al., 2004). A high score indicates a greater degree of complexity. Although the average score in other patient samples is 6.7 (Eskedal, 2008), the mean Aristotele score in our sample of CHD at age 36 months was 9. Moreover, the degree of self-selection bias in the MoBa has been previously investigated, and analyses have shown that even though the MoBa study does not yield exact prevalence estimates owing to self-selection biases, the associations between exposure and outcome seem to be reliable and valid (Nilsen et al., 2009).
Second, owing to small sample size in the group of mothers of children with moderate CHD, it is important to interpret the P-value with care. Hence, the groups with mild and severe CHD with higher statistical power did not reach significance in our analysis, and we did not have any marginally nonsignificant findings in the overall group effects. Moreover, as the power calculation and the effect size estimates revealed, if the differences were significant, they would have been small.
Third, we only had one short measure of relationship satisfaction. Even though this scale had good psychometric properties, we cannot rule out the possibility that a full version testing with additional assessments could have been more sensitive.
Fourth, we did not have the exact timing of the CHD diagnosis. As prenatal detection rates are higher for children with severe CHD (Meberg, Otterstad, Froland, Hals, & Sorland, 1999; Offerdal, Blaas, & Eik-Nes, 2008; Tegnander, Williams, Johansen, Blaas, & Eik-Nes, 2006), some of these mothers probably knew about the defects during pregnancy, and this information may affect the family dynamics and the relationship quality at an earlier time point. In this study, the mothers of children with different severity of CHD did not differ from the cohort with respect to relationship satisfaction before birth, suggesting that prenatal diagnosis had no major impact on relationship satisfaction at that point in time.
Fifth, we did not examine the mothers’ RSS levels before pregnancy, and overall decreased RSS can represent a return to prepregnancy levels rather than represent absolute declines in satisfaction. A final limitation is that we did not have information on the fathers’ relationship satisfaction, and their opinion would make it possible to contrast and compare both parents’ satisfaction when having a child with illness.
Despite these limitations, our study has unique features that buttress our findings. These are the prospective design following mothers from pregnancy, the sheer size of the cohort, and the fact that the children with CHD were of the same age and represented different severities of CHD. Further, this study allowed us to include relevant confounders that might have influenced our findings.
Implications and Conclusions
Our findings highlight the importance of including a cohort control group and having baseline data. Pregnancy and the birth of a child are major life transitions for most couples, and as children enter the romantic union, parents’ relationship satisfaction is found to suffer over time and return to pre-event levels or below 3 years after birth. In our study, mothers of children with CHD appear not to be significantly different from our study population of mothers in Norway with regard to relationship satisfaction and the prevalence of separation and divorce. Our findings suggest that having a child with CHD is not alone a sufficient factor on which to base conclusions on reduced maternal relationship satisfaction. However, given the power restrictions with relatively small sample sizes, especially in the moderate CHD group, we cannot rule out that some scores could have reached significance, particularly at 6 months postpartum, with more statistical power.
Moreover, these findings do not disprove research, concluding that mothers of children with CHD experience emotional stress and that having a child with chronic illness affects the family. In particular, attention should be paid to stressors and caregiver burdens experienced by mothers of children with the most severe diagnoses, as the mothers with severe CHD in our sample experienced significant distress at 6 months postpartum, supporting that CHD severity may be a helpful indicator of who might need intervention during the first years after birth.
There are some questions that need future exploration. First, additional follow-up studies are required to explore whether the possible finding of relationship stability up to 36 months after delivery is sustained in the long-term. Parental relationship quality and satisfaction related to the child’s illness and the accompanying stressors during the first years after birth may be different from those occurring when the children are older. Second, more specific information might be brought to light by studies that incorporate parents’ appraisal, coping styles, family support, network size, and parity to better capture and understand how this impact relationship satisfaction and family dynamics.
Acknowledgments
The authors thank Bo Engdahl for his expert contribution to the statistical discussion before the final analysis of the data. They also thank the Department of Pediatric Cardiology at Oslo University Hospital for sharing knowledge and letting us be a part of the clinical environment. A special thanks to the parents whom the authors met in the hospital who voluntarily took the time to give them insights into their everyday lives and the challenges for their children with CHD.
Funding
The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health, National Institutes of Health/National Institute of Environmental Health Sciences (grant number N01-ES-85433); National Institutes of Health/National Institute of Neurological Disorders and Stroke (grant number 1 UO1 NS 047537-01); and the Norwegian Research Council/Functional Genomics (grant number 151918/S10). This study is supported by the Norwegian Research Council (grant number 186031/V50).
Conflicts of interest: None declared.
References
- Allen S W, Gauvreau K, Bloom B T, Jenkins K J. Evidence-based referral results in significantly reduced mortality after congenital heart surgery. Pediatrics. 2003;112:24–28. doi: 10.1542/peds.112.1.24. [DOI] [PubMed] [Google Scholar]
- Amato P R, James S. Divorce in Europe and the United States: Commonalities and differences across nations. Family Science. 2010;1:2–13. [Google Scholar]
- Barakat L P, Alderfer M A, Kazak A E. Posttraumatic growth in adolescent survivors of cancer and their mothers and fathers. Journal of Pediatric Psychology. 2006;31:413–419. doi: 10.1093/jpepsy/jsj058. [DOI] [PubMed] [Google Scholar]
- Benson B A, Gross A M. The effect of a congenitally handicapped child upon the marital dyad: A review of the literature. Clinical Psychology Review. 1989;9:747–758. [Google Scholar]
- Blum J S, Mehrabian A. Personality and temperament correlates of marital satisfaction. Journal of Personality. 1999;67:93–125. [Google Scholar]
- Brandlistuen R E, Stene-Larsen K, Holmstrøm H, Landolt M A, Eskedal L T, Vollrath M E. Motor and social development in 6-month-old children with congenital heart defects. The Journal of Pediatrics. 2010;156: 265–269. doi: 10.1016/j.jpeds.2009.08.035. [DOI] [PubMed] [Google Scholar]
- Clemente C, Barnes J, Shinebourne E, Stein A. Are infant behavioural feeding difficulties associated with congenital heart disease? Child: Care Health and Development. 2001;27:47–59. doi: 10.1046/j.1365-2214.2001.00199.x. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Dale M T G, Solberg O, Holmstrøm H, Landolt M A, Eskedal L T, Vollrath M E. Mothers of infants with congenital heart defects: Well-being from pregnacy through the child’s first six months. Quality of Life Research. 2012;21:115–122. doi: 10.1007/s11136-011-9920-9. [DOI] [PubMed] [Google Scholar]
- Dempster A P, Laird N M, Rubin D B. Maximum likelihood from incomplete data via the EM algorithm. Journal of the Royal Statistical Society Series B (Methodological) 1977;39:1–38. [Google Scholar]
- Dyrdal G, Røysamb E, Nes R, Vittersø J. Can a happy relationship predict a happy life? A population-based study of maternal well-being during the life transition of pregnancy, infancy, and toddlerhood. Journal of Happiness Studies. 2011;12:947–962. doi: 10.1007/s10902-010-9238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskedal L. Survival in surgical congenital heart defects (Doctoral thesis) 2008 University of Oslo, Oslo, Norway. ISBN 978-82-8072-908-8. [Google Scholar]
- Eskedal L, Hagemo P S, Eskild A, Aamodt G, Seiler K S, Thaulow E. Survival after surgery for congenital heart defects: Does reduced early mortality predict improved long-term survival? Acta Paediatica. 2005;94:438–443. doi: 10.1111/j.1651-2227.2005.tb01915.x. [DOI] [PubMed] [Google Scholar]
- Finley J, Putherbough C, Cook D, Netley C, Rowe R. Effect of congenital heart disease on the family: Divorce, separation, and stability in families of children with tetralogy of fallot. Pediatric Cardiology. 1979;1:9–13. [Google Scholar]
- Fisher H R. The needs of parents with chronically sick children: A literature review. Journal of Advanced Nursing. 2001;36:600–607. doi: 10.1046/j.1365-2648.2001.02013.x. [DOI] [PubMed] [Google Scholar]
- Goldbeck L, Melches J. The impact of the severity of disease and social disadvantage on quality of life in families with congenital cardiac disease. Cardiology in the Young. 2006;16:76–75. doi: 10.1017/S1047951105002118. [DOI] [PubMed] [Google Scholar]
- Hendrick S S. A generic measure of relationship satisfaction. Journal of Marriage and the Family. 1988;50:93–98. [Google Scholar]
- Hobbs D F. Transition to parenthood: A replication and an extension. Journal of Marriage and the Family. 1968;30:413–417. [Google Scholar]
- Hoffman J I, Kaplan S. The incidence of congenital heart disease. Journal of the American College of Cardiology. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- Irgens L M. The Medical Birth Registry of Norway: Epidemiological research and surveillance throughout 30 years. Acta Obstetricia et Gynecologica Scandinavica. 2000;79:435–439. [PubMed] [Google Scholar]
- Joesch J M, Smith K R. Children's health and their mothers' risk of divorce or separation. Social Biology. 1997;44:159–169. doi: 10.1080/19485565.1997.9988944. [DOI] [PubMed] [Google Scholar]
- Karsdorp P A, Everaerd W, Kindt M, Mulder B J. Psychological and cognitive functioning in children and adolescents with congenital heart disease: A meta-analysis. Journal of Pediatric Psychology. 2007;32:527–541. doi: 10.1093/jpepsy/jsl047. [DOI] [PubMed] [Google Scholar]
- Kazak A E, Kassam-Adams N, Schneider S, Zelikovsky N, Alderfer M A, Rourke M. An integrative model of pediatric medical traumatic stress. Journal of Pediatric Psychology. 2006;31:343–355. doi: 10.1093/jpepsy/jsj054. [DOI] [PubMed] [Google Scholar]
- Kazak A E, Marvin R S. Differences, difficulties and adaptation: Stress and social networks in families with a handicapped child. Family Relations: An Interdisciplinary Journal of Applied Family Studies. 1984;33:67–77. [Google Scholar]
- Kersh J, Hedvat T T, Hauser-Cram P, Warfield M E. The contribution of marital quality to the well-being of parents of children with developmental disabilities. Journal of Intellectual Disability Research. 2006;50:883–893. doi: 10.1111/j.1365-2788.2006.00906.x. [DOI] [PubMed] [Google Scholar]
- Knafl K. Childhood chronic illness: A comparison of mothers' and fathers' experiences. Journal of Family Nursing. 2000;6:287–302. [Google Scholar]
- Lacour-Gayet F, Clarke D, Jacobs J, Gaynor W, Hamilton L, Jacobs M, Maruszewski B, Pozzi M, Spray T, Tchervenkov C, Mavroudis C, Aristotle Committee The Aristotle score for congenital heart surgery. Seminars in Thoracic and Cardiovascular Surgery: Pediatric Cardiac Surgery Annual. 2004;7:185–191. doi: 10.1053/j.pcsu.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Lawoko S. Factors influencing satisfaction and well-being among parents of congenital heart disease children: Development of a conceptual model based on the literature review. Scandinavian Journal of Caring Sciences. 2007;21:106–117. doi: 10.1111/j.1471-6712.2007.00444.x. [DOI] [PubMed] [Google Scholar]
- Lawoko S, Soares J J. Distress and hopelessness among parents of children with congenital heart disease, parents of children with other diseases, and parents of healthy children. Journal of Psychosomatic Research. 2002;52:193–208. doi: 10.1016/s0022-3999(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Lawoko S, Soares J J. Psychosocial morbidity among parents of children with congenital heart disease: A prospective longitudinal study. Heart and Lung. 2006;35:301–314. doi: 10.1016/j.hrtlng.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Lundeby H, Tøssebro J. Family structure in Norwegian families of children with disabilities. Journal of Applied Research in Intellectual Disabilities. 2008;21:246–256. [Google Scholar]
- Magnus P, Irgens L M, Haug K, Nystad W, Skaerven R, Stoltenberg C, MoBa Study Group Cohort profile: The Norwegian Mother and Child Cohort Study (MoBa) International Journal of Epidemiology. 2006;35:1146–1150. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- Mauldon J. Children's risks of experiencing divorce and remarriage: Do disabled children destabilize marriages? Population Studies. 1992;46:349–362. [Google Scholar]
- McCubbin H I, Thompson A I, McCubbin M A. Resiliency in families: A conceptual model of family adjustment and adaptation in response to stress and crisis. In: McCubbin H I, Thompson A I, McCubbin M A, editors. Familiy assessment: Reciliency, coping and adaptation –inventories for research and practice. Madison, WI: University of Wisconsin Publishers; 1996. pp. 1–64. [Google Scholar]
- Meberg A F, Otterstad J E, Froland G F, Hals J F, Sorland S J. Early clinical screening of neonates for congenital heart defects: The cases we miss. Cardiology in the Young. 1999;9:169–174. doi: 10.1017/s1047951100008398. [DOI] [PubMed] [Google Scholar]
- Miller B C, Sollie D L. Normal stresses during the transition to parenthood. Family Relations. 1980;29:459–465. [Google Scholar]
- Murphy N A, Christian B, Caplin D A, Young P C. The health of caregivers for children with disabilities: Caregiver perspectives. Child: Care Health Development. 2007;33:180–187. doi: 10.1111/j.1365-2214.2006.00644.x. [DOI] [PubMed] [Google Scholar]
- Nilsen R M, Vollset S E, Gjessing H K, Skjærven R, Melve K K, Schreuder P, Alsaker E R, Haug K, Daltveit A K, Magnus P. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatric and Perinatal Epidemiology. 2009;23:597–608. doi: 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- Norton R. Measuring marital quality: A critical look at the dependent variable. Journal of Marriage and the Family. 1983;45:141–151. [Google Scholar]
- Norwegian Institute of Public Health. Norwegian mother and child cohort study: Revised PROTOCOL: End of enrolment - Protocol II. Oslo, Norway: Norwegian Institute of Public Health; 2010. Retrieved from http://www.fhi.no/dokumenter/ce2e768194.pdf. [Google Scholar]
- Offerdal K, Blaas H G, Eik-Nes S H. Prenatal detection of trisomy 21 by second-trimester ultrasound examination and maternal age in a non-selected population of 49 314 births in Norway. Ultrasound in Obstetrics and Gynecology. 2008;32:493–500. doi: 10.1002/uog.5373. [DOI] [PubMed] [Google Scholar]
- Risdal D, Singer G H S. Marital adjustment in parents of children with disabilities: A historical review and meta-analysis. Research and Practice for Persons with Severe Disabilities. 2004;29:95–103. [Google Scholar]
- Rosand G M, Slinning K, Eberhard-Gran M, Roysamb E, Tambs K. Partner relationship satisfaction and maternal emotional distress in early pregnancy. BMC Public Health. 2011;11:161. doi: 10.1186/1471-2458-11-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Røysamb E, Vittersø J, Tambs K. 2012 The relationship satisfaction scale. Reliability, validity and goodness of fit. Manuscript in preparation. [Google Scholar]
- Sabbeth B F, Leventhal J M. Marital adjustment to chronic childhood illness: A critique of the literature. Pediatrics. 1984;73:762–768. [PubMed] [Google Scholar]
- Saenz R B, Beebe D K, Triplett L C. Caring for infants with congenital heart disease and their families. American Family Physician. 1999;59:1857–1868. [PubMed] [Google Scholar]
- Schafer J L, Graham J W. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Silbert A R, Newburger J W, Fyler D C. Marital stability and congenital heart disease. Pediatrics. 1982;69:747–750. [PubMed] [Google Scholar]
- Solberg Ø, Grønning Dale M T, Holmstrøm H, Eskedal L T, Landolt M A, Vollrath M E. Trajectories of maternal mental health: A prospective study of mothers of infants with congenital heart defects from pregnancy to 36 months postpartum. Journal of Pediatric Psychology. 2012;37:687–696. doi: 10.1093/jpepsy/jss044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stene-Larsen K, Brandlistuen R E, Holmstrøm H, Landolt M A, Eskedal L T, Engdahl B, Vollrath M E. Longitudinal analysis of emotional problems in children with congenital heart defects: A follow-up from age 6 to 36 months. Journal of Developmental & Behavioral Pediatrics. 2011;32:461. doi: 10.1097/DBP.0b013e3182202d2b. [DOI] [PubMed] [Google Scholar]
- Strand B H, Dalgard O S, Tambs K, Rognerud M. Measuring the mental health status of the Norwegian population: A comparison of the instruments SCL-25, SCL-10, SCL-5 and MHI-5 (SF-36) Nordic Journal of Psychiatry. 2003;57:113–118. doi: 10.1080/08039480310000932. [DOI] [PubMed] [Google Scholar]
- Tabachnick B G, Fidell L S. Using multivariate statistics. 5th ed. Boston, MA: Pearson/Allyn and Bacon; 2007. [Google Scholar]
- Tegnander E, Williams W, Johansen O J, Blaas H G, Eik-Nes S H. Prenatal detection of heart defects in a non-selected population of 30 149 fetuses: Detection rates and outcome. Ultrasound in Obstetrics and Gynecology. 2006;27:252–265. doi: 10.1002/uog.2710. [DOI] [PubMed] [Google Scholar]
- Twenge J M, Campbell W K, Foster C A. Parenthood and marital satisfaction: A meta-analytic review. Journal of Marriage and Family. 2003;65:574–583. [Google Scholar]
- Wallander J L, Varni J W, Babani L, Banis H T, Wilcox K T. Family resources as resistance factors for psychological maladjustment in chronically ill and handicapped children. Journal of Pediatric Psychology. 1989;14:157–173. doi: 10.1093/jpepsy/14.2.157. [DOI] [PubMed] [Google Scholar]