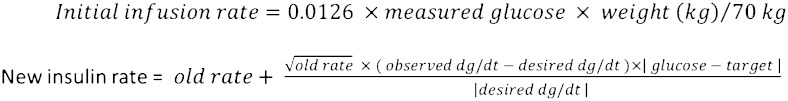Abstract
Background:
Optimal glucose management in the ICU remains unclear. In 2009, many clinicians at Intermountain Healthcare selected a moderate glucose control (90-140 mg/dL) instead of tight glucose control (80-110 mg/dL). We hypothesized that moderate glucose control would affect patients with and without preexisting diabetes differently.
Methods:
We performed a retrospective cohort analysis of all patients treated with eProtocol-insulin from November 2006 to March 2011, stratifying for diabetes. We performed multivariate logistic regression for 30-day mortality with covariates of age, modified APACHE (Acute Physiology and Chronic Health Evaluation) II score, Charlson Comorbidity score, and target glucose.
Results:
We studied 3,529 patients in 12 different ICUs in eight different hospitals. Patients with diabetes had higher mean glucose (132 mg/dL vs 124 mg/dL) and greater glycemic variability (SD = 41 mg/dL vs 29 mg/dL) than did patients without diabetes (P < .01 for both comparisons). Tight glucose control was associated with increased frequency of moderate and severe hypoglycemia (30.3% and 3.6%) compared with moderate glucose control (14.3% and 2.0%, P < .01 for both). Multivariate analysis demonstrated that the moderate glucose target was independently associated with increased risk of mortality in patients without diabetes (OR, 1.36; 95% CI, 1.01-1.84; P = .05) but decreased risk of mortality in patients with diabetes (OR, 0.65; 95% CI, 0.45-0.93; P = .01).
Conclusions:
Moderate glucose control (90-140 mg/dL) may confer greater mortality in critically ill patients without diabetes compared with tight glucose control (80-110 mg/dL). A single glucose target does not appear optimal for all critically ill patients. These data have important implications for the design of future interventional trials as well as for the glycemic management of critically ill patients.
The optimal management for glycemic control in the ICU remains unclear. In 2001, van den Berghe and colleagues1 demonstrated that intensive insulin therapy (maintenance of blood glucose [BG] at a level between 80 and 110 mg/dL) conferred a substantial mortality benefit in patients in the surgical ICU compared with conventional treatment (maintenance of BG between 180 and 200 mg/dL). Subsequent studies evaluating the role of insulin therapy in the ICU either failed to confirm the initial results of the Leuven surgical ICU study or were terminated early because of high hypoglycemia rates.2‐6 The Normoglycemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation (NICE-SUGAR) trial is the largest prospective multicenter trial to date and was designed to compare maintaining a BG target of 80 to 110 mg/dL (intensive insulin therapy) to maintaining a BG target of 144 to 180 mg/dL (termed conventional therapy) in patients in the ICU.7 The NICE-SUGAR algorithm resulted in both decreased hypoglycemia incidence and decreased 90-day mortality in the conventional therapy group. The incidence of hypoglycemia in the 80 to 110 mg/dL group was 6.7% of patients, similar to that reported in the Leuven surgical ICU study,1 and lower than previous comparative studies in medical patients.4‐6 Although NICE-SUGAR results do not causally link severe hypoglycemia with increased mortality, many postulate that the mortality benefit in the 144 to 180 mg/dL group arose from a reduction in hypoglycemia.8,9 Thus, much uncertainty remains.
Some contend that intensive insulin therapy confers greater benefit in certain populations1,2 or that the findings of NICE-SUGAR may be idiosyncratic because of its study protocols.10 There is considerable literature suggesting that patients with diabetes respond differently than patients without diabetes to tight BG control.11 In the 2006 Leuven mixed medical/surgical ICU study, tight BG control with intensive insulin therapy conferred increased survival in patients without diabetes but not in patients with diabetes.12 In the 2006 Stamford mixed medical/surgical ICU study, Krinsley13 demonstrated that hyperglycemia had considerably greater mortality effects on patients without diabetes compared with patients with diabetes. A 2008 study from two Australian ICUs corroborated Krinsley’s13 findings, with a stronger correlation between hyperglycemia and mortality in patients without diabetes than in patients with diabetes.14
Other investigators suggest that glycemic variability may be an important prognostic factor.15‐18 Regardless of its limitations, NICE-SUGAR has resulted in revisions of professional society recommendations for BG control in critically ill patients.19,20 Lack of consensus for the ideal BG target for critically ill patients has led to increased clinician uncertainty. At our institution and at others, the NICE-SUGAR results prompted a shift in the clinical management of insulin infusions, and many intensivists began to endorse a BG target of 90 to 140 mg/dL.
Intermountain Healthcare, the largest health-care provider in the Intermountain West, iteratively developed and refined a point-of-care computerized protocol for insulin (eProtocol-insulin) infusion in the intensive care setting.21 This electronic protocol incorporates the current insulin infusion rate, the difference between current BG and BG target, and the rate of change of BG, and suggests subsequent insulin infusion rates (Fig 1). The protocol then recommends to the clinician a new insulin infusion rate and the time interval for a subsequent BG measurement. Clinician compliance with eProtocol-insulin recommendations is high, and the implementation of eProtocol-insulin has resulted in clinical reproducibility of BG metrics across multiple environments.22
Figure 1.
eProtocol-insulin algorithm.
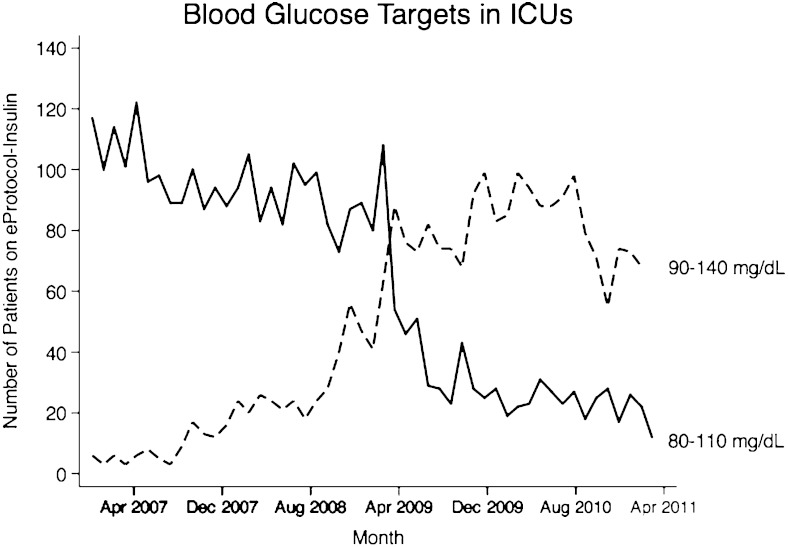
eProtocol-insulin is applied throughout the clinical setting in ICUs in the Intermountain Healthcare network. The protocol operates by evaluating the abovementioned variables and the BG value distance from the BG mean of the chosen target range. The rules for insulin titration remain unchanged, and protocol implementation is identical regardless of chosen BG target range. eProtocol-insulin allows clinicians to select either a target range of 80 to 110 mg/dL or 90 to 140 mg/dL. These BG targets were derived from expert recommendations and local clinician consensus.23,24 Because the mathematical rules of the protocol ignore BG target ranges, these two BG target ranges actually represent a mean BG target of 95 mg/dL for 80 to 110 mg/dL and 115 mg/dL for 90 to 140 mg/dL. Thus, the effective comparison BG targets are 80 to 110 mg/dL and 100 to 130 mg/dL. eProtocol-insulin provides a unique investigation into the effects of BG target on patients, because all other features of protocol implementation remain unchanged. The history of high clinician compliance and clinical reproducibility further support an experimental design in which only the BG target range differs. Clinicians’ implementation of the 80 to 110 mg/dL target decreased and implementation of the 90 to 140 mg/dL target increased throughout Intermountain Healthcare System in March of 2009 (Fig 2). The inflection point coincides with the publication of NICE-SUGAR trial. We hypothesized that the patient population would mimic that of NICE-SUGAR and we would observe decreased hypoglycemia and decreased mortality in patients on the moderate glucose target. We expected that patients without diabetes would have greater glycemic variability on the 90 to 140 mg/dL glucose target compared with 80 to 110 mg/dL, whereas patients with diabetes would have greater glycemic variability on 80 to 110 mg/dL target compared with 90 to 140 mg/dL.
Figure 2.
Selection of glucose targets in eProtocol-insulin throughout the Intermountain Healthcare ICUs from November 2006 to March 2011.
Materials and Methods
Data Collection
We performed a retrospective cohort analysis of all patients treated with eProtocol-insulin. We used the highly detailed electronic medical record from Intermountain Healthcare, which captures data prospectively. This study was approved by the Intermountain institutional review board (#1008548), with waiver of informed consent. The electronic medical record was queried for all instances of eProtocol-insulin use in an ICU from November 2006 to March 2011. We excluded patients who presented with diabetic ketoacidosis, as we believe they represented a different patient experience than the typical ICU patient on intensive insulin. We also excluded patients who were on the protocol for fewer than 10 BG readings (average 10 h). For patients who had multiple ICU admissions during the study period, we counted only the first ICU admission and excluded subsequent ICU admissions. The study population was stratified by presence or absence of diabetes mellitus and by receipt of 80 to 110 mg/dL or 90 to 140 mg/dL eProtocol-insulin therapy.
Determination of diabetes was performed by query of the medical record for International Statistical Classification of Diseases and Related Health Problems, Ninth Revision (ICD-9) code (249.x-250.x). The electronic medical record and Intermountain Medical Center can calculate an APACHE II (Acute Physiology and Chronic Health Evaluation II)25 score automatically from patients based on data entered into the record. We included only patients who had sufficient data to calculate an APACHE II score at the time of initiation of the eProtocol-insulin. The Charlson Comorbidity index was calculated using ICD-9 code, according to previously published methodology.26,27
Glycemic management in the ICUs throughout Intermountain Healthcare include standardized institutional processes. All ICUs used the OneTouch SureStep (LifeScan, Inc) bedside glucometer during the study period until 2010, when all facilities switched to the HemoCue (Quest Diagnostics) glucometer. All glucometers are calibrated nightly according to industry and product standards. Similarly, all Intermountain Healthcare systems use the smart pump in plastic tubing with a 1 unit/mL concentration of IV insulin infusion bag. The time interval of BG measurements is explicitly determined by the protocol, based on glucose stability. Nursing ratio per patient is standardized throughout all Intermountain Healthcare ICUs (2:1). eProtocol-insulin does not contain an explicit nutritional protocol, although it adjusts recommendations based on the amount of glucose calories a patient is receiving and responds to changes in glucose administration. The protocol is discontinued if patients are on bolus feeds. BG values include only those obtained while the patient was on protocol. We did not include BG values obtained after the protocol was discontinued for bolus feeds. Selection of glucose target, as well as whether to use eProtocol-insulin, was at the treating clinician’s discretion.
Statistical Analysis
We tested group comparisons with Mann-Whitney U test for nonnormally distributed continuous values and Student t test for normally distributed data. For comparison of proportions, we used χ2 test or the Fisher exact test when sample size was small. Survival analyses were performed using logistic regression to assess the impact of eProtocol-insulin on 30-day mortality in the diabetic and nondiabetic populations, respectively. Covariates adjusted for in the regression models include age, modified APACHE II score, Charlson Comorbidity score, and target BG. The modified APACHE II score excludes the age or chronic health components in order to avoid collinearity with the Charlson Comorbidity score or age. Secondary analyses applying Cox regression techniques yielded similar results. All displayed P values are double sided. Data were analyzed using Stata-12 statistical software (StataCorp LP).
Results
Study Population
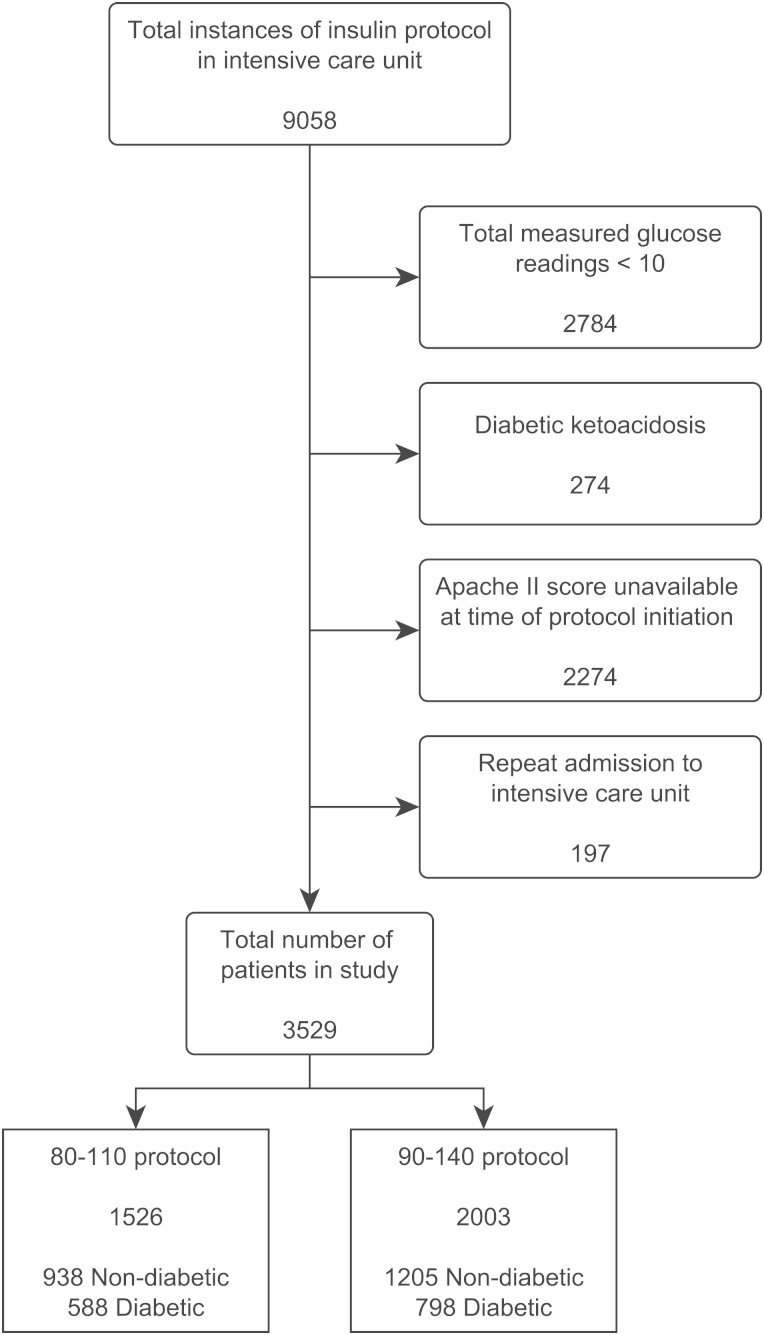
The initial query returned a total of 9,058 instances of eProtocol-insulin in the study period. The breakdown of the exclusion criteria is displayed in Figure 3. The final study population is composed of 3,529 patients drawn from 12 different ICUs in eight different hospitals. These units included medical, surgical, and mixed ICUs. The mix of hospitals included tertiary care, teaching hospitals, and private community hospitals.
Figure 3.
Database query method. Apache II = Acute Physiology and Chronic Health Evaluation II.
Patient Demographics
There was no significant association between the presence of diabetes and selection of insulin protocol, with patients with diabetes composing approximately 40% of both groups. Patient demographics, glucose metrics, and overall mortality are displayed in Table 1. The groups were similar in composition, although the 90 to 140 mg/dL group had a slightly higher proportion of women and slightly higher APACHE II scores than did the 80 to 110 mg/dL group. There was no difference between groups regarding Charlson comorbidity scores. Inclusion of sex in the regression models yielded no change in estimate of coefficients.
Table 1.
—Patient Demographics, Glucose Metrics, and Unadjusted Mortality
| Measure | 80-110 mg/dL (n = 1,526) | 90-140 mg/dL (n = 2,003) | P Value |
| Age, y | 65 (54-75) | 64 (53-74) | .09 |
| Female, % | 38.6 | 42.7 | .02 |
| Diabetic, % | 38.7 | 40.1 | .43 |
| APACHE II score | 23 (18-29) | 25 (19-31) | < .01 |
| Charlson comorbidity score | 2 (1-5) | 2 (1-5) | .67 |
| Patient mean blood glucose, mg/dL | 118 (109-131) | 131 (123-143) | < .01 |
| Patients with diabetes | 124 (113-139) | 138 (127-151) | < .01 |
| Patients without diabetes | 115 (107-126) | 128 (121-137) | < .01 |
| Patient SD of glucose, mg/dL | 25 (33-46) | 24 (33-45) | .78 |
| Patients with diabetes | 42 (31-59) | 39 (30-53) | < .01 |
| Patients without diabetes | 31 (24-40) | 28 (22-38) | < .01 |
| Incidence of hypoglycemia | |||
| < 60 mg/dL, % | 30.3 | 14.3 | < .01 |
| < 40 mg/dL, % | 3.6 | 2.0 | < .01 |
| 30-d mortality, % | 9.7 | 11.1 | .20 |
| Patients with diabetes, % | 12.3 | 9.8 | .12 |
| Patients without diabetes, % | 8.1 | 11.9 | < .01 |
| Patients with hypoglycemia < 60 mg/dL, % | 14.4 | 17.4 | .33 |
| Patients with hypoglycemia < 40 mg/dL, % | 14.5 | 20.0 | .58 |
All continuous or ordinal data are median values followed by interquartile ranges. Significance testing calculated with Fisher exact, χ2 with Yates correction, or Wilcoxon-Mann-Whitney U test, where appropriate. APACHE II = Acute Physiology and Chronic Health Evaluation II.
Hypoglycemia
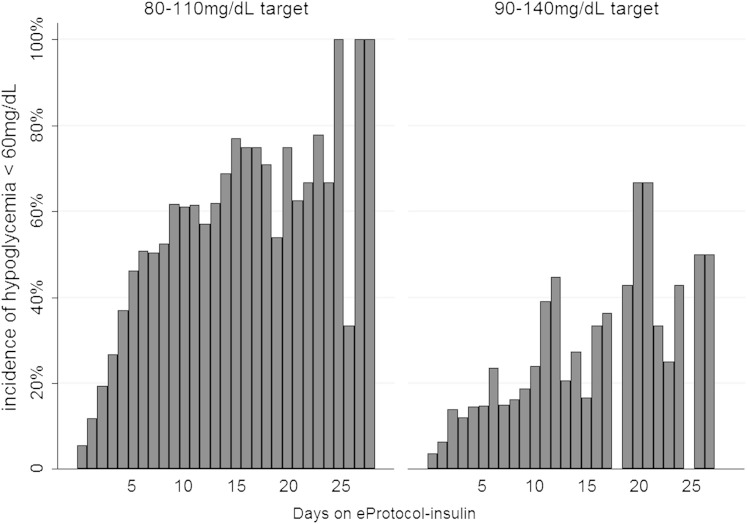
As expected, patients on the 90 to 140 mg/dL target were less likely to experience hypoglycemia compared with patients on the 80 to 110 mg/dL target. Table 1 demonstrates that patients on the 80 to 110 mg/dL target sustained higher rates of moderate and severe hypoglycemia. Hypoglycemia was associated with mortality. Among patients whose BG level never went below 60 mg/dL, 30-day crude mortality was 10.3%. Thirty-day mortality was 15.9% in patients who had at least one glucose measurement < 60 mg/dL and 17.3% if they had at least one incidence of glucose < 40 mg/dL (P < .001). The number of days a patient was managed with eProtocol-insulin was independently associated with hypoglycemia, after adjusting for age, modified APACHE II score, Charlson comorbidity score, and presence of diabetes (OR, 1.14; 95% CI, 1.12-1.16; P < .01) (Fig 4). However, the duration of time a patient was managed with eProtocol-insulin was also highly collinear with the number of days a patient spent in the ICU, a predictor that was also independently associated with hypoglycemia, after adjusting for age, modified APACHE II score, Charlson comorbidity score, and presence of diabetes (OR, 1.08; 95% CI, 1.06-1.10; P < .01).
Figure 4.
Incidence of moderate hypoglycemia while on eProtocol-insulin, stratified by target glucose. Days on protocol was independently associated with incidence of hypoglycemia in both glucose target groups (P < .01).
Mortality
There was no statistically significant difference in overall crude 30-day mortality between the 80 to 110 mg/dL group and the 90 to 140 mg/dL group. Crude mortality, stratified by the presence or absence of diabetes, showed a statistically significant trend: In patients without diabetes, mortality was significantly higher on the 90 to 140 mg/dL BG target than on the 80 to 110 mg/dL target. Crude mortality stratified by patient mean glucose is depicted in e-Figures 1 (343.1KB, pdf) and 2 (343.1KB, pdf) , although inferences from these data may be limited by the small number of patients with mean BG readings < 80 mg/dL or > 180 mg/dL.
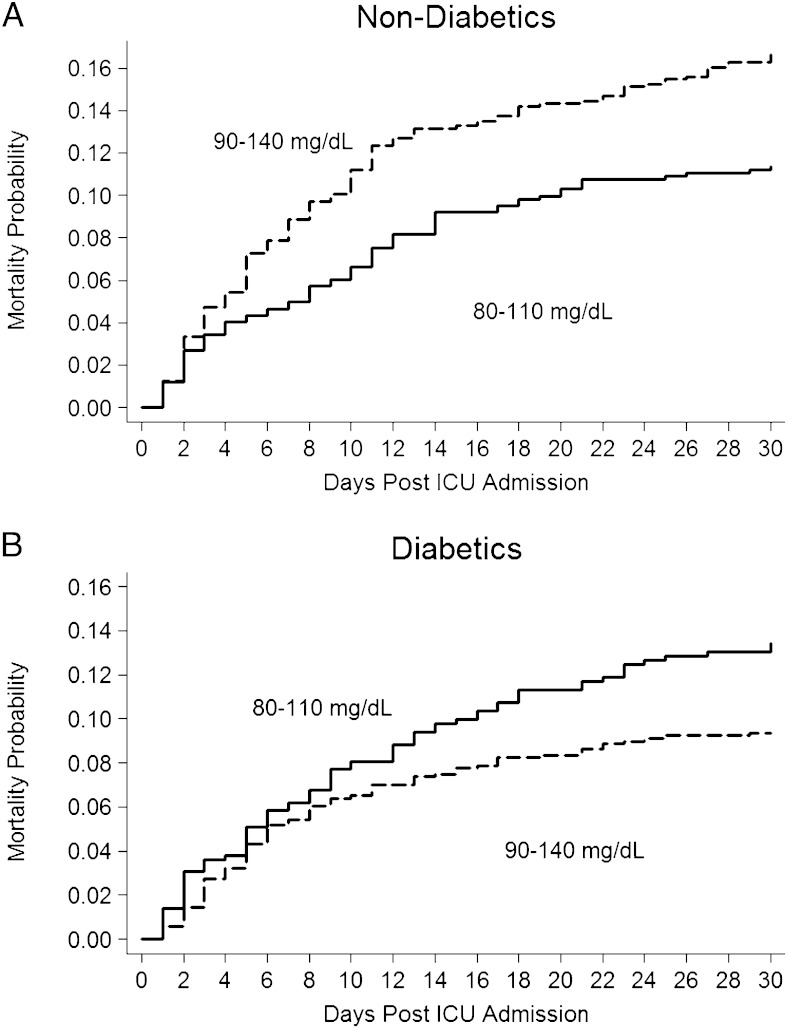
The logistic regression model demonstrated that, among patients without diabetes, use of the 90 to 140 mg/dL target was independently associated with an increased risk of mortality compared with use of the 80 to 110 mg/dL target (Table 2). In patients with diabetes, use of the 90 to 140 mg/dL target was independently associated with decreased risk of mortality compared with use of the 80 to 110 mg/dL target. Kaplan-Meier mortality graphs were calculated from Cox regression models, which yielded similar results (Fig 5).
Table 2.
—Logistic Regression for 30-d Mortality, Comparing 90 to 140 mg/dL Glucose Target to the 80 to 110 mg/dL Target
| Predictor | OR | 95% CI | P Value |
| Patients without diabetes (n = 2,143) | |||
| 90-140 mg/dL | 1.36 | 1.01-1.84 | .05 |
| Modified APACHE II scorea | 1.09 | 1.07-1.11 | < .01 |
| Charlson comorbidity score | 1.06 | 1.01-1.13 | .01 |
| Age, y | 1.02 | 1.01-1.03 | < .01 |
| Patients with diabetes (n = 1,386) | |||
| 90-140 mg/dL | 0.65 | 0.45-0.93 | .02 |
| Modified APACHE II scorea | 1.07 | 1.05-1.10 | < .01 |
| Charlson comorbidity score | 1.05 | 1.00-1.10 | .06 |
| Age, y | 1.04 | 1.02-1.06 | < .01 |
See Table 1 legend for expansion of abbreviation.
APACHE II score was modified to exclude age and measures of chronic health.
Figure 5.
Cox failure curve for mortality in patients without diabetes. A, Patients without diabetes were more likely to die if they received 90 to 140 mg/dL (dashed line); P = .05. B, Patients with diabetes were more likely to die if they received 80 to 110 mg/dL (solid line); P = .01.
Mortality and Hypoglycemia
As expected, patients on the 90 to 140 mg/dL target were less likely to experience hypoglycemia compared with patients on the 80 to 110 mg/dL target. However, if a patient had a hypoglycemic event (both the 60 mg/dL and 40 mg/dL thresholds were examined) at any time on either BG target protocol, there was no mortality effect between glucose targets in terms of 30-day mortality for patients with diabetes or patients without diabetes. The increased mortality associated with the 90 to 140 mg/dL BG target in patients without diabetes persisted in patients who were never hypoglycemic (OR, 1.61; 95% CI, 1.09-2.38; P = 0.02).
Discussion
We analyzed a large patient cohort experience that compares two BG target ranges with an identical electronic insulin titration protocol with documented high clinician compliance. We demonstrated that a BG target of 90 to 140 mg/dL (target mean BG of 115 mg/dL), although independently associated with decreased risk of mortality in patients with diabetes, is independently associated with greater risk of mortality in patients without diabetes when compared with an 80 to 110 mg/dL BG target (target mean BG of 95 mg/dL). These findings persisted after adjusting for age, sex, disease severity, and comorbidities. Our results raise questions about the relationship between hypoglycemia, BG target, diabetic status, and mortality.
This study supports several other studies that demonstrate that patients with diabetes have different glucose metrics and different clinical outcomes than patients without diabetes when managed by intensive insulin therapy.12‐14,28,29 We cannot definitively explain the reason that mortality differs between patients with diabetes and patients without diabetes on eProtocol-insulin. We believe diabetic adaptation to chronic hyperglycemia and increased glycemic variation may provide a rationale for this finding. Glycemic variation correlates with mortality in critically ill patients.11,15‐17 This correlation is exceptionally strong in euglycemic patients without diabetes.30 Patients with diabetes have chronic glycemic dysregulation. In our study, patients with diabetes had higher mean BG levels and higher standard deviations of BG than did patients without diabetes (Table 3). We speculate that the patient with established diabetes may develop a relative tolerance to the complications of hyperglycemia and increased glycemic variability. The GLUT4 transporter, a signaling molecule that can affect myocardial function, is upregulated with exogenous insulin administration and downregulated with chronic hyperglycemia.31,32 Perhaps the moderate glucose target better approximates the diabetic patient’s chronic glycemic dysregulation than does the 80 to 110 mg/dL. In contrast, hyperglycemia appears to have a greater impact on mortality in critically ill patients without diabetes than in patients with diabetes.11,13,14,28,30 Perhaps a person with diabetes may experience deleterious inflammatory effects of hyperglycemia when managed with a moderate BG target. This notion is supported by animal studies.33,34
Table 3.
—Patient Characteristics of Patients With Diabetes vs Patients Without Diabetes
| Characteristic | Nondiabetic (n = 2,143) | Diabetic (n = 1,386) | P Value |
| Age, y | 63 (50-74) | 67 (58-76) | < .01 |
| Female, % | 38.0 | 45.2 | < .01 |
| APACHE II score | 24 (19-30) | 24 (19-30) | .32 |
| Charlson comorbidity score | 1 (0-3) | 5 (3-8) | < .01 |
| Patient mean blood glucose, mg/dL | 124 (113-134) | 132 (120-147) | < .01 |
| Patient SD of glucose, mg/dL | 29 (23-39) | 41 (30-55) | < .01 |
| 30-d mortality, % | 10.2 | 10.8 | .65 |
All continuous or ordinal data are median values followed by interquartile ranges. Significance testing calculated with χ2 with Yates correction, or Wilcoxon-Mann-Whitney U test, where appropriate. See Table 1 legend for expansion of abbreviation.
Strengths of this study include its generalizability, given the large sample size and heterogenous population drawn from community and referral hospitals, from medical and surgical ICUs, and from private and academic hospitals. The use of a well-validated protocol with identical implementation in both glucose targets is a significant strength of the study. To our knowledge, this is the only study to date comparing two different BG targets using an otherwise identical protocol. Additionally, all patients analyzed were treated with insulin for glycemic control. This retrospective study has several limitations: Physicians were not required to use eProtocol-insulin, which raises the possibility of selection bias, in that the patients on eProtocol-insulin may be substantially different than those who were not managed on it. We excluded a large number of patients. Some patients were excluded because they had diabetic ketoacidosis or were on the protocol for a rather short period of time (approximately 10 h or less). Some patients were excluded because an APACHE II score was not able to be determined from the clinical data at the time of protocol initiation. This exclusion may bias the study toward patients who have greater severity of illness. Another limitation of the study is that the method of obtaining BG measurement incorporated glucometers, with their known analytic inaccuracies,35 although the glucometers were calibrated daily according to industry standards. Last, although our data do not demonstrate a change in mortality over the study period, our model is unable to account for changes in clinical practice that occurred during the 4-year study period that might have effects on patient mortality.
Although NICE-SUGAR was a large randomized controlled trial, its results are perhaps more divisive than decisive. Multiple criticisms have been raised, including its analysis of patients initially treated with intensive insulin therapy who switched to conventional therapy and the lack of differences in other clinical outcomes (length of stay, organ dysfunction).36 Although NICE-SUGAR’s Web-based insulin dose calculation was standardized across 42 centers, there were very high rates of clinician error (failure to follow protocol) contributing to hypoglycemia.10 Additionally, glucose measures were taken at 1- to 4-h intervals with no reminder system to reinforce timely glucose measurement. BG measurements were performed with point-of-care glucometers, compared with the first Leuven study, which used blood gas analyzers. NICE-SUGAR compared two separate protocols, each with separate instructions for insulin adjustments and glucose checks. Only 25% of patients in the conventional arm received insulin. The insulin delivery protocols were not explicit and also offered optional instructions for the clinicians to administer 1 to 2 units of insulin as a bolus dose. The presence of optional instructions and the open-label nature of the trial make it impossible to eliminate interclinician variability. Consequently, study outcomes from NICE-SUGAR may arise more from idiosyncrasies of protocol performance or clinician compliance than from comparing glucose targets.
The eProtocol-insulin avoids many of the aforementioned problems. It is an electronic protocol with excellent compliance and reproducibility.21,22 As eProtocol-insulin was well established in the Intermountain Healthcare ICUs at the time of the study, it is unlikely that the decrease in mortality is attributable to the Hawthorne effect. This study comparing glucose targets is unique, in that the only difference between groups, regarding application of the protocol, is the selection of target BG. All other rules for insulin titration remain unchanged, including rules for BG checks. Unlike other studies, which compare two different insulin protocols,1,4,6,7 this study compares two different glucose targets in an otherwise identical protocol.
The interaction between hypoglycemia and mortality may be more complex than it first appears. Our study confirms previous investigations that demonstrated that hypoglycemia was independently associated with increased risk of mortality in critically ill patients.37‐39 However, it is unknown whether hypoglycemia is the result of the intensive insulin protocol, the result of physiologic stress during critical illness, or, more likely, an interaction of the two. In our study, we observed that duration of time a patient spent in the ICU was independently associated with incidence of hypoglycemia. Some authors have suggested that hypoglycemia may be a marker of disease severity rather than an independent predictor.39 In a cohort of cardiac patients, spontaneous hypoglycemia was predictive of mortality, whereas iatrogenic hypoglycemia was not.38 In NICE-SUGAR, the hazard ratio for mortality was significantly greater in patients who had hypoglycemia and were not being treated with insulin compared with those who had hypoglycemia and were receiving insulin.40 Although glucose dysregulation from physiologic stress is very likely to increase mortality, less is known about the effects of iatrogenic hypoglycemia from an insulin protocol. In our protocol, there is little difference in standard deviation between different glucose targets, so it is reasonable to expect that the group with the lower target glucose will have increased hypoglycemia. However, of those who became hypoglycemic, there was no mortality difference between glucose targets. We speculate that a hypoglycemic patient in the 90 to 140 mg/dL target group may be more likely to have glucose dysregulation from critical illness, whereas a hypoglycemic patient in the 80 to 110 mg/dL group may be more likely to have iatrogenic hypoglycemia. Certainly, the group of hypoglycemic patients in the 90 to 140 mg/dL group had greater disease severity (APACHE II score, 29 vs 26; P < .01) and had a longer ICU length of stay (4.8 vs 2.5 days, P < .01), but it is difficult to make inferences about the reasons for hypoglycemia from the available data.
Another feature often ignored in the conflicting literature of intensive insulin therapy is the duration of time on the study protocol. Patients in the NICE-SUGAR trial were on protocol an average of 4.2 days, whereas those in the Leuven medical ICU trial were on protocol for 12.5 days.4,7 These two studies had different findings, perhaps partially due to differences in the duration of time on protocol. In fact, in the second (medical ICU) Leuven study, van den Berghe et al4 noted that in patients on the protocol < 3 days, mortality was increased on intensive insulin therapy. Our study excluded patients with diabetic ketoacidosis and those on the protocol for a very short time; hence, our study may represent a different patient population than studies that included patients on intensive insulin therapy for a short time.
How should these data from Intermountain be interpreted? Although the ORs are rather large, this study is insufficient to change practice, as our findings may be limited to the idiosyncrasies of eProtocol-insulin. In our study, it appeared that although targeting a moderate glucose reduced hypoglycemia, it did not reduce mortality when compared with a tight glucose target. The higher glucose target was associated with increased mortality risk in patients without diabetes and reduced mortality risk in patients without diabetes. Although we found that the presence of diabetes had very different glucose metrics and mortality risks for each glucose target, there may be other patient characteristics that we failed to incorporate that also may have large effects on glycemic control and clinical outcomes. This study should serve as a reminder that the optimal management of glucose in the ICU remains unknown. The selection of the optimal target BG level remains controversial, with even greater uncertainty regarding other glucose parameters, such as glycemic variability. Multiple studies have returned with often disparate findings. We contend that the reason for conflicting studies is that these studies are products of comparing different protocols, using different rules, applied to different populations (ie, surgical vs medical). Our study is the only one to our knowledge that uses an identical protocol to compare two glucose targets. Future attempts to answer the question of glucose control in the critically ill patient should target specific ICU populations and adhere to a prospective study design that uses an explicit, electronic protocol with documented high clinician compliance.
Conclusions
In comparing selected BG targets in an otherwise identical insulin protocol, there was a large survival benefit in patients without diabetes on tight target glucose compared with moderate target glucose. This finding was reversed in patients with diabetes. Although this finding is provocative and hypothesis generating, further inquiry will require randomized interventional trials comparing target glucose levels, implementing an insulin protocol that performs similarly in both arms.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: Dr Lanspa takes responsibility for the integrity of the work as a whole, from inception to published article.
Dr Lanspa: contributed to study design, data analysis, statistical analysis, and writing the manuscript and read and approved final manuscript.
Dr Hirshberg: contributed to study design and writing the manuscript and read and approved final manuscript.
Mr Phillips: contributed to study design, statistical analysis, and revision of the manuscript for important intellectual content and read and approved final manuscript.
Dr Holmen: contributed to data analysis and revision of the manuscript for important intellectual content and read and approved final manuscript.
Mr Stoddard: contributed to study design, statistical analysis, and revision of the manuscript for important intellectual content and read and approved final manuscript.
Dr Orme: contributed to study design and revision of manuscript for important intellectual content, and read and approved final manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: We thank Rick Carlson, PharmD, for clarification of the eProtocol-insulin equation.
Additional information: The e-Figures can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- APACHE II
Acute Physiology and Chronic Health Evaluation II
- BG
blood glucose
- NICE-SUGAR
Normoglycemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation
Footnotes
Funding/Support: This investigation was partly supported with funding from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health [Grant 8UL1TR000105 (formerly UL1RR025764)].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359-1367. [DOI] [PubMed] [Google Scholar]
- 2.Griesdale DE, de Souza RJ, van Dam RM, et al. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180(8):821-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ingels C, Debaveye Y, Milants I, et al. Strict blood glucose control with insulin during intensive care after cardiac surgery: impact on 4-years survival, dependency on medical care, and quality-of-life. Eur Heart J. 2006;27(22):2716-2724. [DOI] [PubMed] [Google Scholar]
- 4.van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449-461. [DOI] [PubMed] [Google Scholar]
- 5.Preiser JC, Devos P, Ruiz-Santana S, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med. 2009;35(10):1738-1748. [DOI] [PubMed] [Google Scholar]
- 6.Brunkhorst FM, Engel C, Bloos F, et al. ; German Competence Network Sepsis (SepNet). Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125-139. [DOI] [PubMed] [Google Scholar]
- 7.Finfer S, Chittock DR, Su SY, et al. ; NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283-1297. [DOI] [PubMed] [Google Scholar]
- 8.Cefalu WT. Mortality and glycemic targets in the intensive care unit: another paradigm shift?. Diabetes. 2009;58(7):1469-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellomo R, Egi M. What is a NICE-SUGAR for patients in the intensive care unit?. Mayo Clin Proc. 2009;84(5):400-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juneja R, Roudebush CP, Nasraway SA, et al. Computerized intensive insulin dosing can mitigate hypoglycemia and achieve tight glycemic control when glucose measurement is performed frequently and on time. Crit Care. 2009;13(5):R163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krinsley JS, Meyfroidt G, van den Berghe G, Egi M, Bellomo R. The impact of premorbid diabetic status on the relationship between the three domains of glycemic control and mortality in critically ill patients. Curr Opin Clin Nutr Metab Care. 2012;15(2):151-160. [DOI] [PubMed] [Google Scholar]
- 12.van den Berghe G, Wilmer A, Milants I, et al. Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm. Diabetes. 2006;55(11):3151-3159. [DOI] [PubMed] [Google Scholar]
- 13.Krinsley JS. Glycemic control, diabetic status, and mortality in a heterogeneous population of critically ill patients before and during the era of intensive glycemic management: six and one-half years experience at a university-affiliated community hospital. Semin Thorac Cardiovasc Surg. 2006;18(4):317-325. [DOI] [PubMed] [Google Scholar]
- 14.Egi M, Bellomo R, Stachowski E, et al. Blood glucose concentration and outcome of critical illness: the impact of diabetes. Crit Care Med. 2008;36(8):2249-2255. [DOI] [PubMed] [Google Scholar]
- 15.Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med. 2008;36(11):3008-3013. [DOI] [PubMed] [Google Scholar]
- 16.Egi M, Bellomo R, Stachowski E, French CJ, Hart G. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology. 2006;105(2):244-252. [DOI] [PubMed] [Google Scholar]
- 17.Bagshaw SM, Bellomo R, Jacka MJ, Egi M, Hart GK, George C; ANZICS CORE Management Committee. The impact of early hypoglycemia and blood glucose variability on outcome in critical illness. Crit Care. 2009;13(3):R91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hermanides J, Vriesendorp TM, Bosman RJ, Zandstra DF, Hoekstra JB, Devries JH. Glucose variability is associated with intensive care unit mortality. Crit Care Med. 2010;38(3):838-842. [DOI] [PubMed] [Google Scholar]
- 19.Qaseem A, Humphrey LL, Chou R, Snow V, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;154(4):260-267. [DOI] [PubMed] [Google Scholar]
- 20.Moghissi ES, Korytkowski MT, DiNardo M, et al. ; American Association of Clinical Endocrinologists; American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353-369. [DOI] [PubMed] [Google Scholar]
- 21.Morris AH, Orme J, Jr, Truwit JD, et al. A replicable method for blood glucose control in critically Ill patients. Crit Care Med. 2008;36(6):1787-1795. [DOI] [PubMed] [Google Scholar]
- 22.Thompson BT, Orme JF, Zheng H, et al. ; Reengineering Critical Care Clinical Research Investigators. Multicenter validation of a computer-based clinical decision support tool for glucose control in adult and pediatric intensive care units. J Diabetes Sci Tech. 2008;2(3):357-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garber AJ, Moghissi ES, Bransome ED, Jr, et al. ; American College of Endocrinology Task Force on Inpatient Diabetes Metabolic Control. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;10(suppl 2):4-9. [DOI] [PubMed] [Google Scholar]
- 24.Bode BW, Braithwaite SS, Steed RD, Davidson PC. Intravenous insulin infusion therapy: indications, methods, and transition to subcutaneous insulin therapy. Endocr Pract. 2004;10(suppl 2):71-80. [DOI] [PubMed] [Google Scholar]
- 25.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818-829. [PubMed] [Google Scholar]
- 26.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. [DOI] [PubMed] [Google Scholar]
- 27.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245-1251. [DOI] [PubMed] [Google Scholar]
- 28.Rady MY, Johnson DJ, Patel BM, Larson JS, Helmers RA. Influence of individual characteristics on outcome of glycemic control in intensive care unit patients with or without diabetes mellitus. Mayo Clin Proc. 2005;80(12):1558-1567. [DOI] [PubMed] [Google Scholar]
- 29.Meyfroidt G, Keenan DM, Wang X, Wouters PJ, Veldhuis JD, van den Berghe G. Dynamic characteristics of blood glucose time series during the course of critical illness: effects of intensive insulin therapy and relative association with mortality. Crit Care Med. 2010;38(4):1021-1029. [DOI] [PubMed] [Google Scholar]
- 30.Krinsley JS. Glycemic variability and mortality in critically ill patients: the impact of diabetes. J Diabetes Sci Tech. 2009;3(6):1292-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klip A, Tsakiridis T, Marette A, Ortiz PA. Regulation of expression of glucose transporters by glucose: a review of studies in vivo and in cell cultures. FASEB J. 1994;8(1):43-53. [DOI] [PubMed] [Google Scholar]
- 32.Huang JP, Huang SS, Deng JY, Hung LM. Impairment of insulin-stimulated Akt/GLUT4 signaling is associated with cardiac contractile dysfunction and aggravates I/R injury in STZ-diabetic rats. J Biomed Sci. 2009;16:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weekers F, Giulietti AP, Michalaki M, et al. Metabolic, endocrine, and immune effects of stress hyperglycemia in a rabbit model of prolonged critical illness. Endocrinology. 2003;144(12):5329-5338. [DOI] [PubMed] [Google Scholar]
- 34.Ling PR, Mueller C, Smith RJ, Bistrian BR. Hyperglycemia induced by glucose infusion causes hepatic oxidative stress and systemic inflammation, but not STAT3 or MAP kinase activation in liver in rats. Metabolism. 2003;52(7):868-874. [DOI] [PubMed] [Google Scholar]
- 35.Hoedemaekers CW, Klein Gunnewiek JM, Prinsen MA, Willems JL, Van der Hoeven JG. Accuracy of bedside glucose measurement from three glucometers in critically ill patients. Crit Care Med. 2008;36(11):3062-3066. [DOI] [PubMed] [Google Scholar]
- 36.Inzucchi SE, Siegel MD. Glucose control in the ICU—how tight is too tight?. N Engl J Med. 2009;360(13):1346-1349. [DOI] [PubMed] [Google Scholar]
- 37.Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med. 2007;35(10):2262-2267. [DOI] [PubMed] [Google Scholar]
- 38.Kosiborod M, Inzucchi SE, Goyal A, et al. Relationship between spontaneous and iatrogenic hypoglycemia and mortality in patients hospitalized with acute myocardial infarction. JAMA. 2009;301(15):1556-1564. [DOI] [PubMed] [Google Scholar]
- 39.Kagansky N, Levy S, Rimon E, et al. Hypoglycemia as a predictor of mortality in hospitalized elderly patients. Arch Intern Med. 2003;163(15):1825-1829. [DOI] [PubMed] [Google Scholar]
- 40.Finfer S, Liu B, Chittock DR, et al. ; NICE-SUGAR Study Investigators. Hypoglycemia and risk of death in critically ill patients. N Engl J Med. 2012;367(12):1108-1118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement