Abstract
We assessed the contribution of the paraventricular nucleus (PVN) in the heat stress-mediated changes in sympathetic nerve activity and blood flow redistribution from the core to the skin surface. Renal sympathetic nerve activity (RSNA), mean arterial pressure (MAP), heart rate (HR), body and tail temperatures were recorded in anesthetized rats after bilateral microinjection of CSF, lidocaine or L-NMMA into the PVN during heat stress. Heat stress was induced by a graded increase in the temperature of a heating pad for 30 min. Heat stimulus after blockade of the PVN with lidocaine resulted in a blunted RSNA response (ΔRSNA: 117.6 ± 17.0 % vs. 11.3 ± 7.3 %), as well as blunted MAP and HR (ΔMAP: 22 ± 2 mmHg vs. −0.04 ± 7.2 mmHg; ΔHR: 93.4 ± 9.3 bpm vs. 43.4 ± 18.8 bpm). Body temperature threshold for tail vasodilation was unaffected by lidocaine treatment. The increase in RSNA, MAP and HR due to heat stress in L-NMMA-treated rats reached similar levels as CSF treated control rats. However, a higher body temperature threshold for tail vasodilation was observed after L-NMMA injection (37.3 ± 0.1 °C vs. 37.8 ± 0.2°). In conclusion, an intact PVN contributes to an increase in renal sympathetic activity provoked by heat stress, resulting in cardiovascular adjustments that influence core blood redistribution to the periphery. Furthermore, during heat stress, the effect of the PVN on cutaneous vasodilation is dependent on a nitric oxide mechanism.
Keywords: Renal sympathetic nerve activity, nitric oxide, thermoregulation, vasodilation threshold
Introduction
In order to maintain body temperature constant at ~37° C, the balance between heat production and heat loss is accurately adjusted by coordinated autonomic outflow dictated by the central nervous system to produce the appropriate thermoregulatory responses.1 As a result of increases in body temperature, heat loss mechanisms are initiated mainly through modulation of sympathetic activity.2,3 During heat stress, the elevation of body temperature is proportionally followed by an increase in renal sympathetic nerve activity (RSNA), heart rate (HR), mean arterial pressure (MAP), as well as sympathetic inhibition to the cutaneous circulation, resulting in vasodilation in the skin.2,3 These autonomic adjustments are critical for heat dissipation through blood flow redistribution from the body core to the skin surface, as they are a consequence of sympathetically controlled vasoconstriction of the viscera, including the kidneys, and vasodilation of the skin.4,5
The paraventricular nucleus of the hypothalamus (PVN) is a central site for the integration of sympathetic nerve activity,6,7 and is shown to be involved in the control of body temperature.8,9 The PVN contains thermosensitive neurons that are activated during heat stress.10,11 Additionally, there is evidence to suggest that projections arising from the PVN project to other thermoregulatory centers, like the rostral ventrolateral medulla, to influence the sympathetic activity to thermoregulatory effector organs like brown adipose tissue, vasculature of the rat's tail, as well as the kidneys and gut.12,13 It has also been demonstrated that neuronal inhibition of the PVN with the GABAA agonist muscimol prevents the reduction in renal blood flow in response to increased body temperature.4 Such evidence suggests an important regulatory role for the PVN in adjusting renal blood flow in situations of hyperthermia. However, there is no evidence suggesting a contributing role for the PVN in changes in autonomic outflow, such as RSNA and cardiovascular adjustments for heat dissipation through blood flow redistribution from the core to the skin surface in response to heat stress.
Nitric oxide, which is diffusely found in the PVN,14 was implicated to exert thermoregulatory effects characterized by hypothermia.15,16 Acting centrally, nitric oxide plays a tonic role in reducing body temperature due to increased heat loss through cutaneous vasodilation.17 Under thermoneutral conditions, the injection of the nitric oxide synthase inhibitor, L-NMMA within the PVN induces an increase in RSNA, vasoconstriction of the kidneys, as well as increased MAP and HR, while treatment with the nitric oxide donor sodium nitroprusside elicits the opposite responses.18 These results are in agreement with the general idea that nitric oxide decreases sympathetic tone,14,19 and indicate that nitric oxide may be a potential signaling molecule involved in the control of blood flow redistribution that enables heat dissipation during heat stress.
In order to evaluate the involvement of the PVN on the control of sympathetic activity that influences cardiovascular adjustments responsible for blood flow redistribution induced by changes in body temperature, we determined the effect of PVN blockade with lidocaine during heat stress on RSNA, MAP, HR, body temperature and tail temperatures. Modification in blood vessel resistance and, consequently, blood flow are mostly controlled by the sympathetic system. Therefore, RSNA was considered an indicator of sympathetic outflow to the kidneys and a reliable and consistent representation of renal vasomotor tonus and, consequently, renal blood flow. Tail temperature was measured simultaneously as an indirect index of cutaneous heat loss. The magnitude of vasodilation of the tail artery was reflected by skin temperature.20,21 Since nitric oxide is thought to be a signaling molecule in thermoregulatory heat dissipation, as well as sympathoinhibitory,14,19 we also investigated contribution of endogenous nitric oxide in the PVN on autonomic adjustments induced by heat stress.
Materials and Methods
Ethical Approval
All procedures and experimental protocols used in this study were performed in accordance with approved protocol by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center and conformed to the guidelines for the care and use of laboratory animals of the National Institutes of Health and the American Physiological Society.
Animals
Eighteen male Sprague-Dawley rats (290–320 g body wt; Sasco Breeding Laboratories, Omaha, NE, USA) were allowed to acclimate to our animal care facility for 1 wk before use. On the day of the experiment, each rat was anesthetized with urethane (0.75 g/kg ip) and α-chloralose (70 mg/kg ip), and the left femoral vein was cannulated with polyethylene (PE-50) tubing for injection of supplemental anesthesia, as required. Adequacy of anesthesia was monitored throughout the experimental procedures by absence of a withdrawal reflex, as well as by absence of a corneal reflex. The left femoral artery was also cannulated and connected to a computer-driven data-recording and -analyzing system (MacLab; AD Instruments, Mountainview, CA, USA) via a pressure transducer (model P231D; Gould) for recording of arterial blood pressure and HR. The trachea was intubated to facilitate spontaneous ventilation.
Placement of Microinjection Cannula in the PVN
The anesthetized rat was placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). A longitudinal incision was made on the head and the bregma was exposed. The coordinates for the PVN (1.5 mm posterior to bregma, 0.4 mm lateral to the midline, and 7.8 mm ventral to the dura) were determined from the atlas of Paxinos & Watson (1986).22 A small burr hole was made in the skull. For the microinjections, a thin needle (0.5 mm OD, 0.1 mm ID) connected to a microsyringe (0.5 μL; model 7000.5; Hamilton, Reno, NV, USA) was lowered into the PVN bilaterally.
Recording of Renal Sympathetic Nerve Activity
The left kidney was exposed through a retroperitoneal flank incision. A branch of the renal nerve was isolated from the fat and connective tissue. The nerve was placed on a pair of thin bipolar platinum electrodes. The nerve-electrode junction was insulated electrically from the surrounding tissue with silicone gel (Sil-Gel 604 AB; Wacker). The electrical signal was amplified (10,000 times) with a Grass amplifier (model P55) with high- and low-frequency cutoff of 1,000 and 100 Hz, respectively. The output signal from the amplifier was rectified and integrated (20-ms time constant) and stored for later analysis using a computer-run data acquisition system (MacLab). The signal recorded at the end of the experiment (after the rat was killed with anesthesia overdose) was considered background noise. Nerve discharge was calculated by subtraction of the background noise from the actual recorded value. Basal nerve discharge was defined by subtraction of the background noise from the actual nerve discharge before administration of drugs into the PVN. The responses of RSNA to heat stress during the experiment were subsequently expressed as percent change from baseline.
Recording of Colonic Temperature and Tail Skin Temperature
Colonic temperature was used as body temperature and was measured using a thermistor probe (model 401, Yellow Springs Instruments, Yellow Springs, OH, USA). The thermistor probe was inserted 4 cm past the anal sphincter after fecal pellets had been removed from the colon by gentle external massage. Tail temperature was measured using a probe (series 409-B, Yellow Springs Instruments, Yellow Springs, OH, USA) taped to the dorsal surface of the skin, 10 mm from the base of the tail. The tail skin temperature provides reliable measurement of heat transferred by the vasodilating blood vessels to the surface. The parameters mentioned above were used to determine body temperature threshold for tail skin vasodilation, i.e., the body temperature at the moment at which tail temperature begun to increase.20,23
Experimental Protocol
After the surgical procedures, the animals were allowed to stabilize for at least 30 min before being submitted to the experimental protocol. RSNA, MAP, HR, body and tail temperatures of the rats were recorded continuously during heat stress. Heat stress was produced in rats by increasing the temperature of a heating pad (Model 3PN 1010BV, Staco Energy Products Company, Dayton, OH, USA) from 37 to 43°C at a rate of 1.2°C every 6 minutes in an interval of 30 minutes. Heat stress was applied to the ventral side of the animal. The rats were randomly assigned to groups receiving bilateral microinjection into the PVN of artificial cerebroespinal fluid (CSF; 100 nL/side; control group), lidocaine (1%; 100 nL/side) or L-NMMA (200 pmol, 100nL/side) immediately before the beginning of heat stress (n=6/group). Each rat received only one of these drugs. Throughout the experimental procedure, the rat's tail was maintained away from the surface of the heating pad.
Brain Histology
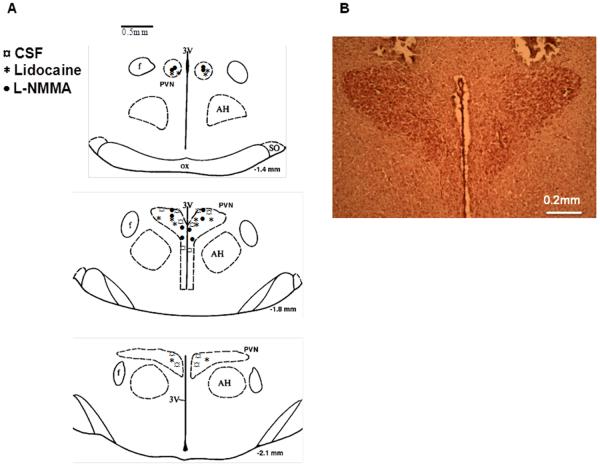
After the experiment, the rats were killed and the brains removed and fixed in 10% formalin for at least 24 h. The brains were then frozen, and serial transverse sections (30 μm) were cut with a cryostat (−18°C). The sections were mounted on microscope slides and stained using 1% neutral red. The location of the injection within the PVN was verified under a microscope with X 40 magnification (Figure 1). The microinjections that terminated in the boundaries of the PVN were considered effective. The 100 nL injection volumes targeting the PVN would be expected to distribute the drug in or within < 0.5 mm from the rostrocaudal and mediolateral boundaries of the PVN. A total of 6 rats were excluded from the study due to misplacement of the injections (i.e. outside the boundaries of the PVN).
Figure 1.
A: Schematic transverse sections of the rat hypothalamic PVN showing the center of the microinjection sites. B: representative photomicrograph of an injection into the PVN of a rat.
Data Analysis
Responses of RSNA to heat stress are expressed as percent change from baseline. Responses of MAP, HR, body and tail temperatures are expressed as the difference between the basal value and the value during heat stress. These data are reported as mean ± S.E.M. and were subjected to a two-way ANOVA, followed by the Newman–Keuls test. The body temperature threshold for tail skin vasodilation was compared using unpaired Student's t-test. Significance level was set at p<0.05.
RESULTS
Table 1 shows that after microinjection of CSF, lidocaine or L-NMMA bilaterally within the PVN of anesthetized rats during 30 min without heat stress, body and tail temperatures remained stable in all experimental groups.
Table 1.
Effect of bilateral injection within the PVN of CSF, lidocaine or L-NMMA on body (Tb) and tail (Ttail) temperatures over time, without changes in temperature in anesthetized rats.
| CSF (n=4) |
Lindocaine (n=3) |
L-NMMA (n=3) |
||||
|---|---|---|---|---|---|---|
| Tb (°C) | Ttail (°C) | Tb (°C) | Ttail (°C) | Tb (°C) | Ttail (°C) | |
| Baseline | 37.0 ± 0.1 | 28.9 ± 0.6 | 37.1 ± 0.0 | 28.2 ± 0.7 | 36.8 ± 0.1 | 28.4 ± 0.6 |
| 6 min | 36.9 ± 0.1 | 23.9 ± 0.6 | 37.2 ± 0.1 | 27.9 ± 0.7 | 36.9 ± 0.1 | 28.2 ± 0.5 |
| 12 min | 36.9 ± 0.1 | 29.0 ± 0.6 | 37.2 ± 0.1 | 27.7 ± 0.5 | 36.8 ± 0.1 | 28.1 ± 0.5 |
| 18 min | 36.9 ± 0.1 | 29.1 ± 0.6 | 37.2 ± 0.1 | 27.3 ± 0.5 | 36.9 ± 0.1 | 28.3 ± 0.6 |
| 24 min | 36.9 ± 0.1 | 29.2 ± 0.6 | 37.2 ± 0.0 | 27.8 ± 0.4 | 36.9 ± 0.1 | 28.1 ± 0.5 |
| 30 min | 37.0 ± 0.1 | 29.3 ± 0.6 | 37.2 ± 0.0 | 27.9 ± 0.5 | 36.9 ± 0.1 | 28.2 ± 0.6 |
Values are expressed as mean ± SEM.
Heat stress induced an increase in RSNA response after PVN injection of CSF, starting at 6 min (Δ1.2°C) and sustained until the end of the stimulus (Figure 2 and 3A) (p<0.05). As seen in Figure 3A, lidocaine injection resulted in blunted RSNA response throughout heat stress. Such response was significantly lower than CSF group from 12 min (Δ2.4°C) (34.9 ± 4.8 % vs. 10.5 ± 6.3 %, p<0.05) of heat stress until the end of the protocol. L-NMMA injection resulted in increased RSNA response, observed from 12 min (Δ2.4°C) and maintained high until the end of the stimulus (Figure 3A) (p<0.05). There was no significant difference in RSNA response to heat stress between CSF and L-NMMA groups. On the other hand, a significant difference in RSNA response was observed between L-NMMA and lidocaine groups from 24 min (Δ4.8°C) of heat stress (75.1 ± 10.2% L-NMMA vs. 7.2 ± 4.4% lidocaine, p<0.05). The greatest difference between the CSF or L-NMMA groups and lidocaine occurred at the end of heat stress (117.6 ± 17.0 % CSF or 105.4 ± 12.6 % L-NMMA vs. 11.3 ± 7.3 % lidocaine, p<0.05).
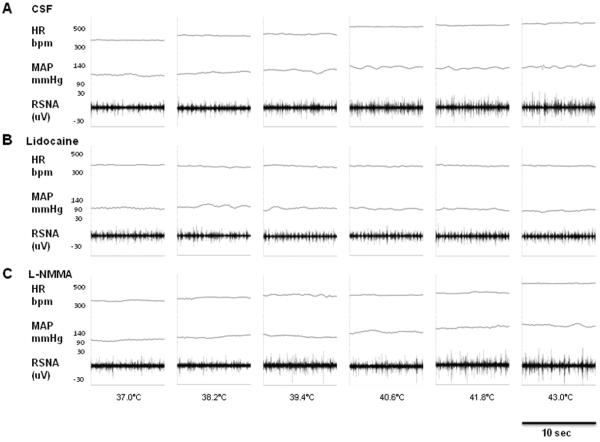
Figure 2.
Segments of original recordings from individual rats demonstrating the effect of heat stress on changes in heart rate (HR), mean arterial pressure (MAP) and renal sympathetic nerve activity (RSNA) after bilateral PVN injection of CSF (A), lidocaine (B) and L-NMMA (C).
Figure 3.
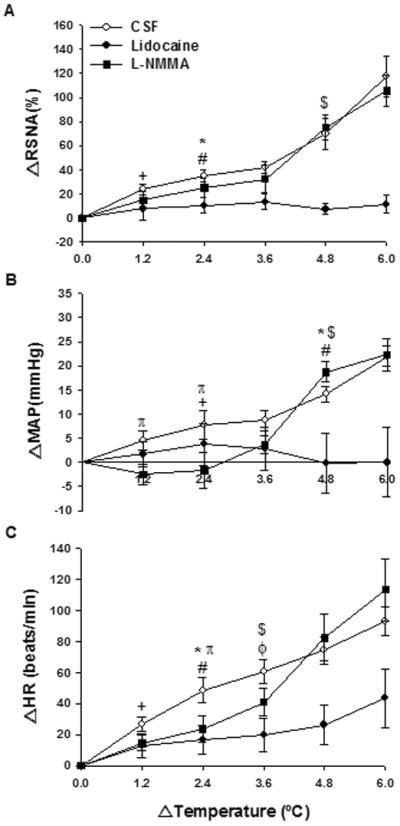
Effect of heat stress over time on changes in renal sympathetic nerve activity (ΔRSNA; A), mean arterial pressure variation (ΔMAP; B) and heart rate variation (ΔHR; C) after bilateral PVN injection of CSF, lidocaine or L-NMMA. Values expressed as mean ± SEM. n = 6/group. Significantly different from basal value until the end of heat stress: + CSF; ϕ lidocaine; # L-NMMA. Significant difference between groups until the end of heat stress: * CSF X lidocaine, $ L-NMMA X lidocaine. π Significant difference between CSF and L-NMMA groups, p<0.05.
MAP, following PVN injection of CSF, increased after 12 min (Δ2.4°C) of heat stress (Figure 2 and 3B) (p<0.05). Again, lidocaine injection resulted in blunted MAP response throughout heat stress (Figure 3B). MAP was only significantly different between CSF and lidocaine groups after 24 min (Δ4.8°C) (14.9 ± 1.6 mmHg CSF vs. −0.2 ± 1.6 mmHg lidocaine, p<0.05) until the end of heat stress. MAP responses in the L-NMMA group reached values similar to those in the CSF group. However, this increase in MAP was slower as seen by the fact that such response was significantly lower in this group in comparison with CSF group between 6 min (Δ1.2°C) (4.5 ± 2.0 mmHg CSF vs. −2.5 ± 2.1 mmHg L-NMMA, p<0.05) and 12 min (Δ2.4°C) (7.8 ± 3.0 mmHg CSF vs. −1.6 ± 3.7 mmHg L-NMMA, p<0.05) of heat stress (Figure 3B). Moreover, in L-NMMA rats, MAP was only significantly greater than baseline value after 24 min (Δ4.8°C) of heat stress (Figure 3B) (p<0.05). The MAP response to L-NMMA was greater than the response to lidocaine after 24 min (Δ4.8°C) (18.8± 2.1 mmHg L-NMMA vs. −0.2 ± 1.6 mmHg lidocaine, p<0.05) until the end of heat stress.
HR values increased progressively with elevations in temperature in all groups of animals (Figure 2 and 3C). HR was already elevated after 6 min (Δ1.2°C) of heat stress in the CSF group (Figure 3C) (p<0.05). Although lidocaine injection induced an increase in HR after body heating for 18 min (Δ3.6 °C) until the end of heat stress (p<0.05), this increase was blunted compared to the CSF group after 12 min (Δ2.4°C) (48.5 ± 8.2 beats/min CSF vs. 16.5 ± 9.4 beats/min lidocaine, p<0.05) until the end of heat stress. Figure 3C shows that L-NMMA injection resulted in elevated HR values after 12 min (Δ2.4°C) of heat stress until the end of the stimulus (p<0.05). HR responses in the L-NMMA group were attenuated compared to CSF only at 12 min (Δ2.4°C) (48.5 ± 8.2 beats/min CSF vs. 23.6 ± 8.8 beats/min L-NMMA, p<0.05) (Figure 3C). Moreover, a significant difference in HR response was observed between L-NMMA and lidocaine groups from 18 min (Δ3.6 °C) of heat stress (40.9 ± 9.1 beats/min L-NMMA vs. 19.7 ± 10.7 beats/min lidocaine, p<0.05) (Figure 3C).
The effect of heat stress on body temperature is shown in Figure 4A. Heat stress induced a rapid increase in body temperature in all groups, which was observed 9 min after injection of CSF within the PVN (Figure 4A) (p<0.05). After 8 min of heat stress, body temperature was higher than baseline values in the lidocaine group (Figure 4A) (p<0.05). Similar to the response observed with CSF injection, L-NMMA induced an increase in body temperature after 9 min until the end of heat stress (Figure 4A). There were no significant differences in body temperature between the CSF and lidocaine or L-NMMA groups, as well as between the L-NMMA and lidocaine groups, throughout heat stress (Figure 4A).
Figure 4.
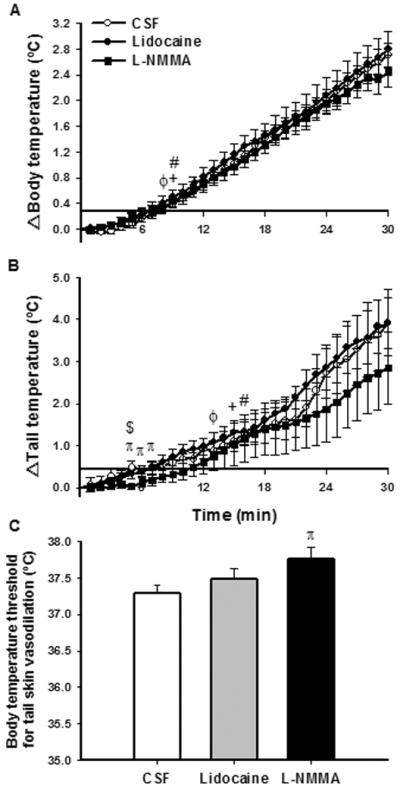
Effect of heat stress over time on changes in body temperature (A) and skin tail temperature (B) and body temperature threshold for tail skin vasodilation (C) after bilateral PVN injection of CSF, lidocaine or L-NMMA. Values expressed as mean ± SEM. n = 6/group. Significantly different from basal value until the end of heat stress: + CSF; ϕ lidocaine; # L-NMMA. Significant difference between groups: π CSF X L-NMMA; $ L-NMMA X lidocaine, p<0.05.
As illustrated in Figure 4B, tail temperature increased within 15, 13 and 16 min of heat stress in CSF, lidocaine and L-NMMA groups, respectively, indicating that heat-loss mechanisms had been activated. The increase in tail temperature remained similar between the CSF and lidocaine groups (Figure 4B). Although tail temperature responses in L-NMMA treated rats increased in a similar fashion as the CSF group, between 5 min (0.48 ± 0.16 °C CSF vs. 0.03 ± 0.06 °C LNMMA, p<0.05) and 7 min (0.51 ± 0.10 °C CSF vs. 0.18 ± 0.13 °C L-NMMA, p<0.05) of heat stress, tail temperature of these rats was significantly lower (Figure 4B). The only difference in tail temperature value between L-NMMA and lidocaine groups was seen at 5 min (0.03 ± 0.06 °C L-NMMA vs. 0.32 ± 0.09 °C lidocaine, p<0.05). To assess whether cutaneous heat loss mechanism was affected by PVN injection of the drugs, the body temperature at the moment of tail vasodilation (threshold for tail vasodilation) was determined (Figure 4C). Body temperature threshold for tail vasodilation was not different between rats that received CSF and lidocaine injections within the PVN. However, results showed that the value was 0.5°C higher in L-NMMA injected rats compared with CSF injected animals (37.3 ± 0.1°C CSF vs. 37.8 ± 0.2°C L-NMMA, p<0.03) (Figure 4C). There were no differences in body temperature until tail vasodilation between L-NMMA and lidocaine rats (Figure 4C).
DISCUSSION
In the present study, blockade of the PVN with lidocaine resulted in blunted RSNA, MAP and HR responses to heat stress, suggesting a contributing role for the PVN in autonomic responses to heat stress. However, such blockade of the PVN did not interfere with body temperature threshold for tail vasodilation. On the other hand, blockade of nitric oxide synthase within the PVN, led to higher body temperature threshold for tail vasodilation, without affecting the increase in RSNA, MAP and HR. Taken together, these data indicate that the PVN contributes to the responsiveness of sympathetic pathways that mediate autonomic adaptations related with body temperature control during heat stress, specifically by modulating RSNA that enables renal blood flow redistribution. This effect is not mediated by a nitric oxide mechanism. However, heat loss through skin vasodilation, depends on a nitric oxide mechanism within the PVN.
Regulation of sympathetic outflow is necessary to facilitate heat dissipation in response to heat stress responses and to maintain body temperature within safe limits.1,3 During heat stress, the sympathetic nervous system is capable of altering blood flow to multiple organs by selectively altering the pattern of sympathetic outflow, non-uniformly and independently, according to specific vascular beds.2,3 It is well known that redistribution of blood flow from the viscera to the cutaneous vasculature is the major cardiovascular response for restoration of thermal homeostasis after an elevation in body temperature. This is a consequence of sympathetically-controlled vasoconstriction of the viscera and vasodilation of the skin.3,4 During heat stress, up to 60% of cardiac output is redistributed to the surface of the skin to improve heat loss, this effect being primarily dependent on decreased vascular resistance to the blood vessels in the skin.24,25 Such vasodilation in the vasculature of the skin is initiated when the body temperature threshold for skin vasodilation is reached, corresponds to the moment when sympathetic outflow is overcome by active vasodilation, leading to increased heat dissipation.20,21
It has been shown that the PVN influences sympathetic outflow that controls cardiovascular responses elicited by heat stress.4,9 These studies proposed that efferent projections from the PVN could influence sympathetic tone via connections with spinally projecting neurons, as well as with the rostral ventrolateral medulla. 26,27 This region contains populations of pre-motor neurons influencing sympathetic activation, including to the kidneys and the skin. 26,27 In context of this background, in the present study, lidocaine was bilaterally injected to block traffic, both neuronally generated and that carried by fibers of passage in the PVN during heat stress. This design was expected to elucidate the contribution of the PVN in processing thermal adjustments. Blockade of the PVN blunted RSNA response to heat stress, consistent with decreased renal blood flow. Although the blood flow to other viscera, such as the gut, liver etc, are also important for blood redistribution to the skin during heat stress, the current findings demonstrate one visceral bed, the kidney is affected. Therefore, renal vasoconstriction during heat stress could be induced by the PVN directly through spinally projecting neurons and indirectly through the rostral ventrolateral medulla. The fact that injection of L-NMMA within the PVN did not affect RSNA response suggests that these effects may not be mediated by a nitrergic mechanism. However, the overall consensus is that nitric oxide acts as a sympathoinhibitory substance within the PVN.14 It may well be that blocking an inhibitory system may not be involved in the excitatory response observed in the RSNA during heat stress. It is of interest to note that blockade of the nitric oxide system was able to block the heat loss through skin vasodilation, an inhibitory mechanism.
The same pattern of response observed in RSNA was observed with MAP and HR responses after lidocaine and L-NMMA treatment. In rats treated with lidocaine, heat stress also resulted in blunted MAP and HR responses. In contrast, after injection of L-NMMA within the PVN, HR remained unaltered. The increase in MAP was delayed after L-NMMA injection, but still reached the values observed in control rats. Hyperthermia is usually followed by increased MAP and HR responses. This hemodynamic characteristic is the result of sympathetic activation, which increases cardiac output, due to enhanced HR, in combination with a greater redistribution of blood flow from visceral vasculature beds 25,28 In fact, during heat stress, the maintenance of blood pressure depends particularly on vasoconstriction because a large percentage of the cardiac output is redistributed to the periphery. 25. The decreased MAP responses in lidocaine-treated rats may be a consequence of the lower sympathetic activation, which not only decreased artery resistance of the kidneys, but also of other vascular beds. Thus, it is reasonable to suggest that the PVN blockade with lidocaine results in attenuation of sympathetic nervous system activation to heat stress and an inability to elicit appropriate cardiovascular adjustments.
In the case of rodents, tail skin vasodilation is the primary route of heat loss from the body, being responsible for the dissipation of an equivalent of 25% of resting heat production. 29,30 Heat stress-induced skin vasodilation is a consequence of tail sympathetic activity withdrawal, which is primarily activated by body heating stimulation over heat loss thermoregulatory centers. 31 Most of the thermoregulatory studies suggest that brain nitric oxide enhances heat dissipation by diminishing such sympathetic outflow to cutaneous vascular beds.15,16,17 The present data demonstrated that L-NMMA injection within the PVN attenuated the increase in tail temperature during heat stress. This probably resulted in an elevated body temperature threshold for tail vasodilation as exhibited by L-NMMA rats, i.e., tail skin vasodilation was induced at a higher body temperature than control animals, delaying heat dissipation. Taken together, these data suggest that the PVN may contribute to inhibition of sympathetic outflow to the skin, especially in situations of high demand such as heat stress, since no effect was seen in tail temperature of rats treated with LNMMA within the PVN under thermoneutral condition. Based on evidences that the rostral ventrolateral medulla contains neurons controlling cutaneous vasomotion whose activity are tonically modulated by GABAergic inhibition,26 we propose that the PVN sends efferent pathways to this site, modulating cutaneous vasodilation with GABA. 32 Utilizing these routes, it is possible that increased nitrergic transmission within the PVN during heating may facilitate the sympathoinibition to the skin and increased heat dissipation by activating the GABA system to the rostral ventrolatral medulla. 26 This is supported by evidence showing that heating significantly increases the number of activated nitrergic PVN neurons. 27 As changes in absolute values of body and tail temperatures did not differ between L-NMMA and CSF groups, and the PVN and nitric oxide are involved in the regulation of metabolic rate, 17,33 we cannot exclude the possibility that the PVN microinjection of L-NMMA may have also resulted in metabolic rate adjustments, affecting the rate of heat production. This issue needs to be examined further for more conclusive results. It should be pointed out that after blockade of the PVN with lidocaine during heat stress, the thermal reflex for tail vasodilation was still present. At least under the conditions of our experiments, such heat defense response was probably stimulated by direct reflex inhibition of nucleus controlling cutaneous vasomotion such as the rostral ventrolateral medulla. 1,9,31
When analyzing the current results, we cannot disregard that the possible interference of the anesthesia on the data, even though this method is widely used in the study of the sympathetic activity and cardiovascular adjustments during heat stress. 2,34 It is possible that anesthesia may influence sympathetic nerve discharge responses and, consequently, thermoregulatory effectors during heat stress. Although this cannot be disregarded, the fact that heat stress results in increased sympathetic discharge in anesthetized rats suggest that anesthesia has only a slight affect if any on sympathetic activity during heat stress. 2,34 Additionally, it is recognized that anesthesia eliminates behavioral modifications that could also alter sympathetic nerve discharge during heat stress.
In summary, our results show that the PVN participates in increasing renal sympathetic activity in response to heat stress, contributing to cardiovascular adjustments that influence core blood flow redistribution from the visceral organs like the kidneys to the periphery. Furthermore, the PVN modulates heat loss through cutaneous vasodilation induced by body heating through nitric oxide mechanisms.
ACKNOWLEDGMENTS
The technical assistance of Lirong Xu and Xuefei Liu is greatly appreciated. This work was supported by National Institutes of Health Grant HL6222 and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Footnotes
AUTHOR CONTRIBUTIONS All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript; LHRL conducted the experiments and wrote the manuscript with inputs from HZ, CCC and KPP.
REFERENCES
- 1.Webb P. The physiology of heat regulation. Am J Physiol. 1995;268:R838–50. doi: 10.1152/ajpregu.1995.268.4.R838. [DOI] [PubMed] [Google Scholar]
- 2.Gisolfi CV, Matthes RD, Kregel KC, Oppliger R. Splanchnic sympathetic nerve activity and circulating catecholamines in the hyperthermic rat. J Appl Physiol. 1991;70:1821–6. doi: 10.1152/jappl.1991.70.4.1821. [DOI] [PubMed] [Google Scholar]
- 3.Morrison SF. Differential regulation of sympathetic outflows to vasoconstrictor and thermoregulatory effectors. Ann N Y Acad Sci. 2001;940:286–98. doi: 10.1111/j.1749-6632.2001.tb03684.x. [DOI] [PubMed] [Google Scholar]
- 4.Cham JL, Badoer E. Hypothalamic paraventricular nucleus is critical for renal vasoconstriction elicited by elevations in body temperature. Am J Physiol Renal Physiol. 2008;294:F309–15. doi: 10.1152/ajprenal.00488.2007. [DOI] [PubMed] [Google Scholar]
- 5.Kanosue K, Yanase-Fujiwara M, Hosono T. Hypothalamic network for thermoregulatory vasomotor control. Am J Physiol. 1994;267:R283–8. doi: 10.1152/ajpregu.1994.267.1.R283. [DOI] [PubMed] [Google Scholar]
- 6.Li YF, Patel KP. Paraventricular nucleus of the hypothalamus and elevated sympathetic activity in heart failure: the altered inhibitory mechanisms. Acta Physiol Scand. 2003;177:17–26. doi: 10.1046/j.1365-201X.2003.01043.x. [DOI] [PubMed] [Google Scholar]
- 7.Patel KP. Role of paraventricular nucleus in mediating sympathetic outflow in heart failure. Heart Fail Rev. 2000;5:73–86. doi: 10.1023/A:1009850224802. [DOI] [PubMed] [Google Scholar]
- 8.Nagashima K, Nakai S, Tanaka M, Kanosue K. Neuronal circuitries involved in thermoregulation. Auton Neurosci. 2000;85:18–25. doi: 10.1016/S1566-0702(00)00216-2. [DOI] [PubMed] [Google Scholar]
- 9.Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol. 2007;292:R37–46. doi: 10.1152/ajpregu.00668.2006. [DOI] [PubMed] [Google Scholar]
- 10.Bratincsak A, Palkovits M. Activation of brain areas in rat following warm and cold ambient exposure. Neuroscience. 2004;127:385–97. doi: 10.1016/j.neuroscience.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Inenaga K, Osaka T, Yamashita H. Thermosensitivity of neurons in the paraventricular nucleus of the rat slice preparation. Brain Res. 1987;424:126–32. doi: 10.1016/0006-8993(87)91201-7. [DOI] [PubMed] [Google Scholar]
- 12.Kazuyuki K, Hosono T, Zhang YH, Chen XM. Neuronal networks controlling thermoregulatory effectors. Prog Brain Res. 1998;115:49–62. doi: 10.1016/s0079-6123(08)62029-4. [DOI] [PubMed] [Google Scholar]
- 13.Smith JE, Jansen AS, Gilbey MP, Loewy AD. CNS cell groups projecting to sympathetic outflow of tail artery: neural circuits involved in heat loss in the rat. Brain Res. 1998;786:153–64. doi: 10.1016/s0006-8993(97)01437-6. [DOI] [PubMed] [Google Scholar]
- 14.Patel KP, Li YF, Hirooka Y. Role of nitric oxide in central sympathetic outflow. Exp Biol Med (Maywood) 2001;226:814–24. doi: 10.1177/153537020122600902. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson S, Hjelmqvist H, Keil R, Gerstberger R. Central application of a nitric oxide donor activates heat defense in the rabbit. Brain Res. 1997;774:269–73. doi: 10.1016/s0006-8993(97)81719-2. [DOI] [PubMed] [Google Scholar]
- 16.Osaka T. Hypoxia-induced hypothermia mediated by noradrenaline and nitric oxide in the rostromedial preoptic area. Neuroscience. 2011;179:170–8. doi: 10.1016/j.neuroscience.2011.01.056. [DOI] [PubMed] [Google Scholar]
- 17.Lacerda AC, Marubayashi U, Coimbra CC. Nitric oxide pathway is an important modulator of heat loss in rats during exercise. Brain Res Bull. 2005;67:110–6. doi: 10.1016/j.brainresbull.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Zhang K, Mayhan WG, Patel KP. Nitric oxide within the paraventricular nucleus mediates changes in renal sympathetic nerve activity. Am J Physiol. 1997;273:R864–72. doi: 10.1152/ajpregu.1997.273.3.R864. [DOI] [PubMed] [Google Scholar]
- 19.Simon E. Nitric oxide as a peripheral and central mediator in temperature regulation. Amino Acids. 1998;14:87–93. doi: 10.1007/BF01345248. [DOI] [PubMed] [Google Scholar]
- 20.Horowitz M, Kaspler P, Simon E, Gerstberger R. Heat acclimation and hypohydration: involvement of central angiotensin II receptors in thermoregulation. Am J Physiol. 1999;277:R47–55. doi: 10.1152/ajpregu.1999.277.1.R47. [DOI] [PubMed] [Google Scholar]
- 21.Leite LH, Lacerda AC, Marubayashi U, Coimbra CC. Central angiotensin AT1-receptor blockade affects thermoregulation and running performance in rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R603–7. doi: 10.1152/ajpregu.00038.2006. [DOI] [PubMed] [Google Scholar]
- 22.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Orlando: 1986. [Google Scholar]
- 23.Wanner SP, Guimaraes JB, Rodrigues LO, Marubayashi U, Coimbra CC, Lima NR. Muscarinic cholinoceptors in the ventromedial hypothalamic nucleus facilitate tail heat loss during physical exercise. Brain Res Bull. 2007;73:28–33. doi: 10.1016/j.brainresbull.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc. 2003;78:603–12. doi: 10.4065/78.5.603. [DOI] [PubMed] [Google Scholar]
- 25.Rowell LB. Cardiovascular aspects of human thermoregulation. Circ Res. 1983;52:367–79. doi: 10.1161/01.res.52.4.367. [DOI] [PubMed] [Google Scholar]
- 26.Cerri M, Zamboni G, Tupone D, Dentico D, Luppi M, Martelli D, et al. Cutaneous vasodilation elicited by disinhibition of the caudal portion of the rostral ventromedial medulla of the free-behaving rat. Neuroscience. 2010;165:984–95. doi: 10.1016/j.neuroscience.2009.10.068. [DOI] [PubMed] [Google Scholar]
- 27.Cham JL, Badoer E. Exposure to a hot environment can activate rostral ventrolateral medulla-projecting neurones in the hypothalamic paraventricular nucleus in conscious rats. Exp Physiol. 2008;93:64–74. doi: 10.1113/expphysiol.2007.039560. [DOI] [PubMed] [Google Scholar]
- 28.Kenney WL. Human cardiovascular responses to passive heat stress. J Physiol. 2008;586:3. doi: 10.1113/jphysiol.2007.147215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shellock FG, Rubin SA. Temperature regulation during treadmill exercise in the rat. J Appl Physiol. 1984;57:1872–7. doi: 10.1152/jappl.1984.57.6.1872. [DOI] [PubMed] [Google Scholar]
- 30.Young AA, Dawson NJ. Evidence for on-off control of heat dissipation from the tail of the rat. Can J Physiol Pharmacol. 1982;60:392–8. doi: 10.1139/y82-057. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura K, Morrison SF. A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci U S A. 2010;107:8848–53. doi: 10.1073/pnas.0913358107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang K, Patel KP. Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: role of GABA. Am J Physiol. 1998;275:R728–34. doi: 10.1152/ajpregu.1998.275.3.R728. [DOI] [PubMed] [Google Scholar]
- 33.Lechan RM, Fekete C. The TRH neuron: a hypothalamic integrator of energy metabolism. Prog Brain Res. 2006;153:209–35. doi: 10.1016/S0079-6123(06)53012-2. [DOI] [PubMed] [Google Scholar]
- 34.Kregel KC, Gisolfi CV. Circulatory responses to vasoconstrictor agents during passive heating in the rat. J Appl Physiol. 1990;68:1220–7. doi: 10.1152/jappl.1990.68.3.1220. [DOI] [PubMed] [Google Scholar]