Abstract
The Pur proteins are an ancient family of sequence-specific single-stranded nucleic acid-binding proteins. They bind a G-rich element in either single- or double-stranded nucleic acids and are capable of displacing the complementary C-rich strand. Recently several reports have described Pur family member knockouts, mutations and disease aberrations. Together with a recent crystal structure of Purα, these data reveal conserved structural features of these proteins that have been adapted to serve functions unique to higher eukaryotes. In humans Pur proteins are critical for myeloid cell development, muscle development, and brain development, including trafficking of mRNA to neuronal dendrites. Pur family members have been implicated in diseases as diverse as cancer, premature aging and fragile-X mental retardation syndrome.
Keywords: RNA dendritic transport, DNA replication and repair, PURA, transcription, translation
INTRODUCTION
The Pur family constitutes a unique structural class of nucleic acid-binding proteins, sequences of which are conserved in evolution from bacteria through humans. (6, 7) (40). Purα makes contact primarily with guanine bases, thus binding the G-rich single strand of the PUR element and displacing the complementary C-rich strand (6). Because Purα binds the guanine base, and not sugar or phosphate moieties, it is capable of binding both DNA and RNA (29, 31, 32) and disrupting G base pairing. By doing so Purα induces local DNA strand separation (17, 116). All vertebrate Pur proteins contain three repeats of an approximately 80 amino acid (aa) sequence termed the Pur domain. Bacterial Pur proteins possess one such domain. It was recognized upon sequencing that each Pur domain contains an aromatic-basic section and a leucine-acidic section (7). It is this unique, signature Pur domain that is so strongly conserved and that confers the ability to bind single-stranded nucleic acids. This review will consider all of the human Pur proteins in terms of their known functions. In light of recent structural data, we shall formulate a generalized principle of Pur protein function comprising its dual interaction with nucleic acids and multiple proteins.
Recent elucidation of the crystal structure of a segment of Drosophila Purα helps provide a structural basis for nucleic acid binding by the protein (38, 39). X-ray crystallography has revealed the structure of each Pur domain to be that of a so-called Whirly-like fold with three beta sheets and an alpha helix in the configuration N-βββα-C (39). Wortman and colleagues (116) had earlier demonstrated that mutation of arginine 71 to glutamic acid of mouse Purα (R72 of human Purα) inhibits ssDNA binding and dsDNA strand separation. This R is part of a KR duo that corresponds to RK 102–103 of Pur repeat 2 (Fig. 1A) and KR 283–4 of repeat 3 (Fig. 1B). A basic aa in this duo, preceding aromatic aa’s, is a defining feature of Pur repeats from bacteria through humans. Mutations by Graebsch et al. (39) have added further evidence that the β-sheets and connecting residues of Pur domains are the interaction surfaces for ss-nucleic acid binding. Pur domain II of hPurα (Fig. 1A) contains sequences highly conserved in evolution. These observations strongly implicate the aromatic-basic motif of the β-sheet region of Pur domains in nucleic acid binding. It can be seen in Fig. 1B that the alpha helical region of human Pur repeat 3, containing the Psycho motif and amphipathic helix, is less conserved among repeats than is the beta sheet region. These helical motifs are involved in protein binding, and X-ray crystallography suggests that repeat 3 is specifically involved in external protein-protein interactions (39).
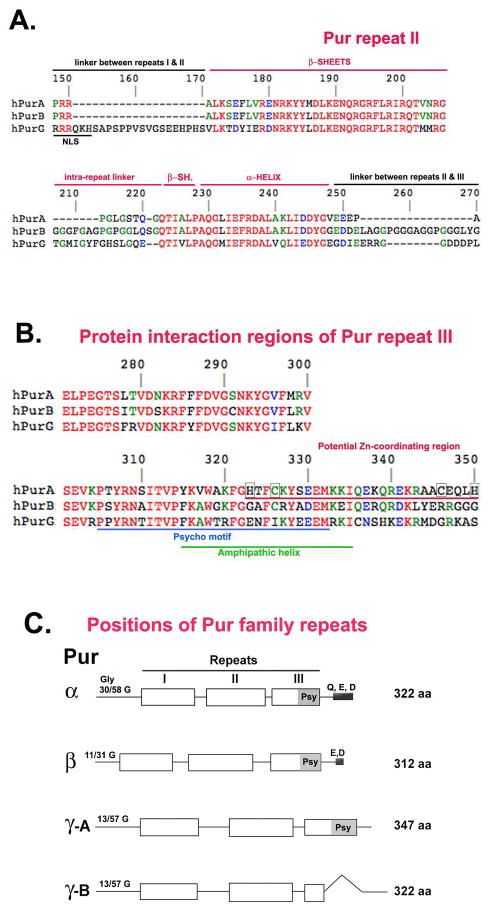
Figure 1. Amino acid repeat structures in the human Pur family.
A. Comparison of aa’s of repeat II, a typical, evolutionarily-conserved Pur repeat among all human Pur family members. Repeat II is identical in Purγ-A and Purγ-B. In panels A and B red letters denote identity, blue denotes strong conservation, and green denotes partial conservation. Lines above the sequence refer to beta sheet and alpha helical portions of the Whirly fold described (39). B. All Pur repeats can bind both nucleic acids and proteins. Repeat III is known to bind to a variety of different regulatory proteins. The amino-terminal portion of the repeat, constituting primarily beta sheets, is involved in nucleic acid binding. Certain C-terminal sequences implicated in protein binding are underlined. C. Positions of the Pur repeats (white boxes) in all four known human Pur family members. The repeat homology used for this drawing extends from R at aa 149 in repeat II (panel A) to V, G or E at aa 248. Drawings in panel C are to scale. Note that in Purγ-B Repeat III is truncated so that only beta sheet sequences remain and the alpha helical portions are eliminated. The N-terminal glycine (C)-rich region and the C-terminal glutamine (Q), glutamic acid (E) and aspartic acid (D) regions are indicated.
All human Pur proteins contain three repeats of a unique and signature-conferring amino acid sequence domain (Fig. 1C). Although these vary in length from 64 to 80 aa, they all contain sequence tracts that are immediately recognizable as Pur repeats from bacteria through humans. The four human protein family members each possess an N-terminal glycine-rich domain. This is most prominent in Purα, in which G comprises more than 50% of the first 58 aa. Purγ possess the least N-terminal G residues, with 13 of the first 57 aa (Fig. 1C). The C-terminus is also a distinguishing feature among Pur family members. Purα has a span of 7 Q residues followed by an acid rich E–D region. Purβ lacks the glutamines but has the N-terminal E–D region. Purγ lacks both of these regions, which have been implicated in binding of Purα to proteins (32, 47, 114). The Psycho motif (Psy, Fig. 1C) is a portion of the alpha helical region of repeat III. It is also present in several other known nucleic acid-binding proteins and has been implicated in binding the tumor suppressor, Rb (74). Note that Psy is absent in Purγ-B, in which repeat III has been truncated (Fig. 1C).
The prototypical Pur family member, Purα, has been best studied in relation to its roles in the initiation of DNA replication, cell cycle regulation, and in the regulation of transcription and mRNA translation. Indeed, Purα has been firmly established as a regulator of transcription for several cellular promoters through its direct interaction with viral promoters and with many transcription factors, both cellular and viral. Therefore, this review will not focus on these aspects. See Supplemental Table 1 and references therein as well as previous review articles (32, 47, 114). The Purα crystalized structure elucidates how Pur family members may interact with both their partner protein(s) along with target nucleic acids. The essential nature of Purα function in normal development has been highlighted by knockout of the PURA gene in mice and by mutations of the gene in human disease. These aspects will be reviewed here with special emphasis on those functions impaired in disease and defective development.
GENETIC INVOLVEMENT OF PUR FAMILY MEMBERS IN HUMAN DISEASE
PUR genes in neoplasia
There are four known members of the Pur protein family in humans, encoded by genes at three chromosome loci. The proteins are Purα, encoded at chromosome band 5q31 (75), Purβ, at 7p13 (65), and two isoforms of Purγ, at 8p11 (71). The respective genes are termed PURA, PURB, and PURG. In this review protein names are not italicized, and the first letter is capitalized. Gene names are all upper case letters and italicized. In the case of Purγ, the two isoforms, Purγ-A and Purγ-B, are generated by differences in termination of transcription of PURG resulting in a splice variant, Purγ-B, that has a distinct C-terminus (71). Aberrations in all three of the PUR gene loci are associated with disease, although evidence for involvement of the encoded proteins varies. At this time evidence is strongest for necessity of PURA expression in a developmentally regulated fashion, with aberrations leading to several defects, including neoplasias. The strength of this evidence is due primarily to statistical association of the PURA gene with human disease states and concurrence with genetic inactivation studies in mice.
All three PUR gene loci have been implicated in genetic aberrations related to multiple neoplasias. Chromosome band 5q31, the location of PURA, is one of the most frequently deleted chromosome regions in malignant myeloid disorders (63). Although the role of Purα in myeloid development will be discussed separately below, certain aspects of PURA chromosomal positioning are relevant to neoplasia in general. 5q31 contains genes encoding multiple cytokines, including several interleukins (63, 103) and the early growth response gene-1 (81). PURA is located approximately 1 Mb telomeric to the EGR-1 gene and segregates with it in several translocations in myelodysplastic syndrome (MDS), as determined by fluorescence in-situ hybridization (FISH) (65). MDS frequently progresses to acute myelogenous leukemia (AML). 5q- syndrome, i.e., the loss of the long arm of chromosome 5, is recognized as a common and separate category of MDS (100, 107, 108, 113). When 5q- occurs as the sole genotypic aberration, without accompanying chromosome changes, the clinical course of MDS is relatively mild, and the progression to AML less frequent (108). When, however, 5q31 deletion is accompanied by an additional 5q31 alteration or alterations involving other PUR gene loci, for example PURB, progression to AML is seen at increased frequency (65). In addition to deletions, translocations of the PURA locus are also seen in both MDS and AML (65). Although deletions or alterations of a single PURA allele are detected by FISH in MDS and AML, there have been no detected mutations in the Purα coding sequence, and there is no evidence at this time that PURA fits the profile of a classical tumor suppressor gene. If PURA is the primary target of chromosome rearrangements at 5q31, it is likely that haploinsufficiency is a factor in the resulting oncogenic phenotype.
Chromosomal alteration is a characteristic uniting all three PUR genes in both myeloid diseases and aspects of CNS development and aging. In addition to those in MDS and AML, chromosome alterations at 5q31 have been reported in primitive neuroectodermal tumors and pineoblastomas (76) as well as intracranial ependymomas (78). At this point, however, specific involvement of the PURA locus in these brain tumors has been unexplored. The ability of enhanced levels of Purα protein to inhibit proliferation of tumor cells in culture (5, 96), including glioblastoma cells (18), has been established. Chromosome band 7p13, the location of PURB, is associated with abnormalities linked to myeloproliferative disorders, particularly when occurring in conjunction with alterations involving PURA at 5q31 (65). Monosomy 7 is a well-known aberration associated with MDS and AML (37). Although evidence is lacking for a mechanistic link, aberrations at 7p13 have been reported for brain tumors, including glioblastoma (73). A common involvement of PURA and PURB genes in specific disease states may be anticipated because of the reported functional interaction of the two encoded proteins. Purα forms multimeric complexes in the presence of nucleic acids (30). For example, Purα and Purβ, which are approximately 70% homologous, reportedly function as a heterodimer to regulate the vascular smooth muscle alpha-actin promoter (55).
The PURG locus at 8p11 is very intriguing with regard to neoplasia. In general the presence of increased levels of the Purα protein confer tumor suppressive (5, 18, 65, 66) and antiproliferative (5, 21, 96) activities. In contrast, increased expression of Purγ, at 8p11, is associated with several tumor types and enhanced cell proliferation. Alterations at 8p11 are the basis of a recognized myeloproliferative syndrome (8, 44). Although many of these cases involve translocations at various breakpoints, in many cases the PURG gene copy number is increased by trisomy of chromosome 8 (57, 65, 69, 86, 88). It is particularly interesting that reports have linked simultaneous occurrence of trisomy 8, monosomy 7 and del 5q to AML (86, 88), thereby potentially involving all three PUR gene members in specific cases of myeloid neoplasia. Although the convergence of these three chromosome alterations is undoubtedly a rare event, neither the copy numbers nor expression of each of the PUR genes has been determined, and there has been no statistical linkage of these events to leukemia. Selective amplification of 8p11 has been reported for several tumor types other than leukemia. Breast cancer amplification of 8p11-12 has been most thoroughly examined (1, 25, 34, 36, 85, 118). Amplification of 8p11-12 is one of the most commonly reported chromosome aberrations in breast carcinoma, occurring in approximately 15% of all cases and associated with a poor prognosis (118). Thus far there is no consensus on which gene(s) are responsible for transforming properties of such amplification, but several genes in close proximity to PURG have been implicated, and the phenomenon may be complex, involving more than one gene.
PUR genes in diseases of development and degeneration of the central nervous system: a role for Pur proteins in dendritic transport of mRNA
A major category in which PUR genes have gathered recent attention comprises diseases involving abnormal development and degeneration of the central nervous system (CNS). This may reflect recent heightened attention investigators have paid to these diseases, but it also reflects the importance of Pur protein functions to genome maintenance and specialized aspects of gene expression in specific organs. A common characteristic of cells in the immune system and the CNS is that they both involve plasticity in response to a multitude of exquisitely differential extracellular signals. That is, in response to external stimuli, these organ systems form adaptable signal transduction pathways and entire pathways of cell differentiation to strengthen or dampen future responses. Neurons and glia of the CNS extend cell outgrowths that must respond very quickly to signals that act at great distances from the cell nucleus. In terms of protein synthesis it is thus efficient for such cells to develop specialized mechanisms of mRNA transport and translation that can respond at sites of signal transduction distal from the genetic apparatus of the nucleus. Several recent advances in elucidating such mechanisms have been derived from studies of fragile-X mental retardation syndrome and the fragile-X-related tremor/ataxia syndrome, in which Purα has been identified as a major contributor (35, 39, 45, 47, 49, 52, 68, 82).
Purα in fragile X mental retardation syndrome and fragile X-associated tremor/ataxia syndrome
Fragile X syndrome (FXS) is the most common cause of inherited mental disability, manifest by cognitive defects, hyperactivity, attention deficits and diverse behavioral characteristics with aspects of autism. Expansion of a trinucleotide CGG repeat in the 5′-untranslated region of the Fragile X Mental Retardation gene (FMR1) has been identified as a primary cause of FXS. This expansion leads to chromosomal instability and loss of expression of the encoded protein, FMRP (22, 23, 93–95, 109, 115). FMRP is an RNA-binding protein potentially involved in mRNA transport in neurons (26, 49, 52, 64, 82). FMRP has been detected in large intraneuronal, dendritic mRNA-containing complexes together with Purα and non-coding RNA molecules implicated in RNA transport (49, 52, 59, 60, 82). Purα binds to mouse BC1 RNA (49, 59) and to its human counterpart, BC200 (49). BC1 RNA has been implicated in targeting mRNA to sites of translation in neurites (49, 80, 101, 102), as has BC200 (27). Purα is specifically localized in dendrites, not axons, of rat hippocampal neurons, and it colocalizes with FMRP at specific dendritic junction sites associated with translation (49). Although both FMRP and BC1 are implicated in mRNA binding at sites of translation, the association of FMRP with its target mRNAs is reportedly independent of the presence of BC1 in vivo (43), suggesting that FMRP and BC1 act separately to exert effects on translation. Because Purα interacts with both FMRP and BC1 RNA, Purα may be involved in separate translational activities of each factor. On the other hand, because Purα is capable of interacting independently with both FMRP and BC1, the presence of Purα may allow formation of a complex including all three factors. Double RNA immunoprecipitation experiments have indicated that FMRP and Purα interact simultaneously with mRNA encoding Map2, a dendrite-specific protein (49).
The evidence that Purα is present with FMRP in complexes regulating transport of mRNA to sites of translation in dendrites of CNS neurons raises the question: are changes in Purα structure or function associated with FXS? The evidence thus far is derived from observations of patients with fragile X-associated tremor/ataxia syndrome (FXTAS). FXTAS is considered a premutation stage of FXS, in which the number of rCGG repeats in the FMRP mRNA is intermediate between that of normal individuals (<60 repeats) and FXS patients (>200 repeats). It has been reported that a total of 90 rCGG repeats alone is sufficient to cause neurodegeneration in Drosophila (46). It has not escaped notice of investigators that CGG repeats are ideal Purα-binding elements (7, 116), and the interaction of Purα and associated RNA-binding proteins with the FMRP mRNA repeat sequence has been documented (45, 84, 97, 99). Binding of Purα to rCGG repeats has been observed to modulate the repeat-mediated neurodegeneration in a Drosophila model of FXTAS (45). It was found that expression of the Purα-binding protein Rm62, an RNA helicase, is influenced by the number of rCGG repeats (84). The role of Purα in binding to rCGG repeats may extend to mRNA transport systems outside of neurons. It has recently been reported that Purα binds to rCGG repeats in mRNA of Drosophila oocytes and mediates nuclear export of the mRNP complex in a manner analogous to the role of Purα in neurons (3). The mechanism of involvement of rCGG repeats in neurodegeneration has yet to be elucidated. Nonetheless, an intriguing multilayered feedback pathway has been revealed in which Purα directly interacts with the mRNA encoding a protein, FMRP, which itself interacts with Purα to regulate the transport and translation of mRNAs in neuronal dendrites.
GENETIC INACTIVATION OF PURα IN THE MOUSE: CORRELATIONS WITH HUMAN DISEASE
The mouse PUR family genes are remarkably syntenic with their human counterparts. For example, non-coding sequences adjacent to the PURA gene in humans, including promoter sequences, 5′-UTR and 5′ intron sequences, are all highly conserved in the mouse, as are genes in the vicinity of PURA (unpublished observations). Similarly, PURG is located head-to-head with WRN in both human and mouse chromosomes with intergenic bidirectional control sequences highly conserved (D. Daniel, unpublished observations). There are presently two recorded homozygous knockouts of the PURA gene in mice and no homozygous knockouts of the other PUR family genes. In the first of the reported PURA knockouts it was found that mice lacking PURA, and consequently expression of the Purα protein, proceed through embryogenesis to produce live births, but that the mice so born do not survive beyond 21 to 25 days post-birth (56). The mice are born with near normal appearance, but by day 10 they begin to exhibit uncoordinated movement, tremor, lack of activity and wasting, all despite eating and sleeping nearly normally. At death the mice are abnormally small, all measured organs so affected while the brain upon gross examination displays some evidence of sparing compared to the other organs. Blood cells were carefully assessed in the knockout mice because of the prominent myeloid defects in humans with PURA defects. While every major differentiated hematopoietic cell was represented in the knockout mice at time of death, the relative numbers of different cell types were skewed toward reduction of monocyte development vs. lymphoid cell development, a feature particularly notable in histology of the spleen, in which the percentage of replicating myeloid cells was <1/10 that recorded in wild type mice of the same age. Defects were also histologically apparent in the developing brains of knockout mice, consistent with a critical role proposed for Purα in the CNS (49). There was mislamination of the cerebellum and cerebrum in the knockout mice, and the numbers of differentiated Purkinje cell neurons, cerebral, and hippocampal neurons were distinctly low. The number of synaptic connections, as measured by number of PSD95 foci in the hippocampus, was low in the PURA −/− mice (56). Purα levels are low in the embryonic brains of developing wild-type mice but increase to peak at approximately 20 days after birth (56), at about the time when the PURA −/− mice die. PURA +/− heterozygous mice display a near normal phenotype that still includes myeloid and neurological defects. Purα levels in measured tissues of the heterozygotes are intermediate between those of the homozygotes and the wild type mice. The numbers of myeloid cells undergoing DNA replication in the spleens of heterozygotes are accordingly intermediate between homozygotes and wild types (56). The heterozygote mice survive normally but are subject to sudden death with seizure-like symptoms. Cross-species comparisons indicate that disruption of PURA gene expression may be correlated with defects in aspects of myeloid and neural development in both mice and humans.
A PURA mouse knockout construct has recently been reported in which defects in postnatal brain development have also been found (41). The homozygous mice in this model displayed a persistent tremor, although the level of quantification of this symptom was descriptive. A minor but significant megalencephaly, in terms of overall brain weight, was reported. The Purkinje cell density in the cerebellum was, however, significantly reduced. In the more recent PURA knockout model there was a marked reduction in the expression of dendritic protein, Map2 (41), the translation of which was observed to be Purα-dependent in the earlier PURA −/− mouse model (49). Details of the extent to which Purα amino acid sequences have been deleted in the more recent PURA knockout system (41) have not been disclosed, making it difficult to correlate Purα protein expression levels with symptomatic quantification. It is notable that the homozygous knockout mice in the recent model did not die prematurely. Because there are four Pur family proteins, including two isoforms of Purγ, expressed in both humans and mice, the possibility of selection of revertant mice based on gene redundancy must be carefully evaluated in all PUR knockout models to prevent dilution of phenotypes. It is conceivable that another Pur family member, or a combination of them, given altered expression, could provide compensatory effects to revert a knockout Purα phenotype. Recent data from mouse brain support a role for Purα in dendritic protein translation and dendrite maturation. Purα regulates the neuronal levels of RhoA, a GTPase critical for mRNA translation and dendritic maturation, at several points, including subcellular compartmentalization and turnover. In PURA knockout mice RhoA levels are reduced and dendritic maturation impaired (77).
STRUCTURAL FEATURES OF PUR PROTEINS AND COOPERATIVE FUNCTIONS OF PUR FAMILY MEMBERS DURING DEVELOPMENT
Pur protein interaction with non-coding RNAs in nucleic acid transport
Purα binds not only to BC1 and BC200 RNAs, but to several non-coding RNA sequences with regulatory properties influencing mRNA synthesis and translation (47, 49). These non-coding RNAs include 7SL RNA, a component of the signal recognition particle derived from a segment of the human Alu sequence element (106, 110). 7SL RNA participates in cotranslational translocation of proteins, whereby a protein traverses a membrane during translation in order to enter a separate cellular or extracellular compartment (90). The association of Purα with these non-coding RNAs reveals a striking theme in Pur-nucleic acid functional interaction: these RNA molecules have similar hairpin structures containing PUR elements, and they all participate in the transport or kinetic processing of nucleic acids, particularly mRNA. Furthermore, all of these molecules possess sequences and a hairpin structure in common with the HIV-1 TAR RNA element (49), responsible for the processive transactivation of HIV-1 transcription. HIV-1 has in a sense co-opted these mRNA regulatory activities through binding of the viral protein, Tat, to Purα, which cooperates with Tat to stimulate HIV-1 transcription (14) (Wright, C.A. and Johnson, E.M., 2012, unpublished) and DNA replication (15, 16). The co-opting of Pur activities by HIV-1 may extend to more than the Tat-Purα interaction. It has recently been reported that HIV-1 packages 7SL RNA into virions as determined by cis-acting elements in the RNA (53). These observations emphasize the importance to HIV-1 infection of subverting the cellular mRNA transport mechanism and highlight the critical role of Purα in this process.
Cooperation between Pur family members, Purα and Purβ: examples in muscle development
The single-stranded DNA binding properties of Purβ have been implicated in transcriptional repression of genes encoding muscle-specific isoforms of actin and myosin in heart, skeletal muscle and vascular smooth muscle (58, 87, 120). Studies on the mouse smooth muscle alpha actin gene have revealed an interaction between Purα, Purβ and the Y-box protein, MSY1 (10, 55). Recent observations implicate interactions of the Pur proteins with another Y-box binding protein, YB-1 (24). Purα and Purβ bind to each other through specific protein-protein interactions, bind to the purine-rich strand of their regulatory DNA element as dimers and multimers. As does YB-1 (12), MSY1 has an affinity for the complementary pyrimidine-rich strand of the PUR element. This complex interaction is linked to the ability of Purα to participate in mRNA transport and compartmentalization through the interaction of the Pur proteins with MSY1. MSY1 is present in an mRNP complex implicated in the storage, transport and translation of mRNAs in mouse germ cells (98). The studies on muscle development thus suggest a cooperative interaction between Purα and Purβ. This familial interaction may extend to mRNA transport in neuronal processes. Both Purα and Purβ have been reported as present in the mRNP complex transported along microtubules by a kinesin motor in neurites (52).
Distinct gene regulatory mechanisms govern Purα and Purγ
At this time, Purγ is the least studied of the Pur family members. Purγ is notable for certain very unusual gene expression features. Humans possess a single PURG gene that encodes two isoforms of the Purγ protein, as determined by differential termination/polyadenylation (71). Purγ-A is expressed as a single, intronless coding sequence, as are family members Purα and Purβ. Purγ-B, however, is expressed as a very long transcript that reads through the Purγ-A transcriptional termination site. Instead, an intron of >30 kb is spliced out of the Purγ-B transcript, removing the Purγ-A translational stop codon and resulting in a different Purγ-B C-terminus (71). This highly unusual switching mechanism is utilized in human development and is characteristic of certain neoplasias. PURG-B is the predominant PURG transcript found in human testis, but it was undetectable in members of a non-cancerous adult tissue cDNA panel. PURG-A levels were low or undetectable in the non-tumor tissue panel, but they were increased in members of a tumor tissue panel. PURG-B was detected in several of the tumor tissues (71).
There is at least a temporal separation in the expression of Pur family members, Purα and Purγ, if not also a functional separation. Purγ protein levels are high in the developing mouse embryo brain from day e14 through day p1, whereas Purα levels in the brain are nearly undetectable at this stage (49). Purγ protein levels in the brain decline to reach a nadir at 18–25 days after birth while Purα levels increase to a near maximum during this time. These results have suggested that Purγ may be an embryonic or fetal Pur family member supplanted by Purα as development ensues. This notion is complicated in humans, however, by the finding that Purγ is highly expressed in certain tumor cells. For example, Purγ and Purα levels are both significantly expressed in KG-1C oligodendroglioma cells (Daniel, D.C. and Johnson, E.M., unpublished observations). There is a single mouse PURG gene, but it is not known whether two isoforms of Purγ can be expressed in the mouse as they can in human cells.
Data regarding the PURG gene suggests a link between Purγ and prevention of aging. The PURG gene is located head-to-head with WRN, the gene encoding the WRN helicase (71). The transcription units of each gene initiate at multiple points, the major clusters of which are approximately 90 bp apart. Mutations in the WRN gene have been linked to several phenotypic changes characteristic of premature aging (28, 72, 89, 119). The WRN protein possesses both helicase and nuclease activities (9, 42, 79, 83, 117) and has been implicated in repair of DNA double-strand breaks (2, 9, 72, 91, 117), a process also involving Purα (50, 111, 112). WRN has also been implicated in telomere maintenance (4, 62, 67). Thus the known functions of WRN suggest that it plays a role in prevention of genomic degeneration due to accumulating damage during aging. The promoter region is shared between PURG and WRN. Both promoters lack TATA and CAAT elements, and both are positively regulated by Sp1 elements. Although promoter elements for the two genes overlap in contrapodal directions, various elements important for transcription of each gene are distinct (71). Due to the near identity of the DNA-binding β-sheet elements in Purγ and Purα repeats (Fig. 1), Purγ is likely to be a DNA strand-separating protein, as is Purα (17, 116). As a helicase, WRN would processively unwind regions of single-stranded DNA. It will be of great interest to determine whether there is any functional association between these two proteins and whether their shared promoter regions may affect this.
SUMMARY: STRUCTURES OF PUR FAMILY MEMBERS AFFECT FUNCTION AND DISEASE PREVENTION
Biochemical analyses indicate that Purα (30, 116) and Purβ (55) interact with nucleic acids as dimers or multimers. X-ray crystallographic analyses provide a structural basis for such a dimeric interaction (39). Each Pur amino acid repeat consists of a β-sheet domain and an α-helical domain (Fig. 1) arranged in a structure, termed a “Whirly fold,” in which convex β-sheets form a surface for interaction with nucleic acids (39). The remaining portions of the Whirly structure are engaged in protein-protein interactions. In a model proposed by Graebsch et al., (39) Pur repeats I and II form intramolecular protein-protein bonds while repeat III forms bonds with a second Purα molecule. Given the similarity in amino acid sequences of repeat III among the Pur protein family members, we propose (Fig. 2) that repeat III is capable of forming dimers involving any combination of two of the three Pur family members. Furthermore, because there are important differences in aa sequence in the α-helical regions of repeats III of the different Pur family members, (Fig. 2), these domains may be capable of forming bonds with proteins other than Pur family members. Evidence summarized in Fig. 2 has already implicated motifs within Pur repeat III in interaction with several cellular and viral regulatory proteins. The C-terminal, α-helical region of Pur repeat III may be important in targeting protein interactions that disrupt the normal cellular functions of Pur protein family members.
Figure 2. Diagrammatic representation of Pur multimerization, nucleic acid-binding and protein-binding domains.
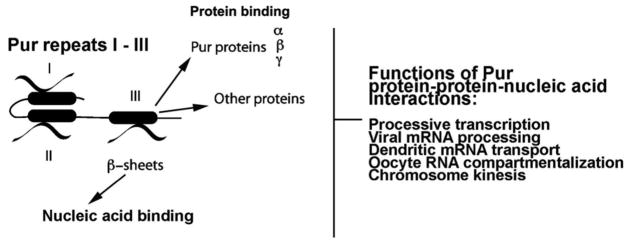
Nucleic acid beta sheet domains believed to be involved in nucleic acid binding are shown as curled lines. Pur multimerization and protein-binding domains are shown as filled ovals. Refer to Table 1, presented as supplemental data, for a list of nucleic acids and proteins reported as bound by human Purα.
Table 1.
Sequence- and structure-specific binding of Purα to nucleic acids and to nucleic acid-binding proteins
| PROTEINS, DNA ELEMENTS, AND RNAs BOUND TO PURα | |
|---|---|
| Protein | Reference |
| HIV-1 Tat | Krachmarov et al. (61), Gallia et al. (33) |
| Rb | Johnson et al. (48) |
| YB-1 | Chen et al. (12, 13) |
| JCV large T-antigen | Gallia et al. (31) |
| Purβ | Kelm et al. (55) |
| Cyclin A | Barr and Johnson (5) |
| Cdk2 | Liu et al. (70) |
| Cdk1 | Liu et al. (70) |
| Cdk4 | Liu et al. (70) |
| Cyclin E/Cdk2 | Barr and Johnson (5) |
| Cyclin B/Cdk1 | Barr and Johnson (5) |
| Cyclin T1/Cdk9 | Darbinian et al. (20) |
| E2F-1 | Darbinian et al. (19) |
| SP-1 | Tretiakov et al. (105) |
| RARα | Chen et al. (11) |
| DNA element | Reference |
| (GGN)n (ssDNA≫dsDNA) | Bergemann et al. (6, 7) |
| c-MYC zone of replication initiation | Bergemann et al. (6, 7) |
| JC virus non-coding control region | Chen and Khalili (13), Krachmarov (61) |
| HIV-1 5′-LTR promoter | Chepenik et al. (14) |
| Myelin basic protein promoter | Haas et al. (40) |
| Smooth muscle alpha-actin promoter | Kelm et al. (54, 55) |
| CD11c beta 2 integrin promoter | Shelley et al. (92) |
| TNF-alpha promoter | Darbinian et al. (20) |
| Non-coding RNA | Reference |
| HIV-1 TAR | Chepenik et al. (14) |
| Ribosomal RNA | Gallia et al. (29) |
| 7SL RNA | Tretiakova et al. (104) |
| Mouse BC1 RNA | Johnson et al. (49) |
| Human BC200 RNA | Johnson et al. (49) |
| HIV-1 Rev response element RRE | Kaminsky et al. (51) |
| mRNA | Reference |
| Map2 | Johnson et al. (49) |
| Map1B | Johnson et al. (49) |
| HIV-1 transcript | Kaminsky et al. (51) |
| NUCLEIC ACID-PROTEIN COMPLEXES FORMED WITH PURα | ||
|---|---|---|
| Nucleic acids | Proteins | Reference |
| General mRNA | Kinesin, FMRP, Staufen | Ohashi et al. (82) |
| CaMKIIalpha mRNA, Arc mRNA | Kinesin (KIF5), Purβ, hnRNP-U, SYNCRIP FMRP, Staufen | Kanai, et al. (52) |
| Map2 mRNA, BC1 RNA | FMRP, Staufen | Johnson, et al. (49) |
| Map1B mRNA, BC1 RNA | FMRP, Staufen | Johnson, et al. (49) |
Acknowledgments
We thank Kamel Khalili for valuable advice and critical evaluation of the manuscript.
Footnotes
CONFLICT OF INTEREST DISCLOSURE
The authors have no conflicts of interest to declare.
References
- 1.Adelaide J, Chaffanet M, Imbert A, Allione F, Geneix J, Popovici C, van Alewijk D, Trapman J, Zeillinger R, Borresen-Dale AL, Lidereau R, Birnbaum D, Pebusque MJ. Chromosome region 8p11-p21: refined mapping and molecular alterations in breast cancer. Genes Chromosomes Cancer. 1998;22:186–99. doi: 10.1002/(sici)1098-2264(199807)22:3<186::aid-gcc4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal M, Sommers JA, Shoemaker RH, Brosh RM., Jr Inhibition of helicase activity by a small molecule impairs Werner syndrome helicase (WRN) function in the cellular response to DNA damage or replication stress. Proc Natl Acad Sci U S A. 2011;108:1525–30. doi: 10.1073/pnas.1006423108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aumiller V, Graebsch A, Kremmer E, Niessing D, Forstemann K. Drosophila Pur-alpha binds to trinucleotide-repeat containing cellular RNAs and translocates to the early oocyte. RNA Biol. 2012:9. doi: 10.4161/rna.19760. [DOI] [PubMed] [Google Scholar]
- 4.Bai Y, Murnane JP. Telomere instability in a human tumor cell line expressing a dominant-negative WRN protein. Hum Genet. 2003;113:337–47. doi: 10.1007/s00439-003-0972-y. [DOI] [PubMed] [Google Scholar]
- 5.Barr SM, Johnson EM. Ras-induced colony formation and anchorage-independent growth inhibited by elevated expression of Puralpha in NIH3T3 cells. J Cell Biochem. 2001;81:621–38. doi: 10.1002/jcb.1099. [DOI] [PubMed] [Google Scholar]
- 6.Bergemann AD, Johnson EM. The HeLa Pur factor binds single-stranded DNA at a specific element conserved in gene flanking regions and origins of DNA replication. Molecular and Cellular Biology. 1992;12:1257–1265. doi: 10.1128/mcb.12.3.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergemann AD, Ma ZW, Johnson EM. Sequence of cDNA comprising the human pur gene and sequence-specific single-stranded-DNA-binding properties of the encoded protein. Mol Cell Biol. 1992;12:5673–5682. doi: 10.1128/mcb.12.12.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brizard A, Guilhot F, Huret JL, Benz-Lemoine E, Tanzer J. The 8p11 anomaly in “monoblastic” leukaemia. Leuk Res. 1988;12:693–7. doi: 10.1016/0145-2126(88)90105-1. [DOI] [PubMed] [Google Scholar]
- 9.Brosh RM, Jr, Opresko PL, Bohr VA. Enzymatic mechanism of the WRN helicase/nuclease. Methods Enzymol. 2006;409:52–85. doi: 10.1016/S0076-6879(05)09004-X. [DOI] [PubMed] [Google Scholar]
- 10.Carlini LE, Getz MJ, Strauch AR, Kelm RJ., Jr Cryptic MCAT enhancer regulation in fibroblasts and smooth muscle cells: Suppression of TEF-1 mediated activation by the single-stranded DNA-binding proteins, Pur{alpha}, Pur{beta}, and MSY1. J Biol Chem. 2001;21:21. doi: 10.1074/jbc.M109754200. [DOI] [PubMed] [Google Scholar]
- 11.Chen N, Onisko B, Napoli JL. The nuclear transcription factor RARalpha associates with neuronal RNA granules and suppresses translation. J Biol Chem. 2008;283:20841–7. doi: 10.1074/jbc.M802314200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen NN, Chang CF, Gallia GL, Kerr DA, Johnson EM, Krachmarov CP, Barr SM, Frisque RJ, Bollag B, Khalili K. Cooperative action of cellular proteins YB-1 and Purα with the tumor antigen of the human JC polymovirus determines their interaction with the viral lytic control element. Proc Natl Acad Sci USA. 1995;92:1087–1091. doi: 10.1073/pnas.92.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen NN, Khalili K. Transcriptional regulation of human JC polyomavirus promoters by cellular proteins YB-1 and Pur alpha in glial cells. J Virol. 1995;69:5843–8. doi: 10.1128/jvi.69.9.5843-5848.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chepenik LG, Tretiakova AP, Krachmarov CP, Johnson EM, Khalili K. The single-stranded DNA binding protein, Pur-alpha, binds HIV-1 TAR RNA and activates HIV-1 transcription. Gene. 1998;210:37–44. doi: 10.1016/s0378-1119(98)00033-x. [DOI] [PubMed] [Google Scholar]
- 15.Daniel DC, Kinoshita Y, Khan MA, Del Valle L, Khalili K, Rappaport J, Johnson EM. Internalization of exogenous human immunodeficiency virus-1 protein, Tat, by KG-1 oligodendroglioma cells followed by stimulation of DNA replication initiated at the JC virus origin. DNA Cell Biol. 2004;23:858–67. doi: 10.1089/dna.2004.23.858. [DOI] [PubMed] [Google Scholar]
- 16.Daniel DC, Wortman MJ, Schiller RJ, Liu H, Gan L, Mellen JS, Chang CF, Gallia GL, Rappaport J, Khalili K, Johnson EM. Coordinate effects of human immunodeficiency virus type 1 protein Tat and cellular protein Puralpha on DNA replication initiated at the JC virus origin. J Gen Virol. 2001;82:1543–53. doi: 10.1099/0022-1317-82-7-1543. [DOI] [PubMed] [Google Scholar]
- 17.Darbinian N, Gallia GL, Khalili K. Helix-destabilizing properties of the human single-stranded DNA- and RNA-binding protein Puralpha. J Cell Biochem. 2001;80:589–95. [PubMed] [Google Scholar]
- 18.Darbinian N, Gallia GL, King J, Del Valle L, Johnson EM, Khalili K. Growth inhibition of glioblastoma cells by human Pur(alpha) J Cell Physiol. 2001;189:334–40. doi: 10.1002/jcp.10029. [DOI] [PubMed] [Google Scholar]
- 19.Darbinian N, Gallia GL, Kundu M, Shcherbik N, Tretiakova A, Giordano A, Khalili K. Association of Pur alpha and E2F-1 suppresses transcriptional activity of E2F-1. Oncogene. 1999;18:6398–402. doi: 10.1038/sj.onc.1203011. [DOI] [PubMed] [Google Scholar]
- 20.Darbinian N, Sawaya BE, Khalili K, Jaffe N, Wortman B, Giordano A, Amini S. Functional interaction between cyclin T1/cdk9 and Puralpha determines the level of TNFalpha promoter activation by Tat in glial cells. J Neuroimmunol. 2001;121:3–11. doi: 10.1016/s0165-5728(01)00372-1. [DOI] [PubMed] [Google Scholar]
- 21.Darbinian N, White MK, Gallia GL, Amini S, Rappaport J, Khalili K. Interaction between the pura and E2F-1 transcription factors. Anticancer Res. 2004;24:2585–94. [PubMed] [Google Scholar]
- 22.Darnell JC, Fraser CE, Mostovetsky O, Darnell RB. Discrimination of common and unique RNA-binding activities among Fragile X mental retardation protein paralogs. Hum Mol Genet. 2009;18:3164–77. doi: 10.1093/hmg/ddp255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darnell JC, Jensen KB, Jin P, Brown V, Warren ST, Darnell RB. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell. 2001;107:489–99. doi: 10.1016/s0092-8674(01)00566-9. [DOI] [PubMed] [Google Scholar]
- 24.David JJ, Subramanian SV, Zhang A, Willis WL, Kelm RJ, Jr, Leier CV, Strauch AR. Y-box binding protein-1 implicated in translational control of fetal myocardial gene expression after cardiac transplant. Exp Biol Med (Maywood) 2012;237:593–607. doi: 10.1258/ebm.2012.011137. [DOI] [PubMed] [Google Scholar]
- 25.Dib A, Adelaide J, Chaffanet M, Imbert A, Le Paslier D, Jacquemier J, Gaudray P, Theillet C, Birnbaum D, Pebusque MJ. Characterization of the region of the short arm of chromosome 8 amplified in breast carcinoma. Oncogene. 1995;10:995–1001. [PubMed] [Google Scholar]
- 26.Dictenberg JB, Swanger SA, Antar LN, Singer RH, Bassell GJ. A direct role for FMRP in activity-dependent dendritic mRNA transport links filopodial-spine morphogenesis to fragile X syndrome. Dev Cell. 2008;14:926–39. doi: 10.1016/j.devcel.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duning K, Buck F, Barnekow A, Kremerskothen J. SYNCRIP, a component of dendritically localized mRNPs, binds to the translation regulator BC200 RNA. J Neurochem. 2008;105:351–9. doi: 10.1111/j.1471-4159.2007.05138.x. [DOI] [PubMed] [Google Scholar]
- 28.Ellis NA. DNA helicases in inherited human disorders. Curr Opin Genet Dev. 1997;7:354–63. doi: 10.1016/s0959-437x(97)80149-9. [DOI] [PubMed] [Google Scholar]
- 29.Gallia GL, Darbinian N, Jaffe N, Khalili K. Single-stranded nucleic acid-binding protein, Pur alpha, interacts with RNA homologous to 18S ribosomal RNA and inhibits translation in vitro. J Cell Biochem. 2001;83:355–63. doi: 10.1002/jcb.1247. [DOI] [PubMed] [Google Scholar]
- 30.Gallia GL, Darbinian N, Johnson EM, Khalili K. Self-association of Puralpha is mediated by RNA. J Cell Biochem. 1999;74:334–48. [PubMed] [Google Scholar]
- 31.Gallia GL, Darbinian N, Tretiakova A, Ansari SA, Rappaport J, Brady J, Wortman MJ, Johnson EM, Khalili K. Association of HIV-1 Tat with the cellular protein, Puralpha, is mediated by RNA. Proc Natl Acad Sci U S A. 1999;96:11572–7. doi: 10.1073/pnas.96.20.11572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallia GL, Johnson EM, Khalili K. SURVEY AND SUMMARY: Puralpha: a multifunctional single-stranded DNA- and RNA-binding protein. Nucleic Acids Res. 2000;28:3197–3205. doi: 10.1093/nar/28.17.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallia GL, Safak M, Khalili K. Interaction of the single-stranded DNA-binding protein Puralpha with the human polyomavirus JC virus early protein T-antigen. J Biol Chem. 1998;273:32662–9. doi: 10.1074/jbc.273.49.32662. [DOI] [PubMed] [Google Scholar]
- 34.Garcia MJ, Pole JC, Chin SF, Teschendorff A, Naderi A, Ozdag H, Vias M, Kranjac T, Subkhankulova T, Paish C, Ellis I, Brenton JD, Edwards PA, Caldas C. A 1 Mb minimal amplicon at 8p11-12 in breast cancer identifies new candidate oncogenes. Oncogene. 2005;24:5235–45. doi: 10.1038/sj.onc.1208741. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Arocena D, Hagerman PJ. Advances in understanding the molecular basis of FXTAS. Hum Mol Genet. 2010;19:R83–9. doi: 10.1093/hmg/ddq166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gelsi-Boyer V, Orsetti B, Cervera N, Finetti P, Sircoulomb F, Rouge C, Lasorsa L, Letessier A, Ginestier C, Monville F, Esteyries S, Adelaide J, Esterni B, Henry C, Ethier SP, Bibeau F, Mozziconacci MJ, Charafe-Jauffret E, Jacquemier J, Bertucci F, Birnbaum D, Theillet C, Chaffanet M. Comprehensive profiling of 8p11-12 amplification in breast cancer. Mol Cancer Res. 2005;3:655–67. doi: 10.1158/1541-7786.MCR-05-0128. [DOI] [PubMed] [Google Scholar]
- 37.Gerritsen WR, Donohue J, Bauman J, Jhanwar SC, Kernan NA, Castro-Malaspina H, O’Reilly RJ, Bourhis JH. Clonal analysis of myelodysplastic syndrome: monosomy 7 is expressed in the myeloid lineage, but not in the lymphoid lineage as detected by fluorescent in situ hybridization. Blood. 1992;80:217–24. [PubMed] [Google Scholar]
- 38.Graebsch A, Roche S, Kostrewa D, Soding J, Niessing D. Of bits and bugs--on the use of bioinformatics and a bacterial crystal structure to solve a eukaryotic repeat-protein structure. PLoS One. 2010;5:e13402. doi: 10.1371/journal.pone.0013402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graebsch A, Roche S, Niessing D. X-ray structure of Pur-{alpha} reveals a Whirly-like fold and an unusual nucleic-acid binding surface. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0907990106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haas S, Gordon J, Khalili K. A developmentally regulated DNA-binding protein from mouse brain stimulates myelin basic protein gene expression. Mol Cell Biol. 1993;13:3103–12. doi: 10.1128/mcb.13.5.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hokkanen S, Feldmann HM, Ding H, Jung CK, Bojarski L, Renner-Muller I, Schuller U, Kretzschmar H, Wolf E, Herms J. Lack of Pur-alpha alters postnatal brain development and causes megalencephaly. Hum Mol Genet. 2012;21:473–84. doi: 10.1093/hmg/ddr476. [DOI] [PubMed] [Google Scholar]
- 42.Huang S, Beresten S, Li B, Oshima J, Ellis NA, Campisi J. Characterization of the human and mouse WRN 3′-->5′ exonuclease. Nucleic Acids Res. 2000;28:2396–405. doi: 10.1093/nar/28.12.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iacoangeli A, Rozhdestvensky TS, Dolzhanskaya N, Tournier B, Schutt J, Brosius J, Denman RB, Khandjian EW, Kindler S, Tiedge H. On BC1 RNA and the fragile X mental retardation protein. Proc Natl Acad Sci U S A. 2008;105:734–9. doi: 10.1073/pnas.0710991105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jackson CC, Medeiros LJ, Miranda RN. 8p11 myeloproliferative syndrome: a review. Hum Pathol. 2010;41:461–76. doi: 10.1016/j.humpath.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Jin P, Duan R, Qurashi A, Qin Y, Tian D, Rosser TC, Liu H, Feng Y, Warren ST. Pur alpha Binds to rCGG Repeats and Modulates Repeat-Mediated Neurodegeneration in a Drosophila Model of Fragile X Tremor/Ataxia Syndrome. Neuron. 2007;55:556–64. doi: 10.1016/j.neuron.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin P, Zarnescu DC, Zhang F, Pearson CE, Lucchesi JC, Moses K, Warren ST. RNA-mediated neurodegeneration caused by the fragile X premutation rCGG repeats in Drosophila. Neuron. 2003;39:739–47. doi: 10.1016/s0896-6273(03)00533-6. [DOI] [PubMed] [Google Scholar]
- 47.Johnson EM. The Pur protein family: clues to function from recent studies on cancer and AIDS. Anticancer Res. 2003;23:2093–100. [PubMed] [Google Scholar]
- 48.Johnson EM, Chen PL, Krachmarov CP, Barr SM, Kanovsky M, Ma ZW, Lee WH. Association of human Pur alpha with the retinoblastoma protein, Rb, regulates binding to the single-stranded DNA Pur alpha recognition element. J Biol Chem. 1995;270:24352–60. doi: 10.1074/jbc.270.41.24352. [DOI] [PubMed] [Google Scholar]
- 49.Johnson EM, Kinoshita Y, Weinreb DB, Wortman MJ, Simon R, Khalili K, Winckler B, Gordon J. Role of Pur alpha in targeting mRNA to sites of translation in hippocampal neuronal dendrites. J Neurosci Res. 2006;83:929–43. doi: 10.1002/jnr.20806. [DOI] [PubMed] [Google Scholar]
- 50.Kaminski R, Cheeseboro L, Amini S, Johnson EM, White MK, Khalili K, Darbinyan A. Role of Puralpha in the cellular response to ultraviolet-C radiation. Cell Cycle. 2010:9. doi: 10.4161/cc.9.20.13456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaminski R, Darbinian N, Sawaya BE, Slonina D, Amini S, Johnson EM, Rappaport J, Khalili K, Darbinyan A. Puralpha as a cellular co-factor of Rev/RRE-mediated expression of HIV-1 intron-containing mRNA. J Cell Biochem. 2008;103:1231–45. doi: 10.1002/jcb.21503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanai Y, Dohmae N, Hirokawa N. Kinesin transports RNA: isolation and characterization of an RNA-transporting granule. Neuron. 2004;43:513–25. doi: 10.1016/j.neuron.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 53.Keene SE, Telesnitsky A. Cis-acting determinants of 7SL RNA packaging by HIV-1. J Virol. 2012 doi: 10.1128/JVI.00856-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kelm JRJ, Elder PK, Strauch AR, Getz MJ. Sequence of cDNAs encoding components of vascular actin single-stranded DNA-binding factor 2 establish identity to Purα and Purβ. The Journal of Biological Chemistry. 1997;272:26727–26733. doi: 10.1074/jbc.272.42.26727. [DOI] [PubMed] [Google Scholar]
- 55.Kelm RJ, Jr, Cogan JG, Elder PK, Strauch AR, Getz MJ. Molecular interactions between single-stranded DNA-binding proteins associated with an essential MCAT element in the mouse smooth muscle alpha-actin promoter. J Biol Chem. 1999;274:14238–45. doi: 10.1074/jbc.274.20.14238. [DOI] [PubMed] [Google Scholar]
- 56.Khalili K, Del Valle L, Muralidharan V, Gault WJ, Darbinian N, Otte J, Meier E, Johnson EM, Daniel DC, Kinoshita Y, Amini S, Gordon J. Puralpha is essential for postnatal brain development and developmentally coupled cellular proliferation as revealed by genetic inactivation in the mouse. Mol Cell Biol. 2003;23:6857–75. doi: 10.1128/MCB.23.19.6857-6875.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kibbelaar RE, Mulder JW, Dreef EJ, van Kamp H, Fibbe WE, Wessels JW, Beverstock GC, Haak HL, Kluin PM. Detection of monosomy 7 and trisomy 8 in myeloid neoplasia: a comparison of banding and fluorescence in situ hybridization. Blood. 1993;82:904–13. [PubMed] [Google Scholar]
- 58.Knapp AM, Ramsey JE, Wang SX, Godburn KE, Strauch AR, Kelm RJ., Jr Nucleoprotein interactions governing cell type-dependent repression of the mouse smooth muscle alpha-actin promoter by single-stranded DNA-binding proteins Pur alpha and Pur beta. J Biol Chem. 2006;281:7907–18. doi: 10.1074/jbc.M509682200. [DOI] [PubMed] [Google Scholar]
- 59.Kobayashi S, Agui K, Kamo S, Li Y, Anzai K. Neural BC1 RNA associates with pur alpha, a single-stranded DNA and RNA binding protein, which is involved in the transcription of the BC1 RNA gene [In Process Citation] Biochem Biophys Res Commun. 2000;277:341–7. doi: 10.1006/bbrc.2000.3683. [DOI] [PubMed] [Google Scholar]
- 60.Kobayashi Y, Suzuki K, Kobayashi H, Ohashi S, Koike K, Macchi P, Kiebler M, Anzai K. C9orf10 protein, a novel protein component of Puralpha-containing mRNA-protein particles (Puralpha-mRNPs): characterization of developmental and regional expressions in the mouse brain. J Histochem Cytochem. 2008;56:723–31. doi: 10.1369/jhc.2008.950733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krachmarov CP, Chepenik LG, Barr-Vagell S, Khalili K, Johnson EM. Activation of the JC virus Tat-responsive transcriptional control element by association of the Tat protein of human immunodeficiency virus 1 with cellular protein Pur alpha. Proc Natl Acad Sci U S A. 1996;93:14112–7. doi: 10.1073/pnas.93.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laud PR, Multani AS, Bailey SM, Wu L, Ma J, Kingsley C, Lebel M, Pathak S, DePinho RA, Chang S. Elevated telomere-telomere recombination in WRN-deficient, telomere dysfunctional cells promotes escape from senescence and engagement of the ALT pathway. Genes Dev. 2005;19:2560–70. doi: 10.1101/gad.1321305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Le Beau MM. Deletions of chromosome 5 in malignant myeloid disorders. Cancer Surv. 1992;15:143–59. [PubMed] [Google Scholar]
- 64.Levenga J, Buijsen RA, Rife M, Moine H, Nelson DL, Oostra BA, Willemsen R, de Vrij FM. Ultrastructural analysis of the functional domains in FMRP using primary hippocampal mouse neurons. Neurobiol Dis. 2009;35:241–50. doi: 10.1016/j.nbd.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lezon-Geyda K, Najfeld V, Johnson EM. Deletions of PURA, at 5q31, and PURB, at 7p13, in myelodysplastic syndrome and progression to acute myelogenous leukemia. Leukemia. 2001;15:954–62. doi: 10.1038/sj.leu.2402108. [DOI] [PubMed] [Google Scholar]
- 66.Lezon-Geyda K, Najfeld V, Johnson EM. The PURA gene, encoding the single-stranded-DNA-binding protein Purα, as a marker for 5q31 alteration in myeloproliferative disorders, a potential early step in induction of AML. FASEB. 1997;11:A100. [Google Scholar]
- 67.Li B, Jog SP, Reddy S, Comai L. WRN controls formation of extrachromosomal telomeric circles and is required for TRF2DeltaB-mediated telomere shortening. Mol Cell Biol. 2008;28:1892–904. doi: 10.1128/MCB.01364-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Y, Koike K, Ohashi S, Funakoshi T, Tadano M, Kobayashi S, Anzai K, Shibata N, Kobayashi M. Pur alpha protein implicated in dendritic RNA transport interacts with ribosomes in neuronal cytoplasm. Biol Pharm Bull. 2001;24:231–5. doi: 10.1248/bpb.24.231. [DOI] [PubMed] [Google Scholar]
- 69.Liapis K, Kousiafes D, Papanikolaou A, Pagratis P, Kokkini G. The 8p11 myeloid and lymphoid neoplasm. Eur J Haematol. 2011;87:471–2. doi: 10.1111/j.1600-0609.2011.01672.x. [DOI] [PubMed] [Google Scholar]
- 70.Liu H, Barr SM, Chu C, Kohtz DS, Kinoshita Y, Johnson EM. Functional interaction of Puralpha with the Cdk2 moiety of cyclin A/Cdk2. Biochem Biophys Res Commun. 2005;328:851–7. doi: 10.1016/j.bbrc.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 71.Liu H, Johnson EM. Distinct proteins encoded by alternative transcripts of the PURG gene, located contrapodal to WRN on chromosome 8, determined by differential termination/polyadenylation. Nucleic Acids Res. 2002;30:2417–26. doi: 10.1093/nar/30.11.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lombard DB, Beard C, Johnson B, Marciniak RA, Dausman J, Bronson R, Buhlmann JE, Lipman R, Curry R, Sharpe A, Jaenisch R, Guarente L. Mutations in the WRN gene in mice accelerate mortality in a p53-null background. Mol Cell Biol. 2000;20:3286–91. doi: 10.1128/mcb.20.9.3286-3291.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lopez-Gines C, Cerda-Nicolas M, Gil-Benso R, Pellin A, Lopez-Guerrero JA, Benito R, del Rey J, Miro R, Roldan R, Barbera J. Primary glioblastoma with EGFR amplification and a ring chromosome 7 in a young patient. Clin Neuropathol. 2006;25:193–9. [PubMed] [Google Scholar]
- 74.Ma ZW, Bergemann AD, Johnson EM. Conservation in human and mouse Purα of a motif common to several proteins involved in initiation of DNA replication. Gene. 1994;149:311–314. doi: 10.1016/0378-1119(94)90167-8. [DOI] [PubMed] [Google Scholar]
- 75.Ma ZW, Pejovic T, Najfeld V, Ward DC, Johnson EM. Localization of PURA, the gene encoding the sequence-specific single-stranded-DNA-binding protein Pur alpha, to chromosome band 5q31. Cytogenet Cell Genet. 1995;71:64–7. doi: 10.1159/000134065. [DOI] [PubMed] [Google Scholar]
- 76.Miller S, Rogers HA, Lyon P, Rand V, Adamowicz-Brice M, Clifford SC, Hayden JT, Dyer S, Pfister S, Korshunov A, Brundler MA, Lowe J, Coyle B, Grundy RG. Genome-wide molecular characterization of central nervous system primitive neuroectodermal tumor and pineoblastoma. Neuro Oncol. 2011;13:866–79. doi: 10.1093/neuonc/nor070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mishra M, Del Valle L, Otte J, Darbinian N, Gordon J. Pur-alpha regulates the developmental expression of RhoA and affects its downstream signaling ability in the mouse brain. J Cell Physiol. 2012 doi: 10.1002/jcp.24105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Modena P, Lualdi E, Facchinetti F, Veltman J, Reid JF, Minardi S, Janssen I, Giangaspero F, Forni M, Finocchiaro G, Genitori L, Giordano F, Riccardi R, Schoenmakers EF, Massimino M, Sozzi G. Identification of tumor-specific molecular signatures in intracranial ependymoma and association with clinical characteristics. J Clin Oncol. 2006;24:5223–33. doi: 10.1200/JCO.2006.06.3701. [DOI] [PubMed] [Google Scholar]
- 79.Moser MJ, Kamath-Loeb AS, Jacob JE, Bennett SE, Oshima J, Monnat RJ., Jr WRN helicase expression in Werner syndrome cell lines. Nucleic Acids Res. 2000;28:648–54. doi: 10.1093/nar/28.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Muslimov IA, Iacoangeli A, Brosius J, Tiedge H. Spatial codes in dendritic BC1 RNA. J Cell Biol. 2006;175:427–39. doi: 10.1083/jcb.200607008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nagarajan L, Zavadil J, Claxton D, Lu X, Fairman J, Warrington JA, Wasmuth JJ, Chinault AC, Sever CE, Slovak ML, et al. Consistent loss of the D5S89 locus mapping telomeric to the interleukin gene cluster and centromeric to EGR-1 in patients with 5q- chromosome. Blood. 1994;83:199–208. [PubMed] [Google Scholar]
- 82.Ohashi S, Koike K, Omori A, Ichinose S, Ohara S, Kobayashi S, Sato TA, Anzai K. Identification of mRNA/protein (mRNP) complexes containing Puralpha, mStaufen, fragile X protein, and myosin Va and their association with rough endoplasmic reticulum equipped with a kinesin motor. J Biol Chem. 2002;277:37804–10. doi: 10.1074/jbc.M203608200. [DOI] [PubMed] [Google Scholar]
- 83.Orren DK, Theodore S, Machwe A. The Werner syndrome helicase/exonuclease (WRN) disrupts and degrades D-loops in vitro. Biochemistry. 2002;41:13483–8. doi: 10.1021/bi0266986. [DOI] [PubMed] [Google Scholar]
- 84.Qurashi A, Li W, Zhou JY, Peng J, Jin P. Nuclear accumulation of stress response mRNAs contributes to the neurodegeneration caused by Fragile X premutation rCGG repeats. PLoS Genet. 2011;7:e1002102. doi: 10.1371/journal.pgen.1002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ray ME, Yang ZQ, Albertson D, Kleer CG, Washburn JG, Macoska JA, Ethier SP. Genomic and expression analysis of the 8p11-12 amplicon in human breast cancer cell lines. Cancer Res. 2004;64:40–7. doi: 10.1158/0008-5472.can-03-1022. [DOI] [PubMed] [Google Scholar]
- 86.Ripperger T, Tauscher M, Praulich I, Pabst B, Teigler-Schlegel A, Yeoh A, Gohring G, Schlegelberger B, Flotho C, Niemeyer CM, Steinemann D. Constitutional trisomy 8p11.21-q11.21 mosaicism: a germline alteration predisposing to myeloid leukaemia. Br J Haematol. 2011;155:209–17. doi: 10.1111/j.1365-2141.2011.08817.x. [DOI] [PubMed] [Google Scholar]
- 87.Rumora AE, Steere AN, Ramsey JE, Knapp AM, Ballif BA, Kelm RJ., Jr Isolation and characterization of the core single-stranded DNA-binding domain of purine-rich element binding protein B (Purbeta) Biochem Biophys Res Commun. 2010;400:340–5. doi: 10.1016/j.bbrc.2010.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schoch C, Kohlmann A, Dugas M, Kern W, Hiddemann W, Schnittger S, Haferlach T. Genomic gains and losses influence expression levels of genes located within the affected regions: a study on acute myeloid leukemias with trisomy 8, 11, or 13, monosomy 7, or deletion 5q. Leukemia. 2005;19:1224–8. doi: 10.1038/sj.leu.2403810. [DOI] [PubMed] [Google Scholar]
- 89.Schonberg S, Niermeijer MF, Bootsma D, Henderson E, German J. Werner’s syndrome: proliferation in vitro of clones of cells bearing chromosome translocations. Am J Hum Genet. 1984;36:387–97. [PMC free article] [PubMed] [Google Scholar]
- 90.Shan SO, Walter P. Co-translational protein targeting by the signal recognition particle. FEBS Lett. 2005;579:921–6. doi: 10.1016/j.febslet.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 91.Sharma S, Otterlei M, Sommers JA, Driscoll HC, Dianov GL, Kao HI, Bambara RA, Brosh RM., Jr WRN helicase and FEN-1 form a complex upon replication arrest and together process branchmigrating DNA structures associated with the replication fork. Mol Biol Cell. 2004;15:734–50. doi: 10.1091/mbc.E03-08-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shelley CS, Teodoridis JM, Park H, Farokhzad OC, Bottinger EP, Arnaout MA. During differentiation of the monocytic cell line U937, Pur alpha mediates induction of the CD11c beta 2 integrin gene promoter. J Immunol. 2002;168:3887–93. doi: 10.4049/jimmunol.168.8.3887. [DOI] [PubMed] [Google Scholar]
- 93.Sheridan SD, Theriault KM, Reis SA, Zhou F, Madison JM, Daheron L, Loring JF, Haggarty SJ. Epigenetic characterization of the FMR1 gene and aberrant neurodevelopment in human induced pluripotent stem cell models of fragile X syndrome. PLoS One. 2011;6:e26203. doi: 10.1371/journal.pone.0026203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Snow K, Doud LK, Hagerman R, Pergolizzi RG, Erster SH, Thibodeau SN. Analysis of a CGG sequence at the FMR-1 locus in fragile X families and in the general population. Am J Hum Genet. 1993;53:1217–28. [PMC free article] [PubMed] [Google Scholar]
- 95.Snow K, Tester DJ, Kruckeberg KE, Schaid DJ, Thibodeau SN. Sequence analysis of the fragile X trinucleotide repeat: implications for the origin of the fragile X mutation. Hum Mol Genet. 1994;3:1543–51. doi: 10.1093/hmg/3.9.1543. [DOI] [PubMed] [Google Scholar]
- 96.Stacey DW, Hitomi M, Kanovsky M, Gan L, Johnson EM. Cell cycle arrest and morphological alterations following microinjection of NIH3T3 cells with Pur alpha. Oncogene. 1999;18:4254–61. doi: 10.1038/sj.onc.1202795. [DOI] [PubMed] [Google Scholar]
- 97.Swanson MS, Orr HT. Fragile X tremor/ataxia syndrome: blame the messenger! Neuron. 2007;55:535–7. doi: 10.1016/j.neuron.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 98.Tafuri SR, Familari M, Wolffe AP. A mouse Y box protein, MSY1, is associated with paternal mRNA in spermatocytes. J Biol Chem. 1993;268:12213–20. [PubMed] [Google Scholar]
- 99.Tan H, Qurashi A, Poidevin M, Nelson DL, Li H, Jin P. Retrotransposon activation contributes to fragile X premutation rCGG-mediated neurodegeneration. Hum Mol Genet. 2012;21:57–65. doi: 10.1093/hmg/ddr437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tefferi A, Mathew P, Noel P. The 5q- syndrome: a scientific and clinical update. Leuk Lymphoma. 1994;14:375–8. doi: 10.3109/10428199409049692. [DOI] [PubMed] [Google Scholar]
- 101.Tiedge H, Chen W, Brosius J. Primary structure, neural-specific expression, and dendritic location of human BC200 RNA. J Neurosci. 1993;13:2382–90. doi: 10.1523/JNEUROSCI.13-06-02382.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tiedge H, Fremeau RT, Jr, Weinstock PH, Arancio O, Brosius J. Dendritic location of neural BC1 RNA. Proc Natl Acad Sci U S A. 1991;88:2093–7. doi: 10.1073/pnas.88.6.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tindall EA, Severi G, Hoang HN, Ma CS, Fernandez P, Southey MC, English DR, Hopper JL, Heyns CF, Tangye SG, Giles GG, Hayes VM. Comprehensive analysis of the cytokine-rich chromosome 5q31.1 region suggests a role for IL-4 gene variants in prostate cancer risk. Carcinogenesis. 2010;31:1748–54. doi: 10.1093/carcin/bgq081. [DOI] [PubMed] [Google Scholar]
- 104.Tretiakova A, Gallia GL, Shcherbik N, Jameson B, Johnson EM, Amini S, Khalili K. Association of Puralpha with RNAs homologous to 7 SL determines its binding ability to the myelin basic protein promoter DNA sequence. J Biol Chem. 1998;273:22241–7. doi: 10.1074/jbc.273.35.22241. [DOI] [PubMed] [Google Scholar]
- 105.Tretiakova A, Steplewski A, Johnson EM, Khalili K, Amini S. Regulation of myelin basic protein gene transcription by Sp1 and Puralpha: evidence for association of Sp1 and Puralpha in brain. J Cell Physiol. 1999;181:160–8. doi: 10.1002/(SICI)1097-4652(199910)181:1<160::AID-JCP17>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 106.Ullu E, Tschudi C. Alu sequences are processed 7SL RNA genes. Nature. 1984;312:171–2. doi: 10.1038/312171a0. [DOI] [PubMed] [Google Scholar]
- 107.Van den Berghe H, David G, Michaux JL, Sokal G, Verwilghen R. 5q-Acute myelogenous leukemia. Blood. 1976;48:624–6. [PubMed] [Google Scholar]
- 108.Van den Berghe H, Vermaelen K, Mecucci C, Barbieri D, Tricot G. The 5q-anomaly. Cancer Genet Cytogenet. 1985;17:189–255. doi: 10.1016/0165-4608(85)90016-0. [DOI] [PubMed] [Google Scholar]
- 109.Verkerk AJMH, Pieretti M, Sutcliffe JS, Kuhl DPA, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang F, Eussen BE, van Ommen GB, Blonden LAJ, Riggins GJ, Chastain JL, Kunst CB, Galjaard H, Caskey CT, Nelson DL, Oostra BA, Warran ST. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 110.Walter P, Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982;299:691–8. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]
- 111.Wang H, Wang M, Reiss K, Darbinian-Sarkissian HN, Johnson EM, Iliakis G, Amini S, Khalili K, Rappaport J. Evidence for the Involvement of Puralpha in Response to DNA Replication Stress. Cancer Biol Ther. 2007:6. doi: 10.4161/cbt.6.4.3889. [DOI] [PubMed] [Google Scholar]
- 112.Wang H, White MK, Kaminski R, Darbinian N, Amini S, Johnson EM, Khalili K, Rappaport J. Role of Puralpha in the modulation of homologous recombination-directed DNA repair by HIV-1 Tat. Anticancer Res. 2008;28:1441–7. [PMC free article] [PubMed] [Google Scholar]
- 113.Washington LT, Jilani I, Estey E, Albitar M. Less apoptosis in patients with 5q-syndrome than in patients with refractory anemia. Leuk Res. 2002;26:899–902. doi: 10.1016/s0145-2126(02)00039-5. [DOI] [PubMed] [Google Scholar]
- 114.White MK, Johnson EM, Khalili K. Multiple roles for Puralpha in cellular and viral regulation. Cell Cycle. 2009;8:1–7. doi: 10.4161/cc.8.3.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wohrle D, Hennig I, Vogel W, Steinbach P. Mitotic stability of fragile X mutations in differentiated cells indicates early post-conceptional trinucleotide repeat expansion. Nat Genet. 1993;4:140–2. doi: 10.1038/ng0693-140. [DOI] [PubMed] [Google Scholar]
- 116.Wortman MJ, Johnson EM, Bergemann AD. Mechanism of DNA binding and localized strand separation by Pur alpha and comparison with Pur family member, Pur beta. Biochim Biophys Acta. 2005;1743:64–78. doi: 10.1016/j.bbamcr.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 117.Yang Q, Zhang R, Wang XW, Spillare EA, Linke SP, Subramanian D, Griffith JD, Li JL, Hickson ID, Shen JC, Loeb LA, Mazur SJ, Appella E, Brosh RM, Jr, Karmakar P, Bohr VA, Harris CC. The processing of Holliday junctions by BLM and WRN helicases is regulated by p53. J Biol Chem. 2002;277:31980–7. doi: 10.1074/jbc.M204111200. [DOI] [PubMed] [Google Scholar]
- 118.Yang ZQ, Liu G, Bollig-Fischer A, Giroux CN, Ethier SP. Transforming properties of 8p11-12 amplified genes in human breast cancer. Cancer Res. 2010;70:8487–97. doi: 10.1158/0008-5472.CAN-10-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yu CE, Oshima J, Fu YH, Wijsman EM, Hisama F, Alisch R, Matthews S, Nakura J, Miki T, Ouais S, Martin GM, Mulligan J, Schellenberg GD. Positional cloning of the Werner’s syndrome gene. Science. 1996;272:258–62. doi: 10.1126/science.272.5259.258. [DOI] [PubMed] [Google Scholar]
- 120.Zhang A, David JJ, Subramanian SV, Liu X, Fuerst MD, Zhao X, Leier CV, Orosz CG, Kelm RJ, Jr, Strauch AR. Serum response factor neutralizes Pur alpha- and Pur beta-mediated repression of the fetal vascular smooth muscle alpha-actin gene in stressed adult cardiomyocytes. Am J Physiol Cell Physiol. 2008;294:C702–14. doi: 10.1152/ajpcell.00173.2007. [DOI] [PubMed] [Google Scholar]