Abstract
Objective
To assess the association between lifestyle practices (cognitive and physical activity) and β-amyloid deposition, measured with positron emission tomography using carbon 11–labeled Pittsburgh Compound B ([11C]PiB), in healthy older individuals.
Design
Cross-sectional clinical study.
Setting
Berkeley, California.
Participants
Volunteer sample of 65 healthy older individuals (mean age, 76.1 years), 10 patients with Alzheimer disease (AD) (mean age, 74.8 years), and 11 young controls (mean age, 24.5 years) were studied from October 31, 2005, to February 22, 2011.
Main Outcome Measures
Cortical [11C]PiB average (frontal, parietal, lateral temporal, and cingulate regions) and retrospective, self-report scales assessing participation in cognitive activities (eg, reading, writing, and playing games) and physical exercise.
Results
Greater participation in cognitively stimulating activities across the lifespan, but particularly in early and middle life, was associated with reduced [11C]PiB uptake (P <.001, accounting for age, sex, and years of education). Older participants in the highest cognitive activity tertile had [11C]PiB uptake comparable to young controls, whereas those in the lowest cognitive activity tertile had [11C]PiB uptake comparable to patients with AD. Although greater cognitive activity was associated with greater physical exercise, exercise was not associated with [11C]PiB uptake.
Conclusions
Individuals with greater early- and middle-life cognitive activity had lower [11C]PiB uptake. The tendency to participate in cognitively stimulating activities is likely related to engagement in a variety of lifestyle practices that have been implicated in other studies showing reduced risk of AD-related pathology. We report a direct association between cognitive activity and [11C]PiB uptake, suggesting that lifestyle factors found in individuals with high cognitive engagement may prevent or slow deposition of β-amyloid, perhaps influencing the onset and progression of AD.
The recent development of the radiopharmaceutical carbon 11–labeled Pittsburgh Compound B ([11C]PiB)1 has made it possible to image fibrillar forms of the β-amyloid (Aβ) protein, which is the major constituent of the amyloid plaque in Alzheimer disease (AD). Studies applying this technique have demonstrated [11C]PiB accumulation throughout cortex in most patients with AD. More important, 20% to 30% of healthy, cognitively normal older individuals2,3 also display significant PiB uptake, which is consistent with evidence that some older individuals who were cognitively intact during life show substantial numbers of Aβ plaques post mortem.4 Greater understanding of the factors associated with Aβ variability in the healthy older population could have important consequences for disease prevention.
Recent evidence indicates that lifestyle practices, such as increased physical exercise, are associated with reduced Aβ deposition based on [11C]PiB positron emission tomography (PET) and cerebrospinal fluid Aβ42 measurements.5 Participation in cognitively stimulating activities has also been linked to reduced risk of late-life cognitive decline and AD.6–8 An individual’s tendency to engage in physically and cognitively stimulating activities is likely related to a broad set of lifestyle factors that are difficult to quantify but include occupational, social, community, and recreational practices. We assessed engagement in cognitive and physical activities and hypothesized that greater levels of engagement may be associated with less Aβ later in life.
We investigated this hypothesis by performing [11C]PiB PET and neuropsychological testing in a sample of cognitively normal older participants. Aβ deposition, characterized as mean cortical [11C]PiB PET uptake, was examined in healthy older participants and comparison samples of young participants and patients with AD. A variety of neuropsychological and lifestyle measurements were also obtained and assessed in relation to Aβ deposition for healthy older participants only, including frequency of engagement in cognitively demanding activities, frequency of engagement in physical and leisure activities, and current episodic memory function.
METHODS
STUDY PARTICIPANTS
Sixty-five cognitively normal older participants (mean [SD] age, 76.1 [6.3] years) were recruited from the Berkeley, California, community (via newspaper advertisements, flyers, and public lectures and events) and enrolled in the ongoing Berkeley Aging Cohort, which assesses individuals at an approximately yearly interval. During this assessment, participants completed an extensive neuropsychological battery. For participants who had completed more than 1 neuropsychological evaluation, only data obtained during the evaluation closest to the imaging sessions were used in this study. Inclusion criteria were age of 50 years or older, Mini-Mental State Examination (MMSE) score of 26 or higher,9 living independently in the community, and normal performance on cognitive tests (≥2 SDs below age-, years of education–, and sex-adjusted means). Exclusion criteria included major neurologic, psychiatric, or medical illness; depression (assessed with the Geriatric Depression Scale)10; use of medications that affect cognition; and magnetic resonance imaging (MRI) contraindications.
Ten patients with AD (mean [SD] age, 74.8 [8.7] years) and 11 young control participants (mean [SD] age, 24.5 [3.7] years) were included for comparison of [11C]PiB uptake only. Young participants were recruited from the University of California, Berkeley, campus and the surrounding community via advertisements. Inclusion criteria were age of 20 to 30 years, normal performance on cognitive tests (≥2 SDs below age-, years of education–, and sex-adjusted means), MMSE score of 26 or higher, and Geriatric Depression Scale score less than 10. Exclusion criteria were identical to those for older controls.
Patients with AD were recruited from the Memory and Aging Center at the University of California, San Francisco, met the age criterion of 60 years or older, and volunteered for MRI at the University of California, San Francisco, and [11C]PiB PET at Lawrence Berkeley National Laboratory from October 31, 2005, to February 22, 2011. The clinical evaluation included a history and physical examination by a neurologist, a structured caregiver interview by a nurse, and a battery of neuropsychological tests.11 Clinical diagnosis was assigned by consensus at a multidisciplinary conference by investigators masked to [11C]PiB status, using standard research criteria.12 Patients with comorbid medical or psychiatric illnesses or other neurologic diagnoses were excluded. Apolipoprotein E ε4 (ApoE4) carrier status was determined for 64 of 65 healthy older participants, 8 of 11 young participants, and 9 of 10 patients with AD.
This study was approved by the institutional review boards of all participating institutions, and all participants or their surrogates gave written informed consent.
COGNITIVE AND PHYSICAL ACTIVITY
Frequency of cognitive stimulation throughout life was assessed in healthy older individuals only using a previously reported 25-item interview in which participants were asked to report how often they engaged in common cognitively demanding activities that depend minimally on socioeconomic status, such as reading books or newspapers, writing letters or e-mails, going to the library, and playing games, at 5 age epochs: 6, 12, 18, and 40 years and the current age. Responses for each item were made using a 5-point frequency scale: 5, every day or almost every day; 4, several times a week; 3, several times a month; 2, several times a year; and 1, once a year or less.13 Because the number of test items varied slightly across the age epochs, 5 separate epoch means were calculated for each study participant so that the age epochs with more test items did not contribute disproportionately to the overall mean. We then created 3 composite scores that were used in subsequent analyses: past cognitive activity, which was an average of epoch means across ages 6, 12, 18, and 40 years; lifetime cognitive activity, which was an average across all 5 epoch means; and current cognitive activity, which was the average of interview items from the current epoch only.
Previous studies13 have described this instrument in detail and reported that the cognitive activity interview has high internal consistency and is positively associated with educational and cognitive performance. In this study, the test-retest correlation of the cognitive activity interview was 0.81 based on 41 cognitively normal older individuals who completed 2 consecutive annual evaluations that included the interview. This is similar to the test-retest correlation reported previously for this scale.13
Participants also completed a physical and leisure activity interview in which they indicated all physical and leisure activities (eg, cycling, walking, dancing, yoga) they participated in during a typical, recent 2-week period. A physical activity measurement (kilocalories burned during a recent 2-week period) was calculated by multiplying the time spent on the activity by an intensity index.14,15
NEUROPSYCHOLOGICAL TESTING
As described, healthy older controls completed an extensive neuropsychological battery, administered on average 0.37 years before or after PET (SD,0.32 years) to screen for impaired cognition. Episodic memory was assessed by the immediate free recall portion of the California Verbal Learning Test (sum of 5 recall trials of the same 16-word list).16 To determine subjective memory function, participants were asked to rate their memory function compared with other people their age and compared with their memory ability 20 years ago, using a 4-point scale (1, better; 2, the same; 3, a bit worse; and 4, much worse).
STRUCTURAL MRI AND ANALYSIS
High-resolution structural MRIs were used to identify brain regions used for the [11C]PiB PET analysis. Structural MRIs in young and healthy older controls were conducted at the Lawrence Berkeley National Laboratory on a 1.5-T Magnetom Avanto System (Siemens Medical Systems) with a 12-channel head coil run in triple mode. The MRI session included an axial, T2-weighted attenuated inversion recovery scan (repetition time, 9730 milliseconds; echo time, 100 milliseconds; flip angle, 150°; 0.80 ×0.80 mm2 in plane resolution; and 3.00-mm thickness with no gap), which was used to screen for stroke, and 3 axial, T1-weighted, volumetric magnetization prepared rapid gradient-echo (MPRAGE) scans (repetition time, 2110 milliseconds; echo time, 3.58 milliseconds; inversion time, 1100 milliseconds; flip angle,15°; 1.00 ×1.00 mm2 in plane resolution; and 1.00-mm thickness with 50% gap), which were averaged together and used to delineate brain regions for the [11C]PiB PET analysis.
For patients with AD, structural MRI was performed at the University of California, San Francisco. For 11 patients, scans were acquired coronally on a 1.5-T VISION System (Siemens Medical Systems) with a quadrature head coil (repetition time, 10 milliseconds; echo time, 7 milliseconds; inversion time, 300 milliseconds; flip angle,15°; 1.00 ×1.00 mm2 in plane resolution; and 1.40-mm section thickness with no gap), and for the remaining 6 participants they were acquired sagittally on a Bruker MedSpec 4T System controlled by a Trio console with an 8-channel head coil (Siemens Medical Systems) (repetition time, 2300 milliseconds; echo time, 3.37 milliseconds; inversion time, 950 milliseconds; flip angle,7°; 1.00 ×1.00 mm2 in-plane resolution; and 1.00-mm section thickness with no gap).
To identify regions of interest, MRIs were processed using Free-Surfer software, version 4.5.0 (http://surfer.nmr.mgh.harvard.edu/), as described previously.17 Briefly, for older controls with multiple MPRAGE images, scans were realigned and averaged to create a single high-contrast structural image. (For young controls and patients with AD, only a single T1-weighted MPRAGE image was acquired.) Regions relevant to PET processing were then derived in each participant’s native space.18–21
To define the spatial transformation from each participant’s native space to MNI template space, the high-resolution structural scan was nonlinearly aligned to the standard MNI 152 brain using FNIRT, a nonlinear image registration tool (http://www.fmrib.ox.ac.uk/fsl/fnirt/). Resulting parameters were used to transform [11C]PiB PET image maps.
[11C]PIB PET IMAGING AND ANALYSIS
The [11C]PiB PET imaging was conducted at the Lawrence Berkeley National Laboratory on a Siemens ECAT EXACT HR PET scanner in 3-dimensional acquisition mode. Approximately 15 mCi of [11C]PiB was injected into an antecubital vein, and 90 minutes of dynamic acquisition frames were obtained (4 ×15 seconds, 8 ×30 seconds, 9 ×60 seconds, 2 ×180 seconds, 8 ×300 seconds, and 3 ×600 seconds). Data were realigned using the SPM8 software package (The MathWorks Inc), and distribution volume ratios were generated using Logan graphical analysis with frames corresponding to 35 to 90 minutes after injection and a gray matter–masked cerebellum reference region.22,23
A [11C]PiB index was derived for each participant representing the average of the mean distribution volume ratios from 4 large regions of interest that were defined using each participant’s native space structural MRIs: prefrontal cortex (all cortex anterior to the precentral sulcus), lateral temporal cortex (middle and superior temporal gyri), parietal cortex (supramarginal gyrus, inferior and superior parietal lobules, and precuneus), and anterior and posterior cingulate gyrus.17
The [11C]PiB PET results presented in this study are not based on data corrected for partial volume effects, but the relationships between our primary variables of interest ([11C]PiB index and past cognitive activity) did not differ when partial volume–corrected data were used.
In addition to the region of interest analysis, [11C]PiB PET maps were transformed to MNI space (see the “Structural MRI and Analysis” subsection). To demonstrate the topography of the relationship between PiB uptake and cognitive activity, voxelwise correlations were performed on spatially normalized [11C]PiB distribution volume ratio maps with past cognitive activity as a regressor (and age, sex, and years of education as nuisance variables), evaluating results at P<.001 and a cluster size of 100 voxels, 2-tailed. These analyses were performed using permutation testing with FSL’s Randomise (http://www.fmrib.ox.ac.uk/fsl/randomise/index.html), which does not assume an underlying distribution of the data and thus accounts for the nonparametric nature of the [11C]PiB data.
STATISTICAL ANALYSIS
Cognitive activity was evaluated as a continuous and categorical variable; cognitively normal older individuals were divided into tertiles (low, middle, and high) based on past cognitive activity, and [11C]PiB was evaluated as a continuous and voxelwise variable. Comparisons of categorical variables (eg, cognitive activity tertiles and ApoE4 carriers and noncarriers) were performed using the Pearson χ2, Kruskal-Wallis, or Mann-Whitney test. Comparisons of pairs of continuous variables were performed using the Spearman rank correlation. The association between PiB and cognitive activity was determined using linear regression to include age, sex, years of education, and episodic memory (all mean centered) as nuisance covariates in the model. Finally, to determine whether physical activity influenced the association between cognitive activity and [11C]PiB, a linear regression model was performed using log-transformed [11C]PiB indices as the dependent variable with past cognitive activity, physical activity, age, sex, and years of education (all mean centered) as independent variables.
RESULTS
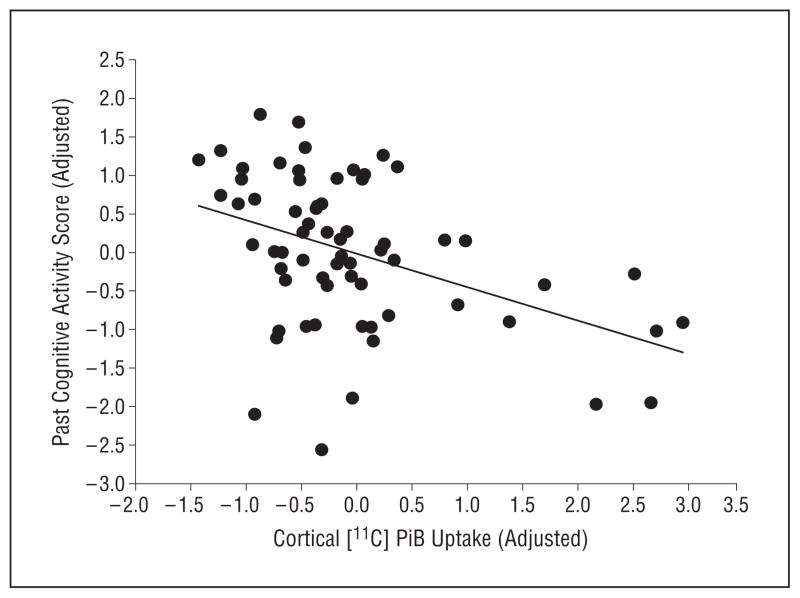
Higher lifetime cognitive activity was significantly associated with lower cortical [11C]PiB uptake (P = .003, Spearman r=−0.37). To investigate the separate contributions of past and present cognitive activity, the lifetime mean cognitive activity score was subdivided into a past cognitive activity score (ages, 6–40 years; see the “Methods” section) and a current cognitive activity score.24 The relationship between higher current cognitive activity and lower [11C]PiB uptake was a nonsignificant trend (P = .09), but higher past cognitive activity was associated with lower [11C]PiB uptake (mean [SD] β= −1.73 [0.47]; P <.001, accounting for age, sex, and years of education; Figure 1), and this association was unchanged by the addition of current episodic memory performance to the model (mean [SD] β= −1.84 [0.45]; P<.001). Because it appeared to be driving the association with [11C]PiB, the past cognitive activity subscore was used in subsequent analyses.
Figure 1.
Individuals with greater cognitive engagement show reduced amyloid burden. Carbon 11–labeled Pittsburgh Compound B ([11C]PiB) in cognitively normal older participants (x-axis) is inversely associated with past cognitive activity (y-axis) (linear regression, β= −1.73 ± 0.47; P <.001). Both variables are residual values after correcting for age, sex, and years of education.
To determine whether the relationship between [11C]PiB and cognitive activity could be explained by any other variables, we examined a number of pairwise associations. We found that [11C]PiB was marginally inversely related to years of education (P=.052, Spearman r=−0.24) but was not related to current episodic memory performance, physical activity, MMSE score, ApoE carrier status, or age. Past cognitive activity was associated with physical activity (P=.001, Spearman r=0.40) and episodic memory (P=.04, Spearman r=0.26) but not years of education, sex, depression, subjective memory ratings, or ApoE4 carrier status.
Because cognitive activity and physical activity were associated with one another, we performed a regression model with past cognitive activity, physical activity, and a cognitive activity × physical activity interaction term as independent variables (along with age, sex, and years of education) and log-transformed [11C]PiB as the dependent variable to determine whether cognitive activity was independently associated with [11C]PiB or whether this association could be explained by physical activity. Cognitive activity alone was a significant predictor of [11C]PiB uptake (P =.002).
Older controls were divided into tertiles based on past cognitive activity. We observed the expected differences in [11C]PiB across young controls, older controls, and patients with AD, with [11C]PiB levels lower in young controls than older controls (P =.04) and higher in patients with AD than older or younger controls (P <.001) (Table 1 and Figure 2). Within older controls, the cognitive activity tertiles differed from one another with respect to [11C]PiB (P =.001) such that the lowest tertile had higher [11C]PiB uptake than the middle tertile (P=.04) and the top tertile (P =.001). The middle and highest tertiles were marginally different (P =.06). Tertiles differed with respect to current physical activity level (P =.004) such that the highest cognitive activity tertile had greater energy expenditure than the middle tertile (P =.02) and lowest tertile (P =.003). However, the tertiles did not differ from one another with respect to subjective memory ratings, age, sex, years of education, depression, episodic memory, MMSE score, or ApoE4 carrier status.
Table 1.
Characteristics of the Patients With AD, Young Controls, and Older Controls, Divided Into Past Cognitive Activity Tertilesa
| Characteristic | AD Patients | Young Controls | Older Controls
|
|||
|---|---|---|---|---|---|---|
| All | Bottom Tertile | Middle Tertile | Top Tertile | |||
| No. of study participants | 10 | 11 | 65 | 22 | 22 | 21 |
| Female, No. (%) | 3 (30) | 6 (55) | 42 (65) | 15 (68) | 12 (55) | 15 (71) |
| ApoE4 carriers, No. (%)b | 6/9 (67) | 5/8 (63) | 19/64 (30) | 8/21 (38) | 4/22 (18) | 7/21 (33) |
| ApoE4 allele frequency, % | 0.44 | 0.31 | 0.16 | 0.23 | 0.09 | 0.17 |
| [11C]PiB uptakec | 1.54 (0.24) | 1.04 (0.24) | 1.11 (0.16) | 1.22 (0.23) | 1.08 (0.08) | 1.03 (0.06) |
| Age, y | 74.8 (8.7) | 24.5 (3.7) | 76.1 (6.3) | 76.3 (5.6) | 76.0 (6.4) | 76.2 (7.0) |
| Years of education | 17.1 (2.8) | 16.2 (1.9) | 17.0 (2.1) | 16.7 (2.3) | 17.0 (2.2) | 17.3 (1.8) |
| MMSE scored | 19.5 (8.0) | … | 29.0 (1.3) | 28.7 (1.5) | 28.9 (1.4) | 29.3 (0.7) |
| Depression score (maximum, 30)e | … | 3.3 (2.7) | 3.6 (2.8) | 3.1 (2.3) | 3.2 (3.0) | |
| Memory score (maximum, 80)f | … | 55.4 (8.2) | 47.5 (10.2) | 45.1 (11.7) | 47.8 (9.5) | 49.9 (9.0) |
| Physical activity interview, kcalg | … | … | 4965 (3792) | 3507 (3059) | 4325 (2127) | 7163 (4856) |
| Cognitive activity interview scoreh | ||||||
| Past (maximum, 5) | … | … | 3.50 (0.63) | 2.80 (0.43) | 3.55 (0.17) | 4.17 (0.20) |
| Current (maximum, 5) | … | … | 3.93 (0.59) | 3.51 (0.48) | 3.98 (0.60) | 4.30 (0.39) |
| Lifetime (maximum, 5) | … | … | 3.55 (0.58) | 2.93 (0.39) | 3.60 (0.23) | 4.16 (0.20) |
Abbreviations: AD, Alzheimer disease; ApoE4, apolipoprotein E4; MMSE, Mini-Mental State Examination; [11C]PiB, carbon 11–labeled Pittsburgh B Compound.
Data are presented as mean (SD) unless otherwise indicated.
ApoE4 carriers are the percentage of individuals who have at least 1 ApoE ε4 allele. ApoE4 carrier status was determined for 64 of 65 healthy older participants, 8 of 11 young participants, and 9 of 10 patients with AD.
[11C]PiB uptake was lower in young controls than older controls (P = .04) and higher in patients with AD than older or younger controls (P <.001). Within older controls, the tertiles differed from one another (P = .001) such that the lowest tertile had higher [11C]PiB uptake than both the middle tertile (P = .04) and the top tertile (P = .001), and the middle and highest tertiles were marginally different (P = .06).
The MMSE score was lower for patients with AD compared with older controls (P <.001).
The depression score is the score on the Geriatric Depression Scale.
The memory score is performance on the free recall portion (sum of trials 1–5) of the California Verbal Learning Test.
Tertiles differed with respect to current physical activity level (P = .004) such that the highest cognitive activity tertile had greater energy expenditure than the middle tertile (P = .02) and lowest tertile (P = .003). The current physical activity interview scores were calculated by multiplying the time reported for each physical or leisure activity (eg, walking, cycling, dancing, or yoga) during a recent 2-week period by an intensity index for that activity.
Items in the cognitive activity interview were scored according to a frequency scale: 5, every day or almost every day; 4, several times a week; 3, several times a month; 2, several times a year; and 1, once a year or less. The total cognitive activity score (average across all age epochs) was subdivided into a past cognitive activity score (average across epoch means for ages 6, 12, 18, and 40 years) and a current cognitive activity score (average of current epoch only).
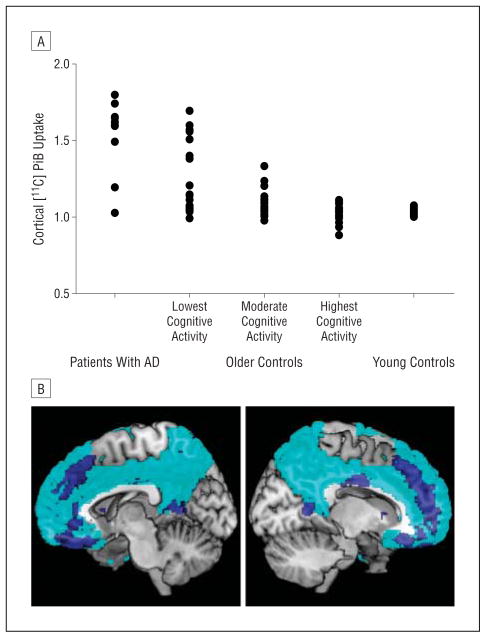
Figure 2.
Cognitively normal older individuals with the lowest cognitive activity have amyloid burden that resembles that of patients with Alzheimer disease (AD). A, Carbon 11–labeled Pittsburgh Compound B ([11C]PiB) indices, reflecting amyloid deposition, in 10 patients with AD and 11 young controls were compared with older controls, who were subdivided into tertiles based on past cognitive activity scores. Within older controls, the cognitive activity tertiles differed from one another (P = .001 by the Kruskal-Wallis test) such that the lowest tertile had higher [11C]PiB uptake than the middle tertile (P = .04 by the Mann-Whitney test) and the top tertile (P = .001 by the Mann-Whitney test). The middle and highest tertiles were marginally different (P = .06). Patients with AD had higher [11C]PiB levels compared with older controls overall (P <.001) and young controls (P <.001). Young controls had lower [11C]PiB levels than older controls overall (P = .04). B, Regions in which past cognitive activity is inversely associated with [11C]PiB (blue; P = .001, cluster size = 100 voxels; controlling for age, sex, and years of education) overlaid, for comparison, on the set of regions used to calculate the mean cortical [11C]PiB indices for each study participant (cyan), which are plotted in A and listed in Table 2.
To visualize the regional distribution of the relationship between past cognitive activity and [11C]PiB, we performed a voxelwise correlation with past cognitive activity scores on spatially normalized [11C]PiB volumes. Regions showing an inverse relationship between [11C]PiB uptake and cognitive activity (P = .001, uncorrected; Table 2 and Figure 2) were located primarily in the lateral and medial prefrontal and parietal cortex and lateral temporal cortex and overlapped with the set of cortical regions used to calculate the mean [11C]PiB index for each participant (Figure 2).
Table 2.
Regions in Which Greater Cognitive Activity Was Associated With Reduced Carbon 11–Labeled Pittsburgh Compound B Uptakea
| Region | Cluster Size, Voxels | x | y | z |
|---|---|---|---|---|
| Right gyrus rectus | 5936 | 2 | 30 | −28 |
| Right insula | 1647 | 38 | 4 | −10 |
| Right middle temporal gyrus | 327 | 56 | −52 | 4 |
| Left insula | 272 | −32 | 14 | −16 |
| Right inferior frontal gyrus | 248 | 40 | 28 | 30 |
| Right inferior temporal gyrus | 223 | 50 | −20 | −36 |
| Right lingual gyrus | 175 | 12 | −56 | 2 |
| Right anterior cingulate gyrus | 174 | 4 | −2 | 26 |
| Left superior temporal gyrus | 145 | −60 | −12 | 14 |
| Left inferior parietal lobule | 122 | −38 | −44 | 44 |
The MNI coordinates of the local maximum of each cluster that showed an inverse relationship between carbon 11–labeled Pittsburgh Compound B uptake and past cognitive activity (see also Figure 2B).
COMMENT
Our data are consistent with the observation that participation in cognitively stimulating activities in early to middle life is associated with lower Aβ accumulation regardless of whether cognitive activity is evaluated as a continuous or categorical variable or whether Aβ is assessed as a global [11C]PiB index or by voxelwise analysis. More important, this association was not affected by the inclusion of possible confounding variables, such as current episodic memory ability, age, sex, and years of education.
When the healthy older participants were divided into tertiles based on past cognitive activity level, the lowest cognitive activity tertile comprised most participants with high Aβ deposition and had levels similar to patients with AD. Although previous epidemiologic studies have shown that cognitive stimulation throughout life7 and in older age6,8 reduces the risk of cognitive decline and AD, our findings suggest a novel mechanism in which increased cognitive activity may play a direct role in reducing Aβ before disease onset. The notion that cognitive activity influences the development of AD pathology is supported by recent findings that cognitively normal older individuals with greater lifelong participation in complex mental activities showed less hippocampal atrophy,25 another biomarker of AD pathology.
An association between [11C]PiB uptake and memory performance has been reported in some studies26 but not others.27 We did not find evidence of this association, perhaps because participants were selected on the basis of intact cognition, thereby restricting the range of memory performance. Thus, our results do not bear directly on whether participation in these lifelong cognitive activities might also protect against the effects of accumulating Aβ through a mechanism of cognitive reserve. It is possible that cognitive activity could play a dual role in preventing the pathology and attenuating the neural response to this pathology.
Our cognitive activity measurement is likely just one of a variety of interrelated lifestyle factors that are difficult to quantify. Cognitive activity and (marginally) years of education were associated with [11C]PiB uptake (although cognitive activity and years of education were not related to one another), suggesting that these measurements may reflect a broader underlying tendency to engage in intellectual, occupational, social, and recreational activities. We also found that physical activity was associated with cognitive activity but not with [11C]PiB, but the addition of physical activity to the model did not reduce the association between cognitive activity and PiB, indicating that cognitive activity was the primary variable driving the association. Physical activity has been linked to Aβ previously,5 although this study differed from ours in that it assessed a 10-year exercise history of walking, jogging, or running, whereas the present study used a recent 2-week assessment of any physical or leisure activity. In addition, the time scale of the physical and cognitive activity indices are important in this study; our physical activity measure assessed current function, whereas the cognitive activity interview assessed lifelong cognitive engagement. The association between [11C]PiB and cognitive (but not physical) activity may thus reflect a time-sensitive neural process in which early- and middle-life practices have a greater influence on AD pathology than later-life practices.
Biologically plausible mechanisms could underlie the association we detected. Recent in vitro, animal, and human data indicate that neural activity regulates the secretion of Aβ.28–32 In addition, the cortical pattern of Aβ deposition overlaps with a set of highly interconnected networks that have been described as cortical hubs.33 These multimodal nodes, which include the posterior cingulate, lateral temporal and parietal, as well as medial and lateral prefrontal regions, are highly active during cognitive activity and rest and therefore may be susceptible to Aβ deposition. Although it is difficult to verify the association between increased neural activation and Aβ deposition in humans, it is conceivable that increased synaptic activity throughout the lifespan within this network of cortical hubs plays a role in the pathogenesis of AD later in life. Individuals who participate in a variety of cognitively stimulating activities during the lifespan may develop more efficient neural processing that results in less Aβ deposition.34 Supporting this idea, transgenic Aβ-expressing mice exposed to enriched environments deposit less Aβ than control animals.35,36
Although the associations we report are cross-sectional, the pattern of the relationships suggests a temporal ordering of events such that cognitive activity (reflecting events occurring in early and middle life) precedes Aβ aggregation, which likely begins in middle life and precedes cognitive decline.2,37 Although the cognitive activity interview is a reliable and well-validated scale, it is based on self-report and could be biased. However, the specificity of the observed association with Aβ for past, but not current, cognitive activity seems to mitigate this concern. Furthermore, past cognitive activity was not associated with other factors that might be expected to reflect recall bias, such as subjective memory ratings or depression. Nevertheless, it remains possible that another unmeasured etiologic factor occurring early in life might both reduce the propensity to participate in cognitively stimulating activities and promote Aβ aggregation.
It is unlikely that our results reflect a single unitary cause of AD, which is a complex disease with many potential pathogenetic processes. Furthermore, cognitive activity is just one component of a complex set of lifestyle practices linked to AD risk that may be examined in future work. However, the present findings extend previous findings that link cognitive stimulation and AD risk (an indirect downstream effect of Aβ) by providing evidence that is consistent with a model in which cognitive stimulation is linked directly to the AD-related pathology itself.
Acknowledgments
Funding/Support: This work was supported by grants AG034570 and AG032814 from the National Institutes of Health and grant ZEN-08-87090 from the Alzheimer’s Association.
Footnotes
Financial Disclosure: None reported.
Additional Contributions: Adi Alkalay, MD, Suzanne Baker, PhD, Amynta Hayenga, BA, Andrea Long, MS, Cindee Madison, MA, Candace Markley, BA/BS, Henry Schwimmer, BA, and Irene Yen, BA, provided assistance with study participant recruitment, neuropsychological testing, scanning, and image analysis.
Author Contributions: Study concept and design: Landau, Marks, Mormino, Rabinovici, Oh, Wilson, and Jagust. Acquisition of data: Mormino, Rabinovici, O’Neil, and Jagust. Analysis and interpretation of data: Landau, Marks, Mormino, O’Neil, and Jagust. Drafting of the manuscript: Landau, Mormino, Oh, and O’Neil. Critical revision of the manuscript for important intellectual content: Landau, Marks, Rabinovici, O’Neil, Wilson, and Jagust. Statistical analysis: Landau, Marks, and Oh. Obtained funding: Jagust. Administrative, technical, and material support: Landau, Mormino, Wilson, and Jagust. Study supervision: Landau, O’Neil, and Jagust.
References
- 1.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 2.Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67(1):122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pike KE, Savage G, Villemagne VL, et al. β-Amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130(pt 11):2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 4.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66(12):1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 5.Liang KY, Mintun MA, Fagan AM, et al. Exercise and Alzheimer’s disease bio-markers in cognitively normal older adults. Ann Neurol. 2010;68(3):311–318. doi: 10.1002/ana.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 7.Wilson RS, Mendes De Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287(6):742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 8.Scarmeas N, Levy G, Tang MX, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology. 2001;57(12):2236–2242. doi: 10.1212/wnl.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 10.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982-1983-83;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 11.Kramer JH, Jurik J, Sha SJ, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16(4):211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 12.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 13.Wilson R, Barnes L, Bennett D. Assessment of lifetime participation in cognitively stimulating activities. J Clin Exp Neuropsychol. 2003;25(5):634–642. doi: 10.1076/jcen.25.5.634.14572. [DOI] [PubMed] [Google Scholar]
- 14.Siscovick DS, Fried L, Mittelmark M, Rutan G, Bild D, O’Leary DH. Exercise intensity and subclinical cardiovascular disease in the elderly: the Cardiovascular Health Study. Am J Epidemiol. 1997;145(11):977–986. doi: 10.1093/oxfordjournals.aje.a009066. [DOI] [PubMed] [Google Scholar]
- 15.Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31(12):741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 16.Delis D. California Verbal Learning Test. 2. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 17.Mormino EC, Kluth JT, Madison CM, et al. Alzheimer’s Disease Neuroimaging Initiative. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132(pt 5):1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis, I: segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 19.Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20(1):70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- 20.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 21.Ségonne F, Dale AM, Busa E, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22(3):1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 22.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16(5):834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Price JC, Klunk WE, Lopresti BJ, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005;25(11):1528–1547. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- 24.Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc. 2005;11(4):400–407. [PubMed] [Google Scholar]
- 25.Valenzuela MJ, Sachdev P, Wen W, Chen X, Brodaty H. Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS One. 2008;3(7):e2598. doi: 10.1371/journal.pone.0002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jack CR, Jr, Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131(pt 3):665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aizenstein HJ, Nebes RD, Saxton JA, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65(11):1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bero AW, Yan P, Roh JH, et al. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat Neurosci. 2011;14(6):750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cirrito JR, Yamada KA, Finn MB, et al. Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron. 2005;48(6):913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 30.Kamenetz F, Tomita T, Hsieh H, et al. APP processing and synaptic function. Neuron. 2003;37(6):925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 31.Brody DL, Magnoni S, Schwetye KE, et al. Amyloid-β dynamics correlate with neurological status in the injured human brain. Science. 2008;321(5893):1221–1224. doi: 10.1126/science.1161591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326(5955):1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buckner RL, Sepulcre J, Talukdar T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29(6):1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jagust WJ, Mormino EC. Lifespan brain activity, β-amyloid, and Alzheimer’s disease. Trends Cogn Sci. 2011;15(11):520–526. doi: 10.1016/j.tics.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costa DA, Cracchiolo JR, Bachstetter AD, et al. Enrichment improves cognition in AD mice by amyloid-related and unrelated mechanisms. Neurobiol Aging. 2007;28(6):831–844. doi: 10.1016/j.neurobiolaging.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Lazarov O, Robinson J, Tang YP, et al. Environmental enrichment reduces Aβ levels and amyloid deposition in transgenic mice. Cell. 2005;120(5):701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 37.Kok E, Haikonen S, Luoto T, et al. Apolipoprotein E-dependent accumulation of Alzheimer disease-related lesions begins in middle age. Ann Neurol. 2009;65(6):650–657. doi: 10.1002/ana.21696. [DOI] [PubMed] [Google Scholar]