Abstract
The perception of the pleasantness of sweet tastes varies widely across individuals. Here, we exploit these differences to isolate brain response to sweet-taste pleasantness while controlling for intensity, quality, and physiological significance. Thirty subjects participated in functional MRI scanning while consuming individually calibrated weak and strong sucrose solutions. All subjects found the weak sweet taste to be neutral in pleasantness, but half of the subjects found strong sweet taste pleasant (likers), whereas half found strong sweet taste unpleasant (dislikers). Greater response was observed in the ventromedial prefrontal cortex (vmPFC) to the sucrose when it was rated pleasant versus neutral compared with unpleasant versus neutral. This suggests that response in the vmPFC underlies sweet-taste preference, this region is preferentially sensitive to affectively positive tastes, and it is the positive value rather than physiological significance, quality, or intensity that drives responses here. Likers versus dislikers did not differ in their diet, alcohol use, body weight, gender, or taq1A allele status, but likers were more likely to report emotional eating. None of these factors influenced response in the vmPFC.
Key words: fMRI, orbitofrontal cortex, sweet taste, taq1A, value, vmPFC
Introduction
It has been well established that sweet-taste preferences vary widely across individuals (Wundt 1897; Mennella et al. 2005, 2011). For example, some people prefer highly concentrated sucrose solutions, whereas others find these same stimuli too sweet (Pepino 2005). The goal of this present study was 2-fold: 1) to exploit this variability to isolate response to sweet-taste pleasantness that cannot be attributed to quality or intensity, and 2) to investigate the effects of other factors that have been implicated in variation in taste responses, including genotype and alcohol use, on brain coding of sweet taste.
Prior studies attempting to isolate brain correlates of the perceived pleasantness of taste have suffered from confounds related to stimulus intensity and/or quality. For example, a bitter stimulus is used to represent a negative valence and a sweet stimulus a positive valence (Zald et al. 2002; Small et al. 2003). Although differential responses were observed, it is unclear whether taste quality (sweet vs. bitter) or valence (pleasant vs. unpleasant) is driving the effect. Given that people can have opposing hedonic reactions to a strong sweet taste, it follows that perceived pleasantness and quality coding can be independent. This possibility is consistent with the fact that calorie ingestion can be reinforcing and drive dopamine response in the absence of sweet taste (De Araujo et al. 2008). Thus, we sought to determine if we could isolate responses that reflect perceived pleasantness, unconfounded by intensity and quality.
Of particular interest to us was the ventromedial prefrontal cortex (vmPFC). This region consistently responds to tastes and flavors as a function of rated pleasantness (Small et al. 2001; De Araujo et al. 2003b; Kringelbach et al. 2003; McClure et al. 2004; Plassmann et al. 2008). The vmPFC has also been proposed to play a general role in calculating the value of food (Hare et al. 2009), which is influenced not only by sensation but also by caloric value (De Araujo et al. 2008). We reasoned that if the magnitude of response in this region reflects the perceived pleasantness of sweet taste, then we should observe greater response to a strong sucrose solution rated as pleasant (likers) compared with an equally strong sucrose solution rated as unpleasant (dislikers). Other regions of interest were the insular cortex, caudolateral orbitofrontal cortex (OFC), and striatum, as hedonic responses to taste and flavor stimuli are consistently isolated in these regions (O’Doherty et al. 2001, 2002; Small et al. 2001; Kringelbach et al. 2003; McClure et al. 2004; McCabe and Rolls 2007; Haase et al. 2009).
In addition, we assessed history of familial and personal alcohol use, as well as taq1a polymorphism genotype, as these factors have been shown to influence response to sweet taste. Specifically, carriers of the A1 allele have reduced brain responses to tasting sweet milk shakes (Felsted et al. 2010), and several studies suggest that links exist between alcohol use and sweet-taste perception or consumption (reviewed in Kampov-Polevoy et al. 1999). For example, rodents (Kampov-Polevoy et al. 1990) and humans (Kampov-Polevoy et al. 1997) who prefer higher concentrations of sucrose consume more alcohol. We also collected information on family history of alcoholism, as there is evidence that those who prefer high concentrations of sweet taste are more likely to have a family history of alcoholism (Pepino and Mennella 2007). We hypothesized that brain response to sweet taste in reward areas would correlate with alcohol use and family history of alcoholism and that A1 carriers would have reduced responses to the sweet tastes.
Materials and methods
Subject selection
This research protocol was approved by the Yale University Human Investigation Committee. Eighty-one subjects were recruited by advertisements posted around Yale University and the greater New Haven area. Only subjects between the age of 18 and 45 were included. Subjects were excluded if they had any nonremovable metal on their body, were currently or recently taking major medications such as antidepressants, were claustrophobic, had a history of food allergies, diabetes, or any psychiatric disorder or drug abuse.
Prior to the experiment, subjects were given a description of the paradigm and provided written informed consent. All subjects completed an initial screening session. Thirty-four subjects were excluded after this session based on ratings falling outside of the target ranges. Those who qualified (47) completed an additional screening and training session on that same day. Data from this session excluded another 12 subjects. Reasons for exclusion included ratings collected in the mock scanner not falling in the target range, discomfort with the scanning environment, or an unwillingness to consume sucrose repeatedly. Thirty-five subjects participated in scanning. After excluding subjects with excessive head movement (greater than 2mm in any direction in more than 2 scanning runs), 30 subjects (18 females, age ± standard deviation [SD] = 24.5±5.8) were used for final functional MRI (fMRI) analysis.
Genotyping procedure
Genotyping procedures were the same as those used in a study by Felsted et al. (2010). Briefly, the Oragene-DNA Self-collection kit was used to collect, preserve, and purify DNA from saliva. The TaqIA single nucleotide polymorphism region of the ANKK1 gene, located near the DRD2 gene (Neville et al. 2004), was amplified using polymerase chain reaction (PCR) for each subject. The amplified DNA was analyzed using gel electrophoresis, and the presence or absence of the taqIA allele was determined based on predicted sizes of PCR products. For details, see Felsted et al. (2010).
Stimuli
Stimuli were solutions of sucrose diluted to 0.045, 0.09, 0.13, 0.18, 0.32, 0.56, 0.7, 0.85, 1.0, 1.25, 1.5, and 2.0. Because water activates taste cortex (De Araujo et al. 2003a), we used dilutions of a 0.0125M KCl and 0.00125M NaHCO3 solution in distilled water as our control stimulus. This solution mimics the ionic components of saliva (O’Doherty et al. 2001), and prior work in our (Small et al. 2003, 2008) and other laboratories (O’Doherty et al. 2001; De Araujo et al. 2003a) has shown that the rinse solution, which is diluted to 3/4, 1/2, or 1/4 strength according to whether the subject perceived it as tasteless, is a good baseline stimulus to control for the somatosensory and motor effects of receiving and swallowing a liquid, without introducing the potentially confounding taste of water.
Screening sessions
An initial screening session was carried out to assess perception of a concentration series of sucrose solutions. After providing written informed consent, subjects completed brief training modules on using the general labeled magnitude scale (gLMS) (Green et al. 1996; Bartoshuk et al. 2003) and Visual Analog Scale (VAS). The gLMS is a vertical-line scale of 100mm with the label “barely detectable” at the lower anchor and the label “strongest imaginable sensation” at the upper anchor. In between these labels, the following words were approximately logarithmically spaced: “barely detectable” (1.4mm), “weak” (5.8mm), “moderate” (17mm), “strong” (35mm), and “very strong” (53mm). No numbers appear on the scale. The VAS was a horizontal 200mm line with the ends labeled “most unpleasant sensation ever” (−100), “most pleasant sensation ever” (100) and the midpoint labeled “neutral” (scored as 0). The scale training included practice trials in which subjects rated the intensity of a range of real and imaginary stimuli. Subjects then completed two series of sucrose sampling and rating, one for rating intensity and one for rating pleasantness, in counterbalanced order. They sampled and swallowed 1mL of each of the sucrose solutions from a pipette, in randomized order. After sampling the solution, they rated its intensity on the gLMS (in one series) or its pleasantness on the VAS (in a different series).
Once the ratings were complete, subjects filled out questionnaires about their diet, eating behavior, and personal and familial alcohol use. These included the Nutrition Questionnaire (Willett et al. 1985), the Dutch Eating Behavior Questionnaire (DEBQ) (Van Strien et al. 1986), the Family Tree questionnaire (Mann et al. 1985), the Alcohol Use questionnaire (Mehrabian and Russell 1978), and the Short Inventory of Problems (SIP-2R) (Forcehimes et al. 2007). Concurrently, the experimenter determined if ratings of sucrose solutions met criterion for assignment to one of two groups: likers or dislikers. Subjects were assigned to the likers group if 1) one stimulus could be identified that was consistently rated as weak in intensity on the gLMS (between 2 and 10) and neutral in liking on the VAS (between −10 and 10) and 2) a second stimulus could be identified that was consistently rated as strong in intensity on the gLMS (between 30 and 40) and at least moderately “liked” on the VAS (25 or above). Subjects were assigned to the dislikers group if 1) one stimulus could be identified that was consistently rated as weak in intensity on the gLMS and neutral in liking on the VAS and 2) a second stimulus was identified that was consistently rated as strong in intensity on the gLMS and at least moderately “disliked” on the VAS (−25 or below). Thus, a weak sucrose solution was identified that was similarly weak and neutral in pleasantness for both groups, and a strong sucrose solution was identified that was similar in intensity but opposite in affective valence. Approximately 45% of subjects screened met criteria for inclusion into one of the groups.
Once assigned to a group, participants were scheduled for an fMRI training session. This session, which took place in a mock fMRI scanner, served to establish the stability of intensity and pleasantness ratings and to ensure that subjects were comfortable in the fMRI environment. In the mock scanner, subjects tasted the three sucrose drinks that they had rated as closest to weak and the three that they had rated as closest to strong on the gLMS. Each solution was delivered 5 times, and ratings were made after each delivery. The experimenter then evaluated these ratings for consistency with session 1 ratings. If responses were inconsistent, subjects were excluded. Subjects were also asked to indicate whether they felt anxious or uncomfortable with the procedure. Those who expressed concern were not asked to participate in the actual scanning session.
Stimulus delivery
In both the training session and the fMRI scan, subjects received the solutions through an fMRI-compatible custom-designed gustometer (Veldhuizen et al. 2007, 2010). The gustometer device operates via infusion pumps (BrainTree Scientific) that released a 0.5-mL aliquot of the appropriate stimulus at a rate of 1L/min in each trial. Pumps were controlled by custom-made software (Matlab, MathWorks) whose operation was linked to scanner pulses. Each pump held a 60-mL syringe connected to a 25-foot length of Tygon beverage tubing that terminated into a specially designed Teflon, fMRI-compatible gustatory manifold that was anchored to the MRI head coil. The manifold was mounted by rigid tubing onto an anchoring block that clamped onto the bars of the head coil. Tastant lines were arrayed around a tasteless line in a circular pattern, and all tastants and rinses are delivered through a 1-mm channel that passes through the entire manifold. These 1-mm channels converged at a central point at the bottom end of the manifold. To prevent the subjects tongue from coming in contact with the 1-mm holes, a 7-mm sphere was positioned directly under the end of the 1-mm channels and rested directly above the subject’s tongue. All subjects were instructed to allow the liquid to roll off the sphere onto the tip of the tongue but to refrain from swallowing until instructed. The sphere also ensured maintenance of constant tactile stimulation across events.
Each taste event consisted of delivery of one of the sucrose solutions as a 1mL bolus over 3 s, a rest period of 13–17 s (a jitter), a cue to swallow the solution, a 1.0-mL bolus rinse of tasteless solution (delivered over 3 s), and a second jitter and swallow cue. Control events (which were separate from the rinses) consisted of delivery of 1.0mL of tasteless solution over 3 s, a 13–17 s jitter and a swallow cue. The subject is trained to hold the taste in their mouth until cued to swallow15 s after delivery. The swallow is delayed so that the blood oxygen level–dependent (BOLD) signal, which peaks 4–5 s after delivery, is not contaminated by movement artifacts related to swallowing.
fMRI session
Upon arrival to the fMRI center, subjects once again rated their 3 “weak” and 3 “strong” sucrose solutions on the VAS and gLMS. These ratings were used to determine the weak and strong stimulus to be used during scanning. The resulting concentrations used in scanning ranged from 0.045M to 0.32M (average 0.086M ± 0.058M) for weak sweet and from 0.7M to 2.0M (average 1.45M ± 0.400M) for strong sweet. Subjects were excluded at this point if stimuli could not be identified, which met all criteria for target ranges of liking and intensity. Subjects underwent 5 functional runs of 9min, 12 s each, as well as a structural scan after the third functional run. The anatomical scan is performed midway into the session to provide a short break from chemosensory stimulation. In each run, subjects received all stimuli 7 times, for a total of 35 repetitions per stimulus (weak, strong, and tasteless) to be used for intertrial averaging. Stimuli were delivered as described earlier. No ratings were collected during scanning because prior work shows that performance of rating tasks alter gustatory responding (Grabenhorst et al. 2008; Bender et al. 2009).
Functional and anatomical images were collected with a 3T Siemens Trio scanner using a 12-channel head coil. Echoplanar imaging was used to measure the BOLD signal as an indication of cerebral brain activation. A susceptibility-weighted single-shot echoplanar method was used to image the regional distribution of the BOLD signal with time repetition [TR] = 2000ms, time echo [TE] = 20ms, flip angle (FA) = 80, matrix = 64×64, slice thickness = 3mm, and acquisition of 40 contiguous axial slices. Slices were acquired in an interleaved mode to reduce the cross talk of the slice selection pulse. At the beginning of each functional run, the MR signal was allowed to equilibrate over 6 scans for a total of 12 s, which were excluded from analysis.
The anatomical scan used a T1-weighted 3D FLASH sequence with TR = 2530, TE = 3.66, FA = 7, matrix = 256×256, acquiring 160 one mm sagittal slices, with a saturation band placed over the neck to reduce distortion in the temporal lobes caused by blood flow.
Data analysis
Group demographics and ratings were compared using unpaired t-tests (for continuous variables) and chi-square tests using Fisher’s Exact test (for nonparametric data including handedness, gender, and taq1A allele status).
Neuroimaging data were pre- and postprocessed using SPM5 (Welcome Department of Cognitive Neurology) on Linux workstations running MATLAB (Mathworks) using standard procedures (Friston et al. 1994; Worsley and Friston 1995; Veldhuizen et al. 2007). Functional images were time acquisition corrected to the slice obtained at 50% of the TR. All functional images were then realigned to the scan immediately preceding the anatomical T1 image. In addition to excluding subjects with excessive head movement (greater than 2mm in any direction during more than 2 separate functional runs) from analysis, we also used the art toolbox to create regressors for each volume in which movement exceeded 1mm and included these regressors and realignment parameters in the design matrix as nuisance regressors. Images were normalized to the Montreal Neurological Institute template (MNI-305), smoothed with a 6-mm full width at half-maximum isotropic Gaussian kernel, and high-pass (128 s) filtered to remove noise. Normalization resulted in a voxel size of 3mm3 for functional images and a voxel size of 1mm3 for structural images. Condition-specific effects at each voxel were estimated using the general linear model. The response to events was modeled by a canonical HRF, consisting of a mixture of 2 gamma functions that emulate the early peak at 5 s and the subsequent undershoot (Friston et al. 1994). The temporal derivative of the hemodynamic function was also included as part of the basis set to account for small deviations in timing from the canonical HRF.
We created 3 events of interest: strong sweet (SS), weak sweet (WS), and tasteless (tls). Statistical parametric maps (SPMs) reflecting the smoothed response (parameter estimate) at each voxel across the whole volume for each event of interest were entered into a random effects model at the second level in order to account for intersubject variability (Strange et al. 1999). We created an analysis of variance (ANOVA) with the following factors: stimulus (3 levels: SS, WS, and tls) and group (2 levels: likers and dislikers). Contrasts between the events of interest were created at the second level. To determine the main effect of taste across all subjects, we weighted the events of interest for both groups as follows ([SS + WS] − tls): 1 + 1 − 2. To determine the main effect of stimulus across all subjects, we created the contrast SS − WS (1 − 1) for both groups. To determine the effect of stimulus between groups, we compared SS − WS (1 − 1) in likers versus SS − WS (1 − 1) in dislikers.
SPMs were thresholded for initial display at P uncorrected < 0.005, with a cluster criterion of 3 voxels. To determine which of the resulting activations were significant, we used the false discovery rate (FDR) correction for multiple comparisons. Predicted peaks were considered significant if their associated P-value was less than 0.05 FDR-corrected across the region of interest (ROI). Unpredicted peaks were considered significant if their associated P-value was less than 0.05 FDR-corrected across the entire brain. The ROI for the main effect analyses included feeding-, taste-, and reward-related areas: insula, OFC, Rolandic operculum, thalamus, amygdala, striatum, and ventral pallidum. These regions were selected using WFU pickatlas (Maldjian et al. 2003) to create a single ROI of all regions. For the main effect of intensity, we created an ROI with WFU pickatlas that included the amygdala and insula (Small et al. 2003). For the between-group analyses, we were interested in testing for differential response in the insula, ventral striatum (Gottfried et al. 2003), and vmPFC (Hare et al. 2009). WFU pickatlas was used to define the insula. However, this software does not include a ventral striatum or vmPFC ROI. Therefore, we drew 10mm spheres with centers defined by peaks reported by Gottfried et al. and Hare et al. Second-level group analyses were also performed with regressors as variables of interest or as covariates to test whether effects were related to eating style, hunger, fullness, genotype, and alcohol use.
To test for the effect of taqIA allele status on brain response to sweet taste, we split subjects into groups of carriers (N = 12) and noncarriers (N = 17) and tested for group differences in the main effect of taste using the same procedure as above, except that the ROI was created from 10mm spheres around the 5 peaks that Felsted et al. (2010) found to be more responsive to milk shake in noncarriers versus carriers.
To test for areas where response to sweet taste was related to personal alcohol use, we regressed SIP-2R scores against whole brain response to (SS + WS − tls) in all subjects. For this analysis, we used the taste- and reward-related areas ROI created earlier.
Results
Group demographics
The 30 subjects included in final analysis (18 females, 27 right-handed) were healthy weight or overweight (body mass index [BMI] average ± SD = 23.4±2.6). Twelve individuals carried at least 1 copy of the taq1A gene. The demographic breakdown of each group is included in Table 1, and the groups did not differ significantly on any of these factors.
Table 1.
Demographics, ratings, and questionnaires by group
| Likers | Dislikers | P | |
|---|---|---|---|
| Gender | 8 females | 10 females | 0.71 |
| Age | 23.1 (4.7) | 25.9 (6.6) | 0.18 |
| BMI | 23.6 (2.4) | 23.2 (2.8) | 0.71 |
| Handedness | 14 right | 13 right | 1.00 |
| Taq1A carriers | 8 (+) | 4 (+)a | 0.26 |
| Hunger VAS | 21.2 (41.8) | −6.7 (43.9) | 0.09 |
| Fullness VAS | −49.2 (37.7) | −15.3 (45.0) | 0.04* |
| WS intensity | 7.8 (5.3) | 7.2 (4.3) | 0.72 |
| SS intensity | 35.3 (11.9) | 32.9 (14.6) | 0.79 |
| Tls intensity | 4.6 (6.0) | 3.9 (6.7) | 0.78 |
| WS pleasantness | −2.9 (17.1) | 3.0 (19.5) | 0.37 |
| SS pleasantness | 55.1 (21.7) | −37.6 (29.4) | <0.0001* |
| Tls pleasantness | −4.4 (13.1) | −3.5 (30.1) | 0.91 |
| SIP-2R | 2.6±3.8 | 1.6±1.5 | 0.35, ns |
| Alcohol Use | 23.2±13.5 | 20.3±12.1 | 0.53, ns |
| DEBQ EE | 2.45±0.84 | 1.83±0.57 | 0.0237* |
| DEBQ Ext. E | 3.3±0.4 | 3.0±0.5 | 0.1217, ns |
| DEBQ RE | 2.4±0.9 | 2.1±0.7 | 0.3018, ns |
| Family Tree alcohol | 7.3±4.3 | 9.9±5.1 | 0.1331, ns |
ns, not significant. EE = Emotional Eating, Ext. E = External Eating, RE = Restrained Eating
1 subject missing data.
aOut of 14 subjects.
*Significant difference.
Stimulus ratings
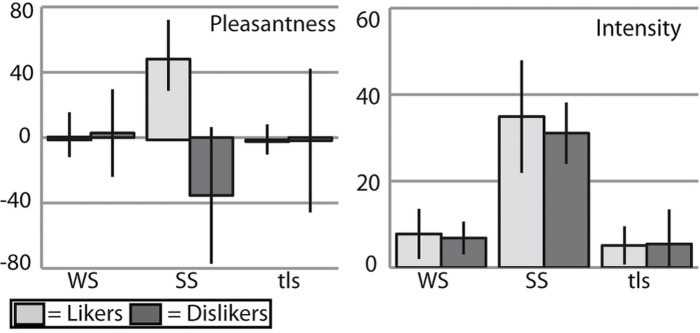
As intended, ratings indicated that participants were neither hungry nor full at the time of scanning (VAS hunger ratings: 11.0±48.4; VAS fullness ratings: −28.0±47.1; see Table 1). However, dislikers showed higher fullness ratings than likers (−15.3±45.0 vs. −49.2±37.7; F 1,28 = 4.85, P = 0.04) and a trend toward lower hunger ratings (−6.7±43.9 vs. 21.2±41.8; F 1,28 =3.08, P = 0.09). Intensity and pleasantness ratings collected on the day of fMRI scanning demonstrated that our groups showed the expected perceptions of sucrose solution (Figure 1). Also as intended, the groups did not differ significantly on their intensity ratings of WS (likers: 7.8±5.3 vs. dislikers: 7.2±4.3; F 1,28 = 0.13, P = 0.72), SS (35.5±11.9 vs. 32.9±14.6; F 1,28 = 0.22, P = 0.79), or tls (4.6±6.0 vs. 3.9±6.7; F 1,28 = 0.14, P = 0.78), or on their pleasantness ratings of WS (−2.9±17.1 vs. 3.0±19.5; F 1,28 = 0.82, P = 0.37), or tls (−4.4±13.1 vs. −3.5±30.1; F 1,28 = 0.01, P = 0.91). In contrast, and as expected, likers found SS significantly more pleasant than the dislikers did (55.1±21.7 vs. −37.6±29.4; F 1,28 = 93.47, P < 0.0001). We also note that the average intensity rating (likers and dislikers) for WS was 7.5. Because “weak” corresponds to 5.8, we infer that subjects consistently experienced a weak sweet sensation in response to the WS stimulus. The average rating for the tasteless solution was 4.3, which is consistently below “weak.” Nevertheless, it was clear that some subjects detected a weak taste in our “tasteless” solution.
Figure 1.
Pleasantness and intensity ratings. Graphs showing stimuli ratings by group. X-axis: stimulus (WS = weak sweet, SS = strong sweet, tls = tasteless). Y axis: group average rating. Pleasantness ratings were made on a VAS scale; intensity ratings were made on the gLMS (see Materials and methods). As expected, all subjects found WS and tls to be weak and SS to be strong in intensity. All subjects found WS and tls to be approximately neutral in pleasantness. Likers found SS strongly pleasant, whereas dislikers found SS strongly unpleasant.
Eating style
Likers scored significantly higher than dislikers on the emotional eating subscale of the DEBQ. No other significant effects were observed. Results are summarized in Table 1.
Analysis of the scores on the Nutrition Questionnaire suggests that likers and dislikers do not habitually consume different amounts or types of food. Although a trend is observed for likers to consume more vegetables than dislikers (25.5±6.5 vs. 19.9±6.6, P = 0.04), significance does not survive Bonferroni correction. No trends for differences in consumption were found for protein (34.7±8.4 vs. 30.3±7.1, P = 0.16), fruits (24.8±7.3 vs. 20.4±6.0, P = 0.11), dairy (26.5±5.0 vs. 22.9±9.0, P = 0.22), starches and grains (44.9±7.5 vs. 43.0±3.2, P = 0.63), sweet foods (37.2±8.1 vs. 38.3±16.2, P = 0.82), salty foods (29.9±6.3 vs. 28.3±12.0, P = 0.69), or fatty foods (65.6±9.3 vs. 67.8±32.7, P = 0.82).
Genotype
Twelve individuals carried 1 or 2 copies of the taq1A A1 polymorphism, with 5 A1/A1 homozygotes (3 likers; 2 dislikers) and 7 A1/A2 heterozygotes (5 likers; 2 dislikers). Eight carriers were likers and 4 were dislikers. However, groups did not differ for total number of carriers (8/15 likers vs. 4/14 dislikers; P = 0.17). In this sample, we find no relationship between taq1A allele status and family history of alcohol use (P = 0.44), personal alcohol use (P = 0.20), BMI (P = 0.40), hunger ratings (P = 0.13), strong sweet-taste pleasantness ratings (P = 0.11), or sucrose concentration chosen as strong (P = 0.33).
Neuroimaging
Main effects
Main effect of sweet taste ([SS + WS] – tls).
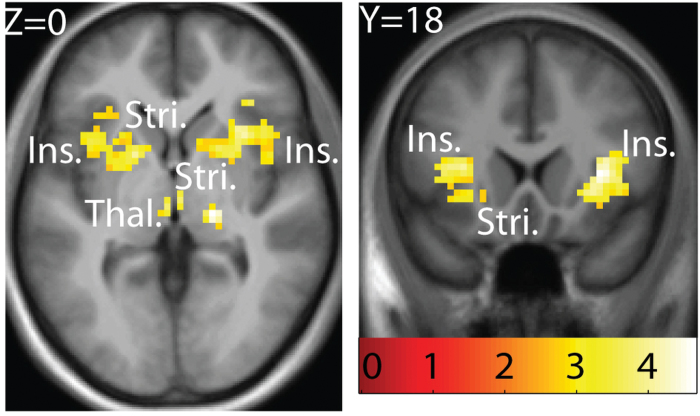
All neuroimaging results are shown in Table 2. Significant responses to sweet taste were observed in the left insula (−36, 0, 12, z = 3.97, P = 0.008, k = 328 including −36, 15, 9, z = 3.96, P = 0.008), extending into the ventral striatum (−18, 6, 0, z = 3.80, P = 0.008), the right insula (33, 18, 6, z = 4.30, P = 0.008, k = 315 including 30, 6, 9, z = 4.18, P = 0.008 and 39, −3, 18, z = 3.81, P = 0.008; as well as 45, −21, 21, z = 3.37, P = 0.009, k = 17), the putamen (18, −21, 0, z = 4.37, P = 0.008, k = 33), the thalamus (−3, −18, 0, z = 3.55, P = 0.009, k = 8 and 3, −15, 0, z = 3.53, P = 0.009, k = 4), and the left striatum (−24, −9, 12, z = 3.22, P = 0.019, k = 9 (Figure 2).
Table 2.
fMRI results based on selected regions of interest
| Area | Coordinates | z | P | k |
|---|---|---|---|---|
| Main effect of sweet taste (SS + WS − tls in all subjects) | ||||
| Right putamen | 18, −21, 0 | 4.37 | 0.008 | 33 |
| Right insula | 33, 18, 6 | 4.30 | 0.008 | 315 |
| 30, 6, 9 | 4.18 | 0.008 | ||
| 39, −3, 18 | 3.81 | 0.008 | ||
| 45, −21, 21 | 3.37 | 0.009 | 17 | |
| Left insula | −36, 0, 12 | 3.97 | 0.008 | 328 |
| −36, 15, 9 | 3.96 | 0.008 | ||
| Left ventral striatum | −18, 6, 0 | 3.80 | 0.008 | |
| Thalamus | −3, −18, 0 | 3.55 | 0.009 | 8 |
| 3, −15, 0 | 3.53 | 0.009 | 4 | |
| Left striatum | −24, −9, 12 | 3.22 | 0.019 | 9 |
| Affective value of sweet taste (SS − WS in likers vs. dislikers) | ||||
| Right vmPFC | 9, 33, −15 | 3.03 | 0.048 | 13 |
| Left ventral striatum | −12, 6, −6 | 3.09 | 0.085 | 8 |
| Greater response to sweet in taq1A noncarriers (SS + WS − tls in noncarriers vs. carriers) | ||||
| Amygdala/insula | −33, −6, −6 | 3.81 | 0.039 | 7 |
* italicized peaks indicate that the peak is part of the cluster of the preceding peak.
Figure 2.
Main effect of sweet taste and intensity. A feeding network including bilateral insula (ins.) and striatum (stri.), as well as thalamus (thal.) responds to sweet taste independent of intensity or pleasantness. T-map is thresholded at P < 0.005 and k > 3 voxels. Activations are significant at P < 0.05 FDR-corrected across ROI. Coordinates and statistics are provided in Results section and in Table 2.
Main effect of intensity (SS – WS).
No significant main effect of intensity was observed.
Between-group comparisons
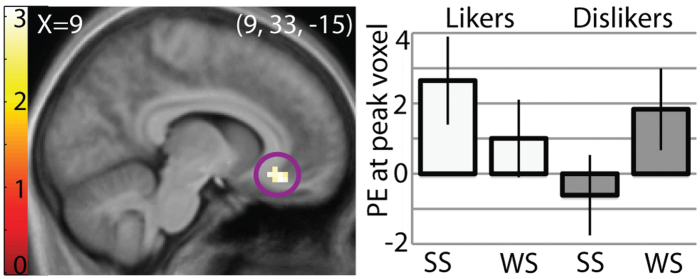
Likers versus dislikers (SS − WS in likers vs. SS − WS in dislikers).
To isolate regions responding to the affective value of the sucrose independently of sweet quality coding, we compared the contrast of SS versus WS between the groups. This analysis capitalizes on the effect of intensity on perceived pleasantness/unpleasantness while ensuring that group differences in intensity do not drive results. It also allows us to remove potential nonspecific effects of group. Likers showed significantly greater responses in the right vmPFC (9, 33, −15, z = 3.03, P = 0.048, k = 13; Figure 3) and a trend toward greater response in the left ventral striatum (−12, 6, −6, z = 3.09, P = 0.085, k = 8). The vmPFC did not respond more to WS versus SS in dislikers. No other significant effects were observed in insula or elsewhere in the brain. Including eating style variables, hunger, fullness, genotype, or SIP-2R scores as covariates in the design did not change these results. No areas responded more to SS − WS in dislikers versus likers.
Figure 3.
Regions coding affective value of a sweet taste. Sagittal section showing the vmPFC responding to strong sweet (SS) versus weak sweet (WS) in likers versus dislikers, suggesting that this area is responsive to the affective value of a sweet taste. Results are from ANOVA contrast of (SS − WS) in likers – (SS − WS) in dislikers. T-map is thresholded at P < 0.005 and k > 3 voxels. Bar graph shows parameter estimates (PE) of the activity in peak voxel within the circled brain region in response to each stimulus in each group, averaged over subjects. Activation in the vmPFC is significant at P = 0.048 after FDR correction across the voxels of the ROI.
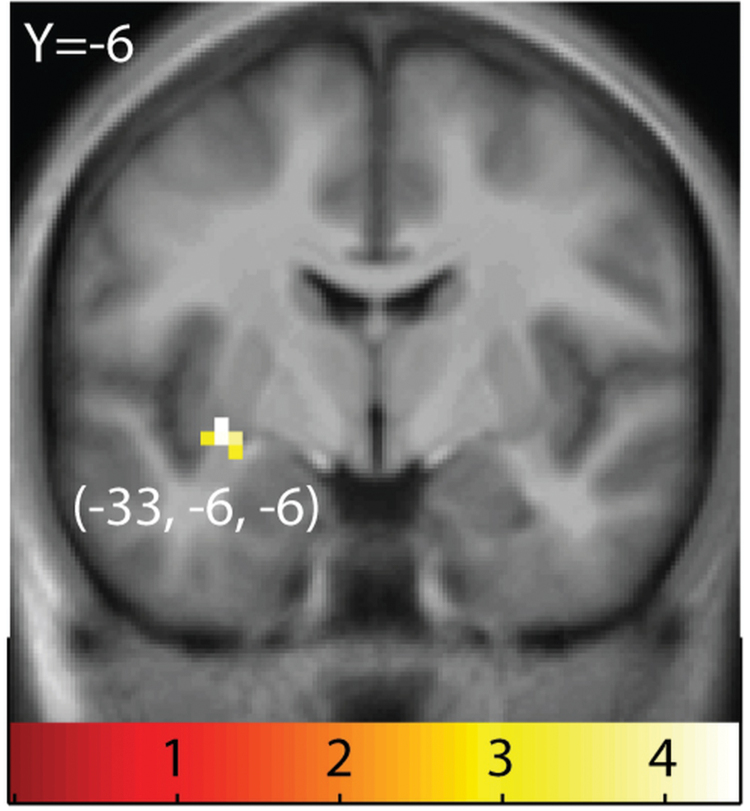
Carriers versus noncarriers (SS − WS in carriers vs. SS − WS in noncarriers).
Carriers showed a significantly decreased response to SS − WS in the ventral striatum (−33, −6. −6; z = 3.81; P = 0.039; k = 7; Figure 4).
Figure 4.
Regions showing greater response to sweet taste in taq1A noncarriers versus carriers. Sagittal section showing greater response to SS + WS − tls in noncarriers versus carriers of the A1 allele in the ventral striatum. The results are from random effects contrast of (SS + WS − tls) in noncarriers – (SS + WS − tls) in carriers. T-map is thresholded at P < 0.005 and k > 3 voxels.
Relationship between alcohol use and brain response to sweet taste.
When we regressed SIP-2R or alcohol use scores against brain response to (SS + WS − tls) or to (SS − tls), we found no significant correlations. We did not have sufficient subjects with a known family history of alcoholism (n = 3) to adequately address its relationship to brain response to sweet taste.
Discussion
In this present study, we used fMRI to test the influence of sweet-taste preference on brain response to the taste of sucrose. Of particular interest was determining whether a similarly intense sucrose solution would result in differential response as a function of whether it was liked or disliked. We found that the only region influenced by preference was the vmPFC and conclude that this region reflects perceived pleasantness irrespective of taste quality. As such, we suggest that processing in this area underlies individual differences in sweet-taste preference. Importantly, the response is not influenced by hunger, fullness, genotype, alcohol use, or eating style.
Influence of preference on brain response to sucrose
In keeping with prior work, sampling a sweet taste produced robust responses in the insula, striatum, and thalamus (Kinomura et al. 1994; Kobayakawa et al. 1996; Faurion et al. 1998; O’Doherty et al. 2001; Small et al. 2003; Haase et al. 2009; Rudenga et al. 2010). Importantly, these responses occurred in sweet likers and dislikers, and for both strong and weak tastes, suggesting that they are independent of intensity and affective value.
In contrast to prior work (Small et al. 2003; Spetter et al. 2010), we did not observe effects of intensity in the amygdala and insula. However, supporting the existence of quality-independent affective responses, we found that the vmPFC response to SS compared with WS was significantly greater in likers compared with dislikers. This is consistent with work demonstrating that odor-evoked responses in the medial OFC are correlated with pleasantness but not intensity (Anderson et al. 2003) and with a diverse literature supporting a role for this area in coding the value of stimuli as diverse as food pictures (Litt et al. 2011), touch (Francis et al. 1999; Rolls et al. 2003; Gordon et al. 2013), music (Blood and Zatorre 2001), money (Weber et al. 2009), and even words (Lewis et al. 2007). Also consistent with past studies is the right lateralized response. A prior meta-analysis demonstrated that the right OFC is more likely to respond to pleasant taste stimuli than the left OFC (Gottfried et al. 2006). Finally, although likers reported less fullness, these ratings were not associated with vmPFC response. Therefore, it is unlikely that group differences in hunger or fullness account for the preferential response in likers. This is an important consideration because vmPFC response to food has been associated with internal state (Small et al. 2001).
Contrary to our prediction, differential responses were not observed in the caudolateral OFC. This higher order gustatory region receives taste information from the anterior insula and the caudomedial OFC (Rolls et al. 1990; Pritchard et al. 2005), and it is believed to play a critical role in associating the sensory properties of foods with their reward value (Rolls and Grabenhorst 2008). Previous neuroimaging work identified differential responses in the vmPFC and caudolateral OFC to sweet versus bitter (Zald et al. 2002; Small et al. 2003) and sweet versus salty (O’Doherty et al. 2001) taste stimuli and attributed these effects to the opposing affective responses evoked (pleasant vs. unpleasant). However, because stimuli differed in both valence and quality, it was not possible to ascribe the differential brain response specifically to valence. The present findings resolve this controversy by showing that value drives responses independently of quality in the vmPFC but not in the caudolateral OFC.
One caveat we note is that it is possible that subjects could have different tongue movement or swallowing patterns in response to pleasant versus unpleasant tastes. Although we cannot rule this out as a potential confound, studies of swallowing (Hamdy et al. 1999; Kern et al. 2001; Martin et al. 2007) and tongue movement (Corfield et al. 1999; Veldhuizen et al. 2007) suggest that these movements are primarily associated with responses in the sensorimotor cortical areas and not in the vmPFC.
Influence of eating style, alcohol use, and genotype on brain response to sucrose
Because eating style, alcohol use, and taq1A allele genotype have been associated with perceptual and/or brain response to food (Kampov-Polevoy et al. 1997; Barnard et al. 2009; Burger and Stice 2011), we evaluated whether these factors were associated with liker status and brain response.
In agreement with previous findings that emotional eating is associated with liking for sweet foods (Nguyen-Michel et al. 2007; Keskitalo et al. 2008), likers scored higher on the emotional eating subscale of the DEBQ than dislikers did. No other group differences were observed. In addition, vmPFC response was not associated with emotional eating.
Taq1A genotype has been associated with food intake, diabetes, and body weight (Blum et al. 1996; Barnard et al. 2009), with individuals who possess a copy of the A1 allele showing increased risk for obesity and diabetes. In addition, Stice et al. (2008) found that the presence of the A1 allele moderates the relationship between striatal response to food intake and obesity. Furthermore, Felsted et al. (2010) found that carriers of the A1 allele show reduced response to a palatable milk-shake drink in the midbrain, thalamus, OFC, caudate, and hippocampus compared with noncarriers. We found no relationship between taq1A genotype status and sweet-taste liking. However, we did observe reduced response to sweet taste in the ventral striatum in carriers compared with noncarriers. This result is in keeping with an influence of taq1A genotype on brain response during consumption of caloric beverages and with the observation that responses are reduced in carriers; however, the brain regions where we observed the effect were different from what was observed in prior studies. One possible explanation for this difference is that in prior work all subjects reported the milk shake as pleasant tasting, whereas in the current sample half of the subjects experienced the strong sweet stimulus as unpleasant.
Finally, rodent and human studies have linked sweet-taste perception to alcohol consumption (Bachmanov et al. 1996; Kampov-Polevoy et al. 1999, 2004; Lemon et al. 2004; Pepino and Mennella 2007; Blednov et al. 2008; Brasser et al. 2010). In general, greater liking or intake of sweet substances is believed to be associated with increased preference and use of alcohol. This effect is mediated in part by central gustatory substrates, with concentration-dependent responses to ethanol present in sucrose-responsive but not in non-sucrose-responsive neurons (Lemon et al. 2004). In this present study, alcohol consumption was unrelated to sweet liking or to brain response to sweet taste. Unfortunately, our sample included an insufficient number of subjects with a known family history of alcoholism to adequately address this question.
Summary
The results from this present study indicate that response in the vmPFC may underlie individual differences in sweet-taste preference and that response to sucrose in this region reflects the affective value of sucrose rather than sweet quality coding. We also demonstrate that this response is unrelated to perceptions of hunger or fullness, eating style, taq1a genotype, or personal alcohol use.
Funding
This work was supported by the National Institutes of Health [R01 DC006706 awarded to D.M.S. and NIH NRSA F31-DC010557-01 awarded to K.J.R.].
Acknowledgements
The authors wish to thank Wambura Fobbs for assistance with pilot testing, Sarah Nolan-Poupart and Lara Fourman for assistance with data collection, and Dr Marga Veldhuizen for assistance with data processing.
Conflict of interest: The authors declare that they have no competing financial conflicts of interest.
References
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Gabrelli JDE, Sobel N. 2003. Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci. 6(2):196–202 [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK. 1996. Ethanol consumption and taste preferences in C57BL/6ByJ and 129/J mice. Alcohol Clin Exp Res. 20(2):201–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard ND, Noble EP, Ritchie T, Cohen J, Jenkins DJ, Turner-McGrievy G, Gloede L, Green AA, Ferdowsian H. 2009. D2 dopamine receptor Taq1A polymorphism, body weight, and dietary intake in type 2 diabetes. Nutrition. 25(1):58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoshuk L, Duffy V, Fast K, Green BG, Prutkin J, Snyder D. 2003. Labeled scales (e.g., category, Likert, VAS) and invalid across-group comparisons: what we have learned from genetic variation in taste. Food Qual Prefer. 14(2):125–138 [Google Scholar]
- Bender G, Veldhuizen MG, Meltzer JA, Gitelman DR, Small DM. 2009. Neural correlates of evaluative compared with passive tasting. Eur J Neurosci. 30(2):327–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Martinez M, Levine M, Damak S, Margolskee RF. 2008. Perception of sweet taste is important for voluntary alcohol consumption in mice. Genes Brain Behav. 7(1):1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ. 2001. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci U S A. 98(20):11818–11823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Braverman ER, Wood RC, Gill J, Li C, Chen TJH, Taub M, Montgomery AR, Sheridan PJ, Cull JG. 1996. Increased prevalence of the Taq I A1allele of the dopamine receptor gene (DRD2) in obesity with comorbid substance use disorder: a preliminary report. Pharmacogenet. 6(4):297–305 [DOI] [PubMed] [Google Scholar]
- Brasser SM, Norman MB, Lemon CH. 2010. T1r3 taste receptor involvement in gustatory neural responses to ethanol and oral ethanol preference. Physiol Genomics. 41(3):232–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger KS, Stice E. 2011. Relation of dietary restraint scores to activation of reward-related brain regions in response to food intake, anticipated intake, and food pictures. Neuroimage. 55(1):233–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfield DR, Murphy K, Josephs O, Fink GR, Frackowiak RS, Guz A, Adams L, Turner R. 1999. Cortical and subcortical control of tongue movement in humans: a functional neuroimaging study using fMRI. J Appl Physiol. 86(5):1468–1477 [DOI] [PubMed] [Google Scholar]
- De Araujo IE, Kringelbach ML, Rolls ET, McGlone F. 2003a. Human cortical responses to water in the mouth, and the effects of thirst. J Neurophysiol. 90(3):1865–1876 [DOI] [PubMed] [Google Scholar]
- De Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MAL, Simon SA. 2008. Food reward in the absence of taste receptor signaling. Neuron. 57(6):930–941 [DOI] [PubMed] [Google Scholar]
- De Araujo IE, Rolls ET, Kringelbach ML, McGlone F, Phillips N. 2003b. Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur J Neurosci. 18(7):2059–2068 [DOI] [PubMed] [Google Scholar]
- Faurion A, Cerf B, Le Bihan D, Pilliasa A. 1998. fMRI study of taste cortical areas in humans. Ann NY Acad Sci. 855(1):535–545 [DOI] [PubMed] [Google Scholar]
- Felsted JA, Ren X, Chouinard-Decorte F, Small DM. 2010. Genetically determined differences in brain response to a primary food reward. J Neurosci. 30(7):2428–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcehimes AA, Tonigan JS, Miller WR, Kenna GA, Baer JS. 2007. Psychometrics of the Drinker Inventory of Consequences (DrInC). Addict Behav. 32(8):1699–1704 [DOI] [PubMed] [Google Scholar]
- Francis S, Rolls ET, Bowtell R, McGlone F, O’Doherty J, Browning A, Clare S, Smith E. 1999. The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. Neuroreport. 10(3):453–459 [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ. 1994. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 2(4):189–210 [Google Scholar]
- Gordon I, Voos AC, Bennett RH, Bolling DZ, Pelphrey KA, Kaiser MD. 2013. Brain mechanisms for processing affective touch. Hum Brain Mapp. 34(4):914–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. 2003. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 301(5636):1104–1107 [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Small DM, Zald DH. 2006. The chemical senses. In: Zald DH, Rauch SL, editors. The orbitofrontal cortex. Oxford: Oxford University Press; p. 125–173 [Google Scholar]
- Grabenhorst F, Rolls ET, Bilderbeck A. 2008. How cognition modulates affective responses to taste and flavor: top-down influences on the orbitofrontal and pregenual cingulate cortices. Cereb Cortex. 18(7):1549–1559 [DOI] [PubMed] [Google Scholar]
- Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. 1996. Evaluating the ‘Labeled Magnitude Scale’ for measuring sensations of taste and smell. Chem Senses. 21(3):323–334 [DOI] [PubMed] [Google Scholar]
- Haase L, Cerf-Ducastel B, Murphy C. 2009. Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. Neuroimage. 44(3):1008–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy S, Mikulis DJ, Crawley A, Xue S, Lau H, Henry S, Diamant NE. 1999. Cortical activation during human volitional swallowing: an event-related fMRI study. Am J Physiol Gastrointest Liver Physiol. 277(1):G219–G225 [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. 2009. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 324(5927):646–648 [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy A, Garbutt JC, Janowsky D. 1997. Evidence of preference for a high-concentration sucrose solution in alcoholic men. Am J Psychiatry. 154(2):269–270 [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Eick C, Boland G, Khalitov E, Crews FT. 2004. Sweet liking, novelty seeking, and gender predict alcoholic status. Alcohol Clin Exp Res. 28(9):1291–1298 [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Garbutt JC, Janowsky DS. 1999. Association between preference for sweets and excessive alcohol intake: a review of animal and human studies. Alcohol Alcohol. 34(3):386–395 [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Kasheffskaya OP, Sinclair JD. 1990. Initial acceptance of ethanol: gustatory factors and patterns of alcohol drinking. Alcohol. 7(2):83–85 [DOI] [PubMed] [Google Scholar]
- Kern M, Birn R, Jaradeh S, Jesmanowicz A, Cox R, Hyde J, Shaker R. 2001. Swallow-related cerebral cortical activity maps are not specific to deglutition. Am J Physiol Gastrointest Liver Physiol. 280(4):G531–G538 [DOI] [PubMed] [Google Scholar]
- Keskitalo K, Tuorila H, Spector TD, Cherkas LF, Knaapila A, Kaprio J, Silventoinen K, Perola M. 2008. The Three-Factor Eating Questionnaire, body mass index, and responses to sweet and salty fatty foods: a twin study of genetic and environmental associations. Am J Clin Nutr. 88(2):263–271 [DOI] [PubMed] [Google Scholar]
- Kinomura S, Kawashima R, Yamada K, Ono S, Itoh M, Yoshioka S, Yamaguchi T, Matsui H, Miyazawa H, Itoh H. 1994. Functional anatomy of taste perception in the human brain studied with positron emission tomography. Brain Res. 659(1-2):263–266 [DOI] [PubMed] [Google Scholar]
- Kobayakawa T, Endo H, Ayabe-Kanamura S, Kumagai T, Yamaguchi Y, Kikuchi Y, Takeda T, Saito S, Ogawa H. 1996. The primary gustatory area in human cerebral cortex studied by magnetoencephalography. Neurosci Lett. 212(3):155–158 [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. 2003. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 13(10):1064–1071 [DOI] [PubMed] [Google Scholar]
- Lemon CH, Brasser SM, Smith DV. 2004. Alcohol activates a sucrose-responsive gustatory neural pathway. J Neurophysiol. 92(1):536–544 [DOI] [PubMed] [Google Scholar]
- Lewis PA, Critchley HD, Rotshtein P, Dolan RJ. 2007. Neural correlates of processing valence and arousal in affective words. Cereb Cortex. 17(3):742–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt A, Plassmann H, Shiv B, Rangel A. 2011. Dissociating valuation and saliency signals during decision-making. Cereb Cortex. 21(1):95–102 [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. 2003. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 19(3):1233–1239 [DOI] [PubMed] [Google Scholar]
- Mann RE, Sobell LC, Sobell MB, Pavan D. 1985. Reliability of a family tree questionnaire for assessing family history of alcohol problems. Drug Alcohol Depend. 15(1-2):61–67 [DOI] [PubMed] [Google Scholar]
- Martin R, Barr A, MacIntosh B, Smith R, Stevens T, Taves D, Gati J, Menon R, Hachinski V. 2007. Cerebral cortical processing of swallowing in older adults. Exp Brain Res. 176(1):12–22 [DOI] [PubMed] [Google Scholar]
- McCabe C, Rolls ET. 2007. Umami: a delicious flavor formed by convergence of taste and olfactory pathways in the human brain. Eur J Neurosci. 25(6):1855–1864 [DOI] [PubMed] [Google Scholar]
- McClure SM, Li J, Tomlin D, Cypert KS, Montague LM, Montague PR. 2004. Neural correlates of behavioral preference for culturally familiar drinks. Neuron. 44(2):379–387 [DOI] [PubMed] [Google Scholar]
- Mehrabian A, Russell JA. 1978. A questionnaire measure of habitual alcohol use. Psychol Rep. 43(3):803–806 [DOI] [PubMed] [Google Scholar]
- Mennella JA, Lukasewycz LD, Griffith JW, Beauchamp GK. 2011. Evaluation of the Monell forced-choice, paired-comparison tracking procedure for determining sweet taste preferences across the lifespan. Chem Senses. 36(4):345–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella JA, Pepino MY, Reed DR. 2005. Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics. 115(2):e216–e222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville MJ, Johnstone EC, Walton RT. 2004. Identification and characterization of ANKK1: a novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Hum Mutat. 23(6):540–545 [DOI] [PubMed] [Google Scholar]
- Nguyen-Michel ST, Unger JB, Spruijt-Metz D. 2007. Dietary correlates of emotional eating in adolescence. Appetite. 49(2):494–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. 2001. Representation of pleasant and aversive taste in the human brain. J Neurophysiol. 85(3):1315–1321 [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. 2002. Neural responses during anticipation of a primary taste reward. Neuron. 33(5):815–826 [DOI] [PubMed] [Google Scholar]
- Pepino MY. 2005. Factors contributing to individual differences in sucrose preference. Chem Senses. 30(Suppl 1):i319–i320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepino MY, Mennella JA. 2007. Effects of cigarette smoking and family history of alcoholism on sweet taste perception and food cravings in women. Alcohol Clin Exp Res. 31(11):1891–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassmann H, O’Doherty J, Shiv B, Rangel A. 2008. Marketing actions can modulate neural representations of experienced pleasantness. Proc Natl Acad Sci U S A. 105(3):1050–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard TC, Edwards EM, Smith CA, Hilgert KG, Gavlick AM, Maryniak TD, Schwartz GJ, Scott TR. 2005. Gustatory neural responses in the medial orbitofrontal cortex of the old world monkey. J Neurosci. 25(26):6047–6056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F. 2008. The orbitofrontal cortex and beyond: from affect to decision-making. Prog Neurobiol. 86(3):216–244 [DOI] [PubMed] [Google Scholar]
- Rolls ET, O’Doherty J, Kringelbach ML, Francis S, Bowtell R, McGlone F. 2003. Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cereb Cortex. 13(3):308–317 [DOI] [PubMed] [Google Scholar]
- Rolls ET, Yaxley S, Sienkiewicz ZJ. 1990. Gustatory responses of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. J Neurophysiol. 64(4):1055–1066 [DOI] [PubMed] [Google Scholar]
- Rudenga K, Green B, Nachtigal D, Small DM. 2010. Evidence for an integrated oral sensory module in the human anterior ventral insula. Chem Senses. 35(8):693–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T. 2003. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 39(4):701–711 [DOI] [PubMed] [Google Scholar]
- Small DM, Veldhuizen MG, Felsted J, Mak YE, McGlone F. 2008. Separable substrates for anticipatory and consummatory food chemosensation. Neuron. 57(5):786–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. 2001. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 124(Pt 9):1720–1733 [DOI] [PubMed] [Google Scholar]
- Spetter MS, Smeets PA, de Graaf C, Viergever MA. 2010. Representation of sweet and salty taste intensity in the brain. Chem Senses. 35(9):831–840 [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Small DM. 2008. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 322(5900):449–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Portas C, Dolan R, Holmes AP, Friston KJ. 1999. Random effects analysis for event-related fMRI. Neuroimage. 9(36):1053–1089 [Google Scholar]
- Van Strien T, Frijters JER, Bergers GPA, Defares PB. 1986. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int J Eat Disord. 5(2):295–315 [Google Scholar]
- Veldhuizen MG, Bender G, Constable RT, Small DM. 2007. Trying to detect taste in a tasteless solution: modulation of early gustatory cortex by attention to taste. Chem Senses. 32(6):569–581 [DOI] [PubMed] [Google Scholar]
- Veldhuizen MG, Nachtigal D, Teulings L, Gitelman DR, Small DM. 2010. The insular taste cortex contributes to odor quality coding. Front Hum Neurosci. 4:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber B, Rangel A, Wibral M, Falk A. 2009. The medial prefrontal cortex exhibits money illusion. Proc Natl Acad Sci U S A. 106(13):5025–5028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. 1985. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 122(1):51–65 [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. 1995. Analysis of fMRI time-series revisited–again. Neuroimage. 2(3):173–181 [DOI] [PubMed] [Google Scholar]
- Wundt W. 1897. Outlines of psychology. Leipzig: Wilhelm Engelman [Google Scholar]
- Zald DH, Hagen MC, Pardo JV. 2002. Neural correlates of tasting concentrated quinine and sugar solutions. J Neurophysiol. 87(2):1068–1075 [DOI] [PubMed] [Google Scholar]