Abstract
Temperature modulates the peripheral taste response of many animals, in part by activating transient receptor potential (Trp) cation channels. We hypothesized that temperature would also modulate peripheral taste responses in larval Manduca sexta. We recorded excitatory responses of the lateral and medial styloconic sensilla to chemical stimuli at 14, 22, and 30 °C. The excitatory responses to 5 chemical stimuli—a salt (KCl), 3 sugars (sucrose, glucose, and inositol) and an alkaloid (caffeine)—were unaffected by temperature. In contrast, the excitatory response to the aversive compound, aristolochic acid (AA), increased robustly with temperature. Next, we asked whether TrpA1 mediates the thermally dependent taste response to AA. To this end, we 1) identified a TrpA1 gene in M. sexta; 2) demonstrated expression of TrpA1 in the lateral and medial styloconic sensilla; 3) determined that 2 TrpA1 antagonists (HC-030031 and mecamylamine) inhibit the taste response to AA, but not caffeine; and then 4) established that the thermal dependence of the taste response to AA is blocked by HC-030031. Taken together, our results indicate that TrpA1 serves as a molecular integrator of taste and temperature in M. sexta.
Key words: aristolochic acid, insect, taste, temperature, TrpA1
Introduction
Temperature modulates the peripheral taste response of mammals, amphibians, and insects to a variety of ecologically relevant compounds (Table 1). In most cases, the response to taste stimuli (e.g., 0.3M sucrose) increased monotonically between 10 and 35 °C, and then decreased at higher temperatures. Temperature dependence is not unique to the taste system, as there are reports of temperature modulating olfactory (Bestmann and Dippold 1983; Bestmann and Dippold 1989; Shoji et al. 1994), auditory (Fonseca and Correia 2007), and visual (Adolph 1973; Aho et al. 1993) responses. These temperature-dependent sensory responses are thought to be mediated in large part by transient receptor potential (Trp) channels, which open in response to temperature changes and permit influx of cations (Venkatachalam and Montell 2007). Trpm5 is the only Trp channel known to modulate peripheral taste responses. In mammalian taste cells, it functions as a molecular integrator of chemical and thermal input, causing peripheral taste responses to a specific concentration of sugars or artificial sweeteners to increase with temperature (Talavera et al. 2005; Ohkuri et al. 2009).
Table 1.
Temperature dependence of the peripheral taste system in 4 mammals, 1 amphibian and 1 insect
| Species | Chemical stimuli | Reference |
|---|---|---|
| Laboratory rat | NaCl, HCl, acetic acid, KCl, NH4Cl, quinine, sucrose, glycine | Yamashita et al. 1964; Yamashita et al. 1970; Nakamura and Kurihara 1991; Breza et al. 2006 |
| Domestic dog | NaCl, NH4Cl, acetic acid, sucrose, fructose, monosodium glutamate | Nakamura and Kurihara 1991 |
| Domestic cat | NaCl, quinine, and HCl | Nagaki et al. 1964 |
| Laboratory mouse | Glucose, sucrose, fructose, maltose, SC-45647, glycine, saccharin, NH4Cl, monosodium glutamate, NaCl, quinine | Talavera et al. 2005; Ohkuri et al. 2009; Lu et al. 2012 |
| Frog | NaCl, CaCl2, quinine, acetic acid | Yamashita 1964 |
| Blowfly | Glucose, sucrose, NaCl | Gillary 1966; Uehara and Morita 1972 |
We show the chemical stimuli that elicited temperature-dependent taste responses in each species.
The functional significance of temperature-dependent chemosensory responses is unclear. This is because it distorts perceptions of stimulus intensity, making plant chemicals appear more concentrated at high temperatures. Poikilothermic animals with a high surface-to-volume ratio (e.g., insects) would be particularly susceptible to these distortions because their body temperature equilibrates rapidly with ambient temperature. In this study, we examined the extent to which temperature modulates peripheral taste responses of an herbivorous caterpillar, Manduca sexta. We hypothesized that M. sexta would have evolved a taste system that functioned largely independently of temperature for 2 reasons. First, free-ranging M. sexta occupy environments that experience large temperature changes across the day and year (Madden and Chamberlin 1945; Casey 1976). Because the body temperature of M. sexta conforms to ambient temperature (Casey 1976) and because M. sexta feeds throughout the day and night (Casey 1976; Reynolds et al. 1986), it follows that its peripheral taste system would have to evaluate the chemical composition of foods across a wide range of temperatures. Second, taste plays a critical role in the life history of M. sexta, helping it identify host plants (Waldbauer and Fraenkel 1961; del Campo et al. 2001; Glendinning et al. 2009) and regulate intake of nutrients and poisons in both host and non-host plants (Glendinning et al. 1999; Kester et al. 2002).
We did not expect the peripheral taste system of M. sexta to operate completely independently of temperature, however. This expectation stemmed from reports 1) that the peripheral taste system of Drosophila melanogaster responds to aristolochic acid (AA; Kim et al. 2010), 2) that the taste response to AA, but not a variety of other aversive compounds (e.g., caffeine), is mediated by the TrpA1 channel (Kim et al. 2010), and 3) that Drosophila TrpA1 (dTrpA1) responds to temperature (Hamada et al. 2008; Kwon et al. 2008). Given that 2 classes of gustatory receptor neuron (GRN) in the peripheral taste system of M. sexta respond vigorously to AA (Figure 1B), we hypothesized that TrpA1 may serve as a molecular integrator of taste and temperature input in M. sexta, in much the same way as Trpm5 does in mammals (Talavera et al. 2005; Ohkuri et al. 2009).
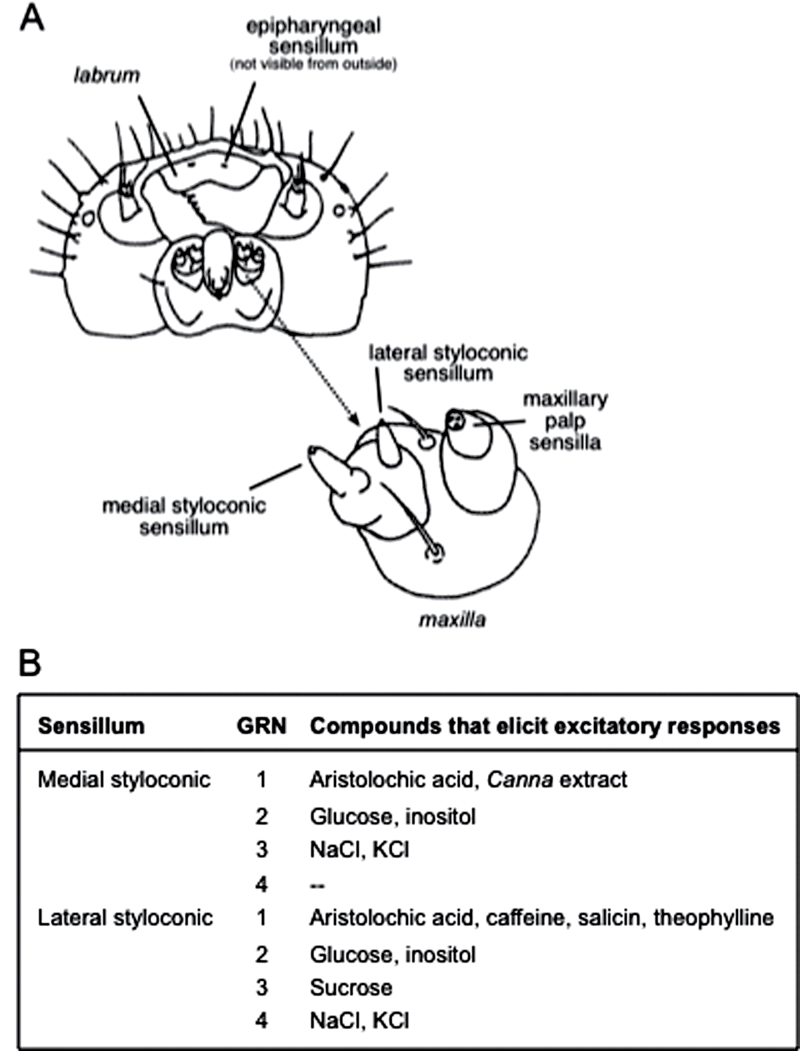
Figure 1.
(A) Cartoon of the head of a M. sexta caterpillar, as viewed from below. An enlargement of the maxilla (indicated with an arrow) is provided to clarify the location of the medial and lateral styloconic sensilla. This cartoon was adapted from Bernays and Chapman 1994; their Fig. 3.4). (B) Chemical stimuli that elicit excitatory responses in GRNs within the lateral and medial styloconic sensilla of M. sexta. These molecular receptive ranges were derived from previous studies (Schoonhoven 1972; Glendinning et al. 2002; Glendinning et al. 2007).
We describe the results of 4 experiments. First, we asked whether 2 classes of taste sensilla (the lateral and medial styloconic sensilla; Figure 1A) exhibit temperature-dependent responses to a diverse range of chemical stimuli. We selected these 2 sensilla because they play a key role in host plant identification and avoidance of potentially toxic plant tissues (Waldbauer and Fraenkel 1961; Glendinning et al. 1999; del Campo et al. 2001; de Boer 2006; Glendinning et al. 2009). Second, we sought to identify the TrpA genes in M. sexta and determine whether TrpA1 is expressed in the lateral and medial styloconic sensilla. Third, we tested the prediction that if the response of the medial and lateral styloconic sensilla to AA is mediated by TrpA1, then we should be able to inhibit it with TrpA1 antagonists. Fourth, we asked whether a highly selective TrpA1 antagonist eliminates the temperature-dependent response of the lateral styloconic sensilla to AA.
Materials and methods
Subjects and rearing conditions
We maintained a colony of tobacco hornworms (M. sexta; Sphingidae) in our laboratory. These insects were derived from eggs purchased from Carolina Biological Supply, reared on a wheat germ-based artificial diet (Bell and Joachim 1976), and maintained in an environmental chamber with a 16:8-h light:dark cycle at 25 °C. The experiments involving caterpillars were conducted during the first or second day of the fifth larval growth stage (instar). All caterpillars were naive to the taste stimuli prior to testing. To control for differences between caterpillars from different egg batches, individuals from each batch were interspersed randomly across treatment levels, according to a blind procedure. Sample sizes are provided in the figure legends.
Tip recording technique
We recorded taste responses with a non-invasive extracellular tip recording technique (Gothilf and Hanson 1994). In brief, this method involved anesthetizing the caterpillar by sealing it in a grounded 15-mL vial containing 0.1M KCl (with its head protruding), and then placing a glass electrode containing a taste stimulus solution over a lateral or medial styloconic sensillum.
To minimize any potential carry-over between successive recordings, we paused at least 1min between stimulations. To minimize the effects of solvent evaporation at the tip of the recording/stimulating electrode, we drew fluid from the tip with a piece of filter paper immediately before stimulation. For each caterpillar, we made recordings from a single lateral and a single medial styloconic sensillum.
We recorded extracellular signals with the Tasteprobe amplifier system (Syntech). We preamplified each recording 10×, ran it through a band-pass filter set at 100–1200 Hz, fed it into a computer through a 16-bit analog-to-digital converter board, and then analyzed it off-line with Autospike software (Syntech). For all electrophysiological analyses described below, we counted total number of spikes over the initial 1000ms of the response.
Controlling body temperature
We manipulated maxilla temperature by immersing the caterpillar (while anesthetized in the 15-mL vial described above) into a temperature-controlled water bath (Digital One; Thermo Scientific), leaving its head protruding from the water. We tested the caterpillars at 3 temperatures: low (14 °C), control (22 °C) and high (30 °C). We selected this temperature range for 2 reasons. First, it reflects the temperature range over which free-ranging M. sexta have been observed feeding in their natural environment (Madden and Chamberlin 1945; Casey 1976). Second, the amount of current flowing through the TrpA1 channel in Drosophila increases with temperature over this range (Kang et al. 2012). In preliminary experiments, we determined that the caterpillar’s maxilla temperature would equilibrate at 14, 22, or 30 °C following 15min of immersion in a water bath set at 5, 22, or 40 °C, respectively.
Does temperature modulate the peripheral taste response? (Experiment 1)
Thermal stability of the maxilla
A key requirement of this experiment was that the temperature of each caterpillar’s maxilla remained relatively stable for at least 5min after it had been removed from the water bath. As a result, we examined thermal stability of the maxilla at the 3 experimental temperatures: 14, 22 and 30 °C. At the beginning of each test, we equilibrated the 15-mL vial (containing a caterpillar) to the target temperature. Then, we removed the vial from the water bath, wrapped foam insulation around it, secured it in a clamp, and immediately began taking maxilla temperature measurements every 30 s over a 5-min period. To measure maxilla temperature, we inserted a small thermister (coupled to a TC-324B; Warner Instruments) into the “neck” of the caterpillar (while it was still inserted in the 15-mL vial), just posterior to the head capsule. The tip of the thermister was positioned so that it was <2mm from the base of a maxilla, providing a reliable measure of maxilla temperature.
Effect of low maxilla temperature on taste response
We measured neural responses of each sensillum to a given taste stimulus 3 times. The first recording was made at 22 °C and provided a premanipulation control measure; the second recording was made at 14 °C and indicated the effect (if any) of decreasing the maxilla temperature; and the third recording was made at 22 °C and indicated whether the temperature effect was reversible.
We recorded neural responses to the following chemical stimuli: KCl (0.6M), glucose (0.3M), inositol (10mM), sucrose (0.3M), caffeine (5mM), and AA (0.1mM). Note that the latter 5 stimuli were dissolved in 0.1M KCl so as to increase electrical conductivity of the stimulation solution. We selected these chemical stimuli because they together activate all of the identified GRNs within the lateral and medial styloconic sensilla (Figure 1B), and because they all (except KCl) modulate feeding, either alone or binary mixture with other compounds (Cocco and Glendinning 2012). We chose the indicated concentrations of each chemical because they produce maximal excitatory responses, and thus enabled us to avoid any confounds associated with a floor effect. We did not stimulate the medial styloconic sensillum with caffeine or sucrose because previous work indicated that it is unresponsive to both chemicals (Glendinning et al. 1999; Glendinning et al. 2007).
Once the maxilla reached the target temperature, we recorded neural responses to each chemical stimulus. Based on results from Experiment 1, we knew that the maxilla would remain at the target temperature (±2 °C) for 5min. Given this time constraint and the fact that we had to pause at least 1min between successive recordings, we could only make 3 recordings within the 5-min time window. As a result, we had to reimmerse the caterpillar in the water bath for 15min (to return its maxilla to the target temperature) before obtaining responses to the remaining chemical stimuli. Note that we systematically varied the order of presentation of stimuli during each 5-min test session. In this manner, we tested 10 lateral and 10 medial sensilla, each from different caterpillars.
Effect of high maxilla temperature on taste response
We used the same electrophysiological procedure as described above, with 2 exceptions. The recordings were made at 22, 30 and 22 °C. Further, we selected concentrations of each chemical stimulus that elicited weak excitatory responses so as to avoid confounds associated with a ceiling effect: KCl (0.1M), glucose (0.1M), inositol (0.3mM), sucrose (0.03M), caffeine (0.1mM), and AA (0.1 µM). We tested 11 lateral and 10 medial styloconic sensilla, each from different caterpillars.
Data analysis
We used a repeated-measures ANOVA to compare neural responses to a given taste stimulus across the 3 temperatures (e.g., 22, 14, and then 22 °C), separately for each chemical stimulus, sensillum type, and temperature manipulation (i.e., decreasing or increasing temperature). If there was a significant effect of temperature, then we ran a Tukey post hoc test to determine which means differed significantly from one another. In this and all subsequent analyses, we used an α level of 0.05. We also calculated the Q10 value, which is a measure of the extent to which the taste response increased in response to a 10 °C increase in temperature. It is defined by the following equation: Q10 = (TR2/TR1) * [10/(T2−T1)], where the asterisk denotes the exponential function and TRn denotes the magnitude of the taste response at temperature Tn. In all cases, T2 > T1.
Identification of M. sexta Trp genes and analysis of TrpA1 expression in chemosensory tissues (Experiment 2)
We used previously reported Trp amino acid sequences (from 5 other insect species) to search the Manduca genome (Matsuura et al. 2009). We used BLASTp to search the Manduca OGS proteins database (June 2012 release) located at the Agricultural Pest Genomics Resource Database (www.agripestbase.org). Phylogenetic analysis was performed with Mega 5.05 (Tamura et al. 2011). We aligned the predicted amino acid sequences with ClustalW (using default parameters) and generated a consensus neighbor-joining cluster (using default parameters) with bootstrap values calculated by resampling 1000 times. Finally, we assigned identities of M. sexta sequences based on clustering. Agripestbase accession numbers for each sequence are listed in Supplementary Table 1.
We performed tissue dissections, RNA extraction, and cDNA synthesis as described previously (Howlett et al. 2012) from larvae 2 days after molting to the fifth instar. In brief, we conducted RT-PCR in 50-µL reactions using Invitrogen Taq polymerase (cat #10342-020) under the following conditions: 2.5U Taq, 20mM Tris pH 8.4, 40mM KCl, 1.5mM MgCl2, 10mM each deoxyribonucleotide triphosphate, 40 pmol each primer, and 0.5 µL cDNA. Primer sequences were forward: 5ʹ-agcaatggtgaccgtttttc-3ʹ and reverse 5ʹ-attagggtgccctggacatt-3ʹ. Temperature conditions were 94 °C for 2min, 30 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, followed by a final extension of 72 °C for 10min. We confirmed the identity of the 204-bp-amplified product by subcloning it into the pDrive vector (Qiagen cat #231224) and sequencing it (Genewiz).
Are taste responses to AA and caffeine inhibited by TrpA1 antagonists? (Experiment 3)
If the temperature-dependent responses to AA in Experiment 1 were mediated by TrpA1, then treatment of the AA-sensitive GRNs with TrpA1 antagonists should inhibit the response to AA. To test this prediction, we asked how 2 TrpA1 antagonists (HC-030031 and mecamylamine) impacted neural responses of the lateral and medial styloconic sensilla to a relatively high concentration of AA (0.1mM) and caffeine (5mM). We did not expect the antagonists to inhibit the response to caffeine because previous studies in D. melanogaster reported that TrpA1 mediates the peripheral taste response to AA, but not caffeine (Kim et al. 2010). The concentration of each TrpA1 antagonist (1 µM HC-030031 and 1mM mecamylamine) was selected based on previous reports (McNamara et al. 2007; Eid et al. 2008; Talavera et al. 2009). Both antagonists were purchased from Sigma-Aldrich.
For the tests involving mecamylamine, the stimuli were dissolved in 0.1M KCl. For the tests involving HC-030031, the stimuli were dissolved in a solution containing 0.1M KCl and 0.1% dimethylsulfoxide (DMSO). The use of DMSO was necessary because the HC-030031 is water insoluble. We initially dissolved the HC-030031 in pure DMSO, and then diluted it with 0.1M KCl to create a solution of 1mM HC-030031 in 0.1% DMSO. Importantly, in the tests involving HC-030031, all test solutions (both with and without antagonist) contained 0.1% DMSO plus 0.1M KCl.
The electrophysiological procedures were identical to those in Experiment 1, except that we made all recordings at room temperature (i.e., 22 °C). To avoid potential carry-over effects between antagonists, we tested only 1 antagonist per caterpillar. The lateral styloconic sensillum was stimulated 6 times with 1) 5mM caffeine, 5mM caffeine + antagonist, and then 5mM caffeine; and 2) 0.1mM AA, 0.1mM AA + antagonist, and then 0.1mM AA. The medial styloconic sensilla was stimulated 3 times with 0.1mM AA, 0.1mM AA + antagonist, and then 0.1mM AA.
We analyzed the effect of each TrpA1 antagonist on neural responsiveness to a given taste stimulus across the 3 successive stimulations with a repeated-measures ANOVA, followed by a post hoc Tukey test (adjusted for repeated measures).
Does a selective TrpA1 antagonist eliminate the effect of temperature on the taste response to AA? (Experiment 4)
In the previous experiment, we discovered that the TrpA1 antagonist, HC-030031, selectively reduced the peripheral taste response to AA. Here, we asked whether 1mM HC-030031 (henceforth, the antagonist) eliminates the temperature-dependent response to AA in the lateral styloconic sensillum. To this end, we used the same procedure outlined in Experiment 3, with a few exceptions. We ran 2 series of tests. In the first series, each lateral styloconic sensillun was subjected to decreasing temperatures under the following conditions: 1) 22 °C without antagonist, 14 °C without antagonist, and 22 °C without antagonist (this served as a positive control for the effect of temperature alone); 2) 22 °C without antagonist, 22 °C with antagonist, and 22 °C without antagonist (this served as a positive control for the effect of the antagonist alone); and 3) 22 °C with antagonist, 14 °C with antagonist, and 22 °C with antagonist (this tested the necessity of TrpA1 in the temperature-dependent taste response to AA). The second series of tests was identical to the first series, except each lateral styloconic sensilla experienced increasing temperatures under the following conditions: 1) 22 °C without antagonist, 30 °C without antagonist, and 22 °C without antagonist; 2) 22 °C without antagonist, 22 °C with antagonist, and 22 °C without antagonist; and 3) 22 °C with antagonist, 30 °C with antagonist, and 22 °C with antagonist. Note that we used different sensilla in the first and second test series. We analyzed the data from a given test series and condition with a repeated measure ANOVA, followed by a post hoc Tukey test (adjusted for repeated measures).
Results
Does temperature modulate the peripheral taste response? (Experiment 1)
Thermal stability of the maxilla
The maxilla temperatures remained relatively stable across the 5-min sessions, irrespective of whether they began at 14, 22 or 30 °C (Supplementary Figure 1). There was, however, a small amount of drift towards room temperature (i.e., 21 °C) over the 5-min session. When the maxilla started the session at 14 °C, it increased to 15.4 °C; when it started at 22 °C, it decreased to 21.5 °C; and when it started at 30 °C, it decreased to 28 °C. Thus, the temperature differential between the maxilla tested at 14 and 22 °C decreased from 8 (at start of session) to 6.1 °C (at end of session). Likewise, the temperature differential between the maxilla tested at 30 and 22 °C decreased from 8 (at start of session) to 6.5 °C (at end of session). Despite this drift, our results establish that large temperature differentials persisted over the 5-min session for sensilla tested at 14, 22 and 30 °C.
Effect of decreasing temperature
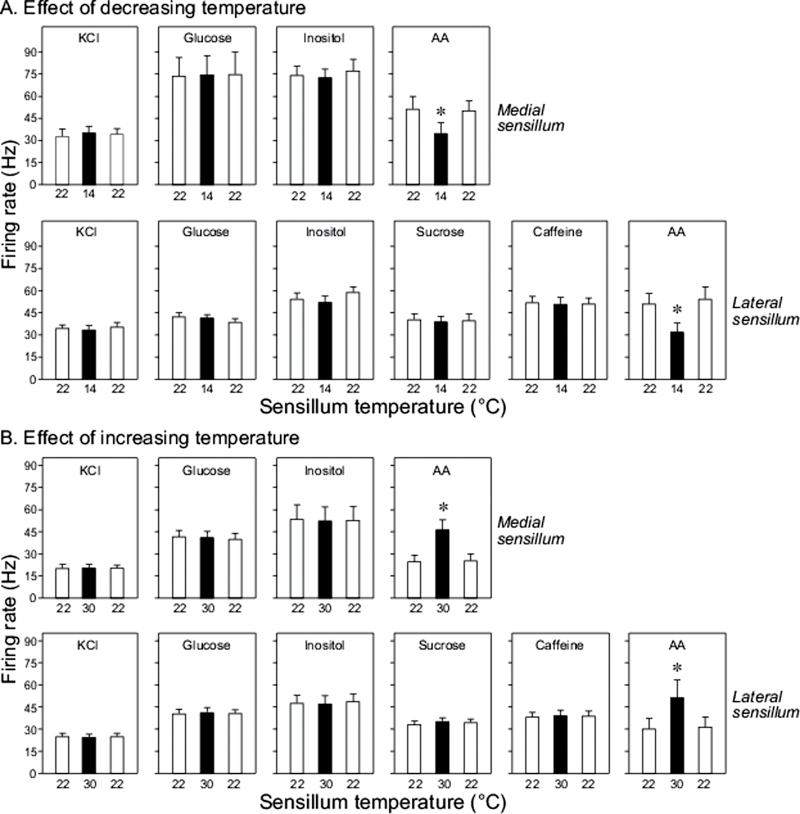
In Figure 2A, we show that lowering sensilla temperature from 22 to 14 °C did not alter the taste response to KCl, glucose, inositol, sucrose, and caffeine in the lateral styloconic sensillum (in all cases, F 2,23 < 2.9, P > 0.05); it also had no effect on the taste response to KCl, glucose, and inositol in the medial styloconic sensillum (in all cases, F 2,29 < 2.8, P > 0.05). In contrast, there was a significant effect of lowering sensilla temperature on the response to AA in both the lateral (F 2,29 = 14.3, P < 0.0003) and medial (F 2,29 = 12.1, P < 0.0006) sensilla. A post hoc Tukey test revealed that the AA response at 14 °C was significantly less than those at 22 °C. These findings demonstrate that decreasing the temperature of both classes of sensilla reduced the neural response exclusively to AA, and that this effect was reversed when the sensilla was returned to 22 °C.
Figure 2.
Effect of decreasing (A) or increasing (B) the temperature of the medial and lateral styloconic sensilla on excitatory responses to KCl (0.6M), glucose (0.3M), inositol (10mM), sucrose (0.3M), caffeine (5mM), and AA (0.1mM). We tested the sensilla at 22, 14, and 22 °C (A); and 22, 30 and 22 °C (B). Within each panel, we indicate when the black bar differed significantly from the white bars (P ≤ 0.05, Tukey multiple comparison test) with an asterisk. Each bar reflects mean ± standard error; n = 10–11/medial and lateral sensilla (each from different caterpillars).
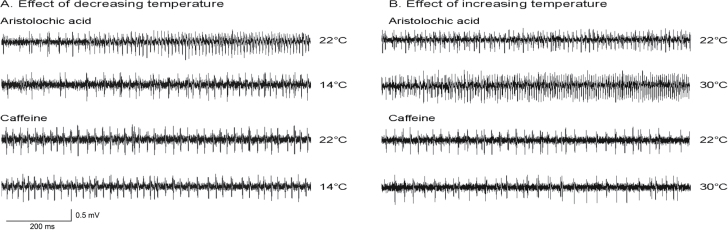
In Figure 3A, we show typical neural responses of the lateral styloconic sensilla to AA and caffeine at 22 and 14 °C. These traces illustrate that the low temperature reduced firing rate, but it did not alter the temporal pattern of spiking during the AA response. It also reveals that there was no effect of temperature on the dynamics of the caffeine response.
Figure 3.
Illustration of how decreasing (A) or increasing (B) sensilla temperature altered the neural responses of a lateral styloconic sensillum to AA (0.1mM), but not caffeine (5mM). Note that both chemicals were dissolved in 0.1M KCl. In A, we show neural responses at 22, 14 and 22 °C; and in B, we show neural responses at 22, 30 and 22 °C.
Effect of increasing temperature
In Figure 2B, we show the response of the medial and lateral sensilla styloconica to each of the taste stimuli at 3 target temperatures: 22, 30 and 22 °C. Increasing sensilla temperature had no effect on the neural response to KCl, glucose, inositol, sucrose, or caffeine in the lateral styloconic sensillum (in all cases, F 2,32 < 1.8, P > 0.05); it also had no effect on the taste response to KCl, glucose, and inositol in the medial styloconic sensillum (in all cases, F 2,29 < 1.9, P > 0.05). On the other hand, there was a significant effect of temperature on the response to AA in both the lateral (F 2,32 = 15.0, P = 0.0001) and medial (F 2,29 = 31.7, P < 0.0001) sensilla. A post hoc Tukey test revealed that the AA response at 30 °C was significantly greater than those at 22 °C. Thus, the high temperature increased firing rate, but this effect was reversed after returning the sensilla to 22 °C.
In Figure 3B, we show typical neural responses of the lateral styloconic sensillum to AA and caffeine at 22 and 30 °C. These traces show that the high temperature increased firing rate but failed to alter the temporal pattern of spiking for AA. On the other hand, the high temperature had no effect on the response to caffeine.
Q10 values for AA responses
We limited the Q10 calculations to the AA responses. Further, because there was a small amount of thermal drift in Supplementary Figure 1, we used the average temperature across the 5-min recording session to determine T1 and T2 in the equation. Accordingly, the Q10 values for the AA response in the medial and lateral styloconic sensilla were, in respective order, 1.9 and 2.2 at the low temperature range (i.e., 14 – 22 °C) and 2.6 and 2.2 at the high temperature range (i.e., 22 – 30 °C).
Identification of M. sexta Trp genes and analysis of TrpA1 expression in chemosensory tissues (Experiment 2)
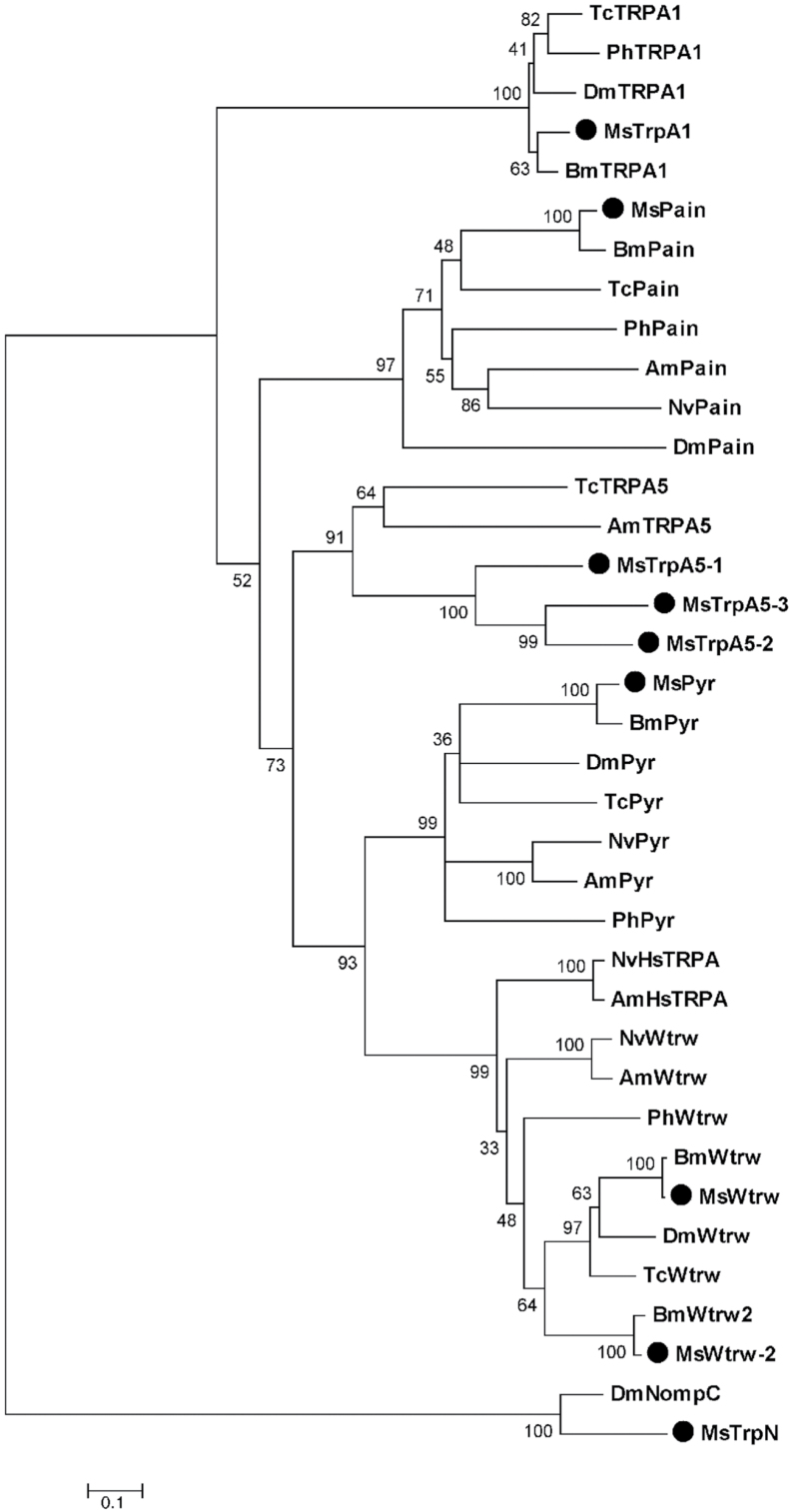
Trp channels are encoded by a large gene family that includes several subfamilies. At least 6 genes belonging to the TrpA subfamily are present in most insect genomes (Matsuura et al. 2009). We BLAST searched the complete predicted protein set generated by the Manduca genome project, using previously reported insect TrpA and TrpN sequences as queries. TrpN is the family most closely related to TrpA (Matsuura et al. 2009). We identified 8 putative TrpA family members and 1 putative TrpN from M. sexta, as shown in the neighbor-joining cluster analysis in Figure 4. Representatives of each TrpA subfamily were present in M. sexta, and 3 putative TrpA5 sequences were found, in contrast to other insects, suggesting duplications in this lineage. A single M. sexta predicted gene clustered with TrpA1 from other insects and shares 59% amino acid identity with dTrpA1. BLAST searches of the M. sexta whole genome and expressed sequence tag databases did not identify any additional TrpA-like sequences (not shown), suggesting that the M. sexta genome likely encodes a single TrpA1 gene (henceforth, MsexTrpA1).
Figure 4.
Neighbor-joining cluster analysis of putative M. sexta TrpA and TrpN sequences and those previously identified in other insects. Putative M. sexta sequences are labeled with a dot. Other insect sequences were obtained from the literature (Matsuura et al. 2009). Bm: Bombyx mori; Ms: Manduca sexta; Dm: Drosophila melanogaster; Tc: Tribolium castaneum; Am: Apis mellifera; Nv: Nasonia vitripennis; Ph: Pediculus humanus. Bootstrap values from 1000 replicates are shown. Scale bar represents number of amino acid substitutions per site.
If MsexTrpA1 mediated the temperature-dependent response to AA in Figure 2, then we predicted that it should be expressed in GRNs within the lateral and medial styloconic sensilla. We used RT-PCR to test this prediction. As shown in Figure 5, we detected expression of TrpA1 in GRNs within the lateral and medial styloconic sensilla. Next, the contribution of TrpA1 to the temperature-dependent response to AA was further evaluated with 2 TrpA1 antagonists.
Figure 5.
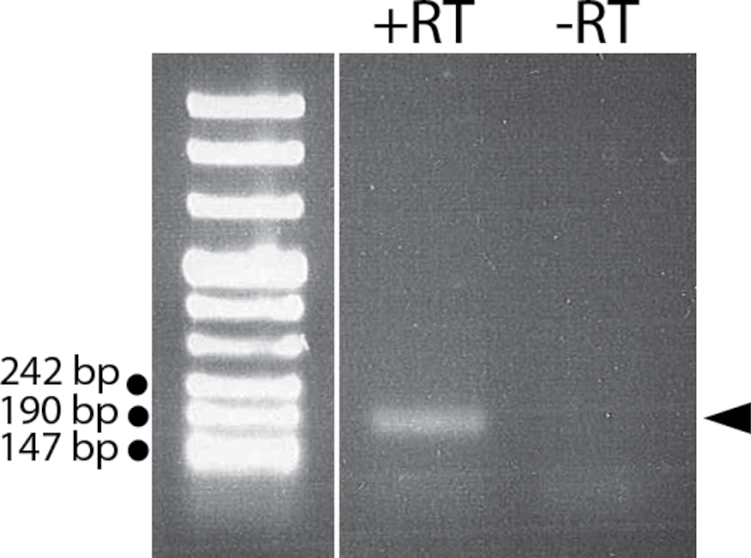
The putative TrpA1 mRNA from M. sexta is expressed in the lateral and medial styloconic sensilla. RT-PCR for TrpA1 was performed on tissue samples containing both classes of sensilla. The expected 205-bp fragment was amplified from tissue samples (arrow; compare with indicated size standards, Roch ME ladder VIII). Reverse transcriptase was omitted in samples labeled –RT and included in those labeled +RT.
Are taste responses to AA inhibited by TrpA1 antagonists? (Experiment 3)
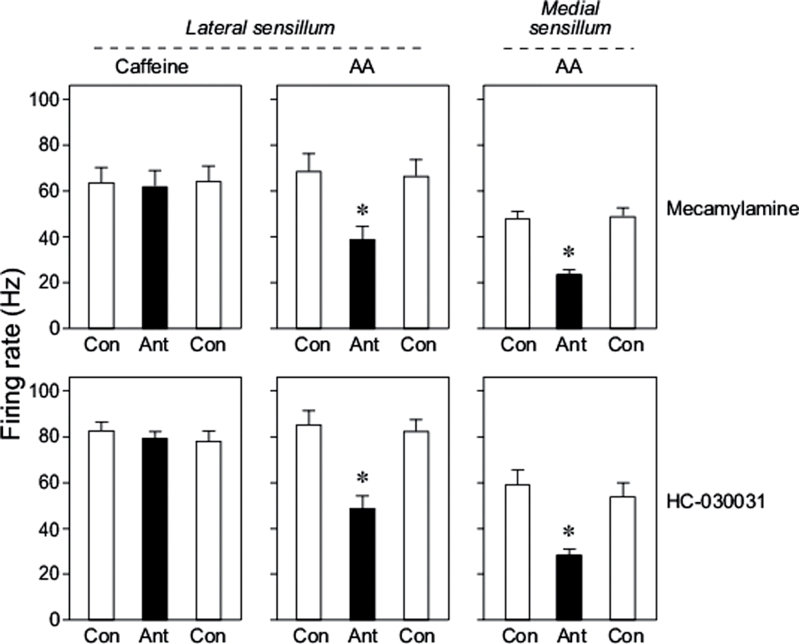
There was no significant main effect of mecamylamine on the response of the lateral styloconic sensillum to caffeine (F 2,29 = 1.2, P > 0.05; Figure 6, top row of panels). In contrast, there was a significant main effect of mecamylamine on the response of both the lateral and medial styloconic sensillum to AA (in both cases, F 2,29 > 24.0, P < 0.0001). A Tukey post hoc test revealed that the neural response to mecamylamine plus AA was significantly smaller than those to AA alone.
Figure 6.
Impact of 2 TrpA1 antagonists (mecamylamine and HC-030031) on excitatory responses of the lateral styloconic sensilla to 5mM caffeine and 0.1mM AA, and of the medial styloconic sensilla to 0.1mM AA. Sensilla temperature was 22 °C for all recordings. We show results for mecamylamine (top row of panels) and HC-030031 (bottom row of panels) separately. In each panel, we show the response to 3 consecutive stimulations: taste stimulus alone (Control or Con), taste stimulus plus a TrpA1 antagonist (Ant), and then Con again. Within each panel, we indicate when the black bar differed significantly from the white bars (P ≤ 0.05, Tukey multiple comparison test) with an asterisk. Each bar reflects mean ± standard error; n = 10/medial and lateral sensilla (each from different caterpillars).
Likewise, there was no significant main effect of HC-030031 on the neural response of the lateral styloconic sensillum to caffeine (F 2,29 = 0.6, P > 0.05; Figure 6, bottom row of panels). However, there was a significant main effect of HC-030031 on the response of both styloconic sensilla to AA (in both cases, F 2,29 > 30.0, P < 0.0001). The post hoc test showed that the response to HC-030031 plus AA was significantly smaller than those to AA alone.
Taken together, these results demonstrate that the 2 TrpA1 antagonists effectively blocked the response to AA but not caffeine, and that the blocking effect recovered within 3min.
Does a selective TrpA1 antagonist eliminate the effect of temperature on the taste response to AA? (Experiment 4)
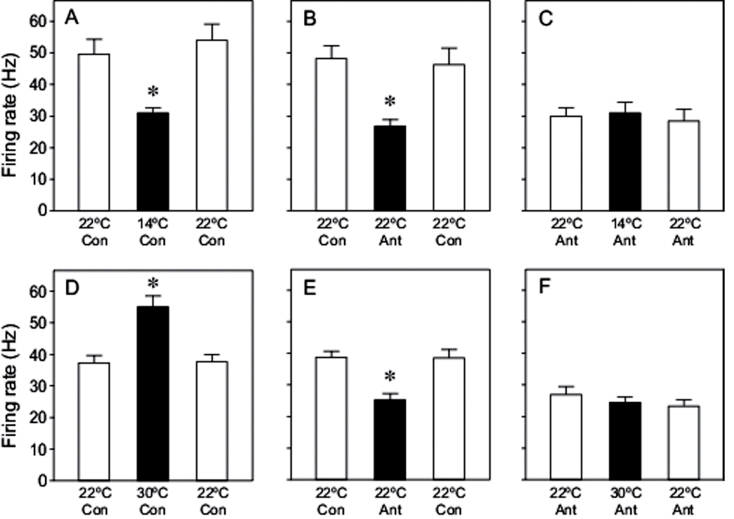
In Figure 7, we illustrate how temperature alone, HC-030031 (a selective TrpA1 antagonist) alone, and temperature plus HC-030031 impacted the excitatory response of lateral styloconic sensilla to AA. In panels 7A and 7D, we show that the excitatory response to AA at 14 °C was significantly less than that at 22 °C (F 2,20 = 24.8, P < 0.0001), whereas the response to AA at 30 °C was significantly greater than that at 22 °C (F 2,20 = 23.2, P < 0.0001). In panels 7B and 7E, we demonstrate that the response of the lateral styloconic sensilla to AA was decreased significantly by HC-030031 (in both comparisons, F 2,20 > 30.6, P < 0.0001). In panels 7C and 7F, we asked whether the modulatory effect of temperature would be blocked in the presence of HC-030031. Our results demonstrate that the HC-030031 completely blocked the thermally dependent response to AA. Irrespective of whether we decreased (F 2,20 < 1.0, P = 0.39) or increased (F 2,20 < 1.9, P = 0.18) the temperature, there was no temperature-dependent change in the firing rate of the lateral styloconic sensillum in the presence of HC-030031.
Figure 7.
Effect of temperature and the TrpA1 antagonist, HC-030031, on the excitatory response of the lateral styloconic sensillum to 0.1mM AA. The top row of panels shows the effect of (A) decreasing sensilla temperature alone, (B) the antagonist alone, and (C) decreasing sensilla temperature in the presence of the antagonist. The bottom row of panels shows the effect of (D) increasing sensilla temperature alone, (E) the antagonist alone, and (F) increasing sensilla temperature in the presence of the antagonist. Note we used 10 sensilla (each from different caterpillars) to generate all of the data in the top row of panels, and a different set of 10 sensilla to generate all of the data in the bottom row of panels. Below each bar within a panel, we indicate sensilla temperature, and whether the TrpA1 antagonist was (Ant) or was not (Con) present in the 0.1mM AA solution. Within each panel, we indicate when the black bar differed significantly from the white bars (P ≤ 0.05, Tukey multiple comparison test) with an asterisk. Each bar reflects mean ± standard error.
Discussion
In all mammals, amphibians, and insects studied to date, temperature modulated peripheral taste responses to salts, sugars, and a bitter alkaloid (quinine; Table 1). This was not the case in M. sexta. Its peripheral taste responses to KCl, 3 carbohydrates (glucose, inositol, and sucrose), and 1 alkaloid (caffeine) were totally unresponsive to large changes in temperature. The only compound that elicited a temperature-dependent increase in responsiveness was the aversive compound, AA. These results indicate that the GRNs in the lateral and medial styloconic sensilla function largely independently of temperature.
Contribution of TrpA1 to temperature-dependent taste responses to AA
In adult D. melanogaster, changes in temperature alone directly activated specific neurons in the brain (Hamada et al. 2008), but not GRNs in taste sensilla (Kang et al. 2012). This led Kang et al. (2012) to conclude that GRNs in D. melanogaster are temperature insensitive. Although this may be the case, our results provide an alternative explanation. We, too, found that temperature alone failed to activate any GRNs in M. sexta, based on the absence of temperature-dependent changes in taste response to KCl, glucose, inositol, sucrose, and caffeine (Figures 2 and 3). However, we discovered that the response of 2 classes of bitter-sensitive GRN to AA was modulated by temperature. This type of temperature sensitivity has not yet been explored in D. melanogaster.
Several lines of evidence indicate that MsTrpA1 mediated the temperature-dependent taste responses to AA in M. sexta. First, investigators established elsewhere that TrpA1 is a necessary component of the taste signaling pathway for AA (but not caffeine) in Drosophila (Kim et al. 2010). Our finding that TrpA1 antagonists, one of which is highly selective for TrpA1 (HC-030031; McNamara et al. 2007), significantly reduced the excitatory response to AA (but not caffeine) is consistent with the previous work in Drosophila and directly implicates TrpA1 in AA taste signaling. Second, we established that the M. sexta genome likely encodes a single TrpA1 gene, and that TrpA1 mRNA is expressed in the lateral and medial styloconic sensilla. Third, dTrpA1 is activated by both temperature (Hamada et al. 2008; Kwon et al. 2008) and AA (Kim et al. 2010). Based on these convergent lines of evidence, we propose that MsexTrpA1 functions as a molecular integrator of chemical and thermal input in the AA-sensitive GRNs within the lateral and medial styloconic sensilla (Figure 1B). Although it is well established that Trpm5 serves this function in mammalian taste cells (Talavera et al. 2005), our results provide the first evidence that TrpA1 does so in insect GRNs.
We reported previously that AA and caffeine stimulate the same GRN in the lateral styloconic sensillum, but do so by activating different signaling pathways (Glendinning and Hills 1997). This inference was corroborated herein by the observation that temperature modulated the peripheral taste response to AA but not caffeine. Previous work in Drosophila provides clues about the nature of the caffeine- and AA-activated transduction pathways in M. sexta. For instance, dTrpA1 is required for the peripheral taste response to AA, but not caffeine in adult D. melanogaster (Kim et al. 2010). AA does not appear to directly activate dTrpA1, but rather appears to activate a G protein (Gq)/phospholipase C signaling pathway that secondarily activates TrpA1 (Kim et al. 2010). However, there is also evidence that the naturally occurring insect repellent citronellal activates TrpA1 directly in the mosquito Anopheles gambiae (Kwon et al. 2010), indicating that there is some variability in the mechanism of action of TrpA1 across species.
Finally, we quantified the temperature dependence of the taste response to AA by calculating Q10 values, separately for each sensillum and temperature manipulation. The Q10 values ranged from 1.9 to 2.6. These values were intermediate, as compared with other taste (Yamashita 1964), visual (Adolph 1973; Aho et al. 1993), and muscular (Rall and Woledge 1990) systems. This indicates that the temperature dependence of the AA taste response was fairly typical.
Ecological relevance
Before discussing the ecological relevance of our findings, it is necessary to highlight 2 caveats about our experimental approach. First, our ability to draw generalizations about the entire taste system of M. sexta is limited because we examined only a subset of taste sensilla. We studied the lateral and medial styloconic sensilla, but not the maxillary palp or epipharyngeal sensilla (see Figure 1A). Given that AA stimulates a GRN within the epipharyngeal sensilla (Glendinning et al. 1999), it is possible that temperature would also modulate the response of this GRN to AA. Second, we focused on the impact of relatively rapid temperature changes (i.e., <20min) on peripheral taste responses. It is possible that more protracted exposure (e.g., several days; Martin et al. 2011) would have altered peripheral taste responses to the nutrients tested herein. Notwithstanding these caveats, our findings have several potential implications for the feeding ecology of M. sexta caterpillars.
We found that the peripheral taste response to KCl, glucose, inositol, and sucrose functioned independently of temperature. Given that all these nutrients occur in the host plant foliage of M. sexta (Nelson and Bernays 1998; Samczyński et al. 2012), it follows that its taste system should generate taste intensity perceptions about nutrient levels that are free of temperature distortions. Because reaction rates in most biological systems increase with temperature, one might expect that the magnitude of taste responsiveness should have done so, irrespective of whether Trp channels were present. Indeed, many physiological and behavioral processes in M. sexta increase with temperature, including biting rate (Casey 1976), contractile rate of flight muscles (George et al. 2012), activity levels (Casey 1976), growth, development and fecundity (Diamond and Kingsolver 2010), and digestive efficiency on diets that are either low in quality (Diamond and Kingsolver 2010) or contain noxious plant compounds (Stamp and Yang 1996). However, temperature had no impact on taste response to the majority of chemical stimuli in this study. This suggests that a buffering mechanism exists in the GRNs of M. sexta to resist thermal effects on most gustatory responses.
It is unclear whether M. sexta benefits from the temperature-modulated signaling pathway for AA. For instance, low temperatures (e.g., such as would be encountered in the morning and afternoon) would diminish its ability to detect (and hence avoid) the noxious and potentially toxic compounds that activate the AA-sensitive pathway. This would increase the insect’s risk of poisoning itself. On the other hand, high temperatures may augment the ability of M. sexta to detect low concentrations of noxious and potentially toxic compounds, and thereby permit it to modulate intake of these compounds until appropriate levels of P450 detoxification enzymes are induced (Snyder and Glendinning 1996). More work is needed to assess the validity of these possibilities.
Conclusion
In conclusion, as compared with other species of omnivores and carnivores studied to date (see Table 1), the peripheral taste system of M. sexta functions relatively independently of temperature. We propose that this temperature insensitivity evolved in response to its herbivorous and ectothermic lifestyle, permitting M. sexta to evaluate the chemical composition of its host plants without temperature-induced perceptual distortions. To determine whether temperature insensitivity is a specific adaptation to herbivory, it will be necessary to examine a variety of species that exemplify different feeding ecologies.
Supplementary material
Supplementary material can be found at http://www.chemse.oxfordjournals.org/
Funding
This work was supported by a grant from the Howard Hughes Medical Institute to Barnard College.
Supplementary Material
Acknowledgements
We thank Frederic Marion-Poll for valuable editorial comments.
References
- Adolph AR. 1973. Thermal sensitivity of lateral inhibition in Limulus eye. J Gen Physiol. 62(4):392–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aho AC, Donner K, Reuter T. 1993. Retinal origins of the temperature effect on absolute visual sensitivity in frogs. J Physiol. 463:501–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RA, Joachim FA. 1976. Techniques for rearing laboratory colonies of tobacco hornworms and pink bollworms. Ann Entomol Soc Am. 69:365–373 [Google Scholar]
- Bernays EA, Chapman RF. 1994. Host-plant selection by phytophagous insects. New York: Chapman & Hall; [Google Scholar]
- Bestmann HJ, Dippold K. 1983. Temperaturabhiingigkeit von elektroantennogrammen bei Lepidopteren. Naturwissensch. 70:47–48 [Google Scholar]
- Bestmann HJ, Dippold K. 1989. Temperature dependence of electrophysiological responses of Lepidopteran antennae. Z Naturforsch. 44c:333–344 [Google Scholar]
- Breza JM, Curtis KS, Contreras RJ. 2006. Temperature modulates taste responsiveness and stimulates gustatory neurons in the rat geniculate ganglion. J Neurophysiol. 95(2):674–685 [DOI] [PubMed] [Google Scholar]
- Casey TM. 1976. Activity patterns, body temperature and thermal ecology in two desert caterpillars (Lepidoptera: Sphingidae). Ecology. 57:485–497 [Google Scholar]
- Cocco N, Glendinning JI. 2012. Not all sugars are created equal: some mask aversive tastes better than others in an herbivorous insect. J Exp Biol. 215(Pt 8):1412–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer G. 2006. The role of the antennae and maxillary palps in mediating food preference by larvae of the tobacco hornworm, Manduca sexta . Entomol Exp Appl. 119:29–38 [Google Scholar]
- del Campo ML, Miles CI, Schroeder FC, Mueller C, Booker R, Renwick JA. 2001. Host recognition by the tobacco hornworm is mediated by a host plant compound. Nature. 411(6834):186–189 [DOI] [PubMed] [Google Scholar]
- Diamond SE, Kingsolver JG. 2010. Environmental dependence of thermal reaction norms: host plant quality can reverse the temperature-size rule. Am Nat. 175(1):1–10 [DOI] [PubMed] [Google Scholar]
- Eid SR, Crown ED, Moore EL, Liang HA, Choong K, Dima S, Henze DA, Kane SA, Urban MO. 2008. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol Pain. 48:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca PJ, Correia T. 2007. Effects of temperature on tuning of the auditory pathway in the cicada Tettigetta josei (Hemiptera, Tibicinidae). J Exp Biol. 210(Pt 10):1834–1845 [DOI] [PubMed] [Google Scholar]
- George NT, Sponberg S, Daniel TL. 2012. Temperature gradients drive mechanical energy gradients in the flight muscle of Manduca sexta . J Exp Biol. 215(Pt 3):471–479 [DOI] [PubMed] [Google Scholar]
- Gillary HL. 1966. Stimulation of the salt receptor of the blowfly. II. Temperature. J Gen Physiol. 50(2):351–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendinning JI, Davis A, Ramaswamy S. 2002. Contribution of different taste cells and signaling pathways to the discrimination of “bitter” taste stimuli by an insect. J Neurosci. 22(16):7281–7287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendinning JI, Foley C, Loncar I, Rai M. 2009. Induced preference for host plant chemicals in the tobacco hornworm: contribution of olfaction and taste. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 195(6):591–601 [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Hills TT. 1997. Electrophysiological evidence for two transduction pathways within a bitter-sensitive taste receptor. J Neurophysiol. 78(2):734–745 [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Jerud A, Reinherz AT. 2007. The hungry caterpillar: an analysis of how carbohydrates stimulate feeding in Manduca sexta . J Exp Biol. 210(Pt 17):3054–3067 [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Tarre M, Asaoka K. 1999. Contribution of different bitter-sensitive taste cells to feeding inhibition in a caterpillar (Manduca sexta). Behav Neurosci. 113(4):840–854 [PubMed] [Google Scholar]
- Gothilf S, Hanson FE. 1994. A technique for electrophysiologically recording from chemosensory organs of intact caterpillars. Entomol Exp Appl. 72:304–310 [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. 2008. An internal thermal sensor controlling temperature preference in Drosophila . Nature. 454(7201):217–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett N, Dauber KL, Shukla A, Morton B, Glendinning JI, Brent E, Gleason C, Islam F, Izquierdo D, Sanghavi S, et al. 2012. Identification of chemosensory receptor genes in Manduca sexta and knockdown by RNA interference. BMC Genomics. 13:211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K, Panzano VC, Chang EC, Ni L, Dainis AM, Jenkins AM, Regna K, Muskavitch MA, Garrity PA. 2012. Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila . Nature. 481(7379):76–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kester KM, Peterson SC, Hanson F, Jackson M, Severson RF. 2002. The roles of nicotine and natural enemies in determining larval feeding site distributions of Manduca sexta L. and Manduca quinquemaculata (Haworth) on tobacco. Chemoecology. 12:1–10 [Google Scholar]
- Kim SH, Lee Y, Akitake B, Woodward OM, Guggino WB, Montell C. 2010. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc Natl Acad Sci USA. 107(18):8440–8445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Kim SH, Ronderos DS, Lee Y, Akitake B, Woodward OM, Guggino WB, Smith DP, Montell C. 2010. Drosophila TRPA1 channel is required to avoid the naturally occurring insect repellent citronellal. Curr Biol. 20(18):1672–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Shim HS, Wang X, Montell C. 2008. Control of thermotactic behavior via coupling of a TRP channel to a phospholipase C signaling cascade. Nat Neurosci. 11(8):871–873 [DOI] [PubMed] [Google Scholar]
- Lu B, Breza JM, Nikonov AA, Paedae AB, Contreras RJ. 2012. Leptin increases temperature-dependent chorda tympani nerve responses to sucrose in mice. Physiol Behav. 107(4):533–539 [DOI] [PubMed] [Google Scholar]
- Madden AH, Chamberlin FS. 1945. Biology of the tobacco hornworm in the southern cigar-tobacco district. USDA Technical Bull. 896:1–51 [Google Scholar]
- Martin F, Riveron J, Alcorta E. 2011. Environmental temperature modulates olfactory reception in Drosophila melanogaster . J Insect Physiol. 57(12):1631–1642 [DOI] [PubMed] [Google Scholar]
- Matsuura H, Sokabe T, Kohno K, Tominaga M, Kadowaki T. 2009. Evolutionary conservation and changes in insect TRP channels. BMC Evol Biol. 9:228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, et al. 2007. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA. 104(33):13525–13530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaki J, Yamashita S, Sato M. 1964. Neural response of cat to taste stimuli of varying temperatures. Jpn J Physiol. 14:67–89 [DOI] [PubMed] [Google Scholar]
- Nakamura M, Kurihara K. 1991. Differential temperature dependence of taste nerve responses to various taste stimuli in dogs and rats. Am J Physiol. 261(6 Pt 2):R1402–R1408 [DOI] [PubMed] [Google Scholar]
- Nelson N, Bernays EA. 1998. Inositol in two host plants of Manduca sexta . Entomol Exp Appl. 88:189–191 [Google Scholar]
- Ohkuri T, Yasumatsu K, Horio N, Jyotaki M, Margolskee RF, Ninomiya Y. 2009. Multiple sweet receptors and transduction pathways revealed in knockout mice by temperature dependence and gurmarin sensitivity. Am J Physiol Regul Integr Comp Physiol. 296(4):R960–R971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall JA, Woledge RC. 1990. Influence of temperature on mechanics and energetics of muscle contraction. Am J Physiol. 259(2 Pt 2):R197–R203 [DOI] [PubMed] [Google Scholar]
- Reynolds SE, Yoemans MR, Timmins WA. 1986. The feeding behavior or caterpillars (Manduca sexta) on tobacco and artificial diet. Physiol Entomol. 11:39–51 [Google Scholar]
- Samczyński Z, Dybczyński RS, Polkowska-Motrenko H, Chajduk E, Pyszynska M, Danko B, Czerska E, Kulisa K, Doner K, Kalbarczyk P. 2012. Two new reference materials based on tobacco leaves: certification for over a dozen of toxic and essential elements. ScientificWorld J. 2012:216380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonhoven LM. 1972. Plant recognition by lepidopterous larvae.In: van Emden HF, editors. Insect/plant relationships. Oxford: Blackwell Scientific Publications; p. 87–99 [Google Scholar]
- Shoji T, Abe Y, Furihata E, Kurihara K. 1994. High sensitivity of the turtle olfactory system to nonvolatile substances: comparison of response properties with those in gustatory systems. Brain Res. 666(1):68–76 [DOI] [PubMed] [Google Scholar]
- Snyder MJ, Glendinning JI. 1996. Causal connection between detoxification enzyme activity and consumption of a toxic plant compound. J Comp Physiol A. 179(2):255–261 [DOI] [PubMed] [Google Scholar]
- Stamp N, Yang Y. 1996. Response of insect herbivores to multiple allelochemicals under different thermal regimes. Ecology. 77:1088–1102 [Google Scholar]
- Talavera K, Gees M, Karashima Y, Meseguer VM, Vanoirbeek JA, Damann N, Everaerts W, Benoit M, Janssens A, Vennekens R, et al. 2009. Nicotine activates the chemosensory cation channel TRPA1. Nat Neurosci. 12(10):1293–1299 [DOI] [PubMed] [Google Scholar]
- Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, Margolskee RF, Nilius B. 2005. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature. 438(7070):1022–1025 [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 28(10):2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara S, Morita H. 1972. The effects of temperature on the labellar chemoreceptors of the blowfly. J Gen Physiol. 59(2):213–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. 2007. TRP channels. Annu Rev Biochem. 76:387–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldbauer GP, Fraenkel G. 1961. Feeding on normally rejected plants by maxillectomized larvae of the tobacco hornworm, Protoparce sexta (Lepidoptera: Sphingidae). Ann Entomol Soc Am. 54:477–485 [Google Scholar]
- Yamashita S. 1964. Chemoreceptor response in frog, as modified by temperature change. Jpn J Physiol. 14:488–504 [DOI] [PubMed] [Google Scholar]
- Yamashita S, Ogawa H, Kiyoara T, Sato M. 1970. Modification by temperature change of gustatory impulse discharges in chorda tympani fibres of rats. Jpn J Physiol. 20(3):348–363 [DOI] [PubMed] [Google Scholar]
- Yamashita S, Yamada K, Sato M. 1964. The effect of temperature on neural taste response of cats. Jpn J Physiol. 14:505–514 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.