Abstract
Glutaminase 1 is the main enzyme responsible for glutamate production in mammalian cells. The roles of macrophage and microglia glutaminases in brain injury, infection, and inflammation are well documented. However, little is known about the regulation of neuronal glutaminase, despite neurons being a predominant cell type of glutaminase expression. Using primary rat and human neuronal cultures, we confirmed that interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), two proinflammatory cytokines that are typically elevated in neurodegenerative disease states, induced neuronal death and apoptosis in vitro. Furthermore, both intracellular and extracellular glutamate levels were significantly elevated following IL-1β and/or TNF-α treatment. Pretreatment with N-Methyl-D-aspartate (NMDA) receptor antagonist MK-801 blocked cytokine-induced glutamate production and alleviated the neurotoxicity, indicating that IL-1β and/or TNF-α induce neurotoxicity through glutamate. To determine the potential source of excess glutamate production in the culture during inflammation, we investigated the neuronal glutaminase and found that treatment with IL-1β or TNF-α significantly upregulated the kidney type glutaminase (KGA), a glutaminase 1 isoform, in primary human neurons. The upregulation of neuronal glutaminase was also demonstrated in situ in a murine model of HIV-1 encephalitis. In addition, IL-1β or TNF-α treatment increased the levels of KGA in cytosol and TNF-α specifically increased KGA levels in the extracellular fluid, away from its main residence in mitochondria. Together, these findings support neuronal glutaminase as a potential component of neurotoxicity during inflammation and that modulation of glutaminase may provide therapeutic avenues for neurodegenerative diseases.
Keywords: Inflammation, glutaminase, glutamate, neurotoxicity
Introduction
Chronic inflammation and neuronal damage are key processes of neurodegenerative diseases (See reviews at (Huang et al. 2005, Viviani et al. 2004)). The neuroinflammation of HIV-1-associated dementia (HAD), multiple sclerosis (MS), Parkinson’s diseases (PD), and Alzheimer’s diseases (AD) (See review at (Smith et al. 2011)), is considered one of the constitutive components of the disease pathogenesis and lesion generation. Studies have suggested a close link between the inflammatory response of the injured brain and neurotoxicity (Boutin et al. 2001, Takikita et al. 2001), however, whether the inflammation is a causative factor for neuronal damage remains unclear. In neurodegenerative diseases, reactive glia shift towards a pro-inflammatory phenotype and release cytokines, chemokines, as well as potentially neurotoxic substances including excess levels of glutamate, nitric oxide, and arachidonic acid (See reviews at (Zindler & Zipp 2011)). Cytokines, especially IL-1β and TNF-α, are typically elevated during neurodegenerative disease states and further promote central nervous system (CNS) inflammation. The increased levels of IL-1β and TNF-α may alter the activity of neurons (Bender et al. 2005, Chao et al. 1995), and increases in IL-1β and TNF-α have been observed before neuronal death (Esser et al. 1996, Guo et al. 1998). Furthermore, prolonged exposure to these cytokines generally lead to chronic inflammation and neuronal degeneration, which culminate into devastating CNS disease.
Glutamate is the most abundant excitatory neurotransmitter in the mammalian CNS (Komuro & Rakic 1996). This neurotransmitter is important in synaptic plasticity, learning, and development under physiological conditions (LoTurco et al. 1991, McEntee & Crook 1993). However, excessive glutamate stimulation induces excitotoxicity and has been linked to the pathological process of various CNS disorders including traumatic brain injury (Rao et al. 1998), ischemia (Benveniste 2009), spinal cord injury (Xu et al. 2004), stroke (Kanellopoulos et al. 2000), AD (Zoia et al. 2005), MS (Killestein et al. 2005), and HIV-1-associated dementia (HAD) (Zhao et al. 2004). Although many residential CNS cell types have been implicated in the increase of extracellular glutamate, the potential sources of excessive glutamate during the aforementioned disease states remain elusive.
Glutaminase, an enzyme localized in the inner membrane of mitochondria, converts glutamine to glutamate. As the predominant glutamine-utilizing and glutamate-producing enzyme in neurons, this enzyme has the potential to elevate glutamate to an excessive level and cause neurotoxicity (See review at (Erdmann et al. 2006)). In astrocytes, de novo glutamate synthesis takes place in the cytosol via pyruvate carboylase entry to the tricarboxylic acid cycle and the activity of aspartate amino transferase. There are two isozymes of glutaminase: kidney-type glutaminase (GLS1) and liver-type glutaminase (GLS2), of which GLS1 is highly expressed in the brain (Baglietto-Vargas et al. 2004). GLS1 has various isofroms through alternative splicing from the same locus, including glutaminase C isoform (GAC) and kidney-type glutaminase isoform (KGA). GAC shares the same functional region with KGA and possesses a unique C-terminal (Porter et al. 2002). Our previous report showed that the upregulation of GAC plays an important role in HIV-1 infection-induced neurotoxicity (Huang et al. 2011, Erdmann et al. 2009), however, the specific function and regulation of each isoform in neurons is still unclear. Glutamine is the most abundant amino acid present in the extracellular fluid of the brain, and as a substrate for glutaminase in vivo (Holcomb et al. 2000), this glutamine may be utilized by glutaminase for the production of extracellular glutamate.
To determine the regulation of glutaminase during neuroinflammation and its functional effects on neurons, we used inflammatory cytokines to stimulate primary cultured neurons (human neurons and rat cortical neurons, RCN). Our data demonstrated that IL-1β and TNF-α induced glutaminase expression and changed subcellular localization of glutaminase from the mitochondria into the cytosol and extracellular space. The upregulation of glutaminase is associated with increases in both intracellular and extracellular concentrations of glutamate and with cell death in neuron cultures. These data suggest that glutaminase and its product glutamate are critical factors in proinflammatory cytokine-induced neurotoxicity. Modulation of glutaminase may serve as a viable therapeutic target to control excess glutamate in neurodegenerative disorders.
Material and methods
Isolation and culture of primary human neurons and rat cortical neurons
Human fetal brain tissue (gestational age 13–16 weeks) was obtained from elective abortions in full compliance with the University of Nebraska Medical Center (UNMC) and NIH ethical guidelines. Human neurons were isolated from human fetal brain tissue as previously described (Zheng et al. 1999). Human neurons were plated on poly-D-lysine-coated 24-well plates at a density of 2 × 105 cells/well. The neuron differentiation medium used for the cultures was neurobasal media (GIBCO, Invitrogen Corp.) supplemented with 2% B27 (GIBCO, Invitrogen Corp.), 1% penicillin/streptomycin (Sigma-Aldrich), and 0.5 mM glutamine. Neurons were deemed mature and used for experiments 14 days after initiation of in vitro cultures. Typically, 70% of the human neuronal-enriched preparations were microtubule associated protein-2 (MAP-2) immunopositive.
Primary rat cortical neurons (RCN) were prepared from cortices of embryonic day 17-18 (E17-18) Sprague-Dawley rat fetuses as previously described (Zheng et al. 2001). Briefly, the cortex was dissected and individual cells were mechanically dissociated in Neurobasal™ medium (GIBCO, Invitrogen Corp.) and filtered through 70 μm sterile nylon. The cells were then differentiated in the aforementioned neuron differentiation medium. Cultured neurons were assumed to be mature 7-12 days after plating. All experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center. Typically, 90% of the rat neuronal-enriched preparations were MAP-2 immunopositive.
In Situ TUNEL Assay
A commercial available Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay kit (In Situ Cell Death Detection kit, Roche, Manheim, Germany) was used to determine the apoptosis of human neurons following treatment with inflammatory cytokines. The percentage of TUNEL-positive cells over the total amount of neurons (DAPI staining of nuclei) was used to indicate the levels of neuronal apoptosis. At least ten images were acquired from each immunostained treatment group using a Nikon Eclipse E800 microscope. Staurosporine (STS, 1 μM) were used as a positive control to induce neuronal apoptosis.
MTT reduction Assay
Cell viability of RCN was assessed by 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay as described previously (Jiang et al. 2001, Zhao et al. 2004). Briefly, 5 × 104 cells/well were plated in poly-D-lysine coated 96-well plates, treated with cytokines, and incubated with 10% MTT (Sigma-Aldrich) solution in Neurobasal media for 30 min at 37 °C. The extent of MTT conversion to formazan by mitochondrial dehydrogenase was determined by measuring absorbance at 490 nm with a microplate reader (Bio-Rad Laboratories). Glutamate (100 μM, Sigma-Aldrich) and the NMDA receptor antagonist MK801 (2 μM, Invitrogen Co., Carlsbad, CA) were used to induce and block excitotoxicity, respectively.
MAP2 ELISA
Human neurons were plated on poly-D-lysine coated 96-well plates at a density of 5 × 104 cells/well. After stimulated with IL-1β (10 ng/ml) or TNF-α (50 ng/ml) for 3 days, cells were fixed in 4% paraformaldehyde (PFA) at room temperature for 15 min. MAP-2 neuronal antigen was determined in human neurons treated with cytokines using colorimetric ELISA as described previously (Zheng et al. 2001, Constantino et al. 2011, Huang et al. 2011).
Severe Combined Immunodeficient HIV-1 encephalitis Mice
Four-week-old male C.B.-17-SCID mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Animals were maintained in sterile microisolator cages under pathogen-free conditions in the Comparative Medicine Animal Facilities at UNMC in accordance with ethical guidelines for care of laboratory animals set forth by the National Institutes of Health. One day after infection, HIV-1ADA-infected monocyte-derived macrophages (MDM, 5 × 105 cells in 5 μL) were injected intracranially by stereotactic methods (Persidsky et al. 1996). Replicate SCID mice received intracranial injections of PBS (sham-operated) served as controls.
Immunohistochemistry and image analysis
Human neurons were plated on poly-D-lysine-coated 15 mm coverslips in 24-well plates at a density of 2 × 105 cells/well. Cells were fixed in 4% PFA, rinsed with PBS, and then incubated overnight with mouse anti-MAP-2 or mouse anti-β-III-tubulin (Sigma-Aldrich, 1:400) antibody for the identification of neurons, with or without rabbit anti-KGA or GAC antibody (Dr. N. Curthoys, Colorado State University), followed by goat anti-mouse IgG Alexa Fluor 488 and goat anti-rabbit IgG Alexa Fluor 594 (Molecular Probes, Eugene, OR, 1:400) secondary antibodies for 1 h at room temperature. All antibodies were diluted in PBS with 0.1% Triton X-100 and 2% BSA. Cells were counterstained with DAPI (Sigma-Aldrich). Morphological changes were visualized and captured with a Nikon Eclipse E800 microscope equipped with a digital imaging system using a 20x objective. All obtained images were imported into Image-ProPlus, version 7.0 (Media Cybernetics, Sliver Spring, MD) for quantification. Ten to fifteen random fields (total 500–1000 cells per culture) of immunostained cells were manually counted.
Western blotting
Cells were rinsed twice with PBS and proteins were collected with M-PER Protein Extraction Buffer (Pierce, Rockford, IL) containing a protease inhibitors cocktail (Roche Diagnostics, Indianapolis, IN). Protein concentrations in the lysates were determined using a BCA Protein Assay Kit (Pierce). Proteins from lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoretic transfer to polyvinyldifluoridene (PVDF) membranes (Millipore and Bio-Rad), proteins were incubated with polyclonal antibodies for GAC and KGA (Dr. N. Curthoys, Colorado State University), or β-actin (Sigma-Aldrich) overnight at 4°C followed by a horseradish peroxidase-linked secondary anti-rabbit or anti-mouse antibody (Cell Signaling Technologies). Antigen-antibody complexes were visualized by Pierce ECL Western Blotting Substrate (Pierce). For data quantification, films were scanned with a CanonScan 9950F scanner; the acquired images were then analyzed on a Macintosh computer using the public domain NIH image program (developed at the U.S. National Institutes of Health and available on the internet at http://rsb.info.nih.gov/nih-image/).
Intracellular and extracellular glutamate analysis
Intracellular glutamate detection was performed with the Amplex® Red Glutamic Acid/Glutamate Oxidase Assay Kit from Invitrogen following the manufacture’s procedure. HPLC analysis for extracellular glutamate was performed as previously described (Zhao et al. 2004, Huang et al. 2011).
Statistical Analyses
Data were evaluated statistically by the analysis of variance (ANOVA), followed by a Tukey’s test for multiple comparisons. Data were shown as mean ± SD, and significance was determined as P < 0.05. To account for any donor-specific differences, all experiments were performed at least three times, with triplicate or quadruplicate samples in each assay.
Results
IL-1β and TNF-α induce neuronal death and apoptosis in vitro
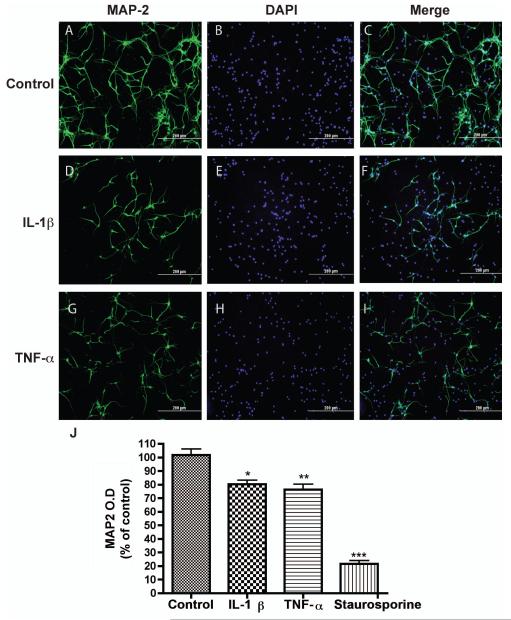
To determine whether IL-1β or TNF-α, the main inflammatory factors increased during neurodegenerative diseases, could induce neurotoxicity, we treated human neurons with 10 ng/ml IL-1β or 50 ng/ml TNF-α for 72 hours and determined the cell death in vitro. First, we described the dendritic damage, which is a characteristic process of neurotoxicity. MAP-2, a marker of neuronal dendrites and cell body, was used to label neurons for morphologic changes after cytokine stimulation. Similar to previous reports (See review at (Block et al. 2007)), treatment with those cytokines had a neurotoxic effect on human neurons (Fig. 1). Specifically, compared with untreated control (Fig. 1A-C), neuronal dendrites were reduced in size following treatment of IL-1β (Fig. 1D-F) or TNF-α (Fig. 1G-I). Notably, there were more nuclei than actual neurons, suggesting other cell type(s) in the neuronal culture. We have used glial fibrillary acidic protein (GFAP) to label astrocytes and found that astrocytes accounted for 20% of the cells in the culture. The culture also contained β-tubulin III-positive immature neurons (data not shown). The levels of MAP-2 were further determined by an ELISA assay that we previously described. The MAP-2 ELISA is a sensitive assay to determine the extent of neuronal injury and cell death (Zheng et al. 2001, Huang et al. 2011, Constantino et al. 2011). Indeed, MAP2 levels were significantly reduced following treatment with IL-1β (10 ng/ml) or TNF-α (50 ng/ml) for 72 hours (Fig. 1J) in human neurons. Together, these data indicate that IL-1β and TNF-α induce dendritic damage and loss.
Figure 1.
IL-1β and TNF-α both mediate neuronal damage in vitro. Human neurons were treated with IL-1β (10 ng/ml) or TNF-α (50 ng/ml) for 2 days. A-I) Neuronal antigen was immunostained with antibody to MAP-2 (A, D, and G). DAPI was used to mark cell nuclei (B, E, and H). Panels C, F, and I are merged pictures of A-B, D-E, and G-H, respectively. J) Levels of MAP-2 antigen after IL-1β, TNF-α, or staurosporine treatment were determined by ELISA. Results shown are the means ± SD. *, P < 0.05, **, P < 0.01, ***, P < 0.001 compared with control.
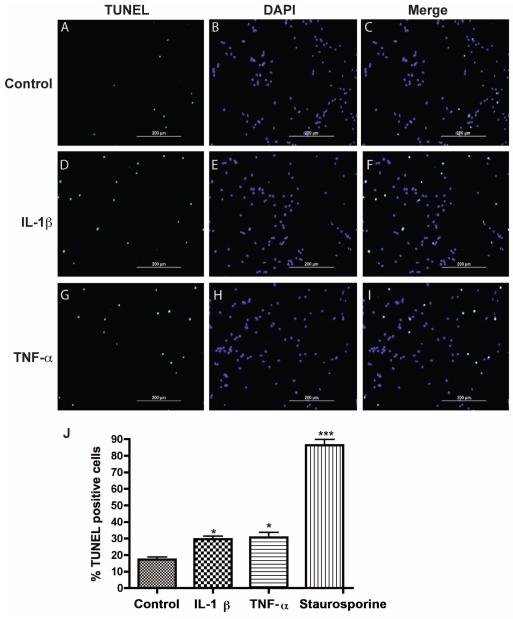
Induction of neuronal apoptosis is one of the mechanisms by which IL-1β induces neurotoxicity (Hu et al. 1997). To test whether IL-1β and TNF-α induce apoptosis in our human neuronal cultures, we treated human neurons with IL-1β or TNF-α for 24 hours and detected the apoptosis by TUNEL assay (Fig. 2, A, D, G). The nuclei of neurons at the TUNEL assay were marked with DAPI (Fig. 2, B, E, H). The specificity of TUNEL immunoreactivity was evident since all of the TUNEL signals were overlapping with DAPI, indicating DNA fragmentation within the apoptotic nuclei (Fig. 2, C, F, I). Based on the quantification of the percentage of TUNEL-positive cells, IL-1β and TNF-α treatment groups had significantly higher numbers of TUNEL-positive cells compared with the untreated control (Fig. 2J). The results suggested that IL-1β and TNF-α induce apoptosis in human neuronal cultures. Together, our data demonstrate that IL-1β and TNF-α induce neuronal dendritic damage and apoptosis in human neurons.
Figure 2.
IL-1β and TNF-α both mediate apoptosis in neuronal cultures. Human neurons were treated with IL-1β (10 ng/ml) or TNF-α (50 ng/ml) for 2 days. A-I) IL-1β- or TNF-α-mediated neuronal apoptosis was determined by TUNEL assays. Apoptotic cells (TUNEL positive, A, D, and G), total cells (DAPI positive, B, E, and H), and their merged pictures (C, F, and I) were shown. d). Quantitative assessments of neuronal apoptosis were determined through counting TUNEL positive cells (green) over total cell number (DAPI, blue). A minimum of 10 photos per treatment group were analyzed. Staurosporine-treated neurons served as positive control for apoptosis. Data represent means ± SD, and *, P < 0.05, ***, P < 0.001 in comparison to control.
IL-1β and TNF-α increase glutamate levels in neuronal cultures
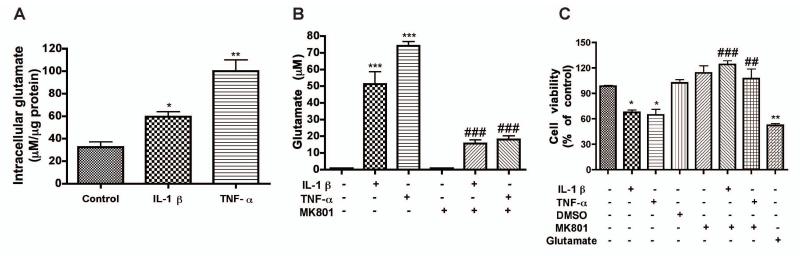
Excessive levels of glutamate are well known to induce neurotoxicity (for review, see (Erdmann et al. 2006)). To determine whether glutamate is a potential mediator of IL-1β- or TNF-α-mediated neurotoxicity, we first measured glutamate levels in the neuronal cultures. We determined intracellular glutamate level using a commercial available glutamate detection kit. As expected, IL-1β and TNF-α induced a 2- and 3-fold increase of intracellular glutamate, respectively, as compared to control (Fig. 3A). Since increased generation of glutamate could be an important cellular source of extracellular glutamate, we next investigated the extracellular levels of glutamate. Similar to the intracellular glutamate, the extracellular glutamate increased following the treatment with IL-1β or TNF-α (Fig. 3B). Both IL-1β (10 ng/ml) and TNF-α (50 ng/ml) also significantly reduced neuronal viability as determined by MTT assay, an effect comparable to the positive control (glutamate, 100 μM) (Fig. 3C). Interestingly, pretreatment with MK801 significantly blocked the increase of extracellular glutamate and inflammatory cytokine-induced neurotoxicity (Fig. 3B, C). Together, these data suggest that glutamate and glutamate-mediated neurotoxicity are critical components of IL-1β- and TNF-α-induced neuronal damage.
Figure 3.
IL-1β and TNF-α both increase intracellular and extracellular glutamate levels in neuronal cultures. RCN were treated with IL-1β (10 ng/ml) or TNF-α (50 ng/ml) for 3 days. A) Intracellular concentration of glutamate was determined by a Glutamic Acid/Glutamate Oxidase Assay Kit. **, P < 0.01 compared with control. B-C) RCN were pre-treated with 2 μM MK801 (NMDA receptor antagonist) for 2 hours then treated with IL-1 β or 50 ng/ml TNF-α for 3 days. The concentration of glutamate in cell-free supernatants was determined by RP-HPLC (B). RCN Cell viability was assessed by MTT assay (C). Results shown are the means ± SD of triplicate samples. *, P < 0.05, **, P < 0.01, ***, P < 0.001 compared with control. ##, P < 0.01, ###, P < 0.001 compared with cytokine treatment alone.
IL-1β and TNF-α regulate glutaminase expression in human neurons
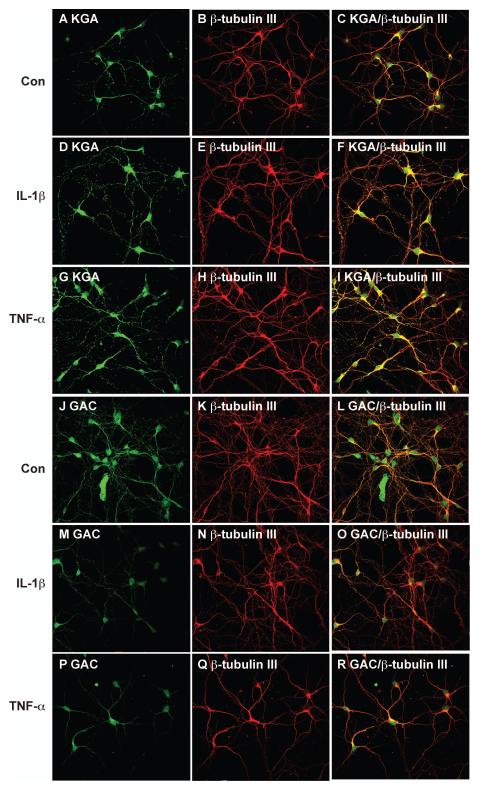
The increase of intracellular and extracelluar glutamate following IL-1β or TNF-α treatment indicates an increase of glutaminase activity in the neuronal culture. We previously demonstrated that the increased glutamate production from HIV-1-infected macrophages and microglia was dependent upon mitochondrial glutaminase (Zhao et al. 2004, Huang et al. 2011). Therefore, we further investigated whether glutaminase, particularly its two main isoforms KGA and GAC, is responsible for glutamate generation and the neurotoxicity induced by IL-1β or TNF-α. The expression of KGA and GAC in human neurons during the process of cytokine-mediated neurotoxicity was first assessed by immunocytochemistry. Neuronal cultures were labeled with KGA (Fig. 4A) and with a neuronal marker β-tubulin III (Fig. 4B). The KGA had a punctate distribution in the cytoplasm and along the dendrites, which parallels the distinct distribution pattern of the mitochondrial network (Fig. 4A). The colocalization of KGA and β-tubulin III suggested that KGA is primarily expressed by the neuronal population (Fig. 4C). After IL-1β or TNF-α treatment, KGA immunoreactivity increased in a pattern similar to mitochondrial distribution when compared with untreated control (Fig. 4D-I). In contrast, the expression of GAC in neurons decreased after IL-1β or TNF-α treatment (Fig. 4J-R). Together, the immunocytochemistry data indicates that the IL-1β and TNF-α regulate the glutaminase isoforms in neuronal cultures.
Figure 4.
IL-1β and TNF-α both regulate glutaminase staining in neurons. Human neurons were treated with IL-1β (10 ng/ml) or TNF-α (50 ng/ml) for 24 hours and then immunostained with KGA (A, D, G), GAC (J, M, P), or β-tubulin III (neuronal marker, B, E, H, K, N, Q). Panels C, F, I, L, O, and R are merged pictures of A-B, D-E, G-H, J-K, M-N, and P-Q, respectively. Images were acquired from a Bio-Rad MRC1024ES LASER scanning confocal microscope. Magnifications: A-R. 600X. Panels are representative of three separate donors.
To further determine the regulation of glutaminase during the cytokine stimulation, we investigated the KGA and GAC mRNA and protein levels in neuronal cultures following IL-1β or TNF-α treatment. Gene expression analysis using real-time RT-PCR revealed a 2.2-fold and 1.7-fold increase of the KGA mRNA following IL-1β and TNF-α treatment, respectively, when compared to untreated human neurons (Fig. 5A). The upregulation of KGA by inflammatory cytokines was further confirmed at the level of protein translation through Western blotting. IL-1β and TNF-α each induced 5-fold increase of KGA compared with untreated control (Fig. 5 C, E). The upregulation of KGA is specific because after inflammatory cytokines stimulation, both the mRNA (Fig. 5B) and protein levels (Fig. 5D, F) of GAC had trended downward. The specific upregulation of KGA suggests that KGA may be the isoform responsible for the IL-1β and TNF-α induced-neurotoxicity.
Figure 5.
IL-1β and TNF-α both regulate glutaminase mRNA and protein expression in neurons. Human neurons were treated with IL-1β (10 ng/ml) or TNF-α (50 ng/ml) for 2 days. A-B) RNA was collected and expression of glutaminase isoforms KGA (A), GAC (B) were analyzed using Real-time RT PCR. Data were normalized to GAPDH and presented as fold change compared to control. * denotes p < 0.05 as compared to control. C-F) Whole cell lysates were collected and protein levels of KGA (C) and GAC (D) were analyzed by Western blot. Actin was used as the loading control. Levels of KGA (E) and GAC (F) were normalized as a ratio to actin after densitometrical quantification and presented as fold change relative to control. Results are expressed as the mean ± SEM of triplicate samples from 3 independent experiments. *, P < 0.05 compared to control.
Glutaminase is elevated in a murine model of HIV-1 encephalitis
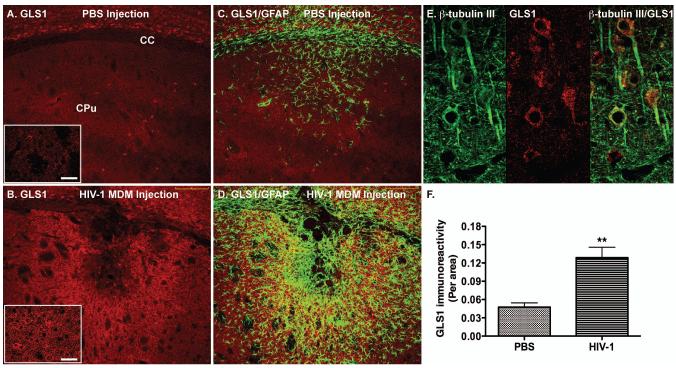
To further determine the relevance of glutaminase during brain inflammation in vivo, we obtained an established murine model of HIV-1 encephalitis. The injection of HIV-1-infected MDM is known to elicit a sustained inflammatory response reminiscent of HIV-1 encephalitis brain tissue (Persidsky et al. 1996). Notably, astrogliosis and inflammatory cytokines TNF-α and IL-1β were evident after MDM inoculation (Persidsky et al. 1996). We have previously correlated the elevation of IL-1β in this animal model to the dysregulation of CXCL12, a chemokine important for leukocyte and stem cell migration (Peng et al. 2006). To determine whether the inflammatory response was related to the glutaminase, we used a polyclonal antibody against glutaminase (Dr. N. Curthoys, Colorado State University) to identify the glutaminase expression in vivo (Fig. 6A, B). The inflammatory area in the HIVE model was defined by the GFAP immunoreactivity, which marked astrocyte activation (Fig 6C, D). Glutaminase expression in the inflammatory area had little colocalization with the GFAP, suggesting lower expression levels of glutaminase in astrocytes. In contrast, a majority of glutaminase immunoreactivity appeared to have morphology characteristic of neuron (Fig. 6A, B lower left inserts). Furthermore, glutaminase was colocalized with β-tubulin III, a neuronal marker, suggesting a predominant expression of glutaminase in neurons (Fig. 6E). Importantly, glutaminase expression in the neuroinflammatory area was significantly increased compared with sham-operated mice (Fig. 6F), suggesting that glutaminase may be associated with inflammatory tissue pathology.
Figure 6.
Glutaminase expression in a murine model of HIV-1 encephalitis. PBS or HIV-1ADA-infected MDM (5 × 105 cells in 5 μL) were intracranially injected into the basal ganglia of SCID mice for 7 days. Brain tissues were collected and subjected to immunohistochemical staining. A-D) Serial floating coronal sections from PBS (A, C) and HIV-1-infected MDM (B, D) injections were immunolabeled with antibodies to GLS 1 (red) and GFAP (green). Higher magnification GLS1 staining images for the PBS (A) and HIV-1-infected MDM (B) affected brain area were shown in the lower left inserts. E, Brain sections were stained with neuronal marker β-tubulin III (green) and GLS1 (red). F: GLS1 expression from panels A and B was quantified by determining the GLS1-positive area as a percentage of the total image area per microscopy field and calculated for a window of tissue immediately surrounding the injection site. Three sections of at least four mice were examined for each group. **, p < 0.01 compared with PBS group. Scale bar represents 200 μM. Scale bar in the lower left inserts represents 25 μM. CC, corpus callosum; CPu, Caudate putamen.
IL-1β and TNF-α regulate subcelluar localization of KGA
We previously reported that glutaminase released from mitochondria contributes to the neurotoxicity of HIV-1 infected macrophages (Erdmann et al. 2009, Tian et al. 2012). To identify changes in glutaminase distribution following cytokine stimulation, particularly at locations away from its main residence in mitochondria, we used subcellular fractionation and subsequently Western blotting for KGA. Mitochondrial and cytosolic fractions from control and cytokine-treated neurons were isolated and the enrichments of mitochondria and cytosol were confirmed by the blotting of Voltage-dependent anion channels (VDAC), a specific outer mitochondrial membrane protein not present in the cytosolic factions (Fig. 7A). Analysis of KGA levels in the mitochondrial and cytosolic fractions revealed that the cytosolic fraction from control human neurons had detectable levels of KGA present, possibly due to the process of in vitro manipulation of the neuronal cultures. Interestingly, in the cytosolic fractions, KGA levels were increased in both IL-1β- and TNF-α-treated neurons (Fig. 7A, B), indicating that proinflammatory cytokines induce a release of KGA from mitochondria into the cytosolic compartment of human neurons.
Figure 7.
IL-1β and TNF-α induce glutaminase release from mitochondria in vitro. Human neurons were treated with IL-1β (10 ng/ml) or TNF-α (50 ng/ml) for 3 days. A) Cells were subjected to subcellular fractionation; the cytosolic and the mitochondrial fractions were separated by SDS-PAGE and blotted for immunodetection of KGA. VDAC and β-actin were used as loading controls for the mitochondrial and cytosolic fractions, respectively. B) Levels of KGA in cytosol and mitochondria were normalized as a ratio to VDAC or β-actin after densitometrical quantification and presented as fold change relative to the untreated control. C) Proteins from equal volumes (30 ml) of conditioned-medium from cultures of control or cytokine-stimulated human neurons were precipitated using TCA. KGA levels in the precipitated protein were determined by Western blotting. D) Results of C were normalized with β-actin and densitometrically quantified as fold change relative to the untreated control. All the data are representative of at least 3 independent experiments with human neurons from 3 different donors.
TNF-α induces glutaminase release into the extracellular space
Glutaminase release into the extracelluar fluid is one of the important mechanisms for excess glutamate production in HIV-1-infected macrophages (Erdmann et al. 2009, Zhao et al. 2004). However, whether there is glutaminase release in neuronal cultures remains unclear. We collected equal amounts (30 ml) of control and cytokines-treated neuron culture supernatants and precipitated the proteins with trichloroacetic acid (TCA). Protein pellets were resuspended in lysis buffer, denatured, and glutaminase was detected via Western blotting (Fig. 7C). Interestingly, there was no evidence of KGA release in control and IL-1β treated samples. In contrast, TNF-α treatment induced a marked increase of KGA levels in the supernatant (Fig. 7C, D). These findings demonstrated that glutaminase was specifically released to the extracellular space in human neurons following TNF-α treatment.
Discussion
One of the most important roles of glutaminase in the CNS is its contribution to excitatory neurotransmitter in neuron. Although the effects of macrophage and microglia glutaminases in brain injury, infection, and inflammation are well studied (Dohmen et al. 2005, Erdmann et al. 2009, Huang et al. 2011, Newcomb et al. 1997, Tian et al. 2008, Zhao et al. 2004), little is known about the regulation of neuronal glutaminase. In this study, we found that IL-1β and TNF-α induced neuronal death and apoptosis in vitro through a mechanism involving the increase of glutamate levels in culture supernatants (Figs. 1-3). Further analysis of glutaminase isoforms revealed that IL-1β and TNF-α upregulated KGA expression in human neurons (Figs. 4, 5). Interestingly, both IL-1β and TNF-α appeared to regulate the subcelluar localization of KGA, inducing KGA into the cytosol and away from its mitochondrial residence (Fig. 7A, B). Furthermore, TNF-α induced glutaminase release into the extracellular space, which is a distinct event not seen in IL-1β-treated or control neuronal cultures (Fig. 7C, D). Together, our data suggest that IL-1β and TNF-α induce neurotoxicity through glutamate, the mechanism of which is through dysregulation and translocation of neuronal glutaminase. These findings provide insights into the critical role of excess glutamate levels and dysregulation of neuronal glutaminase in inflammation induced neurotoxicity.
Through unique primary neuronal cultures, we have determined that IL-1β and TNF-α upregulate neuronal KGA expression and stimulate extensive neuronal KGA release, resulting in increased glutamate production and excitotoxicity. The discovery of this cascade of neurotoxic processes initiated by cytokines is significant because it describes the inflammatory perturbation of brain homeostasis that leads to neuronal deficits. Moreover, the upregulation of cytokines IL-1β and TNF-α is a common feature of glial cell activation in several inflammatory neurodegenerative diseases (Hu et al. 1995, Van Eldik et al. 2007). Although IL-1β and TNF-α alone or in combination with other cytokines have been shown to promote cell death and apoptosis (Bender et al. 2005, Chao et al. 1995), the molecular mechanism remains poorly defined. The identification of the neurotoxic effects of IL-1β and TNF-α through mechanisms involving glutaminase will therefore be important to the understanding of excess glutamate production during brain inflammation.
Little is known about how KGA and GAC, two isoforms of glutaminase 1, are regulated. One recent paper suggests a role for mir-23a/b and NFkB in the regulation of GAC (Gao et al. 2009). Furthermore, we have identified that type I interferon regulates GAC expression through STAT-1 binding (Zhao et al. 2012). In the current study, we have demonstrated that inflammatory cytokines increase KGA isoform in neurons (Figs. 4, 5). Because KGA is expressed at a higher level in cultured neurons than in glia (Data not shown), the upregulation of KGA by inflammatory cytokines indicates neuronal KGA may be more important in cytokine-induced neurotoxicity than GAC. Our study, along with several recent studies, defines the importance of understanding the mechanism of glutaminase-mediated excess glutamate production in disease pathogenesis, including viral infection, cancer development, and neurological diseases (Wang et al. 2010, Maezawa & Jin 2010, Huang et al. 2011). However, the physiological relevance of the novel glutaminase regulation that we identified in neurons remains incompletely understood. Using immunohistochemical technique, we have demonstrated the upregulation of glutaminase in situ in a murine model of encephalitis. The great majority of glutaminase immunoreactivity in the affected mouse brain area was found to be in β-tubulin III-positive neurons (Fig. 6). Further studies are needed to help determine the functional importance of neuronal glutaminase in vivo.
Previous studies in our group showed that in addition to glutaminase expression, glutaminase activity or release mediated by the infective process of HIV-1 may facilitate excess generation of glutamate in the CNS (Erdmann et al. 2009, Tian et al. 2012). Using the subcellular fractionation for neurons, we found that inflammatory cytokines induced neuronal glutaminase translocation from mitochondria to cytosol (Fig. 7 A, B). Interestingly, widespread neuron death could result in glutaminase release, excess glutamate production, and further neurotoxicity in vitro (Newcomb et al. 1997). We further investigated the glutaminase level in culture supernatants. After precipitating protein in culture supernatants, glutaminase was found in TNF-α-stimulated extracellular protein precipitates. However, we were unable to detect glutaminase release in the culture supernatants of IL-1β-stimulated group (Fig. 7C), suggesting that glutaminase release from cells is specific to TNF-α treatment. The specific release of KGA could make promising clinical targets to develop novel therapeutic strategy against excitotoxicity by free glutaminase. For IL-1β, since it increases KGA expression and both the intracellular and extracellular levels of glutamate (Figs. 3-5), it is likely that IL-1β mediates excess glutamate production through a mechanism independent of extracellular glutaminase release.
The interaction between cytokines and the glutamine metabolism is of great importance in inflammatory brain diseases (See review at (Viviani et al. 2004)). Increased glutamate levels have been suggested as responsible for the neuronal death characteristic of many neurodegenerative diseases (See review at (Meldrum 2000)). In this study, we have demonstrated that both IL-1β and TNF-α upregulated intracellular and extracellular glutamate levels (Fig. 3). The cell death in the neuronal cultures is likely a consequence of glutamate increase, since MK801, a NMDA receptor antagonist, blocked the glutamate production and cell death (Fig. 3). The NMDA receptor has a critical role in mediating excitotoxic neuronal cell death in a variety of neurodegenerative conditions (See review at (Leist & Nicotera 1998)). Recent evidence indicates that IL-1 may mediate increased neuronal excitability through direct interaction of its receptor complex with NMDA receptors and inhibition of Ca2+-induced K+ channels (Gardoni et al., Zhang et al. 2008). Whether both IL-1β and TNF-α receptors have direct interaction with NMDA receptors in the neurons remains to be determined.
In summary, we have identified neuronal glutaminase as an important player in mediating glutamate over-production during inflammatory cytokines stimulation. The potential effect of glutaminase on glutamate involves dysregulation, translocation, or release of glutaminase isoforms, which consequently induce neurotoxicity and apoptosis. Uncovering the critical role of glutaminase in inflammation-induced neurotoxicity may provide a potential target for therapeutic intervention in inflammatory brain diseases.
Acknowledgements
We kindly acknowledge Beibei Jia for providing technical support, Dr. N. Curthoys (Colorado State University) for the antibody against KGA, GAC, and GLS1, Randall J Ambroz, Kristin M. Leland Wavrin for editing the manuscript for this work. Tiffany R. Peña, Julie Ditter, Robin Taylor, Johnna Belling, Na Ly, Myhanh Che, Mary Cavell and Emilie Scoggins provided outstanding administrative support. This work was supported in part by research grants by the National Institutes of Health: R01 NS 41858-01, R01 NS 061642-01, 3R01NS61642-2S1, R21 MH 083525-01, P01 NS043985, and P20 RR15635-01 (JZ) and National Natural Science Foundation of China (NSFC) # 81028007. The authors indicate no potential conflicts of interest.
Abbreviations
- RCN
rat cortical neurons
- KGA
kidney type glutaminase
- GAC
glutaminase C
- TCA
trichloroacetic acid
- OD
optical density
- ANOVA
analysis of variance
- STS
staurosporine
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- ELISA
enzyme-linked immunosorbent assay
- HPLC
high performance liquid chromatography
References
- Baglietto-Vargas D, Lopez-Tellez JF, Moreno-Gonzalez I, Gutierrez A, Aledo JC. Segregation of two glutaminase isoforms in islets of Langerhans. Biochem J. 2004;381:483–487. doi: 10.1042/BJ20040523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender LM, Morgan MJ, Thomas LR, Liu ZG, Thorburn A. The adaptor protein TRADD activates distinct mechanisms of apoptosis from the nucleus and the cytoplasm. Cell Death Differ. 2005;12:473–481. doi: 10.1038/sj.cdd.4401578. [DOI] [PubMed] [Google Scholar]
- Benveniste H. Glutamate, microdialysis, and cerebral ischemia: lost in translation? Anesthesiology. 2009;110:422–425. doi: 10.1097/ALN.0b013e318194b620. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nature reviews. Neuroscience. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Boutin H, LeFeuvre RA, Horai R, Asano M, Iwakura Y, Rothwell NJ. Role of IL-1alpha and IL-1beta in ischemic brain damage. J Neurosci. 2001;21:5528–5534. doi: 10.1523/JNEUROSCI.21-15-05528.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, Hu S, Ehrlich L, Peterson PK. Interleukin-1 and tumor necrosis factor-alpha synergistically mediate neurotoxicity: involvement of nitric oxide and of N-methyl-D-aspartate receptors. Brain Behav Immun. 1995;9:355–365. doi: 10.1006/brbi.1995.1033. [DOI] [PubMed] [Google Scholar]
- Constantino AA, Huang Y, Zhang H, Wood C, Zheng JC. HIV-1 clade B and C isolates exhibit differential replication: relevance to macrophage-mediated neurotoxicity. Neurotoxicity research. 2011;20:277–288. doi: 10.1007/s12640-011-9241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohmen C, Kumura E, Rosner G, Heiss WD, Graf R. Extracellular correlates of glutamate toxicity in short-term cerebral ischemia and reperfusion: a direct in vivo comparison between white and gray matter. Brain Res. 2005;1037:43–51. doi: 10.1016/j.brainres.2004.12.046. [DOI] [PubMed] [Google Scholar]
- Erdmann N, Tian C, Huang Y, Zhao J, Herek S, Curthoys N, Zheng J. In vitro glutaminase regulation and mechanisms of glutamate generation in HIV-1-infected macrophage. Journal of neurochemistry. 2009;109:551–561. doi: 10.1111/j.1471-4159.2009.05989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann NB, Whitney NP, Zheng J. Potentiation of excitotoxicity in HIV-1 associated dementia and the significance of glutaminase. Clin Neurosci Res. 2006;6:315–328. doi: 10.1016/j.cnr.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser R, Glienke W, von Briesen H, Rubsamen-Waigmann H, Andreesen R. Differential regulation of proinflammatory and hematopoietic cytokines in human macrophages after infection with human immunodeficiency virus. Blood. 1996;88:3474–3481. [PubMed] [Google Scholar]
- Gao P, Tchernyshyov I, Chang TC, et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardoni F, Boraso M, Zianni E, Corsini E, Galli CL, Cattabeni F, Marinovich M, Di Luca M, Viviani B. Distribution of interleukin-1 receptor complex at the synaptic membrane driven by interleukin-1beta and NMDA stimulation. J Neuroinflammation. 2011;8:14. doi: 10.1186/1742-2094-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Jin YX, Ishikawa M, Huang YM, van der Meide PH, Link H, Xiao BG. Regulation of beta-chemokine mRNA expression in adult rat astrocytes by lipopolysaccharide, proinflammatory and immunoregulatory cytokines. Scandinavian journal of immunology. 1998;48:502–508. doi: 10.1046/j.1365-3083.1998.00422.x. [DOI] [PubMed] [Google Scholar]
- Holcomb T, Taylor L, Trohkimoinen J, Curthoys NP. Isolation, characterization and expression of a human brain mitochondrial glutaminase cDNA. Brain Res Mol Brain Res. 2000;76:56–63. doi: 10.1016/s0169-328x(99)00331-9. [DOI] [PubMed] [Google Scholar]
- Hu S, Peterson PK, Chao CC. Cytokine-mediated neuronal apoptosis. Neurochem Int. 1997;30:427–431. doi: 10.1016/s0197-0186(96)00078-2. [DOI] [PubMed] [Google Scholar]
- Hu S, Sheng WS, Peterson PK, Chao CC. Differential regulation by cytokines of human astrocyte nitric oxide production. Glia. 1995;15:491–494. doi: 10.1002/glia.440150412. [DOI] [PubMed] [Google Scholar]
- Huang Y, Erdmann N, Peng H, Zhao Y, Zheng J. The role of TNF related apoptosis-inducing ligand in neurodegenerative diseases. Cell Mol Immunol. 2005;2:113–122. [PubMed] [Google Scholar]
- Huang Y, Zhao L, Jia B, Wu L, Li Y, Curthoys N, Zheng JC. Glutaminase dysregulation in HIV-1-infected human microglia mediates neurotoxicity: relevant to HIV-1-associated neurocognitive disorders. J Neurosci. 2011;31:15195–15204. doi: 10.1523/JNEUROSCI.2051-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZG, Piggee C, Heyes MP, Murphy C, Quearry B, Bauer M, Zheng J, Gendelman HE, Markey SP. Glutamate is a mediator of neurotoxicity in secretions of activated HIV-1-infected macrophages. Journal of neuroimmunology. 2001;117:97–107. doi: 10.1016/s0165-5728(01)00315-0. [DOI] [PubMed] [Google Scholar]
- Kanellopoulos GK, Xu XM, Hsu CY, Lu X, Sundt TM, Kouchoukos NT. White matter injury in spinal cord ischemia: protection by AMPA/kainate glutamate receptor antagonism. Stroke; a journal of cerebral circulation. 2000;31:1945–1952. doi: 10.1161/01.str.31.8.1945. [DOI] [PubMed] [Google Scholar]
- Killestein J, Kalkers NF, Polman CH. Glutamate inhibition in MS: the neuroprotective properties of riluzole. Journal of the neurological sciences. 2005;233:113–115. doi: 10.1016/j.jns.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Intracellular Ca2+ fluctuations modulate the rate of neuronal migration. Neuron. 1996;17:275–285. doi: 10.1016/s0896-6273(00)80159-2. [DOI] [PubMed] [Google Scholar]
- Leist M, Nicotera P. Apoptosis, excitotoxicity, and neuropathology. Exp Cell Res. 1998;239:183–201. doi: 10.1006/excr.1997.4026. [DOI] [PubMed] [Google Scholar]
- LoTurco JJ, Blanton MG, Kriegstein AR. Initial expression and endogenous activation of NMDA channels in early neocortical development. J Neurosci. 1991;11:792–799. doi: 10.1523/JNEUROSCI.11-03-00792.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maezawa I, Jin LW. Rett syndrome microglia damage dendrites and synapses by the elevated release of glutamate. J Neurosci. 2010;30:5346–5356. doi: 10.1523/JNEUROSCI.5966-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEntee WJ, Crook TH. Glutamate: its role in learning, memory, and the aging brain. Psychopharmacology. 1993;111:391–401. doi: 10.1007/BF02253527. [DOI] [PubMed] [Google Scholar]
- Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130:1007S–1015S. doi: 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- Newcomb R, Sun X, Taylor L, Curthoys N, Giffard RG. Increased production of extracellular glutamate by the mitochondrial glutaminase following neuronal death. The Journal of biological chemistry. 1997;272:11276–11282. doi: 10.1074/jbc.272.17.11276. [DOI] [PubMed] [Google Scholar]
- Peng H, Erdmann N, Whitney N, Dou H, Gorantla S, Gendelman HE, Ghorpade A, Zheng J. HIV-1-infected and/or immune activated macrophages regulate astrocyte SDF-1 production through IL-1beta. Glia. 2006;54:619–629. doi: 10.1002/glia.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Limoges J, McComb R, et al. Human immunodeficiency virus encephalitis in SCID mice. The American journal of pathology. 1996;149:1027–1053. [PMC free article] [PubMed] [Google Scholar]
- Porter LD, Ibrahim H, Taylor L, Curthoys NP. Complexity and species variation of the kidney-type glutaminase gene. Physiological genomics. 2002;9:157–166. doi: 10.1152/physiolgenomics.00017.2002. [DOI] [PubMed] [Google Scholar]
- Rao VL, Baskaya MK, Dogan A, Rothstein JD, Dempsey RJ. Traumatic brain injury down-regulates glial glutamate transporter (GLT-1 and GLAST) proteins in rat brain. Journal of neurochemistry. 1998;70:2020–2027. doi: 10.1046/j.1471-4159.1998.70052020.x. [DOI] [PubMed] [Google Scholar]
- Smith JA, Das A, Ray SK, Banik NL. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull. 2011;87:10–20. doi: 10.1016/j.brainresbull.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takikita S, Takano T, Narita T, Takikita M, Ohno M, Shimada M. Neuronal apoptosis mediated by IL-1 beta expression in viral encephalitis caused by a neuroadapted strain of the mumps virus (Kilham Strain) in hamsters. Experimental neurology. 2001;172:47–59. doi: 10.1006/exnr.2001.7773. [DOI] [PubMed] [Google Scholar]
- Tian C, Erdmann N, Zhao J, Cao Z, Peng H, Zheng J. HIV-infected macrophages mediate neuronal apoptosis through mitochondrial glutaminase. Journal of neurochemistry. 2008;105:994–1005. doi: 10.1111/j.1471-4159.2007.05197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C, Sun L, Jia B, Ma K, Curthoys N, Ding J, Zheng J. Mitochondrial glutaminase release contributes to glutamate-mediated neurotoxicity during human immunodeficiency virus-1 infection. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2012;7:619–628. doi: 10.1007/s11481-012-9364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eldik LJ, Thompson WL, Ralay Ranaivo H, Behanna HA, Martin Watterson D. Glia proinflammatory cytokine upregulation as a therapeutic target for neurodegenerative diseases: function-based and target-based discovery approaches. International review of neurobiology. 2007;82:277–296. doi: 10.1016/S0074-7742(07)82015-0. [DOI] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Corsini E, Galli CL, Marinovich M. Cytokines role in neurodegenerative events. Toxicol Lett. 2004;149:85–89. doi: 10.1016/j.toxlet.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Wang JB, Erickson JW, Fuji R, et al. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer cell. 2010;18:207–219. doi: 10.1016/j.ccr.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu GY, Hughes MG, Ye Z, Hulsebosch CE, McAdoo DJ. Concentrations of glutamate released following spinal cord injury kill oligodendrocytes in the spinal cord. Experimental neurology. 2004;187:329–336. doi: 10.1016/j.expneurol.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Zhang R, Yamada J, Hayashi Y, Wu Z, Koyama S, Nakanishi H. Inhibition of NMDA-induced outward currents by interleukin-1beta in hippocampal neurons. Biochem Biophys Res Commun. 2008;372:816–820. doi: 10.1016/j.bbrc.2008.05.128. [DOI] [PubMed] [Google Scholar]
- Zhao J, Lopez AL, Erichsen D, Herek S, Cotter RL, Curthoys NP, Zheng J. Mitochondrial glutaminase enhances extracellular glutamate production in HIV-1-infected macrophages: linkage to HIV-1 associated dementia. Journal of neurochemistry. 2004;88:169–180. doi: 10.1046/j.1471-4159.2003.02146.x. [DOI] [PubMed] [Google Scholar]
- Zhao L, Huang Y, Tian C, Taylor L, Curthoys N, Wang Y, Vernon H, Zheng J. Interferon-alpha regulates glutaminase 1 promoter through STAT1 phosphorylation: relevance to HIV-1 associated neurocognitive disorders. PloS one. 2012;7:e32995. doi: 10.1371/journal.pone.0032995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Thylin MR, Cotter RL, et al. HIV-1 infected and immune competent mononuclear phagocytes induce quantitative alterations in neuronal dendritic arbor: relevance for HIV-1-associated dementia. Neurotoxicity research. 2001;3:443–459. doi: 10.1007/BF03033203. [DOI] [PubMed] [Google Scholar]
- Zheng J, Thylin MR, Ghorpade A, et al. Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1-associated dementia. Journal of neuroimmunology. 1999;98:185–200. doi: 10.1016/s0165-5728(99)00049-1. [DOI] [PubMed] [Google Scholar]
- Zindler E, Zipp F. Neuronal injury in chronic CNS inflammation. Best Pract Res Clin Anaesthesiol. 2011;24:551–562. doi: 10.1016/j.bpa.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Zoia CP, Tagliabue E, Isella V, Begni B, Fumagalli L, Brighina L, Appollonio I, Racchi M, Ferrarese C. Fibroblast glutamate transport in aging and in AD: correlations with disease severity. Neurobiology of aging. 2005;26:825–832. doi: 10.1016/j.neurobiolaging.2004.07.007. [DOI] [PubMed] [Google Scholar]