Abstract
Background
Bisphenol A (BPA) is a chemical suspected of causing endocrine and metabolic disruption in animals and humans. In rodents, in utero exposure to low-dose BPA is associated with weight gain. Detectable levels of BPA are found in most Americans due to its widespread use in the manufacture of food and drink packaging. We hypothesized that urinary BPA concentrations would be positively associated with general and central obesity.
Methods
Cross-sectional analysis of urinary BPA concentrations, body mass index, and waist circumference in 2747 adults (aged 18–74), using pooled data from the 2003/04 and 2005/06 National Health and Nutrition Examination Surveys.
Results
The creatinine-adjusted geometric mean urinary BPA concentration was 2.05 µg/g creatinine (25th percentile: 1.18, 75% percentile: 3.33). Relative to those in the lowest BPA quartile, participants in the upper BPA quartiles were more likely to be classified as obese (quartile 2 odds ratio (OR): 1.85, 95% confidence interval (CI): 1.22, 2.79; quartile 3 OR: 1.60, 95% CI: 1.05–2.44; quartile 4 OR: 1.76, 95% CI: 1.06–2.94). Higher BPA concentration was also associated with abdominal obesity (quartile 2 OR: 1.62, 95% CI: 1.11, 2.36; quartile 3 OR: 1.39, 95% CI: 1.02–1.90; quartile 4 OR: 1.58, 95% CI: 1.03–2.42).
Conclusions
Higher BPA exposure is associated with general and central obesity in the general adult population of the United States. Reverse causation is of concern due to the cross-sectional nature of this study; longitudinal studies are needed to clarify the direction of the association.
Keywords: Bisphenol A (BPA), Endocrine disruptors, Human, NHANES, Obesity
1. Introduction
The prevention of overweight and obesity is a public health priority in the United States due to its large and increasing prevalence, as well as its strong association with many chronic diseases, including type II diabetes (Hu et al., 2001) and cardiovascular disease (CVD) (Wilson et al., 2002). Approximately two-thirds of Americans are considered overweight or obese and one-third is obese (Wang et al., 2008). If current trends continue, approximately 86% of Americans will be overweight and over half will be obese by 2030 (Wang et al., 2008). Abdominal obesity in particular is thought to be a driving force of insulin resistance and the metabolic syndrome, a constellation of cardiovascular risk factors, which is a strong marker for type II diabetes and CVD (Wilson et al., 2005), as well as for total and cardiovascular mortality (Malik et al., 2004).
Animal and epidemiologic studies support a link between low-dose exposure to endocrine-disrupting chemicals and obesity (Heindel, 2003; Newbold et al., 2007b). Bisphenol A (BPA) is of particular interest because of its widespread human exposure (Calafat et al., 2008). BPA is used in the manufacture of polycarbonate plastic and dental composites and sealants, and is also found in the inner coatings of food and beverage cans. Exposure occurs primarily through the consumption of food and beverages that have been contaminated with BPA during storage (Kang et al., 2006).
Although few studies have been performed in adult animals, studies in rodents suggest that prenatal exposure to low-dose (<50 mg/kg body weight/day) BPA leads to obesity and elevated lipid levels. Pups born to mice exposed to low-dose BPA in drinking water (in combination with a high-fat diet) are heavier in early adulthood compared to pups of untreated mice (Miyawaki et al., 2007); similarly, pups born to perinatally exposed rats are heavier as adults (Rubin et al., 2001). Following a high-fat diet, mice exposed to BPA in utero have also developed elevated total cholesterol and triglycerides (Miyawaki et al., 2007). Although the mechanism by which this occurs is unknown, several pathways have been proposed. Insulin resistance and tissue inflammation may be triggered by the inhibition of adiponectin release, as has been observed in human adipose tissue explants and adipocytes exposed to low-dose BPA (Hugo et al., 2008). Low-dose BPA also stimulates release of IL-6 and TNF-α (Ben-Jonathan et al., 2009), inflammatory cytokines that stimulate lipolysis.
Lang et al. (2008) and Melzer et al. (2010) previously reported positive associations between urinary BPA concentrations and coronary heart disease, myocardial infarction, angina, and diabetes in the National Health and Nutrition Examination Survey (NHANES). However, little attention has been paid to the role of BPA exposure in the development of CVD and type II diabetes precursors, such as obesity and insulin resistance. A closer examination of NHANES data is especially necessary since few other large-scale datasets have information on human BPA exposure. Given the existing evidence from animal and epidemiologic studies, we hypothesized that urinary BPA concentrations would be positively associated with general and central obesity.
2. Materials and methods
We used data from the 2003–2004 (CDC, 2004a) and 2005–2006 (CDC, 2006a) NHANES to examine the association between urinary BPA, body mass index (BMI), and waist circumference. NHANES, administered by the National Center for Health Statistics, Centers for Disease Control and Prevention, is a cross-sectional study that assesses the health and diet of the civilian, non-institutionalized, United States population using a multistage clustered design. Certain demographic groups, including older adults, Mexican-Americans, non-Hispanic blacks, and low-income persons, are over-sampled.
2.1. Study population
Measures of body composition are subjected to different interpretation in the elderly; therefore, we restricted our analyses to participants 18–74 years of age, who were included in the random subsample of participants, who supplied a spot urine sample analyzed for BPA. After excluding pregnant women (N = 207), and participants with missing data on urinary BPA (N = 47) or creatinine (N = 1), our final sample size was 2747.
2.2. Urinary BPA concentrations
A single spot urine sample was collected from each participant. Total (free and conjugated) urinary BPA concentrations were measured at the Division of Environmental Health Laboratory Sciences (National Center for Environmental Health, CDC) using online solid-phase extraction coupled to isotope dilution high-performance liquid chromatography–tandem mass spectrometry. Quality control (QC) procedures included analysis of reagent blanks and samples of pooled human urine spiked with BPA at low- and high-concentrations. Coefficients of variation calculated for low- and high-concentration QC samples were 19% and 12% in 2003–2004 and 13% and 11% in 2005–2006. Additional information on laboratory methods is available online (CDC, 2004b, 2006b). The lower limit of detection (LLOD) was 0.36 ng/ml in 2003/04 and 0.4 ng/ml in 2005/06. For BPA concentrations below the LLOD (2003/04: n = 110/1373 [8%]; 2005/06: n = 114/1374 [8%]) NHANES assigned a value of the LLOD divided by the square root of two. We used this value in our analyses. To calculate creatinine-adjusted urinary BPA concentrations for geometric means, we used the formula 100 × urinary BPA concentration (micrograms per liter) ÷ creatinine concentration (milligrams per deciliter).
2.3. Disease ascertainment
Trained health technicians measured participants’ weight, height, and waist circumference. Waist circumference was measured to the nearest 0.1 cm. A detailed description of laboratory methods, quality assurance, and quality control procedures is available on the NHANES website. Our primary outcomes were general and central obesity, measured using BMI and waist circumference, respectively. Elevated waist circumference was defined according to the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) criteria as ≥ 102 cm in men and ≥ 88 cm in women. BMI was calculated as weight in kilograms divided by height in meters squared and used to define overweight [25.0 < BMI ≤ 29.9] and obesity [BMI ≥ 30.0].
2.4. Potential confounders
Potential confounders were identified based on previous report of an association with urinary BPA (Calafat et al., 2008) and creatinine concentrations (Barr et al., 2005). These covariates included: sex, age (continuous), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, other/multi-racial), education (<high school, high school diploma, >high school), and smoking (never, former, current-everyday, current-some days). To account for variability in the volume of urine, urinary creatinine concentration (continuous mg/dL) was included as a covariate in all analyses (Barr et al., 2005). When urinary creatinine concentrations were omitted from multivariate models, effect estimates were slightly higher than those otherwise observed. The association between exposure quartiles and potential confounders was assessed using a Chi-square test. Model 1 was adjusted for age, sex, and urinary creatinine. Model 2 was adjusted for race, education, and smoking in addition to Model 1 covariates. We used the missing indicator method for covariates with missing data for ≥ 10% of observations, otherwise observations with missing covariate data were excluded.
2.5. Statistical analysis
The sampling design of NHANES necessitates the use of sample weights to make inference to the general United States adult population. Accordingly, we conducted weighted analyses using population weights for the subsample in which BPA was measured. Survey design variables were incorporated in all analyses according to NHANES guidelines.
Associations between BPA exposure and disease outcomes are often nonmonotonic (Vandenberg et al., 2009); therefore, we categorized urinary BPA into quartiles and modeled them using indicator variables to allow for non-linear relations. We used polytomous logistic regression to evaluate the association between urinary BPA and BMI category, logistic regression to investigate the association between urinary BPA and abdominal obesity, and linear regression to assess the association of urinary BPA with the continuous forms of BMI and waist circumference. Tests of trend were performed by modeling the median of each exposure category as a continuous term. We assessed whether the associations between urinary BPA and obesity were modified by sex by examining sex-stratified analyses or by creating cross-product terms between urinary BPA quartiles and sex. Wald tests were used to determine if interactions were statistically significant. Stratified analyses were also performed by NHANES wave, using urinary BPA cutpoints from the pooled data to aid comparison.
All analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC) and SUDAAN (release 8.0; Research Triangle Institute, Research Triangle Park, NC). Results were considered significant at the <0.05 level and all statistical tests were two-sided.
3. Results
In this population of 2747 adult participants in 2003–2006 NHANES, the geometric mean creatinine-adjusted urinary BPA concentration was 2.05 µg/g creatinine (25th percentile: 1.18, 75th percentile: 3.33). Consistent with 2003–2004 NHANES, higher urinary BPA concentrations were observed for men, younger individuals, smokers, and minorities, including African-Americans, Mexican-Americans, and other Hispanics (Table 1).
Table 1.
Sample characteristics across urinary bisphenol A quartiles in pooled 2003–2006 NHANES (N = 2747).
| Urinary BPA concentrations | ||||||
|---|---|---|---|---|---|---|
| Total N (%)a |
Quartile 1 (≤1.1 ng/mL) (%) |
Quartile 2 (1.2–2.3 ng/mL) (%) |
Quartile 3 (2.4–4.6 ng/mL) (%) |
Quartile 4 (≥4.7 ng/mL) (%) |
p-valueb | |
| Sex | 0.01 | |||||
| Men | 1416 (49.6)c | 22.5 | 26.4 | 26.4 | 24.7 | |
| Women | 1331 (50.4) | 30.7 | 25.8 | 22.1 | 21.4 | |
| Age (y) | <.001 | |||||
| 18–29 | 790 (22.4) | 16.9 | 22.4 | 28.7 | 31.9 | |
| 30–39 | 446 (19.8) | 22.8 | 27.0 | 26.8 | 23.5 | |
| 40–49 | 501 (22.9) | 28.2 | 27.0 | 24.7 | 20.1 | |
| 50–59 | 385 (18.5) | 32.5 | 25.7 | 21.7 | 20.1 | |
| 60–74 | 625 (16.4) | 35.8 | 29.3 | 17.1 | 17.8 | |
| Race/ethnicity | 0.01 | |||||
| Mexican American | 574 (8.1) | 21.3 | 29.3 | 25.0 | 24.4 | |
| Other Hispanic | 101 (3.9) | 19.6 | 30.6 | 23.5 | 26.5 | |
| Non-Hispanic White | 1277 (70.3) | 29.0 | 25.8 | 24.0 | 21.2 | |
| Non-Hispanic Black | 674 (11.8) | 15.3 | 22.6 | 27.5 | 34.6 | |
| Other race (including multi-racial) | 121 (6.0) | 32.8 | 29.4 | 19.6 | 18.1 | |
| Level of educationd | 0.01 | |||||
| Less than high school diploma | 746 (16.9) | 23.3 | 25.5 | 21.7 | 29.5 | |
| High school diploma | 696 (25.6) | 23.1 | 26.0 | 25.9 | 25.0 | |
| Some college education | 1304 (57.6) | 29.2 | 26.4 | 24.3 | 20.2 | |
| Cigarette smokingd | 0.02 | |||||
| Never smoker | 1235 (48.4) | 26.5 | 28.2 | 24.7 | 20.6 | |
| Former smoker | 559 (22.1) | 27.1 | 29.9 | 21.3 | 21.6 | |
| Current smoker-some days | 109 (3.8) | 28.7 | 19.2 | 27.2 | 24.8 | |
| Current smoker-every day | 529 (22.0) | 28.8 | 20.0 | 24.1 | 27.2 | |
Abbreviations: BMI, body mass index; BPA, bisphenol A.
All percentages are weighted using population weights for the sample in which BPA was measured.
Calculated using a Chi-square test.
Percentages may not sum to 100 due to rounding and missing data.
1 missing data on education and 315 missing data on smoking.
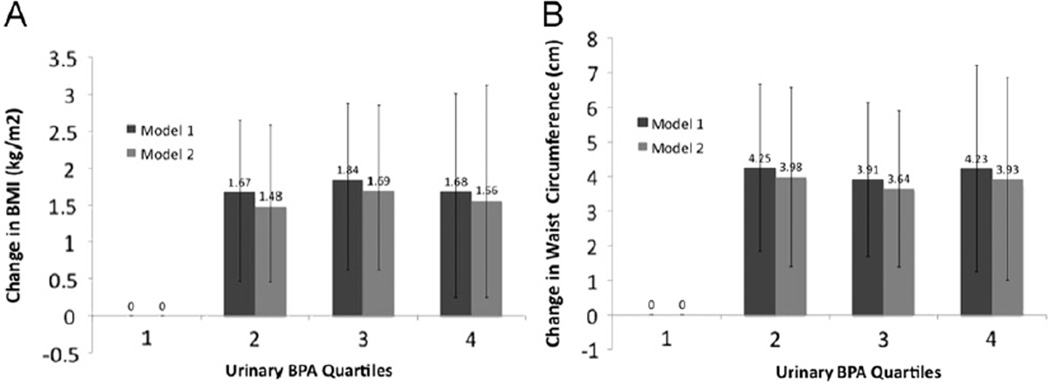
Urinary BPA concentration was associated with both general and central obesity. Relative to participants in the lowest BPA quartile, participants in the upper three BPA quartiles had similar prevalence odds of obesity, with odds ratios ranging from 1.60 to 1.85 (Table 2). In addition, participants in the upper three BPA quartiles had non-significantly increased prevalence odds of overweight relative to participants in the lowest BPA quartile (Table 2). To explore this association further, we examined the relation between urinary BPA quartiles modeled as indicator variables and continuous BMI using linear regression. Consistent with our other findings, we observed a non-linear association (p-trend = 0.18) with an apparent threshold effect; participants in the second, third, and fourth BPA quartiles had a 1.48 kg/m2 (95% CI: 0.46, 2.51), 1.69 kg/m2 (95% CI: 0.62, 2.76), and 1.56 kg/m2 (95% CI: 0.25, 2.87) greater BMI on average, respectively, compared to those in the lowest BPA quartile, after multivariable adjustment (Fig. 1).
Table 2.
Odds ratios (OR) and 95% confidence intervals (CI) of prevalent overweight and obesity across urinary bisphenol A quartiles in pooled 2003–2006 NHANES.
| Overweight (25 ≤ BMI < 30.0) vs. recommended weight (BMI < 25) | Obese (BMI ≥ 30.0) vs. recommended weight (BMI < 25) | |||||
|---|---|---|---|---|---|---|
| 25 ≤ BMI < 30.0/BMI < 25 | OR (95% CI) | BMI ≥ 30.0/BMI < 25 | OR (95% CI) | |||
| Model 1a | Model 2b | Model 1a | Model 2b | |||
| Quartile 1 (≤1.1 ng/mL) | 214/268c | 1.00 | 1.00 | 182/268 | 1.00 | 1.00 |
| Quartile 2 (1.2–2.3 ng/mL) | 242/211 | 1.72 (1.27–2.34) | 1.66 (1.21–2.27) | 240/211 | 1.96 (1.32–2.92) | 1.85 (1.22–2.79) |
| Quartile 3 (2.4–4.6 ng/mL) | 205/215 | 1.30 (0.88–1.92) | 1.26 (0.85–1.87) | 254/215 | 1.69 (1.13–2.52) | 1.60 (1.05–2.44) |
| Quartile 4 (≥4.7 ng/mL) | 203/225 | 1.34 (0.81–2.22) | 1.31 (0.80–2.14) | 256/225 | 1.85 (1.10–3.09) | 1.76 (1.06–2.94) |
Abbreviations: BMI, body mass index (kg/m2); OR, odds ratio; CI, confidence interval.
Model 1 is a polytomous regression model adjusted for age (continuous years), sex, and urinary creatinine (continuous mg/dL).
Model 2 is a polytomous regression model adjusted for race (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, other/ multi-racial), education (<high school, high school, >high school), and smoking (former, current-everyday, current-some days, never) in addition to Model 1 covariates.
32 observations were excluded from both models due to missing data on BMI. 1 additional observation was excluded from Model 2 due to missing education data.
Fig. 1.
Mean change in body mass index (BMI) (A) and waist circumference (cm) (B) across urinary bisphenol A quartiles in pooled 2003–2006 NHANES. Model 1 was adjusted for age, sex, and urinary creatinine. Model 2 was adjusted for Model 1 covariates plus race, education, and smoking. Error bars represent 95% confidence intervals.
The association between urinary BPA and waist circumference was investigated in a similar manner. After adjustment for age, sex, urinary creatinine, race, education, and smoking, participants in the upper three BPA quartiles had a 3.64–3.98 cm greater waist circumference (Fig. 1) and 39–62% higher odds of being classified as abdominally obese (Table 3) relative to participants in the lowest BPA quartile. None of the examined associations were modified by sex; however, when stratified by sex, associations became slightly stronger in men and slightly weakened and lost statistical significance in women (Supplemental Tables 1 and 2). Likewise, relative to 2005–2006 data, associations were attenuated in 2003–2004 data, losing statistical significance (Supplemental Tables 3 and 4).
Table 3.
Odds ratios (OR) and 95% confidence intervals (CI) of elevated waist circumference (≥ 102 cm in men or ≥ 88 cm in women) across urinary bisphenol A quartiles in pooled 2003–2006 NHANES.
| Elevated WC/normal WC | OR (95% CI) | ||
|---|---|---|---|
| Model 1a | Model 2b | ||
| Quartile 1 (≤1.1 ng/mL) | 303/355c | 1.00 | 1.00 |
| Quartile 2 (1.2–2.3 ng/mL) | 356/324 | 1.67 (1.18–2.34) | 1.62 (1.11–2.36) |
| Quartile 3 (2.4–4.6 ng/mL) | 329/328 | 1.43 (1.07–1.92) | 1.39 (1.02–1.90) |
| Quartile 4 (≥4.7 ng/mL) | 342/323 | 1.64 (1.08–2.48) | 1.58 (1.03–2.42) |
Abbreviations: OR, odds ratio; CI, confidence interval; WC, waist circumference.
Model 1 is a logistic regression model adjusted for age (continuous years), sex, and urinary creatinine (continuous mg/dL).
Model 2 is a logistic regression model adjusted for race (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, other/ multi-racial), education (<high school, high school, >high school), and smoking (former, current-everyday, current-some days, never) in addition to Model 1 covariates.
87 observations were excluded from both models due to missing data on BMI. 1 additional observation was excluded from Model 2 due to missing education data.
4. Discussion
In this cross-sectional analysis of pooled 2003–2006 NHANES, urinary BPA was associated with general and central obesity. This study takes advantage of one of the few large-scale datasets with high-quality data on human exposure to BPA.
4.1. Previous studies
Experimental animal studies report that rodent pups exposed to low-dose BPA in utero or during the perinatal period are heavier at weaning and as adolescents and adults than unexposed pups (Howdeshell et al., 1999; Miyawaki et al., 2007; Patisaul and Bateman, 2008; Rubin et al., 2001; Somm et al., 2009). Other studies have reported no difference in the weight of perinatally exposed and unexposed adult animals (Newbold et al., 2007a; Ryan et al., 2010). Only one study has investigated BPA’s effect on body weight in animals exposed during adulthood; however, their finding of no association between low-dose BPA exposure, body weight and fat depots in ovariectomized female adult rats may lack generalizability to male and intact female rats (Seidlova-Wuttke et al., 2005). The comparability of low doses of BPA administered in animal studies to urine concentrations detected in observational human data is unclear. Vandenberg et al. (2007) compared blood levels of unconjugated BPA from 11 studies investigating the pharmacokinetics of orally administered BPA in adult rodents to those reported in human studies. After scaling the administered doses in the animal studies to a single dose of 50 µg/kg, they reported that none of the blood levels reported in the animal studies reached the median level of unconjugated BPA found in human studies, suggesting that humans may be exposed to BPA at levels above the reference dose that defines the threshold of low-dose exposure (50 µg/kg/day). Although BPA dose was not directly related to urinary BPA levels in humans, human blood BPA levels were abstracted from studies of non-occupationally exposed adults, and are unlikely to differ substantially from this study population.
Three previous studies have related BPA biomarkers to measures of general adiposity in human populations. In contrast to our observations, no association was reported between urinary BPA and overweight or obesity in a cross-sectional analysis of Italian adults (Galloway et al., 2010) or an analysis of 2003–2004 NHANES (Lang et al., 2008). The apparent discrepancy between 2003–2004 and 2003–2006 data may reflect the improved statistical power in the pooled data as well as the incorporation of the stronger observed associations in the 2005–2006 data. In contrast, positive associations were reported between serum BPA, obesity, and BMI in a small cross-sectional study in Japanese women (Takeuchi et al., 2004). However, this study did not account for potential confounding by age, smoking, or socioeconomic status, and was restricted to women. Our study confirms the findings of Galloway et al. (2010), who reported an association between daily excreted BPA and waist circumference in Italian adults.
4.2. Biological mechanism
Adult male mice treated with oral or injected low-dose BPA develop hyperinsulinemia and insulin resistance (Alonso-Magdalena et al., 2006). BPA is hypothesized to act on these metabolic processes by binding estrogen receptors located on adipocytes and pancreatic β- and islet cells (Alonso-Magdalena et al., 2006; Ben-Jonathan et al., 2009), and has also been reported to suppress the release of the hormone adiponectin in human adipose tissue explants and adipocytes (Hugo et al., 2008). Other proposed mechanisms include stimulation of inflammatory cytokines (Ben-Jonathan et al., 2009), which stimulate lipolysis and suppress insulin sensitivity, and disrupted peroxisome proliferator-activated receptor gamma signaling (Rubin and Soto, 2009), with potential consequences for adipocyte differentiation and lipid homeostasis (Masuno et al., 2005, 2002; Sakurai et al., 2004).
4.3. Strengths and limitations
The cross-sectional nature of this study precludes us from ruling out reverse causation (e.g. obesity leads to higher urinary BPA concentrations); however, the biological plausibility of this is unclear since the metabolism of BPA in humans is poorly understood. BPA is known to be lipophilic (KOW = 2.2–3.4), but it is unknown whether BPA is stored and slowly released from adipose tissue. Two pharmacokinetic studies reported rapid and complete elimination of orally administered BPA (Volkel et al., 2005, 2002), suggesting that BPA is excreted before it could be delivered to adipose stores. Recently, however, these studies have been criticized for their relatively high limit of detection and inconsistency with epidemiologic studies, in which unconjugated BPA has been detected in the majority of participants in which it has been investigated (reviewed by Vandenberg et al., 2010 and Ginsberg and Rice, 2009). The presence of unconjugated BPA in the blood or urine suggests that first pass metabolism is incomplete, conjugated BPA is later deconjugated, or meaningful non-ingested BPA exposure (e.g., dental composites and sealants, carbonless paper) is present. Under any of these scenarios, BPA enters the systemic circulation before elimination, during which time it could be stored in adipose tissue. This mechanism for BPA metabolism is also supported by recent evidence suggesting a population based half-life for BPA of up to 418 h, which was calculated by relating fasting time and urinary BPA in 2003–2004 NHANES participants assigned to abstain from food or drinks besides water or tea for 6–9.5 h (Stahlhut et al., 2009). Under the premise that the vast majority of BPA exposure occurs through the ingestion of contaminated food and drink and metabolism is rapid and complete, the authors expected that urinary BPA concentrations would decrease with fasting time; however, they observed little decline in urinary BPA between 8.5 and 24 h of fasting. Although these findings suggest slower than expected BPA metabolism or release of BPA from internal stores, they could also be explained by meaningful non-ingested BPA exposure or exposure to BPA from drinking water.
The detection of BPA in human adipose tissue further supports the hypothesis that BPA is delivered to peripheral tissues, although this finding should be confirmed in additional studies (Fernandez et al., 2007). Interestingly, Fernandez et al. reported a non-significant negative association between BMI and adipose BPA concentration, which suggests that even if BPA is stored in adipose tissue, higher BMI may not predict elevated BPA biomarker concentrations. In summary, while early pharmacokinetic studies support the immediate and complete metabolism of BPA, recent epidemiological evidence suggests that unconjugated BPA is circulated in the body and possibly delivered to peripheral tissues, including adipose stores. Hence, a better understanding of BPA metabolism and exposure routes is necessary to fully assess the potential for reverse causation.
A second possible scenario leading to reverse causation is one in which obese individuals have higher urinary BPA levels relative to those of normal weight due to greater overall energy intake, and thus higher consumption of canned food and drinks. However, the difference in total calories reported was small; normal weight individuals reported consuming approximately 50 fewer calories per day than overweight individuals and 90 fewer calories per day than those classified as obese. Moreover, the within-group variability was moderate, with standard errors ranging from 33.8 calories per day (obese) to 44.4 calories per day (overweight). Although data on the impact of canned food and drink consumption on urinary BPA levels is currently lacking, it seems implausible for the observed differences in energy intake between BMI categories to be responsible for the observed association between urinary BPA and BMI category, given that only a fraction of these calories is likely obtained from canned foods and drinks. It is more likely that differences in diet composition between BMI categories, specifically the proportion of the diet derived from canned food and drink, account for differing urinary BPA levels. However, the relationship between canned food consumption and BMI category is difficult to disentagle, as some canned foods are considered healthy (e.g., canned beans and vegetables) and might therefore be more commonly consumed by normal weight individuals, while others (e.g., canned soda) have been associated with increased risk of obesity (Malik et al., 2006). Additional studies are needed to identify populations with relatively high intake of canned goods.
We measured urinary BPA using a single spot urine sample. As previously discussed, BPA metabolism and kinetics is an area of dispute. Under the premise of rapid and complete excretion, considerable temporal variability in urinary BPA concentrations should exist, making a single BPA sample a poor representation of long-term exposure. Concern is somewhat mitigated by the continuous nature of dietary BPA exposure (Kang et al., 2006). If BPA metabolism is slower, a single urine sample may be an acceptable marker of chronic exposure. Indeed, Mahalingaiah et al. (2008) found that a single urinary BPA sample had moderate sensitivity in classifying a participant in the highest BPA tertile several weeks to months later. Although the degree of misclassification is unknown, we expect temporal variability in urinary BPA to result in an underestimate of the true association.
We used BMI to measure general adiposity and waist circumference to measure central adiposity. Although BMI cannot be used to distinguish between fat mass and lean body mass, between-person variation in BMI typically represents variation in fat mass in non-elderly populations after adjustment for age and sex (Willett, 1998). In addition, waist circumference has been validated against other measures of abdominal fat (Clasey et al., 1999). Both these measures are commonly used in epidemiologic research and predict multiple health outcomes (Hu, 2008).
Our results are consistent with previous reports of a positive association of urinary BPA levels with CVD and diabetes (Lang et al., 2008; Melzer et al. 2010). It is likely that BPA acts early in the development of CVD and diabetes, possibly by increasing risk of general and central obesity, strong risk factors for CVD and diabetes.
4.4. Conclusions
Urinary BPA was associated with general and central obesity in this sample of the general adult population of the United States. The potential for reverse causation is of concern due to the cross-sectional nature of the study. Prospective studies are needed to establish the timing of BPA exposure and weight gain, although animal studies have found that BPA exposure precedes weight gain. These findings have important public health implications due to the high and increasing prevalence of obesity, the ubiquity of BPA exposure, and the amenability of this exposure to intervention.
Supplementary Material
Acknowledgments
Ms. Carwile was supported by the Training Program in Environmental Epidemiology under grant T32 ES 007069. We acknowledge the National Center for Health Statistics of the U.S. Centers for Disease Control and Prevention (CDC) for its invaluable work conducting the National Health and Nutrition Examination Survey, and the researchers at the Division of Laboratory Sciences, National Center for Environmental Health of the CDC for conducting the BPA measurements.
Footnotes
Human subjects review was not required for this analysis of secondary data.
Appendix. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.envres.2011.05.014.
References
- Alonso-Magdalena P, et al. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ. Health Perspect. 2006;114:106–112. doi: 10.1289/ehp.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, et al. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ. Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Jonathan N, et al. Effects of bisphenol A on adipokine release from human adipose tissue: implications for the metabolic syndrome. Mol. Cell. Endocrinol. 2009;304:49–54. doi: 10.1016/j.mce.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, et al. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Hyattsville, MD: 2004a. National Health and Nutrition Examination Survey Data 2003–04. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) NHANES 2003–2004. [accessed 30 March 2010];Laboratory Procedure Manual. 2004b Available: 〈 http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/l24eph_c_met_phenols.pdf〉.
- Centers for Disease Control and Prevention (CDC) Hyattsville, MD: 2006a. National Health and Nutrition Examination Survey Data 2005–06. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) NHANES 2005–2006. [accessed 30 March 2010];Laboratory Procedure Manual. 2006b Available: 〈 http://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/eph_d_met_phenols_parabens.pdf〉.
- Clasey JL, et al. The use of anthropometric and dual-energy X-ray absorptiometry (DXA) measures to estimate total abdominal and abdominal visceral fat in men and women. Obes. Res. 1999;7:256–264. doi: 10.1002/j.1550-8528.1999.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Fernandez MF, et al. Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod. Toxicol. 2007;24:259–264. doi: 10.1016/j.reprotox.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Galloway T, et al. Daily bisphenol A excretion and associations with sex hormone concentrations: results from the InCHIANTI adult population study. Environ. Health Perspect. 2010;118:1603–1608. doi: 10.1289/ehp.1002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg G, Rice DC. Does rapid metabolism ensure negligible risk from bisphenol A? Environ. Health Perspect. 2009;117:1639–1643. doi: 10.1289/ehp.0901010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ. Endocrine disruptors and the obesity epidemic. Toxicol. Sci. 2003;76:247–249. doi: 10.1093/toxsci/kfg255. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, et al. Exposure to bisphenol A advances puberty. Nature. 1999;401:763–764. doi: 10.1038/44517. [DOI] [PubMed] [Google Scholar]
- Hu FB. Obesity Epidemiology. New York: Oxford University Press; 2008. [Google Scholar]
- Hu FB, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N. Engl. J. Med. 2001;345:790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- Hugo ER, et al. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ. Health Perspect. 2008;116:1642–1647. doi: 10.1289/ehp.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, et al. Human exposure to bisphenol A. Toxicology. 2006;226:79–89. doi: 10.1016/j.tox.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Lang IA, et al. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. Jama. 2008;300:1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- Mahalingaiah S, et al. Temporal variability and predictors of urinary bisphenol A concentrations in men and women. Environ. Health Perspect. 2008;116:173–178. doi: 10.1289/ehp.10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- Malik VS, et al. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am. J. Clin. Nutr. 2006;84:274–288. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuno H, et al. Bisphenol a accelerates terminal differentiation of 3T3-L1 cells into adipocytes through the phosphatidylinositol 3-kinase pathway. Toxicol. Sci. 2005;84:319–327. doi: 10.1093/toxsci/kfi088. [DOI] [PubMed] [Google Scholar]
- Masuno H, et al. Bisphenol A in combination with insulin can accelerate the conversion of 3T3-L1 fibroblasts to adipocytes. J. Lipid Res. 2002;43:676–684. [PubMed] [Google Scholar]
- Melzer D, et al. Association of urinary bisphenol a concentration with heart disease: evidence from NHANES 2003/06. PLoS One. 2010;5:e8673. doi: 10.1371/journal.pone.0008673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki J, et al. Perinatal and postnatal exposure to bisphenol a increases adipose tissue mass and serum cholesterol level in mice. J. Atheroscler. Thromb. 2007;14:245–252. doi: 10.5551/jat.e486. [DOI] [PubMed] [Google Scholar]
- Newbold RR, et al. Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod. Toxicol. 2007a;24:253–258. doi: 10.1016/j.reprotox.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold RR, et al. Developmental exposure to endocrine disruptors and the obesity epidemic. Reprod. Toxicol. 2007b;23:290–296. doi: 10.1016/j.reprotox.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Bateman HL. Neonatal exposure to endocrine active compounds or an ERbeta agonist increases adult anxiety and aggression in gonadally intact male rats. Horm. Behav. 2008;53:580–588. doi: 10.1016/j.yhbeh.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Rubin BS, et al. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ. Health Perspect. 2001;109:675–680. doi: 10.1289/ehp.01109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS, Soto AM. Bisphenol A: perinatal exposure and body weight. Mol. Cell Endocrinol. 2009;304:55–62. doi: 10.1016/j.mce.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KK, et al. Perinatal exposure to bisphenol-a and the development of metabolic syndrome in CD-1 mice. Endocrinology. 2010;151:2603–2612. doi: 10.1210/en.2009-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai K, et al. Bisphenol A affects glucose transport in mouse 3T3-F442A adipocytes. Br. J. Pharmacol. 2004;141:209–214. doi: 10.1038/sj.bjp.0705520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidlova-Wuttke D, et al. Effects of bisphenol-A (BPA), dibutylphtalate (DBP), benzophenone-2 (BP2), procymidone (Proc), and linurone (Lin) on fat tissue, a variety of hormones and metabolic parameters: a 3 months comparison with effects of estradiol (E2) in ovariectomized (ovx) rats. Toxicology. 2005;213:13–24. doi: 10.1016/j.tox.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Somm E, et al. Perinatal exposure to bisphenol a alters early adipogenesis in the rat. Environ. Health Perspect. 2009;117:1549–1555. doi: 10.1289/ehp.11342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlhut RW, et al. Bisphenol A data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both. Environ. Health Perspect. 2009;117:784–789. doi: 10.1289/ehp.0800376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, et al. Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocr. J. 2004;51:165–169. doi: 10.1507/endocrj.51.165. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, et al. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 2010;118:1055–1070. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, et al. Human exposure to bisphenol A (BPA) Reprod. Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, et al. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr. Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkel W, et al. Quantitation of bisphenol A and bisphenol A glucuronide in biological samples by high performance liquid chromatography-tandem mass spectrometry. Drug Metab. Dispos. 2005;33:1748–1757. doi: 10.1124/dmd.105.005454. [DOI] [PubMed] [Google Scholar]
- Volkel W, et al. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem. Res. Toxicol. 2002;15:1281–1287. doi: 10.1021/tx025548t. [DOI] [PubMed] [Google Scholar]
- Wang Y, et al. Will all Americans become overweight or obese? estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 2008;16:2323–2330. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- Willett W. Nutritional Epidemiology. New York (NY): Oxford University Press; 1998. [Google Scholar]
- Wilson PW, et al. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- Wilson PW, et al. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch. Intern. Med. 2002;162:1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.