Abstract
Background
Vitamin D deficiency or insufficiency is thought to be common among pregnant women. Vitamin D supplementation during pregnancy has been suggested as an intervention to protect against adverse gestational outcomes.
Objectives
To examine whether supplements with vitamin D alone or in combination with calcium or other vitamins and minerals given to women during pregnancy can safely improve maternal and neonatal outcomes.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 October 2011), the International Clinical Trials Registry Platform (ICTRP) (31 October 2011), the Networked Digital Library of Theses and Dissertations (28 October 2011) and also contacted relevant organisations (8 April 2011).
Selection criteria
Randomised and quasi-randomised trials with randomisation at either individual or cluster level, evaluating the effect of supplementation with vitamin D alone or in combination with other micronutrients for women during pregnancy.
Data collection and analysis
Two review authors independently i) assessed the eligibility of studies against the inclusion criteria ii) extracted data from included studies, and iii) assessed the risk of bias of the included studies. Data were checked for accuracy.
Main results
The search strategy identified 34 potentially eligible references. We included six trials assessing a total of 1023 women, excluded eight studies, and 10 studies are still ongoing. Five trials involving 623 women compared the effects of vitamin D alone versus no supplementation/placebo and one trial with 400 women compared the effects of vitamin D and calcium versus no supplementation.
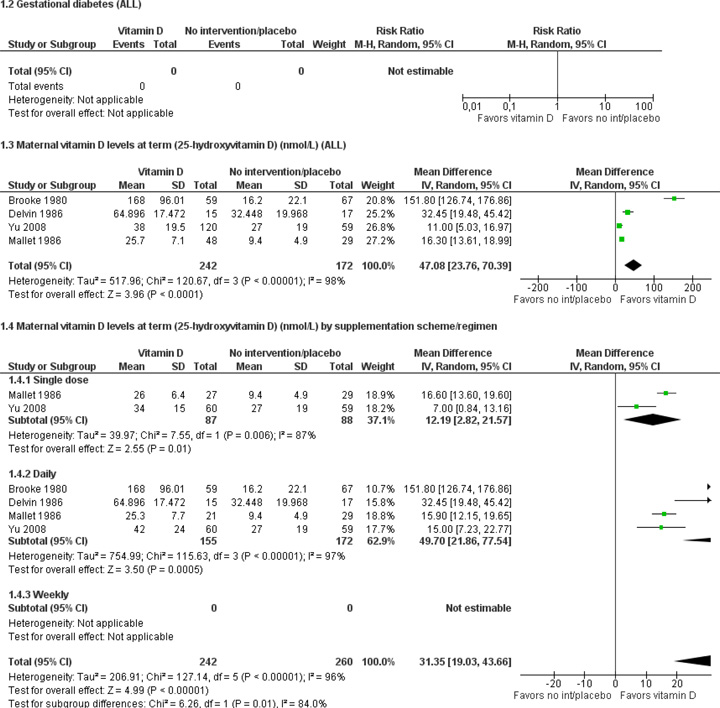
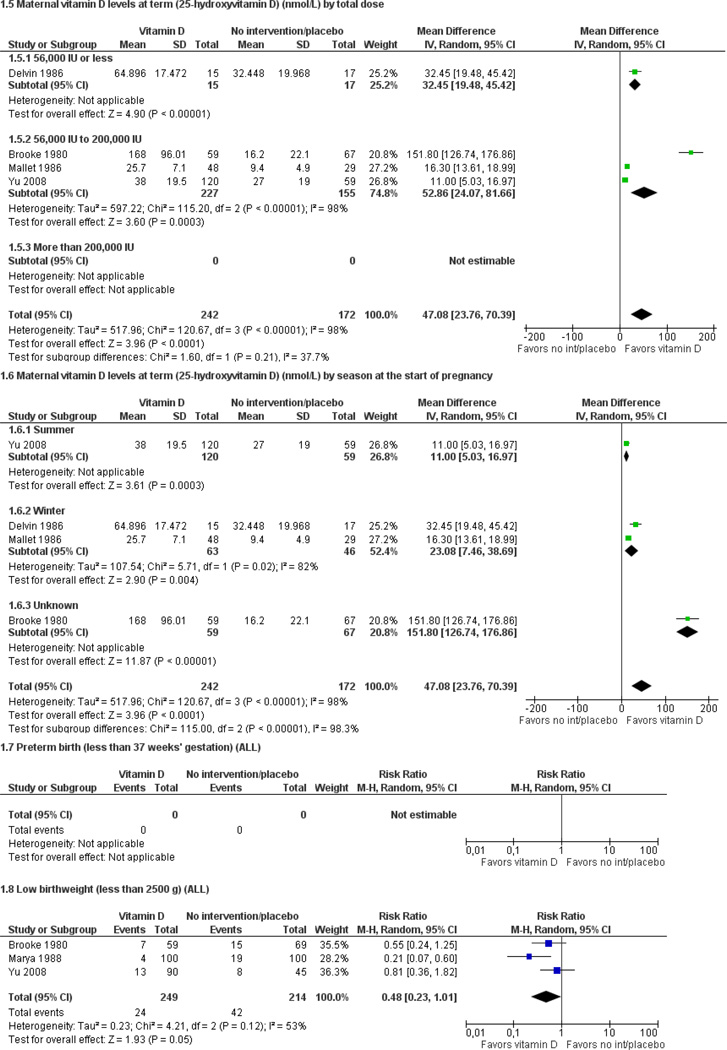
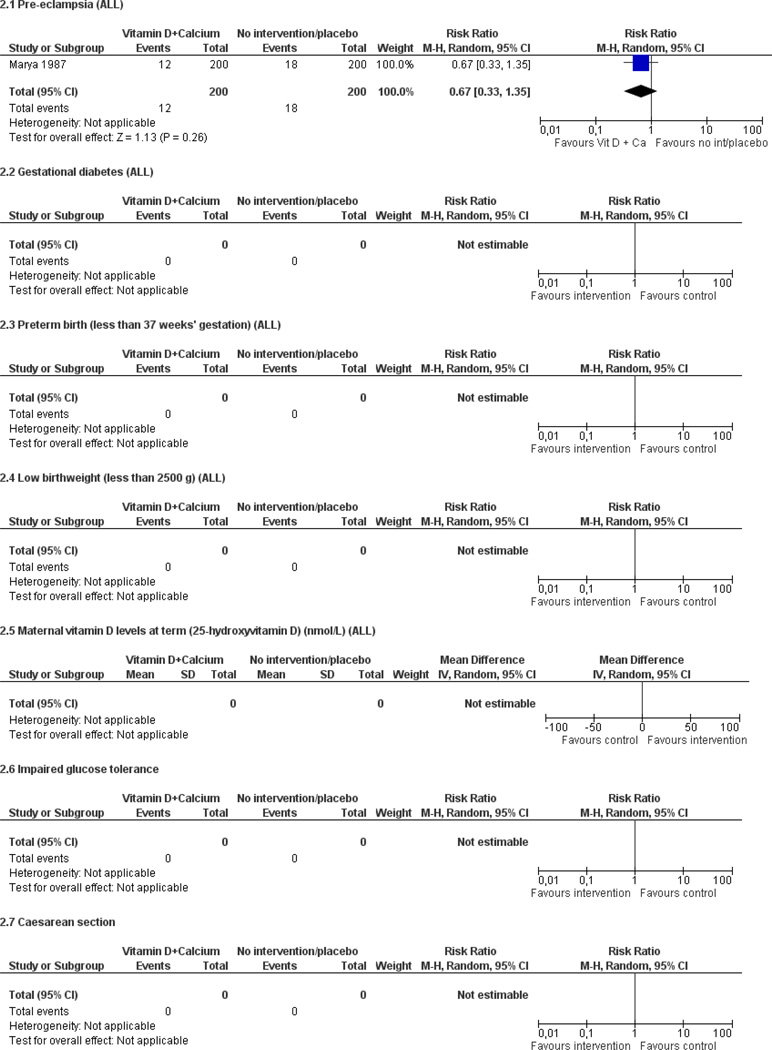
Only one trial with 400 women reported on pre-eclampsia: women who received 1200 IU vitamin D along with 375 mg of elemental calcium per day were as likely to develop pre-eclampsia as women who received no supplementation (average risk ratio (RR) 0.67; 95% confidence interval (CI) 0.33 to 1.35). Data from four trials involving 414 women consistently show that women who received vitamin D supplements had higher concentrations of vitamin D in serum at term than those women who received no intervention or a placebo; however the magnitude of the response was highly heterogenous.
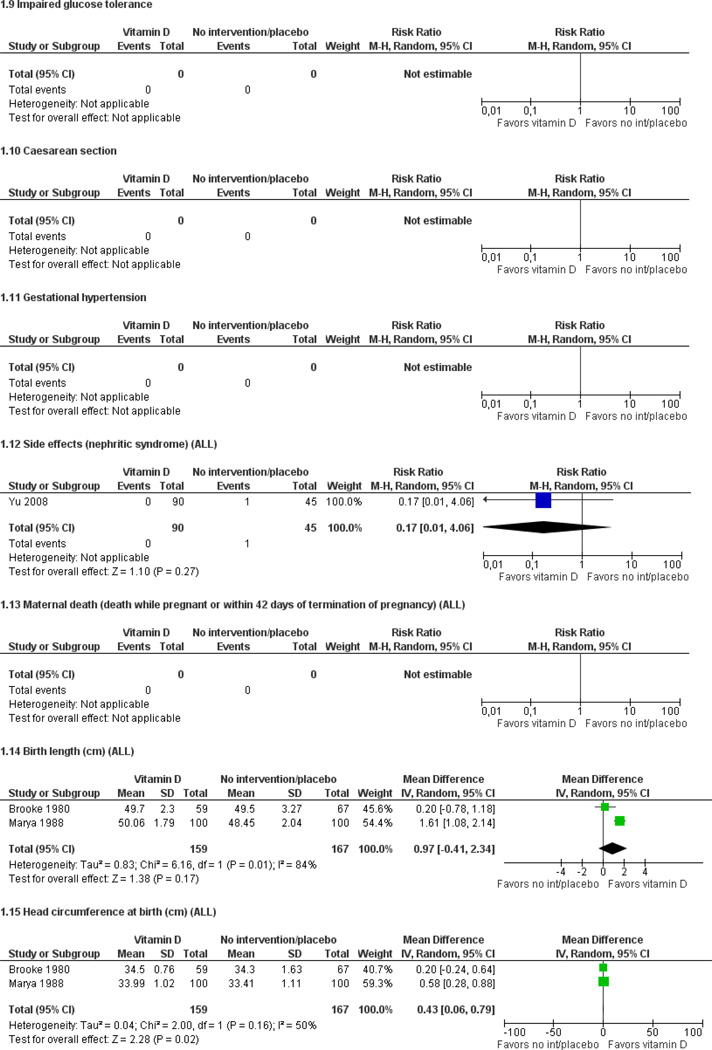
Data from three trials involving 463 women suggest that women who receive vitamin D supplements during pregnancy less frequently had a baby with a birthweight below 2500 grams than those women receiving no treatment or placebo; statistical significance was borderline (RR 0.48; 95% CI 0.23 to 1.01).
In terms of other conditions, there were no significant differences in adverse side effects including nephritic syndrome (RR 0.17; 95% CI 0.01 to 4.06; one trial, 135 women); stillbirths (RR 0.17; 95% CI 0.01 to 4.06; one trial, 135 women) or neonatal deaths (RR 0.17; 95% CI 0.01 to 4.06; one trial, 135 women) between women who received vitamin D supplements in comparison with women who received no treatment or placebo. No studies reported on preterm birth, maternal death, admission to neonatal intensive care unit/special nursery or Apgar scores.
Authors' conclusions
Vitamin D supplementation in a single or continued dose during pregnancy increases serum vitamin D concentrations as measured by 25-hydroxyvitamin D at term. The clinical significance of this finding and the potential use of this intervention as a part of routine antenatal care are yet to be determined as the number of high quality trials and outcomes reported is too limited to draw conclusions on its usefulness and safety. Further rigorous randomised trials are required to evaluate the role of vitamin D supplementation in pregnancy.
Plain language summary
Vitamin D supplementation for women during pregnancy
Vitamin D is produced by the human body from exposure to sunlight and can also be consumed from foods such as fish-liver oils, fatty fish, mushrooms, egg yolks, and liver. Vitamin D has many functions in the body; it helps maintain bone integrity and calcium homeostasis.
During pregnancy, vitamin D deficiency or insufficiency may develop. Vitamin D supplementation during pregnancy has been suggested to safely improve pregnancy and infant outcomes. This review included six randomised controlled trials. Five trials involving 623 women compared the effects of vitamin D alone versus no supplementation or a placebo and one trial with 400 women compared the effects of vitamin D and calcium with no supplementation.
The results show that the provision of vitamin D supplements during pregnancy improves the women’s vitamin D levels, as measured by 25-hydroxyvitamin D levels, at term. However, the clinical significance of this finding is yet to be determined as there is no evidence that vitamin D supplementation prevents pre-eclampsia, gestational diabetes, impaired glucose tolerance, caesarean section, gestational hypertension, or death in the mothers; or preterm birth, stillbirth, neonatal death, neonatal admission to intensive care unit, newborns with low Apgar score or neonatal infection.
Data from three trials involving 463 women show a trend for women who receive vitamin D supplementation during pregnancy to more frequently have a baby with a birthweight below 2500 grams than those women receiving no treatment or placebo, although the statistical significance was borderline.
The number of trials and outcomes reported are too limited, and in general are of low quality, to draw conclusions on the usefulness and safety of this intervention as a part of routine antenatal care. Further rigorous randomised trials are required to evaluate the role of vitamin D supplementation in pregnancy.
Background
Description of the condition
Vitamin D metabolism
Vitamin D is a fat-soluble vitamin which comes primarily from exposure to sunlight, and is found naturally only in a few foods, such as fish-liver oils, fatty fish, mushrooms, egg yolks, and liver (Holick 2007a; Holick 2008). There are two physiologically active forms of vitamin D collectively called calciferol: D2 and D3. Vitamin D2 (also called ergocalciferol) is synthesised by plants while vitamin D3 (also called cholecalciferol) is subcutaneously produced in humans from 7-dehydrocholecalciferol upon exposure to ultraviolet light B (UVB) radiation (DeLuca 2004). Vitamin D in supplements is found as either vitamin D2 or D3. The latter may be three times more effective than vitamin D2 in raising serum concentrations of vitamin D and maintaining those levels for a longer time; also, its metabolites have superior affinity for vitamin D-binding proteins in plasma (Armas 2004; McCullough 2007). As vitamin D has a short half-life, adequate vitamin D intake is necessary in order to ensure sustained circulating levels.
Both D2 and D3 forms share a similar metabolism. They are first hydroxylated in the liver to form 25 hydroxy vitamin D (25(OH)D or calcidiol), and then in the kidney to 1,25 di hydroxyl vitamin D (1,25 (OH)2 D or calcitriol) in response to parathyroid hormone (PTH) levels. Calcitriol is considered an important pre-hormone with active metabolites that are involved in metabolic processes including bone integrity and calcium homeostasis (Wagner 2008).
The major sites of vitamin D action include the skin, intestine, bone, parathyroid gland, immune system, and pancreas as well as the small intestine and colon in the human fetus (Theodoropoulos 2003). Additionally, vitamin D helps maintain normal levels of glucose in the blood, by binding to its receptors in the pancreatic beta cells, regulating the release of insulin in response to the level of circulating glucose (Clifton-Bligh 2008; Maghbooli 2008; Palomer 2008).
There is a unique relationship between vitamin D and calcium. The parathyroid hormone is responsible for raising the calcium concentration in the blood through bone resorption, while calcitriol inhibits PTH and allows an increase of serum calcium concentration from sources other than the bone. In the presence of calcitriol, renal and intestinal calcium and phosphorus absorption is augmented leading to an improved calcium status.
Vitamin D status
Serum calcidiol or 25-hydroxyvitamin D can be used to assess vitamin D status, as it reflects the sum of the vitamin D produced cutaneously and that obtained from foods and supplements (Jones 2008). This metabolite is difficult to measure, with large variations between methods and among laboratories even when the same methods are used (Hollis 2004).
Recently, the Institute of Medicine defined adequate vitamin D status as having serum 25-hydroxyvitamin D concentrations greater than 50 nmol/L (or 20 ng/mL) in both the general population and pregnant women (Institute of Medicine 2010). Some investigators propose that concentrations around 80 nmol/L (32 ng/ml) are optimal, since they suppress PTH levels and lead to the greatest calcium absorption and the highest bone mass, reducing the rates of bone loss, falls, and fractures (Dawson-Hughes 2005; Dawson-Hughes 2008). It is uncertain whether these higher levels proposed for non pregnant adults are also adequate for pregnant women.
Vitamin D status is affected by factors that regulate its production in the skin (i.e. skin pigmentation, latitude, dressing codes, season, aging, sunscreen use, and air pollution) and by factors affecting its absorption or metabolism (Holick 2007b; Maghbooli 2007). Melanin acts as a filter for ultraviolet (UV) rays hence reducing the production of vitamin D by the skin. Hispanic and black populations in the United States may have a higher melanin content, and thus have reduced vitamin D photosynthesis (endogenous synthesis from exposure to sunlight) (Clemens 1982), explaining the variations in vitamin D concentration among ethnic groups living in the same geographical areas (Brooke 1980; Egan 2008; Matsuoka 1991; Nesby-O'Dell 2002; Rockell 2005). An individual's skin phototype reflects the extent of sun-burning versus subsequent tanning after an initial moderate sun exposure after a long period of little or no exposure (Gilchrest 2008). Phototypes I and II have rapid vitamin D photosynthesis after a minimal erythematic dose (MED). In contrast, prototype VI has little vitamin photosynthesis following the same MED dose (Clemens 1982). Differences in latitude have also been shown to influence the concentration of vitamin D, and individuals from countries in high and low latitudes have lower vitamin D levels. The importance of UV rays is further shown by the seasonal variation in the concentration of vitamin D between summer and winter, with higher levels during the summer compared with the winter months (Holick 2007b; Levis 2005). Vitamin D metabolism is also affected in obese individuals, as vitamin D is deposited in body fat stores, making it less bioavailable (Arunabh 2003). It has been shown that low levels of 25-hydroxyvitamin D are more prevalent among overweight and obese individuals compared with normal weight individuals (Vilarrasa 2007; Wortsman 2000). In the same context, sedentary activity is also associated with low vitamin D levels as it may be linked with diminished sunlight exposure (Ohta 2009).
Magnitude of vitamin D deficiency
Vitamin D deficiency (VDD) may be a common health problem worldwide both in children and adults (Bandeira 2006; Holick 2007a). Low concentrations of vitamin D have been found in all age groups in various countries including some in the Middle East (Fuleihan 2001; Sedrani 1984), the United States (Gordon 2004; Lips 2001; Sullivan 2005; Tangpricha 2002), India (Farrant 2009; Marwaha 2005), Japan (Sato 2005) and Australia (McGrath 2001b). It has been estimated that about 40% to 100% of elderly men and women living in the United States and Europe are deficient in vitamin D (Holick 2007a).
In pregnancy, vitamin D deficiency and vitamin D insufficiency are also thought to be common. A study in black and white pregnant women residing in the northern United States found that approximately 29% of black pregnant women and 5% of white pregnant women had VDD (defined as serum 25-hydroxyvitamin D less than 37.5 nmol/L); whereas 54% of black participants and 47% of white participants had vitamin D insufficiency (defined as serum 25-hydroxyvitamin D levels 37.5 to 80 nmol/L) (Bodnar 2007). Similar results have been found in pregnant African-American adolescents (Davis 2010), pregnant Asian women (Alfaham 1995), Iranian pregnant women (Kazemi 2009), veiled or dark-skinned pregnant women (Grover 2001), Indian pregnant women (Sachan 2005), non-Western pregnant women in the Netherlands (Van der Meer 2006), and among pregnant women from Pakistan, Turkey and Somalia (Madar 2009). Recent studies in white pregnant women also show a high prevalence of VDD in the United Kingdom (Holmes 2009) and Ireland (O'Riordan 2008).
Seasonal variation increases the risk of VDD in pregnancy, with a greater prevalence of VDD during the winter months compared with the summer months (Nicolaidou 2006; O'Riordan 2008). Differences in latitude have also been shown to influence the concentration of vitamin D in a majority of pregnant women (Sloka 2009).
Vitamin D status and health outcomes
Vitamin D status and hypertensive disorders during pregnancy
Maternal vitamin D deficiency in pregnancy has been associated with an increased risk of pre-eclampsia (new-onset gestational hypertension and proteinuria after 20 weeks of gestation), a condition associated with an increase in maternal and perinatal morbidity and mortality (Bodnar 2007; Holick 2008; Li 2000; MacKay 2001; Xiong 1999). Women with pre-eclampsia have lower concentrations of 25-hydroxyvitamin D compared with women with normal blood pressure (Diaz 2002; Frenkel 1991; Halhali 1995; Halhali 2000; Tolaymat 1994). The low levels of urinary calcium (hypocalciuria) in women with pre-eclampsia may be due to a reduction in the intestinal absorption of calcium impaired by low levels of vitamin D (August 1992; Halhali 1995). Additionally, pre-eclampsia and vitamin D deficiency are directly and indirectly associated through biologic mechanisms including immune dysfunction, placental implantation, abnormal angiogenesis, excessive inflammation, and hypertension (Bodnar 2007; Cardus 2006; Evans 2004; Hewison 1992; Li 2002).
Vitamin D status and other maternal conditions
Maternal vitamin D deficiency in early pregnancy has been associated with elevated risk for gestational diabetes mellitus, although findings are still not consistent (Farrant 2008; Zhang 2008). Poor control of maternal diabetes in early pregnancy is inversely correlated with low bone mineral content in infants, as is low maternal vitamin D status (Namgunga 2003). VDD may lead to a high bone turnover, bone loss, osteomalacia (softening of the bones) and myopathy (muscle weakness) in the mother in addition to neonatal and infant VDD (Glerup 2000; Lips 2001).
An adequate vitamin D status may also protect against other adverse pregnancy outcomes. For example, maternal vitamin D deficiency has been linked to caesarean section in a single recent study (Merewood 2009) but the mechanisms involved are unclear.
Low prenatal and perinatal maternal vitamin D concentrations can affect the function of other tissues, leading to a greater risk of multiple sclerosis, cancer, insulin-dependent diabetes mellitus, and schizophrenia later in life (McGrath 2001a).
Vitamin D status and preterm birth and low birthweight
A potential inverse association between maternal vitamin D status and preterm birth (less than 37 weeks' gestation) has been reported (Dawodu 2011; Morley 2006). Conversely, not all the studies show significant associations between maternal calcidiol levels and any measure of the child's size at birth or during the first months of life (Bodnar 2010; Farrant 2009; Gale 2008; Morley 2006). There is not much information on maternal vitamin D status and low birthweight or preterm birth in children born from HIV-infected pregnant women (Mehta 2009).
Vitamin D status and postnatal growth
Some observational studies suggest that vitamin D levels during pregnancy influence fetal bone development and children's growth (Bodnar 2010; Brooke 1980; Mahon 2010; Morley 2006). While head circumference in children nine years of age has been significantly associated with maternal calcidiol levels (Gale 2008), there is still inconsistent information about the association of maternal vitamin D status and infants' bone mass (Akcakus 2006; Javaid 2006; Viljakainen 2010).
It is not clear if maternal vitamin D deficiency leads to neonatal rickets, since rickets is usually identified later in childhood. Early studies indicate a possible risk for neonatal rickets in the offspring of women with osteomalacia, abnormal softening of the bone by deficiency of phosphorus, calcium or vitamin D (Ford 1973). More recent studies have found that vitamin D deficiency (serum levels lower than 25 nmol/L) was identified in 92% of rachitic (having rickets) Arab children and 97% of their mothers compared with 22% of nonrachitic children and 52% of their mothers (Dawodu 2005). A positive correlation was found between maternal and child vitamin D levels.
Vitamin D status and immune response
Vitamin D has direct effects on both adaptive and innate immune systems (Miller 2010; Walker 2009). In children, vitamin D insufficiency is linked to autoimmune diseases such as type 1 diabetes mellitus, multiple sclerosis, allergies and atopic diseases (Bener 2009; Miller 2010; Pierrot-Deseilligny 2010). Various studies have also shown that vitamin D deficiency is strongly associated with tuberculosis, pneumonia, and cystic fibrosis (Chocano-Bedoya 2009; Hall 2010; Williams 2008) and both prenatal and perinatal vitamin D deprivation might influence early-life respiratory morbidity as this vitamin is important for lung growth and development (Devereux 2007; Litonjua 2009).
Vitamin D may have positive effects on the immune system by up-regulating the production of the antimicrobial peptides by macrophages and endothelial cells (Wang 2004), which may inactivate viruses and suppress inflammation (Cantorna 2008), and subsequently reduce the severity of infections.
Vitamin D toxicity
Vitamin D excess leads to hypercalcaemia (calcium levels are 10.5 mg/dL or higher) and hypercalciuria (urinary excretion of calcium exceeds 250 mg/day in women), which is associated with renal and kidney stones (Heaney 2008). Toxicity in adults usually appear at doses of vitamin D higher than 10,000 IU/d (250 µg/d), although most of the evidence is based on short-term exposures (less than six months) (Hathcock 2007; Heaney 2008; Institute of Medicine 2010; Vieth 1999). Single-dose supplements providing 7.5 mg (300,000 IU) or more may also be harmful (Roth 2011).
The potential for vitamin D-induced teratogenesis (birth defects) and adverse effects in the offspring (e.g. growth restriction, delayed ossification, craniofacial hypoplasia) has been suggested by a few studies in rats and rabbits (Ariyuki 1987; Chan 1979; Friedman 1969; Ornoy 1968; Ornoy 1969). However, there are considerable limitations in extrapolating such findings to humans, in whom adverse fetal effects have not reportedly occurred following maternal ingestion of maintenance doses as high as 5 mg (200,000 IU) of vitamin D per day. Overall, animal and human studies show that fetal excess of vitamin D metabolites are unlikely to occur when maternal concentrations are within a normal range (Roth 2011).
Description of the intervention
Some health organisations recommend vitamin D supplementation during pregnancy and lactation. However, there are variations in the recommended dose for supplementation ranging from 200 to 400 IU/d (5 to 10 µg/d) (Canadian Paediatric Society 2007; UK Department of Health 2009). The American Academy of Pediatrics (Wagner 2008) suggests that healthcare professionals who provide obstetric care should consider monitoring maternal vitamin D status by measuring its concentrations in pregnant women.
However, there is controversy regarding the 25-hydroxyvitamin D levels that are considered adequate or optimal for overall health. The US Institute of Medicine has determined that concentrations greater than 50 nmol/L or 20 ng/mL are adequate based on the current studies available (Institute of Medicine 2010), although many investigators consider that optimal levels should be higher (greater than 75 nmol/L or 30 ng/mL) (Dawson-Hughes 2005; Hollick 2009). It has been suggested that a supplemental dose of vitamin D of 1000 to 1600 IU (25 to 40 µg/d) might be necessary to achieve the optimal level of this vitamin in the body (Dawson-Hughes 2005). This dose is expected to raise serum 25-hydroxyvitamin D by 1.2 nmol/L for every µg (40 IU) of vitamin D3 given orally to individuals with low 25-hydroxyvitamin D levels; those with higher baseline concentrations would have smaller increments with the same dose (Dawson-Hughes 2005). However, the dose of vitamin D needed to have an effect during pregnancy or to prevent or treat vitamin D deficiency is not clear. Some researchers have suggested that doses around 1000 IU/d may be needed in order for pregnant women to maintain a blood concentration of vitamin D of more than 50 nmol/L (20 ng/mL) (Heaney 2003; Hollis 2004; Hollis 2007; Vieth 2001). Others have suggested providing vitamin D as weekly doses of 5000 IU (125 µg/wk) (Utiger 1998) or a single dose of 200,000 IU (5 mg) or greater (Mallet 1986; Sahu 2009; Yu 2009).
Since vitamin D can also be synthesised by the skin upon exposure to sunlight, increasing casual sun exposure for reaching the optimal serum levels has been recommended (Holick 2002). However, as excessive UV radiation is a carcinogen, it might be worth obtaining additional vitamin D from foods or supplements.
How the intervention might work
Vitamin D supplementation improves maternal vitamin D status during pregnancy (Delvin 1986; Yu 2009), which in turn may have a direct influence on the fetal and neonatal supply of vitamin D (Brooke 1980). The potential effect of gestational vitamin D supplementation in preventing preterm birth (less than 37 weeks 'gestation) and low birthweight (less than 2500 g) has been suggested (Maxwell 1981), although there is limited information on the additional benefit of vitamin D supplementation over other nutritional interventions during pregnancy such as iron and folic acid supplementation on the risk of low birthweight (Christian 2003). There is also a potential effect of maternal vitamin D supplementation on neonatal growth (Marya 1988). Vitamin D supplementation during pregnancy may be necessary to ensure adequate concentrations of vitamin D in breast milk during lactation (Butte 2002).
Why it is important to do this review
This review updates a previous Cochrane review (Mahomed 1999) and incorporates new evidence on the effects and safety of vitamin D supplementation in pregnancy for the well being of the mother and newborn.
Objectives
To examine whether supplements of vitamin D alone or in combination with calcium or other vitamins and minerals given to women during pregnancy can safely improve maternal and neonatal outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We intended to include randomised and quasi-randomised trials with randomisation at either individual or cluster level, but we only found randomised controlled trials with individual randomisation. We did not include crossover trials or any other observational designs (e.g. cohort or case-control studies) in this meta-analysis but we considered such evidence in the discussion, where relevant.
Types of participants
Pregnant women of any gestational or chronological age, parity (number of births) and number of fetuses.
Types of interventions
Vitamin D supplementation during pregnancy irrespective of dose, duration or time of commencement of supplementation. We included trials testing vitamin D alone or in combination with other micronutrients as long as the intervention and the control group were treated similarly. Specifically, we assessed the following comparisons.
Vitamin D alone versus no treatment/placebo (no vitamins or minerals).
Vitamin D + calcium versus no treatment/placebo (no vitamin or minerals).
Vitamin D + calcium versus calcium (but no vitamin D).
Vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D).
Types of outcome measures
Maternal antenatal clinical and laboratory outcomes and infant clinical and laboratory outcomes as described below.
Primary outcomes
Maternal
Pre-eclampsia (as defined by trialists).
Gestational diabetes (as defined by trialists).
Vitamin D status at term (25-hydroxyvitamin D in nmol/L).
Infant
Preterm birth (less than 37 weeks' gestation).
Low birthweight (less than 2500 g).
Secondary outcomes
Maternal
Impaired glucose tolerance (as defined by trialists).
Caesarean section.
Gestational hypertension (as defined by trialists).
Side effects (e.g. hypercalcaemia, kidney stones).
Maternal death (death while pregnant or within 42 days of termination of pregnancy).
Infant
Birth length (cm).
Head circumference at birth (cm).
Birthweight (g).
Admission to intensive care unit during the neonatal period (within 28 days after delivery).
Stillbirth (as defined by trialists).
Neonatal death (within 28 days after delivery).
Apgar score less than seven at five minutes.
Neonatal infection (e.g. respiratory infections within 28 days after delivery).
Very preterm birth (less than 34 weeks' gestation).
Search methods for identification of studies
Electronic searches
The Trials Search Co-ordinator from the Cochrane Pregnancy and Childbirth Group’s Trials Register conducted the search on 31 October 2011.
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co-ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co-ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) for any ongoing or planned trials and the Networked Digital Library of Theses and Dissertations (NDLTD) for grey literature on 28 October 2011 (see: Appendix 1).
Searching other resources
For the identification of ongoing and unpublished studies, we contacted on 8 April 2011 different institutions including the WHO Departments of Reproductive Health and Research and the Department of Nutrition for Health and Development, the WHO regional offices, UNICEF, the Micronutrient Initiative (MI), the Global Alliance for Improved Nutrition (GAIN) and the US Centers for Disease Control and Prevention (CDC).
We did not apply any date or language restrictions but we only found English language papers.
Data collection and analysis
Selection of studies
Two review authors independently assessed for inclusion all the references identified through the search. Cristina Palacios (CP) assessed all the potentially eligible papers and Luz Maria De-Regil (LMD), Regina Kulier (RK) and Ali Ansary (AS) evaluated one-third of the papers each. All the papers were assessed in duplicate and we resolved any disagreements through discussion or, if required, we consulted a third author (Juan Pablo Peña-Rosas (JPR)).
If studies were published only as abstracts, or study reports contained little information on methods, we attempted to contact the authors to obtain further details of study design and results. We were able to screen all the potentially eligible studies.
Data extraction and management
We designed a form to extract data. For included studies, all review authors extracted the data using the agreed form. CP entered data into Review Manager software (RevMan 2011) and JPR and LMD checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
We analysed dichotomous data in terms of average risk ratio and we analysed continuous data in terms of mean difference. There was no need to use the standard mean difference as trials did not report outcomes in different scales.
Assessment of risk of bias in included studies
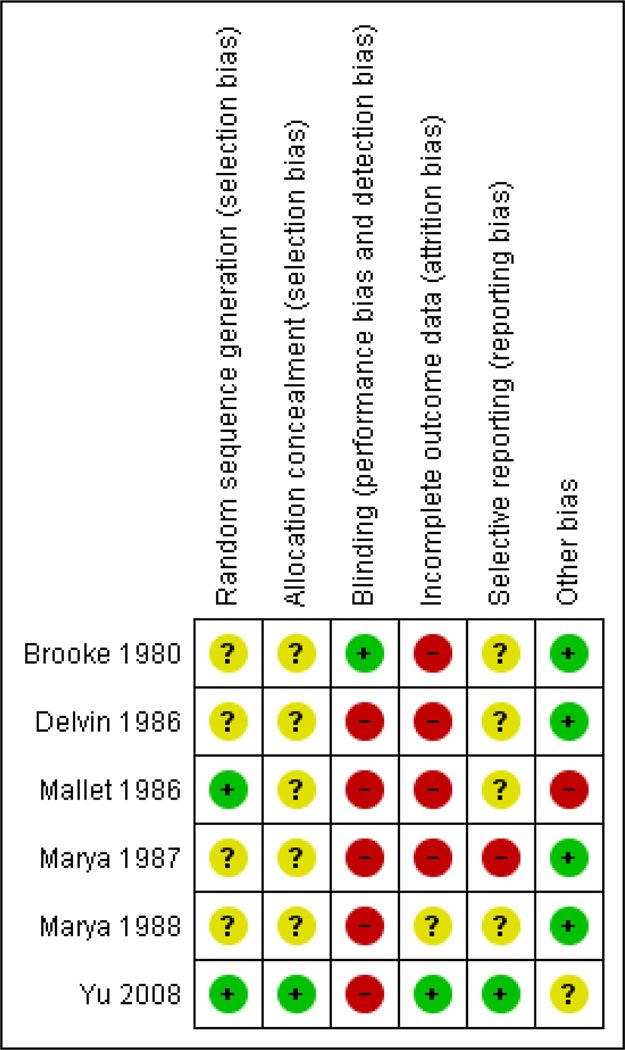
Two authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion.
(1) Sequence generation (checking for possible selection bias)
We have described for each included study the method used to generate the allocation sequence. We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear.
(2) Allocation concealment (checking for possible selection bias)
We have described for each included study the method used to conceal the allocation sequence and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non-opaque envelopes); or
unclear.
(3) Blinding (checking for possible performance bias)
We have described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes and we have noted where there was partial blinding.
We assessed the methods as:
low, high or unclear risk of bias for women;
low, high or unclear risk of bias for clinical staff;
low, high or unclear risk of bias for outcome assessors.
We classified blinding as 'high risk of bias' if the blinding status of a trial was unclear or the trial was open.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We assessed losses to follow-up and post-randomisation exclusions systematically for each trial.
We have described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We have noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. We assessed methods as:
low risk of bias;
high risk of bias; or
unclear.
We considered follow-up to be 'low risk of bias' if more than 80% of participants initially randomised in a trial were included in the analysis and any loss was balanced across groups, unclear if the percentage of initially randomised participants included in the analysis was unclear, and 'high risk of bias' if less than 80% of those initially randomised were included in the analysis or if loss was imbalanced in different treatment groups.
(5) Selective reporting bias
We have described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre-specified outcomes and all expected outcomes of interest to the review had been reported);
high risk of bias (where not all the study’s pre-specified outcomes had been reported; one or more reported primary outcomes were not pre-specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported); or
unclear.
(6) Other sources of bias
We assessed whether each study was free of other problems that could put it at risk of bias: We have noted for each included study any important concerns we had about other possible sources of bias.
low risk of further bias;
high risk of further bias;
unclear whether there is a risk of further bias.
(7) Overall risk of bias
We summarised the risk of bias at two levels: within studies (across domains) and across studies.
For the first, we made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and for primary outcomes, we explored the impact of the level of bias through undertaking a Sensitivity analysis.
For the assessment across studies, the main findings of the review are set out in the Summary of findings table 1 and Summary of findings table 2 (SoF) prepared using GRADE profiler software (GRADEpro 2008). The primary outcomes for each comparison are listed with estimates of relative effects along with the number of participants and studies contributing data for those outcomes, when available. For each outcome, the quality of the evidence was assessed independently by two review authors using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Balshem 2010), which involves consideration of within-study risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias; this results in one out of four levels of quality (high, moderate, low or very low). This assessment was limited only to the trials included in this review.
Summary of findings tables.
1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals)
|
Patient or population: pregnant women Settings: all settings Intervention: supplementation with vitamin D alone Comparison: placebo/no intervention (no vitamins or minerals) | |||
| Outcomes |
Relative effect (95% CI) |
No of Participants (studies) |
Quality of the evidence (GRADE) |
| Pre-eclampsia | Not estimable | 0 (0 studies) |
No trial assessed this outcome |
| Gestational diabetes | Not estimable | 0 (0 studies) |
No trial assessed this outcome |
| Maternal vitamin D status at term (25-hydroxyvitamin D in nmol/L) | MD 47.08 (23.76, 70.39) |
414 (4 studies) |
⊕⊕⊝⊝ low1,2,3 |
| Preterm birth | Not estimable | 0 (0 studies) |
No trial assessed this outcome |
| Low birthweight | 0.48 (0.23 to 1.01) |
463 (3 studies) |
⊕⊕⊝⊝ low1,2,3 |
|
CI: confidence interval; RR: risk ratio; GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||
Two of the included trials have high risk of performance and detection bias as they were not blinded. All trials had unclear allocation concealment.
High statistical heterogeneity but consistency in the direction of the effect.
Wide confidence intervals.
2 Vitamin D + calcium versus no treatment/placebo (no vitamin or minerals)
|
Patient or population: pregnant women Settings: all settings Intervention: supplementation with vitamin D + calcium Comparison: placebo/no intervention (no vitamins or minerals) | |||
| Outcomes |
Relative effect (95% CI) |
No of Participants (studies) |
Quality of the evidence (GRADE) |
| Pre-eclampsia | 0.67 (0.33, 1.35) | 400 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| Gestational diabetes | Not estimable | 0 (0 studies) | No trial assessed this outcome |
| Maternal vitamin D status at term (25-hydroxyvitamin D in nmol/L) | Not estimable | 0 (0 studies) | No trial assessed this outcome |
| Preterm birth | Not estimable | 0 (0 studies) | No trial assessed this outcome |
| Low birthweight | Not estimable | 0 (0 studies) | No trial assessed this outcome |
|
CI: confidence interval; RR: risk ratio; GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||
Wide confidence interval.
Only one study reported on this outcome. It is unclear how the random sequence was generated and it lacks of blinding. The study is also at high risk of selective reporting as the biochemical indicators were reported only for some groups.
Measures of treatment effect
Dichotomous data
For dichotomous data, we present results as average risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference as the outcomes were measured in the same way between trials; there was no need to use the standardised mean difference to combine trials.
Unit of analysis issues
Cluster-randomised trials
We planned to include cluster-randomised trials in the analyses along with individually randomised trials but we did not find eligible studies with this design. We planned to adjust the standard errors of the results from cluster-randomised studies using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) if sufficient information was available to allow for this. We planned to use an estimate of the intra cluster correlation co-efficient (ICC) derived from the trial (if possible), or from another source. If ICCs from other sources were used, we planned to report this and to conduct sensitivity analyses to investigate the effect of variation in the ICC.
If we would have identified both cluster-randomised trials and individually-randomised trials, we would have combined the results from both if there was little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit would be considered as unlikely.
Studies with more than two treatment groups
For studies with more than two intervention groups (multi-arm studies), we combined groups to create a single pair-wise comparison (Higgins 2011) and included the disaggregated data in the corresponding subgroup category. When the control group was shared by two or more study arms, we divided the control group (events and total population) over the number of relevant subgroup categories to avoid double counting the participants. The details are described in the Characteristics of included studies tables.
Characteristics of studies.
Characteristics of included studies
| Brooke 1980 | ||
|---|---|---|
| Methods | Randomised double-blind controlled trial; 2-arm design with individual randomisation. | |
| Participants | 126 pregnant women 28–32 weeks of gestation attending the antenatal clinic at St George's Hospital, London, United Kingdom (latitude: 51°30'N, north of tropic of Cancer). All pregnant women were first-generation immigrants mostly from India, Pakistan, Bangladesh, Sri Lanka, Mauritius and east Africa. Exclusion and elimination criteria: preterm deliveries, congenital malformations and maternal illnesses likely to affect fetal growth (such as diabetes). Pre-gestational body mass index and skin pigmentation not reported. |
|
| Interventions | Participants were randomly allocated to 1 of the following groups. Group 1 (n = 59 at the end of the trial): women received daily 1000 IU/day of calciferol (estimated total dose: 56000–84000 IU). Group 2 (n = 67 at the end of the trial): women received a placebo. Start of supplementation: weeks 28–32 gestation. Length of the intervention/follow-up: 8–12 weeks from supplementation to term. Season: authors report that to avoid distortion of the results due to seasonal variation in sunlight hours the trial was carried out during autumn and winter 1977, the whole of 1978 and spring and summer 1979. |
|
| Outcomes |
Maternal: maternal weight gain, dietary vitamin D intake, 25-hydroxy vitamin D (25-OHD) concentrations in cord blood and at term. Plasma calcium (adjusted for albumin concentration), inorganic phosphate, bilirubin, albumin concentrations and total alkaline phosphatase activity, alanine transaminase and y-glutamyl transferase activities, vitamin D binding globulin concentration, compliance. Infant: weight, crown-heel length, crown-rump length, rump-heel length, occipitofrontal head circumference, forearm length, lower leg length, triceps and subscapular skinfold thickness, fontanelle area, plasma cholecalciferol at day 3 and day 6. Weight, length and head circumference at 3,6,9 and 12 months. |
|
| Notes | There were no significant baseline differences between the groups in maternal age, parity, height, vegetarian: non-vegetarian ratio or the distribution of the various countries of origin. | |
| Bias | Authors' judgement |
Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial reported random allocation to the groups, although the method of sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding (performance bias and detection bias) | Low risk | Trial reported as double blind. |
| Incomplete outcome data (attrition bias) | High risk | Unclear number of randomised participants. Preterm deliveries, congenital malformations, and maternal illnesses likely to affect fetal growth (such as diabetes) were eliminated from the trial. There is not complete documentation of the exclusions. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Delvin 1986 | ||
|---|---|---|
| Methods | Randomised trial; 2-arm design with individual randomisation. | |
| Participants | 40 pregnant women attending their compulsory visit during the third month of pregnancy at the Obstetrical Unit of the Hopital Edouard Herriot, Lyon, France (latitude: 45° 45' 0" N north of tropic of Cancer). Inclusion criterion: singleton pregnancy at term and uneventful vaginal deliveries. Pre-gestational body mass index and skin pigmentation not reported. | |
| Interventions | Participants were randomly assigned to 1 of the following groups at the time of the compulsory visit. Group 1 (n = 20): women received daily 1000 IU vitamin D3 (estimated total dose: 55000 IU). Group 2 (n = 20): women received no supplement during the last trimester of pregnancy. Start of supplementation: week 27 of gestation (third trimester). Length of the intervention/follow-up: 12 weeks from start of supplementation to term. Season: winter-spring. All selections were performed in December, and all deliveries occurred in June. |
|
| Outcomes |
Maternal: serum (during last trimester of pregnancy) and cord blood immunoreactive parathyroid hormone (iPTH), 25-hydroxyvitamin D (25-OHD), 1-alfa,25-dihydroxyvitamin D (1,25(OH)2D), total calcium, ionised calcium, magnesium, inorganic phosphate. Infant: immunoreactive parathyroid hormone (iPTH), 25-hydroxyvitamin D (25-OHD), 1-alfa,25-dihydroxyvitamin D (1,25(OH)2D), total calcium, ionised calcium, magnesium, inorganic phosphate at 4 days of age. |
|
| Notes | Compliance was verified weekly visit by a midwife. | |
| Bias | Authors' judgement |
Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial reported as randomised but the method of sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding (performance bias and detection bias) | High risk | Paper describes that participants were allocated to the intervention by a blind randomisation process. Given that the participants did not receive an intervention it is unlikely that the trial was blind. |
| Incomplete outcome data (attrition bias) | High risk | 1 subject from the control group (5%) and 5 (25%) from the vitamin D supplemented group. Laboratory methods reported for 25 to 30 participants (depending on the outcome) out of 40 originally randomised. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Mallet 1986 | ||
|---|---|---|
| Methods | Randomised controlled trial; 3-arm design with individual randomisation. | |
| Participants | 77 white pregnant women 18–36 years of age in the last trimester of pregnancy living in Northwest of France (latitude: 49° 26' 0" N north of tropic of Cancer). Pre-gestational body mass index not reported. | |
| Interventions | Participants were randomly assigned to 1 of the following groups. Group 1 (n = 21): women received 1000 IU of vitamin D2 for the last 3 months of pregnancy (estimated total dose throughout pregnancy: 90,000 IU). Group 2 (n = 27): women received a single dose of 200,000 IU (5 mg) vitamin D at the 7th month of pregnancy. Group 3 (n = 29): women received no supplement and served as controls. Start of supplementation: week 28 of gestation (third trimester). Length of the intervention/follow-up: 12 weeks from start of supplementation to term. Season: winter pregnancy. Infants born during February and March. |
|
| Outcomes |
Maternal: 24-hour urinary calcium excretion after 6 week supplementation, calcium, 25-Hydroxyvitamin D (25-OHD) and1-alfa,25-dihydroxyvitamin D (1,25(OH)2D) metabolites of vitamin D from serum and cord during labour and delivery. Infant: serum calcium levels at days 2 and 6 of life, birthweight. |
|
| Notes | ||
| Bias | Authors' judgement |
Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by random numbers table. |
| Allocation concealment (selection bias) | Unclear Risk | Method of concealment not described. |
| Blinding (performance bias and detection bias) | High risk | Not reported as blinded. Different interventions were used: daily dose or single dose or no supplement. |
| Incomplete outcome data (attrition bias) | High risk | It is unclear if there was attrition, but given the uneven number of participants reported it is likely that there were losses to follow-up. |
| Selective reporting (reporting bias) | Unclear Risk | There is insufficient information to permit judgement. |
| Other bias | High risk | Groups are reported with notorious different sample size. It is unclear whether the numbers reflect the participants who finished the trial (unclear and uneven losses to follow-up); a non randomised process; or a selection bias in which randomised participants did not received the intervention. |
| Marya 1987 | ||
|---|---|---|
| Methods | Randomised controlled trial; 2-arm design with randomisation at individual level. | |
| Participants | 400 pregnant women 20–35 years of age, attending the antenatal clinic of Medical College Hospital in Rohtak, India (latitude: 76° 34' 0' north of Tropic of Cancer). Pre-gestational body mass index and skin pigmentation not reported. | |
| Interventions | Participants were allocated to 1 of the following groups. Group 1 (n = 200) received a daily supplement containing 1200 IU vitamin D and 375 mg calcium (estimated total dose from week 20–24 of gestation to term:134,400–168,000 IU). Group 2 (n = 200) received no supplement from 20–24 weeks of pregnancy until delivery. Start of supplementation: 20–24 weeks pregnancy (third trimester). Length of the intervention/follow-up: 16–20 weeks from start of supplementation to term. Season: not reported. |
|
| Outcomes | Maternal: pre-eclampsia (defined as blood pressure of 140 mmHg or higher systolic and/or 90 mmHg diastolic along with proteinuria higher than 300 mg/24 hours); systolic and diastolic blood pressure at 24, 28, 32 and 36 weeks of gestation. | |
| Notes | Biochemical analysis were made in those who developed pre-eclampsia (n = 12) and also in a group of women with no pre-eclampsia (n = 25) and a control group of non pregnant women. The results of the stratified analysis are not reported in this review. | |
| Bias | Authors' judgement |
Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | '400 pregnant women, of these 200 were randomly selected and put on a daily supplement of calcium and vitamin D. Method of sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding (performance bias and detection bias) | High risk | It is not reported whether the trial was blinded to participants, outcome assessor or care providers. |
| Incomplete outcome data (attrition bias) | High risk | Only data on biochemical were reported for those who developed pre-eclampsia and some of those with no pre-eclampsia and a group of non pregnant controls. |
| Selective reporting (reporting bias) | High risk | Outcomes reported for some subgroups only. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Marya 1988 | ||
|---|---|---|
| Methods | Randomised clinical trial; 2-arm design with individual randomisation. | |
| Participants | 200 pregnant women, aged 22–35 years old, attending the antenatal clinic of the Medical College Hospital, Rohtak, India (latitude: 76° 34' 0' north of Tropic of Cancer). Inclusion criterion: uncomplicated single pregnancy. Exclusion criteria: pre-eclampsia, antepartum haemorrhage, premature delivery. Pre-gestational body mass index and skin pigmentation not reported. | |
| Interventions | Participants were allocated to 1 of the following groups. Group 1 (n = 100): women received 2 doses of 600,000 IU 1 each at 7th and 8th month of pregnancy (estimated total dose: 1200,000 IU). Group 2 (n = 100): women received no intervention. Start of supplementation: 28 weeks pregnancy (third trimester). Length of the intervention/follow-up: 12 weeks from start of supplementation to term. Season: not reported. |
|
| Outcomes |
Maternal: venous and cord serum calcium, serum proteins, inorganic phosphate, alkaline phosphatase, weight. Radiological examination on women with abnormal biochemistry or osteomalacia symptomatology. Side effects: back age, leg-pains, general weakness, cramps. Infant: birthweight, low birthweight, crown-heel length, head circumference, mid-arm circumference within 24 hours after birth. Skinfold thickness (triceps and infrascapular). |
|
| Notes | ||
| Bias | Authors' judgement |
Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | '200 pregnant women, of these 100 were randomly selected (supplemented group) had been administered two doses of vitamin D'. Method of sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding (performance bias and detection bias) | Unclear risk | Method of concealment not described. |
| Blinding (performance bias and detection bias) | High risk | It is not reported whether the trial was blinded to participants, outcome assessor or care providers. |
| Incomplete outcome data (attrition bias) | Unclear risk | Losses to follow-up are not documented although exclusions included pregnancy complications. Result tables mention that each arm was comprised of 100 women, a number that corresponds to that described for the treatment allocation. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Yu 2008 | ||
|---|---|---|
| Methods | Randomised controlled trial; 4 × 3 block design with randomisation at individual level. | |
| Participants | 180 pregnant women from the following ethnic populations; 45 Indian Asians, 45 Middle Eastern, 45 Black and 45 Caucasian attending the routine antenatal clinic at St Mary’s Hospital, London, United Kingdom (latitude: 51°30'N north of tropic of Cancer). Exclusion criteria: pre-existing sarcoidosis, osteomalacia, renal dysfunction and tuberculosis. Pre-gestational body mass index and skin pigmentation (in addition to ethnicity) not reported. | |
| Interventions | Women were randomised in blocks of 15 within each of the 4 ethnic groups to 3 groups. Group 1: women received a daily dose of vitamin D (ergocalciferol) at 800 IU (estimated total dose 72,800 IU); Group 2: women received a stat dose of 200,000 IU of calciferol. Group 3: women received no treatment. Start of supplementation: 27 weeks' gestation (third trimester). Length of the intervention/follow-up: 13 weeks from start of supplementation to term. Season: April to November 2007; summer. |
|
| Outcomes | Maternal: Maternal and cord 25-hydroxyvitamin D levels at delivery, maternal PTH and corrected calcium levels at delivery. | |
| Notes | Women who did not speak English were only included if a health advocate was able to interpret and a leaflet was provided in their language. | |
| Bias | Authors' judgement |
Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated random number lists were drawn up by an independent researcher, with randomisation in blocks of 15. |
| Allocation concealment (selection bias) | Low risk | The person seeing the pregnant women allocated the next available number on entry to the trial, and each woman collected her tablets directly from the hospital pharmacy department or her local pharmacy. |
| Blinding (performance bias and detection bias) | High risk | All study personnel and participants were not blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) | Low risk | Only 1 loss to follow-up on group 3. |
| Selective reporting (reporting bias) | Low risk | Unlikely. |
| Other bias | Unclear risk | Women were randomised within each ethnic group. It is not clear if the ethnicity can be clearly established as it was self reported. Women who did not speak English were included only if a health advocate was able to interpret and a leaflet was provided in their language (English, Arabic, Bengali and Farsi) although the ability to read was not clearly established. |
IU: international units
Crossover trials
We did not consider crossover trials eligible for inclusion.
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention-to-treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and analyse all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta-analysis using the T2, I2 and Chi2 statistics. We regarded heterogeneity as substantial if I2 was greater than 30% and either T2 was greater than zero, or there was a low P value (less than 0.10) in the Chi2 test for heterogeneity.
Assessment of reporting biases
If we had included 10 or more studies in the meta-analysis, we would have investigated reporting biases (such as publication bias) by using funnel plots. We planned to assess funnel plot asymmetry visually, and use the statistical test proposed by Egger 1997 for continuous outcomes. For dichotomous data, we did not plan to use formal tests to investigate the asymmetry.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2011). We intended to use fixed-effect meta-analysis for combining data where it would be reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
Since we detected substantial statistical heterogeneity, we used random-effects meta-analysis to produce an overall summary of an average treatment effect across trials. We treated the random-effects summary as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
As we used random-effects analyses, we present the results as the average treatment effect with its 95% confidence interval, and the estimates of T2 and I2.
Subgroup analysis and investigation of heterogeneity
We planned to investigate any substantial heterogeneity on the primary outcomes by using subgroup analyses as follows:
by total dose of supplementary vitamin D during pregnancy: 56,000 IU vitamin D or less versus more than 56,000 to 200,000 IU versus more than 200,000 IU of vitamin D (the lowest cut-off is based on the highest daily supplemental dose during pregnancy, 400 IU/d times 140 days in 20 weeks of gestation; the highest cut-off is based on the usual single dose during gestation);
by start of supplementation: less than 20 weeks versus 20 weeks of pregnancy, or more;
by pre-gestational body mass index (kg/m2): underweight (lower than 18.5) versus normal weight (18.5 to 24.9) versus overweight (25 or higher) versus unknown/mixed;
by supplementation scheme/regimen: single versus daily versus weekly;
by skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): three or less versus four or more versus mixed/unknown;
by latitude: between Tropics of Cancer and Capricorn versus north of the Tropic of Cancer or South of the Tropic of Capricorn;
by season at the start of pregnancy: summer versus winter versus unknown.
Pragmatically, we decided not to conduct subgroup analyses in those outcomes with three or less trials. We examined differences between subgroups by visual inspection of the subgroups’ confidence intervals; non-overlapping confidence intervals suggesting a statistically significant difference in treatment effect between the subgroups. We formally investigated differences between two or more subgroup categories (Borenstein 2008). Analyses were conducted in Revman version 5.1.1 (RevMan 2011).
Sensitivity analysis
We intended to conducted a sensitivity analysis based on the quality of the studies, however, as only one study was considered of high quality, we did not perform this analysis. We considered a study to be of high quality if it was assessed as having low risk of bias in both the randomisation and allocation concealment and additionally a low risk of bias in either blinding or losses to follow-up.
Results
Description of studies
In this review, we included six trials involving 1023 women and all of them contributed data to the planned comparisons. We excluded eight studies and we identified 10 ongoing trials (Bisgaard 2009; Das 2010; Goldring 2010; Grant 2010; Habib 2010; Hacker 2010; Judkins 2011; Rasmussen 2009; Roth 2010; Soheilykhah 2011).
Details of these studies are provided in: Characteristics of included studies; Characteristics of excluded studies; Studies awaiting classification tables.
Characteristics of excluded studies
| Ala-Houhala 1986 | |
|---|---|
| Reason for exclusion | 49 healthy, well-nourished mothers delivering in January 1984 in the maternity wards and outpatient clinic of the Department of Paediatrics of the University Central Hospital of Tampere, Finland (latitude 61°N) and exclusively breastfeeding their infants, were divided in succession into 3 groups: group 1 (n = 17): mothers were given 2000 IU vitamin D3 a day, infants not supplemented; group 2 (n = 16): mothers were given 1000 IU vitamin D3 a day, infants not supplemented; group 3 (n = 16): mothers were not supplemented, and their breast fed infants were given 400 IU of vitamin D2 a day. During pregnancy, 33 mothers had no vitamin D supplementation, 8 mothers received 500 IU a day of vitamin D during the second trimester of pregnancy, and 8 mothers received 500 IU a day throughout the pregnancy. The mothers from these 3 groups supplemented in pregnancy were distributed in the postpartum maternal vitamin D supplementation and infant vitamin D supplementation interventions. This is not a randomised trial and the intervention includes mothers at postpartum and their infants. |
| Cockburn 1980 | |
|---|---|
| Reason for exclusion | 1139 pregnant women were assigned to 1 of 2 wards: group 1 (n = 506) Caucasian pregnant women assigned to 1 ward of the Simpson Memorial Maternity Pavilion, Edinburgh, United Kingdom during the 9 months from September to May, were given a daily dietary supplement of 400 IU of vitamin D2 from about the 12th week of pregnancy until delivery; group 2 women (n = 633) were assigned to another ward over the same period and were given a placebo containing no vitamin D. Outcomes included plasma concentrations of calcium, phosphorus, magnesium, total proteins, and 25-hydroxycholecalciferol at 24th and 34th weeks of pregnancy and at delivery. Infant plasma concentrations of calcium, phosphorus, magnesium, total proteins, and 25-hydroxycholecalciferol were measured from umbilical venous blood taken from the infants at birth and on capillary blood on the 6th day. This is not a randomised trial. |
| Das 2009 | |
|---|---|
| Reason for exclusion | 150 consecutive pregnant women pregnant women during their second trimester from 6 villages of a poor socioeconomic region in district Barabanki (latitude 26.8 °N), Uttar Pradesh, north India. The participants were initially randomised to receive either no dose or 1 dose of 60,000 IU cholecalciferol under observation in the 5th gestational month. However, the first few results showed rampant vitamin D deficiency and no improvement at delivery despite good exposure to sun and calcium supplementation. Therefore, this randomisation was abandoned subsequently and 2 comparison groups were followed up, alternate women receiving either 60,000 IU in the 5th month or 120,000 IU, each in the 5th and 7th months of pregnancy. This is not a randomised trial and the comparisons are outside the scope of this review. |
| Ito 1994 | |
|---|---|
| Reason for exclusion | 876 singleton pregnant women with blood pressure lower than 140/90 mmHg at 20 weeks’ gestation, and no evidence of proteinuria, who were attending the obstetric clinic of Kumamoto University Hospital, Japan were divided into 2 groups: group 1 (n = 666) women received conventional antenatal care; group 2 (n = 210 women) were managed under a protocol for the prediction of pre-eclampsia with an angiotensin sensitivity test and prevention of the condition by calcium supplementation. Participants from group 2 were further assigned to 1 of 4 groups according to their risk of developing pre-eclampsia, based on the angiotensin sensitivity test and the effective pressor dose: group A received 156 mg/day of oral elemental calcium (as calcium L-aspartate, Aspara-Ca from 22 weeks’ gestation, followed by 312 mg/day oral elemental calcium and vitamin D3 (0.5 µg for 3 days) from 30 weeks’ gestation to term. Participants in group B received 156 mg/day oral elemental calcium from 22 weeks’ gestation and 312 mg/day oral elemental calcium from 30 weeks’ gestation to term; group C received 312 mg/day oral elemental calcium from 30 weeks’ gestation to term and group D received no supplementation. This is not a randomised trial and the comparisons are outside the scope of this review. |
| MacDonald 1986 | |
|---|---|
| Reason for exclusion | This trial was registered in 1986 on the Oxford Database of Perinatal Trials and reports the recruitment and follow-up completed in 1979. The registration form reports a randomised controlled trial to assess the efficacy of calcium and vitamin D supplementation versus placebo in the prevention of maternal and fetal hypocalcaemia. The reports indicates that the sample size was 55 Asian women with morbidity and laboratory results as primary outcomes but no further information is available. |
| Marya 1981 | |
|---|---|
| Reason for exclusion | 45 Hindu pregnant women were randomly assigned to 1 of 2 groups: group 1 (n = 25) received tablets containing 1200 IU vitamin D and 375 mg calcium daily throughout the 3rd trimester; group 2 (n = 20) received oral single dose of 600,000 IU vitamin D2 once during 7th month and 8th month (total 2 doses). This group was compared with group 3 (n = 75) who had not received vitamin D supplements during pregnancy. The results were also compared with data from 25 non pregnant, non-lactating healthy women. Patients with complications such as pre-eclampsia, antepartum haemorrhage or twin pregnancies were excluded. The randomised study compares 2 doses of vitamin D supplementation. The type of study, type of participants and types of interventions are not eligible for this review. |
| von Hurst 2009 | |
|---|---|
| Reason for exclusion | 235 South Asian women, aged 23–68 years, living in Auckland, New Zealand were recruited for the study and those who were insulin resistant - homeostasis model assessment 1 (HOMA1) >1.93 and had serum 25-hydroxyvitamin D concentration < 50 nmol/L were randomised to receive 100 µg (4000 IU) vitamin D(3) (n = 42) or placebo (n = 39) daily for 6 months. The study participants were non pregnant women. The type of participants is outside the scope of this review. |
| Wagner 2006 | |
|---|---|
| Reason for exclusion | 494 apparently healthy pregnant women (16–45 years of age) with 12–16 weeks' gestation of singletons attending prenatal care in Medical University of South Carolina, Charleston, South Carolina in South Carolina, United States were randomised into 1 of 3 groups stratified by race: group 1 received 400 IU vitamin D3/day; group 2 received 2000 IU vitamin D3/day; and group 3 received 4000 IU vitamin D3/day until delivery. All women received daily multiple micronutrients supplements. 350 women continued until delivery. Outcomes included monthly 25-hydroxyvitamin D; 1,25(OH)2D; intact PTH, serum calcium, creatinine, phosphorus, and urinary calcium/creatinine levels, gestational age at delivery, birth weight, mode of delivery, co-morbidities of pregnancy, pre-eclampsia, gestational diabetes, any infection, preterm labour and premature birth. All women received vitamin D supplementation at different doses. The types of comparison are outside the scope of this review. |
IU: international units
PTH: parathyroid hormone
Characteristics of studies awaiting classification.
Characteristics of ongoing studies
| Bisgaard 2009 | |
|---|---|
| Study name | Vitamin D supplementation during pregnancy for prevention of asthma in childhood: an interventional trial in the ABC (Asthma Begins in Childhood) cohort. |
| Methods | Randomised double-blind, placebo-controlled trial with 2 arms. |
| Participants | Danish-fluent pregnant women 18 years of age or older, with 22–26 week of gestation living in Sealand, Denmark participating in the ABC-cohort. The mothers in ABC also participate in an interventional trial with fish oil supplementation, and the vitamin D randomisation is stratified by fish oil treatment group. Women with intake of more than 400 IU of vitamin D during the previous 6 months, endocrinological disease such as calcium metabolic disorder, parathyroid disorder, thyroid disorder or Diabetes type 1, tuberculosis, sarcoidosis or in need of diuretics or heart medication including calcium channel blockers are excluded. |
| Interventions | Participants are randomised to 1 of 2 groups: group 1 receives a daily supplement with 2400 IU of vitamin D3 from week 24 of gestation to 1 week after delivery ; group 2 receives placebo from week 24 of gestation to 1 week after delivery. |
| Outcomes | Primary: Maternal: none. Infant: recurrent wheeze from 0 to 3 years of age. Secondary: Maternal: 25-OH-vitamin D, PTH, Calcium, alkaline phosphatase concentrations 1 week postpartum. Infant: upper and lower respiratory infections, allergy, eczema from 0–3 years of age. |
| Starting date | Date of start: 03/2009. Status: recruiting participants. |
| Contact information | Hans Bisgaard, MD, DMSc Copenhagen Studies on Asthma in Childhood Copenhagen University Hospital of Copenhagen Gentofte, Denmark, 2820 Tel: +45 39777360 E-mail: bisgaard@copsac.com |
| Notes | Sponsor: Copenhagen Studies on Asthma in Childhood. |
| Das 2010 | |
|---|---|
| Study name | Vitamin D and calcium nutrition in pregnancy-evaluation of optimal supplementation dose of vitamin D during antenatal period. |
| Methods | Randomised, parallel group, multiple-arm trial. |
| Participants | 200 consecutive pregnant women attending antenatal clinic of at Queen Mary Hospital in CSMMU (former KGMC) will be enrolled into the study after taking informed consent. Patients already on calcium or on vitamin D supplementation, on anticonvulsants, on antitubercular treatment or having any medical condition affecting calcium and vitamin D metabolism (e.g. renal or liver disease) will be excluded. |
| Interventions | Participants will be randomly assigned to 1 of 3 groups: group 1 will receive 3 single doses of 120,000 IU vitamin D each provided every 8 weeks apart + 500 mg elemental calcium (as calcium carbonate) and 250 IU vitamin D twice a day, daily throughout pregnancy; group 2 will receive 3 single doses of 60,000 IU vitamin D each provided every 8 weeks apart + 500 mg elemental calcium (as calcium carbonate) and 250 IU vitamin D twice a day, daily throughout pregnancy; group 3 will receive 500 mg elemental calcium (as calcium carbonate) and 250 IU vitamin D twice a day daily throughout pregnancy. |
| Outcomes | Primary: Maternal 25-hydroxyvitamin D, calcium and albumin at baseline, 2nd trimester (14–20 weeks' gestation) and at delivery. Infant: cord blood 25-hydroxyvitamin D and albumin at delivery, neonatal calcium at 4–6 days after delivery. Secondary: Maternal: none. Infant: newborn's anterior fontanelle diameter, birthweight, crown heel length, head circumference within 24 hours after birth, occurrence of neonatal seizures, other morbidity within 1 week of delivery. |
| Starting date | Date of start:18-09-2009. Status: open to recruitment. |
| Contact information | Dr Vinita Das CSM Medical University Chowk, Lucknow, Uttar Pradesh, 226003 , India Tel: +91 522 2257742 Email: fogsiemoc_lko@yahoo.co.in Dr Vijailakshmi Bhatia SGPGI, Lucknow , Uttar Pradesh, 226014 , India Tel: +91 522 2494380 Email: bhatiaviji@gmail.com |
| Notes | Financial Support: Council of Science & Technology, UP. |
| Goldring 2010 | |
|---|---|
| Study name | Effects of prenatal vitamin D supplementation on respiratory and allergic phenotypes and bone density in the first 3 years of life. |
| Methods | Randomised interventional prevention trial. |
| Participants | 180 mothers attending antenatal clinic at St Marys Hospital, London United Kingdom. This is a follow-up trial of the infants of these trial participants. All of the offspring of the 180 mothers recruited in this trial are eligible and are invited to participate in this follow-up study when their children are 3 years of age. |
| Interventions | Participants were randomised at 27 weeks' gestation to 1 of 2 groups: group 1 received no vitamin D (n = 60), group 2: received 800 IU of vitamin D daily for the remainder of pregnancy (n = 60); group 3 (n = 60) received a single oral dose of 200,000 IU vitamin D at 27 weeks' gestation. |
| Outcomes | Primary: Maternal: none. Infant: wheezing episode in the first 3 years of life, measured at 36–48 months. Secondary: Maternal: none. Infant: use of inhaled bronchodilators in the last 12 months, doctor-diagnosed rhinitis, any wheezing episode in the preceding 12 months, doctor-diagnosed asthma, doctor-diagnosed eczema, doctor-diagnosed food allergy, positive skin prick test responses, 25-hydroxyvitamin D levels, bronchodilator responsiveness, exhaled nitric oxide level (in parts per billion), nasal secretions for inflammatory mediators, pulmonary airflow resistance and reactance at a range of frequencies using impulse oscillometry, total number of all wheezing episodes since birth and total number of upper and lower respiratory tract infections since birth, at 36–48 months. |
| Starting date | Date of start: 01/03/2010. Status: ongoing. Anticipated end date: 31/05/2011. |
| Contact information | Dr Stephen Goldring Department of Paediatrics Wright-Fleming Institute Norfolk Place, London W2 1PG, United Kingdom E-mail: sgoldring@nhs.net |
| Notes | Sponsor: Imperial College London (UK). |
| Grant 2010 | |
|---|---|
| Study name | Randomised placebo-controlled study of vitamin D3 during pregnancy and infancy to determine the vitamin D dose in pregnancy and early life that safely and effectively increases serum vitamin D concentration in infants. |
| Methods | Randomised controlled trial, blinded. |
| Participants | 260 pregnant women attending antenatal care and intending to delivery at Middlemore Hospital, in the suburb of Middlemore, Manukau City, New Zealand and who are either public patients attending the antenatal clinics at Middlemore hospital or whose lead maternity caregiver is a member of South Auckland Maternity Care Limited. Pregnant mothers taking vitamin D supplementation that exceeds 200 IU/day and those with a history of renal stones or hypercalcaemia or found to be hypercalcaemic at enrolment, or with any serious complication of pregnancy at the time of enrolment will be excluded. Their infants will be further randomised to vitamin D supplementation regimens or placebo from birth to 6 months of age. |
| Interventions | Participants will be randomly assigned to 1 of 3 groups: group 1 will receive 1000 IU/day of vitamin D3; group 2 will receive 2000 IU/day of vitamin D3; and group 3 will receive a placebo. Each enrolled pregnant woman will receive the intervention from enrolment at approximately 28 gestation until delivery. The infants of these mothers will be randomised to receive placebo, if their mother was randomised to placebo, 400 IU/day (if mother’s dose was 1000 IU/day) or 800 IU/day of vitamin D3 (if mother’s dose was 2000 IU/day). Vitamin D supplementation and placebo will be an oral liquid medicine. Each enrolled infant will receive the supplement from birth until 6 months of age. Vitamin D supplementation and placebo will be an oral liquid medicine (purified components of coconut and palm oil). |
| Outcomes | Primary: Maternal:number of mothers hypercalcaemia at any measurement point, serum calcium concentration at 36 week of gestation. Infant: proportion of infants achieving a serum 25[OH]vitamin D concentration > 75 nmol/L at 6 months of age, serum calcium concentration on an umbilical cord blood sample collected at birth, and on blood samples at 2, 4 and 6 months of age, number of infants with hypercalcaemia at any measurement point. Secondary: Maternal: proportion of mothers achieving a serum 25[OH]vitamin D concentration > 75 nmol/L at 36 weeks' gestation. |
| Starting date | Date of start: 1/04/2010. Status: open to recruitment. |
| Contact information | Associate Professor Cameron Grant cc.grant@auckland.ac.nz 64 9 373 7999. |
| Notes | Financial support: Australian Research Council, Health Research Council of New Zealand Level 3, 110 Stanley Street, Auckland, 1010, New Zealand PO Box 5541, WellesleyStreet, Auckland, 1141, New Zealand Sponsors: University of Auckland, The University of Auckland, Private Bag 92019, Auckland 1142, New Zealand and University of Otago, School of Medicine & Health Sciences, University of Otago, Wellington PO Box 7343, Wellington South, Wellington 6242, New Zealand. |
| Habib 2010 | |
|---|---|
| Study name | Evaluation of the effectiveness of vitamin D supplementation to pregnant women and their infants in Pakistan. |
| Methods | Randomised controlled trial. |
| Participants | 550 apparently healthy pregnant women 15–49 years of age from 20–22 weeks of gestation and their infants in Pakistan. Pregnant women with pre existing type 1 or type II diabetes, multiple fetuses, babies (twins, triplets), with high levels of vitamin D will be excluded. Infants with multiple congenital anomalies, serious birth injury, birth asphyxia, serious infections, very low birthweight, will be excluded. |
| Interventions | Participants will be individually randomised to 1 of 2 groups: group 1 will receive a daily dose of 4000 IU of vitamin D from 20-22 weeks of pregnancy till the time of delivery; group 2 will receive placebo. The infants will be stratified in 2 groups: group 1 will receive 400 IU of vitamin D for 6 months as intervention (if mothers are from group 1); group 2 will receive placebo (if mothers are from group 2). |
| Outcomes | Primary: Maternal: pre-eclampsia, gestational hypertension, poor weight gain during pregnancy. Stillbirth rates, Infant: low birthweight, prematurity, neonatal seizures, infants with growth failure, signs and symptoms of vitamin D deficiency, infections: pneumonia, diarrhoea and receptor polymorphism. Secondary: Maternal: prevalence and risk factors for maternal vitamin D deficiency. Infant: prevalence and risk factors for neonatal vitamin D deficiency. |
| Starting date | Date of start: February 2010. Status: recruiting participants. Estimated study completion date: June 2011. |
| Contact information | Muhammad Atif Habib, MBBS, MPH Project Office Aga Khan University Phone: +92 21 3 4864798 Email: atif.habib@aku.edu Principal investigator: Zulfiqar A Bhutta, FRCPCH, PhD Aga Khan University, Pakistan. |
| Notes | Sponsors: Aga Khan University and John Snow, Inc. |
| Hacker 2010 | |
|---|---|
| Study name | Testing the calcium DRI during pregnancy: a study of bone health in black and white women. |
| Methods | Randomised controlled trial. |
| Participants | 120 African American or Caucasian primigravidae women 19–40 years of age in their first trimester of pregnancy in Children's Hospital & Research Center Oakland, California, USA. Women who are smokers, have a pre-pregnancy body mass index (BMI) higher than 30, have a medical condition that affects bone or taking a medication that affects bone will be excluded. |
| Interventions | Participants will be randomly assigned to 1 of 3 groups: group 1 will receive 1000 mg of calcium; group 2 will receive 2000 IU vitamin D and group 3 will receive a placebo. The intervention will be provided from week 16 of pregnancy until delivery. |
| Outcomes | Primary: Maternal: change in peripheral cortical and trabecular bone loss and gain during a reproductive cycle in black and white women. Infant: none. Secondary: change in bone markers of bone formation and resorption during pregnancy and postpartum, differences in calcium absorption in late pregnancy among black and white women, differences in adaptive immune function tests and markers of inflammation during pregnancy. Infant: none. |
| Starting date | Date of start: 05/2010. Status: currently recruiting participants. Expected study completion date: May 2013. |
| Contact information | Andrea N Hacker, MS, RD Children's Hospital & ResearchCenter Oakland, CA, USA Phone: +1 510 428-3885 Email: efung@mail.cho.org Principal Investigators: Ellen Fung, PhD, RD and Janet King, PhD, RD |
| Notes | Sponsors: Children's Hospital & Research Center Oakland and USDA, Western Human Nutrition Research Center, USA. |
| Judkins 2011 | |
|---|---|
| Study name | A randomised double-blinded interventional trial to determine the effect of 50,000 IU vitamin D supplementation monthly or twice monthly from 20 weeks' gestation. |
| Methods | Randomised double-masked clinical trial with randomisation at the individual level. Method of sequence generation: serial tossing of a coin. Allocation will be not concealed. |
| Participants | Pregnant women seeking maternity care with midwifery services involved in the study. Exclusion criteria: antenatal Vitamin D level is > 75 nmol/L when enrolling in study |
| Interventions | There are two arms in the study. One arm of the study will receive 50,000 IU tablets twice monthly, 2 weeks apart. The other arm of the study will receive 50,000 IU monthly and a placebo monthly, 2 weeks apart from 20 weeks' gestation until delivery of baby. The placebo tablet contains lactose monohydrate, acacia, calcium carbonate, castor oil, maize starch, povidone, sucrose, purified talc, hydrated silica, powdered cellulose, magnesium sterate, shellac, gelatin, beeswax white, titanium dioxide and prepared theobroma. |
| Outcomes | At delivery. Vitamin D levels taken from the cord blood samples at delivery. If emergencies at delivery prevent a cord blood sample being taken then a maternal venous blood sample will be taken for analysis. |
| Starting date | Status: not yet recruiting participants. |
| Contact information | Dr Annie Judkins Newtown Union Health Service 14 Hall Ave Newtown Wellington 6021 New Zealand annie.judkins@nuhs.org.nz |
| Notes | ACTR Number: ACTRN12610001044011 |
| Rasmussen 2009 | |
|---|---|
| Study name | Effects of vitamin D supplement before and during and after pregnancy on complications, birthweight and bone mineral density during lactation. |
| Methods | Double-blind, randomised, placebo-controlled trial. |
| Participants | 400 apparently healthy women 30–35 years of age, all with concentrations of P-25-hydroxyvitamin D (25OHD)- lower than 50 nmol/L. All women included attempts to get pregnant. Visits take place at Clinic of Osteoporosis, Department of Endocrinology, at Aarhus University Hospital, Aarhus, Denmark. Women with infertility, an intake of 400 IU or more vitamin D/day, cancer, history of alcohol or drug abuse, calcium metabolic disturbances or spontaneous abortion within last 6 months will be excluded. |
| Interventions | Participants will be randomly assigned to 1 of 3 groups: group 1 will receive 35 μg per day cholecalciferol; group 2 will receive 70 μg per day cholecalciferol; and group 3 will receive placebo. All women will receive 2 tablets daily from baseline until 16 weeks after delivery. Intervention with cholecalciferol or placebo starts before pregnancy is achieved and continues until 4 months after the women has given birth. |
| Outcomes | Primary: Infant: birthweight. Maternal: none Secondary: Infant: weight, crown-heel length and head circumference, and infections within 16 weeks after birth. Concentration of 25OHD in umbilical cord and venous sample 16 weeks after birth. Maternal: postpartum effects of vitamin D supplement on maternal bone mineral density, concentration of 25OHD in mothers milk, incidence of pre-eclampsia and abortions. |
| Starting date | Date of start: 12/2009. Status: recruiting participants. Estimated study completion date: December 2011. |
| Contact information | Gitte Bloch Rasmussen, MD Department of Endocrinology Aarhus University Hospital University of Aarhus Phone: +45 89 4976 81 Email: gittebr@ki.au.dk |
| Notes | Sponsor: University of Aarhus, Denmark. |
| Roth 2010 | |
|---|---|
| Study name | The effect of antenatal vitamin D supplementation on maternal-fetal vitamin D status and neonatal immune function: a randomised controlled trial in Bangladesh. |
| Methods | Randomised, placebo controlled trial. |
| Participants | 160 pregnant women aged 18–34 years of age with 26–29 weeks of gestation living in Dhaka planning a delivery at the Shimantik maternity centre, Dhaka, Bangladesh and planning to live in this location for at least 1 month postpartum. Women who use any dietary supplement containing more than 400 IU/day (10 mcg/day) of vitamin D within the month prior to enrolment, or refusal to stop taking supplemental vitamin D at any dose after enrolment; currently use of anti-convulsant or anti-mycobacterial (tuberculosis) medications; have severe anaemia (haemoglobin concentration less than 70 g/L); have a complicated medical or obstetric history that may increase the risk of preterm birth or labour/delivery complications; or a prior history of delivery of an infant with a major congenital anomaly, birth asphyxia, or perinatal death (stillbirth or death within first week of life) will be excluded. |
| Interventions | Participants will be randomly assigned to 1 of 2 groups: group 1 will receive a weekly dose of 35,000 IU vitamin D3 (875 μg/week); group 2 will receive placebo (Miglyol® 812). Intervention will start in the third trimester at 26-29 weeks' gestation until delivery. |
| Outcomes | Primary: Maternal: serum 25-hydroxyvitamin D concentration during third trimester. Infant: neonatal serum 25-hydroxyvitamin D concentration (cord blood). Secondary: Maternal: serum calcium concentration, urine Ca:Cr ration during third trimester. Infant: neonatal immune function (cord blood) measured as in vitro stimulated cord blood mononuclear cell (CBMC) LL-37 expression, gene expression related to inflammatory and immunoregulatory pathways, Th1/Th2 cytokine secretion, and bactericidal properties. |
| Starting date | Date of start: 06/2010. Status: ongoing. Estimated study completion date: May 2011. |
| Contact information | Daniel Roth, MD Johns Hopkins Bloomberg School of Public Health, USA. Email: droth@jhsph.edu Principal Investigators: Dr Abdullah Baqui, Dr Daniel Roth, Dr Rubhana Raqib. |
| Notes | Sponsors: Johns Hopkins Bloomberg School of Public Health and International Centre for Diarrhoeal Disease Research, Bangladesh. |
| Soheilykhah 2011 | |
|---|---|
| Study name | Effect of different doses of vitamin D on insulin resistance in pregnant women attending in Shahid Sadoughi and Mojibian prenatal clinics |
| Methods | Randomised clinical trial with randomisation at the individual level. |
| Participants | 150 pregnant women with gestational age less than 12 weeks without gestational diabetes, history of PCO, BMI less than 27kg/m2 before pregnancy, no Vit D supplementation in the past 6 months. Exclusion criteria: diabetes or gestational diabetes treated with insulin, thyroid or parathyroid disorders, hypertension, PCO. |
| Interventions | Women will be randomly allocated to: Intervention group 1: Vit D supplementation, 2000 IU, daily for 6 months Intervention group 2: Vit D supplementation, 4000 IU, daily for 6 months Control group: vit D, 200 IU (conventional dose), daily for 6 months |
| Outcomes | Gestational Diabetes, insulin concentration, vitamin D concentration. |
| Starting date | Irct registration number : IRCT138811203312N1 Status: recruiting |
| Contact information | Dr. Sedigheh Soheilykhah Shahid Sadoughi hospital, Yazd, Islamic Republic of Iran 8917965556 00983518224001 s_soheilykhah@ssu.ac.ir |
| Notes | Funding source: Yazd Diabetes ResearchCenter, Shahid Sadoughi University of Medical Sciences Irct registration number : IRCT138811203312N1 |
BMI: body mass index
DRI: Dietary References Intakes
PCO: polycystic ovary
PTH: parathyroid hormone
Results of the search
The search of the Cochrane Pregnancy and Childbirth Group's Trials Register found 23 reports for possible inclusion and the additional search strategy identified another 13 references. Figure 1 depicts the process for assessing and selecting the studies.
Figure 1.
Study flow diagram.
Included studies
Settings: the studies included in the review were mostly carried out during the 1980s and one trial in 2008. Trials were conducted in the United Kingdom (Brooke 1980; Yu 2008), France (Delvin 1986; Mallet 1986) and India (Marya 1987; Marya 1988). The latitude of the settings was north of the Tropic of Cancer, also referred to as the Northern tropic. The seasons varied among studies with some trials occurring during the winter-spring (Delvin 1986); winter (Mallet 1986); summer (Yu 2008) or not reported (Marya 1987; Marya 1988). One trial was carried out in different seasons to avoid distortion of the results due to seasonal variation in sunlight hours (Brooke 1980).
Participants: in one trial (Brooke 1980), women were first-generation immigrants mostly from India, Pakistan, Bangladesh, Sri Lanka, Mauritius and east Africa; one trial described the participants as being Indian, Asian, Middle Eastern, Black or Caucasian (Yu 2008), and another trial described the participants as white women (Mallet 1986). The remaining trials did not report the characteristics of the participants in terms of ethnicity or skin pigmentation (Delvin 1986; Marya 1987; Marya 1988).
The sample size from all the studies was small and ranged between 40 (Delvin 1986) and 400 women (Marya 1987) and in all the studies women were recruited, and received the supplements during the third trimester of pregnancy, after 28 weeks' gestation (Brooke 1980; Delvin 1986; Mallet 1986; Marya 1987; Marya 1988; Yu 2008). Pre-gestational body mass index of the participants was not reported in any of the trials.
Interventions: five trials compared vitamin D alone versus no treatment or placebo (Brooke 1980; Delvin 1986; Mallet 1986; Marya 1988; Yu 2008) while one trial provided vitamin D plus calcium in comparison with no treatment (Marya 1987). No studies evaluated the effects of vitamin D plus calcium versus calcium nor vitamin D plus calcium and other micronutrients in comparison with other micronutrients (excluding vitamin D).
The dose of vitamin D used on a daily basis ranged from 800 to 1200 IU. One trial provided 800 IU (Yu 2008); three trials provided a dose of 1000 IU in one of their arms (Brooke 1980; Delvin 1986; Mallet 1986) and one trial used 1200 IU (Marya 1987). Three trials evaluated high doses of vitamin D in one of their arms: two of them used a single dose of 200,000 IU at the seventh month (Mallet 1986) or during the third trimester (Yu 2008); and another one used a dose 600,000 IU given twice, during the seventh and eighth month of pregnancy (Marya 1988). The overall supplemental vitamin D dose during pregnancy varied across trials. One trial provided less than 56,000 IU (Delvin 1986); four trials provided 56,000 to 200,00 IU (Brooke 1980; Mallet 1986; Marya 1987; Yu 2008), and only one trial provided more than 200,000 IU of supplemental vitamin D during pregnancy (Marya 1988).
See Characteristics of included studies for a detailed description of the studies, including vitamin D doses used and regimens compared.
Excluded studies
We excluded eight studies. The main reason for exclusion was that they were not randomised trials (Ala-Houhala 1986; Cockburn 1980; Das 2009; Ito 1994) or that the comparisons were among different doses of vitamin D (Marya 1981; Wagner 2006) without placebo or no treatment control. One reference referred to a trial registered in 1986 on the Oxford Database of Perinatal Trials and reports the recruitment and follow-up completed in 1979 but there were no reports available and we were unable to locate the author who registered the trial (MacDonald 1986). One trial (von Hurst 2009) was conducted on non pregnant women. For more detailed descriptions of excluded studies along with the reasons for exclusion, see Characteristics of excluded studies.
Risk of bias in included studies
Allocation (selection bias)
Sequence generation
One study used computer-generated random number sequences (Yu 2008) and one used a random numbers table (Mallet 1986) to randomise the intervention groups. The other trials reported the studies as randomised but the methods used to generate the sequence were not described (Brooke 1980; Delvin 1986; Marya 1987; Marya 1988).
Allocation concealment
One trial (Yu 2008) reported that the person seeing the pregnant women allocated the next available number on entry to the trial (sequence generated by an independent researcher), and each woman collected her tablets directly from the hospital pharmacy department or her local pharmacy. The remaining trials did not report the methods used to conceal the allocation.
Blinding (performance bias and detection bias)
Blinding of participants, staff and outcome assessors
One trial was reported as blinded (Brooke 1980) although it was unclear whether the blinding was specifically for the participants, outcome assessor or care provider. Another trial (Delvin 1986) described that participants were allocated to the intervention by a "blind randomisation process"; however, given that the participants in the control group did not receive an intervention it is unlikely that the trial was blind. Four trials were not reported as blinded (Mallet 1986; Marya 1987; Marya 1988; Yu 2008). While lack of blinding may not represent a serious source of bias for some outcomes (e.g. serum indicators), other outcomes (i.e. reporting of side effects) may have been affected by knowledge of the treatment group.
Incomplete outcome data (attrition bias)
With one exception (Yu 2008), lack of reporting on attrition, missing data and lack of intention-to-treat analyses were serious problems in almost all of the included studies. Two trials excluded participants if they had maternal illness (such as diabetes) or pregnancy complication so that they could receive treatment, but these exclusions are not well-documented (Brooke 1980; Marya 1988). One trial (Marya 1987) only reported biochemical data for those who developed pre-eclampsia and some of the other participants with no pre-eclampsia, but not for all the randomised participants. The attrition rate was unclear in one trial (Mallet 1986) and another one had unbalanced losses between the study arms (Delvin 1986).
Selective reporting (reporting bias)
We did not have access to study protocols and therefore, formally assessing reporting bias was not possible. One study (Marya 1987) reported data only for some subgroups. Insufficient studies contributed data to allow us to carry out exploration of possible publication bias by using funnel plots.
Other potential sources of bias
Full details of 'Risk of bias' assessments are included in the Characteristics of included studies table. We have also included figures which summarise our 'Risk of bias' assessments (Figure 2; Figure 3).
Figure 2.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
In this review we included six trials, involving 1023 women. We organised the summary results by comparison and by primary and secondary outcomes.
In the Data and analyses tables, we set up all four prespecified comparisons but outcome data were only available for two of these. We have not added outcomes to those comparisons without data (comparisons three and four). For the comparisons with data, we set up tables for all primary outcomes (even where no data were available) not only to highlight gaps in the current research evidence, but also to be able to add any data that may become available in future updates.
See Data and analyses for detailed results on primary and secondary outcomes.
For each of the comparisons, we have indicated the number of studies contributing data and the total number of women recruited in these studies. However, for some outcomes only one or two studies provided data and due to loss to follow-up, denominators for particular outcomes may have been considerably less than the randomised sample. Therefore, we have indicated the number of studies contributing data and the number of women included in that analysis.
(1) Vitamin D alone versus no treatment/placebo (no vitamins or minerals) (five studies, 623 participants)
Five studies involving 623 women were included in this comparison (Brooke 1980; Delvin 1986; Mallet 1986; Marya 1988; Yu 2008); all of the contributed data. Only one trial (Yu 2008) was assessed as being at low risk of bias.
Maternal primary outcomes
Pre-eclampsia (as defined by trialists)
No studies reported on this outcome.
Gestational diabetes (as defined by trialists)
No studies reported on this outcome.
Maternal vitamin D levels at term (25-hydroxyvitamin D in nmol/L)
The data from four trials (Brooke 1980; Delvin 1986; Mallet 1986; Yu 2008) involving 414 women consistently show that women who received vitamin D supplements had higher 25-hydroxyvitamin D concentrations than those women who received no intervention or a placebo. The response to supplementation was highly heterogeneous (T2 = 517.96, I2 = 98% and ChI2 test for heterogeneity P < 0.00001) and ranged from 11.00 nmol 25-hydroxyvitamin D per litre (95% confidence interval (CI) 5.03 to 16.97) in Yu 2008 to 151.80 25-hydroxyvitamin D per litre (95% CI 126.74 to 176.86) in Brooke 1980; the large effect reported in this study contributes importantly to the observed heterogeneity. The average mean difference (MD) between groups was 47.08 nmol 25-hydroxyvitamin D per litre (95% CI 23.76 to 70.39) (Analysis 1.3) but this result should be interpreted cautiously.
The subgroup analysis suggests that women who received vitamin supplementation on a daily basis reached a higher concentration of Vitamin D at the end of the pregnancy compared with women who received a single dose (Analysis 1.4). The results did not vary by dose or the season at which the study was conducted (Analysis 1.5; Analysis 1.6). However, all these results should be interpreted cautiously as only one or two trials were included in most of the subgroup categories and the results may be misleading.
Infant primary outcomes
Preterm birth (less than 37 weeks' gestation)
No studies reported on this outcome.
Low birthweight (less than 2500 g)
The data from three trials (Brooke 1980; Marya 1988; Yu 2008) involving 463 women suggest a trend that women receiving vitamin D supplements during pregnancy less frequently had a baby with a birthweight below 2500 g than those women receiving no treatment or placebo; but the statistical significance was borderline (9.6% versus 19.6%; average risk ratio (RR) 0.48; 95% CI 0.23 to 1.01) (Analysis 1.8). There was some variation among trials in terms of the size of the treatment effect (T2 = 0.23, I2 = 53% and ChI2 test for heterogeneity P < 0.012).
Maternal secondary outcomes
Adverse side effects (nephritic syndrome)
A single study including 135 women reported on this outcome (Yu 2008). The data from this trial suggest that the women receiving vitamin D supplementation were as likely to report nephritic syndrome as a side effect than women who did not receive supplementation or placebo (RR 0.17; 95% CI 0.01 to 4.06) (Analysis 1.12) but given the scarcity of data for this outcome and the wide CIs, no firm conclusions can be drawn.
No trials reported on our other pre-specified maternal secondary outcomes: impaired glucose tolerance (as defined by trialists); caesarean section; gestational hypertension (as defined by trialists) or maternal death.
Infant secondary outcomes
Length at birth (cm)
The data from two trials (Brooke 1980; Marya 1988) involving 326 women suggest that infants from women who take vitamin D supplementation during pregnancy have similar length at birth than infants from women taking no treatment or placebo (MD 0.97 cm; 95% CI −0.41 to 2.34 cm) (Analysis 1.14).
Head circumference at birth (cm)
Two trials involving 326 women (Brooke 1980; Marya 1988) reported on this anthropometric measurement. Results suggest that children born to women who received vitamin D supplements during pregnancy have a larger head circumference at birth than infants born to women who did not receive vitamin D supplements (MD 0.43 cm; 95% CI 0.06 to 0.79 cm) (Analysis 1.15). There was some variation among trials in terms of the size of the treatment effect but not in the direction of the effect (T2 = 0.04, I2 = 50% and ChI2 test for heterogeneity P < 0.16).
Birthweight (g)
Three trials involving 403 women (Brooke 1980; Mallet 1986; Marya 1988) reported on this outcome. Results suggest that there was no difference of weight at birth of infants from women who received vitamin D supplements in comparison with women who did not receive vitamin D supplements (MD 39.55 g; 95% CI −240.68 to 319.78 g) (Analysis 1.16). There was some substantial heterogeneity among trials in terms of the size of the treatment (T2 = 58118.23, I2 = 96% and ChI2 test for heterogeneity P < 0.00001).
Stillbirth (as defined by trialists)
A single study (Yu 2008) including 135 women reported this outcome. The data from this trial suggest that the women receiving vitamin D supplementation are as likely to have a stillbirth as women who do not receive supplementation or placebo (RR 0.17; 95% CI 0.01 to 4.06) (Analysis 1.18) but given the scarcity of data for this outcome no firm conclusions can be drawn.
Neonatal death (as defined by trialists)
A single study (Yu 2008) including 135 women reported this outcome. The data from this trial suggest that the neonates from women receiving vitamin D supplementation are as likely to die during the neonatal period as the neonates from women who do not receive supplementation or placebo (RR 0.17; 95% CI 0.01 to 4.06) (Analysis 1.19) but given the scarcity of data for this outcome no firm conclusions can be drawn.
No trials reported on our other pre-specified infant secondary outcomes: admission to intensive care unit during the neonatal period; Apgar score less than seven at five minutes; neonatal infection (e.g. respiratory infections) or very preterm birth (less than 34 weeks' gestation).
(2) Vitamin D + calcium versus no treatment/placebo (no vitamin or minerals) (one study, 400 participants)
Maternal primary outcomes
Pre-eclampsia (as defined by trialists)
A single study (Marya 1987) including 400 women reported on this outcome. The data from this trial suggest that women receiving vitamin D and calcium supplementation combined are as likely to have pre-eclampsia as women who do not receive supplementation or placebo (RR 0.67; 95% CI 0.33 to 1.35) (Analysis 2.1) but given the scarcity of data for this outcome no firm conclusions can be drawn.
Gestational diabetes (as defined by trialists)
No studies reported on this outcome.
Infant primary outcomes
Preterm birth (less than 37 weeks' gestation)
No studies reported on this outcome.
Low birthweight (less than 2500 g)
No studies reported on this outcome.
Maternal vitamin D levels at term (25-hydroxyvitamin D in nmol/L)
No studies reported on this outcome.
Maternal secondary outcomes
No trials reported on our pre-specified maternal secondary outcomes: impaired glucose tolerance (as defined by trialists); caesarean section; gestational hypertension (as defined by trialists); side effects (e.g. hypercalcaemia, kidney stones) or maternal death.
Infant secondary outcomes
No trials reported on our pre-specified infant secondary outcomes: length at birth (cm); head circumference at birth (cm); weight at birth (g); admission to intensive care unit during the neonatal period; stillbirths (as defined by trialists); neonatal death (as defined by trialists); Apgar score less than seven at five minutes; neonatal infection (e.g. respiratory infections) or very preterm birth (less than 34 weeks' gestation).
(3) Vitamin D + calcium versus calcium (but no vitamin D) (no studies)
No studies were included in this comparison.
(4) Vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D) (no studies)
No studies were included in this comparison.
Subgroup analysis
We attempted to conduct a subgroup analysis but in all the outcomes very few studies contributed data. Indeed, for several subgroups all the trials were in the same subgroup category or only one trial was allocated to one of the subgroup categories impeding any judgements.
As more data become available, in updates of the review, we hope to explore possible subgroup differences by carrying out both visual exploration and formal statistical tests.
Discussion
Summary of main results
This review evaluates the effects of vitamin D supplementation alone or in combination with calcium and other vitamins and minerals during pregnancy. It includes six small trials (1023 women), five of which compared vitamin D alone versus no treatment or placebo while one trial provided vitamin D plus calcium in comparison with no treatment. No studies evaluated the effects of vitamin D plus calcium versus calcium nor vitamin D plus calcium and other micronutrients in comparison with other micronutrients (but not vitamin D).
In comparison with the group that received no intervention or a placebo:
vitamin D supplementation during pregnancy did not have significant effects on length and weight at birth. There was a trend to decrease the incidence of low birthweight babies by a half in the vitamin D supplemented group, although the non statistical significance was borderline;
women supplemented with vitamin D during pregnancy had significantly higher concentrations of 25-hydroxyvitamin D at the end of pregnancy. Children born to women who received vitamin D supplements during pregnancy had a larger head circumference at birth than infants born to women who did not receive vitamin D supplements; however, given that only two studies reported on this outcome, this result should be interpreted cautiously.
Overall completeness and applicability of evidence
Vitamin D supplementation during pregnancy aims to improve gestational and neonatal outcomes. However, the scarcity of data was evident, not only from the limited number of trials, but also from the small number of outcomes evaluated. Numerous maternal outcomes (pre-eclampsia, gestational diabetes, impaired glucose tolerance, caesarean section, gestational hypertension, side effects or death) and infant outcomes (preterm birth, stillbirth, neonatal death, admission to intensive care unit during the neonatal period, Apgar score less than seven at five minutes, neonatal infection or very preterm birth) were either not reported or reported only by one trial
Vitamin D supplementation raised the serum concentration of 25-hydroxyvitamin D at the end of pregnancy. However, the clinical significance of this finding still needs to be demonstrated as vitamin D supplementation did not have a clear protective effect on the few maternal and infant outcomes reported in this review.
To our best knowledge there are currently 10 ongoing studies that, once published, will double the body of evidence identified for this review. After their publication and overall assessment, conclusions on the effects and safety of this intervention may be updated.
Quality of the evidence
The methodological quality of five out of the six trials included in this review is poor after considering the methods for allocating the treatment, the blinding and the attrition rates, with many studies being at high risk of bias (see Risk of bias in included studies). In most of the included trials, the methods used to randomly assign participants and conceal allocation were not described. Blinding of participants, care providers and outcome assessors was not generally attempted. Attrition was also a problem in most of the studies.
We evaluated the quality of the body of evidence for the primary outcomes with the GRADE methodology for the first two comparisons (Summary of findings table 1 and Summary of findings table 2). We considered that indirectness or publication bias were unlikely but the poor quality of the trials, the inconsistency (or the lack of studies), and the imprecision resulted in evidence of low quality for low birthweight and maternal vitamin D concentrations and of very low quality for pre-eclampsia.
Potential biases in the review process
We identified several potential biases in the review process. They were minimised in two ways: (1) eligibility for inclusion and data extraction was assessed independently by two review authors and (2) assessments of risk of bias and data entry were also assessed independently by two review authors. However, this type of review requires that we make a number of subjective judgments and others may have reached different decisions regarding assessments of eligibility and risk of bias. We would encourage readers to examine the Characteristics of included studies tables to assist in the interpretation of results.
Agreements and disagreements with other studies or reviews
This review updates the previous Cochrane review on vitamin D supplementation in pregnancy (Mahomed 1999). The previous review included two trials and assessed the following infant outcomes: low birthweight, neonatal hypocalcaemia, craniotabes (softening of the skull) and perinatal mortality. The authors concluded that there was inadequate information about vitamin D supplementation safety due to the lack of information. The findings of the present review are similar, in that there are insufficient data to address the effects of vitamin D on the pre-specified maternal and infant health outcomes.
The Food and Nutrition Board from the US Institute of Medicine conducted a narrative systematic review of randomised and observational studies in order to update the Dietary References Intakes (DRI) values for vitamin D and calcium. The review aimed to assess both the individual and combined effect of these nutrients on a wide range of health outcomes including some pregnancy-related (i.e. pre-eclampsia, pregnancy-induced hypertension, and other non-skeletal reproductive outcomes such as cesarean section, obstructed labor and vaginosis) (Chung 2009; Institute of Medicine 2010). Overall, the findings are in agreement with our review. No placebo-controlled RCTs were identified that examined a causal relationship between vitamin D and preeclampsia or pregnancy-induced hypertension and two observational studies identified associations between supplementary vitamin D and incidence of preeclampsia, but data on associations between serum 25OHD level and preeclampsia were not conclusive. Additionally, authors found that the available evidence for non-skeletal outcomes from three randomised controlled trials and observational studies was limited and conflicting, precluding the ability to use these data to support that pregnant women need an additional intake of vitamin D intake in comparison with other age groups.
Authors' conclusions
Implications for practice
The use of vitamin D supplements during pregnancy improves vitamin D concentrations as measured by 25-hydroxyvitamin D at term. However, the clinical significance of this finding is yet to be determined as there is currently insufficient high quality evidence relating to the clinical effects of vitamin D supplementation during pregnancy.
Good quality studies are needed to determine the usefulness and feasibility of this intervention as a part of routine antenatal care.
Implications for research
Further rigorous randomised trials are required to evaluate the role of vitamin D supplementation in pregnancy. Future research should evaluate if an increase of serum 25-hydroxyvitamin D concentration is associated with improved maternal and infant outcomes in populations with different degrees of body mass index, skin pigmentation and settings. Information on the most effective and safe dosage; supplementation regimen (daily, intermittent or single doses), the timing of initiation of vitamin D supplementation, and the effect of vitamin D when combined with other vitamins and minerals are also needed to inform policy-making.
Supplementary Material
Acknowledgements
The World Health Organization retains copyright and all other rights in the manuscript of this review as submitted for publication, including any revisions or updates to the manuscript which WHO may make from time to time.
As part of the pre-publication editorial process, this protocol has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group’s international panel of consumers and the Group’s Statistical Adviser.
Sources of support
Internal sources
Micronutrients Unit, Department of Nutrition for Health and Development, World Health Organization, Switzerland
Programa de Nutricion, Escuela Graduada de Salud Publica, Universidad de Puerto Rico, Puerto Rico
External sources
-
Micronutrient Initiative (MI), Canada
WHO acknowledges Micronutrient Initiative (MI) for their financial support to the Micronutrients Unit for conducting systematic reviews on micronutrients interventions.
Appendices
1 Search terms used for additional author searching
Authors searched he WHO International Clinical Trials Registry Platform (ICTRP) for any ongoing or planned trials and the Networked Digital Library of Theses and Dissertations (NDLTD) for grey literature on 28 October 2011 using the terms "vitamin D supplementation and pregnancy".
Graphs

Footnotes
Contributions of authors
Ali Ansary prepared a draft of the protocol during an internship with the Micronutrients Unit, Department of Nutrition for Health and Development in the World Health Organization. The other review authors commented and provided extensive feedback. All review authors discussed the document and provided edits and references. Luz Maria De-Regil and Cristina Palacios evaluated the references for eligibility. All authors extracted data from the included trials. All contributed to the preparation of the review.
Disclaimer: Luz Maria De-Regil, Regina Kullier and Juan Pablo Pena-Rosas are currently staff members of the World Health Organization. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of the World Health Organization.
Declarations of interest
We certify that we have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of the review (e.g. employment, consultancy, stock ownership, honoraria, expert testimony).
Differences between protocol and review
- Types of outcome measures: we moved "maternal vitamin D concentrations at the end of pregnancy" from secondary to primary outcomes.
- Subgroup analysis: In addition to the visually examination of the forest plots, we decided to use Borenstein 2008's approach to formally investigate differences between two or more subgroups. We specified that analyses were conducted in Revman version 5.1.1 (RevMan 2011).
- Originally we intended to include randomised crossover trials (their first period), but we decided not to include them as this type of study design is considered inappropriate for the topic under investigation.
References to studies
Included studies
- *.Brooke OG, Brown IRF, Bone CDM, Carter ND, Cleeve HJW, Maxwell JD, et al. Vitamin D supplements in pregnant Asian women: effects on calcium status and fetal growth. British Medical Journal. 1980;1:751–754. doi: 10.1136/bmj.280.6216.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke OG, Butters F, Wood C. Intrauterine vitamin D nutrition and postnatal growth in Asian infants. British Medical Journal. 1981;283:1024. doi: 10.1136/bmj.283.6298.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell JD, Ang L, Brooke OG, Brown IRF. Vitamin D supplements enhance weight gain and nutritional status in pregnant Asians. British Journal of Obstetrics and Gynaecology. 1981;88:987–991. doi: 10.1111/j.1471-0528.1981.tb01686.x. [DOI] [PubMed] [Google Scholar]
- Delvin EE, Salle BL, Glorieux FH, Adeleine P, David LS. Vitamin D supplementation during pregnancy: effect on neonatal calcium homeostasis. Journal of Pediatrics. 1986;109:328–334. doi: 10.1016/s0022-3476(86)80396-1. [DOI] [PubMed] [Google Scholar]
- Mallet E, Gugi B, Brunelle P, Henocq A, Basuyau JP, Lemeur H. Vitamin D supplementation in pregnancy: a controlled trial of two methods. Obstetrics & Gynecology. 1986;68:300–304. doi: 10.1097/00006250-198609000-00002. [DOI] [PubMed] [Google Scholar]
- Marya RK, Rathee S, Manrow M. Effect of calcium and vitamin D supplementation on toxaemia of pregnancy. Gynecologic and Obstetric Investigation. 1987;24(1):38–42. doi: 10.1159/000298772. [DOI] [PubMed] [Google Scholar]
- Marya RK, Rathee S, Dua V, Sangwan K. Effect of vitamin D supplementation during pregnancy on foetal growth. Indian Journal of Medical Research. 1988;88:488–492. [PubMed] [Google Scholar]
- Yu C, Newton L, Robinson S, Teoh TG, Sethi M. Vitamin D deficiency and supplementation in pregnant women of four ethnic groups. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2008;93(Suppl 1):Fa68. [Google Scholar]
- Yu CK, Sykes L, Sethi M, Teoh TG, Robinson S. Vitamin D deficiency and supplementation during pregnancy. Clinical Endocrinology. 2009;70(5):685–690. doi: 10.1111/j.1365-2265.2008.03403.x. [DOI] [PubMed] [Google Scholar]
Excluded studies
- Ala-Houhala M, Koskinen T, Terho A, Koivula T, Visakorpi J. Maternal compared with infant vitamin D supplementation. Archives of Disease in Childhood. 1986;61:1159–1163. doi: 10.1136/adc.61.12.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn F, Belton NR, Purvis RJ, Giles MM, Brown JK, Turner TL, et al. Maternal vitamin D intake and mineral metabolism in mothers and their newborn infants. British Medical Journal. 1980;281(6232):11–14. doi: 10.1136/bmj.281.6232.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia V. A study of the effect of vitamin D supplementation in pregnant women, on the growth and biochemical features of the newborn baby and infant. [accessed 2010];Clinical Trials Registry - India. ( http://ctri.nic.in/Clinicaltrials/login.php)
- *.Das V, Agarwal A, Bhatia V, Pandey A, Agarwal S, Saxena P, et al. Evaluation of vitamin d status and need for supplementation in pregnant women of a rural area of North India. International Journal of Gynecology & Obstetrics. 2009;107(Suppl 2):S151. [Google Scholar]
- Sahu M, Das V, Aggarwal A, Rawat V, Saxena P, Bhatia V. Vitamin D replacement in pregnant women in rural north India: a pilot study. European Journal of Clinical Nutrition. 2009;63(9):1157–1159. doi: 10.1038/ejcn.2009.27. [DOI] [PubMed] [Google Scholar]
- Ito M, Koyama H, Ohshige A, Maeda T, Yoshimura T, Okamura H. Prevention of preeclampsia with calcium supplementation and vitamin D3 in an antenatal protocol. International Journal of Gynecology & Obstetrics. 1994;47(2):115–120. doi: 10.1016/0020-7292(94)90350-6. [DOI] [PubMed] [Google Scholar]
- MacDonald HN. Fetal and maternal benefits from calcium and vitamin D supplementation of pregnant Asians. Personal communication. 1986 [Google Scholar]
- Marya RK, Rathee S, Lata V, Mudgil S. Effects of vitamin D supplementation in pregnancy. Gynecologic and Obstetric Investigation. 1981;12:155–161. doi: 10.1159/000299597. [DOI] [PubMed] [Google Scholar]
- von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient - a randomised, placebo-controlled trial. British Journal of Nutrition. 2010;103(4):549–555. doi: 10.1017/S0007114509992017. [DOI] [PubMed] [Google Scholar]
- von Hurst PR, Stonehouse W, Matthys C, Conlon C, Kruger MC, Coad J. Study protocol--metabolic syndrome, vitamin D and bone status in South Asian women living in Auckland, New Zealand: a randomised, placebo-controlled, double-blind vitamin D intervention. BMC Public Health. 2008;8:267. doi: 10.1186/1471-2458-8-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.von Hurst PR. A thesis presented in partial fulfilment of the requirements for the degree of doctor of philosophy in nutritional science. Vol. 1. Albany: Massey University; 2009. The role of vitamin D in metabolism and bone health. Massey University, Albany New Zealand. [Google Scholar]
Published data only (unpublished sought but not used)
- Appelgren KE, Nietert PJ, Hulsey TC, Hollis BW, Wagner CL. Analyzing adherence to prenatal supplement: does pill count measure up? International Journal of Endocrinology. 2010;2010:1–8. doi: 10.1155/2010/631971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: Double-blind, randomized clinical trial of safety and effectiveness. Journal of Bone and Mineral Research. 2011;26(10):2341–2357. doi: 10.1002/jbmr.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Wagner CL, Johnson D, Hulsey TC, Ebeling M, Shary J, Smith PG, et al. Pediatric Academic Societies Annual Meeting. Canada: Vancouver; 2010. May 1–4, Vitamin D supplementation during pregnancy Part I NICHD/CTSA randomized clinical trial (RCT): safety consideration; p. 2010. [Google Scholar]
- Wagner CL, McNeil R, Hamilton SA, Davis DJ, Prudgen C, Winkler J, et al. Pediatric Academic Societies 2010 Annual Meeting. Canada: Vancouver; 2010. May 1–4, Vitamin D (vitD) supplementation during pregnancy: Thrasher Research Fund RCT in SC community center networks; p. 2010. [Google Scholar]
- Wagner CL. Evaluation of vitamin D requirements during pregnancy (ongoing trial) [accessed 21 March 2006];ClinicalTrials.gov. ( http://clinicaltrials.gov/)
Studies awaiting classification
Ongoing studies
- Bisgaard H. Vitamin D supplementation during pregnancy for prevention of asthma in childhood (ABCvitaminD) [accessed 31 July 2009];ClinicalTrials.gov. ( http://clinicaltrials.gov)
- Das V. Vitamin D and calcium nutrition in pregnancy-evaluation of optimal supplementation dose of vitamin D during antenatal period. [accessed 2010];Clinical Trials Registry - India. ( http://ctri.nic.in/Clinicaltrials/login.php) [Google Scholar]
- Goldring S. Effects of prenatal vitamin D supplementation on respiratory and allergic phenotypes and bone density in the first three years of life. [accessed 6 April 2011];UKCRN. ( http://public.ukcrn.org.uk) [Google Scholar]
- Grant C. Randomised placebo controlled study of vitamin D during pregnancy and infancy. [accessed 17 August 2010];Australian New Zealand Clinical Trials Register. ( www.anzctr.org.au) [Google Scholar]
- Habib MA. Evaluation of the effectiveness of vitamin D supplementation to pregnant women and their infants in Pakistan. [accessed 15 February 2011];ClinicalTrials.gov. ( http://clinicaltrials.gov/)
- Hacker AN. Bone Health in Pregnancy (B-Hip) [accessed 2010];ClinicalTrials.gov. ( http://clinicaltrials.gov)
- Judkins A. A randomised double blinded interventional trial to determine the effect of 50,000 IU vitamin D supplementation monthly or twice monthly from 20 weeks gestation. ACTRN12610001044011. 2011 [Google Scholar]
- Rasmussen G. Additional information on registered trial. Effects of vitamin D supplement before, during and after pregnancy on complications, birth weight and bone mineral density during lactation (gravita) Personal communication. 2011 [Google Scholar]
- Rasmussen GB. Effects of vitamin D supplement before and during pregnancy on birth weight (gravita) [accessed 17 August 2010];ClinicalTrials.gov. ( http://clinicaltrials.gov/) [Google Scholar]
- Roth D. Antenatal vitamin D3 supplementation in Bangladesh: randomized controlled trial (AViDD-2) [accessed 6 April 2011];ClinicalTrials.gov. ( http://clinicaltrials.gov) [Google Scholar]
- Soheilykhah S. Effect of different doses of vitamin D on insulin resistance in pregnant women attending in Shahid Sadoughi and Mojibian prenatal clinics. Iranian registry of clinical trials. 2011 [Google Scholar]
Other references
Additional references
- Akcakus M, Koklu E, Budak N, Kula M, Kurtoglu S, Koklu S. The relationship between birthweight, 25-hydroxyvitamin D concentrations and bone mineral status in neonates. Annals of Tropical Paediatrics. 2006;26(4):267–275. doi: 10.1179/146532806X152782. [DOI] [PubMed] [Google Scholar]
- Alfaham M, Woodhead S, Pask G, Davies D. Vitamin D deficiency: a concern in pregnant Asian women. British Journal of Nutrition. 1995;73(6):881–887. doi: 10.1079/bjn19950093. [DOI] [PubMed] [Google Scholar]
- Ariyuki F. Growth retardation induced in rat fetuses by maternal fasting and massive doses of ergocalciferol. Journal of nutrition. 1987;117(2):342–348. doi: 10.1093/jn/117.2.342. [DOI] [PubMed] [Google Scholar]
- Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. Journal of Clinical Endocrinology and Metabolism. 2004;89(11):5387–5391. doi: 10.1210/jc.2004-0360. [DOI] [PubMed] [Google Scholar]
- Arunabh S, Pollack S, Yeh J, Aloia JF. Body fat content and 25-hydroxyvitamin D levels in healthy women. Journal of Clinical Endocrinology and Metabolism. 2003;88(1):157–161. doi: 10.1210/jc.2002-020978. [DOI] [PubMed] [Google Scholar]
- August P, Marcaccio B, Gertner JM, Druzin ML, Resnick LM, Laragh JH. Abnormal 1,25-dihydroxyvitamin D metabolism in preeclampsia. American Journal of Obstetrics and Gynecology. 1992;166(4):1295–1299. doi: 10.1016/s0002-9378(11)90625-5. [DOI] [PubMed] [Google Scholar]
- Balshem H, Helfanda M, Schunemann HJ, Oxmand AD, Kunze R, Brozek J, et al. GRADE guidelines: rating the quality of evidence: introduction. Journal of Clinical Epidemiology. 2010;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Bandeira F, Griz L, Dreyer P, Eufrazino C, Bandeira C, Freese E. Vitamin D deficiency: a global perspective [Deficiência de vitamina D: uma perspectiva global] Arquivos Brasileiros de Endocrinologia e Metabologia. 2006;50(4):640–646. doi: 10.1590/s0004-27302006000400009. [DOI] [PubMed] [Google Scholar]
- Bener A, Alsaied A, Al-Ali M, Al-Kubaisi A, Basha B, Abraham A, et al. High prevalence of vitamin D deficiency in type 1 diabetes mellitus and healthy children. Acta Diabetologica. 2009;4(2):183–189. doi: 10.1007/s00592-008-0071-6. [DOI] [PubMed] [Google Scholar]
- Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. Journal of Clinical Endocrinology and Metabolism. 2007;92(9):3517–3522. doi: 10.1210/jc.2007-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar LM, Catov JM, Zmuda JM, Cooper ME, Parrott MS, Roberts JM, et al. Maternal serum 25-hydroxyvitamin D concentrations are associated with small-for-gestational age births in white women. Journal of Nutrition. 2010;140(9):999–1006. doi: 10.3945/jn.109.119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis (Statistics in Practice) Chichester (UK): John Wiley & Sons; 2008. [Google Scholar]
- Butte NF, Lopez-Alarcon MG, Garza C. Nutrient adequacy of exclusive breastfeeding of the term infant during the first six months of life. Geneva: World Health Organization; 2002. [Google Scholar]
- Canadian Paediatric Society, First Nations, Inuit and Métis Health Committee. Vitamin D supplementation: recommendations for Canadian mothers and infants. Paediatrics & Child Health. 2007;12(7):583–598. [PMC free article] [PubMed] [Google Scholar]
- Cantorna MT, Yu S, Bruce D. The paradoxical effects of vitamin D on type 1 mediated immunity. Molecular Aspects of Medicine. 2008;29:369–375. doi: 10.1016/j.mam.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardus A, Parisi E, Gallego C, Aldea M, Fernandez E, Valdivielso JM. 1,25-Dihydroxyvitamin D3 stimulates vascular smooth muscle cell proliferation through a VEGF-mediated pathway. Kidney International. 2006;69:1377–1384. doi: 10.1038/sj.ki.5000304. [DOI] [PubMed] [Google Scholar]
- Chan GM, Buchino JJ, Mehlhorn D, Bove KE, Steichen JJ, Tsang RC. Effect of vitamin D on pregnant rabbits and their offspring. Pediatric Research. 1979;13(2):121–126. doi: 10.1203/00006450-197902000-00007. [DOI] [PubMed] [Google Scholar]
- Chocano-Bedoya P, Ronnenberg AG. Vitamin D and tuberculosis. Nutrition Reviews. 2009;67(5):289–293. doi: 10.1111/j.1753-4887.2009.00195.x. [DOI] [PubMed] [Google Scholar]
- Christian P, Khatry SK, Katz J, Pradhan EK, LeClerq SC, Shrestha SR, et al. Effects of alternative maternal micronutrient supplements on low birth weight in rural Nepal: double blind randomised community trial. BMJ. 2003;326(7389):571. doi: 10.1136/bmj.326.7389.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung M, Balk EM, Brendel M, Ip S, Lau J, Lee J, et al. (prepared by Tufts Evidence-based Practice Center under Contract No. 290-2007-10055-I) Rockville, MD: Agency for Healthcare Research and Quality; 2009. Vitamin D and calcium: systematic review of health outcomes. Evidence report/technology assessment No. 183. Vol. Publication No. 09-E015 ( http://www.ahrq.gov/downloads/pub/evidence/pdf/vitadcal/vitadcal.pdf) [Google Scholar]
- Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;319:74–76. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- Clifton-Bligh RJ, McElduff P, McElduff A. Maternal vitamin D deficiency, ethnicity and gestational diabetes. Diabetic Medicine. 2008;25(6):678–684. doi: 10.1111/j.1464-5491.2008.02422.x. [DOI] [PubMed] [Google Scholar]
- Davis LM, Chang SC, Mancini J, Nathanson MS, Witter FR, O'Brien KO. Vitamin D insufficiency is prevalent among pregnant African American adolescents. Journal of Pediatric and Adolescent Gynecology. 2010;23(1):45–52. doi: 10.1016/j.jpag.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Dawodu A, Agarwal M, Sankarankutty M, Hardy D, Kochiyil J, Badrinath P. Higher prevalence of vitamin D deficiency in mothers of rachitic than nonrachitic children. Journal of Pediatrics. 2005;147(1):109–111. doi: 10.1016/j.jpeds.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Dawodu A, Nath R. High prevalence of moderately severe vitamin D deficiency in pre-term infants. Pediatrics International. 2011;53(2):207–210. doi: 10.1111/j.1442-200X.2010.03209.x. [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporosis International. 2005;16:713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B. Serum 25-hydroxyvitamin D and functional outcomes in the elderly. American Journal of Clinical Nutrition. 2008;88(2):527S–540S. doi: 10.1093/ajcn/88.2.537S. [DOI] [PubMed] [Google Scholar]
- DeLuca HF. Overview of general physiologic features and functions of vitamin D. American Journal of Clinical Nutrition. 2004;80(6 Suppl):1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- Devereux G, Litonjua AA, Turner SW, Craig LC, McNeill G, Martindale S, et al. Maternal vitamin D intake during pregnancy and early childhood wheezing. American Journal of Clinical Nutrition. 2007;85(3):853–859. doi: 10.1093/ajcn/85.3.853. [DOI] [PubMed] [Google Scholar]
- Diaz L, Arranz C, Avila E, Halhai A, Vilchis F, Larrae F. Expression and activity of 25-hydroxyvitamin D-1 alpha-hydroxylase are restricted in cultures of human syncytiotrophoblast cells from preeclamptic pregnancies. Journal of Clinical Endocrinology and Metabolism. 2002;87(8):3876–3882. doi: 10.1210/jcem.87.8.8730. [DOI] [PubMed] [Google Scholar]
- Egan KM, Signorello LB, Munro HM, Hargreaves MK, Hollis BW, Blot WJ. Vitamin D insufficiency among African-Americans in the southeastern United States: implications for cancer disparities (United States) Cancer Causes and Control. 2008;19(5):527–535. doi: 10.1007/s10552-008-9115-z. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans KN, Bulmer JN, Kilby MD, Hewison M. Vitamin D and placental-decidual function. Journal of the Society for Gynecologic Investigation. 2004;11(5):263–271. doi: 10.1016/j.jsgi.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Farrant HJ, Krishnaveni GV, Hill JC, Boucher BJ, Fisher DJ, Noonan K, et al. Vitamin D insufficiency is common in Indian mothers but is not associated with gestational diabetes or variation in newborn size. European Journal of Clinical Nutrition. 2008;63(5):646–652. doi: 10.1038/ejcn.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant HJ, Krishnaveni GV, Hill JC, Boucher BJ, Fisher DJ, Noonan K, et al. Vitamin D insufficiency is common in Indian mothers but is not associated with gestational diabetes or variation in newborn size. European Journal of Clinical Nutrition. 2009;63(5):646–652. doi: 10.1038/ejcn.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Archives of Dermatology. 1988;124(6):869–873. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- Ford JA, Davidson DC, McIntosh WB, Fyfe WM, Dunnigan MG. Neonatal rickets in Asian immigrant population. BMJ. 1973;3(5873):211–212. doi: 10.1136/bmj.3.5873.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel Y, Barkai G, Mashiach S, Dolev E, Zimlichmann R, Weiss M. Hypocalciuria of preeclampsia is independent of parathyroid hormone level. Obstetrics & Gynecology. 1991;77:822–825. [PubMed] [Google Scholar]
- Friedman WF, Mills LF. The relationship between vitamin D and the craniofacial and dental anomalies of the supravalvular aortic stenosis syndrome. Pediatrics. 1969;43(1):12–18. [PubMed] [Google Scholar]
- El-Hajj Fuleihan G, Nabulsi M, Choucair M, Salamoun M, Hajj Shahine C, Kizirian A, et al. Hypovitaminosis D in healthy schoolchildren. Pediatrics. 2001;107(4):E54. doi: 10.1542/peds.107.4.e53. [DOI] [PubMed] [Google Scholar]
- Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, et al. Maternal vitamin D status during pregnancy and child outcomes. European Journal of Clinical Nutrition. 2008;62(1):68–77. doi: 10.1038/sj.ejcn.1602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrest BA. Sun exposure and vitamin D sufficiency. American Journal of Clinical Nutrition. 2008;88:570S–577S. doi: 10.1093/ajcn/88.2.570S. [DOI] [PubMed] [Google Scholar]
- Glerup H, Mikkelsen K, Poulsen L, Hass E, Overbeck S, Andersen H, et al. Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcified Tissue International. 2000;66(6):419–424. doi: 10.1007/s002230010085. [DOI] [PubMed] [Google Scholar]
- Gordon CM, DePeter KC, Estherann G, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Archives of Pediatrics and Adolescent Medicine. 2004;158(6):531–537. doi: 10.1001/archpedi.158.6.531. [DOI] [PubMed] [Google Scholar]
- Version 3.2 for Windows. Jan Brozek, Andrew Oxman, Holger Schünemann. GRADEpro; 2008. GRADEpro [Computer program] [Google Scholar]
- Grover SR, Morley R. Vitamin D deficiency in veiled or dark-skinned pregnant women. Medical Journal of Australia. 2001;175(5):251–252. doi: 10.5694/j.1326-5377.2001.tb143558.x. [DOI] [PubMed] [Google Scholar]
- Halhali A, Bourgues H, Carrillo A, Garabedian M. Lower circulating insulin-like growth factor I and 1,25-dihydroxyvitamin D levels in preeclampsia. Revista de Investigacion Clinica. 1995;47(4):259–266. [PubMed] [Google Scholar]
- Halhali A, Tovar AR, Torres N, Bourgues H, Garabedian M, Larrae F. Preeclampsia is associated with low circulating levels of insulin-like growth factor I and 1,25-dihydroxyvitamin D in maternal and umbilical cord compartments. Journal of Clinical Endocrinology and Metabolism. 2000;85(5):1828–1833. doi: 10.1210/jcem.85.5.6528. [DOI] [PubMed] [Google Scholar]
- Hall WB, Sparks AA, Aris RM. Vitamin D deficiency in cystic fibrosis. International Journal of Endocrinology. 2010 doi: 10.1155/2010/218691. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. American Journal of Clinical Nutrition. 2007;85(1):6–18. doi: 10.1093/ajcn/85.1.6. [DOI] [PubMed] [Google Scholar]
- Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. American Journal of Clinical Nutrition. 2003;77(1):204–210. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- Heaney RP. Vitamin D: criteria for safety and efficacy. Nutrition Reviews. 2008;66(10 Suppl 2):S178–S181. doi: 10.1111/j.1753-4887.2008.00102.x. [DOI] [PubMed] [Google Scholar]
- Hewison M. Vitamin D and the immune system. Journal of Endocrinology. 1992;132(2):173–176. doi: 10.1677/joe.0.1320173. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editors. The Cochrane Collaboration. 2009. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. [updated March 2011] Available from www.cochrane-handbook.org. [Google Scholar]
- Holick MF. Too little vitamin D in premenopausal women: why should we care? American Society for Clinical Nutrition. 2002;76(1):3–4. doi: 10.1093/ajcn/76.1.3. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D deficiency. New England Journal of Medicine. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: a D-lightful story. Journal of Bone and Mineral Research. 2007;22(Suppl 2):V28–V33. doi: 10.1359/jbmr.07s211. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D deficiency: a worldwide problem with health consequences. American Journal of Clinical Nutrition. 2008;87(4):1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D status: measurement, interpretation and clinical application. Annals of Epidemiology. 2009;19:73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis BW, Wagner CL. Vitamin D requirements during lactation: high-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. American Journal of Clinical Nutrition. 2004;80(6 Suppl):1752S–1178S. doi: 10.1093/ajcn/80.6.1752S. [DOI] [PubMed] [Google Scholar]
- Hollis B. Vitamin D requirement during pregnancy and lactation. Journal of Bone and Mineral Research. 2007;22(Suppl 2):V39–V44. doi: 10.1359/jbmr.07s215. [DOI] [PubMed] [Google Scholar]
- Holmes VA, Barnes MS, Alexander HD, McFaul P, Wallace JM. Vitamin D deficiency and insufficiency in pregnant women: a longitudinal study. British Journal of Nutrition. 2009;102(6):876–881. doi: 10.1017/S0007114509297236. [DOI] [PubMed] [Google Scholar]
- Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington DC: National Academy Press; 2010. [Google Scholar]
- Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, et al. Princess Anne Hospital Study Group. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367:36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- Jones G. Pharmacokinetics of vitamin D toxicity. American Journal of Clinical Nutrition. 2008;88(2):582S–586S. doi: 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- Kazemi A, Sharifi F, Jafari N, Mousavinasab N. High prevalence of vitamin D deficiency among pregnant women and their newborns in an Iranian population. Journal of Women's Health. 2009;18(6):835–839. doi: 10.1089/jwh.2008.0954. [DOI] [PubMed] [Google Scholar]
- Levis S, Gomez A, Jimenez C, Veras L, Ma F, Lai S, et al. Vitamin D deficiency and seasonal variation in an adult south Florida population. Journal of Clinical Endocrinology and Metabolism. 2005;90(3):1557–1562. doi: 10.1210/jc.2004-0746. [DOI] [PubMed] [Google Scholar]
- Li DK, Wi S. Maternal pre-eclampsia/eclampsia and the risk of sudden infant death syndrome in offspring. Paediatric and Perinatal Epidemiology. 2000;14(2):141–144. doi: 10.1046/j.1365-3016.2000.00245.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Kong J, Wei M, Chen ZF, Liu S, Cao LP. 1,25-Dihydroxyvitamin D3 is a negative endocrine regulator of the reninangiotensin system. Journal of Clinical Investigation. 2002;110(2):229–239. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocrine Reviews. 2001;22(4):477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- Litonjua AA. Childhood asthma may be a consequence of vitamin D deficiency. Current opinion in allergy and clinical immunology. 2009;9(3):202–207. doi: 10.1097/ACI.0b013e32832b36cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstetrics & Gynecology. 2001;97(4):533–538. doi: 10.1016/s0029-7844(00)01223-0. [DOI] [PubMed] [Google Scholar]
- Madar AA, Stene LC, Meyer HE. Vitamin D status among immigrant mothers from Pakistan, Turkey and Somalia and their infants attending child health clinics in Norway. British Journal of Nutrition. 2009;101(7):1052–1058. doi: 10.1017/S0007114508055712. [DOI] [PubMed] [Google Scholar]
- Maghbooli Z, Hossein-Nezhad A, Shafaei AR, Karimi F, Madani FS, Larijani B. Vitamin D status in mothers and their newborns in Iran. BMC Pregnancy and Childbith. 2007;7:1. doi: 10.1186/1471-2393-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghbooli Z, Hossein-Nezhad A, Karimi F, Shafaei AR, Larijani B. Correlation between vitamin D3 deficiency and insulin resistance in pregnancy. Diabetes/Metabolism Research and Reviews. 2008;24(1):27–32. doi: 10.1002/dmrr.737. [DOI] [PubMed] [Google Scholar]
- Mahon P, Harvey N, Crozier S, Inskip H, Robinson S, Arden N, et al. Low maternal vitamin D status and fetal bone development: cohort study. Journal of Bone and Mineral Research. 2010;25(1):14–19. doi: 10.1359/jbmr.090701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwaha RK, Tandon N, Reddy DR, Aggarwal R, Singh R, Sawhney RC, et al. Vitamin D and bone mineral density status of healthy schoolchildren in northern India. American Journal of Clinical Nutrition. 2005;82(2):477–482. doi: 10.1093/ajcn.82.2.477. [DOI] [PubMed] [Google Scholar]
- Matsuoka LY, Wortsman J, Haddad JG, Kolm P, Hollis BW. Racial pigmentation and the cutaneous synthesis of vitamin D. Archives of Dermatology. 1991;127(4):536–538. [PubMed] [Google Scholar]
- Maxwell JD, Ang L, Brooke OG, Brown IR. Vitamin D supplements enhance weight gain and nutritional status in pregnant Asians. British Journal of Obstetrics and Gynaecology. 1981;88(10):987–991. doi: 10.1111/j.1471-0528.1981.tb01686.x. [DOI] [PubMed] [Google Scholar]
- McCullough M. Vitamin D deficiency in pregnancy: bringing the issues to light. Journal of Nutrition. 2007;137:305–306. doi: 10.1093/jn/137.2.305. [DOI] [PubMed] [Google Scholar]
- McGrath J. Does 'imprinting' with low prenatal vitamin D contribute to the risk of various adult disorders? Medical Hypotheses. 2001;56(3):367–371. doi: 10.1054/mehy.2000.1226. [DOI] [PubMed] [Google Scholar]
- McGrath JJ, Kimlin MG, Saha S, Eyles DW, Parisi AV. Vitamin D insufficiency in south-east Queensland. Medical Journal of Australia. 2001;174(3):150–151. doi: 10.5694/j.1326-5377.2001.tb143195.x. [DOI] [PubMed] [Google Scholar]
- Mehta S, Hunter DJ, Mugusi FM, Spiegelman D, Manji KP, Giovannucci EL, et al. Perinatal outcomes, including mother-tochild transmission of HIV, and child mortality and their association with maternal vitamin D status in Tanzania. Journal of Infectious Diseases. 2009;200(7):1022–1030. doi: 10.1086/605699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merewood A, Mehta SD, Chen TC, Bauchner H, Holick MF. Association between vitamin D deficiency and primary cesarean section. Journal of Clinical Endocrinology and Metabolism. 2009;94(3):940–945. doi: 10.1210/jc.2008-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J, Gallo RL. Vitamin D and innate immunity. Dermatologic Therapy. 2010;23(1):13–22. doi: 10.1111/j.1529-8019.2009.01287.x. [DOI] [PubMed] [Google Scholar]
- Morley R, Carlin JB, Pasco JA, Wark JD. Maternal 25-hydroxyvitamin D and parathyroid hormone concentrations and offspring birth size. Journal of Clinical Endocrinology and Metabolism. 2006;91(3):906–912. doi: 10.1210/jc.2005-1479. [DOI] [PubMed] [Google Scholar]
- Namgunga R, Tsang RC. Bone in the pregnant mother and newborn at birth. Clinica Chimica Acta. 2003;333(1):1–11. doi: 10.1016/s0009-8981(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. American Journal of Clinical Nutrition. 2002;76(1):187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- Nicolaidou P, Hatzistamatiou Z, Papadopoulou A, Kaleyias J, Floropoulou E, Lagona E, et al. Low vitamin D status in mother-newborn pairs in Greece. Calcified Tissue International. 2006;78(6):337–342. doi: 10.1007/s00223-006-0007-5. [DOI] [PubMed] [Google Scholar]
- O'Riordan MN, Kiely M, Higgins JR, Cashman KD. Prevalence of suboptimal vitamin D status during pregnancy. Irish Medical Journal. 2008;101(8):240, 242–243. [PubMed] [Google Scholar]
- Ohta H, Kuroda T, Onoe Y, Orito S, Ohara M, Kume M, et al. The impact of lifestyle factors on serum 25-hydroxyvitamin D levels: across-sectional study in Japanese women aged 19–25 years. Journal of Bone and Mineral Metabolism. 2009;27(6):682–688. doi: 10.1007/s00774-009-0095-1. [DOI] [PubMed] [Google Scholar]
- Ornoy A, Menczel J, Nebel L. Alterations in the mineral composition and metabolism of rat fetuses and their placentas induced by maternal hypervitaminosis D2. Israel Journal of Medical Sciences. 1968;4(4):827–832. [PubMed] [Google Scholar]
- Ornoy A, Nebel L, Menczel Y. Impaired osteogenesis of fetal long bones.Induced by maternal hypervitaminosis D2. Archives of Pathology. 1969;87(6):563–571. [PubMed] [Google Scholar]
- Palomer X, González-Clemente JM, Blanco-Vaca F, Mauricio D. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes, Obesity and Metabolism. 2008;10(3):185–197. doi: 10.1111/j.1463-1326.2007.00710.x. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Souberbielle JC. Is hypovitaminosis D one of the environmental risk factors for multiple sclerosis? Brain. 2010;133(Pt 7):1869–1888. doi: 10.1093/brain/awq147. [DOI] [PubMed] [Google Scholar]
- Review Manager (RevMan) [Computer program]. Version 5.1.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2011. [Google Scholar]
- Rockell JE, Green TJ, Skeaff CM, Whiting SJ, Taylor RW, Williams SM, et al. Season and ethnicity are determinants of serum 25-hydroxyvitamin D concentrations in New Zealand children Aged 5–14 y. Journal of Nutrition. 2005;135:2602–2608. doi: 10.1093/jn/135.11.2602. [DOI] [PubMed] [Google Scholar]
- Roth DE. Vitamin D supplementation during pregnancy: safety considerations in the design and interpretation of clinical trials. Journal of Perinatology. 2011;31(7):449–459. doi: 10.1038/jp.2010.203. [DOI] [PubMed] [Google Scholar]
- Sachan A, Gupta R, Das V, Agarwal A, Awasthi PK, Bhatia V. High prevalence of vitamin D deficiency among pregnant women and their newborns in northern India. American Journal of Clinical Nutrition. 2005;81(5):1060–1064. doi: 10.1093/ajcn/81.5.1060. [DOI] [PubMed] [Google Scholar]
- Sahu M, Das V, Aggarwal A, Rawat V, Saxena P, Bhatia V. Vitamin D replacement in pregnant women in rural north India: a pilot study. European Journal of Clinical Nutrition. 2009;63(9):1157–1159. doi: 10.1038/ejcn.2009.27. [DOI] [PubMed] [Google Scholar]
- Sato Y, Iwamoto J, Kanoko T, Satoh K. Amelioration of osteoporosis and hypovitaminosis D by sunlight exposure in hospitalized, elderly women with Alzheimers disease: a randomized controlled trial. Journal of Bone and Mineral Research. 2005;20(8):1327–1333. doi: 10.1359/JBMR.050402. [DOI] [PubMed] [Google Scholar]
- Sedrani SH. Low 25-hydroxyvitamin D and normal serum calcium concentrations in Saudi Arabia: Riyadh region. Annals of Nutrition & Metabolism. 1984;28(3):181–185. doi: 10.1159/000176801. [DOI] [PubMed] [Google Scholar]
- Sloka S, Stokes J, Randell E, Newhook LA. Seasonal variation of maternal serum vitamin D in Newfoundland and Labrador. Journal of Obstetrics and Gynaecology Canada: JOGC. 2009;31(4):313–321. doi: 10.1016/S1701-2163(16)34148-2. [DOI] [PubMed] [Google Scholar]
- Sullivan SS, Rosen CJ, Halteman WA, Chen TC, Holick MF. Adolescent girls in Maine at risk for vitamin D insufficiency. Journal of the American Dietetic Association. 2005;105(6):971–974. doi: 10.1016/j.jada.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. American Journal of Medicine. 2002;112(8):659–662. doi: 10.1016/s0002-9343(02)01091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoropoulos C, Demers C, Delvin E, Ménard D, Gascon-Barré M. Calcitriol regulates the expression of the genes encoding the three key vitamin D3 hydroxylases and the drug-metabolizing enzyme CYP3A4 in the human fetal intestine. Clinical Endocrinology. 2003;58(4):489–499. doi: 10.1046/j.1365-2265.2003.01743.x. [DOI] [PubMed] [Google Scholar]
- Tolaymat A, Sanchez-Ramos L, Yergey AL, Vieira NE, Abrams SA, Edelstein P. Pathophysiology of hypocalciuria in preeclampsia: measurement of intestinal calcium absorption. International Journal of Gynecology & Obstetrics. 1994;83(2):283. [PubMed] [Google Scholar]
- UK Department of Health. [accessed 2009];Maternal nutrition: Vitamin D. UK Department of Health. ( http://www.dh.gov.uk/en/Healthcare/Children/Maternity/Maternalandinfantnutrition/Maternalnutrition/index.htm)
- Utiger RD. The need for more vitamin D. New England Journal of Medicine. 1998;338(12):828–829. doi: 10.1056/NEJM199803193381209. [DOI] [PubMed] [Google Scholar]
- Van der Meer IM, Karamali NS, Boeke AJ, Lips P, Middelkoop BJ, Verhoeven I, et al. High prevalence of vitamin D deficiency in pregnant non-Western women in The Hague, Netherlands. American Journal of Clinical Nutrition. 2006;84(2):350–353. doi: 10.1093/ajcn/84.1.350. [DOI] [PubMed] [Google Scholar]
- Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. American Journal of Clinical Nutrition. 1999;69(5):842–856. doi: 10.1093/ajcn/69.5.842. [DOI] [PubMed] [Google Scholar]
- Vieth R, Chan PCR, MacFarlane GD. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. American Journal of Clinical Nutrition. 2001;73(2):288–294. doi: 10.1093/ajcn/73.2.288. [DOI] [PubMed] [Google Scholar]
- Vilarrasa N, Maravall J, Estepa A, Sánchez R, Masdevall C, Navarro MA, et al. Low 25-hydroxyvitamin D concentrations in obese women: their clinical significance and relationship with anthropometric and body composition variables. Journal of Endocrinological Investigation. 2007;30(8):653–658. doi: 10.1007/BF03347445. [DOI] [PubMed] [Google Scholar]
- Viljakainen HT, Saarnio E, Hytinantti T, Miettinen M, Surcel H, Makitie O, et al. Maternal vitamin D status determines bone variables in the newborn. Journal of Clinical Endocrinology and Metabolism. 2010 doi: 10.1210/jc.2009-1391. Epub: 1391. [DOI] [PubMed] [Google Scholar]
- Wagner CL, Greer FR. American Academy of Pediatrics Section on breastfeeding and American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–1152. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- Walker VP, Modlin RL. The vitamin D connection to pediatric infections and immune function. Pediatrics Research. 2009;65(5 Pt 2):106R–113R. doi: 10.1203/PDR.0b013e31819dba91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. Journal of Immunology. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- Williams B, Williams AJ, Anderson ST. Vitamin D deficiency and insufficiency in children with tuberculosis. Pediatric Infectious Disease Journal. 2008;27(10):941–942. doi: 10.1097/INF.0b013e31817525df. [DOI] [PubMed] [Google Scholar]
- Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. American Journal of Clinical Nutrition. 2000;72(3):690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- Xiong X, Mayes D, Demianczuk N, Olson DM, Davidge ST, Newburn-Cook C, et al. Impact of pregnancy-induced hypertension on fetal growth. American Journal of Obstetrics and Gynecology. 1999;180(1 Pt 1):207–213. doi: 10.1016/s0002-9378(99)70176-6. [DOI] [PubMed] [Google Scholar]
- Yu CK, Sykes L, Sethi M, Teoh TG, Robinson S. Vitamin D deficiency and supplementation during pregnancy. Clinical Endocrinology. 2009;70(5):685–690. doi: 10.1111/j.1365-2265.2008.03403.x. [DOI] [PubMed] [Google Scholar]
- Zhang C, Qiu C, Hu FB, David RM, Van Dam RM, Bralley A, et al. Maternal plasma 25-hydroxyvitamin D concentrations and the risk for gestational diabetes mellitus. PLoS ONE. 2008;3(11):e3753. doi: 10.1371/journal.pone.0003753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Other published versions of this review
- Mahomed K, Gülmezoglu AM. Vitamin D supplementation in pregnancy. Cochrane Database of Systematic Reviews. 1999;(Issue 1) doi: 10.1002/14651858.CD000228. Art. No.: CD000228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.