Abstract
As the traditional cardiovascular control laboratory has disappeared from the first-year medical school curriculum, we have recognized the need to develop another “hands-on” experience as a vehicle for wide-ranging discussions of cardiovascular control mechanisms. Using an echocardiograph, an automatic blood pressure cuff, and a reclining bicycle, we developed protocols to illustrate the changes in cardiac and vascular function that occur with changes in posture, venous return, and graded exercise. We use medical student volunteers and a professional echocardiographer to generate and acquire data, respectively. In small-group sessions, we developed an interactive approach to discuss the data and to make a large number of calculations from a limited number of measurements. The sequence of cardiac events and cardiac structure in vivo were illustrated with the volunteers lying down, standing, and then with their legs raised passively above the heart to increase venous return. Volunteers were then asked to peddle the bicycle to achieve steady-state heart rates of 110 and 150 beats/min. Data were collected in all these states, and calculations were performed and used as the basis of a small-group discussion to illustrate physiological principles. Information related to a surprisingly large number of cardiovascular control mechanisms was derived, and its relevance to cardiovascular dysfunction was explored. This communication describes our experience in developing a new cardiovascular control laboratory to reinforce didactic material presented in lectures and small-group sessions.
Keywords: echocardiography, cardiac dimensions, cardiac output, wall stress, work, bicycle, venous return, blood pressure
for many decades, the hallmark of the cardiovascular section of the first-year medical physiology course was the cardiovascular control laboratory. This, depending on the institution, entailed using an anesthetized dog, pig, or cat to illustrate the principles presented in didactic lectures (5) and more recently in small-group sessions. Due to the cost (7), the limited number of faculty members still capable of conducting the laboratory, the political pressures placed upon the university, and the potential physical danger to faculty members and staff, many medical schools, including our own, no longer use animals in the physiology curriculum (7). The laboratory was unique for a number of reasons, including 1) the presence of faculty members and students in an intimate learning atmosphere where a hands-on approach was encouraged, 2) the lack of predictable textbook responses illustrating that variability and complexity are inherent in physiology and medicine, 3) the group's responsibility to the animal for its humane treatment and death, and 4) the need to develop a professional vocabulary and acquire the thought processes that would be required in subsequent years. Finally, the physiology laboratory was an opportunity for many of the participants to observe a beating and then fibrillating heart (5). For some, this was a profound experience. The history and value of laboratories in physiology courses have been discussed by Davenport (5).
With all this in mind, we developed a laboratory in our medical physiology curriculum that intends to demonstrate many of the principles of human cardiovascular physiology, allows for faculty-student interactions, illustrates the basics of data collection and processing, and finally provides examples of variability in biological responses.
The nature of integrated physiology research currently demands the assessment of cardiovascular function most commonly performed in rodents. The use of an echocardiograph machine outfitted with special probes allows for the collection of data at the high heart rates encountered in small animals, such as rats (heart rate: 300–400 beats/min) or mice (heart rate: 500–700 beats/min). Thus, most physiology departments or institutes already have echo machines adapted for the collection of hemodynamic data in rodents. With the purchase of the echo machine come various data manipulation modules that can process the information obtained not only in animals but also in humans. The limiting factor is the purchase of an echo probe appropriate for medical students and a protocol that can systematically alter human cardiac and vascular function. The use of echocardiography for student instruction was already proposed in 1995 by Brunner et al. (4); this, however, did not include an investigation into physiological control mechanisms but rather focused on the anatomy and calculation of cardiac mass.
Thus, the goal of this article is to present our ongoing efforts to develop a human cardiovascular physiology laboratory that provides a valuable learning experience to our medical students. This laboratory exercise does not entirely substitute for the classic physiology laboratory as the emphasis is on the heart and not the peripheral circulation and is limited by the lack of invasive measures of cardiac and vascular function. On the other hand, it does provide students an opportunity to collect data, manipulate them into common clinical formats, and interpret them using physiological concepts that are course objectives of the first-year medical school physiology course.
METHODS
The methods below describe our experience trying to develop this laboratory and are by no means complete. Our general concept was to use our echo machine to 1) measure the changes in cardiac function in different physiological states, such as position in space, exercise, and altered venous return; and 2) to create a learning paradigm for our students. We did ask the Insitutional Review Board of New York Medical College if approval of our protocols involving human subjects was needed. No approval was required as long as this was a teaching exercise and we did not use the data for medical purposes and did not connect a specific volunteer with specific data.
Gathering of equipment and expenses.
The Physiology Department of New York Medical College has a core facility designed to measure cardiovascular function in mice, rats, rabbits, and dogs for our faculty members and students and to teach our faculty members and graduate students the use of echocardiography in biomedical research. Thus, we already have a Accusan Sequoia 256 (now Seimens) and the necessary probes for use in research. A probe (model 5V2C, 4 MHz, $12,000) for use in adult male humans was purchased. In addition, we purchased a reclining bicycle (Pro-form XP 110R, $450) and an automatically inflating pressure cuff (Prodigy II, model 2200, Colin Medical Instruments, $2,500) to measure arterial pressure and electronically calculate mean arterial pressure (while the pressure cuff also measured heart rate, this was mostly collected from the echo). The total expense was a one-time cost of $15,000.
Experimental setup and the need for a good echocardiographer.
It quickly became obvious to us (from practicing on other faculty members) that we needed an expert in echocardiography if the laboratory was to be successful. Simple things like the lung shadow, slight rotations of the heart, and the difficulty collecting data while standing or during the tachycardia and hyperventilation of exercise and its inherent physical stress are all easily overcome by a professional echocardiographer. Thus, we recruited an echocardiographer from our Division of Pediatric Cardiology, who also gave the introductory didactic lecture to our first-year class emphasizing the practical aspects of echocardiography. This step was invaluable for the success of the laboratory.
We used a small room (the Physiology Department library) and divided the class into 10 groups of ∼20 students/session (200 students total). We attached the echo machine via a video output to a projector and presented the images, measured variables, and calculated data on a large screen. We positioned the students so that they could observe both the test subject and the large screen where the data were displayed. With the volunteer reclining on the bicycle and turned slightly to the left, we placed the pneumatic cuff on the dependent arm. We asked the test subject his height and weight for the calculation of body surface area (for use in calculating cardiac index and stroke index). We developed a data collection tool and distributed it to the students. One of the faculty members kept the master data sheet for the group for later discussion. We also developed a formula sheet (1–3, 6) and made some assumptions that the students were to use in group discussions (Table 1). Once the recordings were complete (1 h), the group and a faculty member went to another room to discuss the data and review the pertinent physiological principles. Our approach during the laboratory involved the echocardiographer discussing the collected images, one faculty member collecting the data and providing instruction to the medical students, and another faculty member asking appropriate fact-seeking questions of the students. Thus, there was a team approach during and after the data collection.
Table 1.
Equations
| Cardiac output | Cardiac output = stroke volume × heart rate |
| Cardiac index | Cardiac index = cardiac output/body surface area |
| Mean arterial blood pressure | Mean arterial blood pressure = 1/3(pulse pressure) + diastolic blood pressure |
| Total peripheral resistance | Total peripheral resistance = (mean arterial blood pressure − right atrial pressure)/cardiac output (assume that right atrial pressure = 0) |
| Cardiac work | Cardiac work = cardiac output × mean arterial blood pressure |
| Ejection fraction | Ejection fraction = stroke volume/end-diastolic volume |
| Myocardial O2 consumption | Myocardial O2 consumption = systolic blood pressure × heart rate |
| Wall stress | Wall stress = (blood pressure × diameter)/4(wall thickness) (assume systolic pressure = systolic arterial pressure and diastolic pressure = 7 mmHg) |
| Index | Index = (X)/body surface area |
| Volume of a sphere | Volume = 4/3(πr3), where r is the radius |
| Volume of a cylinder | Volume = 4r(πr2), where r is the radius (assume the long axis is twice the diameter at the base) |
Illustrating the principles of echocardiography and its quantitative nature and data collection.
The first step was to have the student volunteer lie on the reclining bicycle at an angle of ∼120°, turned slightly to the left. An echo was then recorded of the short axis and long axis illustrating the position of the mitral valve, the papillary muscles, the position of the right ventricle (RV) and left ventricle (LV), and the atria. The sequential contraction of the atria and ventricles was clearly demonstrated. When possible, the positions of the aortic and pulmonic valves were also shown.
The M-mode was then selected, the image at the level of the papillary muscle of the LV was shown, and data were collected for analysis (Fig. 1). Standard use of the M-mode following published guidelines was provided by the echocardiographer (8). Cardiac output and stroke volume were also calculated from the M-mode since the M-mode was usually the easiest (and most reliable) to acquire during the exercise. A discussion of the assumptions used to calculate volumes from dimensions was provided (the formula to calculate the volume of the heart if it is considered to be a sphere or cylinder).
Fig. 1.
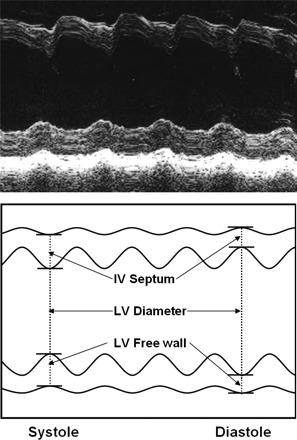
An actual M-mode recording (top) with a schematic diagram (bottom). The measurement of septal and anterior wall thickness and chamber diameter in systole and diastole are shown. Similar data were obtained during standing and sitting, during leg lifting, and during exercise. IV, intraventricular; LV, left ventricular.
The color Doppler feature of the echocardiograph was then selected, and the flows across the mitral valve and down the aorta were examined. The velocity of flow and direction of flow were discussed. Blood pressure was measured via the automatic cuff.
Actual experiments.
Only the M-mode was used for all the protocols since it was the easiest to obtain quickly and reliably (8). Once the data were acquired with the student lying on the bicycle, the student was asked to stand quietly next to the bicycle, i.e., to reduce venous return and cardiac size and increase the heart rate slightly (2, 3). The M-mode was acquired. To examine the effects of an increase in venous return on the heart, the student was asked to lie on the bicycle, and, in one motion, two other students lifted the student's legs passively above the heart level. Data were collected in the M-mode almost immediately.
After a rest period, with the student already lying on the horizontal bicycle, the student was asked to peddle to a heart rate to 110 beats/min, the heart rate that occurs in humans during complete withdrawal of vagal tone to the sinoatrial node (1–3). This was read off the ECG lead used to trigger the echo. Once a steady state had been reached (3–4 min) and data were collected, the student was asked to peddle at a rate that would increase the heart rate to 150 beats/min, a rate at which sympathetic tone to the heart is recruited. Because these students are generally fit and often good athletes, this required adjusting the resistance on the bicycle and a loud level of encouragement by his classmates. Most of the volunteers were athletes, and we gauged the level of their conditioning by low resting heart rates (in the 50s). The compiled data were skewed by one student who had a heart rate of 100 beats/min at rest. At steady state, blood pressure was taken along with echo M-mode images and the bicycle was stopped.
Finding experimental protocols that will work and explaining those that do not.
Thus, our protocol has currently evolved to include the effects of posture (standing and lying down), venous return (reclining and lifting the legs), and two levels of exercise. We did try the Valsalva maneuver [exhaling against a closed glottis (1–3)]. This did not work since the lungs were inflated and the lung shadow obscured the heart. This was illustrative to the students as a limitation, but no data on the heart could be collected. We also tried the cold pressor test by placing the student's hand in a bucket of ice water for 2–3 min (2, 3). Generally, the results were inconsistent, perhaps because the tachycardia tended to reduce cardiac size and the hypertension tended to increase cardiac size. That the student was already lying down also tended to increase cardiac size. Thus, neither the Valsalva maneuver nor the cold pressor test were useful.
Bases for calculations and review of cardiac mechanical function.
M-mode measures of septal wall thickness, LV chamber diameter, and LV free wall thickness were taken for a number of representative heart beats at steady state. The software uses the dimensions and a spherical model to calculate volumes, end diastole and end systole, and their difference as stroke volume. With this information (Table 1) and using the body surface area, we calculated cardiac output, stroke index, and cardiac index. Knowing the arterial pressure, we calculated stroke work and cardiac work. Using the systolic arterial pressure, wall thickness, and chamber diameter, we estimated cardiac wall stress in systole and diastole (estimating the diastolic pressure at various time points). Myocardial oxygen consumption was estimated from the double product, i.e., systolic arterial pressure times heart rate. Peripheral resistance was calculated using the following equation: mean arterial pressure = cardiac output × total peripheral resistance. Right atrial pressure was estimated as zero. The rationale for estimating right arterial pressure and LV end-diastolic pressure was discussed with the students (1–3).
To use a statistical approach or not.
One of the graduate students summarized the data in a tabular form and then in a graphical one, but this is not necessary for group discussions. Not surprisingly, however, careful analyses of the data often lead to a clearer view of the physiology. Units were ignored in the calculations, for instance, of stroke work, for simplicity. However, students with a more biophysical interest could be given the constants.
The basis of small-group discussions and laboratory review.
Once the data were collected, a faculty member took the group (n = 20) to a separate room to discuss and attempt to make sense of the data. This often entailed the faculty member “thinking on his feet” or recognizing the interaction between the opposing actions of heart rate and filling of the heart, for example. The results were not always predictable. Normal biological variability was pointed out.
RESULTS
Description of the heart at rest.
With the medical student lying on the reclining bicycle, the echo was first performed using a short-axis view to identify the papillary muscles. Using a parasternal long-axis view, the obvious landmarks were described, including the LV and right ventricle and the most predominant feature: the mitral valve. The thickness of the LV free wall and septum were pointed out and, if possible, the thickness of the RV free wall. The timing of wall thickening, i.e., when the aortic valve opens, and what happens during isovolumic contraction as well as the relationship between changes in diameter and the conservation of mass (shortening results in wall thickening) during cardiac contraction were discussed.
Next, using apical four- and five-chamber views, the long axis of the heart was again visualized with an emphasis on viewing all four valves of the heart. Using the color Doppler feature of the echo machine, the flow through the aortic valve was seen, and the students were asked to recall representative flow velocity in the aorta (100 cm/s). The relationship between aortic cross-sectional area (in cm2), flow velocity (in cm/s), and blood flow (in cm3/min) were discussed (2, 3). We increased the volume of the sound so that the nature of aortic flow, the high frequency and single phase of forward flow across the aortic valve, was obvious and discussed. Reverse flow in the aorta and the closure of the aortic valve were discussed. In contrast, during Doppler measures of flow across the mitral valve, the complicated nature and low frequency of the sound of mitral flow were shown. Students were asked to recall that there were two forward flows across the mitral valve: one during passive filling of the LV and another during atrial contraction.
The M-mode feature of the sonomicrometer was then selected to measure dimensions at the level of the papillary muscles, the standard landmark (8). Blood pressure was measured, and the heart rate calculated from the R-R interval. It was pointed out that, while the heart is an ellipse of revolution, initially for the sake of simplicity the shape could be estimated as a sphere or cylinder. The software modeled the heart as a sphere. Once the data were collected, the image was projected onto the screen (Fig. 1), and a cursor was used to point out the septum, diameter of the LV, and free wall thickness in systole and diastole. Measures of septal wall thickness, LV chamber diameter, and LV free wall thickness were made with the students watching the projected image. The computer then calculated wall thickening in the septum and free wall, the diameter of the LV, stroke volume, cardiac output, stroke index, and cardiac index. The ejection fraction was then calculated. The data appeared on the projection screen and were recorded on film. The data were stored on the hard drive.
Measurements with the student standing.
The student was asked to stand quietly next to the bicycle. Once heart rate came to some equilibrium, a small tachycardia was observed, and an increase in total peripheral resistance and fall in cardiac output were usually observed (Table 2). The echocardiographer again measured cardiac dimensions at the level of the mitral valve using M-mode. Arterial pressure was measured using the automatic cuff. When the data were projected on the screen, it was most obvious that cardiac output and other indexes of pump function decreased slightly, mostly due to the venous pooling. Calculations were made.
Table 2.
Hemodynamic data collected during sitting and standing
| Sitting | Standing | |
|---|---|---|
| Arterial systolic pressure, mmHg | 142 ± 3.0 | 137 ± 3.1 |
| Arterial diastolic pressure, mmHg | 79 ± 1.9 | 80 ± 2.6 |
| Mean arterial pressure, mmHg | 101 ± 1.9 | 99 ± 1.4 |
| Pulse pressure, mmHg | 62 ± 2.6 | 57 ± 4.6 |
| Heart rate, beats/min | 70 ± 2.8 | 85 ± 6.7 |
| Diameter at diastole, cm | 5.06 ± 0.17 | 4.05 ± 0.17 |
| Diameter at systole, cm | 3.15 ± 0.16 | 2.24 ± 0.30 |
| Wall thickness at diastole, cm | 0.80 ± 0.07 | 1.00 ± 0.10 |
| Wall thickness at systole, cm | 1.46 ± 0.07 | 1.57 ± 0.06 |
| Thickening, cm | 0.67 ± 0.06 | 0.57 ± 0.07 |
| Stroke volume, ml | 82 ± 5.6 | 54 ± 5.5 |
| Stroke index, ml/m2 | 57 ± 3.9 | 37 ± 3.8 |
| Stroke work, units | 8,255 ± 546 | 5,370 ± 557 |
| Cardiac output, l/min | 5.74 ± 0.40 | 4.56 ± 0.20 |
| Cardiac index, l·min−1·m−2 | 3.96 ± 0.28 | 3.14 ± 0.14 |
| Cardiac work, units | 579 ± 28 | 451 ± 28 |
| Ejection fraction, % | 67 ± 2 | 64 ± 6 |
| Myocardial O2 consumption, units | 9,837 ± 408 | 11,635 ± 792 |
| Total peripheral resistance, units | 19 ± 1.4 | 22 ± 1.2 |
Measurements with increased passive filling of the heart (by leg lifting).
The student then lay quietly on the bicycle, and, in one motion, two additional students lifted the legs above heart level, i.e., as high as possible. Blood pressure and the M-mode were recorded immediately to observe the consequences of an increase in venous return. As shown in Fig. 2, there was an increase in systolic and diastolic arterial pressure, pulse pressure, and mean pressure. Heart rate increased slightly. There was an increase in systolic and diastolic wall thickness and calculated wall thickening (Fig. 3). Stroke volume, cardiac output, stroke work, and cardiac work all increased, as did stroke index, cardiac index, and ejection fraction (Fig. 4). The mechanical estimate of myocardial oxygen consumption, the double product, increased, and calculated total peripheral resistance fell. Thus, during passive leg lifting, there appeared to be a slight tachycardia indicative of the activation of the Bainbridge reflex and an increased wall thickening and ejection fraction indicative of a Frank-Starling effect (2, 3).
Fig. 2.
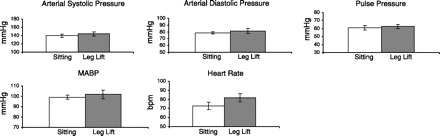
Measured variables. Pressures and heart rate are shown (n = 10 students) for sitting and during an increase in passive filling (leg lift), i.e., venous return. There was a small increase in pressures and in heart rate [in beats/min (bpm)], perhaps indicative of a Bainbridge reflex. MABP, mean arterial blood pressure.
Fig. 3.
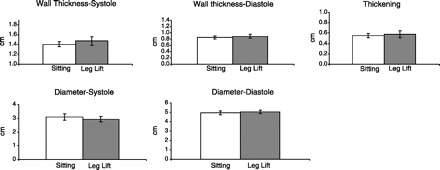
Measured values. There was a small increase in wall thickness (n = 10 students) during systole and no real changes in wall thickening or diameter. This may be indicative of a small inotropic effect, perhaps a Treppe or Bowditch phenomenon.
Fig. 4.
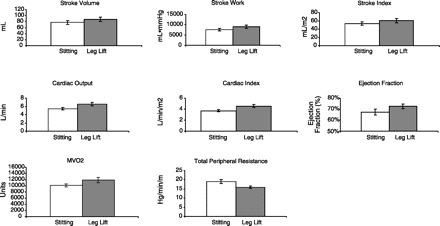
Calculated values. There were increases (n = 10 students) in stroke volume, stroke work, cardiac output, cardiac index, and our estimate of myocardial oxygen consumption (MV̇o2). These all indicate that venous return has increased. Total peripheral resistance fell, although there must be some error in this calculation since we estimated right atrial pressure as zero. Be that as it may, the error is small since atrial pressure was numerically small.
Effects of exercise on cardiac function.
With the student already lying on the bicycle, they were asked to pedal the bicycle to a heart rate of 110 beats/min, the level reached by withdrawal of vagal tone to the sinoatrial node and one with minimal effects upon sympathetic inotropic mechanisms (1). After a steady state was achieved and M-mode and blood pressure taken, the student peddled to increase their heart rate to 150 beats/min to clearly recruit an enhanced adrenergic chronotropic and inotropic state (1).
As shown in Fig. 5, there was an increase in systolic pressure, pulse pressure, and mean arterial pressure with exercise and no change or a slight increase in diastolic pressure at the highest level of exercise. Heart rate increased, and the subjects achieved the desired heart rates of 110 and 150 beats/min. Figure 6 shows that there were no changes in diastolic wall thickness but an increase in systolic wall thickness and wall thickening at the highest level of exercise. In addition, there was no change in diastolic diameter, and systolic diameter actually fell at the highest heart rate. Stroke volume and stroke work increased (Fig. 7), as did stroke index, cardiac output, cardiac index, and ejection fraction. The double product (a measure of myocardial oxygen consumption) increased, and total peripheral resistance fell.
Fig. 5.
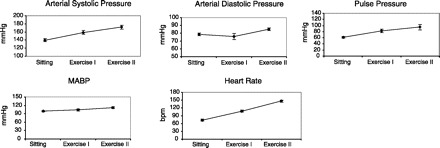
Measured variables. During exercise (n = 10 students) there was an increase in arterial pressure and pulse pressure. Heart rate increased and the volunteers made their goals of heart rates of 110 and 150 b/min during exercise.
Fig. 6.
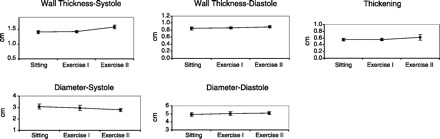
Measured values. The changes (n = 10 students) in diameter and wall thickness during exercise were small, especially during the low level of exercise. Accompanying the tachycardia, wall thickening and wall thickness during systole were increased and systolic diameter was decreased during the highest level of exercise.
Fig. 7.
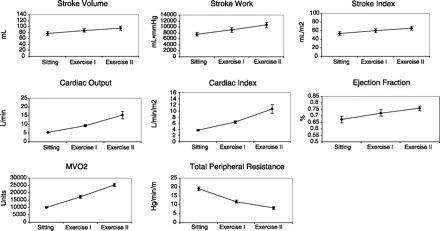
Calculated values. Stroke volume, stroke index, stroke work, cardiac output, cardiac index, and cardiac work (not shown) all (n = 10 students) increased during exercise. Of note, the increase in cardiac output from 5 to 10 to 15 l/min fitted well with values used in lectures and textbooks for moderate levels of exercise. MV̇o2 increased, reinforcing the concept that increased cardiac work is supported by increased metabolism. This also evolved into a discussion that because of a near-maximal oxygen extraction at rest in the heart, coronary blood flow must increase, especially during increased work states, such as exercise. Total peripheral resistance fell during exercise, reflecting increased blood flow to skeletal muscle and the heart.
Calculations of ventricular wall stress.
Ventricular wall stress in systole and diastole (6) are measures of ventricular contraction and preload, respectively. They are also considered the physical stimuli for concentric and eccentric hypertrophy of the heart. In addition, wall stress is a determinant of myocardial oxygen consumption in that coronary blood flow occurs almost entirely during diastole, where the throttling effect of systolic wall stress is low. Finally, diastolic wall stress may limit blood flow to the endocardium and perhaps promote myocardial fibrosis when diastolic wall stress is high (2, 3). Changes in wall stress during changes in posture, venous return, and exercise are shown in Fig. 8. Systolic and diastolic wall stress fell with standing due to a reduction in filling. Diastolic stress increased with leg lifting due to an increase in filling. Systolic and diastolic stress increased due to increased filling and increased systolic pressure during exercise.
Fig. 8.
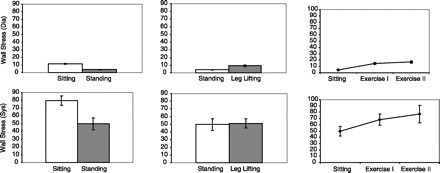
Calculated (n = 10 students) ventricular systolic and diastolic wall stress are shown for changes in posture (left), during leg lifting (middle), and during exercise (right). The units are omitted. Systolic and diastolic wall stress fall during standing due to a reduction in venous return. Diastolic wall stress increases during an increase in venous return during leg lifting. Both diastolic and systolic wall stress increase during exercise due to increased contractility and increased filling. (It is important to note that a putative ventricular diastolic pressure was provided to the students for calculations, and, because systolic wall stress may occur during isovolumic contraction, we estimated pressure in the ventricle as arterial systolic pressure.)
DISCUSSION
The major goal of this project was to develop an interactive laboratory for first-year medical students that would emphasize the changes in cardiac and vascular function that occur in humans during alterations in posture, venous return, and exercise. We measured cardiac function via echocardiography, a technique that all of the students will use in their clinical training regardless of their career choices, and thus familiarized the students with its basic principles. We did emphasize changes in cardiac mechanical function and required the students to use a group of textbook-level equations to make calculations and interpret data. It became obvious with a limited number of measurements, including heart rate, arterial pressure by cuff, and measures of cardiac wall thickness and LV chamber diameter via the echo, that a great number of cardiovascular principles could be discussed and indexes calculated. These include, among many others, calculations of peripheral resistance, work, assessment of mitral valve function, an understanding of timing of cardiac wall thickening, the potential influence of wall stress on the type of cardiac hypertrophy that occurs, and its use as a determinant of cardiac oxygen consumption. Many of these observations occurred after the fact, in that the amount and quality of the data available were not recognized during our initial planning sessions. It should be emphasized here that the quality of the data was, for the most part, determined by the expertise of the echocardiographer.
Cardiac structure and function.
The initial viewing of the heart was exciting to the students. The different views of the heart by the echo (long axis, four chamber, and M-mode) underscored the orientation of the heart within the chest. The echocardiographer or the participating faculty member identified the septum, free wall, LV, RV, and atria. One of the most obvious structures was the mitral valve, and the images showed its complicated opening and closing, like a fish's mouth. The pulmonic, aortic, and tricuspid valves were occasionally seen. The color Doppler feature, assigning different colors to forward (toward the probe, red) and reverse (away from the probe, blue) flows, as well as the difference in the sounds were very impressive to the students. A discussion was initiated related to the timing of events, i.e., that electrical activity precedes mechanical activity. Mechanical contraction results in, first, isovolumic contraction with no change in chamber diameters or wall thickness and, then, once the valve opens, wall thickening and a reduction in chamber dimensions. The flow in the aorta was used to illustrate the reverse flow, which closes the aortic valve.
Quantification of cardiac function.
The M-mode was then presented, and a number of beats were displayed on the projection screen. After some orientation by the echocardiographer and faculty member, systole and diastole were clearly seen and wall thickening was identified. As heart rate changed. the number of beats in a specific time interval also changed. The M-mode tracing was saved to the screen, and a cursor was used to identify and calculate wall thickness and chamber dimensions (Fig. 1). This led to a description of the structure of the heart, including identifying the “bright white” pericardium. A discussion of the effects of restrictive pericardial disease was initiated by the faculty member (2, 3). The wall thickness of the human heart was shown to be ∼1 cm and the diastolic chamber diameter was ∼5 cm. We introduced the concept of cardiac shape and how this was measured or estimated by the echocardiograph. The concept that the heart is twice as long as its diameter at the base not only illustrated, in a simple way, the structure of the heart but also allowed the students to calculate the cardiac volume as a cylinder. We emphasized the usefulness and limitation of ejection fraction as a measure of contractility and pointed out that it is very much load dependent. This led to a discussion of the definitions of afterload, preload, and contractility. From the collected data, we recorded cardiac output and cardiac index, reinforcing the range of the normal cardiac index (2.1–4.2 l·min−1·m−2) and why body surface area is a better way to normalize the data compared with weight or height alone (2, 3).
Standing: the influence of gravity.
While the student stood quietly next to the bicycle, measurements of blood pressure, heart rate, and cardiac dimensions were taken, and calculations were made. The concept of a hydrostatic pressure gradient proportional to the distance above or below the heart was introduced. Furthermore, a discussion of the effects of gravity on cardiac size and function was started and then continued with the effect of spaceflight and zero gravity (2, 3). A discussion of blood volume homeostasis ensued, including the mechanisms, i.e., the consequence and control of the atrial stretch-stimulated release of antidiuretic hormone and atrial natriuretic factor. The tachycardia was attributed to unloading of the baroreceptors, which led to a discussion of the location, structure, and function of the arterial baroreceptor reflexes and to a discussion of the Treppe phenomenon (2, 3).
Regulation of venous return.
The physiological consequences of increasing venous return to the heart were evident from the hemodynamics and discussed. Evidence for an increase in venous return including an increase in stroke volume and cardiac output, leading to the concept that cardiac output must equal venous return over any number of heart beats, was also discussed (2, 3). The measurement of indexes of filling pressure, e.g., end-diastolic diameter, were contrasted with the effects of tachycardia on cardiac filling i.e., a reduction in filling time. Since there was a tachycardia, the possible participation of the Bainbridge reflex and the neural pathways involved were discussed (2, 3).
Effect of exercise on cardiovascular function.
Data were collected at two steady-state levels: one at a heart rate of 110 beats/min and the other at a heart rate of 150 beats/min. This was done in an attempt to uncover an inotropic component to the increase in cardiac output at high heart rates and to contrast this with cardiac function at a heart rate due mostly to a withdrawal of vagal tone when adrenergic influences are of little import. Since vagal efferent fibers do not extend much below the atrioventricular node, they have little direct effect on LV contraction (1–3). Calculations of cardiac output resulted in values commonly seen at moderate levels of exercise and resulted in increased cardiac work and increased double product, i.e., myocardial oxygen consumption. The fall in peripheral resistance supported the increase in output and resulted in a discussion of why mean arterial pressure increased during exercise, the conclusion being that output is greater than the fall in resistance (1). The competition between ventricular filling (increased venous return) and better emptying (the increase in contractitlity) and reduction in filling time (reducing end-diastolic volume) led to some discussion between the faculty member and students.
Limitations.
There are a number of limitations in our proposed student laboratory. These are largely due to the assumptions that we made governing the intracardiac pressures used in calculations. For instance, right atrial pressure and LV systolic and diastolic pressures were assigned values found in most physiology textbooks. Pressures vary from those standards, making the absolute values of our data imprecise. This was also true using the M-mode to estimate cardiac volumes. When the estimates of volume and pressure are combined, for instance, in the calculation of wall stess, the inaccuracy may be compounded. Be that as it may, and due to the noninvasive nature of the student laboratory, the accumulated data still serve as a basis for discussion with first-year medical students. The clinical scenario will undoubtedly be much more complicated than our discussions and calculations indicate.
We recognized the need to replace the old cardiovascular control laboratory historically given to first-year medical students and mostly eliminated from current curricula (7). We wanted to develop a hands-on experience for our students that focused mostly on the heart and that was cost effective. We wanted a high impact factor with clinical relevance and thus decided to use our departmental echocardiograph to illustrate physiological principles. From this, we were able to collect data and to calculate and discuss other indexes of cardiac and vascular function. By the nature of echocardiography, most of our data were on cardiac function. Having performed this laboratory twice now, we have started to cull many physiological principles from the data as a focus for discussion and recognized that with a limited number of measured hemodynamic variables we can acquire a surprisingly large number of measurements to illustrate many cardiovascular principles. We are sure there are more insights to come.
Summary.
So far, we have collected data and engaged in discussions related to the following:
Cardiac structure and anatomy in vivo
The sequence of cardiac events during the cardiac cycle, i.e., the Wiggers Diagram
Pericardial disease
The use and limitations of ejection fraction as an index of contractility
The competition between the Frank -Starling effect and heart rate on ventricular filling
Baroreceptor control
The influence of gravity on the heart and peripheral circulation
Atrial stretch and the Bainbridge reflex
Venous return equals cardiac output
Why mean arterial pressure increases during exercise
The mechanism of tachycardia during exercise
Factors influencing cardiac filling during exercise
The role of physical factors in the control of myocardial oxygen consumption
the collapse of veins in the thorax as a limitation for venous return ala Guyton
The redistribution of peripheral resistance and blood flow during exercise
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants PO1-HL-43023, PO1-HL-74237, RO1-HL-50142, and HL-61290.
REFERENCES
- 1. American College of Sports Medicine. Advanced Exercise Physiology, edited by Tipton CM. New York: Lipincott, Williams & Wilkens, 2006. [Google Scholar]
- 2. Berne RM, Levy MN. (editors). Physiology (4th ed.). New York: Mosby, 1998. [Google Scholar]
- 3. Boron WF, Boulpaep EL. (editors). Medical Physiology. New York: Saunders, 2003. [Google Scholar]
- 4. Brunner M, Moeslinger T, Spieckmann PG. Echocardiography for teaching cardiac physiology in practical student courses. Adv Physiol Educ 13: 2–9, 1995. [DOI] [PubMed] [Google Scholar]
- 5. Davenport HW. The life and death of laboratory teaching of medical physiology: a personal narrative. Adv Physiol Educ 9: 16–23, 1993. [DOI] [PubMed] [Google Scholar]
- 6. Grossman W, Jones D, McLaurin P. Wall stress and patterns of hypertrophy. J Clin Invest 56: 56–64, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ra'anan AW. The evolving role of animal laboratories in physiology instruction. Adv Physiol Educ 20: 144–150, 2005. [DOI] [PubMed] [Google Scholar]
- 8. Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survery of echocardiographic measurements. Circulation 58: 1072–1083, 1978. [DOI] [PubMed] [Google Scholar]


