Abstract
Purpose.
Scanning laser polarimetry (SLP) reveals abnormal retardance of birefringence in locations of the edematous peripapillary retinal nerve fiber layer (RNFL), which appear thickened by optical coherence tomography (OCT), in nonarteritic anterior ischemic optic neuropathy (NAION). We hypothesize initial sector SLP RNFL abnormalities will correlate with long-term regional visual field loss due to ischemic injury.
Methods.
We prospectively performed automated perimetry, SLP, and high definition OCT (HD-OCT) of the RNFL in 25 eyes with acute NAION. We grouped visual field threshold and RNFL values into Garway-Heath inferior/superior disc sectors and corresponding superior/inferior field regions. We compared sector SLP RNFL thickness with corresponding visual field values at presentation and at >3 months.
Results.
At presentation, 12 eyes had superior sector SLP reduction, 11 of which had inferior field loss. Six eyes, all with superior field loss, had inferior sector SLP reduction. No eyes had reduced OCT-derived RNFL acutely. Eyes with abnormal field regions had corresponding SLP sectors thinner (P = 0.003) than for sectors with normal field regions. During the acute phase, the SLP-derived sector correlated with presentation (r = 0.59, P = 0.02) and with >3-month after presentation (r = 0.44, P = 0.02) corresponding superior and inferior field thresholds.
Conclusions.
Abnormal RNFL birefringence occurs in sectors corresponding to regional visual field loss during acute NAION when OCT-derived RNFL shows thickening. Since the visual field deficits show no significant recovery, SLP can be an early marker for axonal injury, which may be used to assess recovery potential at RNFL locations with respect to new treatments for acute NAION.
Keywords: NAION, retardance, retinal birefringence, OCT, scanning laser polarimetry
Scanning laser polarimetry measured loss in RNFL birefringence in acute NAION seems to reveal early axonal injury and permanent field loss at presentation.
Introduction
Optical coherence tomography (OCT) and scanning laser polarimetry (SLP) effectively show loss of peripapillary retinal nerve fiber layer (RNFL) in glaucoma, and following an episode of optic neuritis, nonarteritic anterior ischemic optic neuropathy (NAION), or traumatic optic neuropathy during the chronic atrophic stage.1–11 The relationship between regions of field loss and RNFL thinning using OCT and SLP has been shown after swelling of the optic nerve head (ONH) has resolved in NAION.7,8,12 Previous studies used either OCT or SLP, but not both methods together, to study swollen ONHs due to a variety of etiologies. Some investigators, who used older methodologies to compensate for the corneal birefringence, found SLP was not useful for evaluating thickening of the RNFL associated with the swollen or edematous ONH due to entities, such as NAION.6,13 In acute optic neuritis, reduced birefringence found at presentation generally was transient and most likely reflected temporary increased RNFL water content13 (Kupersmith M, et al. IOVS 2009;50:ARVO E-Abstract 5664). In general, eyes with ONH swelling due to papilledema, NAION, or optic neuritis do not show SLP-derived RNFL thickening due to the increased intra-axonal and extracellular water.14
Multiple studies have demonstrated correlation between functional (visual field threshold) and thinning of the peripapillary RNFL following an episode of optic neuritis or NAION. This is evident particularly when the chronic atrophic stage has been reached. However, to our knowledge, no studies have shown a correlation or predictive capability of optical imaging of the RNFL at presentation with the vision outcome at the chronic stage of injury. Our study explored whether optical imaging of acute injury of the optic nerve could distinguish those eyes at presentation that have irreversible injury from those more likely to recover with therapy. An identified risk factor could be applied in clinical trials that test new experimental interventions to protect injured, but potentially salvageable axons of the optic nerve.
NAION presumably is due to ischemia of the lamina and retrolaminar segments of the optic nerve, and visual field deficits commonly are irreversible and permanent. Recently, we showed that SLP at presentation can reveal decreased retardance of the RNFL in NAION, but not with other disorders of ONH swelling, such as papilledema and optic neuritis, in which permanent visual field loss is minimal.14 This finding suggests that the SLP-demonstrated loss of birefringence could be due to acute axonal injury and the associated vision loss likely would persist. We conducted this study to explore more precisely the correlation between decreased retardance of peripapillary regions in the acute stage NAION with the visual field defects at presentation, and at the chronic stage greater than 3 months.
Methods
We studied subjects prospectively if they had new onset (within 21 days of vision loss) NAION.15 During this acute phase of NAION, all subjects showed optic disc swelling on ophthalmoscopic examination. Subjects could have a prior NAION in a fellow eye, but not the newly affected eye. This research was conducted with New York Eye and Ear Infirmary Institutional Review Board approval and is in compliance with the Declaration of Helsinki.
Each subject underwent a complete clinical evaluation and threshold perimetry using the Humphrey Field Analyzer (Carl Zeiss Meditec, Dublin, CA) with SITA 24-2 Standard protocol using size III. In a small number of cases the damage was so severe that size III showed no response at most locations, and spot size V was used; data were analyzed using probability plots for size V developed for glaucoma.16 We compared the visual threshold results to the corresponding regions of RNFL by grouping data into locations corresponding to the mapping proposed by Garway-Heath,17 and used the methods reported by Hood et al.12 We calculated the average threshold deviation from normal for the superior and inferior regions (composed of Garway-Heath arcuate and nasal step sectors organized into superior and inferior regions, Fig. 1), since superior and inferior field defects are the most common pattern seen with NAION. A region was considered abnormal if the mean deviation (MD) was less than the fifth percentile of normal fellow unaffected eyes of the NAION patients shown below:
Figure 1. .
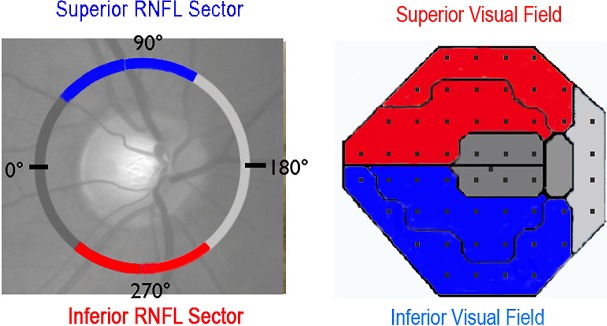
Corresponding peripapillary RNFL sector and regional visual field mapping. Superior RNFL sector—inferior field region is in blue and inferior RNFL sector—superior field region is in red.
Fifth percentile of superior region average threshold deviation: −7.06 dB.
Fifth percentile of inferior region average threshold deviation: −4.98 dB.
The peripapillary RNFL evaluation was performed with SLP in undilated eyes and OCT following pupillary dilation. For SLP (GDx with enhanced corneal compensation, ECC, research software; Carl Zeiss Meditec), an elliptical annulus was adjusted to permit capture of the same peripapillary RNFL measurement between exams and eyes. The SLP retardation values were determined within the annulus. Each annulus was resampled to calculate 64 circumferential measures of RNFL thickness. For the OCT (high definition, Cirrus spectral HD-OCT; Carl Zeiss Meditec), the laser scanned a 6 × 6 mm area, capturing a volume of data consisting of 200 A-scans from 200 linear B-scans (40,000 points). The Cirrus software determined RNFL thickness values at 256 points in the peripapillary circumference. At least two scans were performed for each method on each eye, and only images centered on the optic disc with signal strength scores 6 or greater were analyzed.
We analyzed the SLP retardance and OCT RNFL thickness along the peripapillary circumference. The optical imaging of peripapillary RNFL results for both methods were organized into the Garway-Heath map with attention to superior and inferior sectors (Fig. 1).17 For SLP, we determined the fifth and 95th (not shown) percentile of RNFL thickness (retardance) in normal controls (provided by Carl Zeiss Meditec, Table 1) for 65-year-olds to compare with the study data. For OCT, we determined the fifth and 95th (not shown) percentiles of RNFL thickness in the unaffected fellow eyes as well as for normal control age 65 years (provided by Carl Zeiss Meditec, Table 1).
Table 1. .
Fifth Percentile of RNFL Thickness in 65-Year-Old Normal Subjects by SLP and HD-OCT
|
Average, μm |
Superior Quadrant, μm |
Inferior Quadrant, μm |
|
| Fifth percentile SLP, age 65 | 34 | 50 | 54 |
| Fifth percentile SLP, unaffected eyes |
41 |
48 |
51 |
| Fifth percentile HD-OCT, age 65 |
76 |
87 |
84 |
| Fifth percentile HD-OCT, unaffected eyes |
82 |
88 |
80 |
We correlated (Pearson) superior and inferior visual field regions with their corresponding RNLF sectors at presentation and at a time point 3 months from onset of visual loss or later. We adjusted the P values for calculating multiple comparisons. We also analyzed the number of SLP sectors with values less than the fifth percentile of controls and having an abnormal (<5th percentile) corresponding region of visual threshold.
Results
We evaluated 25 eyes with acute NAION an average of 9.9 + 5.5 days from the time of self-reported vision loss. Five patients had prior NAION in the fellow eye. None had glaucoma or retinal disease that could cause visual field or acuity deficits. The visual field and visual acuity deficits varied, but were typical of NAION (Table 2). The acute visual field was abnormal for the inferior field region in 19 eyes and for the superior field region in 9 eyes (6 eyes had deficits in both regions, one of which was almost complete visual threshold loss). A maculopapillary type defect occurred in 1 eye, and a small superior arcuate defect that did not register as abnormal for the entire region occurred in one eye. The average threshold deviation was −12.89 + 6.62 dB for the affected superior field Garway-Heath region and −11.87 + 5.52 dB for the affected inferior field region. At presentation, the average RNFL measurement (Table 3) varied widely for OCT and for SLP. All eyes had OCT average RNFL measurements that were abnormally thickened, greater than the 95th percentile of controls, and two or more quadrants were thickened in every affected eye, as described previously.10 No eyes had an OCT average or quadrant RNFL measurement less than the fifth percentile of controls. For SLP at presentation, no affected eye had an average RNFL or quadrant measurement greater than the 95th percentile of controls. SLP was below the fifth percentile (for control and unaffected fellow eyes) for the superior quadrant in 12 eyes (11 with abnormal corresponding inferior visual field region; Figs. 2, 3, case example) and for the inferior quadrant in 6 eyes (6 with abnormal superior visual field region; 2 with superior and inferior sectors affected).
Table 2. .
Patient Demographics and Visual Performance of NAION Eyes at Three Time Points
|
Time Point |
Age, y Mean ± SD (Range) |
Sex, Women/Men |
LogMAR Visual Acuity Mean ± SD (Range) |
MD dB Mean ± SD |
Superior Quadrant MD dB Mean ± SD |
Inferior Quadrant MD dB Mean ± SD |
| Presentation | 67.6. ± 12.5 (38–85) | 9/15 | 0.47 ± 0.68 (hand motion–0) | −12.10 ± 5.68 | −5.99 ± 5.98 | −10.10 ± 7.32 |
| 1 Mo | 0.40 ± 0.71 (hand motion–0) | −14.28 ± 9.11 | −10.11 ± 9.40 | −13.37 ± 9.32 | ||
| ≥3 Mo | 0.65 ± 0.84 (hand motion–0) | −14.50 ± 8.53 | −9.09 ± 8.95 | −12.88 ± 9.15 |
Table 3. .
RNFL Thickness by SLP and HD-OCT of AION Eyes at Time Points Measured
|
Diagnosis Imaging Test |
Average, μm |
Temporal Quadrant, μm |
Superior Quadrant, μm |
Nasal Quadrant, μm |
Inferior Quadrant, μm |
| Presentation SLP, mean ± SD | 46 ± 8 | 30 ± 8 | 51 ± 14 | 41 ± 9 | 64 ± 14 |
| 1 mo SLP, mean ± SD | 41 ± 18 | 28 ± 9 | 40 ± 13 | 36 ± 12 | 59 ± 19 |
| Presentation OCT, mean ± SD | 201 ± 98 | 137 ± 52 | 257 ± 115 | 180 ± 77 | 248 ± 106 |
| 1 mo OCT, mean ± SD | 122 ± 57 | 79 ± 48 | 115 ± 52 | 101 ± 39 | 163 ± 95 |
| >3 mo OCT, mean ± SD | 69 ± 14 | 53 ± 7 | 70 ± 18 | 63 ± 8 | 89 ± 31 |
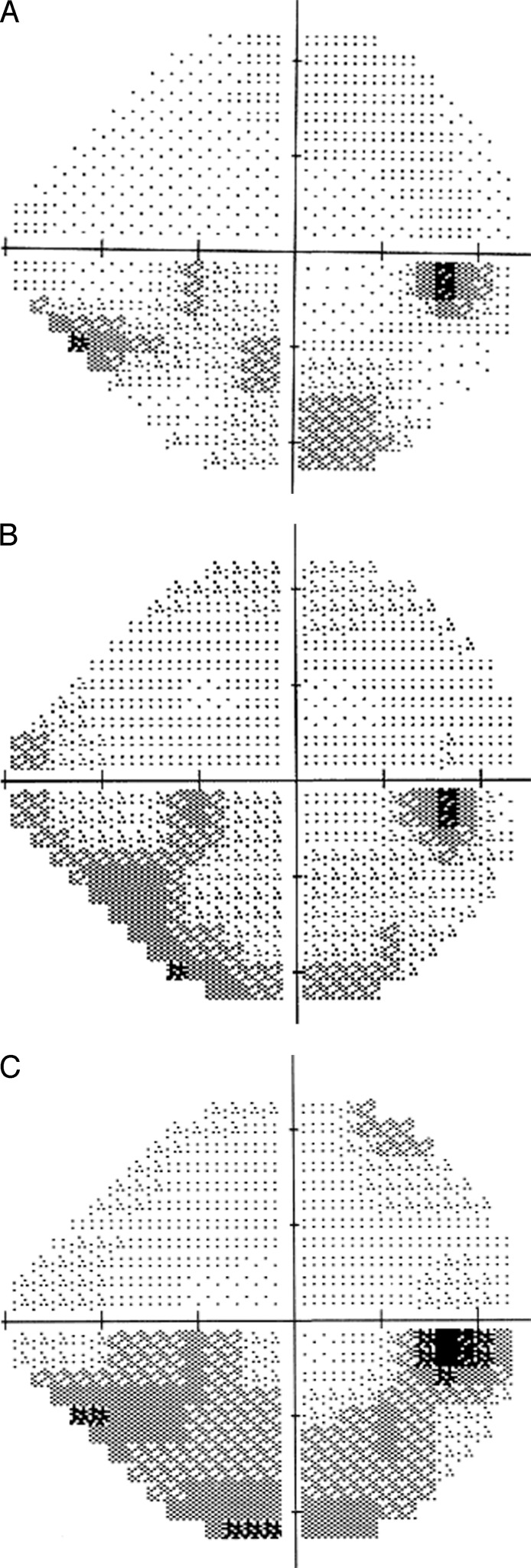
Figure 2.
Case example 24-2 of affected eye. (A) At presentation (MD −2.10 dB). (B) At 2 months (MD −8.45 dB). (C) At 9 months (MD −10.36 dB).
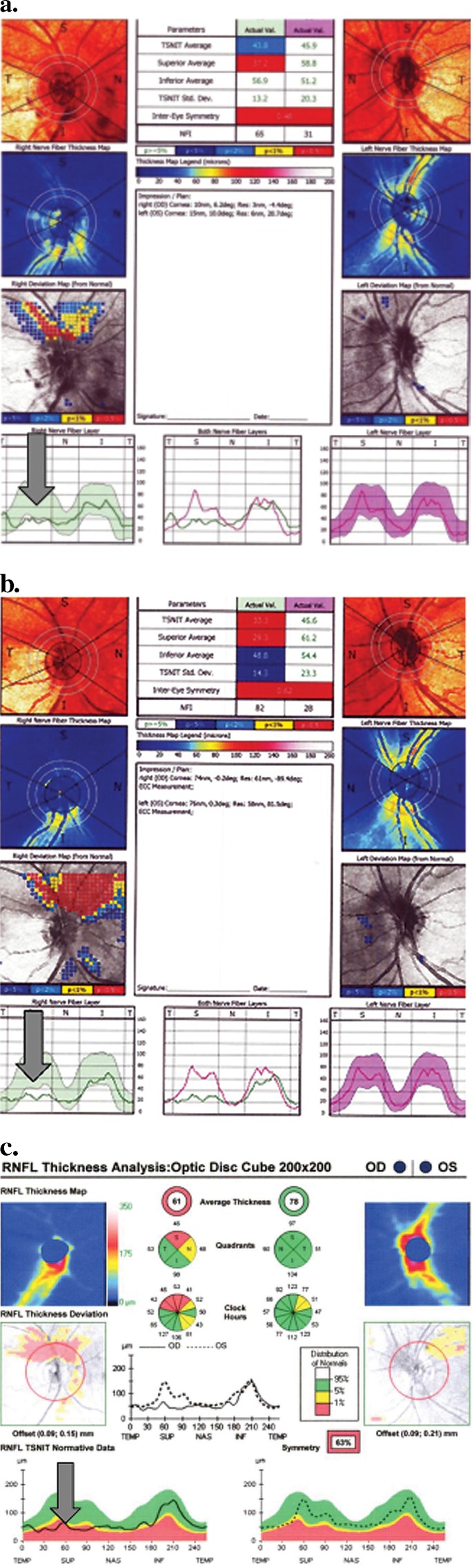
Figure 3.
Case example optical imaging of ONH region. (a) SLP at presentation. (b) SLP at 2 months. (c) OCT at 9 months.
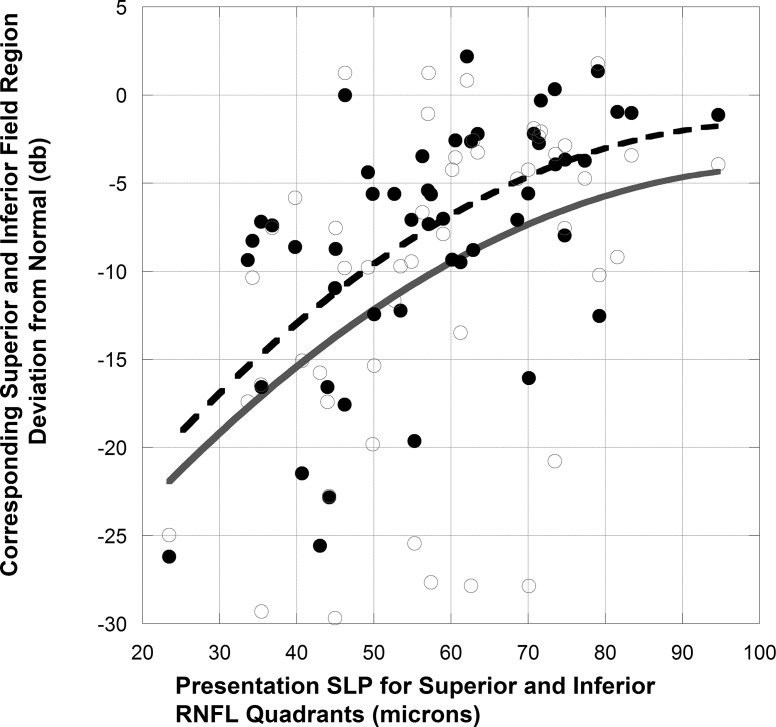
At presentation, for NAION eyes there was no correlation between the visual acuity deficit and sector retardance. Twelve of the 19 eyes with abnormal inferior field region and 6 of 10 eyes with abnormal superior visual field region had retardance values less than the fifth percentile of controls in the corresponding SLP superior and inferior sectors. For eyes with abnormal field regions, the corresponding SLP superior or inferior sector average values were 58 + 16 μm (P = 0.003) compared to 67 + 12 μm for sectors with corresponding normal field regions. The acute SLP retardance values for the superior sector correlated with inferior region threshold (r = 0.45, P = 0.04) and the inferior sector correlated with superior region threshold (r = 0.63, P = 0.002; for SLP values for superior and inferior sectors r = 0.59, P = 0.04, Fig. 4). The eye with complete visual field loss had no sectors with retardance less than the fifth percentile of controls. The values for baseline, acute OCT RNFL values for the same sectors did not correlate with the corresponding area of field loss at baseline.
Figure 4. .
All SLP superior and inferior quadrant RNFL values in acute NAION compared to corresponding presentation and 3-month visual field region. Closed circles and broken fit curve indicate presentation visual field data. Open circles and solid fit curve indicate 3-month visual field data.
At one month, all affected eyes had persistent visual field loss (Table 2). None of the eyes with an abnormal SLP sector retardance at presentation showed recovery of the corresponding field region to the normal threshold range. However, three eyes with normal baseline SLP and abnormal visual regional threshold during the acute stage had improvement of the field region over time. All affected eyes at the second visit at approximately one month later showed less thickening or thinning of the RNFL by SLP and OCT compared to the measurement at presentation (Table 3). None of the RNFL sectors with abnormal retardance by SLP at presentation recovered retardance. Nine eyes had thinning of OCT RNFL less than the control fifth percentile in at least one sector at the one month visit (9 superior, 1 temporal, and 3 inferior quadrants). OCT values for either superior or inferior sectors at one month did not correlate with the field loss in the corresponding quadrant at 3 months.
After three or more months, significant visual threshold loss (<5th percentile) was seen in the inferior quadrant in 19 eyes and in the superior quadrant in 12 eyes. The OCT was below the fifth percentile of controls in the corresponding 19 superior sectors and in 10/12 inferior sectors. Presentation superior sector SLP-derived RNFL values correlated with the >3-month inferior region threshold (r = 0.45, P = 0.04) and the inferior sector SLP-derived RNFL values correlated with the >3-month superior region threshold (r = 0.42, P = 0.08; for SLP values for superior and inferior sectors r = 0.44, P = 0.02, Fig. 4). The >3-month OCT-derived inferior RNFL sector (but not the superior RNFL sector), correlated with the corresponding superior regional threshold value (r = 0.60, P = 0.002).
Discussion
Scanning laser polarimetry showed acutely significant retardance reduction in sectors of the peripapillary RNFL at presentation with ONH ischemia that did not recover, in eyes with recent onset NAION. The affected RNFL regions did not recover retardance over time even as swelling of the RNFL resolved by OCT. Acute segmental SLP-derived RNFL change correlated with acute and subsequent chronic threshold loss in corresponding regions of the visual field. In contrast, the OCT did not show RNFL thinning or loss at presentation. On the contrary, OCT is effective at showing thickening of the RNFL during acute optic nerve edema, but not acute damage.
As reported previously for NAION, by three months, significant RNFL loss, particularly in sectors corresponding to regions of visual field loss, are seen by OCT. Additionally, at one to two months, RNFL thinning below the fifth percentile was seen by OCT in eyes that had other RNFL sectors still swollen. Axonal loss and RNFL thinning, which was not seen acutely in our NAION eyes by OCT, was readily apparent using OCT (24/25 eyes) at 3 months or later. OCT consistently shows RNFL thinning or loss after this ischemic injury.
At presentation, the OCT, but not SLP, showed marked swelling of the RNFL, due to increased intra-axonal and interstitial water content. The lack of increased RNFL thickening by SLP at presentation may reflect a relative inability to detect increases in extracellular water or edema in the peripapillary RNFL in optic neuritis and NAION.13,14,17,18 SLP measurements are based on birefringence properties in the retina, which arise almost exclusively from the RNFL and are due to the array of parallel cylindrical organelles, such as axonal membranes, microtubules, and neurofilaments.19,20 The RNFL birefringence induces reflected light polarized along bundles of nerve fibers to be delayed and this slowing is termed retardance. The calculated RNFL thickness value is proportional to the amount of retardance.
At presentation, SLP showed abnormal retardance in 64% of eyes with NAION. This suggests that not all RNFL segments associated with acute field loss have irreversible ischemic damage or microtubule dissolution at presentation. In support of this concept, three eyes with regional field loss and normal corresponding SLP sectors at presentation showed significant improvement of the regional field defect at one month. Further, some improvement in visual acuity and visual field in NAION has been reported.21 In NAION, ischemia of the optic nerve in the laminar and retrolaminar sections22 typically causes permanent injury of many, but not all, axons. Some axons must be subject to a relative ischemia that causes visual dysfunction, but does not cause infarction. SLP-measured retardance change and derived RNFL thinning is associated with axonal thinning23 or microtubule loss.24 Loss or disruption of the structures that are birefringent most likely accounts for the abnormal retardance, while swelling is seen clinically and is measured as a thickened RNFL by OCT. Thus, SLP retardance reduction suggests an acute disruption in the intra-axonal structures, such as microtubules or axonal membrane at presentation in regions of irreversible damage in NAION eyes. This would reduce or inhibit any intervention from restoring vision or reducing axon loss in SLP-shown abnormal regions. In our study, the abnormal retardance most often was focal, affecting one sector in 13 of the 16 eyes showing this finding.
Our results showed SLP abnormal retardance in sectors at baseline correlated with the baseline and final visual threshold abnormalities for superior and inferior visual fields. These results contrast with a prior report that used older SLP methodology and only found correlation with inferior sector SLP reduction and superior visual field deficits, both obtained when swelling had resolved and optic atrophy was present.8 One might expect that the correlation for baseline SLP sector reduction and 3-month regional visual field deficits would be stronger than the baseline correlations. Two factors could contribute to this finding. Relative ischemia at presentation evolved to infarction in the optic nerve axons. The dynamics of NAION pathophysiology that leads to frequent worsening of the visual field and new regional field deficits were common in our patients.
Conclusions
SLP demonstrated abnormal retardance in specific peripapillary sectors, seen at presentation of NAION, and corresponding to acute and permanent regional visual field deficits, presumably reflects irreversible ischemic axonal injury. SLP could serve as an effective biomarker to be integrated into any study design that tests neuroprotection or other therapies for acute NAION.
Acknowledgments
Supported by 3U10EY017281-01A1S1 and Veterans Administration (Merit Grant; Rehabilitation Division), and Research to Prevent Blindness (New York, New York).
Disclosure: M.J. Kupersmith, None; S. Anderson, None; M. Durbin, Carl Zeiss Meditec (E); R. Kardon, None
References
- 1. Medeiros F, Zangwill L, Bowd C, Weinreb R. Comparison of the GDx VCC scanning laser polarimeter, HRT II confocal scanning laser ophthalmoscope, and Stratus OCT optic coherence tomography for the detection of glaucoma. Arch Ophthalmol. 2004; 122: 827–837 [DOI] [PubMed] [Google Scholar]
- 2. Pro M, Pons M, Liebmann J, et al. Imaging of the optic disc and retinal nerve fiber layer in acute optic neuritis. J Neurol Sci. 2006; 250: 114–119 [DOI] [PubMed] [Google Scholar]
- 3. Trip A, Schlottmann P, Jones S, et al. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol. 2005; 58: 383–391 [DOI] [PubMed] [Google Scholar]
- 4. Costello F, Coupland S, Hodge W, et al. Quantifying axonal loss after optic neuritis with ocular coherence tomography. Ann Neurol. 2006; 59: 963–969 [DOI] [PubMed] [Google Scholar]
- 5. Steel DH, Waldock A. Measurement of the retinal nerve fiber layer with scanning laser polarimetry in patients with previous demyelinating optic neuritis. J Neurol Neurosurg Psychiatry. 1988; 64: 505–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colen T, van Everdinagen J, Lemij H. Axonal loss in a patient with anterior ischemic optic neuropathy as measured with scanning laser polarimetry. Am J Ophthalmol. 2000; 130: 847–850 [DOI] [PubMed] [Google Scholar]
- 7. DeLeon-Ortega J, Carroll K, Arthur S, Girkin C. Correlations between retinal nerve fiber layer and visual fields in eyes with nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 2007; 143: 288–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Danesh-Meyer H, Carroll S, Ku J, et al. Correlation of retinal nerve fiber layer measured by scanning laser polarimeter to visual field in ischemic optic neuropathy. Arch Ophthalmol. 2006; 124: 1720–1726 [DOI] [PubMed] [Google Scholar]
- 9. Contreras I, Rebolleda G, Noval S, Munoz-Negrete F. Optic disc evaluation by optical coherence tomography in nonarteritic anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2007; 48: 4087–4092 [DOI] [PubMed] [Google Scholar]
- 10. Bellusci C, Savini G, Carbonelli M, Carelli V, Sadun A, Barboni P. Retinal nerve fiber layer thickness in nonarteritic anterior ischemic optic neuropathy. Graefes Arch Clin Exp Ophthalmol. 2008; 246: 641–648 [DOI] [PubMed] [Google Scholar]
- 11. Meier F, Bernasconi P, Stürmer J, Caubergh M, Landau K. Axonal loss from acute optic neuropathy documented by scanning laser polarimetry. Br J Ophthalmology. 2002; 86: 285–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hood D, Anderson S, Rouleau J, et al. Retinal nerve fiber structure versus visual field function in patients with ischemic optic neuropathy. A test of a linear model. Ophthalmology. 2008; 115: 904–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Banks M, Robe-Collignon N, Rizzo J, Pasquale L. Scanning laser polarimetry of edematous and atrophic optic nerve heads. Arch Ophthalmol. 2003; 121: 484–449 [DOI] [PubMed] [Google Scholar]
- 14. Kupersmith M, Kardon R, Durbin M, Horne M. Scanning laser polarimetry reveals status of axon integrity in areas of optical coherence tomography revealed thickened retinal nerve fiber layer (RNFL) with optic nerve head swelling. Invest Ophthalmol Vis Sci. 2012; 53: 1962–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. The Ischemic Optic Neuropathy Decompression Trial Group The ischemic optic neuropathy decompression trial (IONDT): design and methods. Control Clin Trials. 1998; 19: 276–296 [DOI] [PubMed] [Google Scholar]
- 16. Wall M, Brito C, Woodward K, Doyle C, Kardon R, Johnson C. Total deviation probability plots for stimulus size V perimetry. Arch Ophthalmol. 2008; 126: 473–479 [DOI] [PubMed] [Google Scholar]
- 17. Garway-Heath D, Poinoosawmy D, Fitzke F, Hitchings R. Mapping the visual field to the optic disc in normal tension glaucoma eyes. Ophthalmology. 2000; 107: 1809–1815 [DOI] [PubMed] [Google Scholar]
- 18. Kupersmith M, Mandel G, Anderson S, Meltzer D, Kardon R. Baseline and one month changes in the peripapillary retinal nerve fiber layer in acute optic neuritis: relation to baseline vision and MRI. J Neurol Sci. 2011; 308: 117–123 [DOI] [PubMed] [Google Scholar]
- 19. Zhou Q, Knighton R. Light scattering and form of parallel cylindrical arrays that represent cellular organelles of the retinal nerve fiber layer. Appl Opt. 1997; 36: 2273–2285 [DOI] [PubMed] [Google Scholar]
- 20. Huang XR, Bagga H, Greenfield D, Knighton R. Variation of peripapillary retinal nerve fiber layer birefringence in normal human subjects. Invest Ophthalmol Vis Sci. 2004; 45: 3073–3080 [DOI] [PubMed] [Google Scholar]
- 21. The Ischemic Optic Neuropathy Decompression Trial Group Ischemic optic neuropathy decompression trial: twenty-four month update. Arch Ophthalmol. 2000; 118: 793–798 [PubMed] [Google Scholar]
- 22. Arnold A. Pathogenesis of nonarteritic anterior ischemic optic neuropathy. J Neuro-Ophthalmol. 2003; 23: 157–163 [DOI] [PubMed] [Google Scholar]
- 23. Weinreb R, Dreher A, Coleman A, Quigley H, Shaw B, Reiter K. Histopathologic validation of Fourier-ellipsometry measurements of retinal nerve fiber layer thickness. Arch Ophthalmol. 1990; 108: 557–560 [DOI] [PubMed] [Google Scholar]
- 24. Huang XR, Knighton R. Microtubules contribute to the birefringence of the retinal nerve fiber layer. Invest Ophthalmol Vis Sci. 2005; 46: 4588–4593 [DOI] [PubMed] [Google Scholar]