Abstract
Purpose.
Deposition of the macular pigment carotenoids lutein and zeaxanthin in the human retina occurs early in life. In this study, we examined the interrelationships of maternal carotenoid status and newborn infant macular pigment levels and systemic carotenoid status. As a secondary measure, we also evaluated the effects of intrauterine growth restriction (IUGR) on carotenoid status in term newborn infants.
Methods.
We measured mother and infant skin carotenoids using resonance Raman spectroscopy (RRS), serum carotenoids by HPLC, and mother breast milk carotenoids by HPLC. We measured infant macular pigment levels using noninvasive blue light reflectometry.
Results.
We enrolled 30 healthy term infants, their mothers, and 10 IUGR infants and their mothers. A subset of 16 infants was imaged for macular pigment optical density (MPOD). Infant serum zeaxanthin levels correlated with MPOD (r = 0.68, P = 0.007). Mother serum zeaxanthin levels correlated with infant MPOD (r = 0.59, P = 0.032). Infant and mother serum lutein did not correlate with MPOD. Mother–infant correlations were found for total serum carotenoids (r = 0.42, P = 0.020) and skin carotenoids (r = 0.48, P = 0.001). No difference was seen between IUGR infants and controls in total serum or skin carotenoids. Mothers of IUGR infants had lower total serum carotenoids (P = 0.019) and breast milk carotenoids than controls (P = 0.006).
Conclusions.
Our findings suggest that maternal zeaxanthin status may play a more important role than lutein status in macular pigment deposition in utero. Controlled trials are needed to determine whether maternal zeaxanthin prenatal supplementation can raise infant macular pigment levels and/or improve ocular function.
Keywords: macular pigment, carotenoid, nutrition, lutein, zeaxanthin
Systemic carotenoid status was measured in mothers and their newborn infants, and macular pigment was measured in a subset of the enrolled infants. Our findings suggest that maternal zeaxanthin status may play a more important role than maternal lutein status in macular pigment deposition in utero.
Introduction
Carotenoids are integral to the functioning and protection of the macula and are also of interest in fetal macular development. Carotenoids are found in fruits and green, leafy vegetables and are important in the physiologic function of skin, adipose, and retinal tissue.1–4 Of the 10 to 15 carotenoids found in human serum, lutein and zeaxanthin are preferentially found in the macula.5–7 Lutein and zeaxanthin play important roles in prevention of AMD,8,9 maintenance of visual acuity,3,9,10 and healthy prenatal and postnatal macular development.3,5 Many studies have examined the relationship between lutein, zeaxanthin, and AMD11–13; however, the effects of carotenoids on macular development have not been studied in depth. Macular development coincides with progressive shifts in the distribution of lutein, zeaxanthin, and meso-zeaxanthin within the developing macula,5 and nutritional status may affect this process. Newborn infants may have low carotenoid levels due to decreased placental nutrient transfer in utero, similar to the decrease in placental transfer of macronutrients, which has been implicated as a mechanism for intrauterine growth restriction or IUGR (defined as a birth weight below the 10th percentile for age).14–16
In a previous study conducted by Bernstein, et al.,17 macular pigment levels were measured in children, infants, and premature infants, and a correlation was demonstrated between age, serum carotenoid levels, and macular pigment optical density (MPOD). Newborn infants were not included in the study by Bernstein et al., so levels of macular pigment at birth were unknown, although linear regression analysis predicted a MPOD at birth of 0.0835. Also, the relationship between maternal carotenoid levels and infant carotenoid status was not addressed in the previous study by Bernstein et al. The purpose of the study reported here is to examine the interrelationships of maternal and infant carotenoid status and macular pigment levels in term newborn infants. As a secondary measure, we also evaluated the effects of IUGR on carotenoid status in term newborn infants.
Methods
Human Subjects
The study enrolled 30 healthy-term infants (37 weeks gestation or older) and their mothers, and 10-term IUGR (birth weight < 10th percentile) infants and their mothers prior to hospital discharge (48–72 hours postdelivery). Informed consent was obtained from all mothers under a University of Utah institutional review board approved protocol and in compliance with the Declaration of Helsinki. A subgroup of 16 infants was imaged to measure macular pigment. This subgroup was selected based on parental consent to join the optional substudy and on imager availability. The infants received 0.2% cyclopentolate-HCl and 1% phenylephrine-HCl (Cyclomydril; Alcon, Fort Worth, TX) for pupil dilation prior to imaging. Due to the young age of the newborn infants in the study, swaddling was sufficient for imaging, and general anesthesia was not necessary to complete the measurements. Demographic information including age, sex, race and ethnicity, and prenatal supplementation was obtained at the time of enrollment and was compiled in the Table.
Table. .
Demographic and Dietary Information
|
Infants |
Mothers |
|||||||||||||||
|
Race/Ethnicity |
Sex |
Gestational Age, Wk |
Race/Ethnicity |
Fruit & Vegetable Intake |
Taking Prenatal Vitamins |
|||||||||||
|
Caucasian |
Latino |
Asian |
Other |
Male |
Female |
37–38 |
39–40+ |
Caucasian |
Latino |
Asian |
Other |
0–2/d |
3–4/d |
>4/d |
||
| Control | 53% | 43% | 3% | 0% | 53% | 47% | 43% | 57% | 53% | 43% | 3% | 0% | 33% | 40% | 23% | 93% |
| IUGR | 33% | 58% | 8% | 0% | 42% | 58% | 75% | 25% | 30%* | 60%* | 10% | 0% | 50% | 33% | 17% | 100% |
Mother IUGR percentages differ from infant IUGR percentages due to two mothers of twins.
Blood and Milk Carotenoid Measurements
Nonfasting blood samples were obtained at the time of a routine blood draw for the infants, and an additional draw was performed on the mothers. Serum was centrifuged at 3500 rpm (1000g) at 4°C for 5 minutes. Carotenoids were then extracted from 200 μL portions of serum into ethylacetate with 0.1% butylated hydroxytoluene (BHT) and dried under nitrogen. The dried film was resuspended in HPLC organic phase and analyzed by HPLC according to previously published methods.11 Total serum carotenoids were the sum of lutein, oxolutein, zeaxanthin, beta-cryptoxanthin, alpha-carotene, beta-carotene, and lycopene. Breast milk was collected via manual expression, separate from normal feeding times, 48 to 72 hours after delivery, and colostrum was not collected. Breast milk samples were analyzed similarly with carotenoids extracted from 1 mL portions of breast milk into ethylacetate with 0.1% BHT and analyzed as described for serum above.
Skin Carotenoid Measurement
Skin carotenoid levels were measured noninvasively via resonance Raman spectroscopy (RRS)18 on the heel of the infants and the palm of the mothers. This was the measurement of choice for infants due to its reliability as a noninvasive biomarker of serum and tissue carotenoid levels.4,19,20 Briefly, a 10 mW, 488 nm, low intensity blue laser light was focused on a 2-mm segment of skin for 30 seconds using a hand-held probe containing light delivery and collection optics (Image Technologies, Inc., Salt Lake City, UT). The collected backscattered light was analyzed by a custom Raman spectrograph. The intensity of the characteristic carbon–carbon double-bond stretch band at 1525 cm−1 was measured after correcting for background skin fluorescence. Signal intensity was reported in arbitrary units (Raman Counts) after calibration at each session against an external industrial diamond calibration standard. A total of three measurements were taken for each subject, and the average value was used for analysis.
Macular Pigment Measurement
The measurement of MPOD was performed according to the same protocol described by Bernstein et al.17 A brief description of the methods is included here as well. Video capture images from each eye, centered on the fovea, were collected on a RetCam II (Clarity Medical Systems, Pleasanton, CA) using the blue light fluorescein angiography light source with the matched barrier filter for fluorescein angiography omitted. Total measurement times were less than 1 minute per eye. The blue light reflectance images were transferred to ImageJ software (National Institutes of Health, Bethesda, MD), and the blue light channel data were converted to grayscale images. ImageJ software was then used to compare pixel intensity at the fovea and background regions, and using a scaling factor of 1.15 to adjust for the peak absorbance of macular pigment at 460 nm versus the RetCam's blue light source peak at 475 to 480 nm, two- and three-dimensional macular pigment plot profiles were obtained. The reference light intensity, Imax, was measured at approximately 10° eccentricity from the fovea (i.e., at a peripheral location where the macular pigment concentration is known to be minimal). Light reflections of superficial layers could be identified in the RetCam images as easily identifiable isolated regions with hyperreflective intensities. We avoided these areas by manually selecting only “normal” peripheral areas (i.e., areas with relatively low and similar light intensities at various suitable locations along the periphery) and using only their intensity average as reference. This processing strategy is similar to the evaluation of autofluorescence-based macular pigment imaging in adults.21
Statistical Analysis
Statistical analyses were performed on SAS version 9.3 (SAS Institute Inc., Cary, NC). MPOD measurements for right and left eyes were averaged.
Results
Subject Data
Over a period of 6 weeks, a total of 30 healthy-term infants were enrolled with their mothers, and 10 IUGR-term infants (two sets of twins) with their mothers were also enrolled.
Serum Carotenoid Measurements
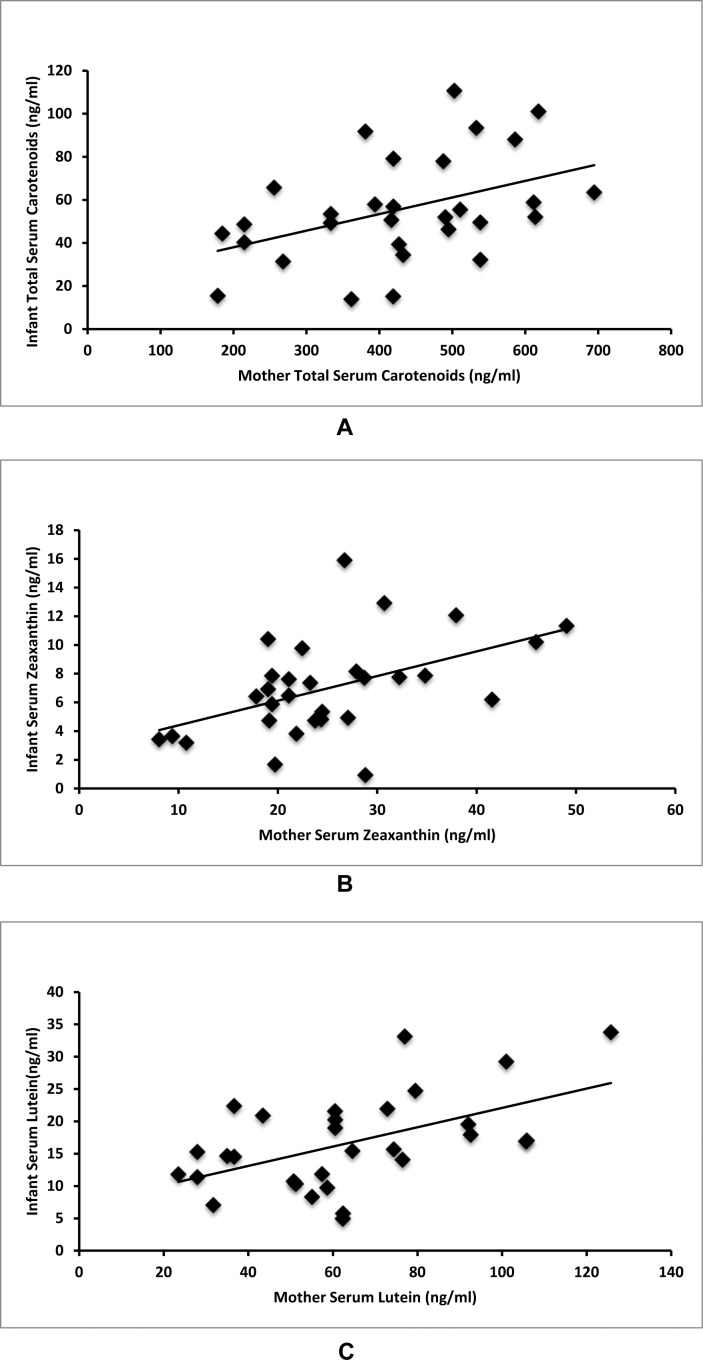
Average mother serum total carotenoids were 428.4 ng/mL (178.3–694.6 ng/mL, SD = 125.3), serum lutein levels were 63.2 ng/mL (23.4–125.7 ng/mL, SD = 26.1), and serum zeaxanthin levels were 24.3 ng/mL (8.1–49.1 ng/mL, SD = 9.4). As shown in Figure 1, correlations were found between mother and infant total serum carotenoids (r = 0.43, P = 0.017), mother and infant serum zeaxanthin (r = 0.49, P = 0.006), and mother and infant serum lutein (r = 0.53, P = 0.003). Infant levels were much lower with average serum total carotenoids of 57.2 ng/mL (13.8–110.6 ng/mL, SD = 24.1), average serum lutein of 17.1 ng/mL (4.9–33.8 ng/mL, SD = 7.3), and average serum zeaxanthin of 7.14 ng/mL (0.9–15.9 ng/mL, SD = 3.3).
Figure 1. .
Maternal–infant serum carotenoid correlations. (A) Mother total serum carotenoids correlate with infant total serum carotenoids (r = 0.43, P = 0.017). (B) Mother serum zeaxanthin correlates with infant serum zeaxanthin (r = 0.49, P = 0.006). (C) Mother serum lutein correlates with infant serum lutein (r = 0.53, P = 0.003).
Skin Carotenoid Levels
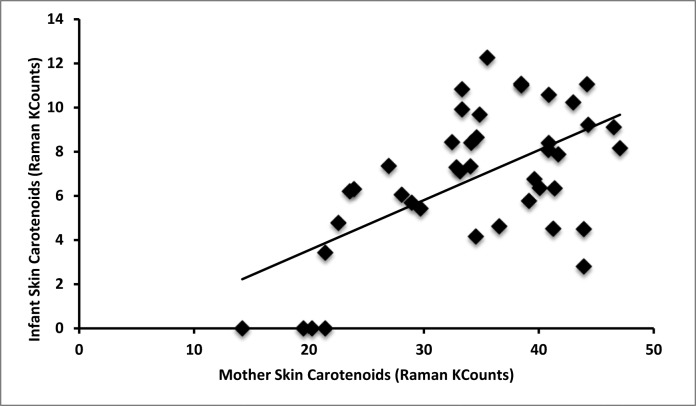
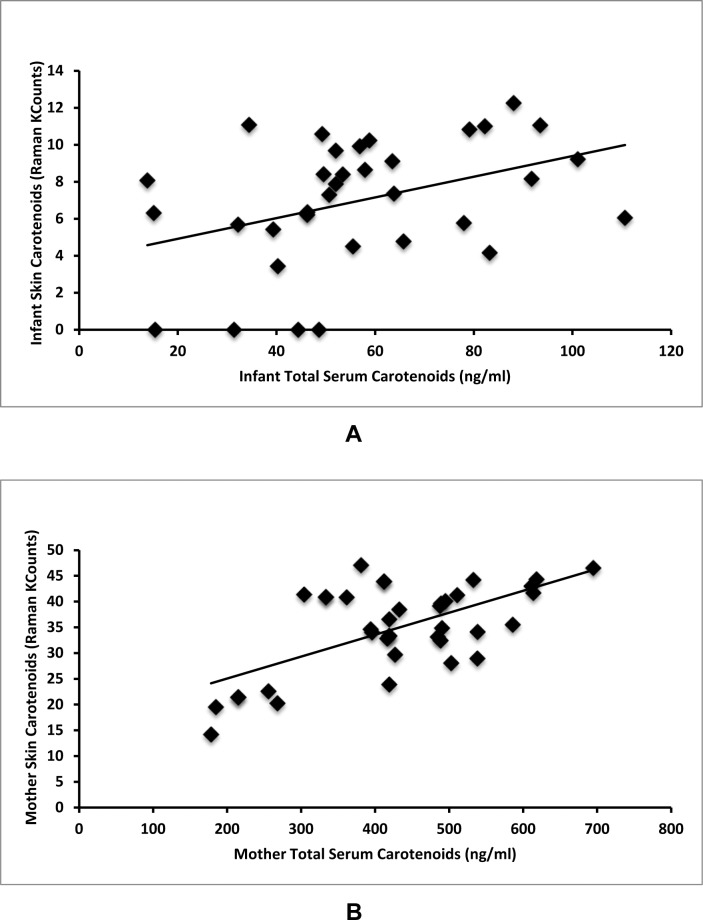
Average mother Raman skin carotenoids were 34400 Raman counts (14200–46500 Raman counts, SD = 8300). Average infant Raman skin carotenoids were much lower than mother levels at 6800 Raman counts (0–12300 Raman counts, SD = 3200). Mother Raman skin carotenoid levels correlate with infant skin carotenoid levels (r = 0.59, P < 0.001, Fig. 2). Infant skin carotenoids correlate with infant serum levels (r = 0.39, P = 0.021, Fig. 3A). Mother Raman skin carotenoids correlate with mother serum levels (r = 0.63, P < 0.001, Fig. 3B). It is notable that four infants had Raman skin measurements quantified as 0 or no difference from background. These infants were all from mothers in the bottom quintile of skin carotenoid levels as seen in Figure 2.
Figure 2. .
Maternal–infant skin carotenoid correlations. Mother skin carotenoids correlate with infant skin carotenoids (r = 0.59, P < 0.001).
Figure 3. .
Serum–skin carotenoid correlations. (A) Infant serum carotenoids correlate with infant Raman skin carotenoids (r = 0.39, P = 0.021). (B) Mother serum carotenoids correlate with mother Raman skin carotenoids (r = 0.63, P < 0.001).
RetCam Measurements of Macular Pigment
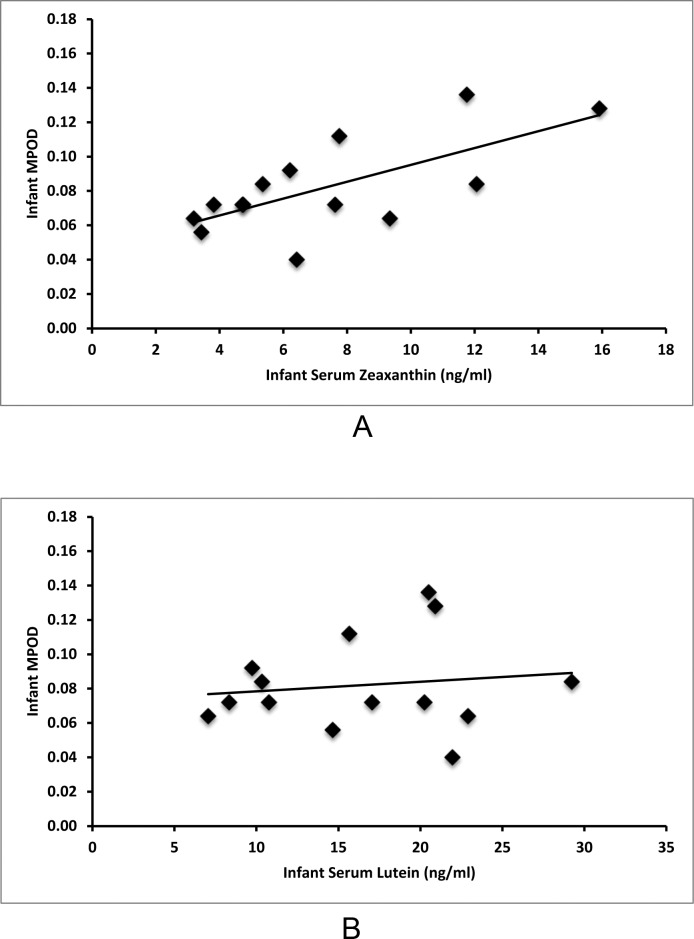
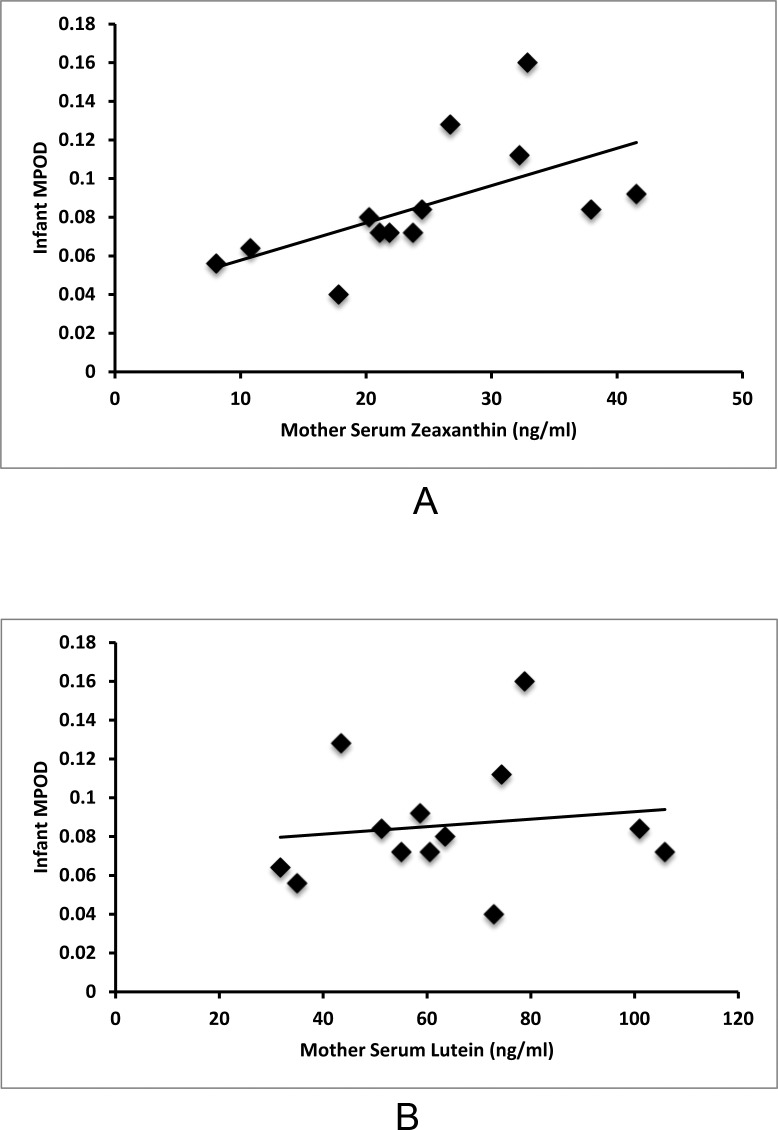
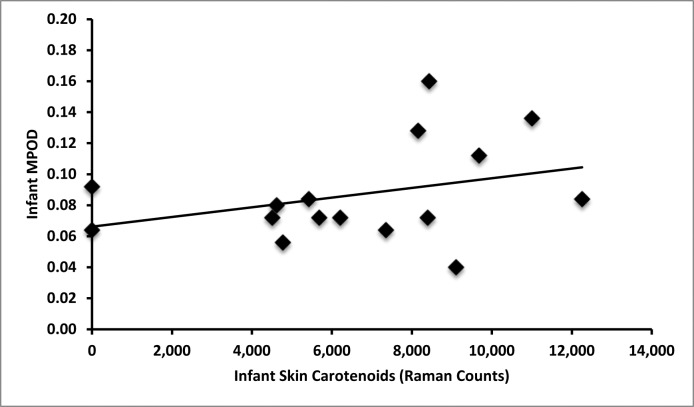
Macular pigment was detectable in all imaged term infants ranging from 0.04 to 0.16 with an average value of 0.087 (SD = 0.032). As shown in Figure 4A, infant serum zeaxanthin levels correlate with MPOD (r = 0.68, P = 0.007), but infant serum lutein levels do not correlate with MPOD (r = 0.13, P = 0.648, Fig. 4B). As shown in Figure 5A, mother serum zeaxanthin levels also correlate with infant MPOD values (r = 0.59, P = 0.03), but mother serum lutein levels do not correlate with infant MPOD (r = 0.14, P = 0.653, Fig. 5B). Infant MPOD was not correlated with infant serum lutein + zeaxanthin (r = 0.36, P = 0.210) or mother serum lutein + zeaxanthin levels (r = 0.31, P = 0.653). Infant Raman skin carotenoids did not correlate with infant MPOD (r = 0.34, P = 0.202, Fig. 6).
Figure 4.
Serum–MPOD carotenoid correlations in infants. (A) Infant serum zeaxanthin correlates with infant MPOD (r = 0.68, P = 0.007). (B) Infant serum lutein does not correlate with infant MPOD (r = 0.13, P = 0.648).
Figure 5.
Maternal serum carotenoid and infant MPOD correlations. (A) Mother serum zeaxanthin correlates with infant MPOD (r = 0.59, P = 0.03). (B) Mother serum lutein does not correlate with infant MPOD (r = 0.14, P = 0.653).
Figure 6. .
Infant skin carotenoid and MPOD correlations. Infant skin carotenoids do not correlate with MPOD (r = 0.34, P = 0.202).
IUGR Comparisons
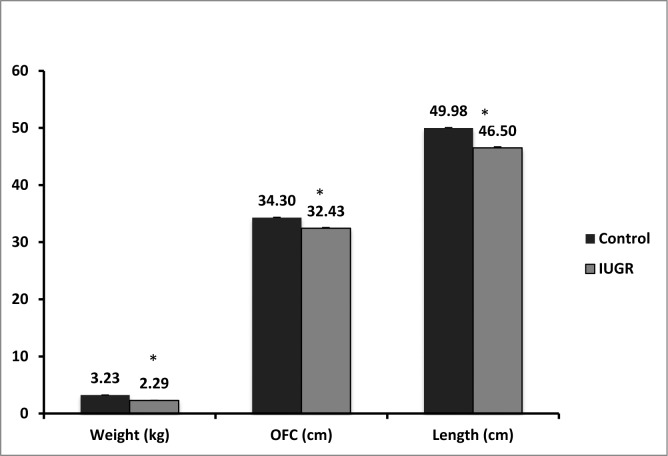
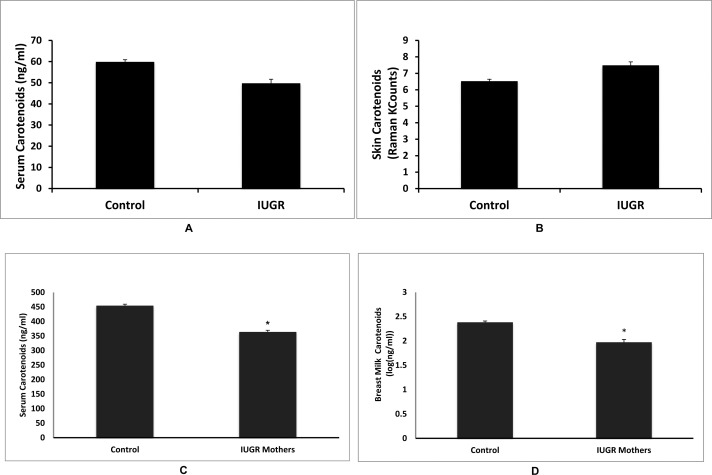
IUGR infants had significantly lower birth weights (P < 0.001), lengths (P = 0.001), and occipitofrontal head circumferences than control infants (P < 0.001, Fig. 7). There was no significant difference between control and IUGR infant total serum carotenoids (P = 0.205), and skin carotenoids (P = 0.327, Figs. 8A, 8B). Mothers of healthy control infants had significantly higher total serum carotenoids (P = 0.008), and total breast milk carotenoids compared with mothers of IUGR infants (P = 0.038, Figs. 8C, 8D). There was no significant difference between control and IUGR mother skin carotenoids (P = 0.680). Of the 10 IUGR infants, only two were consented and imaged for MPOD. Average MPOD for IUGR infants was 0.09, which was lower than the average control MPOD value of 0.11, but this difference was not statistically significant (P = 0.08).
Figure 7. .
Comparisons of healthy and IUGR infants. IUGR infants had lower weights (P < 0.001), occipitofrontal circumferences (P = 0.001), and lengths (P < 0.001) compared with control infants. Asterisks denote statistically significant differences (P < 0.05).
Figure 8. .
Comparisons of carotenoid status between healthy and IUGR infants and their mothers. (A) Infant serum carotenoids were not different between control and IUGR groups (P = 0.205). (B) Infant skin carotenoids were not different between control and IUGR groups (P = 0.327). (C) Mothers of IUGR infants had significantly lower total serum carotenoids compared with control mothers (P = 0.008). (D) Mothers of IUGR infants had significantly lower breast milk total carotenoids compared with control mothers (P = 0.038). Asterisks denote statistically significant differences (P < 0.05).
Discussion
The carotenoids lutein and zeaxanthin are likely to be important in macular development, visual acuity, and protection against AMD.3,22 Lutein and zeaxanthin are obtained from the diet, and systemic maternal stores and breast milk are important fetal and infant carotenoid sources.1,2,14 Most over-the-counter prenatal supplements contain beta-carotene, but lutein and zeaxanthin are not normally added to prenatal multivitamins, and none of the mothers enrolled in our study took prenatal vitamins containing lutein or zeaxanthin. The lack of lutein and zeaxanthin in prenatal supplements leaves a balanced diet with green leafy vegetables and orange and yellow fruits as the primary source of maternal and fetal lutein and zeaxanthin.1,2
Lutein, zeaxanthin, and meso-zeaxanthin are the main carotenoids found in the macula.5,7 Lutein and zeaxanthin levels are dependent on dietary intake, and lutein acts as the metabolic precursor for meso-zeaxanthin.23 Serum carotenoid levels have been shown to correlate with macular levels,12,24 and macular pigment at the fovea consists of lutein, zeaxanthin, and meso-zeaxanthin in a 1:1:1 ratio.25 Although macular pigment in infants has been quantified in autopsy studies,5 prior to our study, noninvasive measurement techniques had not been reported for newborn infants. The use of the blue light reflectometry with the RetCam, a Food and Drug Administration cleared imaging device developed specifically for infants and children, provided us with an accurate and appropriate noninvasive method to measure infant MPOD. This method was first reported by Bernstein et al.,17 who found that macular pigment was undetectable in premature infants and that MPOD rose steadily with age to at least age 7. Although newborn infants were not measured by Bernstein et al., linear regression analysis predicted an MPOD level of 0.0835 at birth, and our MPOD average value of 0.087 in newborns is very close to the predicted value. In addition, serum measurements quantified by HPLC indicate that maternal and infant serum zeaxanthin levels correlate with infant MPOD. This finding strengthens the relationship of zeaxanthin with macular development, and suggests the need for adequate fetal serum carotenoid levels in utero. We also found that serum lutein and lutein + zeaxanthin combination levels did not correlate with MPOD levels in infants. This finding is in contrast to early reports that demonstrated that lutein, not zeaxanthin, was the most abundant carotenoid in the infant retina,25 although the infant macula was not specifically examined. The decreased influence of lutein on macular pigment deposition in utero may be due to inefficiency or incomplete development of the enzymes involved in the conversion of lutein to meso-zeaxanthin in the macula as occurs in healthy adults.23,25 This could explain our finding that zeaxanthin had a stronger correlation with MPOD than lutein in newborn infant maculae. Clinical trials with zeaxanthin supplementation may help to clarify the roles of lutein and zeaxanthin in macular development.
In addition to the relationship of infant serum and MPOD, the relationship between mother serum and infant MPOD is also important in understanding infant macular pigment deposition. Previous studies have shown correlations between carotenoids in maternal serum and cord blood, but our study is unique in examining this relationship with infant serum 24 to 72 hours after birth.26,27 Mother skin and serum carotenoid levels correlated with infant skin and serum levels. Similar to the infant serum–MPOD correlations described above, mother serum zeaxanthin levels also correlated with infant MPOD measurements. These findings suggest that maternal zeaxanthin is important for fetal macular pigment development, and provide unique evidence that maternal nutrition may affect fetal macular carotenoid deposition.
Along with these mother–infant correlations, average mother serum lutein and zeaxanthin levels measured in our study were lower than levels previously reported in healthy adults.11,28 Mother lutein and zeaxanthin levels in our study were similar to values previously reported for pregnant mothers.27–31 The depletion in lutein and zeaxanthin levels in pregnancy suggests an increased maternal need or placental transfer of these carotenoids during gestation. These findings also suggest potential benefits of prenatal lutein and zeaxanthin supplementation and prenatal nutrient monitoring. As evidenced by our maternal and infant skin and serum measurements, RRS is a reliable biomarker of maternal carotenoid levels and indirect biomarker of fetal carotenoid levels. Prenatal lutein and zeaxanthin supplementation and RRS nutrient monitoring need to be studied directly to determine whether lutein and zeaxanthin supplementation and monitoring may be beneficial complements to routine prenatal care.
It is possible that IUGR may be associated with retinopathy in infants and impaired vision later in life,32–34 and this group of infants was of interest in our study. Our results indicate that although IUGR infants did not have decreased serum or skin carotenoids or MPOD levels, their mothers were depleted in serum and breast milk carotenoids when compared with mothers of healthy infants. These results may have several explanations including maintenance of placental carotenoid transfer even in the setting of depleted maternal serum levels. Preferential partitioning of limited nutrients to the fetus, including protein and micronutrients, has been previously documented in the setting of maternal malnutrition.35,36 In our study, mothers of IUGR infants had depleted carotenoid levels compared with controls, and a nutrient sparing mechanism may have maintained placental carotenoid transfer to preserve carotenoid levels in IUGR infants.
This study was limited by various factors. Mothers and infants were enrolled 24 to 72 hours post delivery, and all measurements were made before patients were discharged from the hospital. This limited the number of study-related assessments that could be performed. For example, it was not feasible to measure the mothers' MPOD in a post delivery hospital ward. Due to time constraints and need for proper mother and infant recovery from delivery, serum samples were not taken while fasting, so some variation in these measurements may be due to dietary influences. Finally, this was a cross-sectional study of maternal and infant carotenoid status and does not allow for causality to be determined.
Maternal nutrition is vital to proper fetal development, especially with regards to the macula. Maternal nutritional status correlates with and likely influences infant nutrition status, as evidenced by our serum HPLC and skin RRS measurements. RetCam imaging using blue light reflectometry is a noninvasive measurement that provided evidence for the presence of MPOD within 24 to 72 hours of birth. Infant serum zeaxanthin levels correlate with MPOD measurements indicating a physiologic relationship between zeaxanthin and macular development is present at birth. Maternal serum zeaxanthin levels also correlate with infant MPOD, suggesting an important link between maternal carotenoid status, especially zeaxanthin, and infant macular development. This relationship needs to be explored in greater detail in larger studies to confirm its role in macular physiology early in life. Randomized, controlled trials with maternal zeaxanthin supplementation may also be helpful in determining the effectiveness of prenatal zeaxanthin supplementation on improved macular pigment levels, accelerated foveal development, and enhanced ocular function in infants and later in life.
Acknowledgments
The authors thank Carrie Rau and Bonnie Carlstrom who helped with recruitment and data collection, and Xiaoming Sheng who assisted with statistical analysis.
Supported by grants from National Eye Institute (EY-11600), NID/NIDDK T35 HL007744, Research to Prevent Blindness, and Image Technologies, Inc.
Disclosure: B.S. Henriksen, None; G. Chan, None; R.O. Hoffman, None; M. Sharifzadeh, None; I.V. Ermakov, None; W. Gellermann, None; P.S. Bernstein, Abbott Nutrition (F), Kalsec (C), Kemin Health (C), DSM (C)
References
- 1. Scott KJ, Thurnham DI, Hart DJ, Bingham SA, Day K. The correlation between the intake of lutein, lycopene and beta-carotene from vegetables and fruits, and blood plasma concentrations in a group of women aged 50-65 years in the UK. Br J Nutr. 1996; 75: 409–418 [DOI] [PubMed] [Google Scholar]
- 2. Holden Joanne M, et al. Carotenoid content of US foods: an update of the database. J Food Compos Anal. 1999; 12: 169–196 [Google Scholar]
- 3. Zimmer JP, Hammond BR., Jr. Possible influences of lutein and zeaxanthin on the developing retina. Clin Ophthalmol. 2007; 1: 25–35 [PMC free article] [PubMed] [Google Scholar]
- 4. Scarmo S, Cartmel B, Lin H, et al. Significant correlations of dermal total carotenoids and dermal lycopene with their respective plasma levels in healthy adults. Arch Biochem Biophys. 2010; 504: 34–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bone RA, Landrum JT, Fernandez L, Tarsis SL. Analysis of the macular pigment by HPLC: retinal distribution and age study. Invest Ophthalmol Vis Sci. 1988; 29: 843–849 [PubMed] [Google Scholar]
- 6. Bone RA, Landrum JT, Tarsis SL. Preliminary identification of the human macular pigment. Vision Res. 1985; 25: 1531–1535 [DOI] [PubMed] [Google Scholar]
- 7. Bone RA, Landrum JT, Hime GW, Cains A, Zamor J. Stereochemistry of the human macular carotenoids. Invest Ophthalmol Vis Sci. 1993; 34: 2033–2040 [PubMed] [Google Scholar]
- 8. Bernstein PS. Nutritional interventions against age-related macular degeneration. Acta Hortic. 2009; 841: 103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hammond BR, Jr,, Wooten BR, Curran-Celentano J. Carotenoids in the retina and lens: possible acute and chronic effects on human visual performance. Arc Biochem Biophys. 2001; 385: 41–46 [DOI] [PubMed] [Google Scholar]
- 10. Stringham JM, Bovier ER, Wong JC, Hammond BR., Jr. The influence of dietary lutein and zeaxanthin on visual performance. J Food Sci. 2010; 75: R24–R29 [DOI] [PubMed] [Google Scholar]
- 11. Bernstein PS, Ahmed F, Liu A, et al. Macular pigment imaging in AREDS2 participants: an ancillary study of AREDS2 subjects enrolled at the Moran Eye Center. Invest Ophthalmol Vis Sci. 2012; 53: 6178–6186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Landrum JT, Bone RA, Joa H, Kilburn MD, Moore LL, Sprague KE. A one year study of the macular pigment: the effect of 140 days of a lutein supplement. Exp Eye Res. 1997; 65: 57–62 [DOI] [PubMed] [Google Scholar]
- 13. Beatty S, Chakravarthy U, Nolan JM, et al. Secondary outcomes in a clinical trial of carotenoids with coantioxidants versus placebo in early age-related macular degeneration. Ophthalmology. 2012; 120: 600–606 [DOI] [PubMed] [Google Scholar]
- 14. Song BJ, Jouni ZE, Ferruzzi MG. Assessment of phytochemical content in human milk during different stages of lactation. Nutrition. 2013; 29: 195–202 [DOI] [PubMed] [Google Scholar]
- 15. Jewell VC, Mayes CB, Tubman TR, Northrop-Clewes CA, Thurnham DI. A comparison of lutein and zeaxanthin concentrations in formula and human milk samples from Northern Ireland mothers. Eur J Clin Nutr. 2004; 58: 90–97 [DOI] [PubMed] [Google Scholar]
- 16. Baschat AA. Pathophysiology of fetal growth restriction: implications for diagnosis and surveillance. Obstet Gynecol Surv. 2004; 59: 617–627 [DOI] [PubMed] [Google Scholar]
- 17. Bernstein PS, Sharifzadeh M, Liu A, et al. Blue-light reflectance imaging of macular pigment in infants and children. Invest Ophthalmol Vis Sci. 2013; 54: 4034–4040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ermakov IV, Sharifzadeh M, Bernstein PS, Gellermann W. Application of resonance Raman spectroscopy to the detection of carotenoids in vivo. In: Landrum JT. ed Carotenoids–Physical, Chemical and Biological Functions and Properties. Atlanta, GA: CRC Press; 2009; 87–109 [Google Scholar]
- 19. Mayne ST, Cartmel B, Scarmo S, et al. Noninvasive assessment of dermal carotenoids as a biomarker of fruit and vegetable intake. Am J Clin Nutr. 2010; 92: 794–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ermakov IV, Ermakova MR, Bernstein PS, Chan GM, Gellermann W. Resonance Raman based skin carotenoid measurements in newborns and infants [published online instead of print November 29, 2012]. J Biophotonics. doi:10.1002/jbio.201200195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sharifzadeh M, Bernstein PS, Gellermann W. Nonmydriatic fluorescence based quantitative imaging of human macular pigment distributions. J Opt Soc Am A Opt Image Sci Vis. 2006; 23: 2373–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weigert G, Kaya S, Pemp B, et al. Effects of lutein supplementation on macular pigment optical density and visual acuity in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011; 52: 8174–8178 [DOI] [PubMed] [Google Scholar]
- 23. Johnson EJ, Neuringer M, Russell RM, Schalch W, Snodderly DM. Nutritional manipulation of primate retinas, III: effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Invest Ophthalmol Vis Sci. 2005; 46: 692–702 [DOI] [PubMed] [Google Scholar]
- 24. Wang W, Connor SL, Johnson EJ, Klein ML, Hughes S, Connor WE. Effect of dietary lutein and zeaxanthin on plasma carotenoids and their transport in lipoproteins in age-related macular degeneration. Am J Clin Nutr. 2007; 85: 762–769 [DOI] [PubMed] [Google Scholar]
- 25. Bone RA, Landrum JT, Friedes LM, et al. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp Eye Res. 1997; 64: 211–218 [DOI] [PubMed] [Google Scholar]
- 26. Yeum KJ, Ferland G, Patry J, Russell RM. Relationship of plasma carotenoids, retinol and tocopherols in mothers and newborn infants. J Am Coll Nutr. 1998; 17: 442–447 [DOI] [PubMed] [Google Scholar]
- 27. Kiely M, Cogan PF, Kearney PJ, Morrissey PA. Concentrations of tocopherols and carotenoids in maternal and cord blood plasma. Eur J Clin Nutr. 1999; 53: 711–715 [DOI] [PubMed] [Google Scholar]
- 28. Curran-Celentano J, Hammond BR, Jr,, Ciulla TA, Cooper DA, Pratt LM, Danis RB. Relation between dietary intake, serum concentrations, and retinal concentrations of lutein and zeaxanthin in adults in a Midwest population. Am J Clin Nutr. 2001; 74: 796–802 [DOI] [PubMed] [Google Scholar]
- 29. Cena H, Roggi C, Turconi G. Development and validation of a brief food frequency questionnaire for dietary lutein and zeaxanthin intake assessment in Italian women. Eur J Nutr. 2008; 47: 1–9 [DOI] [PubMed] [Google Scholar]
- 30. McLernon PC, Wood LG, Murphy VE, Hodyl NA, Clifton VL. Circulating antioxidant profile of pregnant women with asthma. Clin Nutr. 2012; 31: 99–107 [DOI] [PubMed] [Google Scholar]
- 31. Zhang C, Williams MA, Sanchez SE, et al. Plasma concentrations of carotenoids, retinol, and tocopherols in preeclamptic and normotensive pregnant women. Am J Epidemiol. 2001; 153: 572–580 [DOI] [PubMed] [Google Scholar]
- 32. Gupta A, Kaliaperumal S, Setia S, Suchi ST, Rao VA. Retinopathy in preeclampsia: association with birth weight and uric acid level. Retina. 2008; 28: 1104–1110 [DOI] [PubMed] [Google Scholar]
- 33. Martin L, Ley D, Marsal K, Hellström A. Visual function in young adults following intrauterine growth retardation. J Pediatr Ophthalmol Strabismus. 2004; 41: 212–218 [DOI] [PubMed] [Google Scholar]
- 34. Loeliger M, Louey S, Cock ML, Harding R, Rees SM. Chronic placental insufficiency and fetal growth restriction lead to long-term effects on postnatal retinal structure. Clin Experiment Ophthalmol. 2003; 31: 250–253 [DOI] [PubMed] [Google Scholar]
- 35. King JC. The risk of maternal nutritional depletion and poor outcomes increases in early or closely spaced pregnancies. J Nutr. 2003; 133: 1732S–1736S [DOI] [PubMed] [Google Scholar]
- 36. Winkvist A, Habicht JP, Rasmussen KM. Linking maternal and infant benefits of a nutritional supplement during pregnancy and lactation. Am J Clin Nutr. 1998; 68: 656–61 Erratum in: Am J Clin Nutr 1999;69:160 [DOI] [PubMed] [Google Scholar]