Abstract
Background and Aims
A plant investing in reproduction partitions resources between flowering and seed production. Under resource limitation, altered allocations may result in floral trait variations, leading to compromised fecundity. Floral longevity and timing of selfing are often the traits most likely to be affected. The duration of corolla retention determines whether fecundity results from outcrossing or by delayed selfing-mediated reproductive assurance. In this study, the role of pollination schedules and soil water availability on floral longevity and seed production is tested in Collinsia heterophylla (Plantaginaceae).
Methods
Using three different watering regimes and pollination schedules, effects on floral longevity and seed production were studied in this protandrous, flowering annual.
Key Results
The results reveal that soil water status and pollination together influence floral longevity with low soil water and hand-pollinations early in the floral lifespan reducing longevity. However, early pollinations under excess water did not extend longevity, implying that resource surplus does not lengthen the outcrossing period. The results also indicate that pollen receipt, a reliable cue for fecundity, accelerates flower drop. Early corolla abscission under drought stress could potentially exacerbate sexual conflict in this protandrous, hermaphroditic species by ensuring self-pollen paternity and enabling male control of floral longevity. While pollination schedules did not affect fecundity, water stress reduced per-capita seed numbers. Unmanipulated flowers underwent delayed autonomous selfing, producing very few seeds, suggesting that inbreeding depression may limit benefits of selfing.
Conclusions
In plants where herkogamy and dichogamy facilitate outcrossing, floral longevity determines reproductive success and mating system. Reduction in longevity under drought suggests a strong environmental effect that could potentially alter the preferred breeding mode in this mixed-mated species. Extrapolating the findings to unpredictable global drought cycles, it is suggested that in addition to reducing yield, water stress may influence the evolutionary trajectory of plant mating system.
Keywords: Autonomous selfing, Collinsia heterophylla, drought stress, floral longevity, herkogamy, Plantaginaceae, reproductive success, resource allocation, sexual conflict
INTRODUCTION
Floral longevity, defined as the duration of time a flower is open and receptive to pollination, is a crucial measure that influences plant reproductive success and can be invoked to explain the extensive variation in angiosperm reproductive strategies (Primack, 1985; Schoen and Ashman, 1995). Flowers are the reproductive organs that protect male and female structures as well as play a significant role in attracting pollinators to facilitate outcrossing (Darwin, 1876) and they are metabolically expensive as turgor in floral organs needs to be maintained throughout the floral lifespan despite sustained evapo-transpiration (Carroll et al., 2001). Cost–benefit models of longevity, describing the relationship between resource trade-offs and flower maintenance, suggest that long-lived flowers are selectively favoured when their fitness accrual rates and the cost for the maternal plant to maintain them are both low and short-lived flowers are favoured when maintenance costs and fitness accrual rates are both high (Schoen and Ashman, 1995). Flowers should therefore exhibit optimal longevity that maximizes fitness accrual at minimum costs to the maternal plant, an optimum which can be expected to vary with the environment, mating system, pollination success and plant species (Gori, 1983; Primack, 1985; Schoen and Ashman, 1995; van Doorn, 1997).
Physiological cues emanating from the completion of sexual functions induce floral senescence but the specific sex that is responsible for these cues may vary from species to species (van Doorn, 1997; Evanhoe and Galloway, 2002; Rathcke, 2003; Stpiczynska, 2003; Blair and Wolfe, 2007; Castro et al., 2008b; Marques and Draper, 2012; Weber and Goodwillie, 2013). In many protandrous species, successful ovule fertilization resulting from pollen deposition leads to floral senescence (Ashman and Schoen, 1997; Arathi et al., 2002; Evanhoe and Galloway, 2002; Blair and Wolfe, 2007). It has been proposed that pollen receipt (completion of female function) is a reliable cue for corolla abscission because by then, pollen removal (completion of male function) is also accomplished, and the maternal plant having completed both the sexual functions can gain the maximum possible benefit (Primack, 1985; Proctor and Harder, 1995; Stpiczynska, 2003; Abdala-Roberts et al., 2007; Marques and Draper, 2012). In cases where floral longevity is sensitive to pollen deposition, several factors including the attractiveness of flowers (van Doorn, 1997; Blair and Wolfe, 2007; Glaettli and Barrett, 2008) and placement of floral organs (Barrett, 2002; Vallejo-Marin and Barrett, 2009) may affect pollen receipt and therefore successful fertilization. In self-compatible species, pollen receipt could potentially be completed during pollen removal with self-pollen depositing on the stigma, but traits such as herkogamy (spatial separation of stigma and anthers) and dichogamy (temporal segregation of male and female phases) significantly reduce this likelihood (Barrett, 2002).
While many angiosperm species readily self-pollinate, not all of them rely on selfing as the predominant reproductive strategy due to associated costs of inbreeding depression (Schemske and Lande, 1985; Goodwillie, 2000; Cheptou and Mathias, 2001; Kennedy and Elle, 2008a; Johnston et al., 2009). In some self-compatible species, herkogamy and dichogamy have been shown to facilitate cross-pollination, with selfing occurring only towards the end of the floral lifespan (Kalisz et al., 1999; Armbruster et al., 2002; Medrano et al., 2005). Such delayed selfing could be a mechanism for reproductive assurance when flowers fail to attract pollinators (Rathcke, 2003; Kalisz et al., 2004; Kennedy and Elle, 2008b; Busch and Delph, 2012). Considering inbreeding depression costs and reproductive assurance benefits, it is natural to expect selfing to be favoured as flowers age, and outcrossing success is progressively reduced (Jarne and Charlesworth, 1993; Busch and Delph, 2012). Some species have floral traits that augment selfing as the corolla begins to abscise, thus supporting the reproductive assurance hypothesis when inbreeding depression is low (Dole, 1990; Kalisz et al., 1999, 2004; Qu et al., 2007; Brys and Jacquemyn, 2011; Evans et al., 2011). Floral longevity, the period for which corolla is maintained on the plant, is therefore an important parameter that determines both outcrossing and selfing and it is known to be influenced by biotic factors such as pollination (Arathi et al., 2002; Castro et al., 2008a; Marques and Draper, 2012) and by abiotic factors such as a stressful maternal environment (Evans et al., 2011).
Resource status of the environment strongly affects maternal trait expression, and when these traits include floral longevity, plant reproductive success is directly affected (Mousseau and Fox, 1998; Arathi et al., 2002; Evanhoe and Galloway, 2002; Delph and Ashman, 2006). In stressful environments, such as under water limitation, the maternal plant exhibits a plastic corolla maintenance response and considerably reduces floral longevity (Arathi et al., 2002). Circumstances such as seasonal extremes of drought or heat result in the resource-stressed maternal plant allocating resources away from floral maintenance and toward offspring production (Ashman and Schoen, 1997; Abdala-Roberts et al., 2007). Therefore, in addition to successful pollination, resource allocation to floral longevity, a maternally regulated trait, would also greatly depend on maternal resource status.
The genus Collinsia includes mixed-mating species with the large-flowered ones often being predominantly cross-pollinated, resorting to delayed self-pollination under pollinator limitation (Randle et al., 2009; Kalisz et al., 2012). In mixed-mating species, post-pollination reallocation of maternal resources from floral maintenance into seed production has been argued to be the primary cause for post-pollination corolla senescence. However, sexual conflict, where the male function could manipulate the success of female function via floral longevity could provide an alternative explanation for the evolution of post-pollination corolla senescence (Arnqvist and Rowe, 2005; Bernasconi et al., 2006; Lankinen et al., 2006). If the corolla retention period in a protandrous species were to be affected by factors extraneous to pollination, such as abiotic stress, modulations to floral longevity could potentially favour one of the sexes, thus exacerbating sexual conflict. Collinsia heterophylla, one of the large flowered species in the genus, has been a model system for research on sexual conflict (Lankinen et al., 2006, 2007, 2009; Madjidian and Lankinen, 2009). However, studies addressing the effect of environmental stress on floral traits and reproductive strategies are lacking in this species.
The goal of our study was therefore to investigate the effects of soil water status and pollination schedules on floral longevity and seed production in C. heterophylla. This is important in the context of ongoing changes in environmental conditions with unprecedented alterations in precipitation and related phenotypic responses in plants (Nicotra et al., 2010), and can have important implications for our understanding about reproductive success and mating system evolution under extreme drought stress events, similar to what is experienced by endemic populations of C. heterophylla (CNRFC, 2012). In our study, we address the following specific questions: (1) How does soil water status affect floral longevity and seed production? (2) How does pollination schedule affect floral longevity and seed production? (3) Do soil water status and pollination schedules interact to affect floral longevity? (4) How does water-stress affect fecundity under autonomous selfing?
MATERIAL AND METHODS
Study system
Collinsia heterophylla (purple Chinese houses; family Plantaginaceae) is a hermaphroditic, annual flowering plant native to California, USA (Newsom, 1929). Protandrous flowers with short stalks are borne in whorls on a raceme, with the corolla of each flower fused to form upper and lower flaring lips. The lower lip of the corolla is typically deep purple, and in rare cases white, while the upper lip is white to light purple (Fig. 1). Each flower has four epipetalous stamens and one pistil. Of the four stamens, two are short and two are long, all of which have red anthers before dehiscence. Protandry in the flowers facilitates the feature of ‘male-first hermaphrodite-later’ during the floral lifespan. The four anthers mature sequentially, beginning from the day after flower opening (Fig. 2). On the day the corolla opens, none of the four anthers has dehisced, and the stigma, which is not yet receptive, is much shorter than the stamens. As in other species of the genus (Armbruster et al., 2002; Kalisz et al., 2012), the first anther in C. heterophylla typically dehisces 1 d after the flower opens. The remaining three anthers dehisce one at a time on each subsequent day, making the flowers functionally male for the first 1–3 d. The transition in reproductive role from being exclusively male to that of a hermaphrodite occurs around Day 3 when the style elongates and places the now receptive stigma above the stamens (Armbruster et al., 2002; Lankinen et al., 2007). Flowers of C. heterophylla thus exhibit two forms of sexual interference: herkogamy and dichogamy. The onset of stigmatic receptivity is associated with the style elongating past the dehisced anthers, providing ample opportunity for self-pollen deposition. Pollen competitive ability, however, maintains outcrossing as the dominant mating strategy in this mixed-mating species (Lankinen and Armbruster, 2007). The mature fruits explode, releasing the dark brown seeds.
Fig. 1.

Floral biology of Collinsia heterophylla. A tagged experimental flower with white upper lip (white arrow) and a fully flared lower purple lip (yellow arrow).
Fig. 2.
The sequence of dehiscing anthers demonstrating protandry: (A) one anther (black arrow) dehisced on Day 1 after flower opening, style shorter than anthers and stigma not receptive; (B) two anthers dehisced on Day 2 after flower opening, style shorter than anthers and stigma not receptive; (C) three anthers dehisced on Day 3 after flower opening, style beginning to elongate and stigma gaining receptivity; (D) all four anthers dehisced on Day 4 after flower opening, style extended beyond the anthers and stigma fully receptive.
Experimental layout
Seeds collected prior to 2005, from two naturally occurring populations in Sisar Canyon (34·4°N, 119·1°W) and Wheeler Gorge Campground (34·5°N, 119·3°W) in Ventura County, California, were grown under common greenhouse conditions to alleviate maternal effects and the seeds obtained from their inter-crossings were used for this study repeated over two years, autumn 2010 and spring 2011. Two to three outcrossed seeds were planted in each of 30 10-cm square pots with similar amounts by mass of Fafard 4P potting soil and maintained at a day temperature of 23 °C, night temperature of 16 °C and a day length of 16 h. These are conditions close to those occurring during the normal growing season of the species in its native environment (USDA NRCS, 2012). Within 7–10 d after germination, seedlings were thinned to one plant per pot and randomly assigned to one of the three watering frequencies, resulting in ten plants in each of the three water treatments: high (watering all days of the week), medium (watering 2 d each week) and low water (watering once each week). The watering frequencies were chosen to reach soil water levels that represent the range that C. heterophylla plants can tolerate, with high being close to saturated soil moisture and low being the maximum drying down that was still compatible with plant life. All pots with plants were placed in water for 10 min, sufficient to saturate the soil with water. Soil moisture content was measured 1 week after the seedlings were assigned to treatments, and again when each plant began flowering using the soil moisture probe, EC-5 (Decagon Devices Inc., Pullman, WA, USA) to obtain the volumetric water content (% VWC). A two-way ANOVA, with watering frequency and year as direct effects, and % VWC as the dependent variable, indicated a significant effect of watering frequency on % VWC (F2,155 = 146·01, P < 0·0001). A Tukey post-hoc comparison at P < 0·0001 ascertained that soil water status was indeed modulated by watering frequency. High water pots had significantly higher soil water content (21·7 ± 3·9% VWC) than the medium and low water ones and medium water pots had higher soil water content (14·8 ± 4·3% VWC) than the low water ones (7·5 ± 5% VWC).
Longevity experiment
Randomly selected flowers from each of the plants in the three water treatments were subject to three hand-pollination treatments: Early (E), hand-pollinations completed on Days 3 and 4 after anthesis; Middle (M), hand-pollinations on Days 5 and 6 after anthesis; and Late (L), hand-pollinations on Days 7 and 8 after anthesis. Two control treatments per plant in the three water treatments accounted for hand-pollination and emasculation effects: Emasculated Control (Em), flowers were emasculated but not hand-pollinated; and Unmanipulated Control (U), flowers were not handled. Each of the experimental and control flowers was uniquely tagged with coloured tape. One day prior to anthesis, flowers were randomly assigned to the respective hand-pollination treatments or controls (Table 1). The day of anthesis was considered Day 0 and each of the treatment flowers was emasculated 1 d prior to Day 0. To prevent autonomous self-pollination, emasculation was achieved by gently prying apart the petals of a flower and removing all four anthers using a pair of dissection forceps. The stigma received pollen from one flower of a different plant within the same water treatment to minimize pollen donor effects. Pollen from the single donor flower was collected on a sterilized microscope slide using dissection forceps, and hand-pollinations were accomplished by dipping the recipient stigma into the pollen on the slide such that the stigmatic surface was covered with pollen. Experimental and control flowers were monitored daily for corolla wilting or abscission. Floral longevity was determined as the number of days from flower opening to abscission or wilting. Developed ovaries were collected at fruit maturity, as evident from the beginnings of a split in the sutures of the fruit coat. Fruits were allowed to air dry before determining seed number per fruit.
Table 1.
Number of experimental flowers for each pollination schedule and water treatments
| Soil water treatments |
||||
|---|---|---|---|---|
| Low | Medium | High | ||
| Pollination schedules (days after anthesis) | Early: E (3–4) | 30 | 29 | 33 |
| Middle: M (5–6) | 30 | 29 | 32 | |
| Late: L (7–8) | 30 | 28 | 31 | |
| Procedural controls | Emasculated: Em | 18 | 18 | 18 |
| Unmanipulated: U | 18 | 18 | 19 | |
Statistical analyses
Analysis of floral longevity was performed by completing a two-way ANOVA with soil water status and hand-pollination schedules as independent variables, year as the random effect and floral longevity as the dependent variable. Analyses of seed numbers were performed by executing (1) a two-way ANOVA within the hand-pollinated treatments (E, M and L), with soil water treatment and hand-pollination schedules as independent variables, year as the random effect and seed production as the dependent variable and (2) a one-way ANOVA comparing the effect of soil water treatment on seed numbers in the Unmanipulated Control (U). All statistical analyses were executed in IBM SPSS Statistics Version 21.0 (IBM Corp., Armonk, NY, USA), which incorporates Bonferroni-corrected P-values for multiple pairwise comparisons.
RESULTS
Floral longevity of hand-pollinated flowers
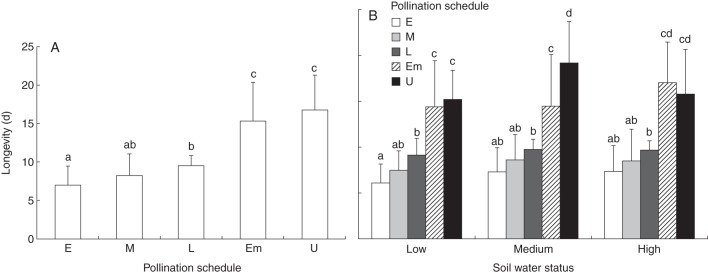
Our results indicate that pollination schedules and soil water status had significant direct and interactive effects on floral longevity in C. heterophylla (Table 2). Pollinations early in the floral lifespan reduced corolla retention period significantly over hand-pollinations late in the floral lifespan while the longevity of flowers pollinated during the middle of the floral lifespan was not different from either early or late hand-pollinations (Fig. 3A). Analysis of the interaction effect of soil water status and pollination schedule on floral longevity indicates that flowers under low soil moisture hand-pollinated early in the floral lifespan retained their corolla for just over 6 d, which is a significant reduction in longevity as compared with late-pollinated flowers in any water status (Fig. 3B). The highest average longevity of over 9 d was seen in flowers receiving hand-pollinations late in the floral lifespan in all the three watering regimes (Fig. 3B). Year did not affect floral longevity.
Table 2.
Effects of soil water and pollination schedules on floral longevity and seed number per fruit in Collinsia heterophylla
| Source | d.f. | Mean SS | F ratio | P |
|---|---|---|---|---|
| Floral longevity | ||||
| Year | 1 | 9·7 | 0·9 | 0·33 |
| Soil water status | 2 | 57·5 | 5·6 | 0·004 |
| Pollination schedules | 4 | 1202·6 | 117·8 | <0·0001 |
| Soil water status × pollination schedules | 8 | 23·5 | 2·7 | 0·007 |
| Error | 351 | 10·2 | ||
| Seed number per fruit (pollinated) | ||||
| Year | 1 | 183·4 | 6·0 | 0·02 |
| Soil water status | 2 | 153·9 | 5·1 | 0·007 |
| Pollination schedules | 2 | 29·9 | 0·9 | 0·3 |
| Soil water status × pollination schedules | 4 | 28·3 | 0·9 | 0·4 |
| Year × pollination schedules | 2 | 7·8 | 0·3 | 0·8 |
| Error | 247 | 30·5 | ||
| Seed number per fruit (unmanipulated) | ||||
| Year | 1 | 0·4 | 0·03 | 0·9 |
| Soil water status | 2 | 38·70 | 3·08 | 0·05 |
| Error | 48 | 12·53 | ||
Fig. 3.
(A) Pollination schedules significantly influenced floral longevity in C. heterophylla. Each bar represents the average days to corolla abscission (± s.d.). Bars with different letters indicate significant differences in floral longevity among pollination treatments by a Tukey post-hoc comparison at P < 0·05. (B) Interactive effect of soil water and pollination schedules on longevity. The x-axis refers to different soil water treatments and individual bars refer to different pollination treatments described in Table 1 and indicated in the key: early (E), middle (M), late (L) hand-pollinations, emasculated (Em) and unmanipulated (U) controls. Bars with different letters indicate statistically significant differences across pollination schedules within a soil water treatment by a Tukey post-hoc comparison at P < 0·05.
Floral longevity of control flowers
Pollination significantly reduced corolla retention periods, as evidenced by the emasculated and unmanipulated flowers exhibiting significantly higher longevity than hand-pollinated flowers (Fig. 3A). Soil water status affected the longevity of the control flowers, with high water plants maintaining corollas for significantly longer periods than low water plants (Fig. 3B). Lack of emasculation and hand-pollinations resulted in the largest values for floral longevity across all water treatments with intact, unmanipulated flowers in medium water retaining their corollas for over 19 d. While the corolla retention periods of unmanipulated flowers in medium water plants were not significantly different from the corresponding values from high soil moisture plants, they were significantly higher than those in plants grown under low soil moisture (Fig. 3B).
Seed production in hand-pollinated and control flowers
Soil water status significantly influenced seed production both in hand-pollinated treatment and in the unmanipulated control (Table 2). Low soil water consistently resulted in a significant reduction in per-capita seed production in all the three hand-pollinated treatments (Fig. 4A). Seed production from autonomous self-pollination in unmanipulated flowers was significantly higher in plants growing under high soil water (Fig. 4A). Per-capita seed production ranged from 9–10 seeds in hand cross-pollinated flowers to two seeds in unmanipulated, autonomously selfed flowers. A non-significant difference between per-capita seed production across pollination schedules suggests that the timing of pollination in the floral lifespan does not determine fecundity. However, seed production by autonomous selfing was marginal at best, as indicated by the significantly lower per-capita seed numbers in the unmanipulated flowers (2·2 ± 3·5 seeds per fruit; Fig. 4B), implying that outcrossing is the predominant mating strategy. Our method of emasculation was effective, as evidenced by the result that emasculated controls did not produce any seeds in a pollinator-free greenhouse. While year did have a significant effect on per-capita seed numbers in pollinated flowers, its effect was not significant in unmanipulated flowers.
Fig. 4.
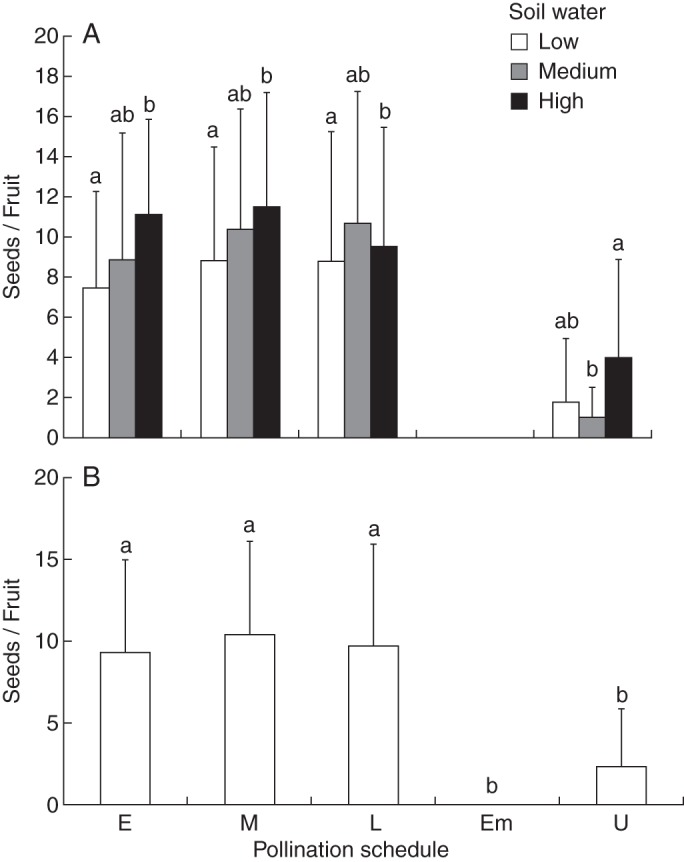
(A) Effect of soil water status on per-capita seed set. The x-axis denotes different pollination schedules described in Table 1: early (E), middle (M), late (L) hand-pollination treatments as well as emasculated (Em) and unmanipulated (U) controls. The individual bars refer to soil water status: low, medium and high soil water, as indicated in the key. Per-capita seed set is compared within each hand-pollination treatment and unmanipulated control across soil water status and the letters on the bars indicate respective significant differences by a Tukey post-hoc comparison at P < 0·05. (B) Autonomous selfing resulted in significantly reduced per-capita seed set. Bars with different letters indicate significant differences in seed number per fruit at P < 0·05 in a Tukey post-hoc comparison.
DISCUSSION
Floral longevity in Collinsia heterophylla appears to be a plastic trait modulated by biotic factors such as when pollination occurs during the floral lifespan, as well as abiotic factors such as soil moisture status. Our results parallel the model of optimal floral longevity, which predicts reduced periods of corolla retention when the cost of floral maintenance and fitness accrual rates therein are both high (Schoen and Ashman, 1995). If the primary function of corolla in angiosperms is associated with pollinator attraction, once pollination is accomplished, resource flow to the corolla may be minimized leading to corolla abscission. Our observations suggest that corolla abscission following pollination can be exacerbated by drought conditions. While extending the period of corolla retention beyond pollination may result in some additional benefits for the plant (van Doorn, 1997), under low soil moisture the cost of maintaining a turgid corolla, which continues to lose water by transpiration, significantly outweighs the benefits (Galen, 2000). Resource constraint appears to determine the duration of corolla retention in C. heterophylla, favouring abscission soon after pollination, as evidenced by substantially reduced floral longevity in the low soil moisture and early pollination treatment. However, high soil water conditions that could be considered as an indication of a resource surplus did not result in increased floral longevity, further supporting our hypothesis that corolla abscission is closely tied to pollination. Although flowers are known to be resource sinks (Galen, 2000), excessive availability of resources does not necessarily enhance the period of flower retention beyond the completion of the flower's sexual function.
In a protandrous species such as C. heterophylla, in which the stigma remains receptive for a few days after anthers have shed pollen, maintaining flowers post-pollination could prolong the period for accrual of female fitness and enhance the diversity of distinct pollen genotypes on the stigma by an extended period for pollinator visitation (van Doorn, 1997; Delph and Havens, 1998). Extrapolating the findings of Lankinen et al. (2007) that delayed stigmatic receptivity facilitates pollen competition on the stigma, we propose that the likelihood of pollen competition could potentially be strengthened, if the period of corolla retention were to be extended. Studies in other species also allude to enhanced offspring fitness, as possible benefits of increased stigmatic pollen competition (Mulcahy and Mulcahy, 1975; Davis et al., 1987; Delph and Havens, 1998; Mazer et al., 2010; Winsor et al., 2000; Armbruster and Rogers, 2004, and references therein). Maintaining pollinated flowers therefore could allow for the possibility of multiple pollinator visits, increasing the number of pollen donors and the resultant benefits of pollen competition (Kalla and Ashman, 2002). While the above studies demonstrate the benefits of increasing floral lifespan, other studies indicate that pollination triggers irreversible changes in physiology, resulting in corolla abscission, within a few hours to a few days after pollen receipt (van Doorn, 1997; Abdala-Roberts et al., 2007; Blair and Wolfe, 2007; Clark and Husband, 2007; Castro et al., 2008a). Our study supports the hypothesis that pollination triggers changes that lead to corolla abscission irrespective of the soil water status. Not only did the unmanipulated flowers in our experiments retain corollas longer than the flowers that received hand-pollinations early in the floral lifespan, they also maintained flowers 5–8 d longer than those that received hand-pollinations late in the floral lifespan.
Given that plants frequently operate under resource constraints, the life history of an annual plant limits the duration for which an unpollinated flower can maintain its corolla. In many plant species, autonomous selfing may be favoured towards the end of the floral lifespan, in order to ensure some amount of reproductive success even as resource flow towards such older flowers may continue to deplete (Dole, 1990; Kalisz et al., 1999; Arathi et al., 2002; Duan et al., 2010; Sicard and Lenhard, 2011; Busch and Delph, 2012). While floral longevity is likely to be highly reduced in predominantly selfing species as compared with closely related outcrossing specie (Weber and Goodwillie, 2013), flowers retain their attractive structures well into the floral lifespan in C. heterophylla, despite ample opportunities to self-pollinate, reiterating that reproductive assurance by delayed selfing is only likely when pollinators are limiting (Dole, 1990; Arathi et al., 2002; Kalisz et al., 2004; Elle et al., 2010). The main purpose of the corolla is for pollinator attraction and successful cross-pollination. Reduced allocation to floral traits that facilitate pollinator attraction is predicted when selfing becomes the predominant breeding mode, due to the redundancy of attractive floral parts, allowing selection to favour reduced corolla retention and, in extreme cases, a highly reduced corolla (Goodwillie et al., 2009). Our results thus lend credence to the growing body of evidence that C. heterophylla, like many other large-flowered species in the genus, is predominantly cross-pollinated, and at best a delayed selfer (Mayer et al., 1996; Kalisz et al., 1999, 2004, 2012). The exact timing and mechanism for selfing have not been described in C. heterophylla but similarity in floral structure allows us to predict that mechanisms similar to that in the other species of the genus may be at play.
In hermaphroditic flowering plants, fitness accrues from both the male function of pollen export, and the female function of pollen receipt and ovule fertilization. Sexual conflict between these two functions could determine the expression of floral traits that benefit one of the sexes at a significant cost to the other (Fetscher, 2001; Prasad and Bedhomme, 2006; Bedhomme et al., 2009). Floral longevity in C. heterophylla could directly relate to such a conflict, as early corolla abscission allows pollen export (male function) in this protandrous species to be accomplished before stigma reaches peak maturity. While there exists a possibility of early deposition of cross-pollen, the benefits of such cross-pollination are likely to be minimal (Lankinen and Kiboi, 2007). Fulfilling the female function (pollen receipt) in this cross-pollinated species, which also represents an indication of successful fecundity for the maternal parent therefore, requires that corolla retention be extended beyond the period of anther functionality in the flower. However, if abiotic stress, such as drought, significantly reduces floral lifespan then the associated advancement of delayed selfing would allow for the fulfilment of female function only via saturation of the stigma with self-pollen while male function may be less compromised given the continued likelihood of pollen export from the early dehiscing anthers. Ongoing studies (H. S. Arathi, Colorado State University, CO, pers. comm.) on the effects of abiotic stress on floral traits in C. heterophylla indicate that patterns of anther dehiscence are not adversely affected, supporting our hypothesis that the male function of pollen export is not compromised under stress. Reduced floral lifespan that largely benefits the male sex could therefore aggravate sexual conflict by limiting opportunities for outcrossed pollen deposition on the stigma and increasing costs of inbreeding depression (Lankinen and Armbruster, 2007), constraining the adaptive significance of delayed stigmatic receptivity in this species (Lankinen et al., 2007). Unless there is a parallel advancement of stigmatic receptivity under abiotic stress, floral longevity could become a male-controlled trait in this protandrous hermaphrodite. Plants have been generally shown to rely on pollen receipt rather than pollen export as a cue for corolla senescence (Ashman and Schoen, 1997; Arathi et al., 2002; Blair and Wolfe, 2007; Castro et al., 2008a; Marques and Draper, 2012), which can be argued as a mechanism favoured to minimize runaway effects of sexual conflict in hermaphrodites (Arnqvist and Rowe, 2005; Bernasconi et al., 2006; Lankinen et al., 2006).
Discussion on the functional significance of the corolla and its longevity is incomplete without information on reproductive success, i.e. per-capita seed production. In our study, seed numbers were affected by pollination and soil moisture status. Previous research with C. heterophylla has shown that early pollination results in a reduced number of seeds per capsule (Lankinen and Kiboi, 2007). This directly contrasts with our finding that timing of pollination did not affect seed production. The discrepancy may lie in the timing of ‘early’ pollinations in Lankinen and Kiboi (2007) where pollinations were done on Day 2 after flower opening, which is before the stigma becomes receptive (Lankinen et al., 2007). In contrast, our earliest hand-pollination treatments were designed to coincide with stigmatic receptivity and probably resulted in higher seed numbers than those observed in Lankinen and Kiboi (2007). While significant differences in seed production between hand-pollinated flowers and emasculated control flowers are to be expected, fewer seeds in the unmanipulated controls support the increased efficacy of cross pollen in C. heterophylla, a predominantly outcrossing species (Mayer et al., 1996; Kalisz et al., 1999, 2004). The reduced seed numbers under autonomous selfing could be due to herkogamy-mediated limitations to self-pollen deposition as the receptive stigma is situated beyond the stamens (Fig. 2D) and/or the possibility of reduced pollen grain viability with pollen age (Arathi et al., 2002). In addition, high amounts of inbreeding depression, known to be prevalent in C. heterophylla, could largely limit fecundity by autonomous selfing in this species (Mayer et al., 1996).
Taken together, our results on the variation in corolla retention and seed production as a function of pollination and soil moisture demonstrate the important role of the maternal plant in allocating resources from one crucial function (e.g. corolla maintenance) to the next (e.g. seed production) through its life cycle. Extending the concept of hierarchical allocation, we argue that once the function of an organ, in our case that of the flower, has been accomplished, plants may be favoured to route resources to the next process in the life cycle, that of seed production (Obeso, 2004). With little information on the genetic control of floral longevity, we can only suggest that the duration of corolla retention has a strong potential to influence the evolution of mating system in a species. Given that flowers are costly structures to maintain (Primack, 1985; Galen, 2000), and that maternal control of floral traits such as herkogamy and dichogamy may respond differently to a limiting resource, water limitation has the potential for multiple and synergistic effects on reproductive output. Soil moisture is a critical resource for reproductive success, making it important to understand the long-term ramifications that water shortage can have on plant fecundity and population size.
ACKNOWLEDGEMENTS
We thank Colorado State University for research support funds, Jennifer Matsuura for greenhouse maintenance, Jim zumBrunnen for statistical consulting, and Amy Angert, Dhruba Naug, Lauren Wilson and two anonymous referees for thoughtful suggestions.
LITERATURE CITED
- Abdala-Roberts L, Parra-Tabla V, Navarro J. Is floral longevity influenced by reproductive costs and pollination success in Cohniella ascendens (Orchidaceae)? Annals of Botany. 2007;100:1367–1371. doi: 10.1093/aob/mcm219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arathi HS, Rasch A, Cox C, Kelly JK. Autogamy and floral longevity in Mimulus guttatus. International Journal of Plant Sciences. 2002;163:567–573. [Google Scholar]
- Armbruster WS, Rogers DG. Does pollen competition reduce the cost of inbreeding? American Journal of Botany. 2004;91:1939–1943. doi: 10.3732/ajb.91.11.1939. [DOI] [PubMed] [Google Scholar]
- Armbruster WS, Mulder CPH, Baldwin BG, Kalisz S, Wessa B, Nute H. Comparative analyses of late floral development and mating system evolution in Tribe Collinsieae (Scrophulariaceae S.L.) American Journal of Botany. 2002;89:37–42. doi: 10.3732/ajb.89.1.37. [DOI] [PubMed] [Google Scholar]
- Arnqvist G, Rowe L. Sexual conflict: monographs in behavior and ecology. Princeton, NJ: Princeton University Press; 2005. [Google Scholar]
- Ashman T-L, Schoen D. The cost of floral longevity in Clarkia tembloriensis: an experimental investigation. Evolutionary Ecology. 1997;11:289–300. [Google Scholar]
- Barrett SCH. Sexual interference of the floral kind. Heredity. 2002;88:154–159. doi: 10.1038/sj.hdy.6800020. [DOI] [PubMed] [Google Scholar]
- Bedhomme SP, Bernasconi G, Koene JM, et al. How does breeding system variation modulate sexual antagonism? Biology Letters. 2009;5:717–720. doi: 10.1098/rsbl.2009.0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernasconi G, Corley LS, Lawniczak MKN. Trick or treat: the battle of the sexes. Journal of Evolutionary Biology. 2006;19:1003–1005. [Google Scholar]
- Blair AC, Wolfe LM. The association between floral longevity and pollen removal, pollen receipt, and fruit production in flame azalea (Rhododendron calendulaceum) Canadian Journal of Botany. 2007;85:414–419. [Google Scholar]
- Brys R, Jacquemyn H. Variation in the functioning of autonomous self-pollination, pollinator services and floral traits in three Centaurium species. Annals of Botany. 2011;107:917–925. doi: 10.1093/aob/mcr032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch JW, Delph LF. The relative importance of reproductive assurance and automatic selection as hypotheses for the evolution of self-fertilization. Annals of Botany. 2012;109:553–562. doi: 10.1093/aob/mcr219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll AB, Pallardy SG, Galen C. Drought stress, plant water status, and floral trait expression in fireweed, Epilobium angustifolium (Onagraceae) American Journal of Botany. 2001;88:438–446. [PubMed] [Google Scholar]
- Castro S, Silveira P, Navarro L. Effect of pollination on floral longevity and costs of delaying fertilization in the out-crossing Polygala vayredae Costa (Polygalaceae) Annals of Botany. 2008a;102:1043–1048. doi: 10.1093/aob/mcn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro S, Silveira P, Navarro L. How flower biology and breeding system affect the reproductive success of the narrow endemic Polygala vayredae Costa (Polygalaceae) Botanical Journal of the Linnean Society. 2008b;157:67–81. [Google Scholar]
- Cheptou PO, Mathias A. Can varying inbreeding depression select for intermediary selfing rates? The American Naturalist. 2001;157:361–373. doi: 10.1086/319320. [DOI] [PubMed] [Google Scholar]
- Clark MJ, Husband BC. Plasticity and timing of flower closure in response to pollination in Chamerion angustifolium (Onagraceae) International Journal of Plant Sciences. 2007;168:619–625. [Google Scholar]
- CNRFC. Southern California Precipitation Data. 2012 http://www.cnrfc.noaa.gov . [Google Scholar]
- Darwin C. The effects of cross and self fertilisation in the vegetable kingdom. London: J. Murray; 1876. [Google Scholar]
- Davis LE, Stephenson AG, Winsor JA. Pollen competition improves performance and reproductive output of the common zucchini squash under field conditions. Journal of the American Society for Horticultural Science. 1987;112:712–716. [Google Scholar]
- Delph LF, Ashman T-L. Trait selection in flowering plants: how does sexual selection contribute? Integrative and Comparative Biology. 2006;46:465–472. doi: 10.1093/icb/icj038. [DOI] [PubMed] [Google Scholar]
- Delph LF, Havens K. Pollen competition in flowering plants. In: Birkhead TR, Møller AP, editors. Sperm competition and sexual selection. San Diego: Academic Press; 1998. pp. 149–173. [Google Scholar]
- Dole JA. Role of corolla abscission in delayed self-pollination of Mimulus guttatus (Scrophulariaceae) American Journal of Botany. 1990;77:1505–1507. [Google Scholar]
- Duan Y-W, Dafni A, Hou Q-Z, He Y-P, Liu J-Q. Delayed selfing in an alpine biennial Gentianopsis paludosa (Gentianaceae) in the Qinghai-Tibetan Plateau. Journal of Integrative Plant Biology. 2010;52:593–599. doi: 10.1111/j.1744-7909.2010.00951.x. [DOI] [PubMed] [Google Scholar]
- Elle E, Gillespie S, Guindre-Parker S, Parachnowitsch AL. Variation in the timing of autonomous selfing among populations that differ in flower size, time to reproductive maturity, and climate. American Journal of Botany. 2010;97:1894–1902. doi: 10.3732/ajb.1000223. [DOI] [PubMed] [Google Scholar]
- Evanhoe L, Galloway LF. Floral longevity in Campanula americana (Campanulaceae): a comparison of morphological and functional gender phases. American Journal of Botany. 2002;89:587–591. doi: 10.3732/ajb.89.4.587. [DOI] [PubMed] [Google Scholar]
- Evans MEK, Hearn DJ, Theiss KE, Cranston K, Holsinger KE, Donoghue MJ. Extreme environments select for reproductive assurance: evidence from evening primroses (Oenothera) New Phytologist. 2011;191:555–563. doi: 10.1111/j.1469-8137.2011.03697.x. [DOI] [PubMed] [Google Scholar]
- Fetscher AE. Resolution of male–female conflict in an hermaphroditic flower. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2001;268:525–529. doi: 10.1098/rspb.2000.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galen C. High and dry: drought stress, sex allocation trade-offs, and selection on flower size in the alpine wildflower Polemonium viscosum (Polemoniaceae) The American Naturalist. 2000;156:72–83. doi: 10.1086/303373. [DOI] [PubMed] [Google Scholar]
- Glaettli M, Barrett SCH. Pollinator responses to variation in floral display and flower size in dioecious Sagittaria latifolia (Alismataceae) New Phytologist. 2008;179:1193–1201. doi: 10.1111/j.1469-8137.2008.02532.x. [DOI] [PubMed] [Google Scholar]
- Goodwillie C. Inbreeding depression and mating systems in two species of Linanthus (Polemoniaceae) Heredity. 2000;84:283–293. doi: 10.1046/j.1365-2540.2000.00656.x. [DOI] [PubMed] [Google Scholar]
- Goodwillie C, Sargent RD, Eckert CG, et al. Correlated evolution of mating system and floral display traits in flowering plants and its implications for the distribution of mating system variation. New Phytologist. 2009;185:311–321. doi: 10.1111/j.1469-8137.2009.03043.x. [DOI] [PubMed] [Google Scholar]
- Gori DF. Post-pollination phenomena and adaptive floral changes. In: Jones CE, Little RJ, editors. Handbook of experimental pollination biology. New York: van Nostrand Reinhold; 1983. pp. 31–49. [Google Scholar]
- Jarne P, Charlesworth D. The evolution of the selfing rate in functionally hermaphrodite plants and animals. Annual Review of Ecology and Systematics. 1993;24:441–466. [Google Scholar]
- Johnston MO, Porcher E, Cheptou P-O, et al. Correlations among fertility components can maintain mixed mating in plants. The American Naturalist. 2009;173:1–11. doi: 10.1086/593705. [DOI] [PubMed] [Google Scholar]
- Kalisz S, Vogler D, Fails B, et al. The mechanism of delayed selfing in Collinsia verna (Scrophulariaceae) American Journal of Botany. 1999;86:1239–1247. [PubMed] [Google Scholar]
- Kalisz S, Vogler DW, Hanley KM. Context-dependent autonomous self-fertilization yields reproductive assurance and mixed mating. Nature. 2004;430:884–887. doi: 10.1038/nature02776. [DOI] [PubMed] [Google Scholar]
- Kalisz S, Randle A, Chaiffetz D, Faigeles M, Butera A, Beight C. Dichogamy correlates with outcrossing rate and defines the selfing syndrome in the mixed-mating genus Collinsia. Annals of Botany. 2012;109:571–582. doi: 10.1093/aob/mcr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalla SE, Ashman TL. The effects of pollen competition on progeny vigor in Fragaria virginiana (Rosaceae) depend on progeny growth environment. International Journal of Plant Sciences. 2002;163:335–340. [Google Scholar]
- Kennedy BF, Elle E. The inbreeding depression cost of selfing: importance of flower size and population size in Collinsia parviflora (Veronicaceae) American Journal of Botany. 2008a;95:1596–1605. doi: 10.3732/ajb.0800322. [DOI] [PubMed] [Google Scholar]
- Kennedy BF, Elle E. The reproductive assurance benefit of selfing: importance of flower size and population size. Oecologia. 2008b;155:469–477. doi: 10.1007/s00442-007-0924-7. [DOI] [PubMed] [Google Scholar]
- Lankinen A, Armbruster WS. Pollen competition reduces inbreeding depression in Collinsia heterophylla (Plantaginaceae) Journal of Evolutionary Biology. 2007;20:737–749. doi: 10.1111/j.1420-9101.2006.01233.x. [DOI] [PubMed] [Google Scholar]
- Lankinen Ã, Kiboi S. Pollen donor identity affects timing of stigma receptivity in Collinsia heterophylla (Plantaginaceae): a sexual conflict during pollen competition? The American Naturalist. 2007;170:854–863. doi: 10.1086/522839. [DOI] [PubMed] [Google Scholar]
- Lankinen A, Hellriegel B, Bernasconi G. Sexual conflict over floral receptivity. Evolution. 2006;60:2454–2466. [PubMed] [Google Scholar]
- Lankinen A, Armbruster WS, Antonsen L. Delayed stigma receptivity in Collinsia heterophylla (Plantaginaceae): genetic variation and adaptive significance in relation to pollen competition, delayed self-pollination, and mating-system evolution. American Journal of Botany. 2007;94:1183–1192. doi: 10.3732/ajb.94.7.1183. [DOI] [PubMed] [Google Scholar]
- Lankinen A, Maad J, Armbruster WS. Pollen-tube growth rates in Collinsia heterophylla (Plantaginaceae): one-donor crosses reveal heritability but no effect on sporophytic-offspring fitness. Annals of Botany. 2009;103:941–950. doi: 10.1093/aob/mcp014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madjidian JA, Lankinen Ã. Sexual conflict and sexually antagonistic coevolution in an annual plant. PLoS ONE. 2009;4:e5477. doi: 10.1371/journal.pone.0005477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques I, Draper D. Pollination activity affects selection on floral longevity in the autumnal-flowering plant, Narcissus serotinus L. Botany. 2012;90:283–291. [Google Scholar]
- Mayer SS, Charlesworth D, Meyers B. Inbreeding depression in four populations of Collinsia heterophylla Nutt (Scrophulariaceae) Evolution. 1996;50:879–891. doi: 10.1111/j.1558-5646.1996.tb03896.x. [DOI] [PubMed] [Google Scholar]
- Mazer SJ, Hove AA, Miller BS, Barbet-Massin M. The joint evolution of mating system and pollen performance: Predictions regarding male gametophytic evolution in selfers vs. outcrossers. Perspectives in Plant Ecology, Evolution and Systematics. 2010;12:31–41. [Google Scholar]
- Medrano M, Herrera CM, Barrett SCH. Herkogamy and mating patterns in the self-compatible daffodil Narcissus longispathus. Annals of Botany. 2005;95:1105–1111. doi: 10.1093/aob/mci129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousseau TA, Fox CW. Maternal effects as adaptations. New York: Oxford University Press; 1998. [Google Scholar]
- Mulcahy DL, Mulcahy GL. The influence of gametophytic competition on sporophytic quality in Dianthus chinensis. Theoretical and Applied Genetics. 1975;46:277–284. doi: 10.1007/BF00281149. [DOI] [PubMed] [Google Scholar]
- Newsom VM. A revision of the genus Collinsia (Scrophulariaceae) Botanical Gazette. 1929;87:260–301. [Google Scholar]
- Nicotra AB, Atkin OK, Bonser SP, et al. Plant phenotypic plasticity in a changing climate. Trends in Plant Science. 2010;15:684–692. doi: 10.1016/j.tplants.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Obeso JR. A hierarchical perspective in allocation to reproduction from whole plant to fruit and seed level. Perspectives in Plant Ecology, Evolution and Systematics. 2004;6:217–225. [Google Scholar]
- Prasad NG, Bedhomme S. Sexual conflict in plants. Journal of Genetics. 2006;85:161–164. doi: 10.1007/BF02935325. [DOI] [PubMed] [Google Scholar]
- Primack RB. Longevity of invidual flowers. Annual Review of Ecology and Systematics. 1985;16:15–37. [Google Scholar]
- Proctor HC, Harder LD. Effect of pollination success on floral longevity in the orchid Calypso bulbosa (Orchidaceae) American Journal of Botany. 1995;82:1131–1136. [Google Scholar]
- Qu R, Li X, Luo Y, Dong M, Xu H, Chen X, Dafni A. Wind-dragged corolla enhances self-pollination: a new mechanism of delayed self-pollination. Annals of Botany. 2007;100:1155–1164. doi: 10.1093/aob/mcm209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle AM, Slyder JB, Kalisz S. Can differences in autonomous selfing ability explain differences in range size among sister-taxa pairs of Collinsia (Plantaginaceae)? An extension of Baker's Law. New Phytologist. 2009;183:618–629. doi: 10.1111/j.1469-8137.2009.02946.x. [DOI] [PubMed] [Google Scholar]
- Rathcke BJ. Floral longevity and reproductive assurance: seasonal patterns and an experimental test with Kalmia latifolia (Ericaceae) American Journal of Botany. 2003;90:1328–1332. doi: 10.3732/ajb.90.9.1328. [DOI] [PubMed] [Google Scholar]
- Schemske DW, Lande R. The evolution of self-fertilization and inbreeding depression in plants. II. Empirical observations. Evolution. 1985;39:41–52. doi: 10.1111/j.1558-5646.1985.tb04078.x. [DOI] [PubMed] [Google Scholar]
- Schoen DJ, Ashman T-L. The evolution of floral longevity: resource allocation to maintenance versus construction of repeated parts in modular organisms. Evolution. 1995;49:131–139. doi: 10.1111/j.1558-5646.1995.tb05965.x. [DOI] [PubMed] [Google Scholar]
- Sicard A, Lenhard M. The selfing syndrome: a model for studying the genetic and evolutionary basis of morphological adaptation in plants. Annals of Botany. 2011;107:1433–1443. doi: 10.1093/aob/mcr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stpiczynska M. Floral longevity and nectar secretion of Platanthera chlorantha (Custer) Rchb. (Orchidaceae) Annals of Botany. 2003;92:191–197. doi: 10.1093/aob/mcg124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA NRCS. The PLANTS Database. Baton Rouge, LA: National Plant Data Center; 2012. http://plants.usda.gov . [Google Scholar]
- Vallejo-Marin M, Barrett SCH. Modification of flower architecture during early stages in the evolution of self-fertilization. Annals of Botany. 2009;103:951–962. doi: 10.1093/aob/mcp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn WG. Effects of pollination on floral attraction and longevity. Journal of Experimental Botany. 1997;48:1615–1622. [Google Scholar]
- Weber JJ, Goodwillie C. Variation in floral longevity in the genus Leptosiphon: mating system consequences. Plant Biology. 2013;15:220–225. doi: 10.1111/j.1438-8677.2012.00595.x. [DOI] [PubMed] [Google Scholar]
- Winsor JA, Peretz S, Stephenson AG. Pollen competition in a natural population of Cucurbita foetidissima (Cucurbitaceae) American Journal of Botany. 2000;87:527–532. [PubMed] [Google Scholar]