Abstract
Background and Aims
The harvesting method of wild and cultivated cereals has long been recognized as an important factor in the emergence of domesticated non-shattering ear genotypes. This study aimed to quantify the effects of spike brittleness and threshability on threshing time and efficiency in emmer wheat, and to evaluate the implications of post-harvest processes on domestication of cereals in the Near East.
Methods
A diverse collection of tetraploid wheat genotypes, consisting of Triticum turgidum ssp. dicoccoides – the wild progenitor of domesticated wheat – traditional landraces, modern cultivars (T. turgidum ssp. durum) and 150 recombinant (wild × modern) inbred lines, was used in replicated controlled threshing experiments to quantify the effects of spike brittleness and threshability on threshing time and efficiency.
Key Results
The transition from a brittle hulled wild phenotype to non-brittle hulled phenotype (landraces) was associated with an approx. 30 % reduction in threshing time, whereas the transition from the latter to non-brittle free-threshing cultivars was associated with an approx. 85 % reduction in threshing time. Similar trends were obtained with groups of recombinant inbred lines showing extreme phenotypes of brittleness and threshability.
Conclusions
In tetraploid wheat, both non-brittle spike and free-threshing are labour-saving traits that increase the efficiency of post-harvest processing, which could have been an incentive for rapid domestication of the Near Eastern cereals, thus refuting the recently proposed hypothesis regarding extra labour associated with the domesticated phenotype (non-brittle spike) and its presumed role in extending the domestication episode time frame.
Keywords: Triticum turgidum ssp. dicoccoides, T. turgidum ssp. durum, emmer wheat, conscious selection, labour trap, post-harvest processing, protracted domestication, spike brittleness (br), threshability
INTRODUCTION
Plant domestication was a major aspect of the Neolithic Revolution in the Levant (Redman, 1978). The reliance on food production, by means of growing plants, rather than on a hunting–gathering subsistence triggered the emergence of new social and cultural adaptations and required agro- (and other) technological innovations, as a consequence of the newly emerging farming way of life (e.g. Bar-Yosef, 2002).
Cereal and legume grains appear as part of the archaeobotanical record in several sites pre-dating the emergence of agriculture (e.g. Kislev and Bar Yosef, 1988; Lev et al., 2005), thereby attesting to the ancient tradition of harvesting and using these plant species in their wild state. As with recent hunter-gatherers, prehistoric humans are assumed to have developed strategies to optimize the foraging, processing and storage of their food-stuffs (e.g. Kelly, 1995). The (old) hunter-gatherers' technologies, however, did not necessarily fit the new situation in which cultivated plots were harvested, and the respective labour requirements of post-harvest processing (e.g. Fuller et al., 2010).
Indeed, some of the human behavioural aspects, in terms of labour investment, have been considered alongside the biological and archaeological data in the context of the origin of agriculture in the Near East. These include three reports on wild cereal harvesting (Harlan, 1967; Ladizinsky, 1975; Kislev et al., 2004), three reports on experimental wild legume harvesting (Abbo et al., 2008a, b, 2013), and a single report on wild pea growing (Abbo et al., 2011a). The reports on wild harvesting focused on the grain yield from the wild population per gathering-time unit and the effect of the spatial organization of the wild plants on potential yield, while the pea growing report emphasized the need to have a free-germinating (non-dormant) seed stock as a prerequisite for profitable pea growing (Abbo et al., 2011a). Other important experiments involved the growing of wild wheat populations in Cardiff, Wales (Hillman and Davies, 1990a, b), and in Jales, France (Willcox, 1992, 2007, and references therein). These seminal works served to answer several questions concerning the dynamics of ‘cultivated’ populations of wild einkorn and resulted in several papers discussing the implications of different harvest methods and crop husbandry on cereal domestication (e.g. Hillman and Davies, 1999; Harris, 2007, and references therein).
The harvesting method of both wild and cultivated cereals and legumes has long been recognized as an important factor in the emergence of domesticated types with non-shattering ears and indehiscent pods, respectively (e.g. Bohrer, 1972; Harlan et al., 1973). However, post-harvest processing and husbandry operations are also expected to have exerted specific selection pressures on the plant populations grown in human-managed fields. For example, deep or shallow sowing, shifting between fields or sowing the same plots year after year, are expected to have driven the nascent crop populations via different evolutionary trajectories. Indeed, Fuller et al. (2010) recognized the need to incorporate such management and post-harvest factors into crop evolutionary modelling. A major component of Fuller et al.'s (2010) model is based on their assumption that non-shattering (phenotypically domesticated) cereals require additional post-harvest labour investment as compared with wild-type cereals in which the mature spikes (or panicles) disarticulate upon ripening. According to this reconstruction, farmers may have selected against the non-brittle types to avoid the extra labour required to extract their grains, thus suggesting a slow emergence of domesticated plant communities (Fuller et al., 2010, p. 18 and fig. 4).
Unlike Fuller et al.'s (2010) assumption and model, and based on experimentation with cultivated wild einkorn populations, Willcox (1992, p. 167) suggested that non-brittle rachis is a labour-saving trait, which corresponds with our hands-on experience with threshing of wild and domesticated emmer wheat (e.g. Peleg et al., 2011). The underlying rationale for the present work was that the time required for threshing and the amount of grain produced per time unit of threshing are good indications of the labour involved in post-harvest processing of any given wheat genotype. Accordingly, the aim of the present work was to quantify the effects of spike brittleness and spike threshability on threshing time and efficiency.
MATERIALS AND METHODS
Plant material
A total of 198 tetraploid wheat genotypes consisting of 22 accessions of Triticum turgidum ssp. dicoccoides (brittle, hulled) – the wild progenitor of domesticated wheat – 12 traditional landraces (non-brittle, either hulled or free-threshing), 14 modern cultivars (T. turgidum ssp. durum, non-brittle, free-threshing) and a population of 150 F7 recombinant inbred lines (RILs), each derived from an individual F2 plant via single seed descent (SSD) from a cross between the modern cultivar ‘Langdon’ and the wild accession G18-16 (segregating for spike brittleness and free-threshing/hulled characters), were used in the current study. The landraces were categorized for spike brittleness and hulled/free-threshing based on their GenBank description and confirmed by our own observations. A full listing of the genotypes used is given in Table 1.
Table 1.
Tetraploid wheat genotypes used in the study
| Species | Genotype | Origin |
|---|---|---|
| Wild accessions | ||
| T. turgidum ssp. dicoccoides | dic137 | Jordan |
| T. turgidum ssp. dicoccoides | J28 | Jordan |
| T. turgidum ssp. dicoccoides | 8736 | Iraq |
| T. turgidum ssp. dicoccoides | 10-8 | Israel |
| T. turgidum ssp. dicoccoides | 12-3 | Israel |
| T. turgidum ssp. dicoccoides | 1-22 | Israel |
| T. turgidum ssp. dicoccoides | 28-6 | Israel |
| T. turgidum ssp. dicoccoides | 13-B-53 | Israel |
| T. turgidum ssp. dicoccoides | 15-T-25 | Israel |
| T. turgidum ssp. dicoccoides | 16-34 | Israel |
| T. turgidum ssp. dicoccoides | 24-39 | Israel |
| T. turgidum ssp. dicoccoides | dic47 | Israel |
| T. turgidum ssp. dicoccoides | dic52 | Israel |
| T. turgidum ssp. dicoccoides | G18-16 | Israel |
| T. turgidum ssp. dicoccoides | KH 4/1 | Israel |
| T. turgidum ssp. dicoccoides | MM 1/1 | Israel |
| T. turgidum ssp. dicoccoides | MM 5/3 | Israel |
| T. turgidum ssp. dicoccoides | 1082 | Syria |
| T. turgidum ssp. dicoccoides | dic131 | Syria |
| T. turgidum ssp. dicoccoides | dic110 | Turkey |
| T. turgidum ssp. dicoccoides | dic55 | Turkey |
| T. turgidum ssp. dicoccoides | dic90 | Turkey |
| Hulled landraces | ||
| T. turgidum L. ssp. turgidum | WIR 50943 | Uzbekistan |
| T. turgidum L. ssp. dicoccum Thell. | G 581 | * |
| T. turgidum L. ssp. dicoccum Thell. | G 929 | * |
| T. timopheevii Zhuk. ssp. armeniacum | G 926 | * |
| T. ispahanicum Heslot | G 805 | * |
| Free-threshing landraces | ||
| T. turgidum L. ssp. turgidum | 340-TR90 | Ethiopia |
| T. turgidum L. ssp. turgidum | 340-TR92 | Ethiopia |
| T. turgidum L. ssp. turgidum | 340-TR93 | Ethiopia |
| T. turgidum L. ssp. turgidum | WIR 39351 | Kazakhstan |
| T. turgidum L. ssp. polonicum (L.) Thell. | G 992 | * |
| T. tirgidum L. ssp. abyssinicum | G 799 | Ethiopia |
| T. militinea | G 5144 | Georgia |
| Modern cultivars | ||
| T. turgidum ssp. durum | C-43 | Israel |
| T. turgidum ssp. durum | C-61 | Israel |
| T. turgidum ssp. durum | C-9 | Israel |
| T. turgidum ssp.durum | P9 | Israel |
| T. turgidum ssp. durum | ‘Eliav’ | Israel |
| T. turgidum ssp. durum | ‘Givati’ | Israel |
| T. turgidum ssp. durum | ‘Inbar’ | Israel |
| T. turgidum ssp. durum | ‘Uzan’ | Israel |
| T. turgidum ssp. durum | ‘Svevo’ | Italy |
| T. turgidum ssp. durum | ‘Saricanak 98’ | Turkey |
| T. turgidum ssp. durum | ‘Kizilitan’ | Turkey |
| T. turgidum ssp. durum | ‘Kofa’ | USA |
| T. turgidum ssp. durum | ‘Langdon’ | USA |
| T. turgidum ssp. durum | UC 1113 | USA |
| 150 F7 recombinant inbred lines (RILs) | ||
| T. turgidum ssp. durum × T. turgidum ssp. dicoccoides | ‘Langdon’ × G18-16 | |
*Obtained from the Lennart Johnson collection, Riverside, California. No specific details on country of origin are available to us.
Experimental procedures
Plants were grown during the winter–spring of 2010/11. The seeds were placed on moist germination paper, and kept at 4 °C for vernalization. Fourteen days later, seedlings were transferred to room temperature for 2 days, and then planted in a 50-mesh screen house at the experimental farm of the Hebrew University, in Rehovot, Israel (31°54′N, 34°47′E; 54 m above sea level). A fully randomized block design with three replicates was employed. Each plot (experimental unit) consisted of five 8-cm spaced plants in a single row. The plants received natural rainfall (440 mm) and supplemental drip irrigation (450 mm) throughout the season until the grain-filling stage of the late-flowering genotypes, to allow appropriate spike and grain development. Upon stem elongation, the central three plants of each plot were supported by a bamboo stick and a wire ring to avoid lodging, whereas the two border plants of each plot remained unsupported. At the early ripening stage, the upper part of the three central plants was wrapped in a cone of perforated polypropylene while keeping the top open (like a flower bouquet), to avoid loss of spikelets from the brittle-spike genotypes, and allowing air circulation. Upon full maturation, the three central plants from each plot were harvested and placed in a paper bag until processing.
The spikes and spikelets from each plot were manually sorted on open trays into two fractions, namely a brittle fraction and a non-brittle fraction. The non-brittle fraction held intact spikes and spike fragments with four or more jointed spikelets. The brittle fraction held single dispersal units (spikelets) and spike fragments with two or three jointed spikelets. Each fraction was weighed separately and remixed to restore the sample's genetic constitution.
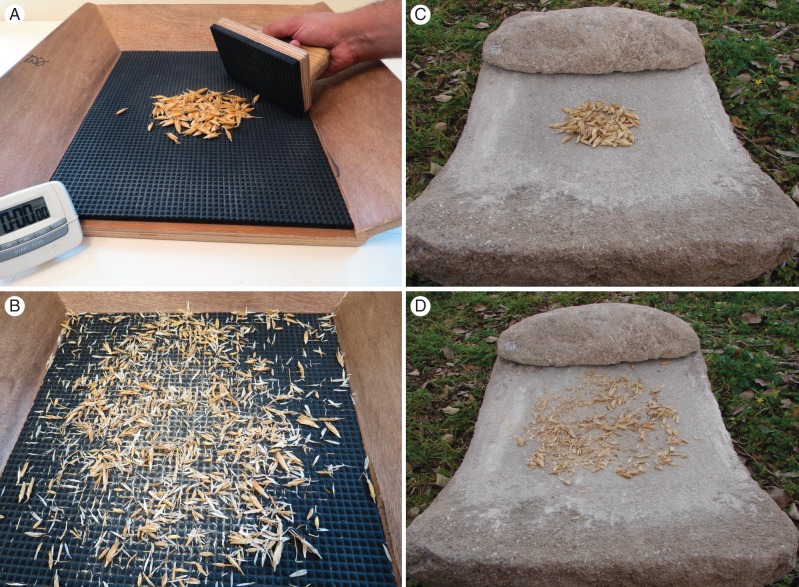
Prior to threshing of the experimental materials, three laboratory workers were trained using non-experimental samples to coordinate their threshing procedure and minimize possible inconsistencies. In addition, each of the three experimental blocks was assigned to a different lab. worker, so that individual differences in handling could be accounted for by the block effect in the ANOVA. A 20-g sub-sample of spikes and/or spikelets was taken from the material harvested from each plot and scissors were used to remove the awns from the individual spikes and/or spikelets. Based on observations of traditional farming practices, it was assumed that awn removal by brief firing could have been part of the ancient post-harvest handling. Regardless, because both our germplasm collection and the RIL population hold a wide array of awn lengths and toughnesses, this procedure served to minimize any possible awn effect on our threshing procedures. Thereafter, the awnless spike/spikelets were placed on a threshing cradle covered by a rough rubber surface and manually threshed with a similar rubber panel (Fig. 1A) until all grains seemed to be released (Fig. 1B). The time required to release the kernels from the chaff in the threshing cradle was measured using a stopwatch. Following the threshing, the material was winnowed to separate the kernels from the chaff, and the grain fraction was weighed.
Fig. 1.
Threshing cradle panel covered by rough rubber used in the current study with a sample before (A) and after threshing with all grains released (B). A similar ‘saddle-shaped’ grinding/threshing slab made of stone approx. 3000 years old used to simulate ancient threshing methods with a sample before (C) and after (D) threshing.
A complete ‘saddle-shaped grinding slab’ made of beach rock from a coastal plain site in Israel dated to some 3000 years BP was used to simulate ancient threshing methods (Fig. 1C, D) assuming that such slabs have been used as grinding/threshing stones. Several samples of different spike/spikelet types were threshed using a similar procedure as with our ‘modern’ experimental threshing cradle. A comparison between the two threshing devices exhibited similar trends in terms of threshing times of the various samples.
Data processing and analyses
Spike brittleness was calculated based on pre-threshing sorting as the weight ratio of the brittle fraction to total material (spikelets/spikes). Spike harvest index (SpHI) was calculated as the weight ratio between the free kernels and the threshed sub-sample. Threshing efficiency was calculated as the weight of kernels divided by the time (in minutes) required for threshing, thus reflecting the weight of kernels extracted per minute.
Groups of 15 RILs (10 % of the total RIL population size) that showed extreme values of spike brittleness and SpHI were selected from the RIL population and subjected to detailed statistical analysis.
The JMP 7.0 software package was used for statistical analyses. A nested factorial model was employed for the analyses of variance with groups, genotypes nested within groups and blocks considered as random effects. Tukey's honestly significant difference (HSD) test was used to compare between treatment means.
RESULTS
Genotypic groups
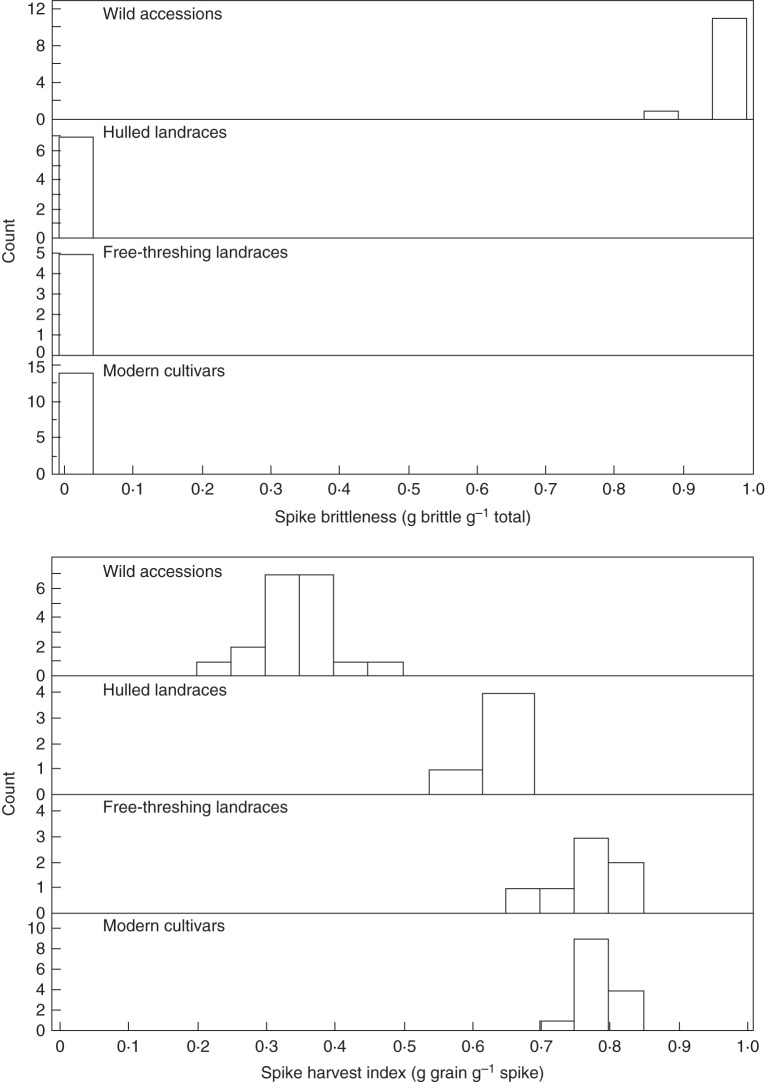
Spike brittleness did not distribute normally and hence it was not subjected to ANOVA. Nevertheless, the various genotypic groups exhibited clearly distinct brittleness values, with wild genotypes showing 0·86–1.0 brittleness vs. 0·0–0·02 brittleness in the modern cultivars and both landrace groups (Fig. 2, Table 2). SpHI, reflecting the hulled/free-threshing character, also exhibited distinct values in the genotypic groups, with the wild genotypes showing the lowest values, nearly double that in the hulled landraces and even higher in the free-threshing landraces and modern cultivars, with no significant difference between the latter two groups.
Fig. 2.
Distributions of spike brittleness and spike harvest index in each of the Emmer wheat genotypic groups studied.
Table 2.
Spike brittleness, spike harvest index (SpHI), threshing time and threshing efficiency in wild, traditional and modern genotypes of tetraploid wheat germplasm
| Genotypic group | d.f. | Spike brittleness† | SpHI | Threshing time (s per 20 g spikes) | Threshing efficiency (g grain min−1) |
|---|---|---|---|---|---|
| Wild accessions | 0·98 | 0·32c | 115·9a | 3·1c | |
| Hulled landraces | 0·01 | 0·62b | 79·3b | 10·9c | |
| Free-threshing landraces | 0·00 | 0·76a | 13·6c | 99·2b | |
| Modern cultivars | 0·00 | 0·77a | 7·9c | 132·8a | |
| Source of variation | |||||
| Genotypic group (F value) | 3 | 750·9*** | 194·4*** | 149·9*** | |
| Genotype (group) (F value) | 41 | 2·9*** | 1·7*** | 2·4*** | |
| Block (F value) | 2 | 1·0n.s. | 9·4*** | 3·3*** | |
† Brittleness values did not distribute normally and hence were not analysed.
a,b,c Different letters indicate significant differences between group means according to Tukey's HSD test at P < 0·05.
***, n.s., Significant F values at P < 0·001 or non-significant values, respectively.
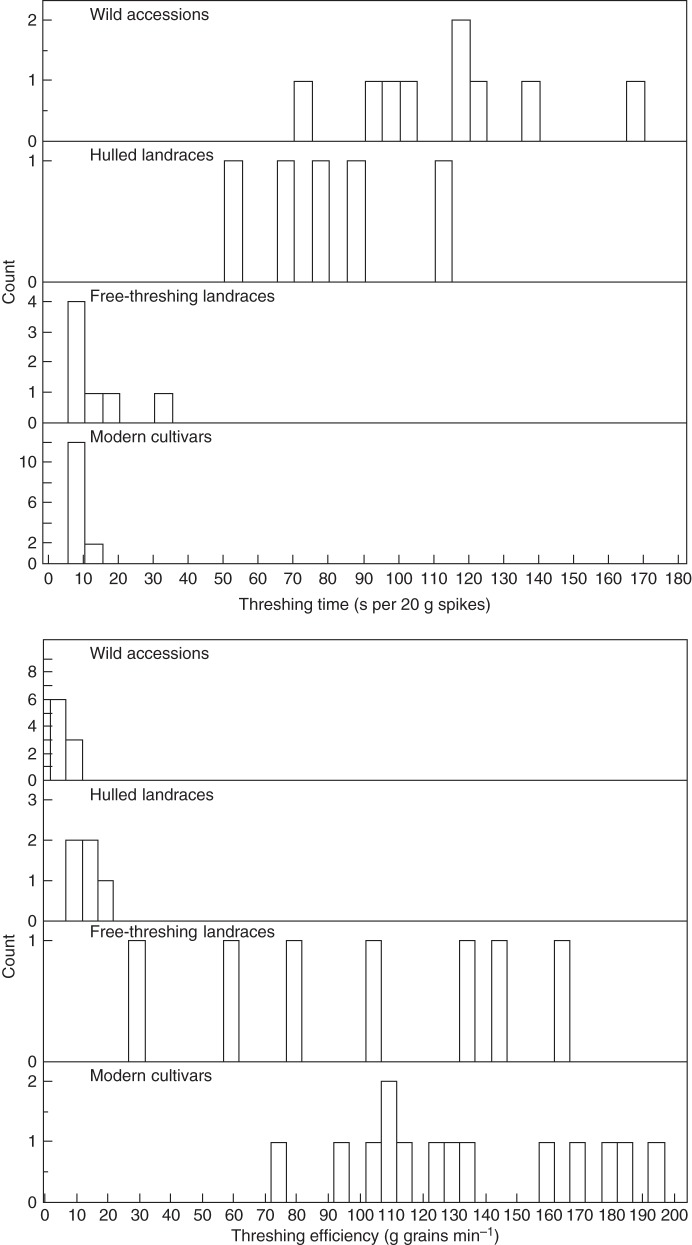
The time required to thresh 20 g of spikes or spikelets varied widely within the wild genotypes (74–165 s) as well as within the hulled landraces (50–112 s), while the free-threshing landraces and modern cultivars exhibited lower values and narrower ranges (Fig. 3). The average threshing time for the wild genotypes was nearly 2 min, about 80 s for the hulled landraces and much shorter (8–14 s) for the free-threshing landraces and modern germplasm (Table 2). Threshing efficiency exhibited a narrow range within each of the hulled genotypic groups and a wide range within each of the free-threshing groups (Fig. 3). The average threshing efficiency of the wild genotypes was about 3 g grains min−1, it was more than three times greater in the hulled landraces, over 30 times greater in the free-threshing landraces and over 40 times greater in the modern cultivars (Table 2).
Fig. 3.
Distributions of threshing time and threshing efficiency in each of the Emmer wheat genotypic groups studied.
Recombinant inbred lines
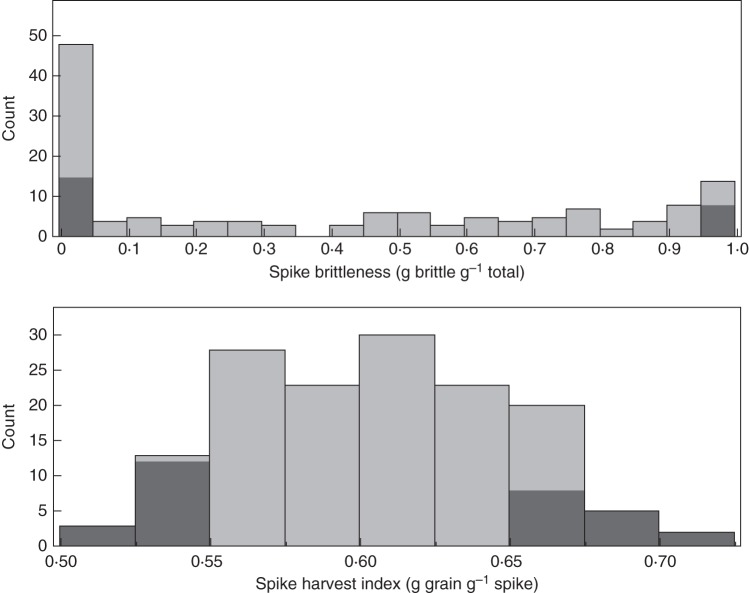
Among the 150 RILs tested, most exhibited either brittle (0·9–1.0 brittleness) or non-brittle (0·0–0·1 brittleness) phenotypes (Fig. 4). Of particular interest, however, are the remaining 64 RILs (43 %), showing intermediate values (0·1–0·9 brittleness) with a fairly uniform continuous distribution. This pattern suggests that, in addition to the major loci controlling spike brittleness, this trait is affected by additional minor genes (modifiers); these genes are currently under study in our laboratory. SpHI of the RILs exhibited a normal distribution, ranging between 0·51 and 0·71.
Fig. 4.
Distributions of spike brittleness and spike harvest index (SpHI) in the 150 F7 recombinant inbred lines (RILs), derived from a cross between the modern free-threshing cultivar ‘Langdon’ and the wild accession G18-16. Groups of 15 (10 %) RILs that showed extreme values in spike brittleness and SpHI and were selected for detailed statistical analysis are marked in grey.
To examine the effects of spike brittleness and SpHI on threshing time and efficiency on a recombinant modern-wild background, groups of 15 RILs that showed extreme values in spike brittleness and SpHI (marked with dark colour in Fig. 4) were selected and subjected to ANOVA. The two groups representing extreme brittleness values exhibited a relatively small but significant difference in SpHI (Table 3). Greater differences were manifested between these two groups in threshing time and threshing efficiency, with the non-brittle genotypic group requiring 61 % threshing time and having 179 % threshing efficiency relative to the brittle genotypic group. The two groups representing extreme SpHI values exhibited a 14 % difference in SpHI (significant) but only a minor difference in spike brittleness (Table 4). These two groups differed significantly in threshing time and threshing efficiency, with the high SpHI group requiring 66 % threshing time and representing 191 % threshing efficiency relative to the low SpHI genotypic group.
Table 3.
Spike brittleness, spike harvest index (SpHI), threshing time and threshing efficiency in two groups of recombinant inbred lines showing extreme values of spike brittleness
| Genotypic group | d.f. | Spike brittleness† | SpHI | Threshing time (s per 20 g spikes) | Threshing efficiency (g grain min−1) |
|---|---|---|---|---|---|
| Brittle (Br) | 0·985 | 0·594b | 41·3a | 19·2b | |
| Non-brittle (br) | 0·001 | 0·623a | 25·4b | 34·4a | |
| Source of variation | |||||
| Genotypic group (F-value) | 1 | 38·4*** | 63·1*** | 58·6*** | |
| Genotype (group) (F-value) | 29 | 12·2*** | 2·2*** | 2·04** | |
| Block (F-value) | 2 | 6·7*** | 23·5*** | 12·1*** | |
† Brittleness values did not distribute normally and hence were not analysed.
a,b Different letters indicate significant differences between group means according to Tukey's HSD test at P < 0·05.
***, ** Significant F values at P < 0·001 or P < 0·01, respectively.
Table 4.
Spike brittleness, spike harvest index (SpHI), threshing time and threshing efficiency in two groups of recombinant inbred lines showing extreme values of SpHI
| Genotypic group | d.f. | Brittleness† | SpHI | Threshing time (s per 20 g spikes) | Threshing efficiency (g grain min−1) |
|---|---|---|---|---|---|
| Low SpHI | 0·37 | 0·53b | 42·2a | 17·9b | |
| High SpHI | 0·38 | 0·67a | 27·7b | 34·2a | |
| Source of variation | |||||
| Genotypic group (F value) | 1 | 617·5*** | 46·1*** | 81·8*** | |
| Genotype (group) (F value) | 29 | 0·7n.s. | 3·2*** | 1·8** | |
| Block (F value) | 2 | 3·1n.s. | 25·3*** | 22·6*** |
† Brittleness values did not distribute normally and hence were not analysed.
a,b Different letters indicate significant differences between group means according to Tukey's HSD test at P < 0·05.
***, **, n.s. Significant F values at P < 0·001 or P < 0·01 or non-significant values, respectively.
DISCUSSION
Spike brittleness and spike harvest index as labour-saving traits
Rachis disarticulation and tough glumes are major adaptive mechanisms for wild wheat under natural conditions (Zohary, 1969). The arrow-like morphology of the dispersal units of the large-grained Near Eastern wild cereals (barley, einkorn and emmer) and the motility mechanism of their awns (Elbaum et al., 2007) facilitate their burial in the ground, under stones and in rocky crevices, while tough glumes protect the grains from predation by rodents, ants and birds. Under domestication, however, a brittle-spike phenotype might be associated with yield losses. Hence, harvest of partially mature crops (Hillman and Davies, 1999) as well as spikelet collection from the ground (Kislev et al., 2004) have been suggested as possible measures to minimize the yield losses associated with spike disarticulation. While it is widely accepted that the emergence of free-threshing genotypes simplified the post-harvest processes, the classical convention that automatic selection in favour of non-brittle spikes occurred under cultivation (sensu Harlan et al., 1973) has recently been challenged (Fuller et al., 2010).
The results of our controlled threshing experiments show that threshing of domesticated modern wheat cultivars is 15 times less time-consuming and 40 times more efficient than that of wild wheat (Table 2). However, these differences reflect the combined effects of spike brittleness and threshability (represented here by SpHI). In this respect, threshing efficiency, which was calculated based on the extracted grains, is inherently associated with SpHI. A comparison between the brittle wild genotypes (hulled) and the non-brittle hulled landraces showed a 30 % reduction in threshing time presumably reflecting the effects of both lower spike brittleness and greater SpHI (reduced glume toughness). A comparison between the two hulled genotypic groups (wild and hulled landraces) and the two free-threshing groups (modern cultivars and free-threshing landraces) showed an approx. ten-fold reduction in threshing time that could be fully attributed to the effect of glume toughness. Note that threshing time was correlated with SpHI across the four genotypic groups (R2 = −0·86, n = 45, P < 0·001).
The extreme phenotypic groups of RILs enabled further distinction between the effect of spike brittleness and that of tough glumes. Among the two RIL groups differing in spike brittleness, the non-brittle genotype exhibited slightly greater SpHI but a considerably shorter threshing time and greater threshing efficiency. Likewise, the two RIL groups differing in SpHI exhibited similar brittleness values, with the high SpHI group showing shorter threshing time and greater threshing efficiency. Thus, the results from the extreme groups of RILs are in full accordance with those from the phenotypic groups, showing that both traits, non-brittle spike and free-threshing, are in fact labour-saving traits that simplify and increase the efficiency of post-harvest processing in tetraploid emmer wheat.
Spike brittleness in relation to recent modelling of plant domestication
The distinct morphology of the non-brittle spike in the Near Eastern cereals can be identified in archaeobotanical material (e.g. Hillman and Davies, 1999). For this reason, and due to its clear agronomic advantage, it is widely accepted that breakdown of the seed-dispersal mechanism (e.g. the emergence of non-brittle spike, non-shattering panicle) is the major trait underlying cereal domestication (e.g. Zohary, 1969; Harlan et al., 1973; Hillman and Davies, 1990a, b). The floral biology of the Near Eastern cereals (Zohary, 1999) and simple population genetic considerations led several authors to suggest that the domesticated non-brittle alleles could have been selected for and fixed in the nascent crop populations in a rather rapid manner (e.g. Hillman and Davies, 1999; Ladizinsky, 1998). Recently, however, a claim for a millennia-long domestication process has been put forward with suggested time frames ranging from 1000 years (Tanno and Willcox, 2006) to 4000 years (Purugganan and Fuller, 2011). This model, referred to as ‘protracted domestication’ (Allaby et al., 2008), was recently criticized in a review by Heun et al. (2012).
Given the advantage of the non-brittle spike in preventing yield losses, several explanations have been offered to account for the presumed ‘slow’ cereal domestication rate in the Near East and elsewhere (Fuller, 2007; Purugganan and Fuller, 2011). Fuller et al. (2010) argued that the domesticated non-brittle ear phenotype is associated with extra post-harvest labour required to release the grains from the non-brittle spike. It was also suggested that due to the extra labour associated with the increase of non-brittle genotypes in the nascent crop populations, farmers may have had to resort to the wild cereal populations for sampling new wild types to renew their seed corn, apparently as a measure to ‘repair’ or in fact ‘de-domesticate’ their seed stocks (Fuller et al., 2010, p. 18). According to this model, the increased yield reliability of the non-brittle types and the selection (in the opposite direction) against the presumed extra (post-harvest) labour resulted in a protracted meta-stable equilibrium in the Neolithic cereal crop populations, in which the non-brittle genotypes were maintained for thousands of years as a fairly stable minority (Fuller et al., 2010).
Although often termed a ‘seed-dispersal mechanism’, spike brittleness in wild wheat and barley results in spikelet shattering rather than seed dispersal. Therefore, extracting the grains from both wild-type and domesticated Near Eastern cereals requires threshing to release them from the glumes and florets (lemma and palea), often collectively termed ‘chaff’. In wild barley, the dispersal unit holds a single-hulled seed in which the chaff is invested in the seed coat, which also necessitates threshing to separate the seeds from the inedible awns and lignified chaff. It is therefore not surprising that our results clearly show no extra labour whatsoever associated with the transition from wild to domesticated non-brittle wheat phenotype. By contrast, our hands-on experience showed that threshing non-brittle spikes is more efficient than threshing disarticulated spikelets. This was also illustrated by a simple, straightforward, small-scale experiment using an ancient stone-made (assumed threshing) device (Fig. 1C, D). Therefore, it is most likely that in addition to its agronomic advantage in minimizing yield losses, the extra labour involved in threshing the brittle wheat types facilitated the transition to non-brittle morphotypes.
The wide variation in threshing time among both the landraces and the wild wheat accessions (Fig. 3) is typical of a quantitative trait. Such naturally occurring variation within the wild gene pool was most probably available to the early Neolithic ‘cultivators’ when approaching wild populations to prepare their initial seed corn stocks, and later on as a raw material to select for the desired phenotypes in their fields. The variation observed in our small collection of traditional domesticated wheat landraces probably represents the gradual transition from difficult (hulled) to easy threshing (free-threshing) domesticated types. Although a very small fraction of spike disarticulation can be observed in domesticated materials, both the landraces and the modern cultivars can be classified as having a non-brittle rachis, whereas all of the wild accessions tested exhibited strong and nearly complete spike disarticulation. Contrary to the discrete classification of brittle vs. non-brittle rachis phenotypes, mostly treated as a monogenic Mendelian trait (e.g. Sood et al., 2009), glume toughness can be analysed as a classical quantitative trait, as done here and by other groups (e.g. Simonetti et al., 1999). These two distinct polymorphism patterns, (nearly) discrete (spike disarticulation) and continuous (glume toughness), suggest that brittle rachis was the first domestication trait in wheat, while glume toughness and its associated SpHI and grain weight underwent a gradual change under domestication (sensu Abbo et al., 2012). While threshing the hundreds of samples analysed in this work, another drawback associated with the threshing of wild wheat was observed. Due to the tough glumes, wild wheat genotypes require much more forceful movement of the threshing implement to achieve full release of the kernels. Often, this resulted in massive kernel breakage (data not shown), directly affecting their viability and consequently reducing their contribution to the next generation after each harvest and processing cycle. This may have served as another selective force in favour of the domesticated (free-threshing) genotypes, at the expense of the wild types from the very early days of agriculture.
The classical treatment of Near Eastern cereal domestication attributes a major role to unconscious (automatic) selection exerted by the early ‘cultivators’ on the managed plant populations (e.g. Harlan et al., 1973; Hillman and Davies, 1999; Zohary, 2004). However, in our view, there is ample experimental (and other types of) evidence suggesting that the early agriculturists were highly conscious in their species (and phenotype within species) selections for domestication (e.g. Ladizinsky, 1987; Kerem et al., 2007; Abbo et al., 2009, 2010, 2011a, b). In this context, taking into account the labour involved in post-harvest processing, the selection in favour of non-brittle spike phenotypes among the Near Eastern cereals can be seen as yet another compelling example of the role of conscious selection in plant domestication.
Concluding remarks
While discussing their ‘labour trap’ assumption, Fuller et al. (2010, p. 17) suggested that the notion of ‘domestication events’ (namely, rapid domestication) be discarded and the concept of ‘protracted pathways towards domestication’ be adopted instead. Having no work experience with wild or domesticated forms of the African and Far Eastern crops (e.g. pearl millet, sorghum, rice) to which Fuller et al.'s (2010) assumption may apply (although still awaiting experimental verification), this cannot be commented upon here. However, as far as the Near Eastern cereals are concerned and with respect to the labour associated with their threshing, it seems more prudent to discard the notion of the ‘extra labour trap’ and its presumed role in affecting the pace of cereal domestication.
ACKNOWLEDGEMENTS
This study was supported by Israel Science Foundation grant # 800-2010. R.T. is indebted to the Matanel Foundation fellowship. We thank Shebolet Muntz and Nitzan Wigoda for their skilful technical assistance. We thank the National Small Grains Collection (NSGC), USA, Institute of Plant Genetics and Crop Plant Research (IPK) Genebank, Gatersleben, Germany, Professor F. Salamini, Max-Plank Institute, Germany, and Professor Ismail Cakmak of Sabanci University, Turkey, for providing some of the germplasm used in this study.
LITERATURE CITED
- Abbo S, Zezak I, Schwartz E, Lev-Yadun S, Kerem Z, Gopher A. Wild lentil and chickpea harvest in Israel: bearing on the origins of Near East farming. Journal of Archaeological Science. 2008a;35:3172–3177. [Google Scholar]
- Abbo S, Zezak I, Schwartz E, Lev-Yadun S, Gopher A. Experimental harvesting of wild peas in Israel: implications for the origins of Near East farming. Journal of Archaeological Science. 2008b;35:922–929. [Google Scholar]
- Abbo S, Saranga Y, Peleg Z, Lev-Yadun S, Kerem Z, Gopher A. Reconsidering domestication of legumes versus cereals in the ancient Near East. Quarterly Review of Biology. 2009;84:29–50. doi: 10.1086/596462. [DOI] [PubMed] [Google Scholar]
- Abbo S, Lev-Yadun S, Gopher A. Yield stability: an agronomic perspective on the origin of Near Eastern agriculture. Vegetation History and Archaeobotany. 2010;19:143–150. [Google Scholar]
- Abbo S, Rachamim E, Zehavi Y, Zezak I, Lev-Yadun S, Gopher A. Experimental growing of wild pea in Israel and its bearing on Near Eastern plant domestication. Annals of Botany. 2011a;107:1399–1404. doi: 10.1093/aob/mcr081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbo S, Lev-Yadun S, Gopher A. Origin of Near Eastern plant domestication: homage to Claude Levi-Strauss and ‘La Pensée Sauvage. Genetic Resources and Crop Evolution. 2011b;58:175–179. [Google Scholar]
- Abbo S, Lev-Yadun S, Gopher A. Plant domestication and crop evolution in the Near East: on events and processes. Critical Reviews in Plant Science. 2012;31:241–257. [Google Scholar]
- Abbo S, Zezak I, Zehavi Y, Schwartz E, Lev-Yadun S, Gopher A. Six seasons of wild pea harvest in Israel: bearing on Near Eastern plant domestication. Journal of Archaeological Science. 2013;40:2095–2100. [Google Scholar]
- Allaby RG, Fuller DQ, Brown TA. The genetic expectations of a protracted model for the origins of domesticated crops. Proceedings of the National Academy of Science USA. 2008;105:13982–13986. doi: 10.1073/pnas.0803780105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Yosef O. The Natufian culture and the early Neolithic: Social and economic trends in southeastern Asia. In: Bellwood P, Renfrew C, editors. Examining the farming/language dispersal hypothesis. Cambridge: McDonald Institute Monographs; 2002. pp. 113–126. [Google Scholar]
- Bohrer VL. On the relations of harvest methods to early agriculture in the Near East. Economic Botany. 1972;26:145–155. [Google Scholar]
- Elbaum R, Zaltzman L, Burgert I, Fratzl P. The role of wheat awns in the seed dispersal unit. Science. 2007;316:884–886. doi: 10.1126/science.1140097. [DOI] [PubMed] [Google Scholar]
- Fuller DQ. Contrasting patterns in crop domestication and domestication rates: recent archaeological insights from the Old World. Annals of Botany. 2007;100:903–924. doi: 10.1093/aob/mcm048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DQ, Allaby RG, Stevens C. Domestication as innovation: the entanglement of techniques, technology and chance in the domestication of cereal crops. World Archaeology. 2010;42:13–28. [Google Scholar]
- Harlan JR. A wild wheat harvest in Turkey. Archaeology. 1967;20:197–201. [Google Scholar]
- Harlan JR, de Wet JMJ, Price EG. Comparative evolution of cereals. Evolution. 1973;27:311–325. doi: 10.1111/j.1558-5646.1973.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Harris DR. Agriculture, cultivation and domestication: exploring the conceptual framework of early food production. In: Denham T, Iriarte J, Vrydaghs L, editors. Rethinking agriculture: archaeological and ethnoarchaeological perspectives. Walnut Creek, CA: Left Coast Press; 2007. pp. 16–35. [Google Scholar]
- Heun M, Abbo S, Lev-Yadun S, Gopher A. A critical review of the protracted domestication model for Near-Eastern founder crops: linear regression, long-distance gene flow, archaeological, and archaeobotanical evidence. Journal of Experimental Botany. 2012;63:4333–4341. doi: 10.1093/jxb/ers162. [DOI] [PubMed] [Google Scholar]
- Hillman CG, Davies MS. Domestication rates in wild type wheats and barley under primitive cultivation. Biological Journal of the Linnean Society. 1990a;39:39–78. [Google Scholar]
- Hillman CG, Davies MS. Measured domestication rates in wild wheats and barley under primitive cultivation, and their archaeological implications. Journal of World Prehistory. 1990b;4:39–78. [Google Scholar]
- Hillman CG, Davies MS. Domestication rates in wild wheats and barley under primitive cultivation. In: Anderson PC, editor. Prehistory of agriculture. Los Angeles: Institute of Archaeology, University of California; 1999. pp. 70–102. Monograph no. 40. [Google Scholar]
- Kelly RL. The foraging spectrum. Washington, DC: The Smithsonian Institute Press; 1995. [Google Scholar]
- Kerem Z, Lev-Yadun S, Gopher A, Weinberg P, Abbo S. Chickpea domestication through the nutritional perspective. Journal of Archaeological Science. 2007;34:1289–1293. [Google Scholar]
- Kislev ME, Bar-Yosef O. The legumes: the earliest domesticated plants in the Near East? Current Anthropology. 1988;29:175–179. [Google Scholar]
- Kislev ME, Weiss E, Hartmann A. Impetus for sowing and the beginning of agriculture: ground collecting of wild cereals. Proceedings of the National Academy of Science USA. 2004;101:2692–2695. doi: 10.1073/pnas.0308739101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladizinsky G. Collection of wild cereals in the upper Jordan Valley. Economic Botany. 1975;29:264–267. [Google Scholar]
- Ladizinsky G. Pulse domestication before cultivation. Economic Botany. 1987;41:60–65. [Google Scholar]
- Ladizinsky G. How many tough-rachis mutants gave rise to domesticated barley? Genetic Resources and Crop Evolution. 1998;45:411–414. [Google Scholar]
- Lev E, Kislev ME, Bar-Yosef O. Mousterian vegetal food in Kebara Cave, Mt. Carmel. Journal of Archaeological Science. 2005;32:475–484. [Google Scholar]
- Peleg Z, Fahima T, Korol AB, Abbo S, Saranga Y. Genetic analysis of wheat domestication and evolution under domestication. Journal of Experimental Botany. 2011;62:5051–5061. doi: 10.1093/jxb/err206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purugganan MD, Fuller DQ. Archaeological data reveal slow rates of evolution during plant domestication. Evolution. 2011;65:171–183. doi: 10.1111/j.1558-5646.2010.01093.x. [DOI] [PubMed] [Google Scholar]
- Redman CL. The rise of civilizations. San Francisco: Freeman & Co; 1978. [Google Scholar]
- Simonetti MC, Bellomo MP, Laghetti G, Simeone R, Blanco A. Quantitative trait loci influencing free-threshing habit in tetraploid wheat. Genetic Resources and Crop Evolution. 1999;46:267–271. [Google Scholar]
- Sood S, Kuraparthy V, Bai G, Gill BS. The major threshability genes soft glume (sog) and tenacious glume (Tg) of diploid and polyploidy wheat, trace their origin to independent mutation at non-orthologous loci. Theoretical and Applied Genetics. 2009;119:341–351. doi: 10.1007/s00122-009-1043-0. [DOI] [PubMed] [Google Scholar]
- Tanno K-I, Willcox G. How fast was wild wheat domesticated? Science. 2006;311:1886. doi: 10.1126/science.1124635. [DOI] [PubMed] [Google Scholar]
- Willcox GH. Archaeobotanical significance of growing Near Eastern progenitors of domestic plants at Jales (France) In: Anderson PC, editor. Prehistoire de l'Agriculture: Nouvelles Approches Experimentales et Ethnographiques. Paris: CNRS; 1992. pp. 159–178. Monographie du CRA No. 6. [Google Scholar]
- Willcox G. Agrarian change and the beginning of cultivation in the Near East. In: Denham T, White P, editors. The emergence of agriculture, a global view. London: Routledge; 2007. pp. 217–241. [Google Scholar]
- Zohary D. The progenitors of wheat and barley in relation to domestication and agricultural dispersal in the Old World. In: Ucko PI, Dimbleby GW, editors. The domestication and exploitation of plants and animals. London: Gerald Duckworth & Co; 1969. pp. 47–66. [Google Scholar]
- Zohary D. Speciation under self-pollination. In: Wasser SP, editor. Evolutionary theory and processes: modern perspective, papers in honour of Eviatar Nevo. Dordrecht: Kluwer Academic Publishers; 1999. pp. 301–307. [Google Scholar]
- Zohary D. Unconscious selection and the evolution of domesticated plants. Economic Botany. 2004;58:5–10. [Google Scholar]