Abstract
Background and Aims
Seed dormancy varies within species in response to climate, both in the long term (through ecotypes or clines) and in the short term (through the influence of the seed maturation environment). Disentangling both processes is crucial to understand plant adaptation to environmental changes. In this study, the local patterns of seed dormancy were investigated in a narrow endemic species, Centaurium somedanum, in order to determine the influence of the seed maturation environment, population genetic composition and climate.
Methods
Laboratory germination experiments were performed to measure dormancy in (1) seeds collected from different wild populations along a local altitudinal gradient and (2) seeds of a subsequent generation produced in a common garden. The genetic composition of the original populations was characterized using intersimple sequence repeat (ISSR) PCR and principal co-ordinate analysis (PCoA), and its correlation with the dormancy patterns of both generations was analysed. The effect of the local climate on dormancy was also modelled.
Key Results
An altitudinal dormancy cline was found in the wild populations, which was maintained by the plants grown in the common garden. However, seeds from the common garden responded better to stratification, and their release from dormancy was more intense. The patterns of dormancy variation were correlated with genetic composition, whereas lower temperature and summer precipitation at the population sites predicted higher dormancy in the seeds of both generations.
Conclusions
The dormancy cline in C. somedanum is related to a local climatic gradient and also corresponds to genetic differentiation among populations. This cline is further affected by the weather conditions during seed maturation, which influence the receptiveness to dormancy-breaking factors. These results show that dormancy is influenced by both long-and short-term climatic variation. Such processes at such a reduced spatial scale highlight the potential of plants to adapt to fast environmental changes.
Keywords: Centaurium somedanum, clinal variation, common garden, ecophysiology, endemic, Gentianaceae, germination, ISSR, plant–climate interactions, PCoA, seed dormancy, seed ecology
INTRODUCTION
Dormancy is a quantitative seed trait that defines the range of environmental conditions that must be met before germination can occur (Vleeshowers et al., 1995; Finch-Savage and Leubner-Metzger, 2006). A seed able to germinate over the widest possible range of conditions is non-dormant; any narrowing of these conditions is by definition an increase in the level of seed dormancy. Dormancy levels vary temporally in any individual seed, most markedly in the case of physiological dormancy (sensu Baskin and Baskin, 2004). Upon dispersal, these seeds show primary dormancy, either total (i.e. no germination under any conditions) or conditional (i.e. germination under a restricted set of conditions) (Baskin and Baskin, 1998; Fenner and Thompson, 2005). This dormancy can be alleviated by certain environmental signals, the dormancy-breaking factors (Batlla and Benech-Arnold, 2003, 2004, 2005; Batlla et al., 2003). In response to these factors, the seeds gradually lose dormancy and their range of germination conditions broadens (Steadman and Pritchard, 2004; Orrù et al., 2012) until eventually they become non-dormant. In some cases, the seeds that do not meet adequate germination conditions enter secondary dormancy as a response to further environmental cues (Brandel, 2005; Kepczynski et al., 2006; Leymarie et al., 2008). These dormancy cycles (Batlla and Benech-Arnold, 2007) go on throughout the year, matching the annual temperature cycles, and have been termed the dormancy continuum (Baskin and Baskin, 1985).
At any given time, spatial variation in seed dormancy can be observed among individuals and populations of the same species, as a consequence of different stages in the dormancy continuum. Variation becomes apparent not only in the dormancy levels, but also in the receptiveness to dormancy-breaking factors (Black et al., 2006). Many studies have found dormancy variation among seed collections from different sites and years (Moyer and Lang, 1976; Andersson and Milberg, 1998; Schütz and Rave, 2003; Koutecká and Leps, 2009; Herranz et al., 2010). These investigations have usually considered broad geographical scales, detecting changes in dormancy associated with latitudinal differences (Ren and Abbott, 1991; Skordilis and Thanos, 1995; Wagmann et al., 2012). Similarly, a positive correlation between dormancy and population altitude has often been established (Vickery, 1983; Beardsell and Mullet, 1984; Holm, 1994; Cavieres and Arroyo, 2000), but exceptions to this pattern have also been found (Barclay and Crawford, 1984; Giménez-Benavides et al., 2005). Further dormancy variation has been detected among seed collections from different environments. For example, in Artemisia tridentata, a negative effect of the winter temperature on seed dormancy was described on a wide gradient along western North America (Meyer and Monsen, 1991). Thymelaea hirsuta showed differences in the germinability of seeds collected in six different desert habitats, with lower germination in seeds from the more extreme sites (El-Keblawy et al., 1996). Regional differences in climatic conditions, which vary with latitude/altitude and among habitats, are probably the cause of some of these correlations.
Field studies, however, cannot disentangle the long- and the short-term effects of climate. Long-term effects arise from sustained climatic differences among sites, which may result in inheritable dormancy differences through ecotypes (Hufford and Mazer, 2003) and clinal variation (Montague et al., 2007). On the other hand, short-term effects are produced by the specific weather during the seed maturation season, and are usually termed the parental or maternal environment effect (Fenner, 1991; Donohue, 2009). This issue is partially addressed by common garden experiments, where seeds with controlled genetic origin are matured under controlled conditions. Such an approach has confirmed that low temperatures and water availability during seed maturation result in more dormant seeds (Wright et al., 1999; Allen and Meyer, 2002; Luzuriaga et al., 2006; Qaderi et al., 2006; Hoyle et al., 2008; Figueroa et al., 2010). At the same time, work has been done to unravel the genetic basis of dormancy variation, understood as a complex quantitative trait controlled by a large number of genes, plant hormones and maternal factors (Koornneef et al., 2002). In the model species Arabidopsis thaliana, variation in dormancy-related genes (Bentsink et al., 2006, 2010) has been identified in relation to maturation environment, seed provenance (Chiang et al., 2011) and local adaptation (Kronholm et al., 2012). Therefore, both inheritable genetic differences and the seed maturation environment seem to determine dormancy variation in the field, but understanding the respective roles of these two sources of variation is not trivial. In seasonal climates, germination traits allow a precise timing of seed germination and seedling establishment, are subjected to natural selection, and determine the action of natural selection in subsequent life history traits (Donohue, 2005; Donohue et al., 2005a, b, c). Thus, ascertaining how seed dormancy responds to local climate in the short and the long term is crucial to understand how germination timing and plant regeneration will be affected by environmental change, especially climate warming (Walck et al., 2011; Ooi, 2012).
The interpretation of local adaptation and clinal variation in A. thaliana and other widespread species, however, is often obscured because only part of the species distribution is studied; as a consequence, the influence of biogeographic and historical events (e.g. different colonization events by already adapted ecotypes) cannot be entirely assessed (Montesinos-Navarro et al., 2011). One clear advantage of studying narrow endemic species is that biogeographical and historical influences are not substantial, and local adaptation can be assumed to have taken place in situ along the environmental gradient. Furthermore, the results obtained in plants with widespread distributions should not be directly extrapolated to the reduced geographical scales where some endemic species occur. One such species is the mountain spring specialist Centaurium somedanum, an endemic perennial herb of the Cantabrian Mountains of north-western Spain. The seeds of this species have non-deep simple morphophysiological dormancy; and the physiological component of this dormancy shows within-species variation at a local scale (Fernández-Pascual et al., 2012). In this work, we separate the effects of environmental and genetic variability on the observed physiological dormancy variation of this species to test whether (1) the patterns of intraspecific variation in seed dormancy levels at dispersal and receptiveness to dormancy-breaking factors, shown by the populations in nature, are maintained or altered when the plants and the seeds mature in a common garden environment; (2) such patterns are correlated with the genetic composition of the original wild populations; and (3) such patterns may be explained by the local climate at the population sites.
MATERIALS AND METHODS
Plant material
Centaurium somedanum M.Laínz (Gentianaceae) is confined to a 210-km2 mountain area (Jiménez-Alfaro et al., 2010) where discrete populations occur in close association with calcareous springs (Jiménez-Alfaro et al., 2013). The regional climate is transitional between Oceanic and Continental–Mediterranean zones, and the local climatic differences depend mainly on altitude (600–1700 m a.s.l.) but also on the distance to the sea and the exposure of the mountain range (Rodríguez, 1985). The reproductive output in nature is high, usually 2–4 fruits per individual with approx. 140 seeds each, although the number of fruits can reach up to 15 (Jiménez-Alfaro et al., 2005). The proportion of viable seeds is usually >95 % (Fernández-Pascual et al., 2012). The species is assumed to be a facultative outcrosser, as is common in the genus (Rich, 2005; Brys and Jacquemyn, 2011). Flowering begins in early July, proceeds during summer, and ripe seeds are dispersed in September and October (Jiménez-Alfaro et al., 2010).
In September–October 2009, we visited the 16 known populations of C. somedanum and took a census by direct counting of all the reproductive adults (i.e. individuals with flowers or fruits) living there at that moment (the initial parent generation, P) (Table 1). We collected leaf tissue for genetic analysis from these P individuals, sampling a number of adults proportional to the local population size and area of occupation (Table 1), and evenly distributed across each site. Immediately after collection, we dried the leaves over silica gel in sealed containers and kept them there until further use. From 13 populations covering the entire distribution area of the species (Fig. 1), we collected seeds (the first seed generation, F1) sampling all P individuals with ripe fruits. The seed collections spent a 3 week period at moderate humidity (approx. 22 °C, 50 % relative humidity) in our laboratory to ensure a homogeneous maturation state before being cleaned and used in the germination experiments.
Table 1.
Description of the study sites
| Site | Co-ordinates (N, W) | Alt | T | P | Nt | Ns | G |
|---|---|---|---|---|---|---|---|
| AG | 43 °06′35″, 06 °15′22″ | 830 | 10·1 | 154 | 12 | 12 | 0·75 |
| AR | 43 °05′13″, 06 °06′43″ | 1470 | 7·0 | 214 | 108 | 32 | –1·20 |
| BR | 43 °01′28″, 06 °12′36″ | 1540 | 6·5 | 139 | 46 | 16 | 0·66 |
| CM | 43 °07′05″, 06 °15′05″ | 600 | 11·1 | 143 | 233 | 42 | 0·62 |
| CU | 43 °10′53″, 06 °11′59″ | 1350 | 7·5 | 203 | 11 | 10 | 0·02 |
| FM | 43 °07′11″, 06 °15′08″ | 710 | 10·7 | 148 | 38 | 18 | 0·73 |
| FU | 43 °06′19″, 06 °16′26″ | 870 | 9·9 | 158 | 65 | 16 | 1·37 |
| MA | 43 °00′35″, 06 °06′30″ | 1670 | 5·7 | 147 | 62 | 15 | –2·99 |
| ML | 43 °02′36″, 06 °09′21″ | 1590 | 6·5 | 231 | 142 | 34 | 0·66 |
| PU | 43 °00′37″, 06 °13′29″ | 1420 | 7·2 | 130 | 163 | 21 | 0·38 |
| TE | 42 °59′40″, 06 °06′31″ | 1580 | 6·3 | 140 | 99 | 33 | –1·84 |
| TR | 43 °00′33″, 06 °07′11″ | 1630 | 6·0 | 143 | 48 | 10 | –1·99 |
| VA | 43 °04′19″, 06 °11′49″ | 1280 | 7·9 | 193 | 283 | 44 | 0·83 |
| JBA | 43 °31′15″, 05 °36′49″ | 25 | 14·0 | 175 | – | – | – |
JBA is the common garden. Alt, altitude (m a.s.l.); T, mean annual temperature (°C); P, summer precipitation (mm); Nt, population size (reproductive individuals); Ns, sample size for the genetic analysis; G, position of the populations in the first PCoA axis obtained from the ISSR matrix.
Fig. 1.
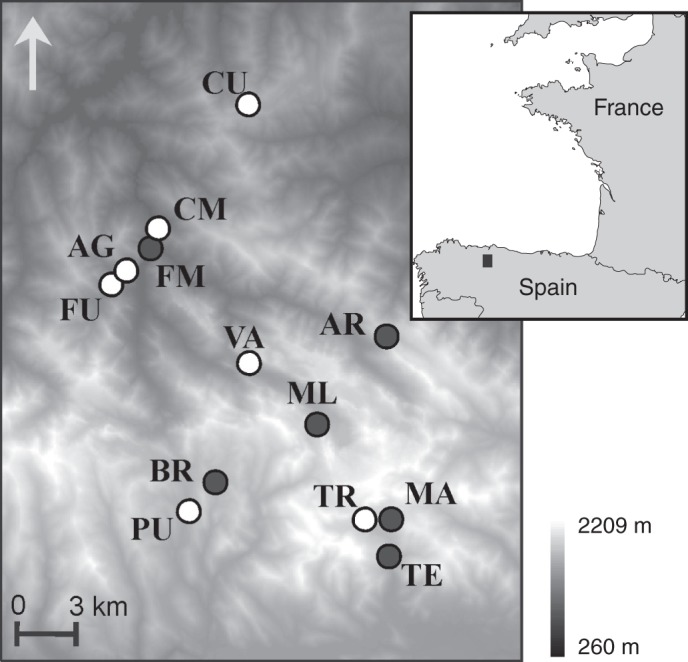
Map of the study area showing the 13 studied population sites. The open circles indicate the seven populations that were also included in the common garden and F2 germination experiments.
Germination and common garden experiments
We sowed seeds from all F1 populations on 1 % distilled water agar held in 6 cm diameter Petri dishes (eight dishes with 25 seeds each per population), sealed with Parafilm to prevent desiccation. Half of the dishes (the fresh seeds) went immediately into the incubation conditions for germination to check dormancy at dispersal; the other half (the stratified seeds) were exposed to wet-cold stratification (12 weeks, 1 % agar, 3 °C, darkness) before being placed in the germination conditions, to assess their receptiveness to dormancy-breaking factors. The germination incubations were conducted in a growth chamber (Grow-S 360, Ing. Climas, Barcelona, Spain) programmed with a 12 h light/12 h darkness photoperiod (approx. 20 µmol m−2 s−1 during the light phase provided by six Philips TLD30W/54-765 cool fluorescent tubes) coupled with a 22/12 °C thermoperiod. These conditions promote C. somedanum germination once dormancy is broken (Fernández-Pascual et al., 2012, 2013). Apart from the population-level germination, we analysed the germination of seeds produced by the same individual plant in four F1 populations (CM, BR, PU and VA; three individuals per population), using the same germination protocol. During the incubations, we counted and discarded germinated seeds three times a week. Radicle protrusion was the criterion for germination. We terminated the experiments after 4 weeks and opened the non-germinated seeds with a scalpel, classifying them as normal, empty or fungus infected. We excluded the empty (1·3 ± 0·3 %) and infected (1·0 ± 0·2 %) seeds from the statistical analyses and the calculation of germination percentages.
At the end of the F1 germination experiment, we chose a reduced sample of seven populations (covering the entire distribution area, Fig. 1) and grew their seedlings in a common garden experiment to produce a second seed generation (the F2). To prevent an artificial selection of less dormant genotypes, we left the F1 stratified seeds for an additional 10 weeks in the germination incubations, until at least 80 % of the seeds had germinated. We planted the seedlings in 5 cm3 plastic pots containing standard growth medium (Sustrato Universal, Pons Agropecuaria S.L., Madrid, Spain), and kept them saturated in water in a greenhouse at approx. 20 °C during their first summer. In September 2010, we randomly selected 20 plants per population, transplanted them to 10 cm3 plastic pots with the same medium and carried them to an open air garden (Jardín Botánico Atlántico, Xixón, Spain) to meet the vernalization requirement for flowering. This garden is 70 km away from the original populations, at sea level, and its climate is warmer (Table 1), making any possible seed maturation environment effects on dormancy more evident. We placed the pots in floating trays (one tray per population) inside a pool protected from the wind and constantly inundated by rain water to represent the original spring habitat. Periodically we changed the position of the trays in a random way. Shortly before flowering (May 2011), we covered each tray with a white nylon mesh to prevent interpopulation pollination. In July 2011, we collected the ripe F2 fruits and used them in a new population-level germination experiment with the same conditions as for the F1.
We analysed the patterns of dormancy variation in the seven populations represented in the common garden by (1) fitting a linear regression between the F1 and F2 germination percentages per population; and (2) fitting a factorial generalized linear mixed model (GLMM, logit link function, binomial distribution) to the germination data with stratification (yes/no) and seed generation as fixed predictors, and population as a random factor. We performed these tests with the SPSS Statistics 20 software (IBM, Armonk, USA).
Genetic analysis
For DNA extraction, we used the cetyltrimethylammonium bromide (CTAB) 2× method as modified by Caujapé-Castells et al. (2011). We quantified the extracted DNA concentration using a Biophotometer (Eppendorf, Hamburg, Germany) and deposited aliquots of the DNA extracts in the DNA Bank of the Jardín Botánico Canario ‘Viera y Clavijo’-Unidad Asociada CSIC, Cabildo de Gran Canaria. Afterwards we conducted an intersimple sequence repeat (ISSR) PCR using eight different 3'-anchored universal primers described in Meimberg et al. (2006) (Table 2), including two primers instead of one in the amplification reactions to increase the number of PCR fragments. Each 25 µL reaction volume contained 2·5 µL of 10× PCR buffer (100 mm Tris–HCl, 15 mm MgCl2, 500 mm KCl), 1 µL of bovine serum albumin (BSA; 20 mg mL−1), 0·5 µL of dNTPs (10 mm each), 0·5 µL of each primer (10 µm), 0·3 µL of Roche Taq DNA polymerase and 1 µL of DNA (20 ng). The amplification reactions were carried out using a C1000 Thermal Cycler (Bio-Rad, Hercules, USA) with the following PCR profile: (1) 94 °C for 2 min; (2) 35 cycles at 94 °C for 1 min, specific annealing temperature for each primer (Table 2) for 1 min, 72 °C for 1 min; and (3) final extension at 72 °C for 5 min. Afterwards we electrophoresed 5 µL of the PCR products on 1·8 % agarose gels stained with SyBr Safe DNA Gel Stain (Life Technologies, New York, USA) during 1·5 h at 100 V in 1× TBE buffer. We visualized and photographed the gels using an AlphaImager EP imaging system (Cell BioSciences Inc., Santa Clara, USA). The 100 bp ladder H3 RTU (Nippon Genetics Europe GmbH, Düren, Germany) provided DNA fragment size verification. Amplification fragments on the gels had a variable length between 150 and 1700 bp. We manually constructed a binary 0/1 matrix based on the absence/presence of the DNA bands; the final matrix included 54 diallelic loci and 324 individuals.
Table 2.
ISSR primers used in this study
| Primer | Sequence 5′–3′ | T (°C) | |
|---|---|---|---|
| 1 | I-GA8C | GAGAGAGAGAGAGAGAC | 53 |
| I-CA9G | CACACACACACACACACAG | ||
| 2 | I-AC9G | ACACACACACACACACACG | 55 |
| I-AC9C | ACACACACACACACACACC | ||
| 3 | I-ACG5G | ACGACGACGACGACGG | 57 |
| I-ACG5C | ACGACGACGACGACGC | ||
| 4 | I-TCG5G | TCGTCGTCGTCGTCGG | 55 |
| I-TCG5C | TCGTCGTCGTCGTCGC |
Two primers were included in one PCR; the annealing temperature (T) is shown for this reaction.
We performed a principal co-ordinate analysis (PCoA) of this binary matrix, based on the Euclidean distance (as implemented in PAST; Hammer et al., 2001), to represent the genetic relationships among populations and individuals (hereafter referred to as genetic composition). This technique reduces the complex multidimensional variability of a given matrix in two or more dimensions or ordination axes (Leps and Smilauer, 2003). Because of their capability to represent genetic differentiation, ordination axes obtained from a neutral loci matrix can be used to assess correlations between genetic composition and other variables (Lee and Mitchell-Olds, 2011; Treier and Müller-Schärer, 2011). Here, we used the population and individual values from the first PCoA axis (Table 1) to test possible correlations between seed dormancy and genetic composition. We fitted binary logistic regressions between the germination results (separately per generation and fresh/stratified seeds) and the mean value of the P populations in the first PCoA axis. When germination data per individual plant were available, we repeated the binary logistic regression using the individual values in the genetic axis, and including the population of origin as a random factor. We performed these tests with SPSS Statistics 20.
Climatic models
To determine if dormancy variation could be predicted from the local environment of the original wild populations, we fitted main effects GLMs to the germination results (separately per generation and fresh/stratified seeds), using the local values of annual temperature and summer precipitation as predictors. Data from neighbouring climate stations were extrapolated to obtain the local values at each site (Table 1; Sánchez Palomares et al., 1999). These variables reflected long-term differences among sites, as they were obtained averaging 50 years of measures. We also included population size (reproductive adults) in the models to control demographic and sample size effects in germinability. The populations were not spatially autocorrelated for any of the three variables, considering either all the populations or only the ones in the common garden; the same was true for the model residuals (in all cases P > 0·05, Moran's I-test computed using ArcGis v9·3, Esri, Redlands, USA). The three explanatory variables were not significantly correlated according to the Pearson test (P > 0·05) and their variance inflation factor was <2. We fitted the models using a forward/backwards stepwise variable selection procedure with Akaike's information criterion (AIC) as implemented in the R-Commander package (v1·5-3) for R (v2·10·1, The R Foundation for Statistical Computing).
RESULTS
Seed dormancy patterns
When analysing the results for the seven populations represented in the common garden, we found the same pattern of dormancy variation in both seed generations, with seeds from higher altitude populations showing higher dormancy (Fig. 2). The patterns of the two generations were significantly correlated (Fig. 3), i.e. the populations that were more dormant in the F1 were also the more dormant ones in the F2. The GLMM nevertheless detected significant differences between the two seed generations (F = 6·512, P = 0·012), as on average the F2 was 9 percentage points (pp) more dormant than the F1 in fresh, but 26 pp less dormant after stratification.
Fig. 2.
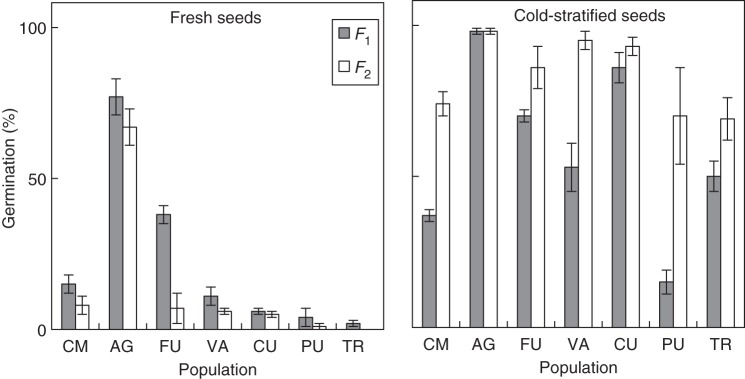
Population germination (mean ± s.e. of four dishes) after 4 weeks of incubation at 22/12 °C of fresh (left) and stratified (right) seeds from the two seed generations (F1, grey bars; F2, white bars). The populations are placed from lowest (left) to highest (right) altitude of origin (see Table 1).
Fig. 3.
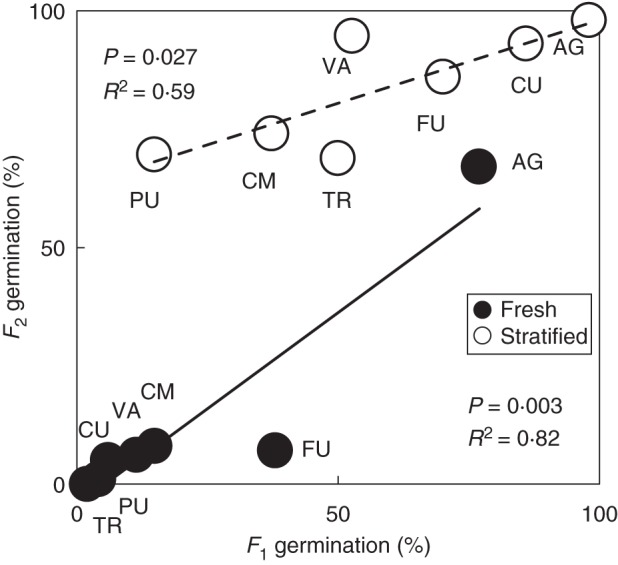
Scatter plot and fitted regression lines between F1 and F2 seed germination after 4 weeks of incubation at 22/12 °C. The symbols represent the percentage germination of a population (mean of four dishes) for fresh and stratified seeds, as indicated in the key. Population codes are indicated next to each symbol, and the results of the linear regressions are given.
Stratification produced a significant dormancy release in both generations (F = 654·541, P < 0·001), but not enough to break dormancy completely in most populations (Fig. 2). The dormancy-breaking effect of the stratification was significantly higher in the F2 (70 pp germination increase vs. 37 pp in the F1), as shown by the significant interaction between seed generation and stratification (F = 107·348, P < 0·001).
Correlation with the genetic composition
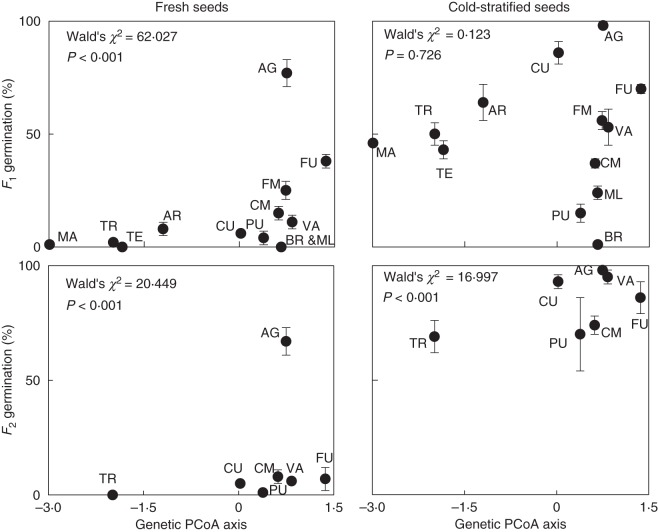
The population-level dormancy patterns of the seeds were significantly correlated with the genetic composition of the wild populations of origin, except in the case of the stratified F1 seeds (Fig. 4). Two population groups could be identified in the scatter plots, especially within F1 plants: (1) the four south-eastern populations (AR, MA, TE and TR), more genetically divergent from the rest, displayed high dormancy in fresh seeds and a parallel decrease after stratification; and (2) the rest of the populations, which formed a genetically closer group, but showed a higher variation in dormancy, both in fresh seeds and after stratification.
Fig. 4.
Scatter plots of population germination (mean ± s.e. of four dishes) vs. population genetic ordination, represented by the value of the first PCoA axis calculated from the ISSR matrix, in fresh and stratified seeds of the F1 (13 populations) and F2 (seven populations) seed generations. Each symbol represents a population, with its code next to to it. The results of the binary logistic regressions are given.
When analyses at the individual level were performed with the F1 seeds, the genetic composition of the mother plants was also significantly correlated with the dormancy of the seeds they produced, even after ruling out the differences due to the population of collection, both in fresh (F = 3·847; P < 0·001) and stratified (F = 2·990; P = 0·004) seeds.
Effect of the local climate
The climatic variables significantly explained the dormancy patterns of fresh and stratified seeds from both generations (Table 3). The selection procedure included all the variables in the final GLMs, except for summer precipitation in the Fresh-F2 model, although population size in the Stratified-F2 model was not significant at the 5 % level (P = 0·053). The models explained a greater portion of the variance and were more informative in the fresh seeds of both generations, where annual temperature and population size were the main variables. In the Stratified-F1 model, all variables shared a similar percentage of explained variance. The Stratified-F2 model was less informative, and in this case summer precipitation became the main explanatory variable. In all cases, annual temperature and summer precipitation had a negative effect on dormancy, while population size had a positive effect.
Table 3.
Explanatory models fitted to the germination data by means of binary logistic regression
| Model | Effect | Coefficient | s.e. | χ2 | P-value | %V |
|---|---|---|---|---|---|---|
| Fresh-F1 | Intercept | –9·74 | 1·33 | 53·509 | <0·001 | |
| 13 populations | Annual temperature | 0·799 | 0·0741 | 116·381 | <0·001 | 17·22 |
| AIC = 789·05 | Population size | –0·00787 | 0·00117 | 45·603 | <0·001 | 21·41 |
| Summer precipitation | 0·0104 | 0·00478 | 4·748 | 0·029 | 21·81 | |
| Fresh-F2 | Intercept | –9·30 | 1·17 | 63·250 | <0·001 | |
| 7 populations | Annual temperature | 0·932 | 0·124 | 56·475 | <0·001 | 9·82 |
| AIC = 403·11 | Population size | –0·0138 | 0·00181 | 57·988 | <0·001 | 22·17 |
| Stratified-F1 | Intercept | –4·05 | 0·443 | 83·686 | <0·001 | |
| 13 populations | Annual temperature | 0·312 | 0·0343 | 83·211 | <0·001 | 4·59 |
| AIC = 1584·2 | Population size | –0·00679 | 7·89 × 10−4 | 74·149 | <0·001 | 9·30 |
| Summer precipitation | 0·0138 | 0·00191 | 51·811 | <0·001 | 13·56 | |
| Stratified-F2 | Intercept | –3·99 | 0·872 | 20·986 | <0·001 | |
| 7 populations | Summer precipitation | 0·0296 | 0·00521 | 32·228 | <0·001 | 6·18 |
| AIC = 579·22 | Annual temperature | 0·156 | 0·0591 | 6·948 | 0·008 | 6·90 |
| Population size | –0·00235 | 0·00121 | 3·733 | 0·053 | 7·43 |
The effects appear in the order they were included by the variable selection procedure (forwards/backwards, AIC).
%V, percentage of the variance explained by the model after the inclusion of the effect.
DISCUSSION
Seed dormancy in C. somedanum exhibits a cline along a local gradient of altitude and climate, and this cline is related both to genetic composition and to the seed maturation environment. Although the general field cline is maintained when the plants and the seeds mature in a common garden, we found certain differences that can be explained by the maturation environment. Being at sea level, the common garden is 6 °C warmer than the original sites as well as frost free for almost the whole year, so the plants and seeds would perceive an extraordinarily warm year. The populations of C. somedanum show a fixed ‘baseline’ level of dormancy at dispersal, as revealed by the high levels of dormancy found in the common garden fresh seeds, even higher than in the natural populations. However, the same populations react to the environment during seed maturation and their dormancy release after stratification is more pronounced in the common garden, i.e. they are more receptive to dormancy-breaking factors. This behaviour agrees well with what was found by Chiang et al. (2011) in A. thaliana, where both the population of origin and the temperature during seed maturation influenced the levels of dormancy and the expression of a gene which controls dormancy variation in nature.
The fact that field and common garden seeds exhibited similar patterns of variation in seed dormancy suggests a genetic basis accounting for dormancy in this set of populations. Such a possibility is further supported by the correlation between the patterns of variation in the phenotypic trait of interest (seed dormancy), and the pattern of underlying neutral genetic variation shown by the first PCoA axis. However, since our genetic analysis characterized neutral variation, and not variation in adaptive dormancy-related genes, support for a genetic basis of seed dormancy must be considered indirect. The lack of correlation precisely in the F1 stratified seeds provides supplementary evidence of the influence of the seed maturation environment on the receptiveness to dormancy-breaking factors. While in the stratified seeds of the F1 the different field maturation environments produced the greatest departures from the fixed dormancy cline, in the F2 the common environment made the cline more evident. A similar correlation between genetic variation and dormancy-related seed traits along a regional climatic gradient has been found in the widespread species A. thaliana (Montesinos-Navarro et al., 2012) and Beta vulgaris L. (Wagmann et al., 2012), and our study suggests that the same adaptive processes operate at reduced geographical scales.
Nevertheless, non-genetic persistent parental effects (Rossiter, 1996) coming from the F1 seed maturation environments in the field cannot be entirely excluded. Alexander and Wulff (1985) reported such behaviour in Plantago lanceolata L., where high temperatures during the F1 seed maturation produced less dormant F1 seeds but more dormant F2 seeds, although their study was inconclusive as this only occurred in one of the genetic lines they studied. In any case, it is difficult to see how the possible F1 parental effects could be so strongly expressed above the homogenizing F2 seed maturation environment without an underlying genetic basis for the cline.
Future work analysing the genetic regulatory pathways for seed dormancy in this species is nonetheless needed to confirm this genetic basis. To this end, new generations must be produced in controlled common garden conditions, in order to minimize undesirable environmental variability and better quantify the effects of the seed maturation environment and the genetic lines. In this work, our goal was to capture phenotypic field variation in conditions close to natural conditions and, as a consequence, environmental influences were considerable. Even so, our results generally agree with those obtained in more controlled experimental settings (Chiang et al., 2011; Kronholm et al., 2012).
The story behind dormancy and genetic variation, however, seems to be more complicated than a linear relationship. Fixed local adaptation is expected when the environmental variation is at a greater spatial scale than gene flow, while the opposite situation would favour plasticity through parental environment effects (Galloway, 2005). It is possible that in C. somedanum local adaptation explains dormancy differences among genetically isolated groups, while the seed maturation environment plays a main role within those groups. The four easternmost populations (AR, MA, TE and TR) are genetically distant from the rest and, in the case of MA, TE and TR, live at the highest altitudes and at the more continental sites, secluded from the majority of the populations by the highest peaks of the area. The germination of these four populations shows similarities, with total or nearly total dormancy at dispersal and a similar receptiveness to dormancy-breaking factors. Meanwhile, the rest of the populations form a genetically homogeneous group and exhibit the altitudinal cline in dormancy at dispersal, but their receptiveness to dormancy-breaking factors varies from a null response in BR to a large germination increase in CU.
Another departure from a linear relationship is the extraordinary low dormancy in AG, which is also one of the smallest populations, with 12 reproductive adults, and may be the consequence of a recent colonization of AG by low dormant genotypes. Indeed, the AG population is largely responsible for the important effect of population size in the predictive models, where bigger population sizes correlate with lower seed germination. This relationship is contrary to what could be expected if the demographic effect was related to a reduction of germinability produced by reduced fitness and higher inbreeding depression in the smaller populations (Heschel and Paige, 1995). If the AG data are removed, the population size effect is much reduced in the F1 models and completely disappears from the F2 models.
Regardless of population size, the dormancy cline found in C. somedanum is related to the local variations in climate. Correlations between higher altitude sites and higher dormancy levels have usually been reported at broader geographical scales (Vickery, 1983; Beardsell and Mullet, 1984; Holm, 1994; Cavieres and Arroyo, 2000). In our case, the sites with lower annual temperatures and summer precipitation produce seeds with higher dormancy levels. The effect of temperature is in agreement with the results reported by Fenner (1991) and Fenner and Thompson (2005) in their reviews of seed maturation under controlled conditions experiments, as well as the behaviour found in the field by Meyer and Monsen (1991). In the case of C. somedanum, the plants growing at lower altitudes, under a generally milder climate, produce seeds that will germinate earlier, benefiting from a longer growing season. Plants from higher altitudes, where winters are harsher, produce seeds which will not germinate until the unfavourable season is over. This work cannot definitely determine that local adaptation is behind the cline, as that would require reciprocal transplant experiments and fitness measures. Nonetheless, a correlation between an environmental factor and a cline in a trait hypothetically related to fitness in response to that factor may be regarded as a clue of such adaptation (Montesinos-Navarro et al., 2011).
The ecological meaning of the summer precipitation effect, on the other hand, is less evident, especially due to its secondary role in comparison with temperature. Most experiments on seed maturation under controlled conditions actually reported a positive correlation between water availability and dormancy (Allen and Meyer, 2002; Hoyle et al., 2008). Studies performed with the weed Sinapis arvensis L. hypothesized that adequate soil moisture during seed maturation results in more dormant seeds because better developed seeds are produced (Wright et al., 1999; Luzuriaga et al., 2006), but in the spring habitats occupied by C. somedanum water availability should not be a limiting factor. However, precipitation may affect the plant through air humidity or the alteration of the water regime and the courses of the springs to which C. somedanum germination is especially adapted (Fernández-Pascual et al., 2012).
In conclusion, our study shows that seed dormancy has the capacity to adapt, even at reduced local scales, to long-term climatic differences through inheritable clinal variation; but also to adjust itself to the weather during seed maturation especially through the receptiveness to dormancy-breaking factors. The processes behind phenotypical plasticity in dormancy which have been detected at broad scales and in widely distributed species (Chiang et al., 2011; Montesinos-Navarro et al., 2012; Wagmann et al., 2012) seem to operate as well at local scales and in narrowly distributed taxa. Therefore, the risk of plants not being able to match their dormancy-breaking requirements as a consequence of the global increase in temperature (Ooi et al., 2009; Orrù et al., 2012) is lessened. The very presence of intraspecific variation on such a reduced scale highlights the great potential of physiological dormancy to adapt to fast environmental changes in time and space.
ACKNOWLEDGEMENTS
We thank R. Álvarez Álvarez, E. Correia Álvarez, A. I. García Torrico, C. Marcenò, D. Rodríguez de la Cruz, J. Sánchez Sánchez and J. I. Sanzo Rodríguez who contributed to leaf and seed collection; I. Pascual González, Y. Peña Abeijón and her students who helped with the common garden; Á. Penas and S. del Río who provided the climatic variables; and J. M. Iriondo, P. Toorop and three anonymous reviewers for their comments and revision of the manuscript. Seed collection was authorized by the regional governments of Asturias and Castile-Leon. This work was supported by the Spanish Ministry of the Environment – Fundación Biodiversidad (project Conservación ex situ de plantas amenazadas de máxima prioridad en el norte peninsular: Aster pyrenaeus y Centaurium somedanum); the Government of Asturias (Programa de Ayudas Predoctorales ‘Severo Ochoa’, Plan de Ciencia, Tecnología e Innovación del Principado de Asturias grant to E.F.P); and the European Social Fund through the Spanish Ministry of Science (PTA2007-0726-I grant to B.J.A.).
LITERATURE CITED
- Alexander HM, Wulff RD. Experimental ecological genetics in Plantago. X. The effects of maternal temperature on seed and seedling characters in P. lanceolata. Journal of Ecology. 1985;73:271–282. [Google Scholar]
- Allen PS, Meyer SE. Ecology and ecological genetics of seed dormancy in downy brome. Weed Science. 2002;50:241–247. [Google Scholar]
- Andersson L, Milberg P. Variation in seed dormancy among mother plants, populations and years of seed collection. Seed Science Research. 1998;8:29–38. [Google Scholar]
- Barclay AM, Crawford RMM. Seedling emergence in the rowan (Sorbus aucuparia) from an altitudinal gradient. Journal of Ecology. 1984;72:627–636. [Google Scholar]
- Baskin CC, Baskin JM. Seeds. Ecology, biogeography, and evolution of dormancy and germination. San Diego: Academic Press; 1998. [Google Scholar]
- Baskin JM, Baskin CC. Annual dormancy cycles in buried weed seeds: a continuum. BioScience. 1985;35:492–498. [Google Scholar]
- Baskin JM, Baskin CC. A classification system for seed dormancy. Seed Science Research. 2004;14:1–16. [Google Scholar]
- Batlla D, Benech-Arnold RL. A quantitative analysis of dormancy loss dynamics in Polygonum aviculare L. seeds: development of a thermal time model based on changes in seed population thermal parameters. Seed Science Research. 2003;13:55–68. [Google Scholar]
- Batlla D, Benech-Arnold RL. A predictive model for dormancy loss in Polygonum aviculare L. seeds based on changes in population hydrotime parameters. Seed Science Research. 2004;14:277–286. [Google Scholar]
- Batlla D, Benech-Arnold RL. Changes in the light sensitivity of buried Polygonum aviculare seeds in relation to cold-induced dormancy loss: development of a predictive model. Seed Science Research. 2005;14:277–286. doi: 10.1111/j.1469-8137.2004.01262.x. [DOI] [PubMed] [Google Scholar]
- Batlla D, Benech-Arnold RL. Predicting changes in dormancy level in weed seed soil banks: implications for weed management. Crop Protection. 2007;26:189–197. [Google Scholar]
- Batlla D, Verges V, Benech-Arnold RL. A quantitative analysis of seed responses to cycle-doses of fluctuating temperatures in relation to dormancy: development of a thermal time model for Polygonum aviculare L. seeds. Seed Science Research. 2003;13:197–207. [Google Scholar]
- Beardsell D, Mullet J. Seed germination of Eucalyptus pauciflora Sieb. ex Spreng. from low and high altitude populations in Victoria. Australian Journal of Botany. 1984;32:475–480. [Google Scholar]
- Bentsink L, Jowett J, Hanhart CJ, Koornneef M. Cloning of DOG1, a quantitative trait locus controlling seed dormancy in Arabidopsis. Proceedings of the National Academy of Sciences, USA. 2006;103:17042–17047. doi: 10.1073/pnas.0607877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentsink L, Hanson J, Hanhart CJ, et al. Natural variation for seed dormancy in Arabidopsis is regulated by additive genetic and molecular pathways. Proceedings of the National Academy of Sciences, USA. 2010;107:4264–4269. doi: 10.1073/pnas.1000410107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black M, Bewley JD, Halmer P. The encyclopedia of seeds. Science, technology and uses. Wallingford, UK: CABI Publishing; 2006. [Google Scholar]
- Brandel M. The effect of stratification temperatures on the level of dormancy in primary and secondary dormant seeds of two Carex species. Plant Ecology. 2005;178:163–169. [Google Scholar]
- Brys R, Jacquemyn H. Variation in the functioning of autonomous self-pollination, pollinator services and floral traits in three Centaurium species. Annals of Botany. 2011;107:917–925. doi: 10.1093/aob/mcr032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caujapé-Castells J, Jaén-Molina R, Cabrera-García N, Curbelo-Muñoz L. Manual del Banco de ADN de la Flora Canaria. 2011 Dpto. Biodiversidad Molecular, Jardín Botánico Canario ‘Viera y Clavijo’, Las Palmas de Gran Canaria. http://www.bioclimac.com/mbdna/index.php/dna-bank/documentation/dna-bank-lab-manual . [Google Scholar]
- Cavieres LA, Arroyo MTK. Seed germination response to cold stratification period and thermal regime in Phacelia secunda (Hydrophyllaceae) Plant Ecology. 2000;149:1–8. [Google Scholar]
- Chiang GC, Bartsch M, Barua D, et al. DOG1 expression is predicted by the seed-maturation environment and contributes to geographical variation in germination in Arabidopsis thaliana. Molecular Ecology. 2011;20:3336–3349. doi: 10.1111/j.1365-294X.2011.05181.x. [DOI] [PubMed] [Google Scholar]
- Donohue K. Niche construction through phenological plasticity: life history dynamics and ecological consequences. New Phytologist. 2005;166:83–92. doi: 10.1111/j.1469-8137.2005.01357.x. [DOI] [PubMed] [Google Scholar]
- Donohue K. Completing the cycle: maternal effects as the missing link in plant life histories. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:1059–1074. doi: 10.1098/rstb.2008.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue K, Dorn L, Griffith C, et al. Environmental and genetic influences on the germination of Arabidopsis thaliana in the field. Evolution. 2005a;59:740–757. [PubMed] [Google Scholar]
- Donohue K, Dorn L, Griffith C, et al. The evolutionary ecology of seed germination of Arabidopsis thaliana: variable natural selection on germination timing. Evolution. 2005b;59:758–770. [PubMed] [Google Scholar]
- Donohue K, Dorn L, Griffith C, et al. Niche construction through germination cueing: life-history responses to timing of germination in Arabidopsis thaliana. Evolution. 2005c;59:771–785. [PubMed] [Google Scholar]
- El-Keblawy AA, Shaltout KH, Lovett Doust J, Lovett Doust L. Maternal effects on progeny in. Thymelaea hirsuta. New Phytologist. 1996;132:77–85. doi: 10.1111/j.1469-8137.1996.tb04511.x. [DOI] [PubMed] [Google Scholar]
- Fenner M. The effects of the parent environment on seed germinability. Seed Science Research. 1991;1:75–84. [Google Scholar]
- Fenner M, Thompson K. The ecology of seeds. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- Fernández-Pascual E, Jiménez-Alfaro B, Díaz TE. The temperature dimension of the seed germination niche in fen wetlands. Plant Ecology. 2013;214:489–499. [Google Scholar]
- Fernández-Pascual E, Jiménez-Alfaro B, García-Torrico AI, Pérez-García F, Díaz TE. Germination ecology of the perennial Centaurium somedanum, a specialist species of mountain springs. Seed Science Research. 2012;22:199–205. [Google Scholar]
- Figueroa R, Herms DA, Cardina J, Doohan D. Maternal environment effects on common groundsel (Senecio vulgaris) seed dormancy. Weed Science. 2010;58:160–166. [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G. Seed dormancy and the control of germination. New Phytologist. 2006;171:501–523. doi: 10.1111/j.1469-8137.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- Galloway LF. Maternal effects provide phenotypic adaptation to local environmental conditions. New Phytologist. 2005;166:93–99. doi: 10.1111/j.1469-8137.2004.01314.x. [DOI] [PubMed] [Google Scholar]
- Giménez-Benavides L, Escudero A, Pérez-García F. Seed germination of high mountain Mediterranean species: altitudinal, interpopulation and interannual variability. Ecological Research. 2005;20:433–444. [Google Scholar]
- Hammer O, Harper DAT, Ryan PD. PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica. 2001;4:1–9. [Google Scholar]
- Herranz JM, Copete MÁ, Ferrandis P, Copete E. Intermediate complex morphophysiological dormancy in the endemic Iberian Aconitum napellus subsp. castellanum (Ranunculaceae) Seed Science Research. 2010;20:109–121. [Google Scholar]
- Heschel MS, Paige KN. Inbreeding depression, environmental stress, and population size variation in scarlet gilia (Ipomopsis aggregata) Conservation Biology. 1995;9:126–133. [Google Scholar]
- Holm SO. Reproductive patterns of Betula pendula and B. pubescens coll. along a regional altitudinal gradient in northern Sweden. Ecography. 1994;17:60–72. [Google Scholar]
- Hoyle GL, Steadman KJ, Daws MI, Adkins SI. Pre- and post-harvest influences on seed dormancy status of an Australian Goodeniaceae species, Goodenia fascicularis. Annals of Botany. 2008;102:93–101. doi: 10.1093/aob/mcn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufford KM, Mazer SJ. Plant ecotypes: genetic differentiation in the age of ecological restoration. Trends in Ecology and Evolution. 2003;18:147–155. [Google Scholar]
- Jiménez-Alfaro B, Bueno Sánchez A, Fernández Prieto JA. Ecología y conservación de Centaurium somedanum M. Laínz (Gentianaceae), planta endémica de la Cordillera Cantábrica (España) Pirineos. 2005;160:45–67. [Google Scholar]
- Jiménez-Alfaro B, Fernández Menéndez S, Bueno SánchezÁ, Fernández Prieto JA. Vegetation and hydrogeology along the distribution range of Centaurium somedanum, an endemic plant of mountain calcareous springs. Alpine Botany. 2013;123:31–39. [Google Scholar]
- Jiménez-Alfaro B, Fernández-Pascual E, García-Torrico AI. Centaurium somedanum M. Laínz. In: Bañares Á, Blanca G, Güemes J, Moreno JC, Ortiz S, editors. Atlas y libro rojo de la flora vascular amenazada de España. Adenda 2010. Madrid: Dirección General de Conservación de la Naturaleza; 2010. pp. 100–101. [Google Scholar]
- Kepczynski J, Bihun M, Kepczynska E. Implication of ethylene in the release of secondary dormancy in Amaranthus caudatus L. seeds by gibberellins or cytokinin. Plant Growth Regulation. 2006;48:119–126. [Google Scholar]
- Koornneef M, Bentsink L, Hilhorst H. Seed dormancy and germination. Current Opinion in Plant Biology. 2002;5:33–36. doi: 10.1016/s1369-5266(01)00219-9. [DOI] [PubMed] [Google Scholar]
- Koutecká E, Leps J. Effect of light and moisture conditions and seed age on germination of three closely related Myosotis species. Folia Geobotanica. 2009;44:109–130. [Google Scholar]
- Kronholm I, Picó FX, Alonso-Blanco C, Goudet J, de Meaux J. Genetic basis of adaptation in Arabidopsis thaliana: local adaptation at the seed dormancy QTL DOG1. Evolution. 2012;66:2287–2302. doi: 10.1111/j.1558-5646.2012.01590.x. [DOI] [PubMed] [Google Scholar]
- Lee C, Mitchell-Olds T. Quantifying effects of environmental and geographical factors on patterns of genetic differentiation. Molecular Ecology. 2011;20:4631–4642. doi: 10.1111/j.1365-294X.2011.05310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leps J, Smilauer P. Multivariate analysis of ecological data using CANOCO. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- Leymarie J, Robayo-Romero ME, Gendreau E, Benech-Arnold RL, Corbineau F. Involvement of ABA in induction of secondary dormancy in barley (Hordeum vulgare L.) seeds. Plant and Cell Physiology. 2008;49:1830–1838. doi: 10.1093/pcp/pcn164. [DOI] [PubMed] [Google Scholar]
- Luzuriaga AL, Escudero A, Pérez-García F. Environmental effects on seed morphology and germination in Sinapis arvensis (Cruciferae) Weed Research. 2006;46:163–174. [Google Scholar]
- Meimberg H, Abele T, Bräuchler C, McKay JK, Pérez de Paz PL, Heubl GN. Molecular evidence for adaptive radiation of Micromeria Benth. (Lamiaceae) on the Canary Islands as inferred from chloroplast and nuclear DNA sequences and ISSR fingerprint data. Molecular Phylogenetics and Evolution. 2006;41:566–578. doi: 10.1016/j.ympev.2006.05.037. [DOI] [PubMed] [Google Scholar]
- Meyer SE, Monsen SB. Habitat-correlated variation in mountain big sagebrush (Artemisia tridentata ssp. vaseyana) seed germination patterns. Ecology. 1991;72:739–742. [Google Scholar]
- Montague JL, Barrett SCH, Eckert CG. Re-establishment of clinal variation in flowering time among introduced populations of purple loosestrife (Lythrum salicaria, Lythraceae) Journal of Evolutionary Biology. 2008;21:234–245. doi: 10.1111/j.1420-9101.2007.01456.x. [DOI] [PubMed] [Google Scholar]
- Montesinos-Navarro A, Wig J, Picó FX, Tonsor SJ. Arabidopsis thaliana populations show clinal variation in a climatic gradient associated with altitude. New Phytologist. 2011;189:282–294. doi: 10.1111/j.1469-8137.2010.03479.x. [DOI] [PubMed] [Google Scholar]
- Montesinos-Navarro A, Picó FX, Tonsor SJ. Clinal variation in seed traits influencing life cycle timing in Arabidopsis thaliana. Evolution. 2012;66:3417–3431. doi: 10.1111/j.1558-5646.2012.01689.x. [DOI] [PubMed] [Google Scholar]
- Moyer JL, Lang RL. Variable germination response to temperature for different sources of winterfat seed. Journal of Range Management. 1976;29:320–321. [Google Scholar]
- Ooi MKJ. Seed bank persistence and climate change. Seed Science Research. 2012;22:53–60. [Google Scholar]
- Ooi MKJ, Auld TD, Denham AJ. Climate change and bet-hedging: interactions between increased soil temperatures and seed bank persistence. Global Change Biology. 2009;15:2375–2386. [Google Scholar]
- Orrù M, Mattana E, Pritchard HW, Bacchetta G. Thermal thresholds as predictors of seed dormancy release and germination timing: altitude-related risks from climate warming for the wild grapevine Vitis vinifera subsp. sylvestris. Annals of Botany. 2012;110:1651–1660. doi: 10.1093/aob/mcs218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaderi MM, Cavers PB, Hamill AS, Downs MP, Bernards MA. Maturation temperature regulates germinability and chemical constituents of Scotch thistle (Onopordum acanthium) cypselas. Canadian Journal of Botany. 2006;84:28–38. [Google Scholar]
- Ren Z, Abbott RJ. Seed dormancy in Mediterranean Senecio vulgaris L. New Phytologist. 1991;117:673–678. [Google Scholar]
- Rich TCG. Could Centaurium scilloides (L. f.) Samp. (Gentianaceae), Perennial Centaury, have colonised Britain by sea? Watsonia. 2005;25:397–401. [Google Scholar]
- Rodríguez Á. El libro de somiedo. Bilbao: Mases Ediciones; 1985. [Google Scholar]
- Rossiter MC. Incidence and consequences of inherited environmental effects. Annual Review of Ecology and Systematics. 1996;27:451–476. [Google Scholar]
- Sánchez Palomares O, Sánchez Serrano F, Carretero Carrero MP. Modelos y cartografía de estimaciones climáticas termopluviométricas para la España peninsular. Madrid: Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria; 1999. [Google Scholar]
- Schütz W, Rave G. Variation in seed dormancy of the wetland sedge, Carex elongata, between populations and individuals in two consecutive years. Seed Science Research. 2003;13:315–322. [Google Scholar]
- Skordilis A, Thanos CA. Seed stratification and germination strategy in the Mediterranean pines Pinus brutia and P. halepensis. Seed Science Research. 1995;5:151–160. [Google Scholar]
- Steadman KJ, Pritchard HW. Germination of Aesculus hippocastanum seeds following cold-induced dormancy loss can be described in relation to a temperature-dependent reduction in base temperature (Tb) and thermal time. New Phytologist. 2004;161:415–425. doi: 10.1046/j.1469-8137.2003.00940.x. [DOI] [PubMed] [Google Scholar]
- Treier UA, Müller-Schärer H. Differential effects of historical migration, glaciations and human impact on the genetic structure and diversity of the mountain pasture weed Veratrum album L. Journal of Biogeography. 2011;38:1776–1791. [Google Scholar]
- Vickery RKJ. Plasticity and polymorphism in seed germination of Mimulus guttatus (Scrophulariaceae) Great Basin Naturalist. 1983;43:470–474. [Google Scholar]
- Vleeshouwers LM, Bouwmeester HJ, Karssen CM. Redefining seed dormancy: an attempt to integrate physiology and ecology. Journal of Ecology. 1995;83:1031–1037. [Google Scholar]
- Wagmann K, Hautekèete NC, Piquot Y, Meunier C, Schmitt SE, Van Dijk H. Seed dormancy distribution: explanatory ecological factors. Annals of Botany. 2012;110:1205–1219. doi: 10.1093/aob/mcs194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walck JL, Hidayati SN, Dixon KW, Thompson K, Poschlod P. Climate change and plant regeneration from seed. Global Change Biology. 2011;17:2145–2161. [Google Scholar]
- Wright KJ, Seavers GP, Peters NCB, Marshall MA. Influence of soil moisture on the competitive ability and seed dormancy of Sinapis arvenis in spring wheat. Weed Research. 1999;39:309–317. [Google Scholar]