Abstract
Objectives
Factor VII activating protease (FSAP) activates FVII as well as pro-urokinase and inhibits platelet-derived growth factor-BB, thus regulating haemostasis- and remodeling-associated processes in the vasculature. A genetic variant of FSAP (Marburg I polymorphism) results in low enzymatic activity and is associated with an enhanced risk for carotid stenosis and stroke. We postulate that there are additional substrates for FSAP that will help to explain its role in vascular biology and have searched for such a substrate.
Results and Methods
Using screening procedures to determine the influence of FSAP on various haemostasis-related processes on endothelial cells we discovered that FSAP inhibited tissue factor pathway inhibitor (TFPI), a major anti-coagulant secreted by these cells. Proteolytic degradation of TFPI by FSAP could also be demonstrated by Western blotting and the exact cleavage sites were determined by N-terminal sequencing. The Marburg I variant of FSAP had a diminished ability to inhibit TFPI. A monoclonal antibody to FSAP, that specifically inhibited FSAP binding to TFPI, reversed the inhibitory effect of FSAP on TFPI.
Conclusions
The identification of TFPI as a sensitive substrate for FSAP increases our understanding of its role in regulating haemostasis and proliferative remodeling events in the vasculature.
Keywords: HABP2, FSAP, Marburg I SNP, Atherosclerosis, thrombosis, TFPI
Introduction
Factor VII activating protease (FSAP) is a protease in human plasma with a broad substrate specificity which includes haemostasis-related proteins like factor VII (FVII) and pro-urokinase (pro-uPA) 1, 2. The addition of exogenous FSAP to plasma and whole blood influences coagulation and fibrinolysis 1, 2. In vitro, the activation of FSAP is mediated by its binding to positively charged polyamines 3 as well as negatively charged polyanions such as heparin, RNA and polyphosphates, resulting in a bi-molecular (auto-) activation 4. Activation of FSAP in plasma can be induced by post-apoptotic/dead cells that presumably release nucleic acids, histones and nucleosomes 5, 6. Hence, tissue damage releases nucleic acids/nucleosomes/polyamines, and platelet activation releases polyphosphates 7 that together can contribute to FSAP activation.
About 5% of the Caucasian population are carriers of a single nucleotide polymorphism (SNP) in the FSAP gene (official name Hyaluronic acid binding protein-2) HABP2 rs080536 that results in an exchange of a single amino acid in the protease domain (G534E) 8. The G534E polymorphism (Marburg I, MI) is only a weak activator of pro-uPA but its ability to activate factor VII is reportedly unchanged 8. Hence, the presence of this SNP may shift the activity profile of FSAP towards a more thrombotic phenotype. This has prompted many investigations into its linkage to venous thrombosis, but with the exception of one study that did find an association 9, the others came to an opposite conclusion 10-14. Other SNP's found in the HABP2 gene have a predictive value in venous thromboembolism in the elderly 15. The MI-SNP is also linked to a higher incidence of carotid stenosis 16, cardiovascular disease in general 17, stroke 18 and liver fibrosis 19 indicating a probable role for FSAP in haemostasis as well as remodeling processes.
We have previously shown that MI-FSAP has a lower proteolytic activity towards chromogenic substrates, pro-uPA and platelet derived growth factor-BB (PDGF-BB) compared to WT-FSAP, and that MI-FSAP did not inhibit neointima formation in vivo 20. In contrast to previous reports that WT and MI-FSAP activate FVII equally well 8 we found that FVII was an extremely poor substrate for WT-FSAP and not activated at all by MI-FSAP (Online supplementary data I). These observations led us to hypothesize that there are probably other substrates for FSAP that can explain its role in vascular biology.
The primary inhibitor of the TF/FXa/FVIIa complex is tissue factor pathway inhibitor (TFPI) 21. TFPI is produced by many cells in the vasculature and its activity in the vessel wall is regulated in numerous ways. It is bound to the vasculature via glycosaminoglycans and can be released by heparin from intracellular stores 22. It is also found in platelets and bound to lipoproteins whereby the latter have a major influence on the levels of circulating TFPI 21. TFPI consists of 3 kunitz domains which allow it to specifically inhibit FXa and FVIIa and dampen the initiation of coagulation. The C-terminal Kunitz domain has strong propensity to bind to heparin21. Multiple transcriptional start sites and alternative splicing lead to expression of different forms of TFPI23. There is a strong species difference in the expression of these forms in that in adult humans the predominant form is the 3 domain form called TFPI-α whereas a truncated transcript coding for TFPI-β, lacking the C-terminal Kunitz domain, is expressed mainly in the adult mouse23. TFPI can be proteolytically inactivated by proteases such as plasmin 24, thrombin 25, elastase 26 as well as activated protein C (APC) 27. Apart from influencing the activity of the extrinsic pathway, TFPI also regulates vascular smooth muscle proliferation 28 and has a role in innate immunity 29. Our studies with isolated proteins and with endothelial cells show that TFPI is indeed an excellent substrate for FSAP and this could account for the effects of FSAP in vascular as well as the extra-vascular compartment.
Materials and methods
Materials
WT- and MI-FSAP as well as PPACK-FSAP were isolated, prepared and characterized as described before 20. Single chain FSAP zymogen is rapidly converted to the active two-chain form within minutes at physiological pH. Recombinant full length TFPI and truncated two-domain TFPI (1-160) were produced as described before 30. Anti-FSAP monoclonal antibodies (Mab 1189, 677, 570), anti-human TFPI and anti-TF polyclonal antibodies (Ab), TFPI-depleted plasmas were from American Diagnostica (Pfungstadt, Germany). Anti-FSAP Mab MA-38C7 was produced in house. Anti-human TFPI polyclonal Ab SC-28861 was obtained from Santa Cruz (Heidelberg, Germany). Tumor necrosis factor α (TNF α) was from R & D Systems (Frankfurt, Germany). Endotoxin levels in FSAP were determined using the limulus amoebocyte lysate (LAL) test from Biowhittaker (Verviers, Belgium).
Cell surface FXa generation
Cell surface functional TF activity was determined by measuring the generation of activated factor X (FXa) from FX (Haemochrom Diagnostica, Essen, Germany) using 200 μM chromogenic substrate N-α-benzyloxycarbonyl-D-arginyl-glycyl-L-arginine-p-nitroaniline (S-2765, Haemochrome Diagnostica). After stimulation of cells in 96-well plates with test substances for the indicated times they were washed with Hepes-buffered saline (HBS) and incubated for 15min at 37°C with FVIIa (2 nM) (Haemochrom Diagnostica), FX (75 nM), aprotinin (1.5 μM) in HBS-CaCl2 (3mM) with 0.3% BSA (w/v). Thereafter the chromogenic substrate for FXa, S-2765 (200 μM) was added and FXa was quantified as increase in absorbance (405 nm) with time (maximal reaction velocity) (BioTek Instruments, Winooski, Vermont). Final reaction volume was 50 ul and standard concentrations of FXa (Haemochrom Diagnostica) were run to quantify the levels of FXa generated.
Measurement of TFPI activity in cell supernatants and solutions
TFPI inhibition of FX activation by FVIIa/TF was measured in a two-stage assay with a chromogenic substrate by a slightly modified method of Sandset et al 31. In the first stage, cell-conditioned medium, with aprotinin (1.5 μM), was incubated for 15 min at 37°C with TF-phospholipid preparation (Innovin or Thromborel S), FVIIa (0.2 nM), FXa (0.8 nM) and CaCl2 (15 mM) in 25mM Tris/HCl, pH 7.5, containing 100 mM NaCl, 10 mM trisodium citrate, 0.2 % BSA (w/v). In the second stage, 25 μl of FX (85 nM) was added and FXa generated in the reaction mixture was measured with S-2765 (200 μM) as a substrate.
TFPI activity released by the HUVECs was also quantified with an Actichrome TFPI activity assay following the manufacturer's instructions (American Diagnostica; Pfungstadt, Germany). In brief, this assay measures, in TFPI-depleted plasma, the ability of TFPI in a test sample to inhibit the activation of FX by the TF/FVIIa complex. A dilution of a TFPI reference standard in TFPI-depleted plasma serves as calibration curve.
Cell culture
Human umbilical vein endothelial cells (HUVEC) were isolated using collagenase and cultivated on collagen-coated dishes (5μg/ml) with endothelial basal medium (PromoCell, Heidelberg, Germany), containing hydrocortisone (1 μg/ml), EGF (10 ng/ml), bFGF (10 ng/ml) and FCS (5% vol/vol). For the complete study cells from about 25 donors at passage 2 to 4 were used. Cells in 96-well plates were stimulated with test substances in serum-free medium. Cells were stimulated with TNFα (25 ng/ml) for 5-6 h.
Western blots
Cells were lysed in SDS-containing buffer and separation of polypeptides was performed by SDS-PAGE under reducing (10 % v/v mercaptoethanol) or non-reducing conditions and electro-transferred onto the PVDF membrane (GE Healthcare, Munich, Germany). Labeling of the transferred protein by primary antibody was done for 16 h at 4°C in 5 % (wt/vol) skim milk in TBS-Tween20 (0.1% vol/vol). Secondary HRP-conjugated antibody against primary antibody was incubated for 1 h at RT. Detection of the HRP signal was performed by ECL Plus Western Blotting Detection Reagents (GE Healthcare).
Processing of TFPI by FSAP in solution
Recombinant (r)TFPI was incubated with FSAP and TFPI was analyzed by Western blotting. In other experiments cleaved TFPI was transferred to PVDF membranes and subjected to N-terminal sequencing (Applied Biosystems, Darmstadt, Germany) for the determination of the cleavage sites.
After treatment of rTFPI (12.5 nM) with WT and MI FSAP, residual TFPI activity was measured with a TFPI chromogenic activity assay (Actichrome, American Diagnostica). Alternatively, the activity of FSAP-treated TFPI was measure as diluted prothrombin time (dPT). The test solution was diluted 1:10 in TFPI-depleted plasma (containing 10 μM aprotinin) and 20 μl was mixed with 100 μl imidazole and 30 μl PTT reagent (Thromborel S, pre-diluted 1:1000 in imidazole). The mixture was incubated for exactly 3 min at 37°C. Finally, 50 μl 20 mM CaCl2 was added and clot formation was measured at 405 nm for 15 min at 37 °C. 10 μM aprotinin was present in all solutions to prevent any FSAP activity during the assay. The diluted pro-thrombin time is defined as the time when 50 % of maximum absorption is reached.
FSAP binding to TFPI
Recombinant full length TFPI or truncated two domain TFPI (1-160) was coated (5 μg/ml) and the binding of FSAP (concentration dependence) was investigated. Binding studies were performed in TBS with Ca2+ (2 mM). In each case control binding to BSA coated wells was determined and accounted for when calculating the specific binding. Binding was detected with specific antibodies against FSAP (Mab 1189) followed by HRP-coupled secondary anti-mouse antibody. Similar results were obtained when biotinylated PPACK-FSAP was used as a ligand and binding was detected with streptavidin-HRP.
Statistical analysis
Results are shown as mean ± SD from triplicate wells. All in vitro data was replicated in at least 3 times and similar results were obtained in 3 independent experiments. Where indicated, statistical significance was analyzed by ANOVA followed by Bonferroni's post test.
Results
Regulation of FXa generation on HUVEC by FSAP
We investigated whether FSAP influences TF/FVIIa mediated FXa formation on activated-endothelial cells using pre-activated FVII (FVIIa). HUVEC were pre-stimulated with TNFα for 5 h to up-regulate TF and then further treated with two-chain FSAP for 1 h. After washing the cells and inhibiting any remaining FSAP activity with excess aprotinin, FXa formation on the cell surface was determined.
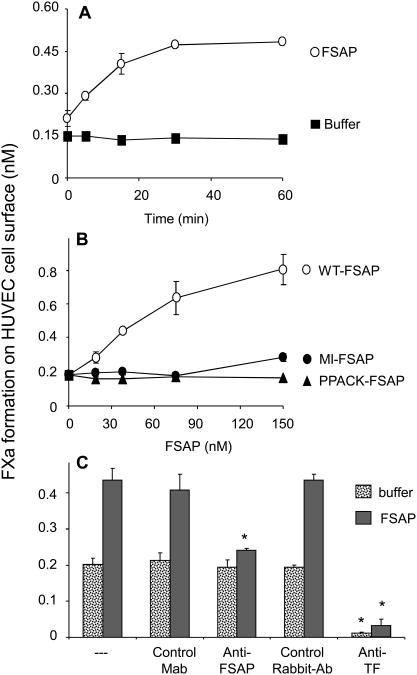
Incubation of TNFα-stimulated cells with FSAP further increased FXa generation (Fig. 1A). The fold-increase with FSAP was identical in the presence or absence of TNFα (data not shown), but in TNFα-stimulated cells the absolute amount of FXa generated was higher. The effect of FSAP on TNFα-stimulated cells was apparent after 5 min and maximal after 30 min. A significant effect was observed already with 15 nM of FSAP (Fig. 1B). MI-FSAP had a much weaker effect than WT-FSAP and was in the same range of active site-blocked FSAP, PPACK-FSAP. In different isolates of TNFα-stimulated HUVEC a 2- to 4-fold increase in FXa generation with FSAP was observed and this variation was intrinsic to the different isolates of cells. The synergistic effect between FSAP and TNFα could be blocked by an anti-TF as well as anti-FSAP blocking antibody which emphasizes the specificity of this effect (Fig. 1C). Measurement of FXa generation required the presence of both FVIIa as well as FX in the incubation mixture and omission of either resulted in no hydrolysis of the FXa substrate (data not shown). These controls indicated that FSAP did not directly activate FX nor cleave the chromogenic substrate S-2765, moreover the activation of cell-bound/serum-derived FVII by FSAP appeared unlikely.
Fig. 1. Effect of FSAP on FXa generation on HUVEC surface.
(A) Cells were activated for 6h with TNFα (25ng/ml) and then treated with FSAP (150 nM) (O) or its control buffer (■) for the indicated times and FXa generation was measured. (B) In TNFα-activated cells the effect of increasing concentrations of WT-FSAP (O), MI-FSAP (●) and PPACK-FSAP (▲), added for 60 min, on FXa generation was measured. (C) In TNFα-activated cells FSAP (150 nM) was added for 60 min and FXa was measured in the presence of an anti-TF Ab or a control Ab or anti-FSAP Mab (#570) or control Mab (all 20 μg/ml). In panels A-C results are shown as mean ± SD (n=3). * indicates a statistical significance p < 0.05.
An increase in TF expression in HUVEC treated with FSAP could account for the above observations. Western blot analysis showed that TF was up-regulated by TNFα but the addition of FSAP did not influence this (Online supplementary data II). FSAP, in the absence or presence of TNFα, did not influence the levels of mRNA for TF, thrombomodulin (TM) or TFPI (Online supplementary data III). An increase in the cell surface content of negatively charged phospholipids by FSAP could also account for the increased FXa generation. However, annexin V binding to FSAP-treated cells was unchanged whereas ionomycin induced a robust increase in annexin V binding (Online supplementary data IV).
Measurable levels of endotoxin (11 ± 1 pg endotoxin per 1 μg FSAP, n = 3 different FSAP preparations) were found in the FSAP preparation, but the use of this concentration of endotoxin did not increase FXa generation on cells (data not shown). All experiments were done in the absence of serum and under these conditions the sensitivity of HUVEC to endotoxin is very low. This together with the antagonistic effect of aprotinin, PPACK or FSAP blocking antibody confirms that the effect of FSAP is not mediated by endotoxin or any other contaminant but by FSAP itself.
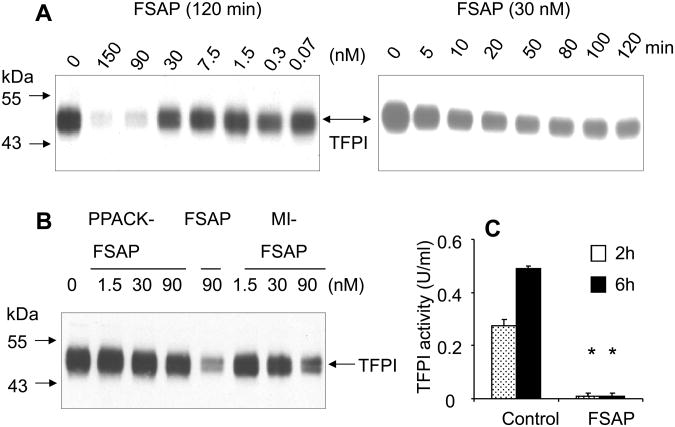
Since the increase in FXa generation was not due to up-regulation of TF or procoagulant phospholipids we considered the possibility that FSAP cleaves and inactivates TFPI. To test this hypothesis, Western blot analysis of cell extracts and supernatants from HUVEC's were performed with anti-TFPI Ab. FSAP decreased TFPI levels in a concentration and time dependent manner in cell supernatants and extracts (Fig. 2A, only data for cell extracts is shown). A significant loss of TFPI was seen with FSAP in a time and concentration-dependent manner (Fig. 2A). PPACK-FSAP did not reduce TFPI levels whereas MI-FSAP had a weaker effect (Fig. 2B). This reduction in the levels of TFPI in cell extracts of FSAP-treated cells (cf. Fig. 2) was not due to FSAP-mediated down-regulation of TFPI mRNA expression (Online supplementary data III). Thus, the influence of FSAP on TF-dependent FXa generation on endothelial cells could be correlated with a loss of TFPI protein.
Fig. 2. Effect of FSAP on TFPI in HUVEC.
(A) Cells were treated with FSAP (0-150 nM) for 120 min (left panels) or with 30 nM FSAP for 0-120 min and the cell extract were used for Western blotting using an anti-TFPI Ab under non-reducing conditions. (B) Cells were treated with PPACK-FSAP, FSAP and MI-FSAP at the indicated concentrations for 120 min and analyzed as above. Migration of MW markers and TFPI is indicated. (C) Cells were treated in the presence or absence of FSAP for 2 h or 6 h respectively, and the TFPI activity was measured in an Actichrome TFPI chromogenic activity assay relative to a TFPI reference standard. * indicates a statistical significance p < 0.05.
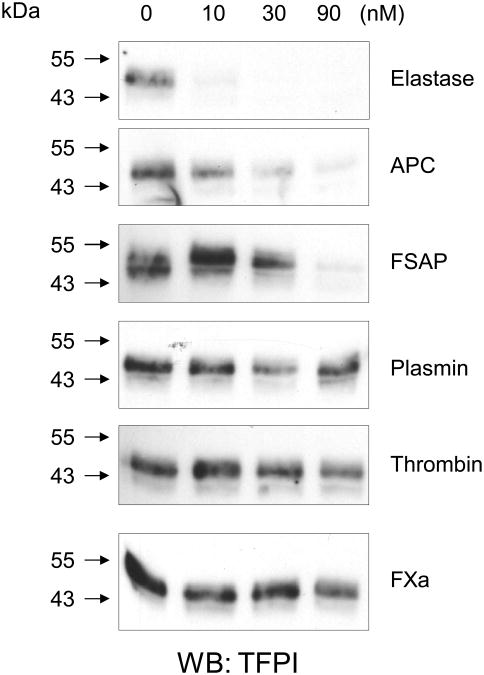
To substantiate this assumption, we investigated whether the loss in TFPI protein leads to the loss of TFPI activity in TFPI-depleted plasma using the Actichrome TFPI chromogenic activity assay. This assay measures the generation of FXa by TF/FVIIa complex in TFPI-depleted plasma relative to a TFPI standard 32. TFPI activity is shown in plasma U/ml (with 1 U/ml corresponding to 55 ng/ml or 1.4 nM). In the conditioned medium of HUVEC a TFPI activity of 0.3 U/ml is seen after 2 h and 0.5 U/ml after 6 h, respectively (Fig. 2C). Treatment of HUVECs with FSAP for 2 h or 6 h prevented the accumulation of TFPI activity, supporting the hypothesis that TFPI released by the cells is inactivated by FSAP. Since other proteases are known to cleave TFPI we compared their relative efficacy. On HUVEC, elastase was very effective in cleaving TFPI followed by APC and FSAP which was more potent than plasminogen, thrombin and FXa (Fig. 3).
Fig. 3. Effect of different proteases on TFPI cleavage in activated HUVEC.
TNFα-stimulated HUVEC were treated for 60 min with neutrophil elastase, APC, FSAP, plasmin, thrombin and FXa (0-90 nM) and cell extracts were examined for TFPI by Western blot analysis with an anti-TFPI Ab.
Characterization of the interaction between FSAP and TFPI
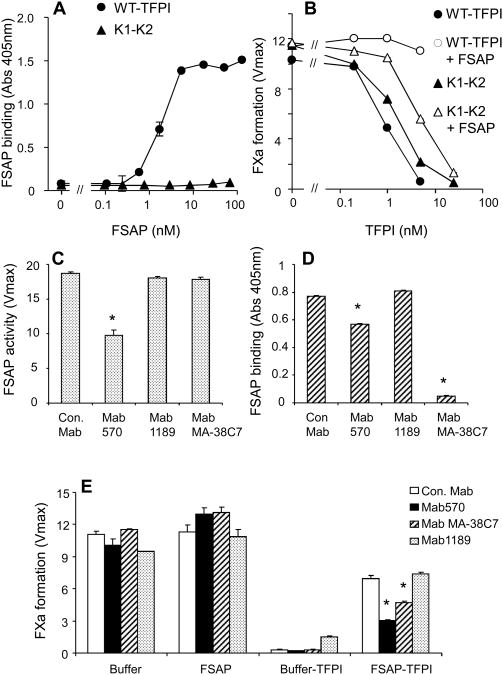
Binding studies with isolated proteins were performed to characterize the interaction between FSAP and TFPI. FSAP bound to full-length TFPI but not to the two domain form (1-160) which does not have the C-terminal heparin binding domain (Fig. 4A). We then compared how the two forms of TFPI were inhibited by FSAP. TFPI activity was determined by a two-step FXa generation assay as described before 31. In a first set of experiments it was confirmed that recombinant TFPI decreased TF/FVIIa-dependent FX activation in a dose dependent manner. With a TFPI-blocking Ab the inhibitory effect of TFPI on FXa generation could be reversed and FX activation was increased (Online supplementary data V). Similarly, in the concomitant presence of FSAP the inhibitory effect of TFPI was reversed and an increase in FX activation was observed. In this experiment FSAP activity was blocked with excess aprotinin so that this did not interfere with the chromogenic substrate assay for FXa. FSAP had no influence on the assay if TFPI was simultaneously neutralized by anti-TFPI Ab. Hence, in a purified system FSAP could regulate TFPI activity (Online supplementary data V).
Fig. 4. Interaction between FSAP and TFPI.
(A) FSAP binding to TFPI. WT-TFPI (●) or the two domain (1-160) variant (▲) was coated (5 μg/ml) and FSAP was added to wells (0-100 nM). Binding was detected with specific antibody against FSAP (Mab677, American Diagnostica) (mean ± SD). (B) Effect of FSAP on TFPI activity. In a FXa generation assay the effect of WT-TFPI (●, ○) or the two domain (1-160) variant (▲, ∆) was determined after pretreatment with FSAP (150 nM) (open symbols) or without any pretreatment (filled symbols). FXa activity is shown as maximal velocity (mean ± SD) in ΔmOD/min. (C) The effect of a panel of anti-FSAP Mabs on FSAP activity. The standard assay system consisted of TBS, 15 nM FSAP and 0.2 mM of the chromogenic substrate S-2288 (H-D-isoleucyl-L-prolyl-L-arginine-p-nitroanilinedihydro-chloride) for FSAP (Haemochrome Diagnostica) and was followed over a period of 60 min at 37°C at 405 nm in a microplate reader. FSAP was was preincubated with the Mabs (10 μg/ml) for 30 min prior to adding the chromogenic substrate. FSAP activity is shown as maximal velocity (mean ± SD) in ΔmOD/min. (D) The effect of a panel of anti-FSAP Mabs on FSAP binding to TFPI was tested. To immobilized TFPI Mabs were added ((10 μg/ml) and then biotinylated PPACK-FSAP binding was determined. (E) Effect of a panel of anti-FSAP Mabs on the modulation of TFPI activity by FSAP. In a FXa generation assay the effect of TFPI as well as FSAP separately or together was determined in the absence or presence of the indicated Mabs as described in the figure legend. * indicates a statistical significance p < 0.05.
In this assay full length TFPI was more effective in inhibiting FXa generation than TFPI (1-160) (Fig. 4B). When both forms were tested in the presence of FSAP then the full length TFPI was completely inhibited whereas the TFPI (1-160) was much less inhibited by FSAP (Fig. 4C). Hence, the C-terminal heparin binding domain of TFPI is crucial for binding to FSAP and for inactivation of TFPI by FSAP.
We then screened a panel of Mabs to identify ones that (i) inhibited the proteolytic activity of FSAP, and (ii) that blocked the binding of FSAP to TFPI. We used one Mab (Mab 570) that inhibited the enzymatic activity of FSAP (Fig. 4C) and another Mab (MA-38C7) that blocked the binding of FSAP to TFPI but not its enzymatic activity (Fig. 4D). Both antibodies could reverse the effect of FSAP on TFPI, indicating that the binding of FSAP to TFPI is important for inhibiting the activity of the latter (Fig. 4E).
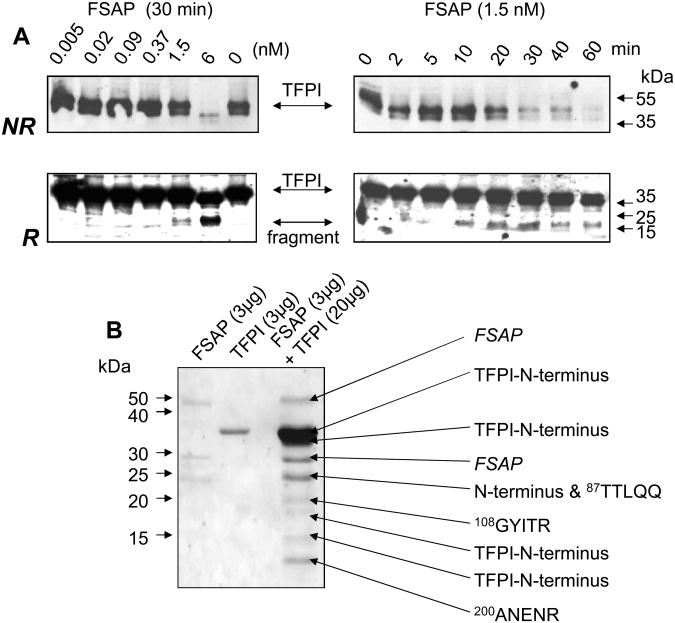
The proteolytic cleavage of TFPI was then characterized in further detail. Incubation of recombinant TFPI with FSAP led to the degradation of TFPI in a time and dose-dependent manner leading to the disappearance of the TFPI band in SDS-PAGE under non-reducing conditions and the appearance of a lower MW band under reducing conditions (Fig. 5A). N-terminal sequencing of the smaller peptides arising from the cleavage showed that many of them contained the original N-terminus of intact TFPI indicating multiple sites of proteolytic cleavage (Fig. 5B). Peptides exhibiting internal TFPI sequences indicated that the cleavage occurred between kringle domains K1 and K2 (K86T87) as well as in the active site of K2 (R107G108) and K3 (R199A200) domains (numbering refers to mature full length TFPI protein). These sites are nearly identical to those reported previously for plasmin 24, thrombin 25 and elastase 26(Online supplementary data VI). Therefore, TFPI is cleaved at multiple sites by FSAP leading to its complete inactivation.
Fig. 5. Characterization of the cleavage of recombinant TFPI by FSAP.
(A) Cleavage of recombinant TFPI by FSAP was followed by Western blotting. In the left panels TFPI (14 nM) was incubated with increasing concentrations of FSAP (0-6 nM) as indicated for 30 min. Western blots were performed with an anti-TFPI polyclonal antibody under non-reducing conditions (NR) or after reduction (R) of samples. In the right panels TFPI (14 nM) was incubated with FSAP (1.5 nM) for the indicated times and Western blots were performed. Migration of MW markers and TFPI as well as a degradation fragment are indicated with arrows. (B) Determination of the cleavage sites in TFPI. TFPI and FSAP were incubated for 30 min at 37°C as indicated and run on SDS-PAGE and blotted on to a PVDF membrane. This was stained with Coomassie blue and the protein bands were subjected to amino terminal sequencing using an automated Edman sequencer (Applied Biosystems). Numbers refer to position of amino acid in full length mature TFPI protein.
Influence of the Marburg I SNP on TFPI anti-coagulant function
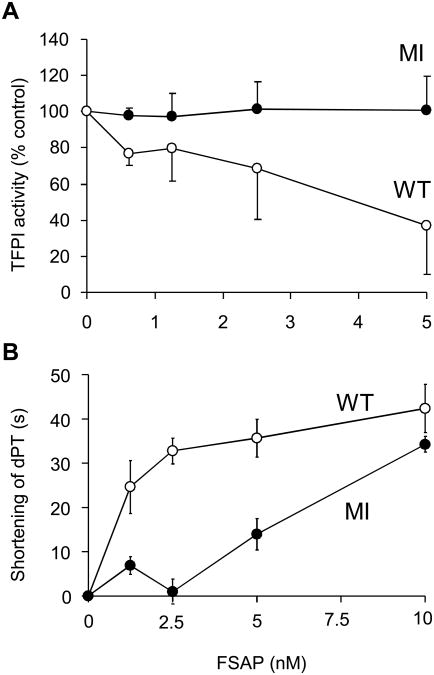
rTFPI was incubated with WT-and MI-FSAP and the residual TFPI activity was measured in TFPI-depleted plasma using a TFPI chromogenic activity assay (Fig 6A). WT-FSAP was much more effective, reducing TFPI activity by 60%, whereas MI-FSAP under the same conditions did not inactivate TFPI. WT-FSAP-treated TFPI, added to TFPI-depleted plasma, accelerated the dPT by 30-40 s compared to untreated TFPI, providing evidence that FSAP can inhibit TFPI activity in plasma (Fig. 6B). As could be expected from the preceding results, the effect of MI-FSAP on TFPI activity in plasma was weaker (Fig. 6B). These results demonstrate that the cleavage of TFPI by FSAP also inhibits TFPI function in plasma leading to accelerated coagulation and show that the MI variant is much weaker in this respect.
Fig. 6. Effect of WT- and MI-FSAP on TFPI activity and clotting time in human plasma.
(A) Residual TFPI activity of FSAP-treated TFPI (12.5 nM) (WT-FSAP ○; MI-FSAP ●) was measured with the Actichrome TFPI chromogenic activity assay (American Diagnostica). The result is shown as % residual TFPI activity compared to untreated TFPI. Data are the mean of 3 experiments ± SD. (B) The effect of FSAP-treated TFPI (WT-FSAP ○; MI-FSAP ●) on the clotting time of TFPI depleted plasma was measured in the presence of 1:6600 diluted TF (Thromborel S) as dilute prothrombin time (dPT). The absolute dPT of untreated TFPI in TFPI-depleted plasma was 185 + 13 s (n = 6); the dPT of TFPI-depleted plasma alone was 139 ± 18 s (n = 6). Data are the mean of 3 experiments ± SD.
Discussion
About 10 years ago FVII activation has been shown to be a key function of FSAP 2. However, there have been no further studies confirming these findings. We found a concentration-dependent increase in FVIIa upon treatment of purified FVII with the WT-FSAP but not more than 4 % of FVII could be activated, similar to previously published data 8. Supraphysiological concentrations of FSAP were needed for FVII activation confirming that FVII is indeed a weak substrate of FSAP. MI-FSAP was even worse and did not activate FVII, in contrast to earlier reports 8. This pattern was exactly similar to what we had previously reported for pro-uPA activation 20. Our search for another substrate relevant in extrinsic coagulation lead to the finding that TFPI is a novel substrate for FSAP. Direct comparison shows that the EC50 for FVII activation by FSAP was in the range 100 nM whereas the EC50 for TFPI inactivation was about 3 nM. TFPI is a key factor in determining the threshold for the initiation of the extrinsic pathway of blood coagulation 21. FSAP can inactivate TFPI, thus altering the balance in favour of FX activation. While procoagulant effects of FSAP have been mainly attributed to FVII activation in plasma 2, the effect of FSAP on TFPI degradation likely plays an equally important role to specifically support the initiation of the TF pathway of coagulation.
Proteolytic inactivation of TFPI by plasmin 24, thrombin 25, elastase 26 and APC 27 has been reported and there is evidence for truncated TFPI in the circulation. The generation of smaller TFPI fragments by FSAP with an intact original N-terminus indicates truncation in the direction of the C-terminus similarly to that described for other proteases. It is likely that cleavage at multiple locations leads to a cumulative loss of TFPI activity. A comparison of these proteases showed that elastase and APC were more effective than FSAP which in turn was more effective than plasmin, thrombin and FXa. There was a difference in the sensitivity of TFPI to FSAP in Western blots using purified proteins compared to that of TFPI in activity assays in HUVEC. It is likely that protease inhibitors bound to the HUVEC surface and in the conditioned medium may also account for the inhibition of exogenously added FSAP and increase the effective concentration required for TFPI inactivation on cells as compared to the purified system.
FSAP binds strongly to nucleic acids 33, histones 6 and post apoptotic cells/nucleosomes 5 and is activated in plasma by factors released from dead cells 5, 6. Recently, TFPI was shown to bind strongly to neutrophil-derived nucleic acid/nucleosomes (neutrophil extracellular trap) and was inactivated by neutrophil elastase that also binds to nucleosomes 29. In such circumstances the restricted localization of the substrate (TFPI) and the protease (FSAP) on a common surface may achieve conditions which favour TFPI cleavage by FSAP. Although the concentrations of other TFPI inactivating proteases such as thrombin, plasmin or elastase are higher in the circulation, in specialized niches that are rich in nucleic acids/nucleosomes active FSAP may reach significant levels and contribute to TFPI inactivation.
Our observation that FSAP inhibits the full length 3-domain TFPI efficiently but not the 2-domain form has fundamental implications for future work in this area. Strong species differences in the expression of different TFPI isoforms exist in humans and mice such that adult mice express predominantly the 2-domain TFPI-β and humans the 3-domain TFPI-α 23, 29. This would suggest that any influence of FSAP on mouse TFPI is not to be expected.
While the presented data show that FSAP regulates the activity of TFPI in clotting assays, FSAP may also regulate the signalling functions of TF. TFPI is also involved in regulating restenosis and atherosclerosis 28, 34 and the same is true for FSAP 20. It is intriguing that TF-dependent angiogenesis is dependent on PDGF-BB 35 and suppressed by inhibitors with a functional mechanism analogous to TFPI 36. Since FSAP directly regulates PDGF-BB and activates the TF-associated proteases involved in cell signalling, it is conceivable that FSAP plays a critical role in fine tuning these non-coagulant aspects of the hemostatic system in vascular remodelling remodeling processes.
Supplementary Material
Acknowledgments
The excellent technical assistance of Susanne Tannert-Otto, Thomas-Schmidt Wöll, Nicole Beer, Angela Schulze-Corda and Haibaier Huojiaaihemati is appreciated. We would like to thank Dr. Gunter Lochnit (Proteomics facility, University of Giessen) for the N-terminal sequencing and Herbert König (Paul Ehrlich Institute, Langen) for the tissue factor. Grant support was from the Deutsche Forschungsgemeinschaft (Bonn, Germany), Boehring Röntgen Stiftung (BRS) and Excellence Cluster in Cardiopulmonary Sciences (ECCPS) to SMK.
Footnotes
Conflict of interest disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Romisch J, Feussner A, Vermohlen S, Stohr HA. A protease isolated from human plasma activating factor VII independent of tissue factor. Blood Coagul Fibrinolysis. 1999;10:471–479. [PubMed] [Google Scholar]
- 2.Romisch J, Vermohlen S, Feussner A, Stohr H. The FVII activating protease cleaves single-chain plasminogen activators. Haemostasis. 1999;29:292–299. doi: 10.1159/000022515. [DOI] [PubMed] [Google Scholar]
- 3.Yamamichi S, Nishitani M, Nishimura N, Matsushita Y, Hasumi K. Polyamine-promoted autoactivation of plasma hyaluronan-binding protein, a serine protease involved in extracellular proteolysis. J Thromb Haemost. 2010;8:559–566. doi: 10.1111/j.1538-7836.2009.03641.x. [DOI] [PubMed] [Google Scholar]
- 4.Kanse SM, Etscheid M. Factor VII activating protease (FSAP): caught in the crossfire between polyanions and polycations. J Thromb Haemost. 2010;8:556–559. doi: 10.1111/j.1538-7836.2009.03718.x. [DOI] [PubMed] [Google Scholar]
- 5.Stephan F, Hazelzet JA, Bulder I, Boermeester MA, van Till JO, van der Poll T, Wuillemin WA, Aarden LA, Zeerleder S. Activation of factor VII-activating protease in human inflammation: a sensor for cell death. Crit Care. 2011;15:R110. doi: 10.1186/cc10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamichi S, Fujiwara Y, Kikuchi T, Nishitani M, Matsushita Y, Hasumi K. Extracellular histone induces plasma hyaluronan-binding protein (factor VII activating protease) activation in vivo. Biochem Biophys Res Commun. 2011 doi: 10.1016/j.bbrc.2011.05.030. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Muller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renne T. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romisch J. Factor VII activating protease (FSAP): a novel protease in hemostasis. Biol Chem. 2002;383:1119–1124. doi: 10.1515/BC.2002.121. [DOI] [PubMed] [Google Scholar]
- 9.Hoppe B, Tolou F, Radtke H, Kiesewetter H, Dorner T, Salama A. Marburg I polymorphism of factor VII-activating protease is associated with idiopathic venous thromboembolism. Blood. 2005;105:1549–1551. doi: 10.1182/blood-2004-08-3328. [DOI] [PubMed] [Google Scholar]
- 10.van Minkelen R, de Visser MC, Vos HL, Bertina RM, Rosendaal FR. The Marburg I polymorphism of factor VII-activating protease is not associated with venous thrombosis. Blood. 2005;105:4898. doi: 10.1182/blood-2005-02-0576. author reply 4899. [DOI] [PubMed] [Google Scholar]
- 11.Gulesserian T, Hron G, Endler G, Eichinger S, Wagner O, Kyrle PA. Marburg I polymorphism of factor VII-activating protease and risk of recurrent venous thromboembolism. Thromb Haemost. 2006;95:65–67. [PubMed] [Google Scholar]
- 12.Franchi F, Martinelli I, Biguzzi E, Bucciarelli P, Mannucci PM. Marburg I polymorphism of factor VII-activating protease and risk of venous thromboembolism. Blood. 2006;107:1731. doi: 10.1182/blood-2005-09-3603. [DOI] [PubMed] [Google Scholar]
- 13.Pecheniuk NM, Elias DJ, Xu X, Griffin JH. Failure to validate association of gene polymorphisms in EPCR, PAR-1, FSAP and protein S Tokushima with venous thromboembolism among Californians of European ancestry. Thromb Haemost. 2008;99:453–455. doi: 10.1160/TH07-10-0607. [DOI] [PubMed] [Google Scholar]
- 14.Weisbach V, Ruppel R, Eckstein R. The Marburg I polymorphism of factor VII-activating protease and the risk of venous thromboembolism. Thromb Haemost. 2007;97:870–872. doi: 10.1160/th06-12-0739. [DOI] [PubMed] [Google Scholar]
- 15.Reiner AP, Lange LA, Smith NL, Zakai NA, Cushman M, Folsom AR. Common hemostasis and inflammation gene variants and venous thrombosis in older adults from the Cardiovascular Health Study. J Thromb Haemost. 2009;7:1499–1505. doi: 10.1111/j.1538-7836.2009.03522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willeit J, Kiechl S, Weimer T, Mair A, Santer P, Wiedermann CJ, Roemisch J. Marburg I polymorphism of factor VII--activating protease: a prominent risk predictor of carotid stenosis. Circulation. 2003;107:667–670. doi: 10.1161/01.cir.0000055189.18831.b1. [DOI] [PubMed] [Google Scholar]
- 17.Ireland H, Miller GJ, Webb KE, Cooper JA, Humphries SE. The factor VII activating protease G511E (Marburg) variant and cardiovascular risk. Thromb Haemost. 2004;92:986–992. doi: 10.1160/TH04-05-0275. [DOI] [PubMed] [Google Scholar]
- 18.Trompet S, Pons D, Kanse SM, de Craen AJM, Ikram MA, Verschuren JJW, Zwinderman AH, Doevendans PAFM, Tio RA, Winters RB, Slagboom PE, Westendorp RGJ, Jukema JW. Factor VII activating protease (FSAP) polymorphism (G534E) is associated with increased risk for stroke and mortality. Stroke, Research and Treatment. 2011 doi: 10.4061/2011/424759. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wasmuth HE, Tag CG, Van de Leur E, Hellerbrand C, Mueller T, Berg T, Puhl G, Neuhaus P, Samuel D, Trautwein C, Kanse SM, Weiskirchen R. The Marburg I variant (G534E) of the factor VII-activating protease determines liver fibrosis in hepatitis C infection by reduced proteolysis of platelet-derived growth factor BB. Hepatology. 2009;49:775–780. doi: 10.1002/hep.22707. [DOI] [PubMed] [Google Scholar]
- 20.Sedding D, Daniel JM, Muhl L, Hersemeyer K, Brunsch H, Kemkes-Matthes B, Braun-Dullaeus RC, Tillmanns H, Weimer T, Preissner KT, Kanse SM. The G534E polymorphism of the gene encoding the factor VII-activating protease is associated with cardiovascular risk due to increased neointima formation. J Exp Med. 2006;203:2801–2807. doi: 10.1084/jem.20052546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crawley JT, Lane DA. The haemostatic role of tissue factor pathway inhibitor. Arterioscler Thromb Vasc Biol. 2008;28:233–242. doi: 10.1161/ATVBAHA.107.141606. [DOI] [PubMed] [Google Scholar]
- 22.Lupu C, Poulsen E, Roquefeuil S, Westmuckett AD, Kakkar VV, Lupu F. Cellular effects of heparin on the production and release of tissue factor pathway inhibitor in human endothelial cells in culture. Arterioscler Thromb Vasc Biol. 1999;19:2251–2262. doi: 10.1161/01.atv.19.9.2251. [DOI] [PubMed] [Google Scholar]
- 23.Maroney SA, Ellery PE, Mast AE. Alternatively spliced isoforms of tissue factor pathway inhibitor. Thromb Res. 2011;125(1):S52–56. doi: 10.1016/j.thromres.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kothari H, Kaur G, Sahoo S, Idell S, Rao LV, Pendurthi U. Plasmin enhances cell surface tissue factor activity in mesothelial and endothelial cells. J Thromb Haemost. 2009;7:121–131. doi: 10.1111/j.1538-7836.2008.03218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohkura N, Enjyoji K, Kamikubo Y, Kato H. A novel degradation pathway of tissue factor pathway inhibitor: incorporation into fibrin clot and degradation by thrombin. Blood. 1997;90:1883–1892. [PubMed] [Google Scholar]
- 26.Higuchi DA, Wun TC, Likert KM, Broze GJ., Jr The effect of leukocyte elastase on tissue factor pathway inhibitor. Blood. 1992;79:1712–1719. [PubMed] [Google Scholar]
- 27.Schuepbach RA, Velez K, Riewald M. Activated protein C up-regulates procoagulant tissue factor activity on endothelial cells by shedding the TFPI Kunitz 1 domain. Blood. 2011;117:6338–6346. doi: 10.1182/blood-2010-10-316257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh R, Pan S, Mueske CS, Witt T, Kleppe LS, Peterson TE, Slobodova A, Chang JY, Caplice NM, Simari RD. Role for tissue factor pathway in murine model of vascular remodeling. Circ Res. 2001;89:71–76. doi: 10.1161/hh1301.092508. [DOI] [PubMed] [Google Scholar]
- 29.Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, Brinkmann V, Lorenz M, Bidzhekov K, Khandagale AB, Konrad I, Kennerknecht E, Reges K, Holdenrieder S, Braun S, Reinhardt C, Spannagl M, Preissner KT, Engelmann B. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16:887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 30.Han X, Girard TJ, Baum P, Abendschein DR, Broze GJ., Jr Structural requirements for TFPI-mediated inhibition of neointimal thickening after balloon injury in the rat. Arterioscler Thromb Vasc Biol. 1999;19:2563–2567. doi: 10.1161/01.atv.19.10.2563. [DOI] [PubMed] [Google Scholar]
- 31.Sandset PM, Larsen ML, Abildgaard U, Lindahl AK, Odegaard OR. Chromogenic substrate assay of extrinsic pathway inhibitor (EPI): levels in the normal population and relation to cholesterol. Blood Coagul Fibrinolysis. 1991;2:425–433. [PubMed] [Google Scholar]
- 32.Bognacki J, Hammelburger J. Functional and immunologic methods for the measurement of human tissue factor pathway inhibitor. Blood Coagul Fibrinolysis. 1995;6(1):S65–72. doi: 10.1097/00001721-199506001-00011. [DOI] [PubMed] [Google Scholar]
- 33.Altincicek B, Shibamiya A, Trusheim H, Tzima E, Niepmann M, Linder D, Preissner KT, Kanse SM. A positively charged cluster in the epidermal growth factor-like domain of Factor VII-activating protease (FSAP) is essential for polyanion binding. Biochem J. 2006;394:687–692. doi: 10.1042/BJ20051563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan S, White TA, Witt TA, Chiriac A, Mueske CS, Simari RD. Vascular-directed tissue factor pathway inhibitor overexpression regulates plasma cholesterol and reduces atherosclerotic plaque development. Circ Res. 2009;105:713–720. doi: 10.1161/CIRCRESAHA.109.195016. 718 p following 720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belting M, Ahamed J, Ruf W. Signaling of the tissue factor coagulation pathway in angiogenesis and cancer. Arterioscler Thromb Vasc Biol. 2005;25:1545–1550. doi: 10.1161/01.ATV.0000171155.05809.bf. [DOI] [PubMed] [Google Scholar]
- 36.Uusitalo-Jarvinen H, Kurokawa T, Mueller BM, Andrade-Gordon P, Friedlander M, Ruf W. Role of protease activated receptor 1 and 2 signaling in hypoxia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2007;27:1456–1462. doi: 10.1161/ATVBAHA.107.142539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.