Abstract
Chronic hepatitis C virus (HCV) infection is a serious disease that can result in numerous long-term complications leading to liver failure or death. Approximately 80% of people fail to clear their infection, largely as the result of weak, narrowly targeting or waning antiviral T cell responses. While professional antigen presenting cells (APCs) like dendritic cells (DCs) might serve as targets for modulation of T cell immunity, the particular role of DCs in immunity to HCV is not known. Moreover the identity, phenotype and functional characteristics of such populations in the liver, the site of HCV replication, have proven difficult to elucidate. Using a multicolor flow-based approach, we identified six distinct populations of professional APCs among liver interstitial leukocytes isolated from uninfected and HCV-infected patients. While a generalized enrichment of DCs in the liver compared to blood was observed for all patients, HCV infection was characterized by a significant increase in the frequency of intrahepatic myeloid DCs (both CD1c+ and CD141+). Phenotypic analyses of liver plasmacytoid (pDC) and myeloid DCs (mDC) further revealed the HCV-induced expression of maturation molecules CD80, CD83, CD40 and PD-L1. Importantly pDC and mDCs from HCV-infected liver were capable of secreting effector cytokines, IFN-α and IL-12 respectively, in response to TLR stimulation in vitro.
Conclusion
Chronic HCV infection facilitates the “customized” recruitment of liver DC subsets with established functional roles in antigen presentation. These DCs are characterized by a mature, activated phenotype and are functionally responsive to antigenic stimulation in vitro. Such findings highlight an important paradox surrounding liver DC recruitment during HCV infection, where despite their activation these cells do not provide adequate protection from the virus.
Keywords: antigen presentation, liver, transplantation, antiviral immunity, cross presentation, plasmacytoid DC, myeloid DC, inhibitory molecules
Introduction
Approximately 170 million people worldwide are infected with hepatitis C virus (1). Of those infected, eighty percent will develop lifelong infection characterized by progressive liver disease with significant risk for developing cirrhosis, hepatocellular carcinoma and liver failure (2). For these individuals, chronic HCV infection is thought to be a consequence of immune failure, where delayed, weak and narrowly targeting CD4+ T cell responses are associated with an antiviral CD8+ T cell response that fails (3-6). One possibility is that the observed defects in T cell function are a result of inefficient priming. For this reason there is increasing interest in the innate activation of professional antigen presenting cells (APCs) such as dendritic cells (DCs) during infection, and the context with which these cells present HCV-derived antigens to T cells (7). Importantly, DCs represent potential therapeutic targets for HCV and other chronic diseases where novel strategies for immune modulation would prove beneficial (8-10).
Evidence modestly supports the notion that impairment in DC fate or function might contribute to HCV pathogenesis. There is a clear decline in the frequency of circulating plasmacytoid (pDC) and myeloid DC (mDC) populations during the acute and chronic phases of HCV infection (11). Observations of pDC function also suggested that pDCs from HCV-infected patients produce less IFN-α when compared to healthy subjects (12). Conflicting reports, however, have suggested that such alterations in pDC function may be due to decreased frequencies of circulating DCs, as similar levels of IFN production were seen when pDCs were evaluated on a per-cell basis (13, 14). Similarly, while several initial studies have demonstrated HCV-associated defects in mDC maturation, cytokine secretion and allostimulation, other studies have failed to corroborate such findings to a similar degree (11, 13, 15-19).
It is possible that reduced DC frequencies and the seemingly contradictory assessment of DC function in some studies reflect aberrant trafficking patterns, or the HCV-induced migration of functionally mature cells from blood to liver (20). Unfortunately, our current understanding of intrahepatic DC fate and function during HCV infection remains somewhat rudimentary, stagnating largely as a consequence of the technological hurdles associated with the study of human intrahepatic DCs in general. Nevertheless, early immunohistochemical and PCR-based studies have demonstrated DC accumulation in the liver during HCV infection, an observation consistent with a study by Wertheimer et al. also showing that DCs are enriched in the liver compared to peripheral blood (14, 20). The latter study also showed that this enrichment was not specific to HCV-infection per se, because liver DC enrichment was observed in uninfected and HCV-infected individuals (14). Such studies suggest that in order to understand a role for DCs in local immunity to HCV, one must distinguish virus-specific effects on DC recruitment, differentiation and function from the potentially enigmatic effects of generalized liver inflammation (21). To date, limited studies exist which address this issue.
Here we used a flow cytometry based approach to identify APC populations present in human liver, and to highlight those with a role in the antiviral response to HCV. In total, six phenotypically distinct populations of HLA-DR+ professional APCs were identified in the liver of uninfected and HCV-infected individuals with progressive liver disease. While a generalized enrichment for mDCs in the liver compared to blood was observed for all patients, an enrichment of mDCs in the livers of HCV-infected individuals was much more profound. In addition, HCV infection also correlated with a significant increase in the frequency of intrahepatic mDCs (both CD1c+ and CD141+ DCs), and the expression of accessory molecules CD80, CD83, CD40 and PD-L1 by intrahepatic pDC and mDC subsets. Importantly, intrahepatic DCs from HCV-infected patients showed no defect in their ability to secrete effector cytokines IFN-α and IL-12 in response to antigenic stimulation in vitro. Taken together, these findings highlight dual potential roles for intrahepatic DCs, in nonspecific liver disease progression, and in the local initiation and/or regulation of HCV-specific host responses.
Experimental Procedures
Subjects
A total of 33 patients undergoing orthotopic liver transplantation at Emory University Hospital were enrolled in the study in accordance with the Emory University Immune Monitoring Protocol (IRB# 00006248). Patient characteristics are summarized in Tables 1 and 2 (Supplemental Table 1, Table 2). Informed consent was obtained in writing from each patient and IRB#00006248 conforms to the guidelines of the 1975 Declaration of Helsinki.
Peripheral blood (PBMC) and liver interstitial mononuclear cell (LIMC) isolation
PBMCs. 50-70ml of blood was collected in Cell Preparation Tubes (BD Vaccutainer, Franklin Lakes, NJ) and processed for PBMCs according to the manufacturer’s instructions. LIMCs. Intact corner lobes of explanted recipient liver specimens (100g) were excised at the time of recipient hepatectomy. Lobes were perfused with PBS, and the tissue was cut into small pieces (<0.5cm in diameter) and digested for 60 minutes at 37°C in DMEM/F12 (Lonza, Allendale NJ) containing .2% collagenase (Worthington Biochemical Corporation, Lakewood, NJ). The digested material was strained through sterile cheesecloth, pelleted and washed in RPMI 1640+ 10% FCS/PS. Purified LIMCs were extracted by Percoll (GE Healthcare, Pitascataway, NJ) density centrifugation.
Phenotypic analysis
Four overlapping panels with antibodies to CD3 (Pacific Blue, HIT3a, Biolegend, San Diego, CA), CD19 (Pacific Blue, HIB19, Biolegend), CD56 (Pacific Blue, MEM-188, Biolegend), CD11c (Biotin, 3.9, Biolegend), CD14 (PerCPCy5.5, HCD14, Biolegend), CD16 (APCH7, 3G8, BD Biosciences, San Diego, CA), HLA-DR (PECy7, L243, BD Biosciences, San Diego, CA), CD123 (FITC, 6H6, Biolegend), CD34 (PE, 4H11, Biolegend) eBioscience, CD34 (FITC, 581, BD Biosciences), CD1c (APC, AD5-8E7, Miltenyi Biotec, Auburn, CA), CD141 (APC, AD5-14H12, Miltenyi Biotec), and CD33 (APC, WM53, Biolegend), and CD303 (APC, AC144, Miltenyi Biotec) were used simultaneously. PBMCs and LIMCs were blocked in 20% NAB human serum (Sigma-Aldrich) for 20 minutes at 4°C, then incubated with fluorophore-conjugated and biotinylated primary antibodies for 30 minutes at 4°C. After two washes, the cells were incubated with streptavidin-conjugated Qdot-605 (Life Technologies, Grand Island, NY) for 30 minutes at 4°C. After two washes, the stained cells were fixed in 4% PFA for 30 minutes at 4°C.
PE-conjugated antibodies to CD40 (53C, Biolegend), CD80 (Clone L307.4, BD Pharmingen), CD83 (HB15e, BD Pharmingen), CD86 (FUN-1, BD Pharmingen), PD-L1 (MIH-1, BD Pharmingen), and IgG1 and IgG2a isotypes (BD Pharmingen), were used with the panels described above. Fluorescence minus one controls were used in each experiment to aid in phenotyping autofluorescent cell populations. A 407nm laser and a 475LP 525/50 BP filter set were used to identify autofluorescent cell populations and extraneous cellular debris. Samples were run on a BD LSR II flow cytometer and analyzed using Flow Jo Software (Treestar, Ashland, OR).
Intracellular cytokine staining
PBMCs and LIMCs were cultured at a concentration of 10 million cells/mL in RPMI 1640 solution +10% FCS/PS. Each sample was unstimulated, or stimulated with 1ug/mL of LPS, or 5ug/mL of R-837 (InvivoGen, San Diego, CA) for 12 hours at 37°C. 1uL/mL Golgi plug (BD Biosciences) was added after 1 hour. After 12 total hours, cells were stained with antibodies to surface molecules CD3 (Pacific Blue, HIT3a), CD19 (Pacific Blue, HIB19), CD56 (Pacific Blue, MEM-188), HLA-DR (PECy7, L243), CD14 (PerCPCy5.5, HCD14), CD11c (Biotin, 3.9), CD1c (APC, AD5-8E7) and CD123 (FITC, 6H6), allowing for subset identification, followed by incubation with streptavidin-conjugated Qdot-605 in a secondary staining step. Intracellular detection of IFN-α (PE, Clone LT27:295, Miltenyi Biotec), IL-12/23 p40/70 (PE, Clone C8.6, eBioscience), or isotype control antibodies (BD Biosciences) was done according to Inside Stain Kit manufacturer’s instructions (Miltenyi Biotec). The toxicity of individual stimulation conditions was assessed using Live/dead fixeable aqua (Life Technologies).
Results
The intrahepatic APC compartment is distinct and comprised by six primary subsets
Postulating that local defects in antigen presentation might be contributing to immune failure in HCV-infected patients, we set out to identify intrahepatic APCs with potential involvement in this process. Given the heterogeneous nature of human APC subsets and an overall lack of knowledge concerning liver-resident APCs, we used a flow cytometry-based characterization strategy that would identify intrahepatic APCs based on the phenotypic expression of HLA-DR. We then dissected the HLA-DR+ parent population it into smaller, phenotypically distinct subpopulations for further study.
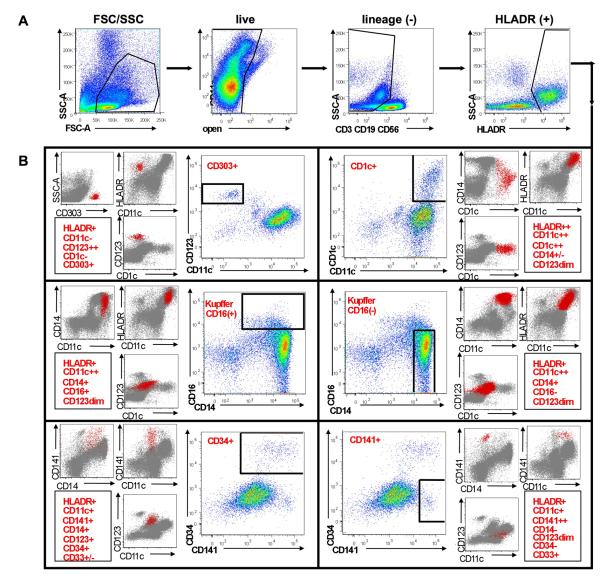
Surface staining of LIMCs from an HCV-infected patient revealed a large population of HLA-DR+ cells lacking lymphocyte lineage markers CD3, CD56 and CD19 (Figure 1). Within this parent population, six distinct subpopulations of professional APCs were identified, defined by their expression of CD303 (CD303+ plasmacytoid DCs, CD303+ CD123++ CD11c− CD1c−), CD1c (CD1c+ myeloid DCs, CD1c++, CD11c++, CD14+/−, CD123+), CD16 (CD16+CD14+ cells, CD16+, CD14+, CD11c++, CD123+), CD14 (CD16-negative CD14+, CD14++, CD16−, CD11c++, CD123+), CD141 (CD141+ myeloid DCs, CD141++, CD11c+, CD14−, CD123+, CD34−, CD33+) and CD34 (CD34+ cells, CD34+, CD141+/−, CD11c+, CD14+, CD123+, CD33+/−)(Figure 1).
Figure 1. Identification of six intrahepatic antigen presenting cell (APC) subsets by multi-parameter flow cytometry.
A. Interstitial mononuclear cells isolated from an HCV-infected liver were simultaneously stained with three, overlapping, 10-color antibody panels differing from each other by an individual marker. Antibodies were directed against CD3, CD56, CD19, CD14, CD16, HLA-DR, CD11c, CD123, CD303, CD1c, CD141, CD34, and CD33. APC subsets were identified among a parental population of HLA-DR+ cells following exclusion of dead cells and those cells expressing lineage markers CD3, CD56 and CD19. B. Six dominant subsets of intrahepatic APCs identified among HLA-DR+ cells are depicted, including: CD303+ plasmacytoid DCs (CD303+, CD11c−, CD123++, CD1c−, CD303+, HLA-DR+), CD1c+ myeloid DCs (CD1c+, CD11c++, CD14+/−, CD123+, HLA-DR++), CD16+ CD14+ cells (CD16+, CD14+, CD11c++, CD123+, HLA-DR+), CD16− CD14+cells (CD14++, CD16−, CD11c++, CD123+, HLA-DR+), CD34+ cells (CD11c+, CD141+/−, CD14+, CD123+, CD34++, CD33+/−, HLA-DR+) and CD141+ myeloid DCs (CD141++, CD11c+, CD14−, CD123+, CD34−, CD33+, HLA-DR+). Phenotypic characterization of each population is shown in the corresponding red (APC subset) and grey (total lineage negative cells) overlays.
Following identification of the six liver subsets, we noted a number of similarities and differences between peripheral blood and liver APC compartments. Five of the six liver populations were phenotypically identical to peripheral blood monocyte and DC subsets. These included CD303+ pDCs, CD16+/− CD14+ cells (monocyte/Kupffer), and two populations of mDCs (CD1c+ and CD141+). The remaining population, termed “CD34+”, was phenotypically distinct from the well-characterized population of CD34+ cells commonly observed in peripheral blood (22-24).
While peripheral blood CD34+ cells express HLA-DR but lack all lineage markers, the intrahepatic CD34+ population demonstrated phenotypic similarities to a number of myeloid lineage cells through their expression of CD14, CD11c, CD141 and CD33 (CD33 expression not shown). In fact, lineage negative CD34+ cells were largely undetectable in the liver compartment (accounting for <0.5% of the HLA-DR+ parent when observed). Also undetectable in liver was a previously described population of peripheral blood mDCs expressing CD16, noted for their tendency to secret proinflammatory cytokines such as TNFα (tumor necrosis factor-α)(22). Together the data show that the liver APC compartment was distinct from that of peripheral blood, and comprised by numerous interstitial DC subsets with potential involvement in the antiviral response to HCV.
Chronic liver disease is characterized by an abundance of intrahepatic myeloid DCs, irrespective of HCV status
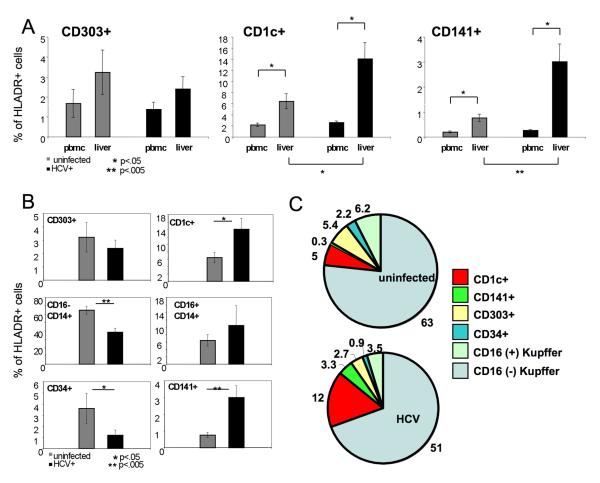
We next sought to better understand the role of the liver microenvironment on local DC recruitment. Assuming that the HCV-infected liver microenvironment would differ from that of uninfected liver during similar stages of advanced liver disease, we compared peripheral blood and liver DC frequencies in uninfected and HCV-infected patients (Figure 2A). Interestingly, we observed a generalized enrichment of CD1c+ and CD141+ mDC subsets in liver for all patients irrespective of HCV status, signifying a potential role for mDCs in liver disease progression, or conversely, a role for liver disease progression (ie. inflammation) in the nonspecific recruitment of mDCs (Figure 2C). Importantly, the most pronounced increase in intrahepatic mDC frequencies compared to blood was observed in HCV-infected patients, consistent with a dual role for these cells in the initiation of adaptive host responses. CD141+ DCs in particular have known functional roles in viral antigen cross-presentation to T cells (25, 26). Taken together, subset analyses of peripheral blood and liver compartments demonstrated an association between progressive liver disease and intrahepatic mDC recruitment, and an HCV-specific enhancement of mDC recruitment.
Figure 2. Comparison of intrahepatic APC compartments from uninfected and HCV-infected individuals.
A. The percentage of peripheral blood and liver HLA-DR+ cells belonging to CD303+(left panel), CD1c+ (center panel) or CD141+ (right panel) DC subsets is shown for uninfected (grey) and HCV-infected individuals (black). Statistical differences in subset frequency (asterisk) between uninfected and HCV-infected patients were analyzed using the Student t test (liver versus liver) and a paired Student t test (PBMC versus liver) (n=9 uninfected, n=8 HCV-infected). B. The percentage of parent HLA-DR+ cells belonging to each of the six identified intrahepatic APC subsets is shown for uninfected (grey) and HCV-infected individuals (black). Statistical differences in subset frequency (asterisk) between uninfected and HCV-infected patients were analyzed using the Student t test (n=9 uninfected, n=8 HCV-infected). C. Pie charts depicting compartmental makeup of HLA-DR+ intrahepatic APC populations from a representative uninfected (upper) and HCV-infected (lower) individuals, n=1.
Chronic HCV infection is characterized by an increase in intrahepatic mDCs over pDCs, CD14+ cells and CD34+ cells
While the enrichment of mDC populations in HCV-infected liver suggested a potential role for these cells in local HCV-specific responses, it remained plausible that mDC enrichment was an indirect manifestation of HCV-related liver pathology rather than virus-mediated DC recruitment. We therefore wanted to identify other HCV-mediated changes in the intrahepatic APC compartment overall, through direct comparisons of the six identified populations in uninfected and HCV-infected patients (Figure 2). When compared, HCV infection was again characterized by a significant increase in the frequency of intrahepatic mDCs (both CD1c+ and CD141+DCs) and a slight decrease in the frequency of CD14+ cells (Figure 2B). Strikingly, we observed ten-fold higher frequency of “cross-presenting” CD141+ mDCs in HCV-infected liver compared to uninfected liver (Figure 2B), suggesting the potential involvement of these cells in the local antiviral response.
In contrast, we observed no difference in the frequency of intrahepatic CD303+ pDCs between cohorts (p= 0.32), consistent with blood to liver comparisons that showed that pDCs were not enriched in the liver compartment of chronically infected individuals (Figure 2A). In addition, while a role for intrahepatic CD34+ cells in host immune responses is not yet known, a drastic decrease in the frequency of CD34+ cells among HLA-DR+ cells was observed in HCV-infected liver (Figure 2B). Importantly, the proportional representation of the six liver APC subsets in individual patients was consistent with the observed differences in frequency between patient cohorts (Figure 2C). It should be noted that a 2-3-fold increase in LIMC numbers per gram of liver tissue was observed in HCV-infected liver compared to uninfected liver (Figures S1, S2). When comparisons of total intrahepatic APC numbers per gram of liver tissue were evaluated, myeloid liver DCs were again observed enriched within HCV-infected liver compared to uninfected liver (Figures S1, S2). In all, the data showed that HCV-infection facilitated the recruitment or differentiation of hepatic mDC populations over pDCs, CD14+ cells and CD34+ cells.
Chronic HCV infection is characterized by an increase of intrahepatic DCs displaying a mature, activated phenotype
Our data show that progressive liver disease and HCV infection alike each result in the enrichment of intrahepatic mDCs over pDCs. We theorized, however, that HCV could still facilitate the virus-specific maturation and/or activation of either subset, when present in liver, regardless of frequency. We therefore assessed the maturation status of pDC and mDC populations in uninfected and HCV-infected liver, and tested whether these cells expressed ligands with accessory function for antigen-presentation to T cells (Figure 3).
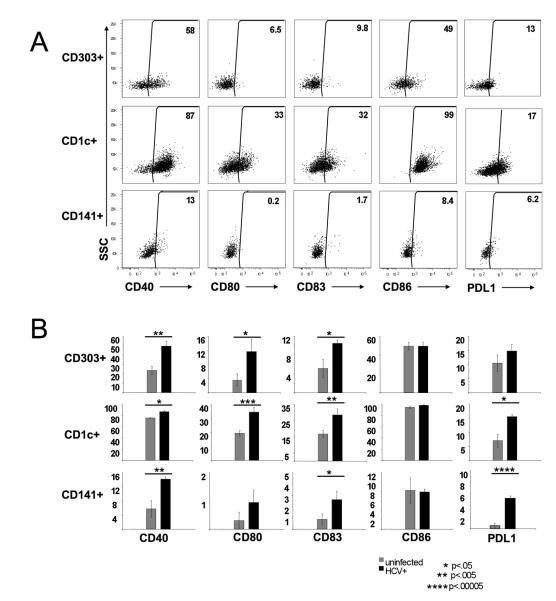
Figure 3. Analysis of maturation status and phenotype of intrahepatic DC subsets during chronic HCV infection.
A. Simultaneous inspection of co-stimulatory molecule expression by three dominant subsets of intrahepatic DCs isolated from an HCV-infected individual. Dot plots gated on individual CD303+, CD1c+ and CD141+ DC subsets according to Figure 1. The percentage of each DC subset expressing co-stimulatory molecules CD40, CD80, CD83 and CD86 is shown. Results from a representative experiment are shown. B. Comparison of intrahepatic DC phenotype between uninfected and HCV-infected individuals. Statistical differences in the frequency of intrahepatic DCs expressing individual co-stimulatory molecules (asterisk) were analyzed using the Student t test (n=6 uninfected, n=6 HCV-infected).
The three liver DC populations were each characterized for the expression of co-stimulatory molecules CD40, CD80, CD83, CD86 and PD-L1 within individual patients (Figure 3A). Interestingly, an increase in the frequency of both pDC and mDC populations expressing CD40, CD80, CD83 and PD-L1 was observed in the liver of HCV-infected patients (Figure 3B). Consistent with our hypothesis, the frequencies of intrahepatic CD303+ pDCs was not found to be substantially different between uninfected and HCV-infected patients yet a higher frequency of these cells expressed CD40, CD80 and CD83 in HCV-infected liver (Figure 3B). Importantly, the frequency of intrahepatic CD1c+ DCs expressing CD40, CD80, CD83 and PD-L1 and of CD141+ DCs expressing CD40, CD83 and PD-L1 was also increased in HCV-infected patients (Figure 3B), demonstrating the HCV-mediated activation of these subsets.
Intrahepatic DCs isolated from HCV-infected liver are functionally responsive
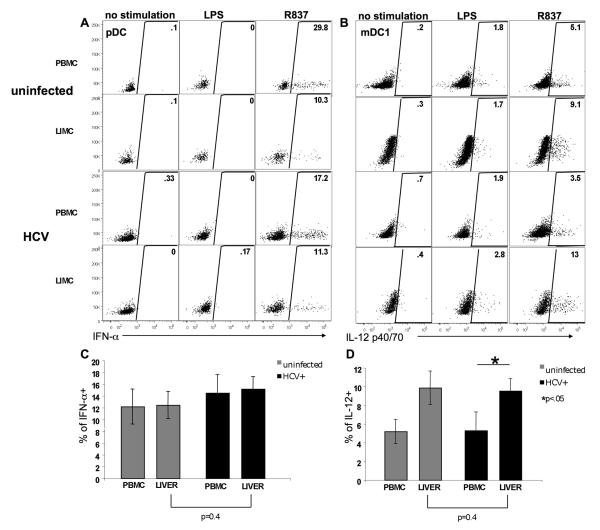
Although it was apparent that intrahepatic DCs from HCV-infected patients were capable of antigen-induced maturation in vivo, whether these cells had the potential to be functionally responsive to antigenic stimulation was not known. To assess their functional potential, we compared the responsiveness of blood and liver DCs to overnight stimulation of TLR-4 by LPS, and TLR-7 stimulation by a synthetic RNA analog, R837. We then compared the ability of pDCs and mDCs from uninfected and HCV-infected individuals to secrete effector cytokines IFN-α and IL-12 (p40/70), respectively (Figure 4A, 4B). In uninfected patients and HCV-infected patients alike, we found the frequency of IFN-α producing pDCs in liver to be comparable to that of blood, demonstrating no decrease in functional responsiveness for intrahepatic pDCs in either cohort. Importantly, when the function of intrahepatic pDCs from uninfected and HCV-infected patients was compared, similar antigen-specific responsiveness to TLR7 stimulation was again observed (Figure 4C). In all, these data show that no differences in intrahepatic pDC function, either by the frequency of IFN-α producing cells or in the amount produced per cell (data not shown), was observed between uninfected and HCV-infected patients (Figure 4C).
Figure 4. Analysis of cytokine secretion potential by peripheral blood and intrahepatic DCs during chronic HCV infection.
A. IFN-α production by CD303+ pDCs isolated from the peripheral blood and the liver of a representative uninfected and HCV-infected patient. Dot plots gated on pDCs according to Figure 1. The percentage of pDCs that produced IFN-α in response to no stimulation or stimulation with TLR agonists LPS, or R837, is depicted. B. IL-12 production by CD1c+ DCs in the same individuals. Dot plots gated on CD1c+ DCs according to Figure 1. C. The percentage of peripheral blood and intrahepatic CD303+ pDCs that produce IFN-α in response to TLR agonist R837 is shown for uninfected (grey) and HCV-infected (black) individuals. Statistical differences in the frequency of IFN-α producing cells (asterisk) were analyzed using the Student t test (liver versus liver) and a paired Student t test (PBMC versus liver) (n=11 uninfected, n=10 HCV-infected). D. The percentage of peripheral blood and intrahepatic CD1c+ DCs that produce IL-12 in response to TLR agonist R837 is shown for uninfected (grey) and HCV-infected (black) individuals. Statistical differences in the frequency of IL-12 producing cells (asterisk) were analyzed using the Student t test (liver versus liver) and a paired Student t test (PBMC versus liver) (n=8 uninfected, n=9 HCV-infected).
IL-12 production by CD1c+ DCs followed similarly to the previously observed enrichment of mDCs in the liver of uninfected and HCV-infected liver patients (Figure 4D). Again, when IL-12 production by CD1c+ DCs was assessed, the proportion of CD1c+ DCs that showed responsiveness to TLR7 agonist R-837 was significantly increased in the liver compared to blood for all patients irrespective of HCV status (Figure 4D). When liver-to-liver comparisons were made, we observed no difference in the percentage of intrahepatic CD1c+ DCs that produced IL-12 in response to stimulation (or in the amount of IL-12 produced per cell, data not shown) (Figure 4D). Together our data demonstrate that intrahepatic pDC and mDC populations alike are responsive to antigenic stimulation in vitro through the secretion of effector cytokines. Importantly, no defect in cytokine production by intrahepatic DCs was observed in chronically infected HCV patients.
Discussion
To our knowledge, this is the first comprehensive description of human liver APCs. The “top down” approach used in this study has allowed us to highlight a number of key differences between peripheral blood and liver APC compartments, and compare the phenotypic and functional properties of these cells between uninfected and HCV-infected patients. Each comparison was necessary for the successful identification of intrahepatic APCs with a potential role in HCV immunity that is separate from a more general role in liver disease. Importantly, our data show that recruitment and activation of liver APCs during chronic HCV infection is not necessarily sufficient for the initiation of successful antiviral responses. Rather, in the case of HCV, the activation of intrahepatic DCs may in fact serve as a double-edged sword, contributing both to the development of cellular immunity (although failing) and to the pathological advancement HCV-mediated liver inflammation.
Overall, we found substantial overlap of blood and liver APC compartments. In both compartments, CD14+ cells (both CD16+ and CD16−) were the dominant population of HLA-DR+ cells, consistent with an important role for these cells in immune surveillance and innate immune activation. Intrahepatic 303+, CD1c+ and CD141+ DC populations were also phenotypically identical to those observed in peripheral blood (23, 27). While we did not readily observe lineage negative CD34+ cells in liver, the potential significance of a partially differentiated population of intrahepatic CD34+ cells that is phenotypically distinct from peripheral blood CD34+ cells is intriguing.
One possibility is that viral or inflammation-mediated differentiation of the lineage negative fraction is occurring in liver, and that the observed intrahepatic CD34+ cells represent ‘precursor’ populations in intermediate stages of hematopoeisis. It is curious that HCV infection was associated with a decrease in the frequency of intrahepatic CD34+ cells, and also an increase in the frequency of intrahepatic mDCs. While there is some evidence that HCV might actually target CD34+ progenitor cells, the idea that HCV itself might promote the local differentiation CD34+ cells to specific APC subsets, however, is nonetheless controversial (28). Differences in trafficking patterns and migratory properties of circulating DCs and CD34+ subsets could also account for the observed differences in compartmentalization. It’s also noteworthy that while the CD34+ cells are proportionally underrepresented by frequency, they are nonetheless still populating HCV-infected liver when total numbers of infiltrating mononuclear cells are taken into account. Efforts by our laboratory to understand the role of this liver-resident population and any genotypic or functional relatedness to other, more characterized populations of liver APCs are ongoing.
Current HCV therapy involves the combined use of antiviral drugs and IFN-α, and the efficaciousness of this approach in some individuals suggests (albeit indirectly) that a deficiency of IFN-α availability in the liver may exist (7). Yet, no apparent deficiency of pDC trafficking to the liver was observed in this study, where the frequencies of pDCs in the liver compartment of both uninfected and HCV-infected individuals were comparable. Even more perplexing is the observation that pDCs isolated from the liver of uninfected and HCV-infected individuals show similar responsiveness to antigenic stimulation in vitro, through the secretion of IFN-α. Taken together, however there is modest phenotypic evidence remaining that would suggest that these cells may have alternative fates following migration to the liver, depending on the presence or absence of HCV infection within that microenvironment. While pDC function was apparently maintained by intrahepatic pDCs during HCV infection, a larger frequency of these cells was also phenotypically mature, suggesting a direct effect of the virus on the activation state of these cells. Whether HCV-specific activation and maintenance of local pDC responsiveness is helpful for low-level control of viremia (ie. maintenance of a virological set point), or the recruitment of cellular responders like CD1c+ DCs, is not known.
We do, however, postulate that CD1c+ DCs have a more general role in the inflammatory process of progressive liver disease (22, 29). Unlike pDCs, CD1c+ cells were enriched in the liver of both uninfected and HCV-infected individuals. Also increasing in both patient cohorts, were the frequencies of CD1c+ DCs in the liver that showed responsiveness to TLR stimulation in vitro through the secretion of IL-12. Yet, two points of evidence suggest a second potential role for these cells in local immunity to HCV. First, the frequency of these cells in HCV-infected liver was significantly higher to that observed in uninfected liver. Secondly, of the three liver DC subsets, CD1c+ DCs expressed the highest number of accessory molecules in the context of HCV infection.
With respect to HCV-specific roles, one might postulate that accessory molecules expressed by intrahepatic CD1c+ DCs could modulate local antiviral T cell responses, through inhibitory receptors such as Programmed Death 1 (PD-1). Previous work from our laboratory and others has shown that the impaired proliferation of HCV-specific CD8+ T cells is associated with, and reversed by antibody blockade of the PD-1 pathway (3, 30). Heightened expression of T cell regulators cytotoxic T lymphocyte attenuator-4 (CTLA-4), natural killer receptor 2B4, and T cell immunoglobulin mucin-3 (TIM-3) has also been associated with T cell exhaustion during chronic HCV infection (31-34), therefore it is logical that CD1c+ DC expression of ligands for these receptors might be culpable in the influence of effector “helper” and cytotoxic T cell responses.
Lastly, the dramatic recruitment of “cross-presenting” CD141+ DCs to HCV-infected liver is perhaps the most compelling evidence to suggest a role for intrahepatic mDCs in the local immune response to HCV. CD141+ DCs would be considered ideal targets for therapeutic intervention given their extremely high frequency in HCV-infected liver (25, 26). Novel strategies employing the use of TLR-agonists, cytokines and DCs as therapy for HCV are currently being sought and may likely serve useful (10, 35). While a higher percentage of intrahepatic CD141+ DCs were found to express costimulatory molecules in the context of HCV-infection, the frequency of these cells displaying a mature, activated phenotype were considerably lower than the other two liver DC populations by comparison. Whether reduced expression of DC maturation markers is a general characteristic of intrahepatic CD141+ DCs, and whether this subset is functionally effective for viral antigen uptake, processing and presentation to CD8+ T cells is not known. Efforts to better understand their potency for modulating CD8+ T cells through antigen presentation are currently ongoing by our laboratory.
In conclusion, it remains to be determined whether the overall function of liver DCs has the potential to be favorable towards the induction of HCV-specific T cell immunity when studied in greater detail. Despite the observed functional potential of intrahepatic pDC and mDC populations in vitro, it should be cautioned that we do not yet know whether DCs residing within the obscure “HCV liver microenvironment” function to stimulate immunity, tolerance, or exacerbate inflammation. It may also be difficult to interpret their relevance with respect to HCV pathogenesis, or protection, with cross-sectional analysis alone. Given that our cohort of HCV patients were already chronically infected, it is likely that such seemingly functional populations could be rendered impaired or functionally tolerogenic in vivo, through effects of the local cytokine milieu or cross-talk with other liver-resident cell types. An alternative possibility is that intrahepatic DCs in HCV-infected liver are both functional and immunogenic in character, but temporal delays in their recruitment does not allow for them to keep up with an already established infection. For this reason we feel that our study would be complimented well by longitudinal studies of intrahepatic DCs capable of elucidating these possibilities, although we understand such studies are difficult to perform. Nevertheless any future studies that can make distinctions between liver DC potential and function, and elucidate their relevance to tolerance, immunity, and liver disease progression, would be infinitely beneficial for the design of therapeutic strategies targeting such populations.
Supplementary Material
Acknowledgements
We thank Hannah Scarborough, Elizabeth Elrod, Beth Begley, Shine Thomas and the Emory Transplant clinical group for technical assistance and patient coordination. We are indebted to the Emory Pathology Department, especially Palin Bagci, Alton B. Farris III, and Volkan Adsay for help with the acquisition of liver biopsies. We thank John Altman, Kiran Gill and the Immunology Core for flow cytometry services and the maintenance of equipment. We also thank the study subjects that generously agreed to participate.
This study was supported in part from the National Research Service Award Institutional Research Training Grants T32 AI007470, T32 AI007610, and American Liver Foundation (VMV); EVC/CFAR Immunology Core P30 AI050409 (CI, AG); Yerkes Research Center Base Grant RR-00165, and Public Health Service DK083356 and AI070101 (AG).
List of Abbreviations
- HCV
(hepatitis C virus)
- APC
(antigen presenting cell)
- DC
(dendritic cell)
- pDC
(plasmacytoid dendritic cell)
- mDC
(myeloid dendritic cell)
- programmed death ligand-1
(PD-L1)
- IFN-α
(Interferon alpha)
- IL-12
(Interleukin-12)
- PCR
(polymerase chain reaction)
- HLA
(human leukocyte antigen)
- PBMC
(peripheral blood mononuclear cells)
- LIMC
(liver interstitial mononuclear cells)
- TNF-α
(tumor necrosis factor alpha)
- TLR
(toll-like receptor)
- PD-1
(programmed death-1)
- CTLA-4
(cytotoxic T lymphocyte attenuator-4)
- TIM-3
(T cell immunoglobulin mucin-3)
- FACS
fluorescence activated cell sort
- ALT
alanine aminotransferase
- MELD
Model for End-Stage Liver Disease
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 2.Chinnadurai R, Velazquez V, Grakoui A. Hepatic transplant and HCV: a new playground for an old virus. Am J Transplant. 2012;12:298–305. doi: 10.1111/j.1600-6143.2011.03812.x. [DOI] [PubMed] [Google Scholar]
- 3.Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81:2545–2553. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radziewicz H, Ibegbu CC, Hon H, Osborn MK, Obideen K, Wehbi M, et al. Impaired hepatitis C virus (HCV)-specific effector CD8+ T cells undergo massive apoptosis in the peripheral blood during acute HCV infection and in the liver during the chronic phase of infection. J Virol. 2008;82:9808–9822. doi: 10.1128/JVI.01075-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uebelhoer L, Han JH, Callendret B, Mateu G, Shoukry NH, Hanson HL, et al. Stable cytotoxic T cell escape mutation in hepatitis C virus is linked to maintenance of viral fitness. PLoS Pathog. 2008;4:e1000143. doi: 10.1371/journal.ppat.1000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946–952. doi: 10.1038/nature04079. [DOI] [PubMed] [Google Scholar]
- 7.Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest. 2009;119:1745–1754. doi: 10.1172/JCI39133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 9.Palucka K, Banchereau J, Mellman I. Designing vaccines based on biology of human dendritic cell subsets. Immunity. 2010;33:464–478. doi: 10.1016/j.immuni.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salem ML, El-Demellawy M, El-Azm AR. The potential use of Toll-like receptor agonists to restore the dysfunctional immunity induced by hepatitis C virus. Cell Immunol. 2010;262:96–104. doi: 10.1016/j.cellimm.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Kanto T, Inoue M, Miyatake H, Sato A, Sakakibara M, Yakushijin T, et al. Reduced numbers and impaired ability of myeloid and plasmacytoid dendritic cells to polarize T helper cells in chronic hepatitis C virus infection. J Infect Dis. 2004;190:1919–1926. doi: 10.1086/425425. [DOI] [PubMed] [Google Scholar]
- 12.Szabo G, Dolganiuc A. Subversion of plasmacytoid and myeloid dendritic cell functions in chronic HCV infection. Immunobiology. 2005;210:237–247. doi: 10.1016/j.imbio.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 13.Longman RS, Talal AH, Jacobson IM, Rice CM, Albert ML. Normal functional capacity in circulating myeloid and plasmacytoid dendritic cells in patients with chronic hepatitis C. J Infect Dis. 2005;192:497–503. doi: 10.1086/431523. [DOI] [PubMed] [Google Scholar]
- 14.Wertheimer AM, Bakke A, Rosen HR. Direct enumeration and functional assessment of circulating dendritic cells in patients with liver disease. Hepatology. 2004;40:335–345. doi: 10.1002/hep.20306. [DOI] [PubMed] [Google Scholar]
- 15.Della Bella S, Crosignani A, Riva A, Presicce P, Benetti A, Longhi R, et al. Decrease and dysfunction of dendritic cells correlate with impaired hepatitis C virus-specific CD4+ T-cell proliferation in patients with hepatitis C virus infection. Immunology. 2007;121:283–292. doi: 10.1111/j.1365-2567.2007.02577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Longman RS, Talal AH, Jacobson IM, Albert ML, Rice CM. Presence of functional dendritic cells in patients chronically infected with hepatitis C virus. Blood. 2004;103:1026–1029. doi: 10.1182/blood-2003-04-1339. [DOI] [PubMed] [Google Scholar]
- 17.Averill L, Lee WM, Karandikar NJ. Differential dysfunction in dendritic cell subsets during chronic HCV infection. Clin Immunol. 2007;123:40–49. doi: 10.1016/j.clim.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanto T, Inoue M, Miyazaki M, Itose I, Miyatake H, Sakakibara M, et al. Impaired function of dendritic cells circulating in patients infected with hepatitis C virus who have persistently normal alanine aminotransferase levels. Intervirology. 2006;49:58–63. doi: 10.1159/000087264. [DOI] [PubMed] [Google Scholar]
- 19.Miyazaki M, Kanto T, Inoue M, Itose I, Miyatake H, Sakakibara M, et al. Impaired cytokine response in myeloid dendritic cells in chronic hepatitis C virus infection regardless of enhanced expression of Toll-like receptors and retinoic acid inducible gene-I. J Med Virol. 2008;80:980–988. doi: 10.1002/jmv.21174. [DOI] [PubMed] [Google Scholar]
- 20.Nattermann J, Zimmermann H, Iwan A, von Lilienfeld-Toal M, Leifeld L, Nischalke HD, et al. Hepatitis C virus E2 and CD81 interaction may be associated with altered trafficking of dendritic cells in chronic hepatitis C. Hepatology. 2006;44:945–954. doi: 10.1002/hep.21350. [DOI] [PubMed] [Google Scholar]
- 21.Szabo G, Mandrekar P, Dolganiuc A. Innate immune response and hepatic inflammation. Semin Liver Dis. 2007;27:339–350. doi: 10.1055/s-2007-991511. [DOI] [PubMed] [Google Scholar]
- 22.Piccioli D, Tavarini S, Borgogni E, Steri V, Nuti S, Sammicheli C, et al. Functional specialization of human circulating CD16 and CD1c myeloid dendritic-cell subsets. Blood. 2007;109:5371–5379. doi: 10.1182/blood-2006-08-038422. [DOI] [PubMed] [Google Scholar]
- 23.Autissier P, Soulas C, Burdo TH, Williams KC. Evaluation of a 12-color flow cytometry panel to study lymphocyte, monocyte, and dendritic cell subsets in humans. Cytometry A. 2010;77:410–419. doi: 10.1002/cyto.a.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512–4520. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- 25.Bachem A, Guttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1273–1281. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med. 2010;207:1247–1260. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ju X, Clark G, Hart DN. Review of human DC subtypes. Methods Mol Biol. 2010;595:3–20. doi: 10.1007/978-1-60761-421-0_1. [DOI] [PubMed] [Google Scholar]
- 28.Sansonno D, Lotesoriere C, Cornacchiulo V, Fanelli M, Gatti P, Iodice G, et al. Hepatitis C virus infection involves CD34(+) hematopoietic progenitor cells in hepatitis C virus chronic carriers. Blood. 1998;92:3328–3337. [PubMed] [Google Scholar]
- 29.Crozat K, Guiton R, Guilliams M, Henri S, Baranek T, Schwartz-Cornil I, et al. Comparative genomics as a tool to reveal functional equivalences between human and mouse dendritic cell subsets. Immunol Rev. 2010;234:177–198. doi: 10.1111/j.0105-2896.2009.00868.x. [DOI] [PubMed] [Google Scholar]
- 30.Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249–9258. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J, Suh WI, Shin EC. T-cell dysfunction and inhibitory receptors in hepatitis C virus infection. Immune Netw. 2010;10:120–125. doi: 10.4110/in.2010.10.4.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, et al. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol. 2009;83:9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, et al. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Invest. 2010;120:4546–4557. doi: 10.1172/JCI43127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bengsch B, Seigel B, Ruhl M, Timm J, Kuntz M, Blum HE, et al. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 2010;6:e1000947. doi: 10.1371/journal.ppat.1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zabaleta A, Llopiz D, Arribillaga L, Silva L, Riezu-Boj JI, Lasarte JJ, et al. Vaccination against hepatitis C virus with dendritic cells transduced with an adenovirus encoding NS3 protein. Mol Ther. 2008;16:210–217. doi: 10.1038/sj.mt.6300333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.