Abstract
It was recently shown that immunization of hamsters with DNA plasmids coding LJM19, a sand fly salivary protein, partially protected against a challenge with Leishmania chagasi, whereas immunization with KMP11 DNA plasmid, a Leishmania antigen, induced protection against L. donovani infection. In the present study, we evaluated the protective effect of immunization with both LJM19 and KMP11 DNA plasmid together. Concerning the protection against an infection by L. chagasi, immunization with DNA plasmids coding LJM19 or KMP11, as well as with both plasmids combined, induced IFN-γ production in draining lymph nodes at 7, 14 and 21 days post-immunization. Immunized hamsters challenged with L. chagasi plus Salivary Gland Sonicate (SGS) from Lutzomyia longipalpis showed an enhancement of IFN-γ/IL-10 and IFN-γ/TGF-β in draining lymph nodes after 7 and 14 days of infection. Two and five months after challenge, immunized animals showed reduced parasite load in the liver and spleen, as well as increased IFN-γ/IL-10 and IFN-γ/TGF-β ratios in the spleen. Furthermore, immunized animals remained with a normal hematological profile even five months after the challenge, whereas L. chagasi in unimmunized hamsters lead to a significant anemia. The protection observed with LJM19 or KMP11 DNA plasmids used alone was very similar to the protection obtained by the combination of both plasmids.
Keywords: Hamster, Leishmania chagasi, Visceral leishmaniasis, Saliva, DNA plasmids, Protection
1. Introduction
Leishmania are transmitted by sand flies and are the etiological agents of cutaneous, mucocutaneous or visceral leishmaniasis. Saliva of sand flies and other blood feeders contains potent pharmacologic components that facilitate blood meals and also plays a role in pathogen transmission (Andrade et al., 2007; Ribeiro, 1995). Small amount of vector saliva can also exacerbate parasite infectivity (Belkaid et al., 1998; Lima and Titus, 1996; Theodos et al., 1991; Titus and Ribeiro, 1988). On the other hand, immune response to arthropod bites or to its saliva precludes establishment of the pathogen in the vertebrate host (Belkaid et al., 1998; Silva et al., 2005). Recent reports have shown the importance of salivary proteins from sand flies as potential targets for vaccine development to control Leishamania infection (Kamhawi et al., 2000; Morris et al., 2001; Valenzuela et al., 2001).
L. chagasi causes VL in Latin America and Lutzomyia longipalpis is its natural vector. Recently, we developed a model for VL in hamsters infecting the animals intradermally in the ear, with parasites plus SGS of Lu. longipalpis. In this model, hamsters developed the main symptoms of the disease such as visceral parasite burden, massive splenomegaly, bone marrow dysfunction, cachexia, pancytopenia, hypergammaglobulinaemia, and ultimately death (Melby et al., 2001). However, hamsters immunized with DNA plasmid coding LJM19, a Lu. longipalpis salivary protein, protected them from disease development and the fatal outcome of visceral leishmaniasis (Gomes et al., 2008). Immunization of hamsters with DNA plasmids coding KMP11 induced a mixed Th1/Th2 T cellular immune response, with high levels of IFN-γ, TNF-α, IL-4 and IL-12 but a lack of IL-10 and protected the animals against disease development (Basu et al., 2005). It was also demonstrated that human CD8 T cells could recognize KMP11epitopes associated to MCH I, pointing out the importance of this protein in the human immune response (Basu et al., 2007). Immunization with either LJM19 or KMP11 DNA plasmid led to a marked reduction in parasite load and to a less severe disease, but neither one induced parasite cure as some parasites remained during the whole period of observation (Basu et al., 2005; Gomes et al., 2008). In the present study, we evaluated the combination of DNA plasmids coding LJM19 and KMP11 as potent inducers of protective immunity against L. chagasi infection in hamsters.
2. Methods
2.1. Sand flies and salivary gland lysates
Laboratory colonies of Lu. longipalpis were reared at Centro de Pesquisa Gonçalo Moniz. Salivary gland from adult female flies were dissected and transferred to 10 or 20 μl Hepes 10 mM pH 7.0 NaCl 0.15 in 1.5 polypropilene vials, usually in groups of 20 pairs of glands in 20 μl of Hepes saline. Salivary glands were kept at −70 °C until used, when they were disrupted by sonication using a Branson Sonifier 450 homogenizer (Branson, Danbury, CT) (Ribeiro and Modi, 2001). Salivary homogenates were centrifuged at 10,000 × g for 2 min and the supernatants were used in the experiments.
2.2. Leishmania parasites
L. chagasi (MCAN/BR/00/BA262) promastigotes were cultivated in Schineider’s insect Medium (Sigma Chemical Co., St Louis, MO, USA) supplemented, with 20% of inactivated FCS, L-glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 μg/ml) at 23 °C for 5–7 days when parasites reached the stationary-phase. These parasites were washed 3 times with saline at 3000 rpm for 10 min, resuspended in saline and adjusted to 5 × 107–108 per ml.
2.3. Construction of DNA plasmids coding for Lu. longipalpis salivary proteins and KMP11. Immunization of hamsters
Male Golden Syrian Hamsters (Mesocricetus auratus) at 10–12 weeks-old from Centro de Pesquisa Gonçalo Moniz were used for experimental purposes with previous approval of the Animal Ethics Committee of the Fundação Oswaldo Cruz-Fiocruz, Bahia- Brazil number 17/2007. DNA plasmids coding for Lu. longipalpis salivary proteins were cloned into the VR2001-TOPO vector and purified as previously described (Oliveira et al., 2006). KMP-11 encoding plasmids were provided by Dr. Manoel Soto of Universidad Autónona de Madrid, Spain (Fuertes et al., 2001). Groups of hamsters were immunized intradermally (i.d.) (Belkaid et al., 1998) in the left ear with 10 μg/animal of LJM19 and/or 100 μg/animal of KMP11 plasmids for three times at 14-day intervals. Control hamsters were immunized with saline or empty plasmid constructions VR2001-TOPO for LJM19 and pcDNA3 for KMP11. Each hamster received plasmid constructions in 20 μl of saline in different situations: I –injected with saline; II – injected with DNA containing LJM19, III –injected with DNA containing KMP11, IV – injected with two different plasmids of DNA containing LJM19 and KMP11 and V – injected together the combination of two different empty plasmid (CT). Two weeks after the last immunization, hamsters were challenged i.d. in the right ear with 105 stationary phase of promastigotes of L. chagasi plus equivalent of 0.5 salivary gland pairs in 20 μl of saline (Gomes et al., 2008).
2.4. Anti-saliva and anti-Leishmania antigen serology by ELISA
ELISA plates were coated with 5 pairs of salivary glands/ml (approximately 5 μg protein/ml) or 10 μg/ml soluble Leishmania antigen (SLA) overnight at 4 °C. After three washes with PBS-0.05% Tween 20, the plates were blocked for 1 h at 37 °C with PBS-0.1% Tween 20 plus 0.05% BSA. Sera were diluted with 1:100 with PBS-0.05%Tween 20, then incubated overnight at 4 °C. After further washes, the wells were incubated with alkaline-phosphatase-conjugated anti-hamster IgG (Jackson Immuno Research) at a 1:1000 dilution for 45 min at 37 °C. Following another washing cycle, the color was developed for 30 min with p-nitrophenylphosphate in sodium carbonate buffer pH 9.6 with 0.2 mM of MgCl2. The absorbance was recorded at 405 nm.
2.5. Limiting dilution assay to determine parasites loads in infected tissues
Parasite load was determined using the quantitative limiting dilution assay as described by Lima et al. (1997). Briefly, infected ears, lymph nodes, liver and spleen were aseptically removed from each hamster at the completion of the experiments. Tissues were homogenized and diluted in Schneider’s insect cell culture medium (Sigma, St. Louis, MO) supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml of penicillin and 100 μg/ml of streptomycin. Homogenate samples were serially diluted in microtiter 96-wells and incubated for one week at 23 °C. Wells with positive growth were noted at specific dilutions and these results were analyzed by ELIDA (Lima et al., 1997) to determine the parasite burden in the samples. Results were expressed as mean parasite titer ± SD.
2.6. RNA isolation and quantitative real-time PCR of cytokine
Total RNA was extracted from the spleen and liver of infected hamsters using Trizol reagent (Invitrogen). First strand of cDNA synthesis was performed with approximately 1–2 μg of RNA in a total volume of 20 μl using the SuperScriptTM III reverse transcriptase. DNA was amplified adding 2 μg of RNA in 30 μl of a mix containing primers oligo (dT), 2.5 μM, dNTPs, 1 mM (Invitrogen), buffer 1× (Tris–HCl 20 mM, pH 8.4, KCl 50 mM, MgCl2 2 mM), 20 U of ribonuclease inhibitor and 50 U of Superscript II reverse transcriptase (Gibco). Amplification conditions consisted of an initial pre-incubation at 42 °C for 50 min, followed by amplification of the target DNA for 40 cycles at 95 °C for 5 min. A standard curve was generated for each set of primers and the efficiency of each reaction was determined. The expression levels of the genes were normalized to GAPDH levels. The results are expressed in fold change over control. Oligonucleotide primers used for real time PCR were: GAPDH (5′CTGACATGCCGCCCTGGAG; 3′TCAGTGTAGCCCAGGATGCC); IFN-γ (5′GAAGCTCACCAAGATTCCGGTAA; 3′TTTTCGTGACAGGTGAGGC AT); IL-10 5′AGACGCCTTTCTCTTGGAGCTTAT; 3′GGCAACTGCAGCG CTGTC); TGF-β (5′GCTACCACGCCAACTTCTGTC; 3′TGTTGGTAGA GGGCAAGG). Primers for IL10, TGF-β, IFN-γ and GAPDH was obtained from Applied Biosystem, EUA. The real time reaction was performed in 96 well plates using SYBER-Green PCR Master Mix and Perkin-Elmer ABI Prism 7500 sequence detection system. Forty cycle reactions with 15 s at 94 °C and 1 min at 60 °C were performed according with ABI-Prism 7500 manufacturater’s instructions.
2.7. Hematological analyses
Blood samples were collected from different animal groups for hematological evaluation, two and five months after challenge. Blood smears were stained by Giemsa and cell counts were performed by a blood cell counter. Healthy hamsters were used as control for immunized and challenged animals.
2.8. Statistical analysis
Results were expressed as medians ± SD. Comparisons among the experimental groups were done by one-way ANOVA (Kruskal–Wallis) test with Dunn’s post test using Graphpad 5 software program. Differences with p < 0.05 were considered significant..
3. Results
3.1. Immunized hamsters showed reduced parasite load and increased pro-inflammatory cytokines profile in early stages of infection
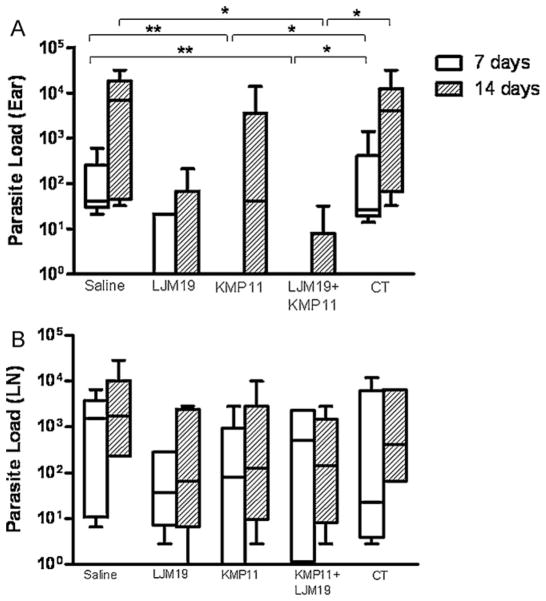
The parasite load was evaluated 7 and 14 days after the infection in LJM19, KMP11 and KMP11 plus LJM19 DNA plasmid immunized hamsters. Seven days after challenge with L. chagasi plus Lu. longipalpis saliva, KMP11 and KMP11 plus LJM19 DNA plasmid immunized hamsters showed significantly reduced parasite load in the ear (Fig. 1A). However, there were no differences concerning parasite loads in the draining lymph nodes (dLN) between immunized and control groups (p > 0.05) (Fig. 1B). To examine the production of pro-inflammatory and anti-inflammatory cytokines in immunized and infected hamsters, real time PCR was performed in dLN samples at 7 and 14 days after challenge. LJM19 DNA plasmid immunized hamsters showed a significantly higher IFN-γ/IL-10 and IFN-γ/TGF-β ratios than control animals (p < 0.05) at both time points. KMP11 or LJM19 plus KMP11 DNA plasmid immunized hamsters showed an enhancement of 3 and 2.5 times in the IFN-γ/IL-10 and IFN-γ/TGF-β ratios respectively, compared to control unimmunized animals. However, these ratios in the dLN did not show significant differences compared to the control groups (Fig. 2A and B). Another alternative is that immunization LJM19 DNA plasmid induced a faster recall response, whereas KMP11 did not. At the same time, it seems that immunization with KMP11 DNA plasmid could exert an inhibitory effect since the immunization with the combination of KMP11 plus LJM19 DNA plasmids resulted in lower IFN-γ/IL-10 and IFN-γ/TGF-β ratios. These results suggest that in the initial events after the infection, other mechanisms such as innate immunity might be responsible for the reduction in the parasite load observed at the site of infection and not only the adaptive immune response against parasite or saliva.
Fig. 1.
Ear and dLN parasite loads in early moments post-infection by L. chagasi. Hamsters were immunized three times in the ear dermis. Groups of animals were immunized with saline, LJM19, KMP11, KMP11 plus LJM19 and empty control plasmids. Two weeks after the last immunization hamsters were challenged with 105 L. chagasi plus 0.5 salivary gland par equivalent in the lateral ear. Ears and draining lymph nodes (dLN) were collected 7 and 14 days after challenge. Parasites loads of the ears and dLN were evaluated by limiting dilution assay. (A) Ear parasite load. (B) dLN parasite load. n = 6. *p < 0.05, **p < 0.01.
Fig. 2.
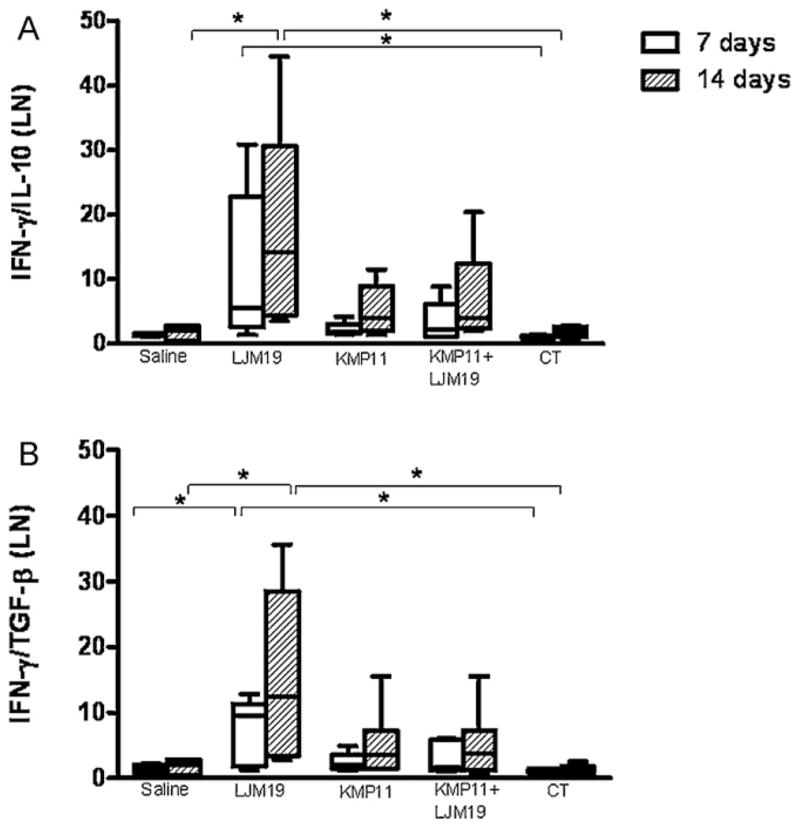
Cytokines expression by dLN of hamsters immunized with DNA plasmids coding KMP11 and LJM19 and challenged with L. chagasi plus saliva. Hamsters were immunized for three times in the ear dermis. Groups of animals were immunized with saline, LJM19, KMP11, KMP11 plus LJM19 and empty control plasmids. Two weeks after the last immunization hamsters were challenged with 105 L. chagasi plus 0.5 salivary gland par equivalent in the lateral ear. Retromandibulars dLN were collected 7 and 14 days after challenge. IFN-γ, IL-10 and TGF-β expression was evaluated by real time PCR. The relative quantification (RQ) was obtained using non-immunized control hamsters and the ratio was obtained dividing IFN-γ expression by IL-10 or TGF-β expression. (A) IFN-γ/IL-10. (B) IFN-γ/TGF-β. n = 6. *p < 0.05.
3.2. Late stages of infection showed reduced visceral parasite load in immunized hamsters
Parasite loads in the liver and spleen were quantified two and five months after infection by L. chagasi plus SGS. Two months after infection hamsters immunized with LJM19, KMP11 or LJM19 plus KMP11 DNA plasmid showed a 105 fold reduction in the parasite load in the spleen compared with control unimmunized group (p < 0.05) (Fig. 3A). The liver of LJM19 or LJM19 plus KMP11 DNA plasmid immunized hamsters did not show parasites after two months of infection (Fig. 3B). Parasite loads in the spleen and liver remained lower five months after infection in immunized hamsters. Spleens and livers from LJM19 and LJM19 plus KMP11 DNA plasmid immunized hamsters showed a 107 fold reduction at this time point and were significantly lower than saline and empty plasmid control groups (p < 0.01 and p < 0.05, respectively) (Fig. 3). At two months post-infection no significant differences were observed in IFN-γ/IL-10 ratio in immunized and non-immunized hamster groups (Fig. 4A). However, the group of KMP11 DNA plasmid immunized hamsters showed a significant increased IFN-γ/TGF-β ratio (Fig. 4B). At five months post-infection immunized groups showed an increase in IFN-γ/IL-10 and IFN-γ/TGF-β ratio. However, control group (saline) also showed an increase in this ratio as well as empty vectors, suggesting that the high number of parasites at this point could be responsible for this alteration in cytokine profile.
Fig. 3.
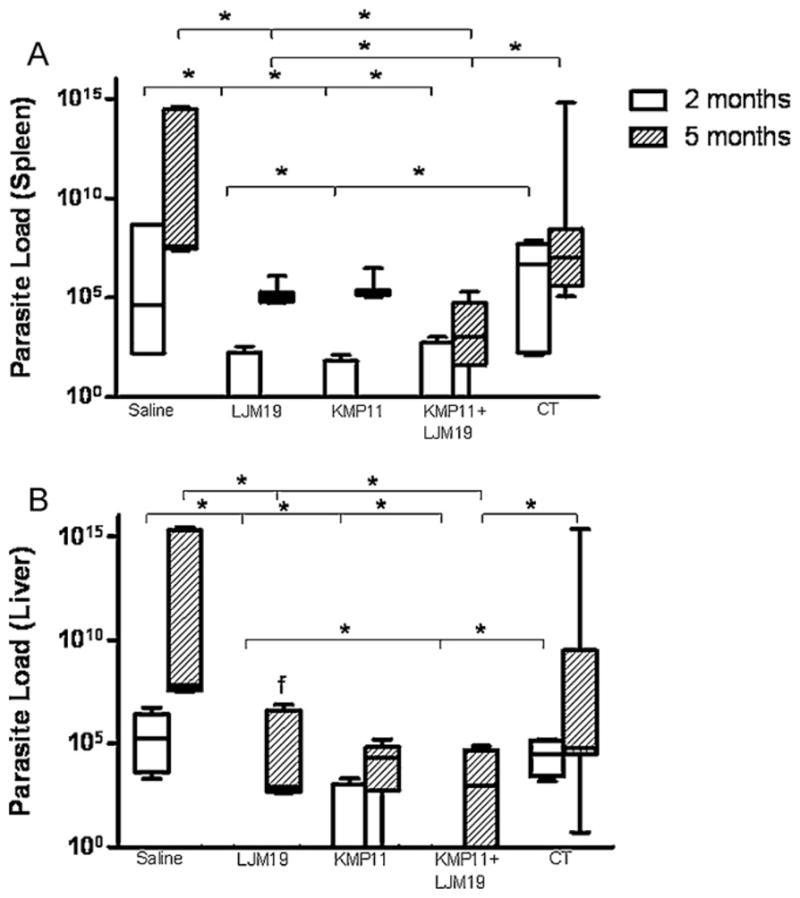
Liver and spleen parasite loads of hamsters immunized with DNA plasmids coding KMP11 and LJM19 and challenged with L. chagasi plus saliva. Hamsters were immunized for three times in the ear dermis. Groups of animals were immunized with saline, LJM19, KMP11, KMP11 plus LJM19 and control plasmids. Two weeks after the last immunization hamsters were challenged with 105 L. chagasi plus 0.5 salivary gland par equivalent in the lateral ear. Two and five months after challenge spleen and liver were collected and weighted. Parasites loads of the spleen and liver were evaluated by limiting dilution assay. (A). Spleen parasite load. (B). Liver parasite load. n = 5–9. *p < 0.05.
Fig. 4.
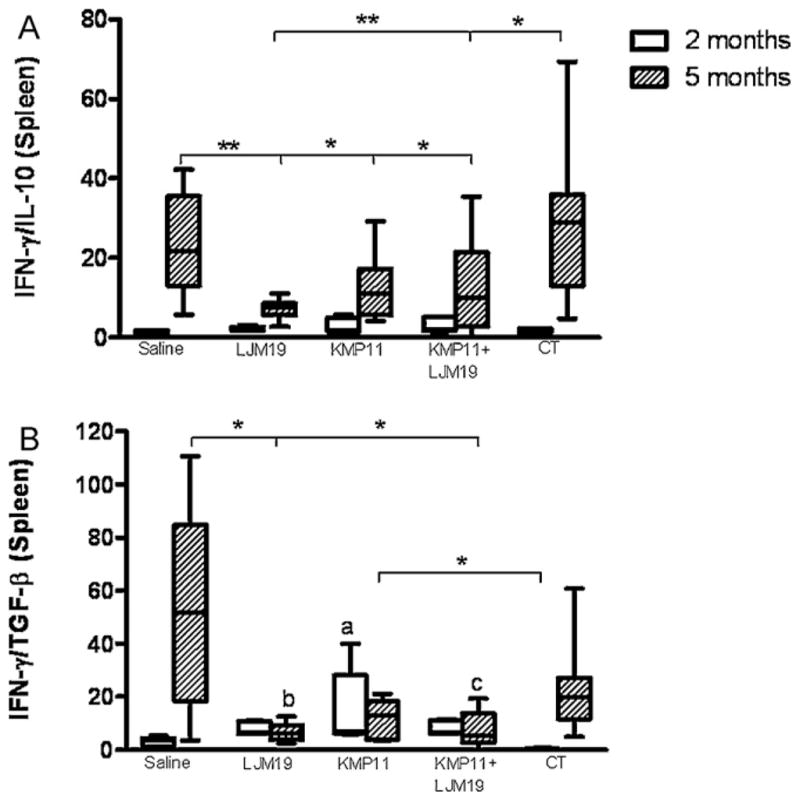
Spleen cytokine expression in immunized hamsters challenged with L. chagasi plus saliva. Hamsters were immunized for three times in the ear dermis. Groups of animals were immunized with saline, LJM19, KMP11, KMP11 plus LJM19 and empty control plasmids. Two weeks after the last immunization hamsters were challenged with 105 L. chagasi plus 0.5 salivary gland par equivalent in the lateral ear. Two and five months after challenge spleen samples were collected. IFN-γ, IL-10 and TGF-β production was evaluated by real time PCR. The relative quantification (RQ) was obtained using non-immunized control hamsters and the ratio was obtained dividing IFN-γ expression by IL-10 or TGF-β expression. (A) IFN-γ/IL-10. (B) IFN-γ/TGF-β. n = 5–9. *p < 0.05, **p < 0.01.
3.3. Immunized hamsters do not develop hematological disorders after challenge with L. chagasi
It is well known that patients with visceral leishmaniasis develop hematological disorders (Fernandez-Guerrero et al., 2004). Hamsters can reproduce many of these clinical features and develop anemia, leukopenia and pancytopenia. To examine if immunization with DNA plasmids could preclude anemia development, blood samples from different groups of hamsters were evaluated two and five months after challenge. We observed that immunization of hamsters with DNA coding plasmids abrogated the development of anemia. Healthy hamsters were used as controls. Compared with healthy hamsters, our control animals showed a decreased number of red blood cells (p < 0.05), a decreased hematocrit (p < 0.05) and a reduced amount of hemoglobin (p < 0.05). However, immunized hamsters did not develop reduction in these hematological parameters and did not show significant differences compared to the healthy control animal group (p > 0.05) (Table 1).
Table 1.
Eritrogram from hamsters immunized with DNA plasmids coding KMP11 and LJM19 and challenged with L. infantum chagasi plus saliva of Lutzomyia longipalpis.
| Healthy | Saline
|
Ljm19
|
Kmp11
|
Kmp11 + Ljm19
|
CT
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 Months | 5 months | 2 Months | 5 months | 2 Months | 5 months | 2 Months | 5 months | 2 Months | 5 months | ||
| Red blood cells (106) | 8.5 ± 0.1 | 8.1 ± 0.1 | 6.8 ± 0.4* | 8.4 ± 0.2 | 8.4 ± 0.1 | 8.2 ± 0.1 | 7.7 ± 0.4 | 7.8 ± 0.2 | 8.5 ± 0.3 | 7.8 ± 0.2 | 6.9 ± 0.4* |
| Hemoglobin (g%) | 16.1 ± 0.4 | 14.9 ± 0.3 | 13.2 ± 1.1* | 16.2 ± 0.4 | 14.9 ± 0.5 | 16.1 ± 0.2 | 14.1 ± 0.7 | 14.4 ± 0.4 | 15.8 ± 0.4 | 14.5 ± 0.7 | 13.1 ± 0.6* |
| Hematocrit (%) | 47.5 ± 0.6 | 45.1 ± 0.5 | 37.3 ± 1.7* | 45.8 ± 1.0 | 47.6 ± 1.6 | 46.4 ± 0.6 | 47.7 ± 2.1 | 43.7 ± 1.2 | 47.9 ± 1.9 | 43.6 ± 1.2 | 37.6 ± 2.2* |
n = 6.
p < 0.05.
4. Discussion
Previous studies from other groups as well as ours have demonstrated protection against experimental visceral leishmaniasis using plasmids coding KMP11, a parasite product, or LJM19, a product from the vector saliva (Basu et al., 2005; Gomes et al., 2008). In this report, we show that such effects are not addictive as the protection obtained with a combination of KMP11 + LJM19 was similar to the levels obtained with either product used separately. Accordingly, the production of IFN-γ, TGF-β as well as IL-10 was similar in all immunized groups. Although no significant differences in parasite load were found in the draining lymph nodes in the early stages of infection (7 and 14 days), hamsters immunized with KMP11 and LJM19 plus KMP11, did not showed a significant reduction in parasite load at 7 and 14 days after infection in the ears. This reduction persisted in the spleen and liver in later stages of infection, possibly, reflecting the initial control in the number of parasites through innate immunity and/or increase in levels of IFN-γ produced in the early stages of the infection.
An enhancement of IFN-γ early production in lymph nodes could be important for parasite control at the inoculation site and could be related with lower visceralization in immunized groups. Many studies show the important role of IL-10 as anti-inflammatory cytokine, deactivating macrophages, diminishing NO production and facilitating Leishmania survival inside phagocytes (Olivier et al., 2005). Similarly, TGF-β is an important cytokine for parasite survival and disease progression (Gantt et al., 2003). Interestingly, our control hamsters showed higher ratios of IFN-γ/IL-10 and IFN-γ/TGF-β in the spleen five months after challenge. These ratios are in agreement with previous studies using cells from human VL patients, which show elevated parasite load even in a pro-inflammatory environment (Nylen et al., 2007). The level of IFN-γ/TGF-β probably increased as a response to an excessive pathology caused by sustained infection (Gomes et al., 2008). Interestingly, immunized hamsters did not show sterilizing infection, however they did not develop clinical manifestations of disease neither hematological alterations, The role of phlebotomine sand fly saliva has been shown both in animal and human models (Barral et al., 2000; Costa et al., 2004; Guilpin et al., 2002) The formal demonstration that mice immunized with DNA plasmids coding SP15, a P. papatasi salivary component, and hamsters immunized with DNA plasmid coding LJM19, a Lu. longipalpis salivary protein, protect these animals against L. major and L. chagasi infection respectively, pointing out for new topics in vaccination against Leishmania infection (Gomes et al., 2008; Valenzuela et al., 2001). These studies showed that DTH development at the site bites promote protective immunity against Leishmania.
Recently, it was demonstrated that immunization with DNA plasmid coding KMP11, a conserved protein in different parasite species, protected hamsters against L. donovani infection (Basu et al., 2005; Fuertes et al., 2001; Ramirez et al., 2001). This protection was related with an enhancement of inducible nitric oxide synthetase, IFN-γ and IL-4 production, a reduction of IL-10 and anti-Leishmania antibodies production (Basu et al., 2005). Our data confirmed the protection conferred by KMP11, using L. chagasi plus saliva as challenge.
Blood disorders are frequently observed both in humans and animal models for visceral leishmaniasis (Caldas et al., 2006; Fernandez-Guerrero et al., 2004; Moreno et al., 2007). Similarly to human patients, hamsters develop anemia and leucopenia (Melby et al., 1998). Parasitism and higher cytokine production can induce alterations in hematopoetic cell behavior conducting to pancytopenia (Pastorino et al., 2002; Yarali et al., 2002). Interestingly, KMP11 and/or LJM19 DNA plasmid immunized hamsters did not show hematological disorders even 5 months after challenge when compared with healthy controls, suggesting that the control of parasite number also correlated to the better condition of the animals.
Interestingly, in our study the combination of DNA plasmids coding KMP11 and LJM19 did not enhance the protection observed in immunized hamsters. Different immunization protocols can be used to reach higher protection. Heterelogous prime/booster immunization, using DNA and protein, has shown excellent results in vaccine development protocols (Dondji et al., 2005). Similarly, Leishmania antigens entrapment in liposomes and nanoparticles has demonstrated to induce Th1 profiles in different animal models, contributing to the control of intracellular microoganisms (Greenland and Letvin, 2007; Sharma et al., 2006).
In summary, we have confirmed the ability of KMP11 DNA plasmid to induce a protective immune response against L. chagasi infection in the hamster model. We confirm the previous results showing protection against this Leishmania specie in hamsters immunized with LJM19 DNA plasmid. This protection is followed by an enhancement of IFN-γ production in the dLN and a reduction in parasites load in the liver and spleen. Interestingly, we demonstrated for the first time that the immunization with these plasmids can abrogate hematological disorders. However, the combination of DNA plasmids coding KMP11 or LJM19 does not enhance these protective effects probably because the immune response elicited by these plasmid immunization could be acting more in the initial phase of infection (presence of saliva) and in promastigotes that are more abundant in the early infection stages, conferring protection in this model.
Acknowledgments
We thank Edivaldo Passos for technical assistance. This research was supported by FAPESB, CNPq and CAPES. CB, AB, CIO and MB-N are senior investigators of CNPq-Brasil. RAAS received a fellowship from CNPq.
Abbreviations
- DTH
delayed-type hypersensitivity
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- SGH
salivary gland homogenate
- VL
visceral leishmaniasis
- i.d
intradermal
References
- Andrade BB, de Oliveira CI, Brodskyn CI, Barral A, Barral-Netto M. Role of sand fly saliva in human and experimental leishmaniasis: current insights. Scand J Immunol. 2007;66:122–127. doi: 10.1111/j.1365-3083.2007.01964.x. [DOI] [PubMed] [Google Scholar]
- Barral A, Honda E, Caldas A, Costa J, Vinhas V, Rowton ED, Valenzuela JG, Charlab R, Barral-Netto M, Ribeiro JM. Human immune response to sand fly salivary gland antigens: a useful epidemiological marker? Am J Trop Med Hyg. 2000;62:740–745. doi: 10.4269/ajtmh.2000.62.740. [DOI] [PubMed] [Google Scholar]
- Basu R, Bhaumik S, Basu JM, Naskar K, De T, Roy S. Kinetoplastid membrane protein-11 DNA vaccination induces complete protection against both pentavalent antimonial-sensitive and -resistant strains of Leishmania donovani that correlates with inducible nitric oxide synthase activity and IL-4 generation: evidence for mixed Th1- and Th2-like responses in visceral leishmaniasis. J Immunol. 2005;174:7160–7171. doi: 10.4049/jimmunol.174.11.7160. [DOI] [PubMed] [Google Scholar]
- Basu R, Roy S, Walden P. HLA class I-restricted T cell epitopes of the kinetoplastid membrane protein-11 presented by Leishmania donovani-infected human macrophages. J Infect Dis. 2007;195:1373–1380. doi: 10.1086/513439. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Kamhawi S, Modi G, Valenzuela J, Noben-Trauth N, Rowton E, Ribeiro J, Sacks DL. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J Exp Med. 1998;188:1941–1953. doi: 10.1084/jem.188.10.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldas AJ, Costa J, Aquino D, Silva AA, Barral-Netto M, Barral A. Are there differences in clinical and laboratory parameters between children and adults with American visceral leishmaniasis? Acta Trop. 2006;97:252–258. doi: 10.1016/j.actatropica.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Costa DJ, Favali C, Clarencio J, Afonso L, Conceicao V, Miranda JC, Titus RG, Valenzuela J, Barral-Netto M, Barral A, Brodskyn CI. Lutzomyia longipalpis salivary gland homogenate impairs cytokine production and costimulatory molecule expression on human monocytes and dendritic cells. Infect Immun. 2004;72:1298–1305. doi: 10.1128/IAI.72.3.1298-1305.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondji B, Perez-Jimenez E, Goldsmith-Pestana K, Esteban M, McMahon-Pratt D. Heterologous prime-boost vaccination with the LACK antigen protects against murine visceral leishmaniasis. Infect Immun. 2005;73:5286–5289. doi: 10.1128/IAI.73.8.5286-5289.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Guerrero ML, Robles P, Rivas P, Mojer F, Muniz G, de Gorgolas M. Visceral leishmaniasis in immunocompromised patients with and without AIDS: a comparison of clinical features and prognosis. Acta Trop. 2004;90:11–16. doi: 10.1016/j.actatropica.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Fuertes MA, Perez JM, Soto M, Lopez MC, Alonso C. Calcium-induced conformational changes in Leishmania infantum kinetoplastid membrane protein-11. J Biol Inorg Chem. 2001;6:107–117. doi: 10.1007/s007750000175. [DOI] [PubMed] [Google Scholar]
- Gantt KR, Schultz-Cherry S, Rodriguez N, Jeronimo SM, Nascimento ET, Goldman TL, Recker TJ, Miller MA, Wilson ME. Activation of TGF-beta by Leishmania chagasi: importance for parasite survival in macrophages. J Immunol. 2003;170:2613–2620. doi: 10.4049/jimmunol.170.5.2613. [DOI] [PubMed] [Google Scholar]
- Gomes R, Teixeira C, Teixeira MJ, Oliveira F, Menezes MJ, Silva C, de Oliveira CI, Miranda JC, Elnaiem DE, Kamhawi S, Valenzuela JG, Brodskyn CI. Immunity to a salivary protein of a sand fly vector protects against the fatal outcome of visceral leishmaniasis in a hamster model. Proc Natl Acad Sci USA. 2008;105:7845–7850. doi: 10.1073/pnas.0712153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland JR, Letvin NL. Chemical adjuvants for plasmid DNA vaccines. Vaccine. 2007;25:3731–3741. doi: 10.1016/j.vaccine.2007.01.120. [DOI] [PubMed] [Google Scholar]
- Guilpin VO, Swardson-Olver C, Nosbisch L, Titus RG. Maxadilan, the vasodilator/immunomodulator from Lutzomyia longipalpis sand fly saliva, stimulates haematopoiesis in mice. Parasite Immunol. 2002;24:437–446. doi: 10.1046/j.1365-3024.2002.00484.x. [DOI] [PubMed] [Google Scholar]
- Kamhawi S, Belkaid Y, Modi G, Rowton E, Sacks D. Protection against cutaneous leishmaniasis resulting from bites of uninfected sand flies. Science. 2000;290:1351–1354. doi: 10.1126/science.290.5495.1351. [DOI] [PubMed] [Google Scholar]
- Lima HC, Titus RG. Effects of sand fly vector saliva on development of cutaneous lesions and the immune response to Leishmania braziliensis in BALB/c mice. Infect Immun. 1996;64:5442–5445. doi: 10.1128/iai.64.12.5442-5445.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima HC, Bleyenberg JA, Titus RG. A simple method for quantifying Leishmania in tissues of infected animals. Parasitol Today. 1997;13:80–82. doi: 10.1016/s0169-4758(96)40010-2. [DOI] [PubMed] [Google Scholar]
- Melby PC, Tryon VV, Chandrasekar B, Freeman GL. Cloning of Syrian hamster (Mesocricetus auratus) cytokine cDNAs and analysis of cytokines mRNA expression in experimental visceral leishmaniasis. Infect Immun. 1998;66:2135–2142. doi: 10.1128/iai.66.5.2135-2142.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melby PC, Chandrasekar B, Zhao W, Coe JE. The hamster as a model of human visceral leishmaniasis: progressive disease and impaired generation of nitric oxide in the face of a prominent Th1-like cytokine response. J Immunol. 2001;166:1912–1920. doi: 10.4049/jimmunol.166.3.1912. [DOI] [PubMed] [Google Scholar]
- Moreno J, Nieto J, Masina S, Canavate C, Cruz I, Chicharro C, Carrillo E, Napp S, Reymond C, Kaye PM, Smith DF, Fasel N, Alvar J. Immunization with H1, HASPB1 and MML Leishmania proteins in a vaccine trial against experimental canine leishmaniasis. Vaccine. 2007;25:5290–5300. doi: 10.1016/j.vaccine.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RV, Shoemaker CB, David JR, Lanzaro GC, Titus RG. Sandfly maxadilan exacerbates infection with Leishmania major and vaccinating against it protects against L. major infection. J Immunol. 2001;167:5226–5230. doi: 10.4049/jimmunol.167.9.5226. [DOI] [PubMed] [Google Scholar]
- Nylen S, Maurya R, Eidsmo L, Manandhar KD, Sundar S, Sacks D. Splenic accumulation of IL-10 mRNA in T cells distinct from CD4+CD25+ (Foxp3) regulatory T cells in human visceral leishmaniasis. J Exp Med. 2007;204:805–817. doi: 10.1084/jem.20061141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira F, Kamhawi S, Seitz AE, Pham VM, Guigal PM, Fischer L, Ward J, Valenzuela JG. From transcriptome to immunome: identification of DTH inducing proteins from a Phlebotomus ariasi salivary gland cDNA library. Vaccine. 2006;24:374–390. doi: 10.1016/j.vaccine.2005.07.085. [DOI] [PubMed] [Google Scholar]
- Olivier M, Gregory DJ, Forget G. Subversion mechanisms by which Leishmania parasites can escape the host immune response: a signaling point of view. Clin Microbiol Rev. 2005;18:293–305. doi: 10.1128/CMR.18.2.293-305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino AC, Jacob CM, Oselka GW, Carneiro-Sampaio MM. Visceral leishmaniasis: clinical and laboratorial aspects. J Pediatr (Rio J) 2002;78:120–127. [PubMed] [Google Scholar]
- Ramirez JR, Gilchrist K, Robledo S, Sepulveda JC, Moll H, Soldati D, Berberich C. Attenuated Toxoplasma gondii ts-4 mutants engineered to express the Leishmania antigen KMP-11 elicit a specific immune response in BALB/c mice. Vaccine. 2001;20:455–461. doi: 10.1016/s0264-410x(01)00341-3. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM. Blood-feeding arthropods: live syringes or invertebrate pharmacologists? Infect Agents Dis. 1995;4:143–152. [PubMed] [Google Scholar]
- Ribeiro JM, Modi G. The salivary adenosine/AMP content of Phlebotomus argentipes Annandale and Brunetti, the main vector of human kalaazar. J Parasitol. 2001;87:915–917. doi: 10.1645/0022-3395(2001)087[0915:TSAACO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Sharma G, Anabousi S, Ehrhardt C, Ravi Kumar MN. Liposomes as targeted drug delivery systems in the treatment of breast cancer. J Drug Target. 2006;14:301–310. doi: 10.1080/10611860600809112. [DOI] [PubMed] [Google Scholar]
- Silva F, Gomes R, Prates D, Miranda JC, Andrade B, Barral-Netto M, Barral A. Inflammatory cell infiltration and high antibody production in BALB/c mice caused by natural exposure to Lutzomyia longipalpis bites. Am J Trop Med Hyg. 2005;72:94–98. [PubMed] [Google Scholar]
- Theodos CM, Ribeiro JM, Titus RG. Analysis of enhancing effect of sand fly saliva on Leishmania infection in mice. Infect Immun. 1991;59:1592–1598. doi: 10.1128/iai.59.5.1592-1598.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus RG, Ribeiro JM. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science. 1988;239:1306–1308. doi: 10.1126/science.3344436. [DOI] [PubMed] [Google Scholar]
- Valenzuela JG, Belkaid Y, Garfield MK, Mendez S, Kamhawi S, Rowton ED, Sacks DL, Ribeiro JM. Toward a defined anti-Leishmania vaccine targeting vector antigens: characterization of a protective salivary protein. J Exp Med. 2001;194:331–342. doi: 10.1084/jem.194.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarali N, Fisgin T, Duru F, Kara A. Myelodysplastic features in visceral leishmaniasis. Am J Hematol. 2002;71:191–195. doi: 10.1002/ajh.10200. [DOI] [PubMed] [Google Scholar]