Abstract
Importance
Over 225,000 surgeries are performed annually in the U.S. for pelvic organ prolapse (POP). Abdominal sacrocolpopexy is considered the most durable POP surgery, but little is known about long-term effectiveness and adverse events.
Objective
To describe anatomic and symptomatic outcomes up to 7 years after abdominal sacrocolpopexy, and to determine whether these are affected by concomitant anti-incontinence surgery (Burch urethropexy).
Design, setting, participants
Long-term follow-up of the randomized, masked 2-year CARE trial (Colpopexy And urinary Reduction Efforts). Participants were stress continent women undergoing abdominal sacrocolpopexy between 2002–5 for symptomatic POP randomized to concomitant urethropexy or not. 92% (215/233) of eligible 2-year CARE trial completers enrolled into this extended study with 181 (84%) and 126 (59%) completing 5 and 7 years follow-up, respectively. Median follow-up was 7 years.
Main Outcome Measures
POP: Symptomatic failure: POP retreatment or reporting bulge on Pelvic Floor Distress Inventory (PFDI); Anatomic failure: POP retreatment or Pelvic Organ Prolapse Quantification demonstrating descent of the vaginal apex descend below upper third of the vagina or anterior or posterior vaginal wall prolapse beyond the hymen.
Urinary incontinence (UI): Stress UI: more than 1 stress urinary incontinence symptom on PFDI or interval treatment; Overall UI: score ≥ 3 on Incontinence Severity Index.
Results
By year 7, the estimated probabilities of failure (POP, SUI, UI) from parametric survival modeling for the urethropexy and no urethropexy groups respectively were were 0.27 and 0.22 for anatomic POP (difference 0.050; 95% CI −0.161, 0.271), 0.29 and 0.24 for symptomatic POP (0.049; −0.060, 0.162), 0.48 and 0.34 for composite POP (0.134; −0.096, 0.322), 0.62 and 0.77 for SUI (−0.153; −0.268, 0.030) 0.75 and 0.81 for overall UI (−0.064; −0.161, 0.032). Mesh erosion probability estimated by Kaplan-Meier method was 10.5% (95% CI 6.8, 16.1) at 7 years.
Conclusion and Relevance
Over seven years, abdominal sacrocolpopexy failure rates increased in both randomized groups. Urethropexy prevented SUI longer than no urethropexy. Abdominal sacrocolpopexy effectiveness must be balanced with long-term risks of mesh and /or suture erosion.
Introduction
Pelvic organ prolapse (POP) occurs when the uterus or vaginal walls bulge into or beyond the vaginal introitus. It is common in women and 7–19% undergo surgical repair.1, 2 Abdominal sacrocolpopexy is the most durable operation for advanced POP and serves as the gold standard against which other operations are compared.3 Abdominal sacrocolpopexy involves attaching the vaginal apex to the sacral anterior longitudinal ligament reinforced with a synthetic mesh graft.
Little is known about long-term durability, complications, and pelvic floor symptoms after abdominal sacrocolpopexy. The few studies assessing outcomes beyond two years are limited by small sample sizes, inconsistent outcome assessment, potentially biased examiners, and non-standardized follow-up.4 Knowing the long term outcomes for the surgical gold standard in treating POP is important since 225,000 women in the U.S. undergo POP surgeries annually. The direct costs for these procedures exceed 1 billion dollars per year. As the population ages, it is anticipated that POP and UI will become more frequently seen in primary care practices.,5,6,7,8
Between 2001 and 2006, the NIH-funded Pelvic Floor Disorders Network (PFDN) conducted a multicenter randomized masked trial in women without stress urinary incontinence (SUI) undergoing abdominal sacrocolpopexy for POP (CARE: Colpopexy And urinary Reduction Efforts) to study whether adding a prophylactic anti-incontinence procedure (Burch urethropexy) impacts denovo SUI, a common adverse event after POP surgery.9 At 2 years, the incidence of SUI was 32.0% (47/147) after urethropexy versus 45.2% (70/155) after no urethropexy, P=.026.10 To understand the balance between positive and negative outcomes and the impact of a Burch urethropexy over time, we invited women that completed their final 2-year visit in the CARE trial to participate in this extended study (E-CARE).
The primary aims of E-CARE are to compare the long-term anatomic success rates, stress continence rates, and overall pelvic floor symptoms and pelvic-floor specific quality of life and to describe mesh-related adverse events in women undergoing abdominal sacrocolpopexy who were or were not randomized to undergo urethropexy.
Methods
Institutional Review Boards at sites approved E-CARE (clinicaltrials.gov NCT00099372). CARE enrollment occurred between March, 2002 and February, 2005. NIH funding decisions limited E-CARE in-person visits to fully funded Pelvic Floor Disorders Network sites, whereas only QOL follow up occurred at non-funded sites and there was no research follow-up for participants at sub-contracted sites. Women completing the in-person 2-year CARE visit were recruited into E-CARE beginning May 2004 and were followed up to 9 years after abdominal sacrocolpopexy. However, years 8 and 9 are excluded from analyses because of small numbers. E-CARE included annual in-person examinations and centralized quality of life (QOL) telephone interviews at fully funded sites and only QOL telephone interviews for participants from sites that did not continue in the PFDN after July, 2007. We contacted all participants midyear from index surgery to update information.
Outcome measures were similar to CARE.11 At in-person visits, research personnel conducted vaginal examinations to identify mesh erosion and assess vaginal support using the Pelvic Organ Prolapse Quantification evaluation (POP-Q), in which the lowest level of vaginal descent during strain is measured relative to its distance in cm from the hymen; points above the hymen are negative while those below are positive.12 While performing checks on POP-Q responses, we discovered several discrepant values. Since data collection had ended and it was not possible to contact investigators to correct data entry, an adjudication committee consisting of three Principal Investigators performed independent data reviews. RTI reconciled the reviews and made manual data adjustments where appropriate.
At visits or by telephone, we asked about surgical complications, comorbidities, and interval treatments for pelvic floor disorders and reviewed pertinent operative reports. Trained interviewers from a centralized facility administered by telephone a battery of instruments including the 46-item Pelvic Floor Distress Inventory (PFDI), which assesses symptom distress in women with pelvic floor disorders, and the Incontinence Severity Index (ISI), a 2-item index in which numerical responses for incontinence frequency and volume are multiplied to yield a score between 0 and 12.13, 14 The PFDI has 3 scales: Urinary Distress, Colorectal-anal Distress, and POP Distress; its scoring is explained in eTable 1. Telephone interviewers administered the 10-item Short Portable Mental Status Questionnaire (SPMSQ) to women ≥ 75 years and withdrew women with scores ≥ 5, indicating moderate to severe cognitive impairment.
POP outcome definitions for E-CARE include: Anatomic failure: re-operation or pessary for POP or POP-Q measurements, as follows: C > [−2/3 x total vaginal length] (i.e., the vaginal apex descends below upper third of the vagina) or one of points Ba, Bp is > 0 cm (i.e., the anterior (Ba) or posterior (Bp) vaginal wall prolapses beyond the hymen). This updated definition differs from our original definition because in the decade since planning ECARE, emerging data revealed that symptoms increase and satisfaction decreases once prolapse descends past the hymen (i.e. > 0 cm).15,16,17 Therefore, after approval by the Steering Committee and Data Safety Monitoring Board, and before any data analysis, we planned analyses for both the above updated anatomic failure definition as well as the following original definition: C> Stage 0 (i.e., vaginal apex descends ≥ 2 cm) or Ba or Bp > +1 cm (i.e., anterior or posterior vaginal wall descends ≥ 1 cm beyond hymen). Symptomatic failure: symptom of bulge with endorsement of ≥ 1 bulge questions on the POPDI (referring to seeing or feeling a bulge) or re-operation or pessary for POP; Composite failure: meets criteria for anatomic (updated definition) or symptomatic failure. We defined SUI failure as report of ≥ 1 SUI symptom on the PFDI; failure of SUI prevention as report of ≥ 1 SUI symptom on the PFDI or interval anti-incontinence surgery or urethral bulking agent for SUI; and overall urinary incontinence as an ISI score ≥ 3. Erosion was defined as exposed suture or mesh material in the vagina or viscera.
Sample Size
CARE sample size and analyses are described in the primary report from that study.11 We assumed that at least 250 women would enroll in E-CARE with approximately 100 in the smallest randomization group, which would provide sufficient power (>80%) to detect treatment differences between randomized groups up to 20% in dichotomous outcomes and 0.4 standard deviation in continuous outcomes.
Statistical analysis
We compared demographic characteristics between groups using t tests and chi-square tests as appropriate. We calculated cumulative POP rates for each year of follow-up, classifying women who experienced POP as failures. Numerators included all failures up to the follow up time point, and denominators included all failures and known successes at that time point (women lost to follow-up without previously experiencing a failure were censored).
We analyzed differences between treatment groups in change from baseline of symptom bother scores using general linear mixed models adjusted for 21 unique surgeons and intent to perform paravaginal procedures. Interactions between visit and treatment group were tested. Model fit was assessed using log likelihood and the Akaike information criterion (AIC). To ensure that we did not enhance results of the index surgery, we conducted sensitivity analyses replacing post-treatment values of scores with last pre-treatment data (if worse) for women undergoing POP or SUI treatment.
To test for differences in failure rates between treatment groups, accounting for interval censoring (where failure times were known to have occurred in a certain interval, for example, between two clinic visits, but the exact failure date was unknown) and stratification by surgeon, we used frailty parametric survival models (the survival data analog to regression models with frailty being analogous to random effects. Where appropriate, interval censoring was used to account for uncertainty about exact failure times. Data from women not known to have experienced a failure were right censored at the last known time of success. Best model fit, and the need to include a frailty to account for unmeasured sources of variation between surgeons in the model, was determined through graphical information, the AIC, and the Deviance Information Criterion (DIC) for model selection. We excluded intent to perform paravaginal repair from final survival models because this variable was statistically insignificant in survival models, and the number with planned paravaginal procedures in the subset of women with in-person examinations was small. Parametric survival models were created using WinBUGS software, and corresponding survival curves were graphed in R (survival package, version 2.36–14).. Kaplan-Meier curves were created with R for selected outcomes; these did not account for surgeon variation or interval censoring (interval midpoints were used). We estimated the rate of mesh erosion over time in all women enrolled in CARE and those that continued into E-CARE using the Kaplan-Meier method in SAS. All other analyses were implemented in SAS. Two-sided p values <0.05 were considered statistically significant.
RESULTS
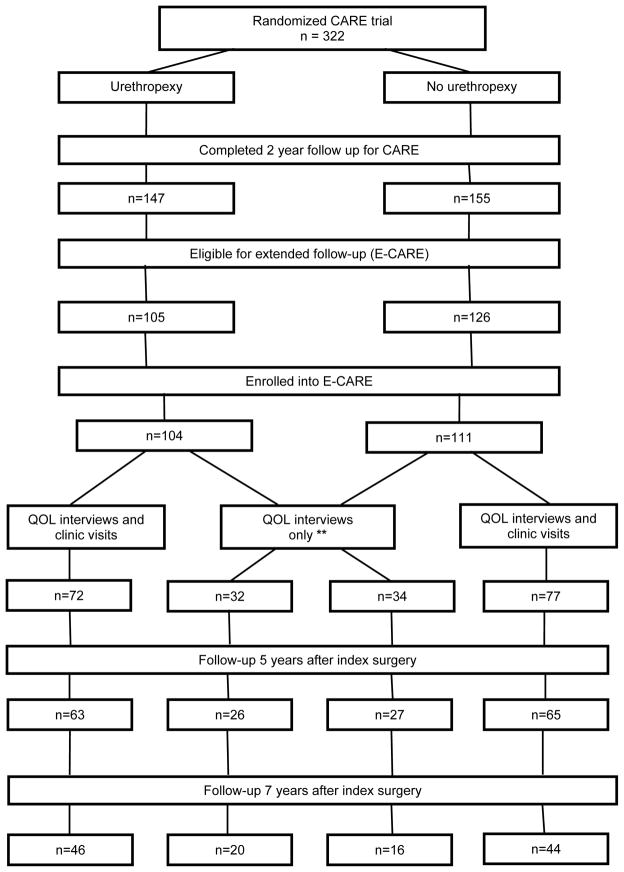
Study participation is summarized in Figure 1. Of 322 randomized women, 302 completed 2-year follow-up; of these, 231 were from sites participating in E-CARE. Ninety-three percent (215 of 231) of eligible participants enrolled in E-CARE of whom 84% (181 women) completed 5-year outcome assessments. Two were excluded from ongoing participation based on cognitive impairment. POP-Q measurements were adjudicated in 57 instances. Baseline demographic and clinical characteristics and 2- to 5-year follow-up rates did not differ by original randomization assignment and were similar between women that did or did not participate in E-CARE (Table 1). E-CARE participants that did only QOL telephone interviews were older and more likely to be married than women completing interviews and in-person examinations (mean (standard deviation) 64.3 (10.6) versus 60.8 (9.6) years, p=0.02; 83.8% and 69.8%, p=0.03, respectively).
Figure 1.
Participant flow. At the conclusion of the 2-year CARE study, 71 women were not eligible for E-CARE as they were participants from 2 sub-contract sites and 1 original PFDN site that did not participate in E-CARE. Of the 215 E-CARE participants, 66 women underwent their index study surgery at 3 sites that, after completion of the CARE study, did not continue into the next funding cycle; these women only had the opportunity to participate in the centralized telephone QOL interviews..
Table 1.
Baseline Characteristics of Study Population
| CHARACTERISTIC | ENROLLED IN E-CARE (N=215) | NOT ENROLLED IN E-CARE (N=107) |
|---|---|---|
| Age - yr | 61.9 ± 10.0 | 60.3 ± 10.6 |
| Race – no./total no. (%) | ||
| White | 198/215 (92.1) | 101/107 (94.4) |
| Black | 13/215 (6.0) | 4/107 (3.7) |
| Other | 4/215 (1.9) | 2/107 (1.9) |
| Ethnic group – no./total no. (%) | ||
| Hispanic | 8/215 (3.7) | 1/107 (0.9) |
| Marital status – no./total no. (%) | ||
| Married or living as married | 160/215 (74.4) | 79/107 (73.8) |
| Education level – no./total no. (%) | ||
| Less than high school | 19/215 (8.8) | 8/107 (7.5) |
| Completed high school or equivalent | 80/215 (37.2) | 45/107 (42.1) |
| Some college or higher | 116/215 (54.0) | 54/107 (50.5) |
| No. of previous vaginal deliveries | ||
| Median (Range) | 3 (0–8) | 3 (1–11) |
| No. of previous cesarean deliveries | ||
| Median (Range) | 0 (0–5) | 0 (0–2) |
| Total no. of previous births | ||
| Median (Range) | 3 (1–10) | 3 (1–11) |
| Prior surgery for incontinence – no./total no (%) | 16/215 (7.4) | 6/107 (5.6) |
| Prior surgery for POP* – no./total no (%) | 84/215 (39.1) | 42/107 (39.3) |
| Prior hysterectomy – no./total no (%) | 159/215 (74.0) | 69/107 (64.5) |
| POP-Q** Stage – no./total no (%) | ||
| II | 24/215 (11.2) | 20/107 (18.7) |
| III | 153/215 (71.2) | 65/107 (60.7) |
| IV | 38/215 (17.7) | 22/107 (20.6) |
| Body Mass Index | ||
| Mean | 26.7 ± 4.4 | 27.7 ± 4.6 |
| BMI >=30 (obesity) – no./total no (%) | 45/215 (20.9) | 29/107 (27.1) |
P-values were obtained through 2-sample t-tests for continuous variables and chi-square tests for categorical variables.
Plus-minus values are means +/− SD.
Race was self-reported
The P value for race omits the category of “other.”
POP, Pelvic Organ Prolapse
The stages of Pelvic Organ Prolapse quantification system (POP-Q) are as follows: in stage II prolapse, the vagina is prolapsed between 1cm above the hymen and 1 cm below the hymen; in stage III, the vagina is prolapsed more than 1 cm beyond the hymen but is less than totally everted; and in stage IV, the vagina is everted to within 2 cm of its length.
POP and UI failure rates gradually increased over the follow-up period (Table 2, Figures 2 and 3; eFigures 1, 2 and 3). By year 7, the estimated probabilities of failure (POP) for the urethropexy and no urethropexy group respectively were0.27 and 0.22 for anatomic (updated ) POP (difference 0.050; 95% CI −0.161, 0.271), 0.29 and 0.24 for symptomatic POP (0.049; −0.060, 0.162), and 0.48 and 0.34 for composite POP (0.134; −0.096, 0.322).
Table 2.
Estimated probability of failure from parametric survival models of primary outcomes at 2–7 years after abdominal sacrocolpopexy
| Pelvic Organ Prolapse Failure Definition | Treatment Group | Estimated Probability of Failure (SE)1, Number2/Denominator2, Difference in Treatment Estimates and 95% Confidence Intervals | |||||
|---|---|---|---|---|---|---|---|
| 2 Years | 3 Years | 4 Years | 5 Years | 6 Years | 7 Years | ||
| Symptomatic3 | Burch | 0.14 (0.026) | 0.18 (0.031) | 0.21 (0.034) | 0.24 (0.038) | 0.27 (0.041) | 0.29 (0.043) |
| 15/104 | 16/101 | 19/94 | 25/91 | 27/86 | 27/73 | ||
| No Burch | 0.12 (0.023) | 0.15 (0.027) | 0.18 (0.031) | 0.20 (0.034) | 0.22 (0.036) | 0.24 (0.038) | |
| 16/111 | 19/108 | 20/103 | 21/96 | 21/90 | 22/71 | ||
| Treatment Difference | 0.026 (−0.032, 0.087) | 0.032 (−0.040, 0.108) | 0.038 (−0.046, 0.124) | 0.042 (−0.051, 0.139) | 0.046 (−0.056, 0.151) | 0.049 (−0.060, 0.162) | |
| Anatomic (Original)4 | Burch | 0.08 (0.027) | 0.12 (0.39) | 0.16 (0.049) | 0.20 (0.059) | 0.23 (0.068) | 0.26 (0.075) |
| 6/69 | 9/61 | 10/49 | 12/47 | 15/44 | 15/33 | ||
| No Burch | 0.10 (0.029) | 0.13 (0.034) | 0.16 (0.039) | 0.19 (0.043) | 0.21 (0.047) | 0.23 (0.050) | |
| 9/76 | 10/67 | 12/63 | 13/59 | 14/47 | 14/37 | ||
| Treatment Difference | −0.017 (−0.098, 0.061) | −0.010 (−0.112, 0.093) | −0.001 (−0.123, 0.125) | 0.009 (−0.133, 0.154) | 0.019 (−0.140, 0.182) | 0.029 (−0.148, 0.208) | |
| Anatomic (Updated)5 | Burch | 0.09 (0.036) | 0.13 (0.052) | 0.16 (0.066) | 0.20 (0.078) | 0.24 (0.089) | 0.27 (0.099) |
| 5/69 | 8/60 | 10/48 | 14/46 | 18/44 | 18/35 | ||
| No Burch | 0.09 (0.028) | 0.12 (0.033) | 0.15 (0.037) | 0.17 (0.041) | 0.20 (0.045) | 0.22 (0.049) | |
| 8/76 | 10/66 | 12/63 | 12/59 | 13/48 | 13/38 | ||
| Treatment Difference | −0.005 (−0.093, 0.087) | 0.005 (−0.110, 0.131) | 0.016 (−0.125, 0.170) | 0.028(−0.139, 0.207) | 0.039(−0.150, 0.240) | 0.050(−0.161, 0.271) | |
| Composite6 | Burch | 0.22 (0.056) | 0.29 (0.066) | 0.34 (0.074) | 0.39 (0.081) | 0.44 (0.087) | 0.48 (0.092) |
| 14/70 | 16/63 | 19/52 | 27/51 | 30/49 | 30/42 | ||
| No Burch | 0.18 (0.038) | 0.23 (0.042) | 0.26 (0.046) | 0.29 (0.049) | 0.32 (0.052) | 0.34 (0.054) | |
| 15/76 | 18/66 | 19/62 | 20/59 | 21/51 | 21/45 | ||
| Treatment Difference | 0.035 (−0101, 0.164) | 0.060 (−0.102, 0.207) | 0.082 (−0.101, 0.244) | 0.102 (−0.099, 0.276) | 0.119 (−0.098, 0.304) | 0.134 (−0.096, 0.322) | |
| Urinary Incontinence Failure Definition | Treatment Group | Estimated Probability of Failure (SD)1, Number2/Denominator2, Difference in Treatment Estimates and 95% Confidence Intervals | |||||
| 2 Years | 3 Years | 4 Years | 5 Years | 6 Years | 7 Years | ||
| Overall UI7 | Burch | 0.59 (0.050) | 0.65 (0.048) | 0.68 (0.047) | 0.71 (0.045) | 0.73 (0.044) | 0.75 (0.043) |
| 48/104 | 52/101 | 55/99 | 58/98 | 59/97 | 62/91 | ||
| No Burch | 0.67 (0.039) | 0.72 (0.038) | 0.76 (0.036) | 0.78 (0.035) | 0.80 (0.033) | 0.81 (0.032) | |
| 66/111 | 70/109 | 70/105 | 72/104 | 73/102 | 73/92 | ||
| Treatment Difference | −0.082 (−0.203, 0.041) | −0.077 (−0.192, 0.038) | −0.073 (−0.183, 0.036) | −0.070 (−0.174, 0.034) | −0.067 (−0.168, 0.033) | −0.064 (−0.161, 0.032) | |
| SUI Prevention Failure7 | Burch | 0.44 (0.052) | 0.50 (0.053) | 0.54 (0.053) | 0.57 (0.052) | 0.60 (0.052) | 0.62 (0.051) |
| 39/104 | 47/102 | 49/99 | 53/98 | 55/95 | 57/89 | ||
| No Burch | 0.61 (0.039) | 0.67 (0.038) | 0.71 (0.037) | 0.73 (0.036) | 0.75 (0.035) | 0.77 (0.034) | |
| 63/110 | 69/109 | 76/107 | 77/105 | 78/105 | 79/96 | ||
| Treatment Difference | −0.175 (−0.296, −0.043) | −0.172 (−0.291, −0.042) | −0.168 (−0.284, −0.041) | −0.163 (−0.278, −0.039) | −0.158 (−0.271, −0.038) | −0.154 (−0.266, −0.037) | |
| SUI7 | Burch | 0.44 (0.053) | 0.49 (0.054) | 0.54 (0.054) | 0.57 (0.053) | 0.60 (0.053) | 0.62 (0.052) |
| 39/104 | 47/102 | 49/99 | 53/98 | 55/95 | 57/89 | ||
| No Burch | 0.61 (0.039) | 0.67 (0.038) | 0.70 (0.037) | 0.73 (0.036) | 0.75 (0.035) | 0.77 (0.034) | |
| 63/110 | 68/109 | 75/107 | 76/105 | 78/105 | 79/96 | ||
| Treatment Difference | −0.173 (−0.298, −0.035) | −0.170 (−0.295, −0.034) | −0.166 (−0.288, −0.033) | −0.161 (−0.281, −0.032) | −0.157 (−0.275, −0.030) | −0.153 (−0.268, −0.030) | |
Estimated Probability of Failure estimates and corresponding standard errors are from the parametric survival models (demonstrated in eFigures 1–3).
Numerators and denominators at each time point include prior failures and exclude prior drop-outs who had not yet met failure criteria
Symptom of bulge on Pelvic Floor Distress Inventory OR interval prolapse surgery OR pessary used after index surgery
TVL + C > 2 cm OR one of points Ba or Bp > +1 cm
C > ( −2/3 x TVL) OR one of points Ba or Bp > 0 cm
Anatomic (updated definition) OR symptomatic failure
SUI=Stress Urinary Incontinence; UI=Urinary Incontinence
NOTE: The original and updated versions of the anatomic failure could have different denominators for the same time point; this is because a prior anatomic failure means the patient is assigned a failure in all subsequent visits regardless of whether they have completed the visits.
Figure 2.
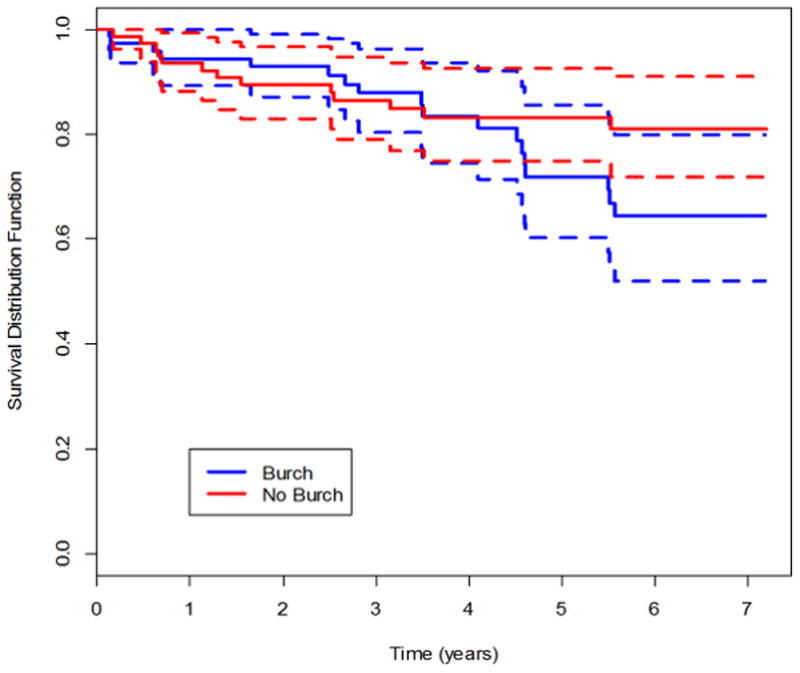
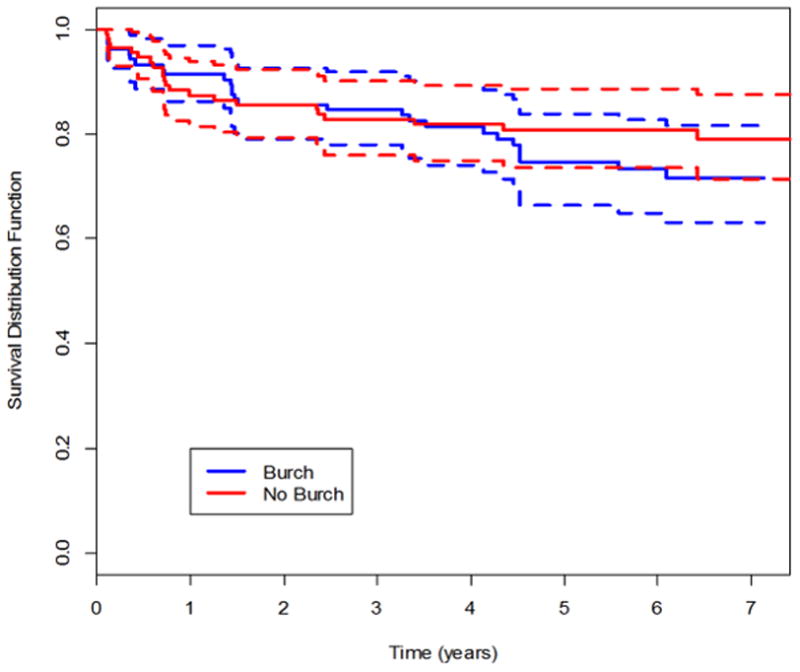
Kaplan-Meier survival curves for success of abdominal sacrocolpopexy in treating pelvic organ prolapse through year 7, using anatomic and symptomatic definitions of success. Figure 2a, updated anatomic success. Figure 2b, symptomatic success.
Figure 3.
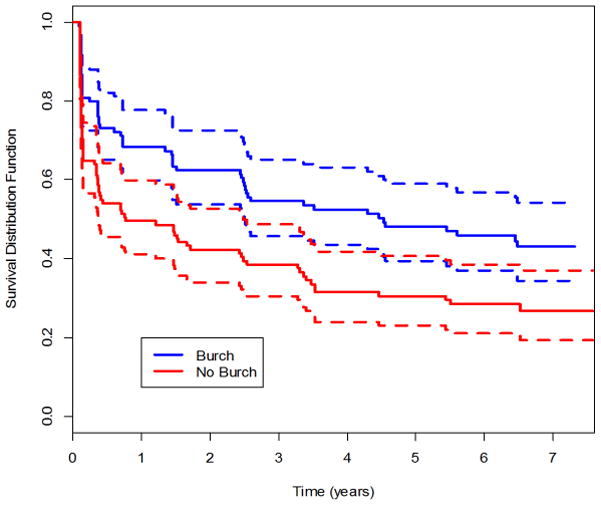
Kaplan-Meier survival curve for success for stress urinary incontinence (absence of SUI) through year 7.
Of the 31 anatomic POP failures, 11 involved the vaginal apex. Half (16 of 31) of anatomic failures denied POP symptoms and were not retreated. Similarly, about half (27 of 49) of symptomatic failures were not retreated and did not meet anatomic failure criteria.
The estimated probability of SUI was 0.62 for the urethropexy and 0.77 for the no urethropexy group (difference −0.153; −0.268, 0.030). The expected time to failure (incontinence) for the SUI outcome in the urethropexy group was 3.3 times (95% CI 1.27, 8.00) that of the no urethropexy group, 131.3 vs 40.2 months, respectively. (eFigure 3)
Patient reported outcomes are summarized in eTable 1. There were no significant interactions between treatment and visit. Five years after surgery, Urinary Distress Inventory scores for the stress and irritative subscales were lower, reflecting better outcomes, in the abdominal sacrocolpopexy with urethropexy than abdominal sacrocolpopexy without urethropexy group with no significant differences between groups for the obstructive subscale or for the POP Distress or Colo-rectal Distress Inventories. Sensitivity analyses produced results consistent with the initial analyses.
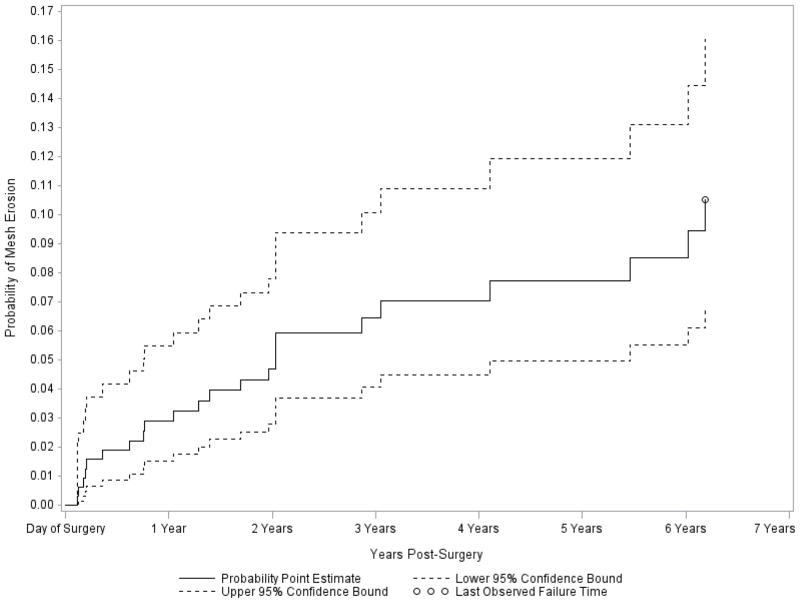
By year 2, three of the 322 women enrolled in CARE had suture and 17 had mesh erosion.18 There were six additional cases of mesh and one suture erosion in the E-CARE population by year 7. Erosions occurred with all mesh types placed. The estimated probability of mesh erosion in CARE and E-CARE at the time of the last known failure (6.18 years) was 10.5% (95% CI 6.8, 16.1) when the right censoring time of women without known mesh erosion was the last clinic visit (Figure 3); when the right censoring time was either the last clinic visit or last telephone interview (whichever came later), the estimated probability of mesh erosion was 9.9% (95% CI 6.5, 15.0). Of the 23 women with mesh erosions (11 in the urethropexy and 12 no urethropexy groups) in the CARE and E-CARE populations, 15 underwent excision in the operating room (13 via the vaginal and 2 via the abdominal route), 4 were given estrogen cream and 4 were asymptomatic. All suture erosions were managed by excision in the office. Seven and 13 women in the urethropexy and no urethropexy groups, respectively, underwent either surgery or urethral bulking agent injection for SUI surgery and 7 and 5 women in the urethropexy and no urethropexy groups, respectively, underwent either surgery or pessary for POP. By year 7, at least 36 of 215 women in E-CARE (16.7%) had additional surgery related to pelvic floor disorders, 11 for recurrent POP, 14 for SUI and 11 for mesh complications.
DISCUSSION
Three key points emerge from these data. First, as a gold standard for the surgical treatment of POP, abdominal sacrocolpopexy is less effective than desired. There is no consensus on defining cure after POP surgery and depending on definition, 2-year cure rates for abdominal sacrocolpopexy range from 19%, (“perfect” anatomic support) to 97% (no re-treatment for POP).19 For this study, we chose a clinically relevant definition of anatomic failure that some would argue is still not stringent enough, yet by 5 years, nearly one-third of women met our composite failure definition. However, 95% had no retreatment for POP. Despite progressive loss of anatomic support, abdominal sacrocolpopexy generally provides relief of prolapse symptoms. The low reoperation rate for POP may imply that women found the treatment adequate, but for older women other health and social concerns may assume primacy over vaginal bulge symptoms. Our ability to interpret the increased anatomic failure rate between 2 and 7 years is limited, given scant information about the natural history of vaginal support and our poor understanding of the pathophysiology of POP.
Second, surgical prevention of SUI at the time of abdominal POP surgery involves no clinically significant trade-offs identified to date. Our study is one of the few available that examine a surgical preventive strategy using a level 1 study design.20,21
Finally, we found that complications related to synthetic mesh continue to occur over time. Long-term follow-up is mandatory to understand the long-term patient burden associated with surgical materials and devices.
Generalizability of our findings is supported by the fact that 21 surgeons from 7 sites performed the study surgeries. Our 2-year anatomic failure and reoperation rates are consistent with those of a large body of literature22, 5 though our 5-year failure rates are higher than cited in the few smaller longer-term studies.23, 20 Indeed, we were surprised by the magnitude of failure rates after what is considered the “gold standard” reconstructive pelvic floor procedure. The lower success rate may in part be explained by the rigor of our data collection, with unbiased outcome assessment and use of validated outcome measures, or by non-standardized aspects of surgical technique. In addition, knowledge about the natural history of POP would allow us to further refine our concepts of “surgical failure” versus “progression of underlying POP disorder”.
Strengths of this study include the randomized design with masking of participants and outcome assessors to randomization assignment, ≥ 5 year follow-up, the multi-center nature of the study, and use of validated measures to assess anatomic and symptomatic outcomes. Additionally, all validated outcomes were assessed by trained study personnel and not by the surgeon.
The limitations of this research involve challenges seen in many long-term studies. Because of the nature of typical NIH network funding cycles, some sites were not renewed during the course of E-CARE and a decision was made not to continue follow up at those sites. This decreased our follow-up rate and limited the number of participants that could undergo physical examination after 2 years but patient reported outcomes in those other participants contributed to deflecting the lack of physical exams in all. Many observed differences were smaller than 15% and our study was not powered to detect these. We standardized the urethropexy procedure to which women were randomized but the surgical techniques used for the abdominal sacrocolpopexy reflected the variability of clinical practice. Conceivably, types, sizes and configurations of mesh, types and numbers of sutures, and other variations in techniques may influence success rates. With the shifting of contemporary clinical practice to mid-urethral slings and to abdominal sacrocolpopexy performed using laparoscopic or robotic approaches, it is unclear to what degree we can extrapolate these results to the newer procedures since we only evaluated open ASC and Burch urethropexy. However, short-term POP success rates are similar in case series between open and minimally invasive approaches.24 Our findings also cannot be extrapolated to other surgical techniques for POP.
Placing synthetic mesh transabdominally to treat POP requires balancing a need for greater effectiveness with the probability for more complications. While the popularity of transvaginal mesh kits used to treat POP dropped after the 2011 FDA safety communication, procedures in which mesh is placed transabdominally, as evidenced by our results, are not without problems that become apparent long after the surgery. We anticipate that continued research in mesh development will lead to new materials and applications with fewer adverse events, but our data highlight the importance of careful long-term evaluation of new devices. Comparative effectiveness trials, with long-term follow up of at least 5 years, are needed to compare the current “gold standard”, abdominal sacrocolpopexy, that we describe in this report to vaginal prolapse procedures with and without mesh augmentation.
Based on our results, women considering abdominal sacrocolpopexy can be counseled that this procedure effectively provides relief from POP symptoms; however, the anatomic support deteriorates over time. Adding an anti-incontinence procedure in women continent pre-operatively decreases, but does not eliminate, the risk of de novo stress urinary incontinence. Surgical counseling about the on-going risk of mesh-related events even for abdominal sacrocolpopexy is critical. Women should be aware that symptoms such as vaginal bleeding, discharge and pain may be due to mesh erosion and seek help accordingly.
Supplementary Material
Figure 4.
Kaplan-Meier failure curve for mesh erosion using last clinic visit as right censoring date Point estimate and 95% CI for probability of mesh erosion at the time of the last reported erosion (6.18 years): 0.105 (0.068, 0.161).
Acknowledgments
Supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the Office of Research on Women’s Health at National Institutes of Health (U01 HD41249, U10 HD41250, U10 HD41261, U10 HD41267, U10 HD54136, U10 HD54214, U10 HD54215, U10 HD54241, U10 HD054136, U10 HD054215, U10 HD041261, U10 HD041267, U10 HD069006, U10 HD054214, U10 HD041250, U01 HD069031).
Role of the Sponsors: Author Susan Meikle, employed by the National Institute of Child Health and Human Development played a role in the design and conduct of the study; the collection, analysis, and interpretation of the data; and in the preparation, review, and approval of the manuscript.
The authors gratefully acknowledge the expertise and assistance of Barry Eggleston, M.S. from RTI International, in creating the survival models used in analyses. Mr. Eggleston has provided written approval of this acknowledgement.
Footnotes
Trial Registration:
Trial registry: Clinicaltrials.gov
Registration number: NCT00099372
URL: http://www.clinicaltrials.gov/ct2/show/NCT00099372?term=E-CARE&rank=2
Disclosures within past 3 years:
None-Nygaard, Menefee, Fine, Cundiff, Brubaker, Ridgeway, Zhang, Klein Warren, Gantz, Meikle
Richter: Research Grant: Pelvalon, Astellas, Univ of CA/Pfizer, Pfizer; Consultant: Astellas Advisory Board, GlaxoSmithKline, Uromedica, IDEO, Pfizer, Xanodyne; Education Grant: Warner Chilcott
Visco: Consultant: Intuitive Surgical
Zyczynski: Consultant: Johnson & Johnson; Consultant: Key Tech
Responsibility: Authors Marie Gantz and Lauren Klein Warren had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Contributor Information
Ingrid Nygaard, University of Utah; Salt Lake City, UT.
Linda Brubaker, Loyola University Chicago; Chicago, IL.
Halina M. Zyczynski, University of Pittsburgh; Pittsburgh, PA.
Geoffrey Cundiff, University of British Columbia; Vancouver, BC.
Holly Richter, University of Alabama at Birmingham; Birmingham, AL.
Marie Gantz, RTI International; Research Triangle Park, NC.
Paul Fine, Baylor College of Medicine-Houston; Houston, TX.
Shawn Menefee, Kaiser Permanente San Diego; San Diego, CA.
Beri Ridgeway, Cleveland Clinic; Cleveland, OH.
Anthony Visco, Duke University; Durham, NC.
Lauren Klein Warren, RTI International; Research Triangle Park, NC.
Min Zhang, University of Michigan; Ann Arbor, MI.
Susan Meikle, Eunice Kennedy Shriver National Institute of Child Health and Human Development; Bethesda, MD.
References
- 1.Olsen AL, Smith VJ, Bergstrom JO, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501–506. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 2.Smith FJ, Holman CD, Moorin RE, Tsokos N. Lifetime risk of undergoing surgery for pelvic organ prolapse. Obstet Gynecol. 2010;116:1096–100. doi: 10.1097/AOG.0b013e3181f73729. [DOI] [PubMed] [Google Scholar]
- 3.Maher C, Feiner B, Baessler K, Glazener CMA. Surgical management of pelvic organ prolapse in women. Cochrane Database of Systematic Reviews 2010. (4):Art. No.: CD004014. doi: 10.1002/14651858.CD004014.pub4. [DOI] [PubMed] [Google Scholar]
- 4.Nygaard I, McCreery R, Brubaker L, et al. for the Pelvic Floor disorders network. Abdominal Sacrocolpopexy: A Comprehensive Review. Obstet Gynecol. 2004;104:805–23. doi: 10.1097/01.AOG.0000139514.90897.07. [DOI] [PubMed] [Google Scholar]
- 5.Subak LL, Waetjen LE, van den Eeden S, Thom DH, Vittinghoff E, Brown JS. Cost of pelvic organ prolapse surgery in the United States. Obstet Gynecol. 2001 Oct;98(4):646–51. doi: 10.1016/s0029-7844(01)01472-7. [DOI] [PubMed] [Google Scholar]
- 6.Brown JS, Waetjen LE, Subak LL, Thom DH, Van den Eeden S, Vittinghoff E. Pelvic organ prolapse surgery in the United States, 1997. Am J Obstet Gynecol. 2002 Apr;186(4):712–6. doi: 10.1067/mob.2002.121897. [DOI] [PubMed] [Google Scholar]
- 7.Boyles SH, Weber AM, Meyn L. Procedures for pelvic organ prolapse in the United States, 1979–1997. Am J Obstet Gynecol. 2003 Jan;188(1):108–15. doi: 10.1067/mob.2003.101. [DOI] [PubMed] [Google Scholar]
- 8.Wu JM, Kawasaki A, Hundley AF, Dieter AA, Myers ER, Sung VW. Predicting the number of women who will undergo incontinence and prolapse surgery, 2010 to 2050. Am J Obstet Gynecol. 2011 Sep;205(3):230.e1–5. doi: 10.1016/j.ajog.2011.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brubaker L, Cundiff GW, Fine P, et al. for the Pelvic Floor Disorders Network. Abdominal sacrocolpopexy with Burch colposuspension to reduce urinary stress incontinence. N Engl J Med. 2006;354:1557–66. doi: 10.1056/NEJMoa054208. [DOI] [PubMed] [Google Scholar]
- 10.Brubaker L, Nygaard I, Richter HE, et al. for the Pelvic Floor Disorders Network. Two-Year Outcomes After Sacrocolpopexy With and Without Burch to Prevent Stress Urinary Incontinence. Obstet Gynecol. 2008;112:49–55. doi: 10.1097/AOG.0b013e3181778d2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brubaker L, Cunduff G, Fine P, et al. A randomized trial of colpopexy and urinary reduction efforts (CARE): design and methods. Controlled Clinical Trials. 2003;24:629–642. doi: 10.1016/s0197-2456(03)00073-4. [DOI] [PubMed] [Google Scholar]
- 12.Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10–17. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 13.Barber MD, Kuchibhatla MN, Pieper CF, et al. Psychometric evaluation of 2 comprehensive condition-specific quality of life instruments for women with pelvic floor disorders. Amer J Obstet Gynecol. 2001;185:1388–1395. doi: 10.1067/mob.2001.118659. [DOI] [PubMed] [Google Scholar]
- 14.Sandvik H, Espuna M, Hunskaar S. Validity of the incontinence severity index: comparison with pad-weighing tests. Int Urogynecol J. 2006;17:520–4. doi: 10.1007/s00192-005-0060-z. [DOI] [PubMed] [Google Scholar]
- 15.Bradley CS, Nygaard IE. Vaginal wall descensus and pelvic floor symptoms in older women. Obstet Gynecol. 2005;106:759–66. doi: 10.1097/01.AOG.0000180183.03897.72. [DOI] [PubMed] [Google Scholar]
- 16.Slieker-ten Hove MC, Pool-Goudzwaard AL, Eijkemans MJ, Steegers-Theunissen RP, Burger CW, Vierhout ME. Prediction model and prognostic index to estimate clinically relevant pelvic organ prolapse in a general female population. International urogynecology journal and pelvic floor dysfunction. 2009;20:1013–21. doi: 10.1007/s00192-009-0903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutman RE, Ford DE, Quiroz LH, Shippey SH, Handa VL. Is there a pelvic organ prolapse threshold that predicts pelvic floor symptoms? Am J Obstet Gynecol. 2008;199:683, e1–7. doi: 10.1016/j.ajog.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cundiff GW, Varner E, Visco AG, et al. Risk factors for mesh/suture erosion following sacral colpopexy. Am J Obstet Gynecol. 2008 Dec;199(6):688.e1–5. doi: 10.1016/j.ajog.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barber M, Brubaker L, Nygaard I, et al. Defining “success” after surgery for pelvic organ prolapse. Obstet Gynecol. 2009;114(3):600–609. doi: 10.1097/AOG.0b013e3181b2b1ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neumann UP, Langrehr JM, Kaisers U, Lang M, Schmitz V, Neuhaus P. Simultaneous splenectomy increases risk for opportunistic pneumonia in patients after liver transplantation. Transplant International. 2002;15(5):226–32. doi: 10.1007/s00147-002-0399-8. [DOI] [PubMed] [Google Scholar]
- 21.Yoshizumi T, Taketomi A, Soejima Y, et al. The beneficial role of simultaneous splenectomy in living donor liver transplantation in patients with small-for-size graft. Transplant International. 2008;21(9):833–42. doi: 10.1111/j.1432-2277.2008.00678.x. [DOI] [PubMed] [Google Scholar]
- 22.Costantini E, Lazzeri M, Bini V, Del Zingaro M, Zucchi A, Porena M. Pelvic organ prolapse repair with and without prophylactic concomitant Burch colposuspension in continent women: a randomized, controlled trial with 8-year follow-up. J Urol. 2011;185:2236–2240. doi: 10.1016/j.juro.2011.01.078. [DOI] [PubMed] [Google Scholar]
- 23.Tate SB, Blackwell L, Lorenz DJ, Steptoe MM, Culligan PJ. Randomized trial of fascia lata and polypropylene mesh for abdominal sacrocolpopexy: 5-year follow-up. Int Urogynecol J. 2011;22(2):137–43. doi: 10.1007/s00192-010-1249-3. [DOI] [PubMed] [Google Scholar]
- 24.Siddiqui NY, Geller EJ, Visco AG. Symptomatic and anatomic 1-year outcomes after robotic and abdominal sacrocolpopexy. Am J Obstet Gynecol. 2012 May;206(5):435.e1–5. doi: 10.1016/j.ajog.2012.01.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.