Abstract
OBJECTIVE
To compare three interventions to reduce diabetes distress (DD) and improve self-management among non–clinically depressed adults with type 2 diabetes mellitus (T2DM).
RESEARCH DESIGN AND METHODS
In REDEEM, 392 adults with T2DM and DD were randomized to computer-assisted self-management (CASM), CASM plus DD-specific problem solving (CAPS), or a computer-administered minimal supportive intervention. Primary outcomes were Diabetes Distress Scale (DDS) total, the Emotional Burden (EB) and Regimen Distress (RD) DDS subscales, and diet, exercise, and medication adherence.
RESULTS
Significant and clinically meaningful reductions in DD (DDS, EB, and RD) and self-management behaviors occurred in all three conditions (P < 0.001), with no significant between-group differences. There was, however, a significant group × baseline distress interaction (P < 0.02), in which patients with high baseline RD in the CAPS condition displayed significantly larger RD reductions than those in the other two conditions. RD generated the most distress and displayed the greatest distress reduction as a result of intervention. The pace of DD reduction varied by patient age: older patients demonstrated significant reductions in DD early in the intervention, whereas younger adults displayed similar reductions later. Reductions in DD were accompanied by significant improvements in healthy eating, physical activity, and medication adherence, although not by change in HbA1c.
CONCLUSIONS
DD is malleable and highly responsive to intervention. Interventions that enhance self-management also reduce DD significantly, but DD-specific interventions may be necessary for patients with high initial levels of DD. Future research should identify the minimal, most cost-effective interventions to reduce DD and improve self-management.
Diabetes distress (DD) refers to the often hidden emotional burdens, stresses, and worries that are part of managing a demanding, progressive, chronic disease like diabetes (1). The point prevalence of moderate and high DD is as high as 45.4% of type 2 diabetic (T2DM) adults in community settings (2), and DD has displayed significant linkages with poor glycemic control, self-management, and self-efficacy, independent of clinical depression (3–6). Approximately 70% of high-DD type 2 patients do not reach criteria for clinical depression (4).
DD is a critical, yet often neglected, area of comprehensive care for patients with diabetes. Because at least 70% of these patients are not clinically depressed (3), i.e., do not meet criteria for major depression or dysthymia, traditional behavioral and pharmacological interventions for depression may not be warranted. Interventions to reduce DD directly, based primarily on cognitive-behavioral approaches, have yielded mixed results (7,8). Several meta-analyses of this literature indicate that, even though some studies report reduced DD, the overall effect size on glycemic control is variable across studies and quite modest (9). Thus, few practical interventions directed specifically at adults with type 2 diabetes and DD without clinical depression have been developed and evaluated.
Our overview of the literature indicates that two strategies of DD intervention have been considered. One strategy suggests that, if effective, interventions to improve self-management behavior will also reduce DD, since the worries and concerns regarding poor disease management will be reduced as well. Programs to improve self-management have been shown to be effective, can be inexpensive, and are easily available for large patient populations (10), but their direct effects on reducing DD have not been demonstrated. A second strategy targets remediation of the very diabetes worries and concerns that underlie DD (7,11). Conceptually, this strategy suggests that as the distress that results from unsuccessful diabetes management increases over time (12), attention narrows, behavioral options become reduced, and alternative approaches and creative solutions become limited (13). An increasing spiral of poorer management and increased distress occurs that leads to even poorer coping (14,15). This pattern hampers the acquisition of new knowledge and skills, accelerates the development of unrealistic goals and expectations, and fosters inaccurate personal beliefs and perceptions that become self-defeating—all common reactions of distressed individuals facing a demanding and progressive chronic disease like diabetes (14). Thus, this perspective suggests that addressing the underlying emotional themes directly tied to DD may be crucial. It remains unclear, however, whether an intervention that targets DD directly will add significantly to the effects that result from improvements in disease-management programs alone (16).
Reducing Distress and Enhancing Effective Management (REDEEM) was a 12-month, three-arm comparative, pragmatic randomized clinical trial for distressed, non–clinically depressed adults with type 2 diabetes who reported difficulties with diet, physical activity, or medication taking. We compared the effectiveness of a general, Web-based, behavioral self-management support program, computer-assisted self-management (CASM), with or without problem-solving therapy (PST), a program to reduce DD directly by improving diabetes-specific problem-solving skills (17), relative to a minimum, largely automated (18) general diabetes support and education condition (Leap Ahead). This additive approach was used to determine whether a combined behavioral and targeted DD intervention program, CASM plus PST (CAPS), was necessary to reduce DD or whether improved disease management alone was sufficient. We address the following research questions in this report: 1) Does an intervention that addresses improvements in disease management also reduce DD, relative to a general, minimal intervention diabetes support and education program? 2) Does an intervention that targets DD directly add significantly to a self-management program in reducing DD? 3) Are programs to improve self-management (e.g., healthy eating, exercise) effective for distressed patients with type 2 diabetes?
RESEARCH DESIGN AND METHODS
Patients with type 2 diabetes and DD were recruited from the patient registries of several community medical groups and diabetes education centers. Inclusion criteria were a registry-recorded diagnosis of type 2 diabetes ≥12 months, a mean score of ≥1.5 on the two-item Diabetes Distress Screener (19) (confirmed later by the full scale) to indicate at least moderate DD (2,20), age ≥21 years, ability to read and speak English, at least moderate computer use facility, easy availability of a computer with internet access, comfort with internet use, and self-reported problems with diabetes management (healthy eating or exercise plan not followed in 3 of 4 days during the previous week or medications not taken 2 or more days during the previous week, based on the Summary of Diabetes Self-Care Activities [21]). Exclusion criteria included clinical depression (Patient Health Questionnaire 8 score ≥15 [22]) and severe diabetes complications or functional deficits (e.g., dialysis, blindness).Patients received a letter from their health care facility, signed by a facility clinician and project representative, informing them of the project. They were told that a REDEEM representative would telephone them to explain the project further unless they opted out by calling an 800 number or by returning an enclosed postcard. During a follow-up call, the project was explained, patients were screened, and eligible patients were invited to a meeting at our project office or at another convenient location (e.g., library, patient’s home). At the meeting, eligibility requirements were confirmed, informed consent was obtained, and a 1.5-h baseline assessment was completed (A0) that included height and weight, questionnaires, brief interview, and visit to a community laboratory for collection of biological data. Patients were then randomized individually to one of the three study arms using a computer-generated algorithm, and an intervention visit was scheduled within 2 weeks. In keeping with a pragmatic design and comparative effectiveness research (23), no usual care condition was included because of concerns about maintaining highly distressed patients in a noninterventional study arm. Assessments were repeated at 4 (A4) and 12 (A12) months postintervention. Three nonprofessional college graduate interventionists were trained and closely supervised by the investigators to deliver each of the three interventions and the telephone calls. A separate team of assistants undertook A0, A4, and A12 assessments.
CASM
Patients randomized to CASM were introduced to “My Path To A Healthy Life,” a 40-min, previously validated, Web-based diabetes self-management improvement program (16,24). Patients selected achievable goals for medication adherence, diet, or exercise and were shown how to monitor their daily progress on the site. They received immediate feedback on their success over the past 7 days. The predominately Web-based intervention also provided an ask-the-expert forum to enhance engagement (25). After 6 weeks, patients completed an “action plan” for each previously prioritized management problem. Also included was a list of personalized barriers and strategies to overcome barriers. Patients received four live phone calls from their interventionist at weeks 2, 4, 7, and 12 to check progress and problems regarding their use of CASM and to provide encouragement to continue their efforts. At month 5, patients received an automated “behavior chain” booster program to reduce negative behavioral practices. This interactive component involved illustrative scenarios of prototypic patients experiencing “chains of events,” e.g., negative thinking that triggered overeating, followed by an exercise to help “break” the sequence (7,10). Finally, patients received four more live 15-min phone calls at weeks 24, 28, 34, and 48.
CAPS.
Patients randomized to CAPS received a 60-min in-person intervention that included CASM plus PST. PST is an eight-step process to identify and define DD, establish realistic goals, generate ways to meet these goals, weigh the pros and cons of each, choose and evaluate solutions, create a DD action plan, evaluate outcome, and engage in pleasant activities (see pstnetwork.ucsf.edu) (26,27). As in CASM, CAPS patients received four live phone calls between A0 and A4 and between A4 and A12 to check progress on CASM and PST, respond to problems, and provide encouragement and a live supplemental booster session at month 5 (a review of the PST steps).
Leap Ahead
Patients randomized to Leap Ahead, a minimal intervention in comparison with the other two conditions, received a 20-min, computer-delivered health risk appraisal (e.g., seat belt and sunscreen use) along with diabetes information regarding healthy living, diet, and physical activity (28) preceding each of the eight calls between A0 and A12. The materials delivered diabetes information only, and patients were not directed to use the information to engage in a specific or structured program of self-management or DD change. Patients received a repeat of the risk appraisal at month 5, the same number and sequence of subsequent live phone calls to answer questions about provided diabetes management information, and assessments similar to those of CASM and CAPS.
The University of California, San Francisco, Institutional Review Board and the committees of collaborating institutions approved this study. Data were collected between 2008 and 2011 and analyzed in 2012.
Measures
Patient demographic variables included age (continuous variable), sex, race (white vs. nonwhite), and education (years); diabetes status included use of insulin (yes vs. no), years since diagnosis, and number of comorbidities and complications derived from a list of 22 common diabetes-related health problems.
The primary dependent variable was DD, which was assessed by the 17-item Diabetes Distress Scale (DDS) (20) (α = 0.87), the five-item Regimen Distress (RD) DDS subscale (α = 0.90), and the five-item Emotional Burden (EB) DDS subscale (α = 0.88). RD and EB were selected because they were directly targeted by the interventions. Items that reflect areas of distress are rated on a 6-point Likert scale from “not a problem” to a “very serious problem.” RD items include feeling that I am often failing with my diabetes regimen and feeling that I am not sticking closely enough to a good meal plan; EB items include feeling overwhelmed by the demands of living with diabetes and feeling that diabetes controls my life. Mean item scores from 2.0 to 2.9 reflect moderate distress, and scores ≥3 are considered high distress (2). Physical activity was assessed by the Community Health Activities Model Program For Seniors (29). It measures weekly caloric expenditure of light, moderate, and heavy physical activity. Only the light physical activity variable was used in the present analyses because it most frequently reflected levels reported by patients. Healthy eating was assessed by the NCI Percent Energy From Fat Screener (30), which estimates percent energy (calories) from fat based on consumption of 14 foods. Medication adherence was assessed by the eight-item Hill-Bone Compliance Scale (α = 0.80) (31) that assesses how often and why respondents miss taking medications, rated on a 4-point scale from “none of the time” to “all of the time.” Glycemic control was assessed by HbA1c, which was analyzed in a central laboratory for all participants and natural log transformed to reduce skew and kurtosis.
Data analysis
Descriptive statistics were computed to review score distributions. Missing data were imputed with multiple imputation procedures using NORM, version 2, software (32). NORM imputes data via an expectation-maximization algorithm, which provides efficient estimation of mean, variances, and covariances and uses a data-augmentation procedure that generates multiple imputations of missing values. Variables within a limited range were logit transformed to ensure that imputed values also fell within that range. The same imputed dataset was used for all analyses to ensure continuity of results.
One-way ANOVA and χ2 tests, as appropriate, were conducted to test for baseline differences across the three treatment conditions and to examine differences in outcomes between dropouts and continuing participants. Repeated-measures ANOVA models were used to test for change across time in the outcome variables (DDS, RD, EB, light physical activity, fat intake, medication adherence, and log-transformed HbA1c). Difference scores were calculated for outcomes, with earlier scores subtracted from later scores: A0–A4, A0–A12, and A4–A12. For each difference-score outcome, a series of ANOVA models was specified to test for main treatment effects both in the absence of and with covariates, main effects of treatment and predictors, and interactions between intervention group and baseline demographic variables, disease status variables, and baseline value of outcome. Given the large number of interactions tested, only variables for which a consistent pattern of significant interaction terms emerged were retained in the final models. Statistical analyses were performed using SPSS 19.0 (SPSS, Chicago, IL).
RESULTS
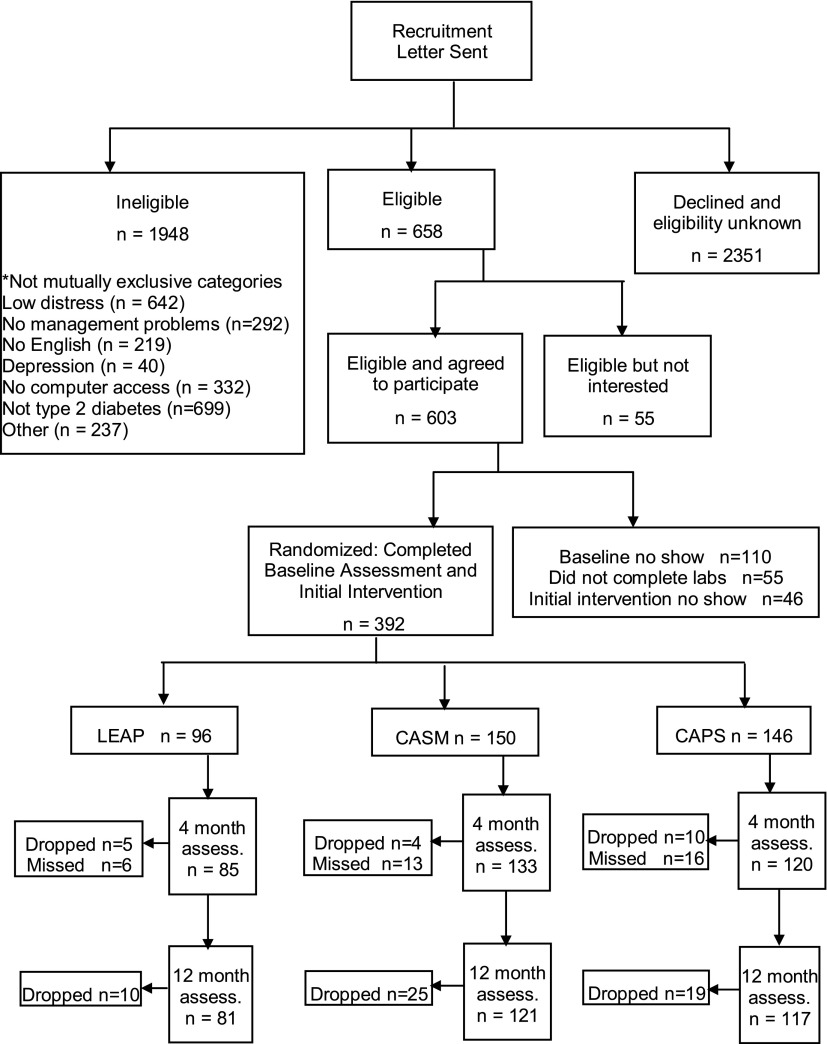
Of 2,606 patients identified from registries whom we could contact, 658 were eligible and 436 agreed to participate (66.6%) (Fig. 1). Problems with time and conflicting life demands were the most frequent reasons for nonparticipation. Of these, 392 completed baseline assessment and intervention (89.5%), with 150 randomized to CASM, 146 to CAPS, and 96 to Leap Ahead. The smaller Leap Ahead sample was built into the randomization allocation algorithm to provide more power to compare the two active arms. Based on telephone screening data, there were no significant differences between those contacted who participated and those who refused.
Figure 1.
Consort diagram of REDEEM study participation and retention. “Missed” indicates the participant missed the stage, but this did not preclude them from participating in a later stage of the project. assess., assessment.
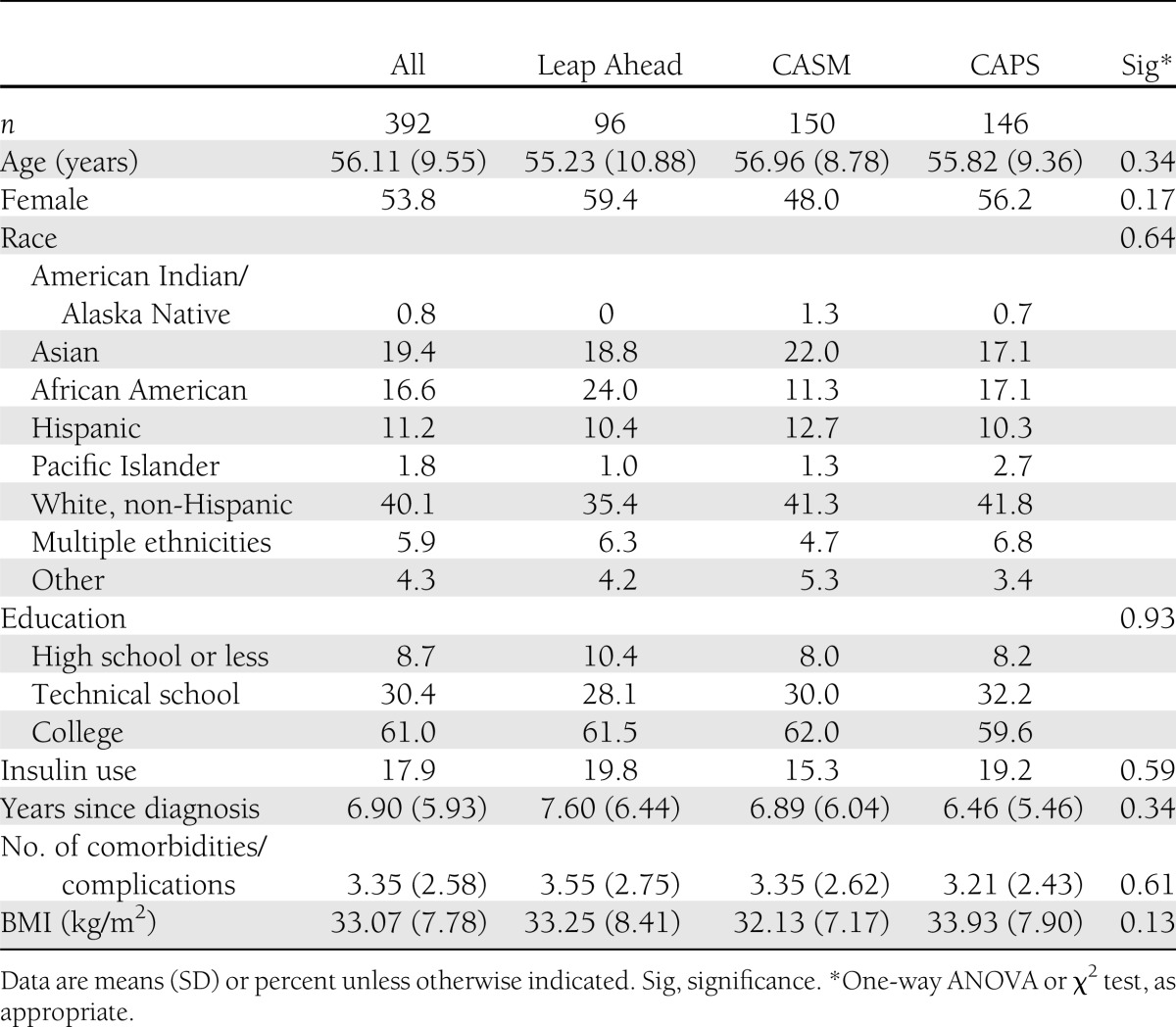
Attrition was 13.8% from A0 to A4, 5.7% from A4 to A12, and 18.7% from A0 to A12. Only 8.4% of patients missed both A4 and A12 follow-up assessments. There were no significant between-group differences in attrition across any time period on any key study variable. There were also no significant baseline differences among the three study groups on any key demographic or diabetes status variable (Table 1). The diverse sample had a mean age of 56 ± 9.6 years (range 21–75), 53.8% of the sample was female, 8.7% of patients had ≤12 years of education, and mean (SD) baseline HbA1c was 7.4% (1.61) (57.0 mm/mol [17.6]).
Table 1.
Baseline characteristics of participants by intervention group (N = 392)
Changes in DD
Significant and clinically meaningful reductions in DDS, RD, and EB occurred across the three study groups from A0 to A4 and A0 to A12 (Table 2). Also, significant reductions in DD scores between A4 and A12 occurred for all groups both after adjusting and not adjusting for changes between A0 and A4, indicating continued improvement over time. There were no significant between-group differences in these reductions at any time point for DDS, RD, or EB. Patients with higher DDS, RD, and EB baseline scores consistently displayed the largest reductions in distress from A0 to A4 and A0 to A12. Older patients showed larger reductions in RD and EB than younger patients from A0 to A4, but younger patients showed larger RD and EB score reductions than older patients from A4 to A12.
Table 2.
Predictors of diabetes distress
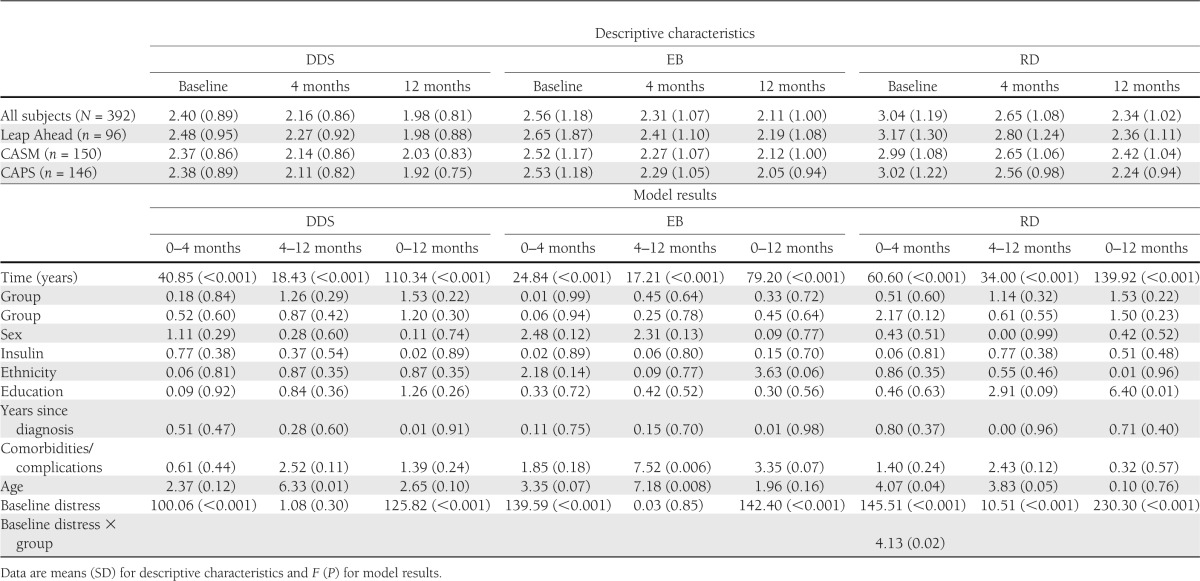
There was a significant group × baseline distress interaction for RD from A0 to A4 such that CAPS patients with high baseline RD displayed significantly larger RD reductions than high-baseline RD patients in Leap Ahead (P = 0.005) and marginally larger reductions than those in CASM (P = 0.08). Of patients classified as high DD on the DDS at baseline (n = 95), by 12 months 27.4% fell into the moderate range and another 34.7% displayed little or no DD. Thus, categorical changes in DDS occurred for 62.1% of initially high DD patients, with no between-group differences (P = 0.92). Among those who displayed moderate DD at baseline (n = 147), 60.5% reported little or no DD at 12 months. Similar reductions occurred for both RD and EB.
Changes in self-management behavior
Healthy eating.
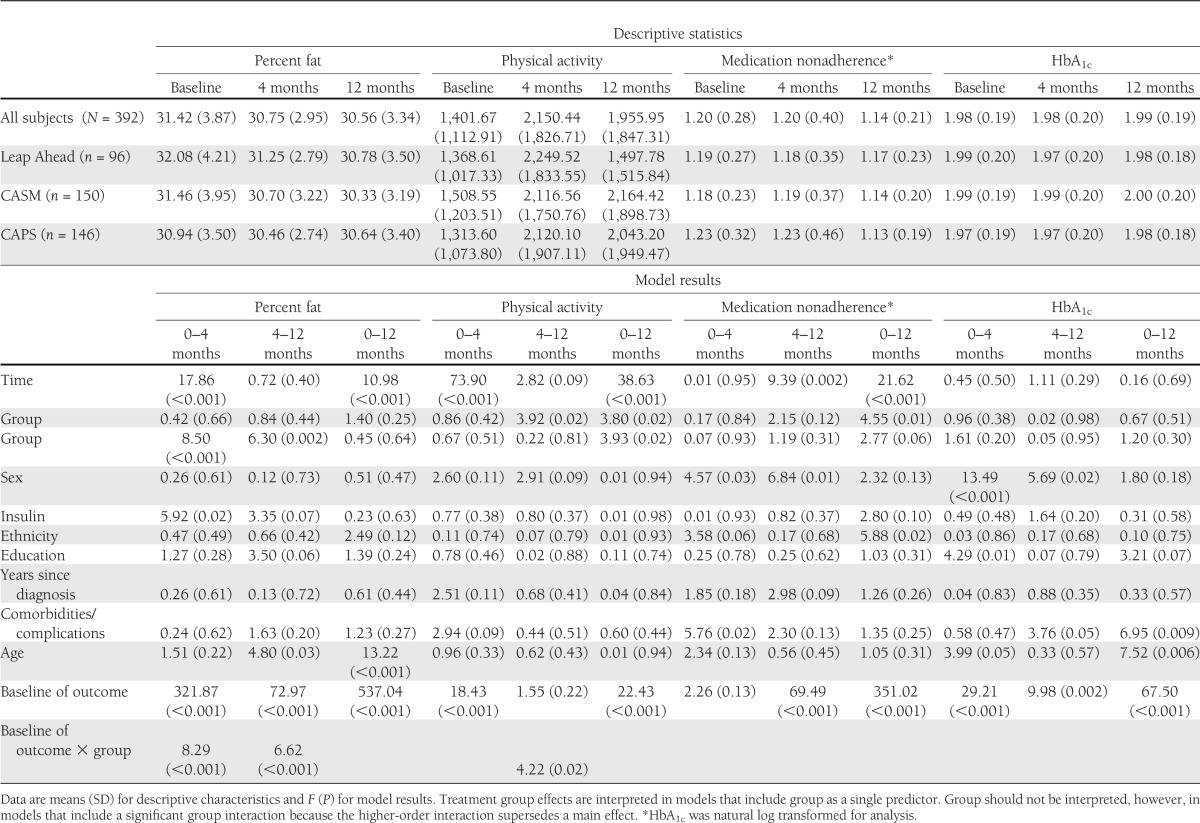
Significant reductions in percent of total calories from fat occurred across the entire sample from A0 to A4 and from A0 to A12 but not from A4 to A12 (Table 3). This suggests that gains that occurred primarily during the initial period of the intervention were maintained over time. There were no significant between-group differences. Older participants and those with higher baseline fat consumption showed the greatest improvements. There was a significant group × baseline percent fat interaction from A0 to A4 and A4 to A12. CAPS and Leap Ahead patients with higher percent fat at baseline demonstrated greater decreases from A0 to A4 than CASM patients. The converse was found from A4 to A12, during which stronger associations were found for CASM than Leap Ahead or CAPS (P = 0.02 and P < 0.001, respectively).
Table 3.
Predictors of behavioral and HbA1c outcomes
Physical activity.
Physical activity increased significantly across the entire sample between A0 and A4 and between A0 and A12 but not between A4 and A12. This suggests that the gains that occurred during the initial part of the intervention were maintained between A4 and A12. Significant treatment group effects were found at A4–A12 and A0–A12, indicating that CASM and CAPS patients maintained their A0–A4 gains through 12 months, whereas Leap Ahead patients reverted back toward baseline levels during this period. Patients with lower baseline physical activity had the largest increases in physical activity from A0 to A4 and A0 to A12. From A4 to A12, there was a significant group × baseline physical activity interaction: among patients with relatively higher baseline physical activity, there were greater reductions in physical activity for patients in Leap Ahead than in CASM (P = 0.005) or CAPS (P = 0.05).
Medication adherence.
Across the full sample, significant improvements in medication adherence occurred between A4 and A12 and A0 and A12 but not A0 and A4. These findings suggest that improvements in medication adherence occurred primarily in the later segments of the study. There was a significant A0–A12 treatment group effect, indicating that patients in CAPS had more improvement in medication adherence than those in CASM (P = 0.02) and Leap Ahead (P = 0.006). Patients who were female and who were nonwhite and those with poor baseline medication adherence showed the largest improvements.
HbA1c.
No significant time or group main effects were found for log-transformed HbA1c. However, patients who were older (from A0–A4 and A0–A12), who had fewer comorbidities/complications (from A4–A12 and A0–A12), and who had higher baseline HbA1c (across all time points) showed the greatest HbA1c improvements over time.
CONCLUSIONS
Regarding our first two research questions, we found that DD and the distress specifically associated with diabetes regimen and emotion management are malleable and highly responsive to intervention. The reductions in DD are most apparent when viewing the categorical data. Approximately 33% of patients who reported high DD at baseline and 60% of patients who reported moderate DD at baseline reported little or no DD at 12 months. More specifically, we find that interventions that target enhanced self-management (CASM) reduce DD significantly but that DD-specific interventions (CAPS) may be necessary for patients with initially high levels of RD. RD generates the most distress and displays the greatest distress reduction as a result of intervention. Thus, for those with initially high RD (mean score ≥3), which is a significant portion of the type 2 diabetes population (31.2%) (33), programs that focus on improving disease management alone may be insufficient; a specific focus on RD may be required.
We also find in general that interventions decrease DD, especially RD, the most for patients with initially high DD. Because high DD is significantly correlated with poor disease management, HbA1c, and functional deficits (1), patients displaying these characteristics are at greatest risk for high DD.
Interestingly, the effects are not moderated by other patient demographic or diabetes-related variables, suggesting that the findings may generalize to most patients with diabetes. Furthermore, the pace of reduction in RD as a result of intervention appears different for older and younger patients: older patients show significant reductions in the early phases of the intervention, whereas younger patients respond later. This suggests that younger patients, who may be managing more non–disease-related stresses and life problems (34), may require greater exposure to distress-reduction programs over time than older patients. Last, we find that reductions in DD are maintained over time. Unlike many behavioral programs, in which return to baseline levels over time is common (35), reductions in DD can be sustained even with limited patient contact.
Where between-group differences emerged, those in CAPS displayed the greatest gains. Although several studies show that improved disease management is accompanied by reduced DD (10,36,37), younger patients, those with many comorbidities and complications, those with poorer initial functioning, and those having less education may require direct DD assistance. The cumulative effects of multiple stressors, life demands, and functional deficits may place substantive ongoing burdens on patients that reduce internal resources and require more distress-focused attention in clinical care (1).
Reductions in DD were accompanied by significant improvements in healthy eating, physical activity, and medication adherence, although not by change in HbA1c, a more distal and nontargeted outcome. Thus, even highly distressed, nondepressed patients with type 2 diabetes respond to disease-management interventions. Interestingly, consistent with other studies (38), improvements in healthy eating and physical activity occur early in the intervention for CASM and CAPS patients and are maintained subsequently without returning to baseline levels, whereas gains in medication adherence occur later in the intervention. Although these findings bear replication, among DD patients it may take longer for the behavioral contingencies that support medication taking to emerge than for diet and exercise. Furthermore, those in CAPS displayed significantly greater improvements in medication adherence than those in the other two groups, underscoring the important contribution of distress-focused interventions for highly distressed patients.
A somewhat surprising finding is that significant reductions in DDS, RD, and EB occur without a significant overall treatment group main effect, suggesting that the pattern of change in DD over time for all three groups is similar. This finding provides evidence that Leap Ahead, a minimal general health and diabetes education intervention (18), is for many patients as effective in reducing distress as CASM and CAPS. Taking about the same time as CASM and CAPS, Leap Ahead did not require, suggest, or encourage patients to change behavior; its goal was only to provide attention and modest health-related information delivery. Excluding initially highly distressed patients, for whom more distressed–focused interventions may be necessary, these findings suggest that most distressed patients are highly responsive to clinical staff attention, concern, and support both with and without the structure of formal programs to change behavior or enhance DD-related problem solving. Reducing distress, therefore, may have less to do with providing patients with programs of action and behavioral change and more to do with health care professionals listening to, understanding, acknowledging, and normalizing DD so that patients’ internal resources can become freer of internal distress–related constraints (1,14,15,39). Thus, programs to reduce DD may be relatively easy to implement and disseminate and require less skilled staff to administer, thus lowering potential costs.
This study had several strengths: a randomized comparative design; a validated, pragmatic, and low-cost Web-based intervention; delivery by nonprofessional staff; high program representativeness and reach (40); and use of a large community sample. Attrition from baseline to 12 months in a highly distressed sample was moderate (18%), was not differential among groups, and did not affect outcomes. Several issues, however, may reduce the impact of the results. First, the study design was additive, comparing CASM to CASM plus PST. We did not test the impact of PST alone (7). It may be that focused interventions to reduce DD may be most efficacious for many patients. We also did not include a usual care arm. Thus, we could not test the effect of attention alone. Finally, inclusion criteria did not include an HbA1C cut point. Mean baseline HbA1c was low (7.4%), making improvement as a result of intervention relatively difficult to demonstrate.
In conclusion, DD is highly prevalent among patients with type 2 diabetes and is linked with poor disease management and glycemic control, independent of clinical depression. This investigation showed that DD is highly responsive to intervention and that although interventions that improve self-management can also reduce distress, distress-specific interventions need to be delivered for patients with initially high levels of DD. Greater attention to DD within the context of self-management improvement programs and general clinical care is warranted.
Acknowledgments
This research was funded by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant DK061937.
No potential conflicts of interest relevant to this article were reported.
L.F., D.H., and R.E.G. reviewed the research data and wrote the manuscript. P.A.A., U.M., and D.N. reviewed and edited the manuscript. L.A.S. reviewed and edited the manuscript, ran the data analyses, and wrote the data analysis section. L.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
Appreciation is expressed to the following for their collaboration: Alta Bates Diabetes Education Center, Brown and Toland Medical Group, California Pacific Medical Center Diabetes Education Center, Hill Physicians Medical Group, and University of California, San Francisco, Lakeshore Medical Group.
Footnotes
Clinical trial reg. no. NCT00714441, clinicaltrials.gov.
The opinions expressed are those of the authors and do not necessarily represent those of the National Institutes of Health, National Cancer Institute, or the National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.Gonzalez JS, Fisher L, Polonsky WH. Depression in diabetes: have we been missing something important? Diabetes Care 2011;34:236–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher L, Hessler DM, Polonsky WH, Mullan JT. When is diabetes distress clinically meaningful?: establishing cut points for the Diabetes Distress Scale. Diabetes Care 2012;35:259–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care 2010;33:1034–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care 2010;33:23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsujii S, Hayashino Y, Ishii H, Diabetes Distress and Care Registry at Tenri Study Group Diabetes distress, but not depressive symptoms, is associated with glycaemic control among Japanese patients with type 2 diabetes: Diabetes Distress and Care Registry at Tenri (DDCRT 1). Diabet Med 2012;29:1451–1455 [DOI] [PubMed] [Google Scholar]
- 6.Zagarins SE, Allen NA, Garb JL, Welch G. Improvement in glycemic control following a diabetes education intervention is associated with change in diabetes distress but not change in doperessive symptoms. Behav Med 2012;35:299–304 [DOI] [PubMed] [Google Scholar]
- 7.van Son J, Nyklicek I, Pop VJ, Pouwer F. Testing the effectiveness of a mindfulness-based intervention to reduce emotional distres in outpatients with diabetes (DiaMind): design of a randomized controlled trial. BMC Public Health 2011;11:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welch G, Zagarins SE, Feinberg RG, Garb JL. Motivational interviewing delivered by diabetes educators: does it improve blood glucose control among poorly controlled type 2 diabetes patients? Diabetes Res Clin Pract 2011;91:54–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winkley K, Landau S, Eisler I, Ismail K. Psychological interventions to improve glycemic control in patients with type 1 diabetes: systematic review and meta-analysis of randomised controlled trials. BMJ 2006;55:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glasgow RE, Kurz D, King DK, et al. Twelve-month outcomes of an Internet-based diabetes self-management support program. Patient Educ Couns 2012;87:81–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartmann M, Kopf S, Kircher C, et al. Sustained effects of a mindfulness-based stress-reduction intervention in type 2 diabetic patients: design and first results of a randomized controlled trial (the Heidelberger Diabetes and Stress-study). Diabetes Care 2012;35:945–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazarus RS, Folkman S. Stress, Appraisal and Coping. New York, Springer, 1984 [Google Scholar]
- 13.Moskowitz JT. Positive affect at the onset of chronic illness: planting the seeds of resilience. In Handbook of Resilience. Reich JW, Zautra AJ, Hall JS, Eds. New York, Guilford, 2010, p. 465–483 [Google Scholar]
- 14.Frederickson BL, Branigan C. Positive emotions broaden the scope of attention and thought-action repertoire. Cogn Emotion 2005;19:313–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reich JW, Zautra A, Davis M. Dimensions of affect relationships: Models and their integrative implications. Rev Gen Psychol 2003;7:66–83 [Google Scholar]
- 16.Glasgow RE, Kurz D, King DK, et al. Outcomes of minimal and moderate support versions of an internet-based diabetes self-management support program. J Gen Intern Med 2010;25:1315–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nezu AM, Nezu CM, Perri MG. Problem solving to promote treatment adherence. In Promoting Treatment Adherence. O'Donohue WT, Levensky ER, Eds. Thousand Oaks, CA, Sage, 2006, p. 135–148 [Google Scholar]
- 18.Gierisch JM, DeFrank JT, Bowling JM, et al. Finding the minimal intervention needed for sustained mammography adherence. Am J Prev Med 2010;39:334–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher L, Glasgow RE, Mullan JT, Skaff MM, Polonsky WH. Development of a brief diabetes distress screening instrument. Ann Fam Med 2008;6:246–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polonsky WH, Fisher L, Earles J, et al. Assesing psychosocial stress in diabetes. Diabetes Care 2005;28:626–631 [DOI] [PubMed] [Google Scholar]
- 21.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care 2000;23:943–950 [DOI] [PubMed] [Google Scholar]
- 22.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zwarenstein M, Treweek S, Gagnier JJ, et al. CONSORT group. Pragmatic Trials in Healthcare (Practihc) group Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 2008;337:a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glasgow RE, Strycker LA, Kurz D, et al. Recruitment for an internet-based diabetes self-management program: scientific and ethical implications. Ann Behav Med 2010;40:40–48 [DOI] [PubMed] [Google Scholar]
- 25.Glasgow RE, Christiansen S, Kurz D, King DK, Wooley T, Faber AJ. Engagement in a diabetes self-management web-site: Usage patterns and generalizability of program use. J Internet Res 2010;13:9–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arean P, Hegel M, Vannoy S, Fan MY, Unuzter J. Effectiveness of problem-solving therapy for older, primary care patients with depression: results from the IMPACT project. Gerontologist 2008;48:311–323 [DOI] [PubMed] [Google Scholar]
- 27.Nezu AM. Problem solving and behavior therapy revisited. Behav Ther 2004;35:1–33 [Google Scholar]
- 28.Masharani U. Diabetes De-Mystified: A Self-Teaching Guide. New York, McGraw-Hill Books, 2007 [Google Scholar]
- 29.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc 2001;33:1126–1141 [DOI] [PubMed] [Google Scholar]
- 30.Thompson FE, Kipnis V, Subar AF, et al. Performance of a short instrument to estimate usual dietary intake of percent calories from fat. Eur J Clin Nutr 1998;52:S63 [Google Scholar]
- 31.Krousel-Wood M, Munter P, Jannu A, Desalvo K. Reliability of a medication adherence measure in an outpatient setting. J Med Sci 2005;330:182–183 [DOI] [PubMed] [Google Scholar]
- 32.Schafer, JL. NORM Users Guide: Multiple Imputation of Incomplete Multivariate Data Under a Normal Model. University Park, Pennsylvania, Methodology Center, Penn State, 1999, p. 1–69 [Google Scholar]
- 33.Fisher L, Mullan J, Skaff M, Glasgow R, Arean P, Hessler D. Predicting Disease Distress Among Primary Care Patients with Type 2 Diabetes: A Longitudinal Study. Diabet Med 2009;26:622–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hessler DM, Fisher L, Mullan JT, Glasgow RE, Masharani U. Patient age: a neglected factor when considering disease management in adults with type 2 diabetes. Patient Educ Couns 2011;85:154–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toobert DJ, Strycker LA, Barrera M, Glasgow RE. Seven-year follow-up of a multiple-health-behavior diabetes intervention. Am J Health Behav 2010;34:680–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorig K, Ritter PL, Villa F, Piette JD. Spanish diabetes self-management with and without automated telephone reinforcement: two randomized trials. Diabetes Care 2008;31:408–414 [DOI] [PubMed] [Google Scholar]
- 37.Zagarins SE, Allen NA, Garb JL, Welch G. Improvement in glycemic control following a diabetes education intervention is associated with change in diabetes distress but not change in depressive symptoms. J Behav Med 2012;35:299–304 [DOI] [PubMed] [Google Scholar]
- 38.Glasgow RE, Nutting PA, Toobert DJ, et al. Effects of a brief computer-assisted diabetes self-management intervention on dietary, biological and quality-of-life outcomes. J Chronic Illness 2006;2:270–283 [DOI] [PubMed] [Google Scholar]
- 39.Fisher L, Hessler DH, Naranjo D, Polonsky WH. AASAP: a program to increase recruitment and retention in clinical trials. Patient Educ Couns 2012;86:372–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glasgow RE. Enhancing the scientific foundation of internet intervention research. Ann Behav Med 2009;38:46–47 [DOI] [PubMed] [Google Scholar]