Abstract
OBJECTIVE
To examine whether chronotype and daily caloric distribution are associated with glycemic control in patients with type 2 diabetes independently of sleep disturbances.
RESEARCH DESIGN AND METHODS
Patients with type 2 diabetes had a structured interview and completed questionnaires to collect information on diabetes history and habitual sleep duration, quality, and timing. Shift workers were excluded. A recently validated construct derived from mid-sleep time on weekends was used as an indicator of chronotype. One-day food recall was used to compute the temporal distribution of caloric intake. Hierarchical linear regression analyses controlling for demographic and sleep variables were computed to determine whether chronotype was associated with HbA1c values and whether this association was mediated by a higher proportion of caloric intake at dinner.
RESULTS
We analyzed 194 completed questionnaires. Multiple regression analyses adjusting for age, sex, race, BMI, insulin use, depressed mood, diabetes complications, and perceived sleep debt found that chronotype was significantly associated with glycemic control (P = 0.001). This association was partially mediated by a greater percentage of total daily calories consumed at dinner.
CONCLUSIONS
Later chronotype and larger dinner were associated with poorer glycemic control in patients with type 2 diabetes independently of sleep disturbances. These results suggest that chronotype may be predictive of disease outcomes and lend further support to the role of the circadian system in metabolic regulation.
The circadian system, controlled by the master circadian clock located in the suprachiasmatic nuclei of the hypothalamus, plays a major role in regulating daily rhythms of sleep/wake and various metabolic outputs, such as feeding behavior, peripheral tissue metabolism, and hormone secretions (1–3). Despite having this genetically regulated master circadian clock, humans living in modern industrialized societies with 24-h access to light often engage in behaviors that are inappropriately timed relative to their endogenous circadian rhythms. This mismatch in timing is termed “circadian misalignment” and has been associated with a number of negative health outcomes. Night shift work is an example of severe circadian misalignment, as workers are awake, active, and eating during their circadian night and trying to sleep and fast during their circadian day. Epidemiologic studies reveal that shift work is associated with health problems including peptic ulcer disease, coronary heart disease, and metabolic syndrome, as well as certain types of cancers (4). In controlled laboratory studies, experimentally induced circadian misalignment in healthy human volunteers resulted in impaired glucose tolerance (5,6). In animal experiments, mice fed a high-fat diet during their inactive period gained significantly more weight than mice fed during their active phase, despite consuming the equivalent amount of calories (7). Taken together, these data suggest that severe circadian misalignment involving eating and sleeping at an abnormal circadian time leads to impaired energy metabolism.
Many individuals in modern society experience a form of mild circadian misalignment, especially during the work or school week as they follow social rhythms imposed by professional obligation, school schedules, family, and other commitments (8). The degree of misalignment is dependent on the individual’s “chronotype” (8). Chronotype is a construct that captures an individual’s preference for being a “morning” or “evening” person. Late chronotype is typically associated with a greater degree of misalignment between social rhythms and the circadian clock (8). This misalignment phenomenon has been termed “social jetlag,” as it resembles the condition experienced after traveling across time zones (8) and can be observed by comparing the difference in sleep timing between work/school days and free days. In a large population study, larger amounts of social jetlag were recently reported to be associated with higher BMI in overweight individuals (9). In addition, a recent study found that patients with type 2 diabetes had significantly later bedtimes and wake times than participants without diabetes, suggesting that chronotype may play a role in glucose metabolism (10).
In addition to chronic circadian misalignment, late chronotypes or “evening types” tend to minimize or skip breakfast (11,12). Therefore, the daily distribution of food intake may be mismatched with circadian-controlled metabolic rhythms. It is well recognized that glucose tolerance is worse in the evening (13), suggesting that eating late may result in adverse metabolic consequences. Indeed, a study of healthy volunteers reported that the amount of calories consumed after 8:00 p.m. predicted a higher BMI after controlling for sleep timing and duration (14), suggesting that the timing of food intake across the waking day is of metabolic relevance.
To date, little is known about chronotypic variations in patients with type 2 diabetes and the potential associations with glycemic control. There is abundant evidence that sleep disturbances such as short sleep duration and poor sleep quality are linked to the risk of diabetes and obesity, as well as glycemic control in subjects with type 2 diabetes (15,16), but little is known about the association between chronotype and metabolism independently of these sleep characteristics. The aim of this study was to examine whether chronotype was independently associated with glycemic control in patients with type 2 diabetes. We hypothesized that late chronotype would be associated with worse glycemic control independently of sleep disturbances. Because the distribution of food intake across the day is associated with chronotype, we also examined whether daily caloric distribution contributed to glycemic control. We hypothesized that a greater percentage of daily calories consumed at dinner would be associated with worse glycemic control.
RESEACH DESIGN AND METHODS
Adults with type 2 diabetes who were being followed in endocrinology or primary care clinics at Rush University Medical Center were invited to participate. Exclusion criteria included pregnancy, inability to understand English or give informed consent, or any neurological or physical impairment that required the participants to depend on others for feeding. Nursing home residents, institutionalized patients, and patients receiving alternate routes of nutrition such as tube feeding or parenteral nutrition were also excluded. All participants gave written informed consent. The protocol was approved by the institutional review board at Rush University Medical Center, Chicago, Illinois.
Assessments
After informed consent was obtained, self-reported age and race were recorded. Weight was measured. Height, current medications, and most recent HbA1c values were extracted from patient medical records. Research personnel interviewed participants about their diabetes history and management using the University of Chicago Diabetes/Quality of Life Survey (17). Depressive symptoms were assessed using the Center for Epidemiologic Studies-Depression (CES-D) Scale (18). Employment status was collected and categorized into employed (working full-time or part-time), retired, or unemployed.
Participants were also asked to complete a 24-h dietary recall to determine the content and time of their meals over the previous day. Specific details (e.g., portion sizes, brand names, etc.) were clarified by the interviewer. Calorie consumption for each meal was calculated using an online dietary database (www.livestrong.com). Participants categorized each entry as breakfast, lunch, dinner, or snack. Breakfast and dinner meals were defined as entries including at least one food item (drink-only entries, such as coffee only, were not defined as a meal). Percent of daily calories consumed at breakfast and dinner were computed and used as indicators of daily caloric distribution. Late evening snack was defined as any caloric intake between last meal of the day and sleep onset. Participants were grouped into those who consumed a late evening snack and those who did not.
Subjective sleep and circadian measures
The Pittsburgh Sleep Quality Index (PSQI) (19) was used to assess sleep quality over the previous month (with scores >5 indicating poor sleep quality). Participants were asked to report usual bedtime, wake-up time, sleep-onset latency, and actual sleep duration on weekdays and weekends over the previous month. Participants were also asked whether they had a diagnosis of obstructive sleep apnea (OSA), and those without a previous diagnosis completed the Berlin questionnaire to assess the risk of OSA, which categorizes respondents as high or low risk of having OSA (18). Participants who had a diagnosis of or were at high risk for OSA were identified together as a group with presence or high risk of OSA (OSA/risk).
The following circadian and sleep parameters were calculated. Mid-sleep time was calculated as the midpoint between sleep onset (bedtime plus sleep latency) and wake time. The primary outcome measure of mid-sleep time on free days (MSF), a metric of chronotype, was derived from mid-sleep time on weekend nights with further correction for calculated sleep debt as previously described (20,21). Specifically, the MSF equals the mid-sleep time on weekend nights subtracting 0.5 times the sleep debt, which is calculated as the difference between sleep duration (duration from sleep onset time until wake time) on weekends minus the weekly average sleep duration. This metric was first proposed by Roenneberg and colleagues with the assumption being that sleep timing on days when unconstrained by the social clock would more accurately reflect the underlying phase of the circadian system (22). Social jetlag, a behavioral indicator of circadian misalignment, was calculated based on the absolute difference between mid-sleep time on weekdays and weekends (9).
Sleep duration was computed using a weighted average of self-reported actual sleep duration between weekdays and weekends [(reported weekday actual sleep duration × 5 + reported weekend actual sleep duration × 2)/7]. In addition, because the perception of not getting enough sleep on weekdays has been shown to be better correlated with HbA1c than the reported sleep duration itself (15), perceived sleep debt was calculated using the difference between participants’ preferred weekday sleep duration (i.e., how many hours they would choose to sleep if their job, family, or other responsibilities did not limit the number of hours they slept) and their self-reported actual weekday sleep duration. Perceived sleep debt is a subjective variable that is likely to combine insufficient sleep duration and poor sleep quality.
Statistical analysis
All study data were checked for normality and presence of potential violations of statistical assumptions. HbA1c values were not normally distributed; therefore, the natural logarithm transformation of HbA1c was used in the analyses. HbA1c values were expressed as median (interquartile range). Diabetes duration was expressed as median (interquartile range) as well as categorized into ≤5, 6–10, 11–20, and >20 years. Diabetes complications were categorized as none or one or more. In order to account for a nonnormal distribution in social jetlag, this variable was categorized dichotomously as >30 min or ≤30 min.
For determination of the factors associated with glycemic control, Pearson correlations were used to explore the associations between the natural logarithm of HbA1c and continuous demographic, sleep, circadian, and dietary variables, while unpaired independent-samples t tests were used to analyze differences in the natural logarithm of HbA1c for dichotomous categorical variables. ANOVA was used to compare differences in the natural logarithm of HbA1c among demographic groups (according to diabetes duration and employment status).
For characterization of the participants according to chronotype, ANOVA was used to analyze the relationship between continuous demographic, metabolic, sleep, and dietary variables among quartiles of MSF. Tukey post hoc analyses were performed. When the variables were not normally distributed (diabetes duration, HbA1c, and social jetlag), Kruskal-Wallis tests were performed, while Mann Whitney U tests were used for post-hoc analyses. χ2 tests were used to analyze differences in categorical variables among quartiles of MSF.
For determination of whether chronotype was independently associated with glycemic control, a hierarchical multiple regression was performed to assess the association between MSF and HbA1c, controlling for demographic and sleep variables. Demographic variables (age, sex [male reference], race [nonwhite reference], BMI, diabetes complications, insulin use, and CES-D) were entered in the first step. The sleep variable significantly associated with HbA1c in the bivariate analyses (perceived sleep debt) was entered in the second step. In the final step, chronotype as assessed by MSF was entered. Collinearity analysis demonstrated no collinearity among the variables.
For determination of whether daily caloric distribution mediated the association between chronotype and glycemic control, four statistical analyses were completed according to the Baron and Kenny test for mediation (23). The first step was a multiple regression analysis to assess the association between MSF and HbA1c, controlling for demographic variables (age, sex [male reference], race [nonwhite reference], BMI, diabetes complications, insulin use, and CES-D). In steps 2 and 3, while controlling for demographics, the regression analyses were performed to assess the association between MSF and percentage of daily calories consumed at dinner and between percentage of daily calories consumed at dinner and HbA1c, respectively. In the final step, while controlling for demographics, both MSF and percentage of daily calories consumed at dinner were entered in multiple regression analysis to assess their associations with HbA1c. All analyses were performed using SPSS Statistics, version 19.0 (Chicago, IL).
RESULTS
A total of 194 participants (ages 18–85) completed the questionnaires. Baseline demographic characteristics, as well as sleep, circadian, and dietary parameters are shown in Table 1.
Table 1.
Descriptive demographic, circadian, sleep, and dietary data (n = 194 unless otherwise noted)

On average, the participants were obese and had a median diabetes duration of 11 years with a median HbA1c of 7.5% (58 mmol/mol). Slightly more than half (55.7%) were using insulin, and 71.1% had at least one diabetes complication. Circadian parameters revealed that average MSF was 3:29 a.m. and 31.4% had social jetlag of >30 min. Sleep quality was generally poor as reflected by the majority of participants (59.7%) having a PSQI score of >5. OSA/risk was present in 61.3% of the participants.
Regarding dietary parameters, 172 participants had breakfast entries, 180 had dinner entries, and 88 consumed a late evening snack. Participants consumed more of their daily calories at the dinner meal (37%) than at the breakfast meal (24%).
Association between HbA1c and demographics and circadian, sleep, and dietary parameters
Pearson correlations between glycemic control and demographics and circadian, sleep, and dietary parameters are shown in Table 2. Higher HbA1c levels were significantly correlated with younger age and more depressive symptoms. There were no differences in HbA1c levels between the sexes. Participants of nonwhite race had significantly higher HbA1c compared with those of white race (7.7% [6.8–9.1] vs. 7.0% [6.4–8.1]; 61 mmol/mol [51–76] vs. 53 mmol/mol [46–65], P = 0.04). There were no differences in HbA1c levels among employment status groups or when data were analyzed according to categories of diabetes duration. As expected, participants using insulin had higher HbA1c compared with noninsulin users (8.1% [7.3–9.4] vs. 6.8% [6.1–7.7]; 65 mmol/mol [56–79] vs. 51 mmol/mol [43–61], P < 0.001]. Participants with at least one diabetes complication had higher HbA1c than those without complications (7.7% [6.8–9.1] vs. 7.1% [6.3–8.3]; 61 mmol/mol [51–76] vs. 54 mmol/mol [45–67], P = 0.04).
Table 2.
Pearson correlation (r) between demographics and circadian, sleep, and dietary parameters and natural log of HbA1c (n = 194 unless otherwise noted)
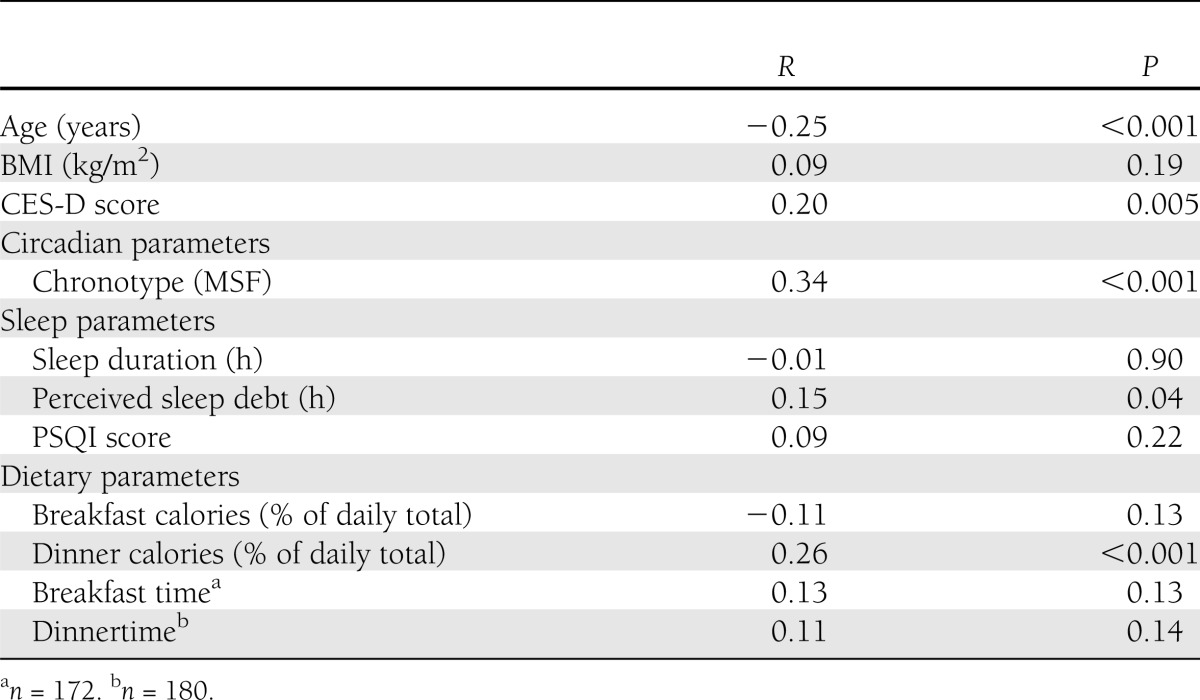
Higher HbA1c levels were significantly correlated with later MSF. HbA1c levels in subjects with social jetlag >30 min tended to be higher than in those with social jetlag ≤30 min, though this result was only a trend (7.7% [6.9–9.5] vs. 7.4% [6.6–8.5]; 61 mmol/mol [52–80] vs. 57 mmol/mol [49–69], P = 0.08).
Analyses of sleep parameters revealed that higher HbA1c levels were correlated with higher perceived sleep debt but not with self-reported sleep duration or PSQI scores. HbA1c levels were similar in participants with or without OSA/risk (7.6% [6.8–8.9] vs. 7.4% [6.6–9.1]; 60 mmol/mol [51–74] vs. 57 mmol/mol [49–76], P = 0.75).
Analyses of caloric distribution showed that percentage of daily calories consumed at dinner, but not breakfast, positively correlated with HbA1c levels. There was no correlation between timing of breakfast or dinner and glycemic control. There were no differences in HbA1c levels between participants who consumed late evening snacks and who did not.
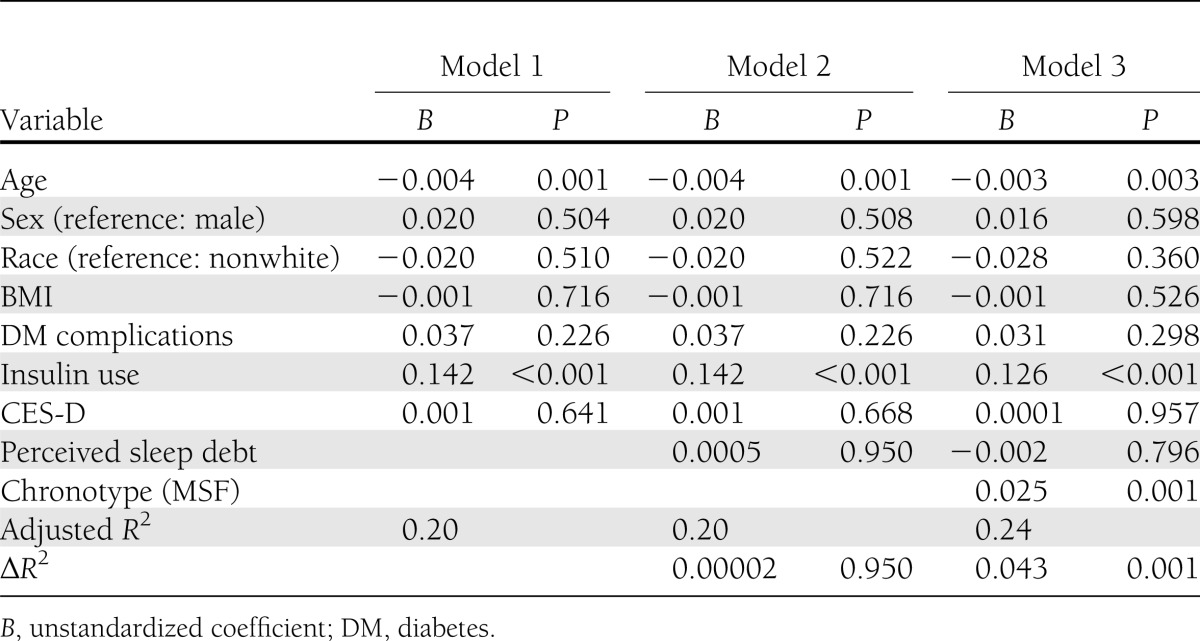
The hierarchical multiple regression to assess the association between MSF and HbA1c controlling for relevant demographic and sleep variables is presented in Table 3. Demographic variables explained 20% of the variance in HbA1c. Of the measured sleep variables, only perceived sleep debt was significantly correlated with glycemic control in bivariate analysis; this was added in the second step. Inclusion of this variable did not improve the explanatory power of the model (ΔR2=0.00002, P = 0.95). Chronotype as assessed by MSF was added in the final model and was significantly associated with HbA1c (unstandardized coefficient, B = 0.025, P = 0.001). Each hour delay in MSF was associated with an increase in HbA1c of 2.5% of its original value. Further, this model explained an additional 4.3% of the variance in HbA1c (ΔR2= 0.043, P = 0.001, total adjusted R2=0.24), which indicated that chronotype contributed significantly to the model’s explanation of the variance of HbA1c above and beyond demographic and sleep variables.
Table 3.
Hierarchical regression analysis with natural log of HbA1c as the outcome (N = 194)
Distribution of calories as a mediator between chronotype and HbA1c
A series of regression models was used to test for mediation, as previously described. In the first regression model, MSF was significantly associated with HbA1c (B = 0.025, P = 0.001), and in the second regression MSF was significantly associated with percentage of daily calories consumed at dinner (B = 0.024, P = 0.006). In the third model, percentage of daily calories consumed at dinner significantly predicted HbA1c (B = 0.0018, P = 0.005). Given that the first three steps were significant, the final and fourth step to examine mediation was completed, in which MSF and percentage of daily calories consumed at dinner were entered in one step and regressed on HbA1c along with covariates. Both MSF (B = 0.021, P = 0.005) and percentage of daily calories consumed at dinner (B = 0.0015, P = 0.02) were significantly associated with HbA1c, indicating that percentage of daily calories consumed at dinner only minimally partially mediated the relationship between chronotype and HbA1c, such that both remained independently associated.
Subjects’ characteristics in relation to chronotype
Since little is known about chronotypic characteristics in patients with type 2 diabetes, we explored the relationship among quartiles of MSF (Table 4). Participants with later chronotype tended to be younger, had a higher BMI, and had more depressive symptoms. There were no differences in sex or race distribution or employment status between groups.
Table 4.
Demographics and circadian, sleep, and dietary parameter comparisons by chronotype quartiles
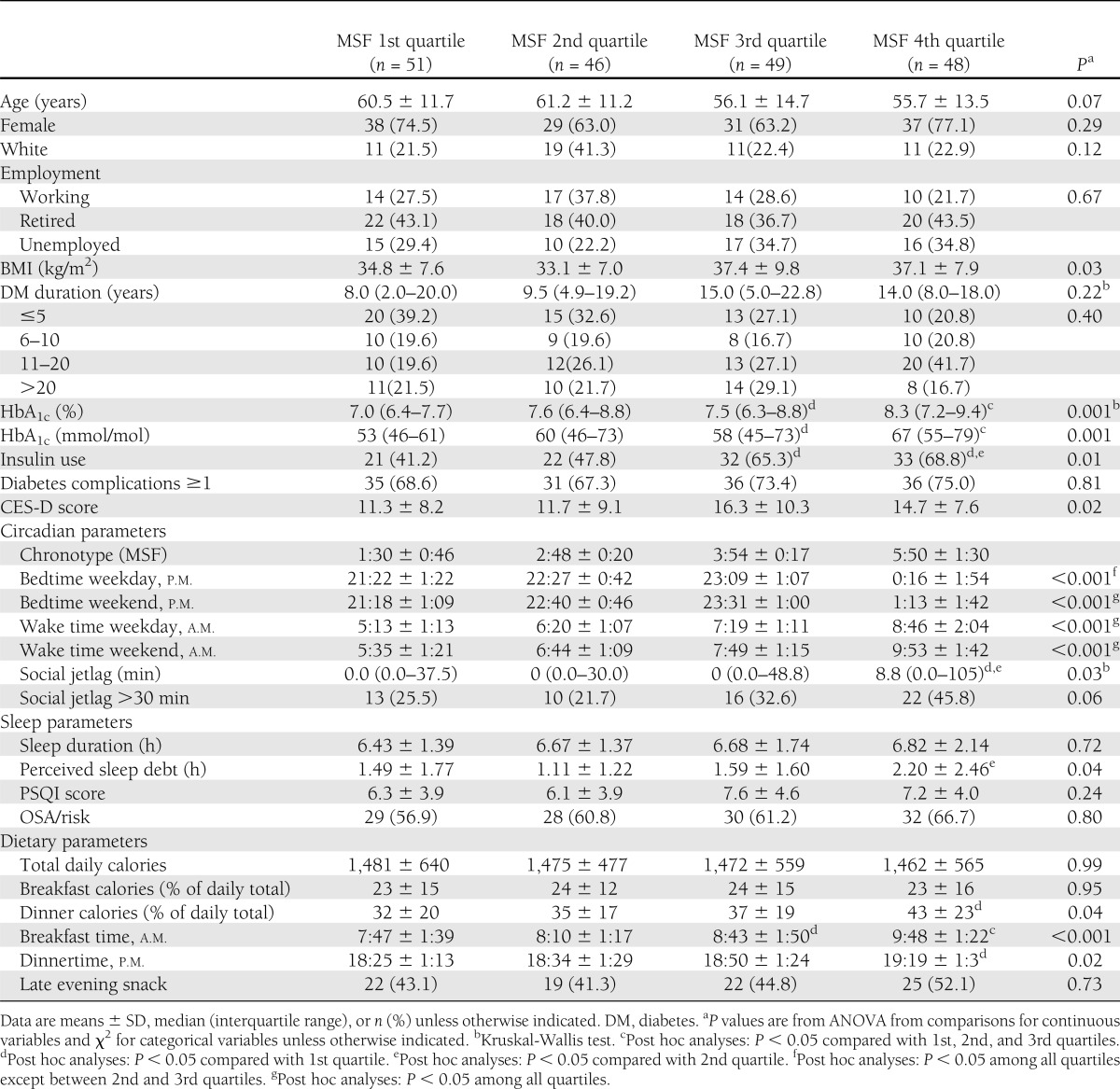
Participants with later chronotype had significantly higher HbA1c levels, and more were using insulin, while there were no differences in diabetes duration or percentage of participants with diabetes complications between groups.
The difference in MSF between 1st quartile of MSF and 4th quartile MSF was ~4.3 h. Participants with later chronotype had significantly later bedtime and wake-up time and more social jetlag than those with earlier chronotype. Interestingly, there were no differences between groups in their sleep duration or PSQI score. However, perceived sleep debt, a subjective measure that likely combines insufficient sleep duration and poor sleep quality, was higher in participants with later chronotype.
While there were no differences in total daily caloric intake between groups, participants with later chronotype ate more of their daily calories at dinner and consumed breakfast and dinner at a significantly later time than those with earlier chronotype.
CONCLUSIONS
The current study demonstrates for the first time that chronotype is associated with glycemic control in patients with type 2 diabetes, independently of perceived sleep debt, a subjective measure of insufficient sleep duration and poor sleep quality. Each hour delay in MSF was associated with a modestly but significantly higher HbA1c of 2.5% of its original value after adjusting for age, sex, race, BMI, depressive symptoms, diabetes complications, insulin use, and sleep variables. For example, given similar demographic characteristics, an HbA1c level of 8% (64 mmol/mol) is expected to increase to 8.8% (73 mmol/mol) if MSF is 4 h later, which was the difference between the earliest and latest chronotype quartiles in this study. In addition, late chronotypes consumed a greater percentage of their daily caloric intake at dinner compared with early chronotypes, but the percentage of daily calories consumed at dinner only minimally mediated the association between chronotype and glycemic control, as the association with chronotype was only slightly reduced after adjustment for daily calorie distribution at dinner. Late chronotypes were associated with later bedtimes and wake-up times and a greater degree of social jetlag than early chronotypes; however, social jetlag itself was not found to be significantly associated with HbA1c.
In addition, subjects with late chronotypes were found to be significantly younger and reported more depressive symptoms. These findings in our participants with type 2 diabetes are consistent with those in larger population-based studies (20,24). Interestingly, participants in the upper (latest) quartile of chronotype ate breakfast only ~2 h later and dinner only 1 h later than participants with an early chronotype despite having MSF that was 4 h later. This may reflect the need to conform to a typical social schedule, especially at dinner, which is generally considered a family meal. The meal timing itself, however, was not found to be associated with glycemic control.
Neurohormonal and metabolic dysregulations due to experimentally induced circadian disruptions have been demonstrated in laboratory studies of healthy volunteers. Severe circadian misalignment, involving sleep-wake and meal schedules ~12 h out of phase from their habitual times, resulted in increased postprandial glucose and insulin levels and elevated mean arterial pressure levels, as well as decreased leptin concentrations and a reversed daily cortisol rhythm (5). Another experimental study in healthy volunteers found that a combination of sleep restriction and circadian disruptions for 3 weeks resulted in impaired glucose tolerance with a 32% reduction in insulin response to a standardized meal (6). These laboratory findings suggest that chronic severe circadian misalignment, such as is found in night and rotating shift workers, may lead to increased cardiometabolic risks. Our study of patients with type 2 diabetes contributes to the emerging body of evidence in support of a link between circadian alignment and metabolic function and suggests that the adverse impact of late chronotypes may be clinically significant for those with impaired glucose metabolism as in diabetes. Patients in the current study were not shift workers; however, having a later chronotype, which placed them at greater risk of chronic mild circadian misalignment, was found to be associated with worse glycemic control.
Previous genetic data suggested the role of circadian genes in metabolic control. In animal experiments, Clock mutant mice were shown to shift their feeding and activity into their normally inactive phase and, as a result, developed obesity and metabolic syndrome (hyperlipidemia, hepatic steatosis, hyperglycemia, and hypoinsulinemia) (25). Interestingly, the findings in our participants with later chronotype mimicked those in Clock mutant mice as they shifted their activities and food intake into normally inactive time (i.e., later in the evening), indicated by later bedtime and larger percentage of daily calories consumed at dinner. In addition, participants with later chronotype were more likely to require insulin treatment despite a tendency of being younger and having had diabetes for about the same duration as those with earlier chronotype. While multiple factors can contribute to the need for insulin treatment, and our study did not assess β-cell function, it is possible that those with late chronotypes were more hypoinsulinemic, similar to the Clock mutant mice. Late chronotype subjects also tended to have a higher BMI. Genetic studies in humans have linked the circadian genotypes to metabolic phenotypes. Certain genotypes of Clock and Brain & Muscle Arnt-like protein-1 (BMAL1) were associated with evening preference, resistance to weight loss, metabolic syndrome, and susceptibility to type 2 diabetes (26–28). In addition, rare MTNR1B variants, a gene encoding melatonin receptor 1B, have been linked to increased type 2 diabetes risk (29).
The current study revealed that eating more at dinner contributed to poorer glycemic control. In humans, glucose tolerance has been shown to decrease from morning to evening from a combination of reduced glucose utilization, decreased insulin sensitivity, and inappropriately low insulin secretion (13). This could explain the independent effect of eating a large dinner on glycemic control.
The current study cannot address the direction of causality of the association between circadian regulation and metabolic function. It is possible that hyperglycemia influences the circadian system, as patients with diabetes exhibit dampened amplitude of rhythms of glucose tolerance and insulin secretion (30). Dietary factors, such as high-fat diet in mice, can modify behavioral circadian rhythms (2,31). Therefore, it is likely that the relationship between circadian disruptions and metabolic derangements is bidirectional (3).
Our study has limitations. We did not have objective measures of sleep or circadian parameters or of OSA severity, all of which could impact glycemic control (32,33). In addition, menopausal status, which could be associated with changes in sleep patterns and HbA1c, was not available. Future studies should obtain objective measures of sleep duration and quality as well as of circadian function. The most important question of whether shifting the circadian system earlier may improve glycemic control remains to be addressed. Simple behavioral modifications, such as going to bed and waking up earlier, keeping a regular sleep/wake schedule, and modifying daily caloric distribution, may lead to improved glycemic control.
In summary, we demonstrated that later chronotype and larger percentage of daily calories consumed at dinner were both associated with worse glycemic control in patients with type 2 diabetes independently of sleep measures. The results support a role of circadian regulation in glycemic control in patients with type 2 diabetes.
Acknowledgments
This study was funded by a departmental grant, Section of Endocrinology, Department of Internal Medicine, Rush University Medical Center.
E.V.C. receives grant support from Philips/Respironics, the ResMed Foundation, and Amylin/Lilly; is a consultant for Pfizer and Viropharma; is Associate Editor for the journal SLEEP and for a volume entitled Sleep Loss and Obesity: Intersecting Epidemics published by Springer Science & Business, LLC; and serves as an expert witness for Lamson, Dugan and Murray, LLP (Omaha, NE). No other potential conflicts of interest relevant to this article were reported.
S.R. conceptualized the study, researched and analyzed data, wrote the manuscript, contributed to discussion, and reviewed and edited the manuscript. M.M.H. and S.J.C. analyzed data, contributed to discussion, and reviewed and edited the manuscript. M.K.M. and M.T. researched data. K.L.K. analyzed data, contributed to discussion, and reviewed and edited the manuscript. E.V.C. contributed to discussion and reviewed and edited the manuscript. S.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Dr. D. Baldwin, Rush University Medical Center, for his support and Dr. F. Meah, Rush University Medical Center, for her assistance with the study.
References
- 1.Aschoff J, Hoffmann K, Pohl H, Wever R. Re-entrainment of circadian rhythms after phase-shifts of the Zeitgeber. Chronobiologia 1975;2:23–78 [PubMed] [Google Scholar]
- 2.Arble DM, Ramsey KM, Bass J, Turek FW. Circadian disruption and metabolic disease: findings from animal models. Best Pract Res Clin Endocrinol Metab 2010;24:785–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang W, Ramsey KM, Marcheva B, Bass J. Circadian rhythms, sleep, and metabolism. J Clin Invest 2011;121:2133–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boivin DB, Tremblay GM, James FO. Working on atypical schedules. Sleep Med 2007;8:578–589 [DOI] [PubMed] [Google Scholar]
- 5.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA 2009;106:4453–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buxton OM, Cain SW, O'Connor SP, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med 4:129ra43, 2012 [DOI] [PMC free article] [PubMed]
- 7.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009;17:2100–2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int 2006;23:497–509 [DOI] [PubMed] [Google Scholar]
- 9.Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol 2012;22:939–943 [DOI] [PubMed] [Google Scholar]
- 10.Nakanishi-Minami T, Kishida K, Funahashi T, Shimomura I. Sleep-wake cycle irregularities in type 2 diabetics. Diabetol Metab Syndr 2012;4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakade M, Takeuchi H, Kurotani M, Harada T. Effects of meal habits and alcohol/cigarette consumption on morningness-eveningness preference and sleep habits by Japanese female students aged 18-29. J Physiol Anthropol 2009;28:83–90 [DOI] [PubMed] [Google Scholar]
- 12.Ostberg O. Circadian rhythms of food intake and oral temperature in “morning” and “evening” groups of individuals. Ergonomics 1973;16:203–209 [DOI] [PubMed] [Google Scholar]
- 13.Van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev 1997;18:716–738 [DOI] [PubMed] [Google Scholar]
- 14.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring) 2011;19:1374–1381 [DOI] [PubMed] [Google Scholar]
- 15.Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med 2006;166:1768–1774 [DOI] [PubMed] [Google Scholar]
- 16.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol 2009;5:253–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meltzer D, Egleston B. How patients with diabetes perceive their risk for major complications. Eff Clin Pract 2000;3:7–15 [PubMed] [Google Scholar]
- 18.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999;131:485–491 [DOI] [PubMed] [Google Scholar]
- 19.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213 [DOI] [PubMed] [Google Scholar]
- 20.Roenneberg T, Kuehnle T, Juda M, et al. Epidemiology of the human circadian clock. Sleep Med Rev 2007;11:429–438 [DOI] [PubMed] [Google Scholar]
- 21.Roenneberg T, Kuehnle T, Pramstaller PP, et al. A marker for the end of adolescence. Curr Biol 2004;14:R1038–R1039 [DOI] [PubMed] [Google Scholar]
- 22.Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms 2003;18:80–90 [DOI] [PubMed] [Google Scholar]
- 23.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986;51:1173–1182 [DOI] [PubMed] [Google Scholar]
- 24.Levandovski R, Dantas G, Fernandes LC, et al. Depression scores associate with chronotype and social jetlag in a rural population. Chronobiol Int 2011;28:771–778 [DOI] [PubMed] [Google Scholar]
- 25.Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005;308:1043–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garaulet M, Esteban Tardito A, Lee YC, Smith CE, Parnell LD, Ordovas JM. SIRT1 and CLOCK 3111T>C combined genotype is associated with evening preference and weight loss resistance in a behavioral therapy treatment for obesity. Int J Obes (Lond) 2012;36:1436–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott EM, Carter AM, Grant PJ. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes (Lond) 2008;32:658–662 [DOI] [PubMed] [Google Scholar]
- 28.Woon PY, Kaisaki PJ, Bragança J, et al. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci USA 2007;104:14412–14417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonnefond A, Clément N, Fawcett K, et al. Meta-Analysis of Glucose and Insulin-Related Traits Consortium (MAGIC) Rare MTNR1B variants impairing melatonin receptor 1B function contribute to type 2 diabetes. Nat Genet 2012;44:297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boden G, Chen X, Polansky M. Disruption of circadian insulin secretion is associated with reduced glucose uptake in first-degree relatives of patients with type 2 diabetes. Diabetes 1999;48:2182–2188 [DOI] [PubMed] [Google Scholar]
- 31.Kohsaka A, Laposky AD, Ramsey KM, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 2007;6:414–421 [DOI] [PubMed] [Google Scholar]
- 32.Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med 2010;181:507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med 2005;165:447–452 [DOI] [PubMed] [Google Scholar]


