Abstract
OBJECTIVE
To investigate the associations between changes in body composition and fitness after exercise training and changes in hemoglobin A1c (HbA1c) in individuals with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Participants (n = 201) were randomized to aerobic, resistance, or combined training for 9 months. HbA1c, waist circumference, total and trunk fat mass, appendicular fat mass, lean body mass, isokinetic leg muscle strength, peak O2 uptake, and estimated METs were assessed at baseline and follow-up. Change in HbA1c was evaluated across quartiles of change in body composition and fitness.
RESULTS
Change in HbA1c was associated with changes in body weight (r = 0.13, P = 0.052), waist circumference (r = 0.17, P = 0.013), trunk fat mass (r = 0.19, P = 0.005), and estimated METs (r = −0.16, P = 0.023). There was a trend in change in HbA1c across quartiles of waist circumference (P = 0.011), trunk fat mass (P = 0.020), and estimated METs (P = 0.011). Participants with increased estimated METs and reduced trunk fat mass had greater odds of having reduced HbA1c after training (3.48, 1.46–8.31). Finally, participants with increased estimated METs and reduced waist circumference were 2.81 (1.13–6.98) times more likely to have reduced HbA1c and type 2 diabetes medication use than those without improved fitness and central adiposity.
CONCLUSIONS
In patients with type 2 diabetes, a reduction in central adiposity and increase in fitness were the most prominent predictors of the change in HbA1c in response to exercise training.
Hemoglobin A1c (HbA1c) is the most widely used indicator of glucose control in individuals with type 2 diabetes, and exercise training is recommended along with standard care to improve glucose control and overall health status (1–5). Strong evidence from our data (6) and that of others (7,8) has established that a combination of aerobic and resistance training is effective in reducing HbA1c, whereas still others have reported that this combination is more effective than aerobic training or resistance training alone (7). However, only few studies specifically address what changes in cardiometabolic factors of exercise training (i.e., changes in cardiorespiratory fitness, muscle strength, body composition) are associated with improvement in HbA1c (9–12).
Previous studies have found that reductions in fat mass (10) and central adiposity (9,11) and increases in fitness (11,12) and muscle strength (12,13) are associated with reductions in HbA1c after exercise training. In a large (n = 251) randomized trial, Larose et al. (12) found that change in cardiorespiratory fitness was the strongest predictor of change in HbA1c after aerobic and aerobic combined with resistance training. However, with resistance training only, the change in muscle strength was the strongest predictor of the change in HbA1c. In a smaller sample (n = 40), Bacchi et al. (11) showed that an increase in fitness and a reduction in trunk fat mass result in the largest decrease in HbA1c after aerobic or resistance training alone in individuals with type 2 diabetes. Of note, previous studies evaluating the effects of body fat distribution, muscle strength, and fitness on long-term glucose control generally are limited by the absence of a control group (11), a small sample size (8), or a homogeneous study population (9). Herein, we performed secondary analyses based on data from the Health Benefits of Aerobic and Resistance Training in Individuals with Type 2 Diabetes (HART-D) trial, which investigated the contribution of 9 months of aerobic training, resistance training, or a combination of both interventions on changes in HbA1c. Although the purpose of the original HART-D trial (6) was to evaluate the effect of exercise, the current study expands on the previous findings by investigating the predictors of change in HbA1c in individuals with type 2 diabetes. Therefore, the primary aim of this study was to investigate the association between changes in body composition and fat distribution, leg muscle strength, and cardiorespiratory fitness and change in HbA1c.
RESEARCH DESIGN AND METHODS
Participants
The full methodology for HART-D has been previously described in the main outcomes paper (6). Briefly, the HART-D study enrolled 262 sedentary individuals aged 30–75 years with type 2 diabetes. Participants were excluded if they had a BMI of ≥48.0 kg/m2, blood pressure ≥160/100 mmHg, fasting triglyceride level ≥500 mg/dL, urine protein level >100 mg/dL, and serum creatinine level >1.5 mg/dL; used an insulin pump; or had a history of stroke, advanced neuropathy or retinopathy, or any serious medical condition that prevented them from adhering to the protocol or exercising safely. Demographic information was collected by self-report questionnaire. Information about type 2 diabetes medications (dose and class) were documented with a detailed questionnaire and confirmed by a visual inspection of the medication bottle. The Pennington Biomedical Research Center institutional review board annually approved the protocol, and all participants provided written informed consent.
Study design
The current study was a secondary analysis of the HART-D data. Of the 262 participants randomized in HART-D, 61 were excluded from this analysis because of either missing baseline (n = 1), follow-up exercise (n = 54), or follow-up body composition (n = 5) data. One participant was excluded from the control group because she gained 6 kg of trunk fat mass. The final analysis included 201 participants (Fig. 1).
Figure 1.
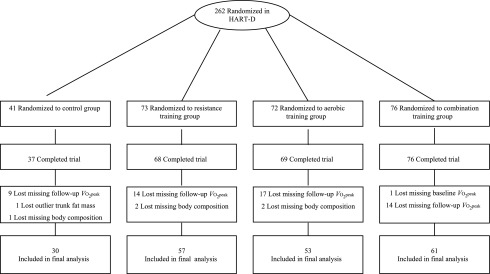
Consort diagram.
Exercise intervention
Aerobic training.
Participants in the aerobic training group exercised 3–5 days per week at an exercise intensity of 50–80% of their maximal cardiorespiratory fitness for a total dose of 12 kcal/kg body weight per week. Participants were weighed weekly to calculate their prescribed caloric dose. During weeks 12 and 24, we reduced the exercise dose by one-third to provide a recuperation week. American College of Sports Medicine equations were used to estimate caloric expenditure rate and, therefore, the time required per session (14).
Resistance training.
Participants exercised 3 days per week, with each session consisting of two sets of four upper-body exercises (bench press, seated row, shoulder press, and lat pull down), three sets of three lower-body exercises (leg press, leg extension, and leg curl), and two sets of abdominal crunches and back extensions. Each set comprised 10–12 repetitions. The prescribed weight was increased when the participant was able to complete 12 repetitions of the final set of each exercise on two consecutive sessions.
Combination training.
We selected an aerobic exercise dose of 10 kcal/kg body weight per week and two sessions of resistance training per week for the combination training group. Resistance training comprised one set of 10–12 repetitions of the same nine exercises, using a similar progressive resistance program as the resistance training group. The combination training group was consistent with federal physical activity guidelines (15) and ensured equal time commitment among all exercise groups.
Control group.
Participants randomized to the nonexercise control group were offered weekly stretching and relaxation classes once per week and were asked to maintain their current lifestyle.
Outcomes and measurements
Cardiorespiratory fitness.
Cardiorespiratory fitness was evaluated by a modified Balke treadmill test (Trackmaster 425; Carefusion, Newton, KS), with respiratory gases sampled by a TrueMax 2400 Metabolic Measurement Cart (ParvoMedics, Salt Lake City, UT). Briefly, participants self-selected a walking pace at a level grade, and the grade increased by 2% every 2 min until they reached volitional exhaustion. Peak O2 uptake (Vo2peak) was reported relative to total and lean body mass.
Estimated METs.
METs are strongly correlated with cardiorespiratory fitness, and measured Vo2 is not available in most cardiology and clinical exercise settings. Therefore, it is relevant to quantify estimated METs from a clinical perspective. Estimated METs were derived from the maximal speed and grade reached during the cardiorespiratory fitness test at baseline and follow-up, using equations from the American College of Sports Medicine (14).
Leg muscle strength.
Concentric isokinetic knee extension torque was assessed with a Biodex System 3 dynamometer (Biodex Medical Systems, Shirley, NY), and peak torque was determined as the highest measured torque over five maximal repetitions.
HbA1c.
Baseline and postintervention HbA1c was obtained by venipuncture after a 10-h fast and analyzed with Beckman Coulter DxC 600 Pro (Brea, CA).
Anthropometric measures and body composition.
Weight was measured on a GSE 450 electronic scale (GSE, Livonia, MI), and height was measured with a standard stadiometer. BMI was calculated as body weight in kilograms divided by height in meters squared. Waist circumference was measured to the nearest 0.1 cm at the level of the iliac crest while the subject was at minimal expiration. Body composition was measured by dual-energy X-ray absorptiometry (DEXA) with the QDR 4500A whole-body scanner (Hologic Inc., Bedford, MA).
Statistical analysis
All analyses were performed with SAS version 9.2 (SAS Institute Inc., Cary, NC) statistical software. Baseline continuous data are presented as mean ± SD, whereas categorical variables are presented as n (%). A one-way ANOVA was performed to identify baseline differences between groups for continuous variables. A χ2 test was used to assess baseline differences among groups in categorical variables. Spearman correlations were performed to determine the associations between changes in body composition, cardiorespiratory fitness, and isokinetic leg muscle strength and change in HbA1c. A multiple linear regression model was performed to test the impact of intervention on changes in HbA1c. Unadjusted and adjusted multiple linear regression models revealed that intervention groups were not significantly associated with changes in HbA1c (unadjusted P = 0.17, adjusted P = 0.18); therefore, a combined analysis strategy was used. Multiple linear regression models were used to identify differences of change in HbA1c across quartiles of change in body composition, cardiorespiratory fitness, and isokinetic leg muscle strength and are presented as adjusted least squares mean with 95% CI.
Multiple logistic regression was used to determine the odds of improving HbA1c (∆ < 0%) on the basis of estimated METs and trunk fat mass as well as estimated METs and waist circumference response after the intervention. A dichotomous variable was coded for estimated METs and trunk fat mass to indicate a favorable response (∆estimated METs > 0, ∆trunk fat mass < 0 kg) and an unfavorable response or no change (∆estimated METs ≤ 0, ∆trunk fat mass ≥ 0 kg) after exercise training. The responses for estimated METs and trunk fat mass were combined to determine the effect on the odds of a favorable response in HbA1c. A similar dichotomous variable was computed for waist circumference and estimated METs. These models were adjusted for age, sex, race/ethnicity, smoking, medication changes, duration of diabetes, and baseline HbA1c value.
Change in HbA1c (≤ −0.5%) or in type 2 diabetes medication use (decrease or discontinuation) suggests an improvement in long-term glucose control (6). Thus, multiple logistic regression was used to determine the odds of reducing HbA1c (≤ −0.5%) or type 2 diabetes medications for glucose control (decrease or discontinuation) on the basis of exercise-induced changes in estimated METs and trunk fat mass as well as estimated METs and waist circumference (see previous paragraph). In this analysis, a dichotomous variable was created for change in HbA1c (∆HbA1c ≤ −0.5% = 1, ∆HbA1c > −0.5% = 0), and a dichotomous variable was created for change in type 2 diabetes medications (reduction/discontinuation = 1, increase = 0, no change = 0). These dichotomous variables were coded into a single dependent variable, and the models were adjusted for age, sex, race/ethnicity, smoking, baseline HbA1c value, and duration of diabetes. Data are presented as odds ratio (OR) (95% CI). Statistical significance was defined as a two-tailed P ≤ 0.05.
RESULTS
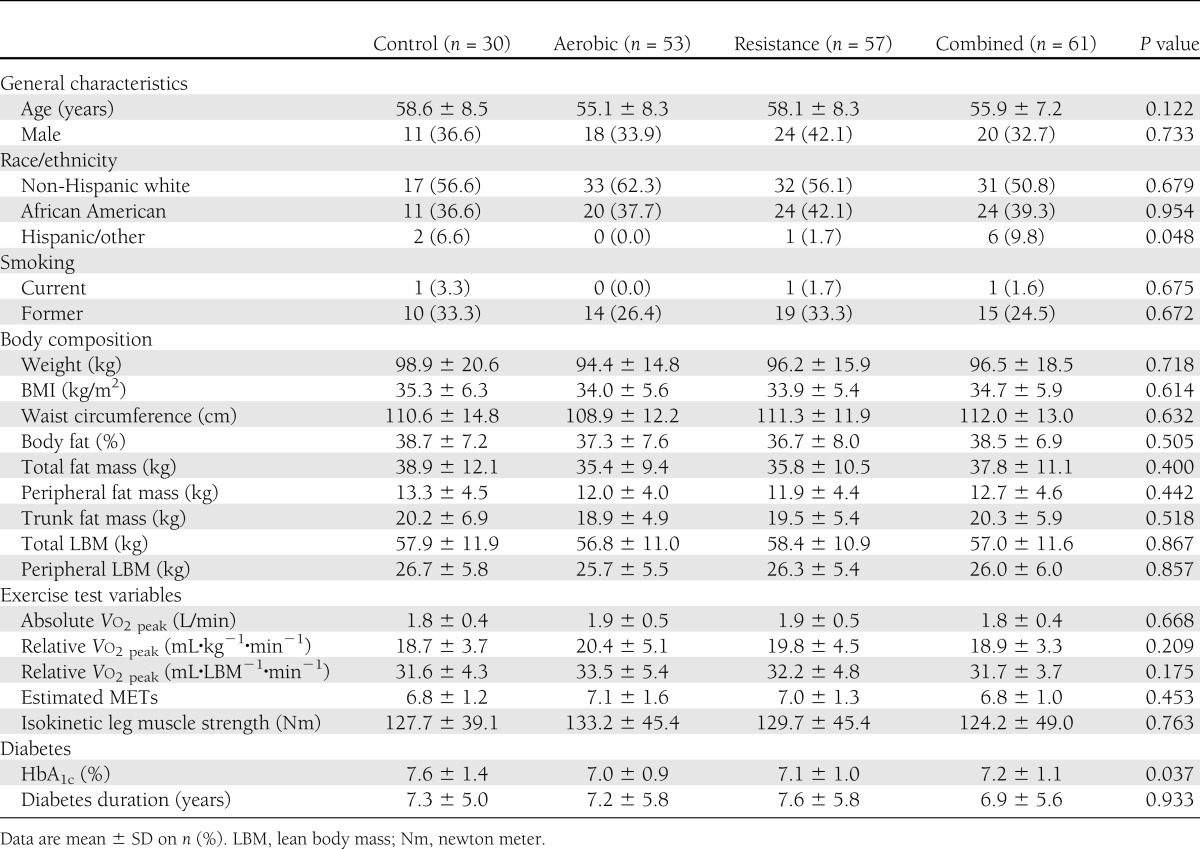
Baseline characteristics are shown in Table 1. The study sample comprised 56.2% non-Hispanic white, 63.7% women, 1.5% current smokers, and 28.9% former smokers. The mean age of the sample was 56.7 ± 8.0 years, and the mean BMI was 34.3 ± 5.7 kg/m2. Relative Vo2peak was 19.5 ± 4.3 mL·kg−1·min−1, and mean type 2 diabetes duration was 7.3 ± 5.6 years. Significant differences were observed among exercise groups at baseline for race/ethnicity (P = 0.048) and HbA1c (P = 0.037).
Table 1.
Baseline characteristics
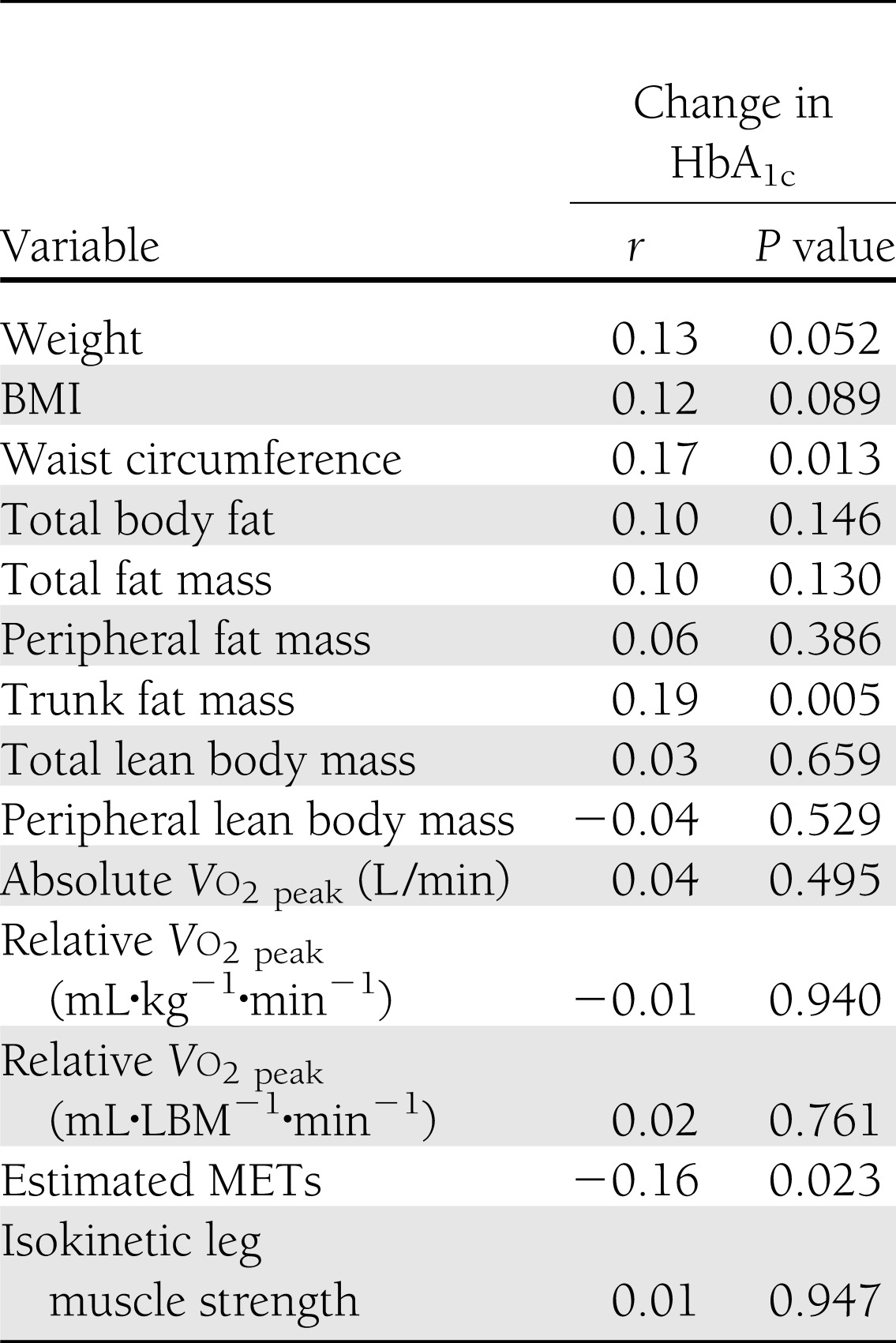
The associations between change in HbA1c and changes in body composition, cardiorespiratory fitness, and isokinetic leg muscle strength after 9 months of intervention are shown in Table 2. Unadjusted change in HbA1c was associated with changes in body weight (P = 0.052), waist circumference (P = 0.013), and trunk fat mass (P = 0.005) and inversely associated with estimated METs (P = 0.023).
Table 2.
Spearman correlation coefficients between change in body composition, fitness measures, and isokinetic leg muscle strength and change in HbA1c
Adjusted change in anthropometry, body composition, fitness, and strength measures across quartiles of changes in HbA1c are shown in Table 3. A significant trend was observed for decrease in body weight (P = 0.013), BMI (P = 0.025), and trunk fat mass (P = 0.020) across quartiles of changes in HbA1c. Similarly, a significant trend was observed for decrease in waist circumference (P = 0.011), with the greatest reduction in HbA1c in the highest quartile. No significant trend was observed for decrease in total fat mass across quartiles of changes in HbA1c (P = 0.154). A significant trend was observed for increase in estimated METs (P = 0.011) across quartiles of changes in HbA1c. No other fitness measures (Vo2peak, muscle strength) were significantly associated with change in HbA1c (P > 0.05 for trend).
Table 3.
Changes in exposure variables across quartiles of change in HbA1c
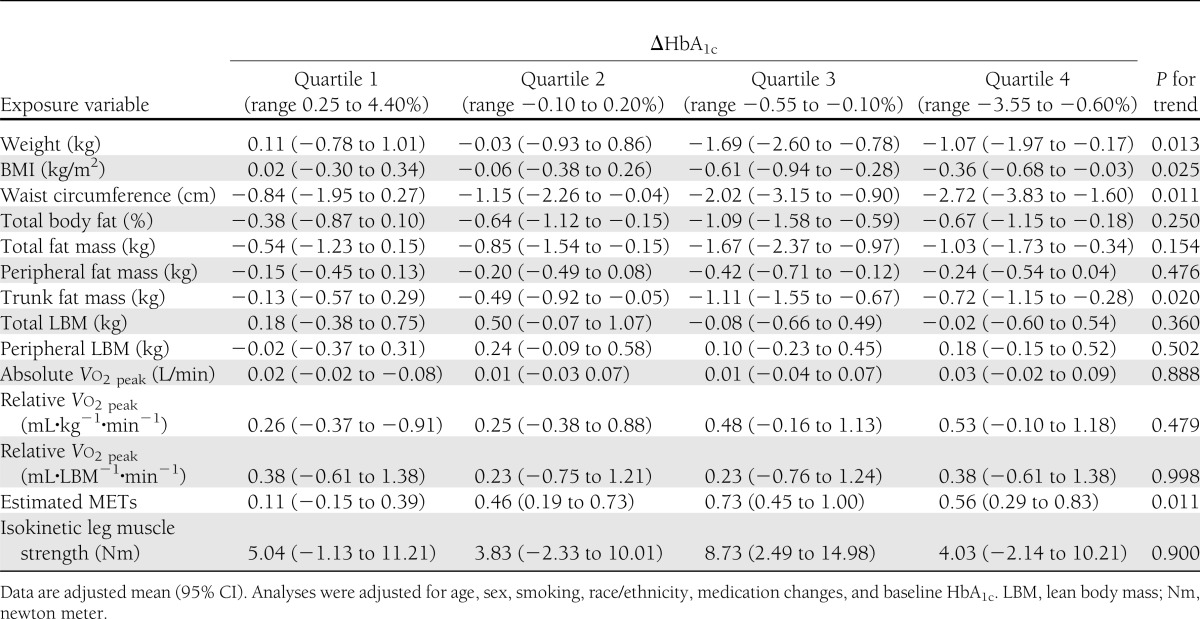
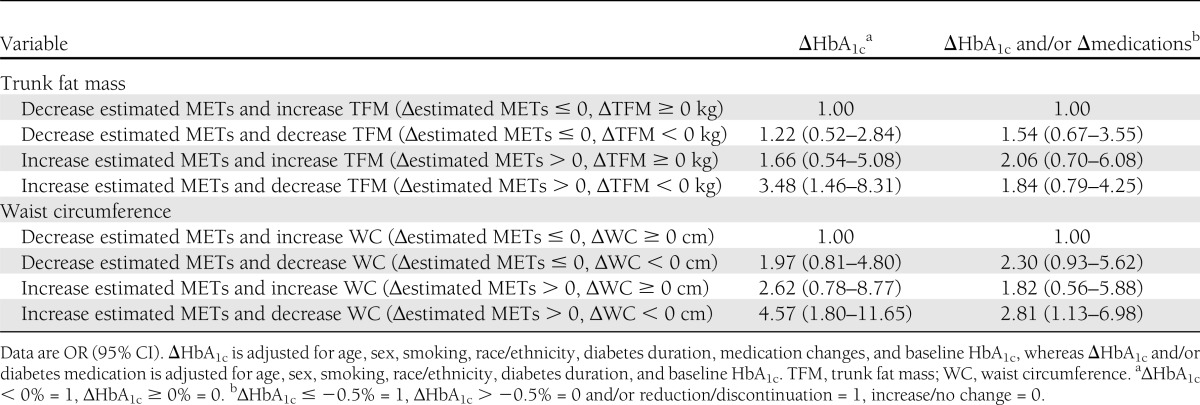
Table 4 describes the odds of reducing HbA1c on the basis of a favorable response for estimated METs and trunk fat mass (∆estimated METs > 0, ∆trunk fat mass < 0 kg) or estimated METs and waist circumference (∆estimated METs > 0, ∆waist circumference < 0 cm). The likelihood of a favorable response (∆HbA1c < 0%) was 3.48 (95% CI 1.46–8.31) in participants who had increased estimated METs and decreased trunk fat mass and 4.57 (1.80–11.65) in participants who had increased estimated METs and decreased waist circumference compared with those with an unfavorable response. However, the interactions between increase in estimated METs and decrease in trunk fat mass (P = 0.435) or decrease in waist circumference (P = 0.863) were not significantly associated with change in HbA1c. Additionally, in participants with increased estimated METs and decreased waist circumference, the likelihood of a favorable response (improve HbA1c, reduced diabetes medications, or both) was 2.81 (1.13–6.98) compared with those with an unfavorable response after the intervention. Similarly, the interaction between an increase in estimated METs and a decrease in trunk fat mass (P = 0.760) or waist circumference (P = 0.884) was not significant.
Table 4.
Odds of improving HbA1c and decreasing type 2 diabetes medications across group of change in fitness and central adiposity
CONCLUSIONS
The primary results of the current study suggest that reducing central adiposity and increasing fitness in a large sample of men and women with type 2 diabetes are critical factors to improving glycemic control (HbA1c) after an exercise training program. Additionally, the data suggest that a decrease in central adiposity and an increase in fitness increase the likelihood of achieving a favorable HbA1c response (>0.5% decrease) or reducing the number of medications required for the treatment of type 2 diabetes. These findings have clinical implications for the design of exercise training programs used to treat individuals with type 2 diabetes because they suggest that a concomitant reduction in central adiposity and increase in fitness should be the main focus of an exercise intervention aiming to improve glycemic control.
The relationship between the change in central adiposity and change in HbA1c is supported by previous studies (9,11,16). Bacchi et al. (11) found that change in trunk fat mass was the strongest predictor for change in HbA1c after 4 months of aerobic or resistance training alone in patients with type 2 diabetes. Castaneda et al. (9) investigated the impact of resistance training on body composition and glycemic control in individuals with type 2 diabetes and found that a decrease in trunk fat mass was accompanied by a reduction of 1.1% in HbA1c after resistance training. Doyon et al. (17) reported an association between trunk fat mass measured by DEXA and visceral fat mass measured by CT scan before (r = 0.61, P ≤ 0.01) and after (r = 0.67, P ≤ 0.01) an intervention of weight loss with or without resistance training in a cohort of obese postmenopausal women. Therefore, loss in visceral fat may, at least in part, be responsible for the reduction in HbA1c observed in the current study because previous research suggests that a reduction in visceral fat improves glycemic control in individuals with type 2 diabetes (18–20). In support of this hypothesis, a study by Giannopoulou et al. (21) suggested that exercise is mandatory to decrease visceral fat in individuals with type 2 diabetes. This decrease in visceral fat mass would decrease free fatty acid flux and improve glucose disposal (22), which would improve glycemic control. Secondary analyses revealed that trunk fat mass measured by DEXA was significantly associated with waist circumference (r = 0.87, P < 0.0001), which supports that waist circumference is an appropriate surrogate measure to evaluate the improvement in central adiposity from exercise training because trunk and visceral fat mass are not evaluated in most clinical and exercise settings. We also found that a reduction in central adiposity (measured by trunk fat mass or waist circumference) was associated with a reduction in HbA1c. Additionally, when this reduction in central adiposity occurred with an improvement in estimated METs, it was associated with a greater odds of improving glucose control or decreasing the number of diabetes medications. Therefore, the current results suggest that a reduction in central adiposity is associated with an improvement in HbA1c, which is optimized by the concomitant increase in fitness, in individuals with type 2 diabetes.
Of note, the results show an association between the change in fitness (measured by estimated METs) and change in HbA1c but not Vo2peak. The change in cardiorespiratory fitness was associated with a change in estimated METs (P < 0.0001), but the variability between the measures was surprisingly low (∼31%, r = 0.56). In contrast, in a similar study, Larose et al. (12) showed that cardiorespiratory fitness measured by a Vo2max treadmill test was a strong predictor of change in HbA1c, whereas treadmill time to exhaustion was only marginally associated with a reduction in HbA1c. This result is controversial because the current study found a strong association between changes in treadmill time to exhaustion and change in HbA1c (data not shown [P = 0.01 for trend]). The discrepancy between the current results and those of Larose et al. may lie on the statistical analysis strategy used (per group versus combined groups), differences in exercise dose in the combined group (full aerobic and full resistance training versus a reduced dose in HART-D for the combined group), and overall intervention design. Although the specific rationale explaining the lack of a relationship between Vo2max and HbA1c is unknown, the estimated METs and workload on a treadmill are associated with cardiovascular and all-cause mortality in individuals with type 2 diabetes (23–25). Furthermore, other exercise training studies found that reductions in HbA1c are associated with an increase in treadmill time to exhaustion (26,27). Considering that treadmill time to exhaustion and estimated METs were associated with changes in HbA1c, the current findings may have relevant clinical applications because these fitness measures typically are evaluated in clinical settings. However, the fitness results are especially relevant to exercise clinicians, who should prescribe training programs that can safely promote the greatest reduction in central adiposity and improvement in fitness in individuals with type 2 diabetes.
In the current study, improvement in muscle strength was not related to reductions in HbA1c, which is in contrast with previous studies (7,12) that found that the combination of resistance and aerobic training were the most beneficial for improvement in glucose control. Perhaps resistance training contributes either to the improvement in fitness or the reduction in central adiposity. In HART-D, the combined training group had the greatest reduction in trunk fat mass, even when compared with the aerobic group, and a significant improvement in treadmill time to exhaustion. In contrast to the current results, Larose et al. (12) reported that change in muscle strength was associated with change in HbA1c. The observed disparity may be explained by the different muscle limb measurement (upper versus lower) or methodology used to quantify change in muscle strength (one repetition maximum versus Biodex). It has been reported that using dissimilar training and testing modalities might compromise the capability to quantify muscle strength changes (28). Nevertheless, previous studies performed in individuals with type 2 diabetes support our observation. For example, Ishii et al. (29) and Ibañez et al. (30) did not observe an association between changes in muscle strength and changes in HbA1c, despite a 16 and 17% increase in muscle strength, respectively.
A potential limitation of our study is the lack of gold standard measures for visceral fat mass and oral glucose tolerance test, which could have given insight into the mechanism underlying the improvement in HbA1c. Strengths of our study were the large sample size and the standardized and tightly supervised exercise training session. Another strength was that HART-D participants had excellent compliance (>80%) to exercise training, and we adjusted the analyses for potential confounding variables that may affect HbA1c. Finally, the sample was diverse in terms of age, sex, and race/ethnicity (39.3% African American), which makes the findings generalizable, although the overall study group was generally middle-aged, obese, and with relatively poor baseline fitness.
In conclusion, this study suggests that trunk fat mass and estimated METs are both associated with changes in HbA1c. From a clinical standpoint, the goals behind physical activity programs in individuals with type 2 diabetes should be to maximize the increase in fitness and decrease in central fat mass (especially waist circumference) to reduce HbA1c. Future research should investigate the specific mechanisms by which exercise training–related increases in fitness and central adiposity reduction improve HbA1c in individuals with type 2 diabetes.
Acknowledgments
This work was supported by National Institutes of Health Grant DK-068298. The National Institutes of Health T32 postdoctoral fellowship program (Obesity from Genes to Man) and the Manitoba Health Research Council supported the salary and training of D.L.S. and M.S., respectively.
No potential conflicts of interest relevant to this article were reported.
The sponsor had no role in the design, protocol development, or conducting of the trial, data collection, data analysis, or preparation of the manuscript.
M.S. analyzed the data and wrote the manuscript. D.L.S. wrote and reviewed the manuscript. N.M.J. researched data, analyzed data, and wrote and reviewed the manuscript. S.N.B., C.P.E., and C.J.L. edited and reviewed the manuscript. T.S.C. edited and reviewed the manuscript and was the primary investigator of the HART-D study. T.S.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT00458133, clinicaltrials.gov.
References
- 1.Colberg SR, Albright AL, Blissmer BJ, et al. American College of Sports Medicine. American Diabetes Association Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: joint position statement. Exercise and type 2 diabetes. Med Sci Sports Exerc 2010;42:2282–2303 [DOI] [PubMed] [Google Scholar]
- 2.Colberg SR, Sigal RJ, Fernhall B, et al. American College of Sports Medicine. American Diabetes Association Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care 2010;33:2692–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pate RR, Pratt M, Blair SN, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA 1995;273:402–407 [DOI] [PubMed] [Google Scholar]
- 4.Zinman B, Ruderman N, Campaigne BN, Devlin JT, Schneider SH, American Diabetes Association Physical activity/exercise and diabetes mellitus. Diabetes Care 2003;26(Suppl. 1):S73–S77 [DOI] [PubMed] [Google Scholar]
- 5.U.S. Department of Health and Human Services Physical Activity and Health: A Report of the Surgeon General. Atlanta, GA, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, 1996 [Google Scholar]
- 6.Church TS, Blair SN, Cocreham S, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA 2010;304:2253–2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sigal RJ, Kenny GP, Boulé NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med 2007;147:357–369 [DOI] [PubMed] [Google Scholar]
- 8.Balducci S, Leonetti F, Di Mario U, Fallucca F. Is a long-term aerobic plus resistance training program feasible for and effective on metabolic profiles in type 2 diabetic patients? Diabetes Care 2004;27:841–842 [DOI] [PubMed] [Google Scholar]
- 9.Castaneda C, Layne JE, Munoz-Orians L, et al. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care 2002;25:2335–2341 [DOI] [PubMed] [Google Scholar]
- 10.Dunstan DW, Daly RM, Owen N, et al. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care 2002;25:1729–1736 [DOI] [PubMed] [Google Scholar]
- 11.Bacchi E, Negri C, Zanolin ME, et al. Metabolic effects of aerobic training and resistance training in type 2 diabetic subjects: a randomized controlled trial (the RAED2 study). Diabetes Care 2012;35:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larose J, Sigal RJ, Khandwala F, Prud’homme D, Boulé NG, Kenny GP, Diabetes Aerobic and Resistance Exercise (DARE) trial investigators Associations between physical fitness and HbA1(c) in type 2 diabetes mellitus. Diabetologia 2011;54:93–102 [DOI] [PubMed] [Google Scholar]
- 13.Tokmakidis SP, Zois CE, Volaklis KA, Kotsa K, Touvra AM. The effects of a combined strength and aerobic exercise program on glucose control and insulin action in women with type 2 diabetes. Eur J Appl Physiol 2004;92:437–442 [DOI] [PubMed] [Google Scholar]
- 14.American College of Sports Medicine ACSM’s Guidelines for Exercise Testing and Prescription. 7th ed. Philadelphia, Lippincott Williams &Wilkins, 2006 [Google Scholar]
- 15.U.S. Department of Health and Human Services. 2008 Physical activity guidelines for Americans [Internet], 2008. Washington, DC, U.S. Department of Health and Human Services. Available from http://www.health.gov/paguidelines/pdf/paguide.pdf Accessed 29 March 2013
- 16.Cuff DJ, Meneilly GS, Martin A, Ignaszewski A, Tildesley HD, Frohlich JJ. Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care 2003;26:2977–2982 [DOI] [PubMed] [Google Scholar]
- 17.Doyon CY, Brochu M, Messier V, et al. Association between abdominal fat (DXA) and its subcomponents (CT scan) before and after weight loss in obese postmenopausal women: a MONET study. J Obes 2011;2011:239516. [DOI] [PMC free article] [PubMed]
- 18.Mourier A, Gautier JF, De Kerviler E, et al. Mobilization of visceral adipose tissue related to the improvement in insulin sensitivity in response to physical training in NIDDM. Effects of branched-chain amino acid supplements. Diabetes Care 1997;20:385–391 [DOI] [PubMed] [Google Scholar]
- 19.Boudou P, De Kerviler E, Vexiau P, Fiet J, Cathelineau G, Gautier J. Effects of a single bout of exercise and exercise training on steroid levels in middle-aged type 2 diabetic men: relationship to abdominal adipose tissue distribution and metabolic status. Diabetes Metab 2000;26:450–457 [PubMed] [Google Scholar]
- 20.Boudou P, Sobngwi E, Mauvais-Jarvis F, Vexiau P, Gautier JF. Absence of exercise-induced variations in adiponectin levels despite decreased abdominal adiposity and improved insulin sensitivity in type 2 diabetic men. Eur J Endocrinol 2003;149:421–424 [DOI] [PubMed] [Google Scholar]
- 21.Giannopoulou I, Ploutz-Snyder LL, Carhart R, et al. Exercise is required for visceral fat loss in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab 2005;90:1511–1518 [DOI] [PubMed] [Google Scholar]
- 22.Park HS, Lee K. Greater beneficial effects of visceral fat reduction compared with subcutaneous fat reduction on parameters of the metabolic syndrome: a study of weight reduction programmes in subjects with visceral and subcutaneous obesity. Diabet Med 2005;22:266–272 [DOI] [PubMed] [Google Scholar]
- 23.Church TS, Cheng YJ, Earnest CP, et al. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care 2004;27:83–88 [DOI] [PubMed] [Google Scholar]
- 24.Church TS, LaMonte MJ, Barlow CE, Blair SN. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Arch Intern Med 2005;165:2114–2120 [DOI] [PubMed] [Google Scholar]
- 25.Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med 2000;132:605–611 [DOI] [PubMed] [Google Scholar]
- 26.Larose J, Sigal RJ, Boulé NG, et al. Effect of exercise training on physical fitness in type II diabetes mellitus. Med Sci Sports Exerc 2010;42:1439–1447 [DOI] [PubMed] [Google Scholar]
- 27.Kadoglou NP, Iliadis F, Angelopoulou N, et al. The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur J Cardiovasc Prev Rehabil 2007;14:837–843 [DOI] [PubMed] [Google Scholar]
- 28.Weiss EP, Racette SB, Villareal DT, et al. Washington University School of Medicine CALERIE Group Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise-induced weight loss. J Appl Physiol 2007;102:634–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishii T, Yamakita T, Sato T, Tanaka S, Fujii S. Resistance training improves insulin sensitivity in NIDDM subjects without altering maximal oxygen uptake. Diabetes Care 1998;21:1353–1355 [DOI] [PubMed] [Google Scholar]
- 30.Ibañez J, Izquierdo M, Argüelles I, et al. Twice-weekly progressive resistance training decreases abdominal fat and improves insulin sensitivity in older men with type 2 diabetes. Diabetes Care 2005;28:662–667 [DOI] [PubMed] [Google Scholar]


