Abstract
OBJECTIVE
To determine whether baseline estimated glomerular filtration rate (eGFR) and albumin-to-creatinine ratio (ACR) independently predict coronary artery calcification (CAC) progression, and to determine how eGFR changes over 6 years in adults with type 1 diabetes compared with nondiabetic adults.
RESEARCH DESIGN AND METHODS
The Coronary Artery Calcification in Type 1 Diabetes study participants (n = 1,066) with complete data for eGFR assessment at baseline and 6 years were included. Three Chronic Kidney Disease Epidemiology Collaboration equations (serum creatinine, cystatin C, and both) were used to estimate eGFR. The association of baseline ACR and eGFR with CAC progression was analyzed using multiple logistic regression.
RESULTS
Increasing categorical baseline ACR (<10, 10–30, and >30 µg/mg) predicted CAC progression in participants with type 1 diabetes (odds ratio [OR], 2.15; 95% CI, 1.50–3.09; 7.19 [3.90–13.26]; and 18.09 [8.48–38.62]), respectively, compared with nondiabetic subjects. Baseline eGFR <60 mL/min/1.73 m2 also predicted CAC progression (OR, 5–7, compared with nondiabetic participants). ORs for CAC progression were higher in women than in men when using the cystatin C–based Chronic Kidney Disease Epidemiology Collaboration equations. Participants with type 1 diabetes had greater eGFR decreases over 6 years than nondiabetic participants using cystatin C–based equations.
CONCLUSIONS
Although increasing ACR or decreasing eGFR predicts CAC progression, coronary atherosclerosis progresses faster in people with type 1 diabetes even in the absence of diabetic kidney disease. These findings emphasize the interaction between kidney disease and cardiovascular disease in type 1 diabetes and highlight the public health importance of lowering cardiorenal risk in people with type 1 diabetes.
Diabetic kidney disease is an important mediator of accelerated cardiovascular disease (CVD) and continues to be a major cause of morbidity and mortality in patients with type 1 diabetes (1–4). The FinnDiane study (5) and the Pittsburgh Epidemiology of Diabetes Complications (EDC) study (6) recently reported no excess mortality, compared with the general population, among adults with type 1 diabetes and free of kidney disease. Both studies, however, reported stepwise increases in mortality with increasing severity of kidney disease. Less is known about the association of diabetic kidney disease with subclinical atherosclerosis, i.e., whether these processes develop sequentially or in parallel.
Although effective treatment exists to prevent or slow decline in renal function, improved early diagnostic (7,8) and treatment (9) methods are needed. Currently, clinical screening for kidney disease is performed by measuring albumin-to-creatinine ratio (ACR) or by measuring estimated glomerular filtration rate (eGFR). The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) recently published three updated glomerular filtration rate (GFR) estimating equations based on serum creatinine, cystatin C, or a combination of these markers (10). Both ACR and eGFR predict CVD and mortality (11,12).
The Coronary Artery Calcification in Type 1 Diabetes (CACTI) study is a longitudinal cohort study designed to investigate the determinants of early and accelerated atherosclerosis in people with and without type 1 diabetes. Our objectives were to determine if ACR and eGFR predicted coronary artery calcification (CAC) progression over a 6-year follow-up, whether differences existed by sex, and how eGFR changed over 6 years in people with and without type 1 diabetes.
RESEARCH DESIGN AND METHODS
The CACTI study enrolled subjects 19–56 years old, with and without type 1 diabetes, who were asymptomatic for CVD at the baseline visit in 2000–2002; they were re-examined 3 and 6 years later, as previously described (13). Subjects with serum creatinine >2 mg/dL were excluded at baseline, unless they were participants in the pilot study. All subjects with data needed to calculate GFR at the baseline and 6-year visits were included in this analysis (n = 489 with type 1 diabetes and n = 577 nondiabetic controls). Among participants with type 1 diabetes, those with missing data had slightly worse baseline renal function (for example, CKD-EPI cystatin C eGFR 99 ± 30 vs. 108 ± 19 mL/min/1.73 m2) and fasting lipid profile than the participants with complete data. In contrast, nondiabetic participants with missing data were younger (34 ± 9 vs. 40 ± 9 years) and had better renal function (for example, CKD-EPI cystatin C eGFR 111 ± 13 vs. 108 ± 12 mL/min/1.73 m2). The study was approved by the Colorado Multiple Institutional Review Board and all participants provided informed consent.
We measured height and weight and calculated BMI in kg/m2. Resting systolic blood pressure (SBP) and fifth-phase diastolic blood pressure (DBP) were measured three times while the patient was seated, and the second and third measurements were averaged (14). Hypertension was defined as current antihypertensive therapy or untreated hypertension (BP ≥140/90 mmHg) at the time of the study visit. Antihypertension medication use was determined by a medication inventory as previously described (13), and use of an ACE inhibitor or an angiotensin receptor blocker (ARB) was combined for these analyses. Dyslipidemia was defined as previously described in CACTI (15).
After an overnight fast, blood was collected, centrifuged, and separated. Plasma was stored at 4°C until assayed. Total plasma cholesterol and triglyceride levels were measured using standard enzymatic methods, HDL cholesterol was separated using dextran sulfate, and LDL cholesterol was calculated using the Friedewald formula. High-performance liquid chromatography was used to measure HbA1c (BioRad variant).
Two timed overnight urine samples were collected from 413 participants with type 1 diabetes in duplicate and urine creatinine and albumin were measured (RIA; Diagnostic Products) and averaged. In those subjects (n = 35) who did not have two timed overnight urine samples, only one timed overnight urine sample was collected. Two subjects did not provide a timed overnight urine sample and, instead, spot urine was collected. There were 39 participants among the 489 with type 1 diabetes without baseline urine samples, but they did not differ for any of the clinical characteristics (presented in Table 1) from those with at least one urine sample.
Table 1.
Subject characteristics
We examined ACR by the cut-points <10, 10–30, and >30 µg/mg based on a meta-analysis including >100,000 adults in which ACR ≥10 µg/mg was an independent predictor of all-cause and CVD mortality (12). This allowed for examination of a group with normal ACR, even by nondiabetic standards.
Cystatin C was measured in the University of Colorado Hospital clinical laboratory using the commercially available Dade-Behring assay and following package insert instructions on a BNII or Prospec instrument. The coefficient of variation was 3.3%. Intra-assay precision is 2.3–4.1% and interassay precision is 2.6–3.3% per the package insert. Results are reported as mg/L, with sensitivity cut-off of 0.23 mg/L. Because of a systematic shift in the Dade-Behring cystatin C assay over the time period of our study, cystatin C levels were standardized to the levels of the third visit using the following Deming regression equations as previously described (16):
Serum creatinine was measured according to package insert instructions using a Roche Mira Plus II analyzer until 2006, and then an Olympus AU400e (r = 0.9999 between methodologies) traceable to the National Institute of Standards and Technology Standard Reference Material in the University of Colorado Clinical Translational Research Laboratory. Intra-assay precision is 1.6% and interassay precision is 3.3%. Because of the longitudinal design of CACTI, and to assess for any potential change over time in the serum creatinine assay, 100 samples each from visits 1, 2, and 3 were remeasured as a single batch. Deming regression equations were used to standardize serum creatinine using the following same methodology as previously described for cystatin C (16):
GFR (mL/min/1.73 m2) was determined using the CKD-EPI serum creatinine, serum cystatin C, and combined equations recently published by Inker for the CKD-EPI Investigators Group (10).
CAC
Two sets of images for scoring of CAC were obtained using an ultrafast Imatron C-150XLP electron beam computed tomography scanner (Imatron, San Francisco, CA). The CAC score using the Agatston method and the total calcium volume score (CVS) using the volumetric method were calculated from the images (17). The volume score was used to identify CAC progression. Scans were repeated in the follow-up examination, which occurred an average of 6.1 years after the baseline scans. A difference between baseline and follow-up square root–transformed CVS of ≥2.5 was used to define significant change in CVS, because a change of this magnitude is <1% likely to be attributable to interscan variability (18,19). The baseline and follow-up CVS were square root–transformed and the difference was calculated for each subject. Individuals were categorized as progressors (n = 337) if the change in square root CVS was ≥2.5 and as nonprogressors (n = 623) if change in square root CVS was <2.5. Among the 1,066 participants with complete eGFR data at both visits, 960 had CAC measurements at both visits. Those without CAC progression data were younger at baseline than those with CAC progression data, but otherwise had similar CVD risk profiles.
Statistical methods
Differences between subjects with and without type 1 diabetes at each visit were compared by t tests or χ2 tests. Baseline renal function was categorized in participants with type 1 diabetes for ACR as <10, 10–30, and >30 µg/mg (12) and for eGFR as <60, 60–90, 90–120, and >120 mL/min/1.73 m2 using each of the three CKD-EPI eGFR equations (10). Nondiabetic participants were considered the reference group, and the frequency of CAC progression in participants with type 1 diabetes by these categories was determined. Next, the odds ratio (OR) for CAC progression (adjusted for age and sex) was calculated for each ACR category and for each eGFR category using each of the three eGFR equations. A sex-by-ACR category or sex-by-eGFR category interaction term was calculated to determine whether sex differences existed in the association of renal function to CAC progression. Univariate associations of CVD risk factors with CAC progression were calculated stratified by diabetes, and variables significantly (P < 0.05) associated with CAC progression were entered into stepwise logistic regression models in which sex, age, and baseline CVS were forced into the models (stratified by diabetes) in addition to each measure of renal function in individual models. Exploratory analyses were performed comparing CVD risk factors in those participants with type 1 diabetes with ACR <10 µg/mg at both visits by CAC progression status and with nondiabetic participants by CAC progression status. In these analyses, additional novel CVD risk factors previously measured in the CACTI study (20–22) were included (adiponectin, waist circumference, visceral fat, high-sensitivity C-reactive protein, fibrinogen, uric acid, and homocysteine). P < 0.05 was considered statistically significant and SAS 9.2 was used for analysis.
RESULTS
At baseline, participants with type 1 diabetes were slightly younger, more likely to be non-Hispanic white, had higher HbA1c, cystatin C, eGFR CKD-EPI serum creatinine, and ACR, and were more likely to have ACR >30 µg/mg and to report ACE or ARB use. Participants with type 1 diabetes also had higher SBP and were more likely to be hypertensive; they had lower DBP, total cholesterol, LDL, and triglycerides and higher HDL than nondiabetic participants (Table 1). At the 6-year visit, all of these differences persisted except that eGFR CKD-EPI serum creatinine and SBP no longer differed, but eGFR CKD-EPI cystatin C was lower in participants with type 1 diabetes compared with nondiabetic participants.
Over 6 years, change in eGFR CKD-EPI serum creatinine did not differ by diabetes group (−6.9 ± 20.0 and −5.5 ± 16.6 mL/min/1.73 m2 for participants with and without type 1 diabetes, respectively; P = 0.22 for comparison of change in GFR over 6 years between groups). However, participants with type 1 diabetes experienced greater declines than nondiabetic participants for both eGFR CKD-EPI cystatin C (change was −5.6 ± 14.2 and −2.6 ± 8.8 mL/min/1.73 m2, respectively; P < 0.0001) and for eGFR CKD-EPI combined (change was −2.2 ± 17.2 and +1.1 ± 14.3 mL/min/1.73 m2, respectively; P = 0.0009).
When baseline eGFR was compared within diabetes strata, eGFR was lower in those with CAC progression than without when using the serum creatinine (99 ± 26 vs. 109 ± 23 mL/min/1.73 m2), cystatin C (103 ± 21 vs. 112 ± 15 mL/min/1.73 m2), and combined equations (96 ± 24 vs. 108 ± 20 mL/min/1.73 m2; P < 0.0001) for each comparison within participants with type 1 diabetes. Similar associations existed for nondiabetic participants with lower eGFR among those with CAC progression compared with those without CAC progression for serum creatinine (98 ± 18 vs. 103 ± 20 mL/min/1.73 m2; P = 0.02), cystatin C (106 ± 12 vs. 109 ± 12 mL/min/1.73 m2; P = 0.03), and combined (97 ± 17 vs. 102 ± 17 mL/min/1.73 m2; P = 0.0009). Participants with type 1 diabetes were more likely to have CAC progression than nondiabetic participants (42 vs. 29%; P < 0.0001).
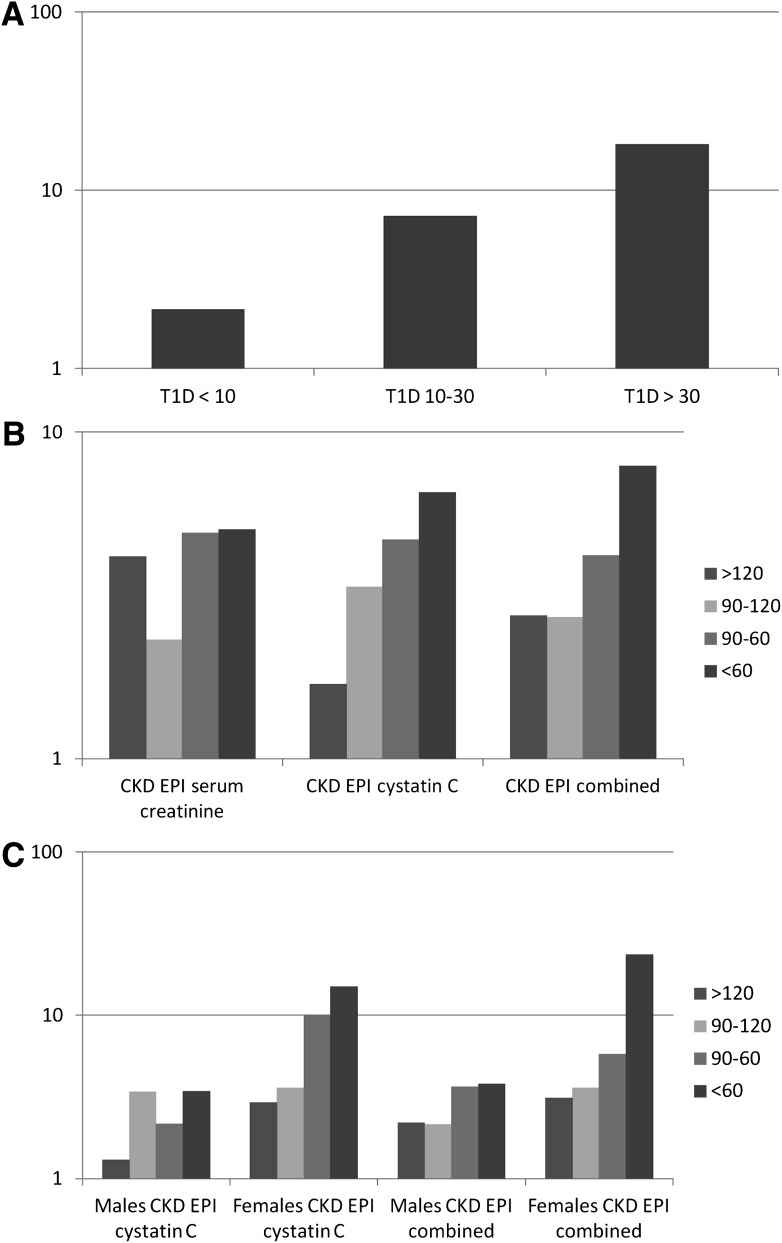
We next examined the association of categorical baseline renal function to CAC progression (Table 2). Participants with type 1 diabetes who had ACR <10, 10–30, and >30 µg/mg at baseline were 2.2-times (OR, 2.15; 95% CI, 1.50–3.09), 7.2-times (7.19 [3.90–13.26]), and 18.1-times (18.09 [8.48–38.62]), respectively, more likely to have CAC progression at 6 years than nondiabetic participants when adjusted for age and sex (Fig. 1A). Restricting the normoalbuminuric group to those who also had ACR <10 µg/mg at the 6-year visit did not change the results (1.92 [CI, 1.31–2.81]).
Table 2.
Rates of CAC progression by GFR and ACR categories for type 1 diabetic and nondiabetic participants
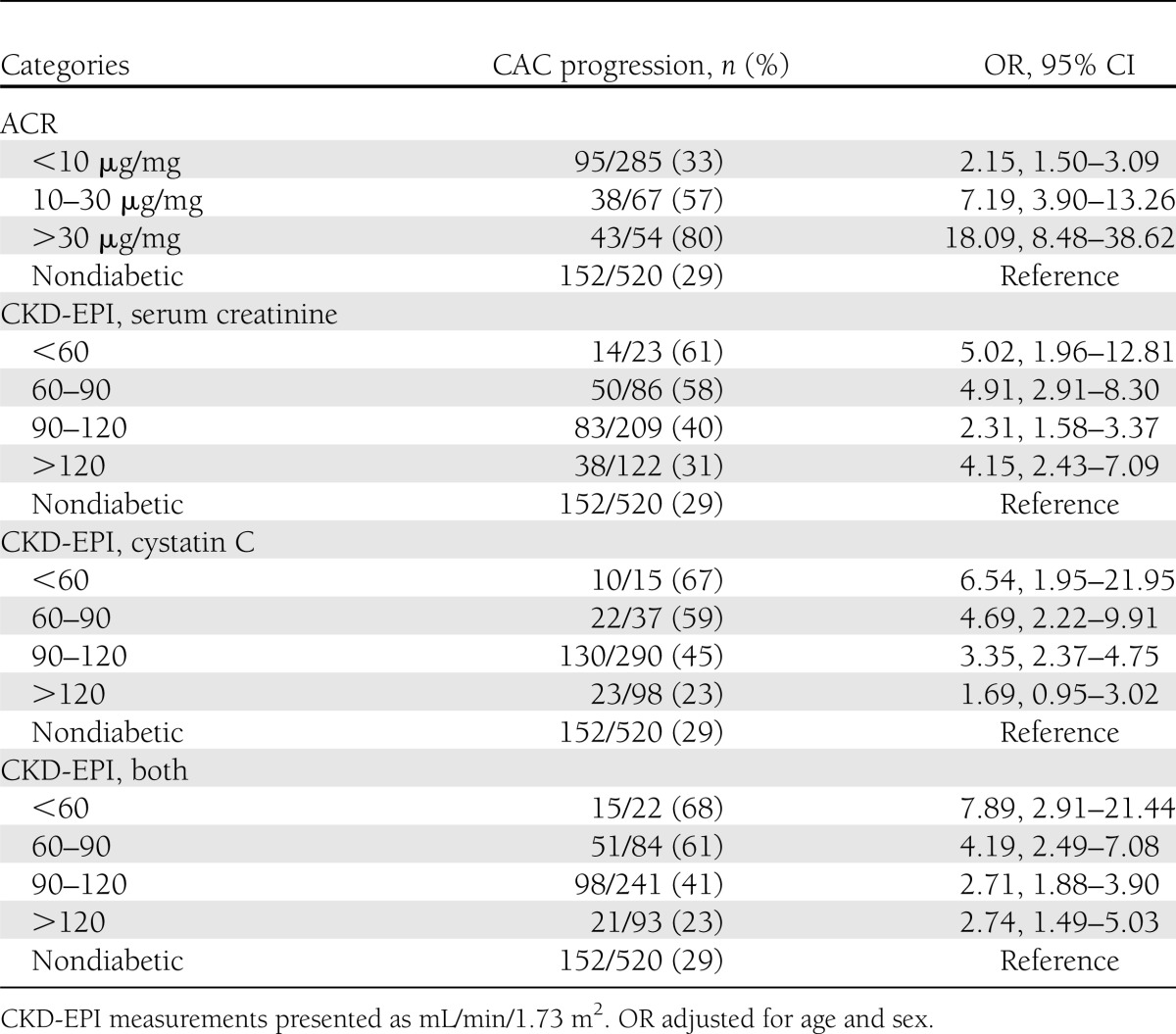
Figure 1.
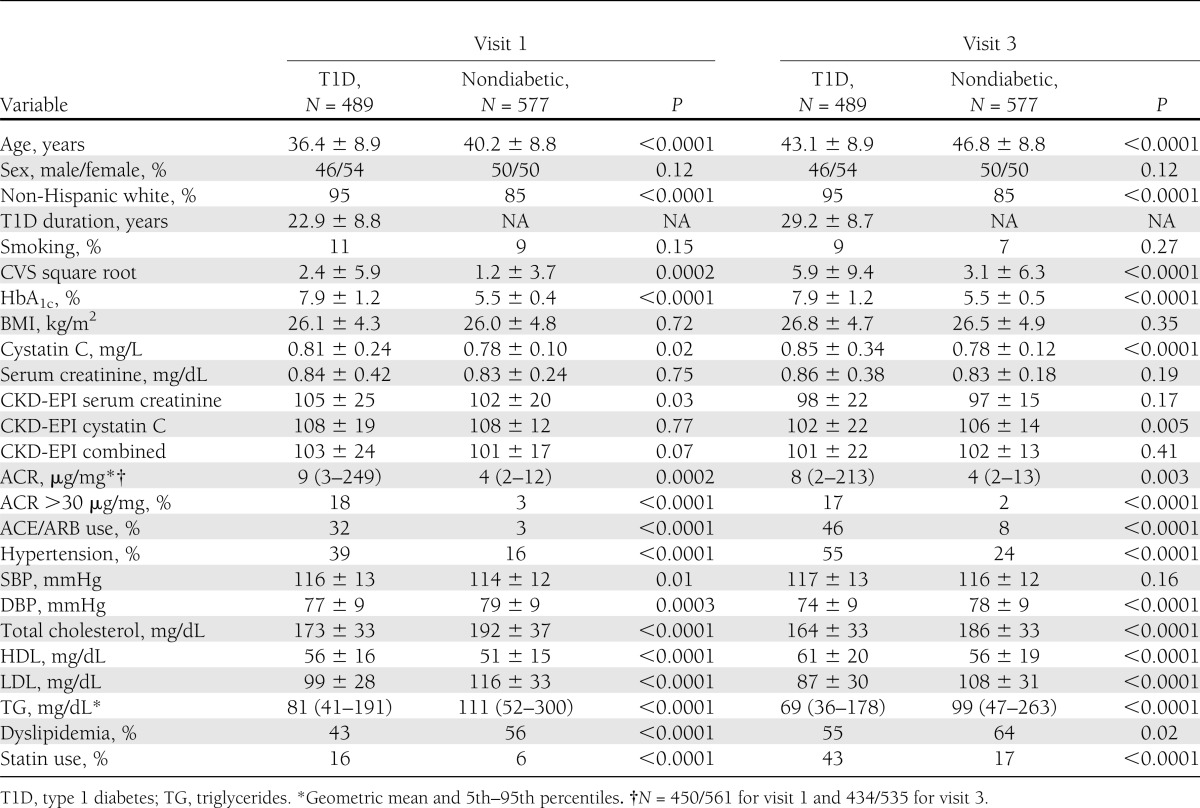
A: OR for CAC progression by ACR status. B: OR for CAC progression by eGFR category (nondiabetic subjects are part of the reference group with OR of 1). C: OR for CAC progression by eGFR category stratified by sex (nondiabetic subjects are the reference group with OR with 1). T1D, type 1 diabetes.
In participants with type 1 diabetes, decreasing renal function as determined by eGFR with all three measures also was associated with increased odds of CAC progression as compared with nondiabetic participants. Baseline eGFR <60 mL/min/1.73 m2 also predicted CAC progression (OR, 5–7, compared with nondiabetic participants). Interestingly, for CKD-EPI serum creatinine there was a J-shape association, whereas for CKD-EPI cystatin C the association was stepwise and for the combined equation the association was intermediate (Fig. 1B). Also of note, for eGFR in both the >120 and 90–120 mL/min/1.73 m2 categories, the odds for CAC progression were still higher than for the nondiabetic participants. Additional adjustment for statin use or smoking status did not change the associations.
We next investigated whether there was a difference in the association of baseline renal function with CAC progression by sex. The interaction terms of sex with baseline ACR status (P = 0.14) and eGFR CKD-EPI serum creatinine (P = 0.11) were not significant, but they were for CKD-EPI cystatin C (P = 0.046) and for CKD-EPI combined (P = 0.014). Data stratified by sex are shown in Table 3 and the odds for CAC progression are significantly higher for women than for men (Fig. 1C). Among participants with type 1 diabetes, men were more likely to have CAC progression (57 vs. 43%; P = 0.0001) and ACR >30 µg/mg (23 vs. 14%; P = 0.01), as were nondiabetic men compared with nondiabetic women (42 vs. 16%; P < 0.0001) for CAC progression and for ACR >30 µg/mg (5 vs. 1%; P = 0.02).
Table 3.
Sex-stratified rates of CAC progression by GFR categories for type 1 diabetic and nondiabetic participants
In univariate analyses stratified by diabetes, age, diabetes duration, BMI, cystatin C, serum creatinine, ACR, SBP, DBP, and triglycerides were positively correlated and eGFR stratified by all three equations and HDL were inversely correlated with CAC progression for participants with type 1 diabetes (P < 0.05). For nondiabetic participants, age, HbA1c, BMI, cystatin C, serum creatinine, SBP, DBP, total cholesterol, LDL, and triglycerides were positively correlated and eGFR stratified by all three equations and HDL were inversely correlated with CAC progression. In multivariate stepwise logistic regression stratified by diabetes, baseline eGFR with each equation (continuous or categorical) was not significantly associated with CAC progression for participants with or without diabetes. Similarly, among nondiabetic participants baseline ACR was not associated with CAC progression. In contrast, among participants with type 1 diabetes, both baseline ACR as a continuous variable (OR, 1.78; 95% CI, 1.36–2.35; P < 0.0001, per SD of ACR) and baseline categorical ACR status (2.00 [1.52–2.63]; P < 0.0001) were significantly associated with CAC progression even with adjustment for age, sex, baseline CVS, HDL, diabetes duration, and BMI. With additional adjustment for ACE or ARB use, the associations were still significant (1.57 [1.18–2.09]; P = 0.002; and (1.79 [1.35–2.39]; P < 0.0001).
In exploratory analyses, baseline characteristics were compared between participants with type 1 diabetes with ACR <10 µg/mg at both visits without and with CAC progression and nondiabetic participants without and with CAC progression. Among participants with type 1 diabetes, those with CAC progression had a longer diabetes duration, were more likely to use ACEs or ARBs, and were more likely to have hypertension and have higher homocysteine (Supplementary Table 1). In contrast, among the nondiabetic participants, those with CAC progression had more uniformly unhealthy CVD risk factors (higher BMI, larger waist, more visceral fat, higher SBP, higher DBP, ACE or ARB use, hypertension diagnosis, higher total cholesterol, higher triglycerides, higher fibrinogen, higher uric acid, and lower HDL.) The participants with type 1 diabetes tended to have "healthier" CVD risk factors compared with the nondiabetic participants by CAC progression status. In logistic regression, results were similar to the analyses with all participants with type 1 diabetes, except that BMI was not significantly associated with CAC progression. When the additional novel risk factors were entered into the stepwise model, only homocysteine was significantly associated with CAC progression.
CONCLUSIONS
CAC is recognized as an important noninvasive measurement of CVD that correlates with increased CVD and mortality. The CACTI study is a unique cohort of participants with and without type 1 diabetes who have been followed-up longitudinally for prospective risk for CAC. Although it is widely accepted that renal dysfunction increases risk of CAC, no prospective study has investigated this in people with and without type 1 diabetes. In this study, we used the three CKD-EPI measurements of eGFR and ACR to evaluate this risk. Our primary finding is a stepwise increased odds for CAC progression in type 1 diabetes with progressive kidney disease and increased odds even with normal renal function. These data emphasize the important interaction between kidney disease and type 1 diabetes on coronary artery disease risk and the importance of renal function in predicting coronary artery disease.
Our data extend and are consistent with recent reports by the FinnDiane (5) and EDC (6) studies in which increasing levels of baseline diabetic kidney disease were predictive of mortality in people with type 1 diabetes. However, in contrast to these studies in which people with type 1 diabetes without diabetic kidney disease had similar standardized mortality rates as the general population (5,6), in CACTI even those participants with normal baseline renal function had increased odds of CAC progression at 6 years compared with nondiabetic participants, even using a lower ACR cut-point of 10 µg/mg. These findings support the public health importance of kidney disease for people with type 1 diabetes (5,6) and highlight the presence of accelerated atherosclerosis even in the absence of diabetic kidney disease.
There are numerous potential explanations and implications of these data. First, in comparison with the FinnDiane and EDC studies, there are not sufficient events in CACTI to calculate mortality outcomes. In contrast, CACTI has used CAC, a marker of subclinical coronary atherosclerosis shown to predict cardiovascular outcomes, as a surrogate marker of CVD (23,24). This contrast between the CACTI data and the previous reports (5,6) may be explained by the use of a surrogate marker of coronary artery disease that captures earlier subclinical atherosclerotic change, whereas even with 7 and 20 years of follow-up the excess risk for CVD in people with type 1 diabetes may not be manifested in clinical events or mortality. For example, the DCCT-EDIC study required 17 years to demonstrate the beneficial effect of intensive insulin therapy on CVD outcomes (25). The data persuade that, for people with type 1 diabetes, increasing diabetic kidney disease increases the risk of CVD and mortality (2–6,26,27). However, the CACTI data demonstrate that even with normal kidney function, people with type 1 diabetes have accelerated progression of atherosclerosis. A recent population-based publication from Scotland of 21,789 adults with type 1 diabetes found the risks for mortality and first CVD event were 2.6- and 2.3-times higher in men and 2.7- and 3.0-times higher in women than in the general population (28). Among the 8,849 individuals with type 1 diabetes with eGFR >90 mL/min/1.73 m2, the risk for incident CVD was 2.1- and 3.7-times higher in men and women than in the general population (28), supporting the concept that even with normal renal function people with type 1 diabetes have increased CVD risk.
To investigate why even the participants with diabetes without renal disease had an increase in CAC progression, exploratory analyses were performed in those with ACR <10 µg/mg at both visits. Progressors with type 1 diabetes were more likely to be hypertensive and report use of ACEs or ARBs and to have higher homocysteine. In contrast, among nondiabetic participants, progressors had the expected differences in multiple CVD risk factors. In fact, the participants with diabetes with ACR <10 µg/mg at both visits had healthier CVD risk profiles when compared with nondiabetic participants by CAC progression status. This suggests unknown pathophysiologic mechanisms may be responsible for progression of early atherosclerosis in young adults with type 1 diabetes and normal ACR. Homocysteine-lowering to reduce CVD risk has been investigated in clinical trials with negative results (29), although not specifically in people with type 1 diabetes.
Although age- and sex-adjusted ACR and eGFR categories were associated with CAC progression in people with type 1 diabetes, in multivariable logistic regression eGFR was not significantly associated with CAC progression, whereas baseline ACR was associated with CAC progression. Different pathophysiologic mechanisms may exist between eGFR with ACR and CAC and its progression. Also, some differences existed for each CKD-EPI eGFR equation and its association with CAC progression. The expected age-related decrease in eGFR over time was seen for participants with type 1 diabetes and nondiabetic participants, except for a minimal increase in eGFR for nondiabetic participants using the CKD-EPI combined equation. A greater difference in eGFR over 6 years was seen for those with type 1 diabetes as compared with nondiabetic participants with the CKD-EPI equations using cystatin C, but not with the CKD-EPI serum creatinine equation. Also, the association of eGFR cystatin C with CAC progression was stepwise but J-shape for eGFR serum creatinine in type 1 diabetic participants. Shlipak (30) previously reported a similar contrast in the association of cystatin C and serum creatinine with mortality and CVD in the elderly. It has been hypothesized that cystatin C may better-capture early GFR change than serum creatinine (31,32). However, the recent article by Inker (10) for the CKD-EPI Investigators suggests that the combined equation may perform better than the individual equations, although they did not specifically address performance of the equations in type 1 diabetes. Moreover, the majority of the CACTI participants had eGFR >60 mL/min/1.73 m2, in which GFR equations are less reliable (33).
We also found that when using the CKD-EPI equations with cystatin C, women with type 1 diabetes had substantially higher odds for CAC progression as compared with nondiabetic women, and that the odds for CAC progression increased more in women than in men with decreasing eGFR. Numerous studies have reported a relative loss of sex protection from CVD (34–36) in women with type 1 diabetes. The recent Scottish registry reported that women with type 1 diabetes have higher relative rates of incident CVD than men with type 1 diabetes as compared with the general population (28). The CACTI data are consistent with these increased CVD rates in women with type 1 diabetes relative to nondiabetic women. One possible explanation for increased CVD in women compared with men is the higher odds of CAC progression with decreasing eGFR; however, such an interpretation should be cautious given the most pronounced effect with eGFR <90 mL/min/1.73 m2 and the relatively small sample size in this category. Data from the EDC study indicate that the incidence of ESRD was higher in men than in women in the 1950–1964 cohort (31 vs. 18%), but is higher in women than in men in the 1965–1980 cohort (8 vs. 14%) at 25 years of diabetes duration (37). In CACTI, men with and without type 1 diabetes were more likely than women to have CAC progression and ACR >30 µg/mg. However, those women with type 1 diabetes with lower eGFR calculated with the cystatin C equations had higher odds for CAC progression than men with type 1 diabetes. We previously reported that cystatin C was associated with CAC progression over 2.5 years in CACTI subjects with type 1 diabetes (26); since this publication, the CKD-EPI equations have been developed and are considered state-of-the-art for eGFR, but they were not associated with CAC progression over 6 years in multivariable analysis.
Our data are novel in that CACTI has longitudinal data regarding a large cohort of participants with and without type 1 diabetes for serum creatinine, cystatin C, ACR, and CAC. However, limitations of our study include no direct measures of GFR using methods such as inulin clearance because ours was a large cohort study. However, we used all three of the recently published CKD-EPI equations that are considered state-of-the-art for eGFR. Incomplete data also may introduce bias. The participants with type 1 diabetes without complete data at baseline had worse renal function and lipids, whereas nondiabetic participants without complete data were younger and had better baseline renal function. These differences may bias our results to the null because of less healthy participants with type 1 diabetes and more healthy nondiabetic participants not being included in the analyses. Also, all nondiabetic participants, even those with elevated ACR, were included. We also examined the ACR data by an even lower cut-point of 10 µg/mg (12), and even below this level at baseline (and at both visits) there were increased odds of CAC progression among people with type 1 diabetes. Other studies have used a lower cut-point for normal ACR in people with type 1 diabetes (38,39), but not (to our knowledge) in respect to CAC progression. Finally, with CACTI samples we performed repeat measures of stored samples to verify and standardize the cystatin C and serum creatinine results (16). Although this strengthens internal validity of our findings, they may differ from measures of other laboratories.
In conclusion, this study demonstrates that people with type 1 diabetes are at increased risk for CAC progression, even in the setting of normal eGFR and normal ACR, but that even subtle worsening of eGFR or ACR amplifies the risk. These data emphasize the importance of eGFR and ACR in the long-term prognosis for people with type 1 diabetes. Pathophysiologic mechanisms responsible for increased CVD risk in type 1 diabetes in the absence of diabetic kidney disease require further investigation. Improving renal and CVD health in people with type 1 diabetes remains an important public health challenge.
Acknowledgments
Support for this study was provided by National Heart, Lung, and Blood Institute grants R01 HL61753 and HL79611, and DERC Clinical Investigation Core P30 DK57516. The study was performed at the Adult Clinical Translational Research Center at University of Colorado Denver, supported by the Grant NIH-M01-RR00051, at the Barbara Davis Center for Childhood Diabetes, and at Colorado Heart Imaging Center in Denver, Colorado. D.M.M. was supported by a grant from National Institute of Diabetes and Digestive and Kidney Diseases (DK075360). J.K.S.-B. was supported by an American Diabetes Association Junior Faculty Award (1-10-JF-50).
No potential conflicts of interest relevant to this article were reported.
D.M.M. researched the manuscript, wrote the manuscript, contributed to discussion, and reviewed and edited the manuscript. D.J. contributed to the discussion and reviewed and edited the manuscript. M.C. contributed to the discussion and reviewed and edited the manuscript. R.J.J. contributed to the discussion and reviewed and edited the manuscript. M.R. designed the CACTI study, performed research, contributed to the discussion, and reviewed and edited the manuscript. J.K.S.-B. performed research, contributed to the discussion, and reviewed and edited the manuscript. D.M.M. and J.K.S.-B. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, Illinois, 21–25 June 2013.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2538/-/DC1.
References
- 1.Maahs DM, Rewers M. Editorial: Mortality and renal disease in type 1 diabetes mellitus—progress made, more to be done. J Clin Endocrinol Metab 2006;91:3757–3759 [DOI] [PubMed] [Google Scholar]
- 2.Andersen AR, Christiansen JS, Andersen JK, Kreiner S, Deckert T. Diabetic nephropathy in type 1 (insulin-dependent) diabetes: an epidemiological study. Diabetologia 1983;25:496–501 [DOI] [PubMed]
- 3.Deckert T, Poulsen JE, Larsen M. Prognosis of diabetics with diabetes onset before the age of thirty-one. II. Factors influencing the prognosis. Diabetologia 1978;14:371–377 [DOI] [PubMed]
- 4.Dorman JS, LaPorte RE, Kuller LH, et al. The Pittsburgh Insulin-dependent Diabetes Mellitus (IDDM) Morbidity and Mortality Study Mortality results. Diabetes 1984;33:271–276 [DOI] [PubMed]
- 5.Groop PH, Thomas MC, Moran JL, et al. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 2009;58:1651–1658 [DOI] [PMC free article] [PubMed]
- 6.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia 2010;53:2312–2319 [DOI] [PMC free article] [PubMed]
- 7.American Diabetes Association Standards of medical care in diabetes—2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maahs DM. Early detection of kidney disease in type 1 diabetes: what do we really know? Diabetes Technol Ther 2012;14:541–544 [DOI] [PubMed] [Google Scholar]
- 9.Maahs DM, Caramori ML, Cherney DZI, et al. ; on behalf of the PERL Consortium Uric acid lowering to prevent kidney function loss in diabetes: The Preventing Early Renal Function Loss (PERL) Allopurinol Study. Curr Diab Rep 2013;13:550–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inker LA, Schmid CH, Tighiouart H, et al. CKD-EPI Investigators Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367:20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ninomiya T, Perkovic V, de Galan BE, et al. ADVANCE Collaborative Group Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 2009;20:1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsushita K, van der Velde M, Astor BC, et al. Chronic Kidney Disease Prognosis Consortium Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010;375:2073–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maahs DM, Kinney GL, Wadwa P, et al. Hypertension prevalence, awareness, treatment, and control in an adult type 1 diabetes population and a comparable general population. Diabetes Care 2005;28:301–306 [DOI] [PubMed] [Google Scholar]
- 14.The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med 1997;157:2413–2446 [DOI] [PubMed] [Google Scholar]
- 15.Wadwa RP, Kinney GL, Maahs DM, et al. Awareness and treatment of dyslipidemia in young adults with type 1 diabetes. Diabetes Care 2005;28:1051–1056 [DOI] [PubMed] [Google Scholar]
- 16.Maahs DM, Jalal D, McFann K, Rewers M, Snell-Bergeon JK. Systematic shifts in cystatin C between 2006 and 2010. Clin J Am Soc Nephrol 2011;6:1952–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snell-Bergeon JK, Hokanson JE, Jensen L, et al. Progression of coronary artery calcification in type 1 diabetes: the importance of glycemic control. Diabetes Care 2003;26:2923–2928 [DOI] [PubMed] [Google Scholar]
- 18.Hokanson JE, MacKenzie T, Kinney G, et al. Evaluating changes in coronary artery calcium: an analytic method that accounts for interscan variability. AJR Am J Roentgenol 2004;182:1327–1332 [DOI] [PubMed] [Google Scholar]
- 19.Jassal SK, Chonchol M, Laughlin GA, et al. Kidney function and progression of coronary artery calcium in community-dwelling older adults (from the Rancho Bernardo Study). Am J Cardiol 2012;110:1425–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maahs DM, Ogden LG, Kinney GL, et al. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation 2005;111:747–753 [DOI] [PubMed] [Google Scholar]
- 21.Jalal DI, Rivard CJ, Johnson RJ, et al. Serum uric acid levels predict the development of albuminuria over 6 years in patients with type 1 diabetes: findings from the Coronary Artery Calcification in Type 1 Diabetes study. Nephrol Dial Transplant 2010;25:1865–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodrigues TC, Maahs DM, Johnson RJ, et al. Serum uric acid predicts progression of subclinical coronary atherosclerosis in individuals without renal disease. Diabetes Care 2010;33:2471–2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Budoff MJ, Hokanson JE, Nasir K, et al. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging 2010;3:1229–1236 [DOI] [PubMed] [Google Scholar]
- 24.Peters SA, Bakker M, den Ruijter HM, Bots ML. Added value of CAC in risk stratification for cardiovascular events: a systematic review. Eur J Clin Invest 2012;42:110–116 [DOI] [PubMed] [Google Scholar]
- 25.Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maahs DM, Ogden LG, Kretowski A, et al. Serum cystatin C predicts progression of subclinical coronary atherosclerosis in individuals with type 1 diabetes. Diabetes 2007;56:2774–2779 [DOI] [PubMed]
- 27.Maahs DM, Snell-Bergeon JK, Kinney GL, et al. ACE-I/ARB treatment in type 1 diabetes patients with albuminuria is associated with lower odds of progression of coronary artery calcification. J Diabetes Complications 2007;21:273–279 [DOI] [PubMed] [Google Scholar]
- 28.Livingstone SJ, Looker HC, Hothersall EJ, et al. Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish registry linkage study. PLoS Med 2012;9:e1001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller ER, 3rd, Juraschek S, Pastor-Barriuso R, Bazzano LA, Appel LJ, Guallar E. Meta-analysis of folic acid supplementation trials on risk of cardiovascular disease and risk interaction with baseline homocysteine levels. Am J Cardiol 2010;106:517–527 [DOI] [PubMed] [Google Scholar]
- 30.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 2005;352:2049–2060 [DOI] [PubMed] [Google Scholar]
- 31.Perkins BA, Nelson RG, Krolewski AS. Cystatin C and the risk of death. N Engl J Med 2005;353:842–844; author reply 842–844 [PubMed] [Google Scholar]
- 32.Perkins BA, Nelson RG, Ostrander BE, et al. Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: results of a 4-year follow-up study. J Am Soc Nephrol 2005;16:1404–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med 2006;354:2473–2483 [DOI] [PubMed] [Google Scholar]
- 34.Laing SP, Swerdlow AJ, Slater SD, et al. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia 2003;46:760–765 [DOI] [PubMed]
- 35.Colhoun HM, Rubens MB, Underwood SR, Fuller JH. The effect of type 1 diabetes mellitus on the gender difference in coronary artery calcification. J Am Coll Cardiol 2000;36:2160–2167 [DOI] [PubMed] [Google Scholar]
- 36.Lloyd CE, Kuller LH, Ellis D, Becker DJ, Wing RR, Orchard TJ. Coronary artery disease in IDDM. Gender differences in risk factors but not risk. Arterioscler Thromb Vasc Biol 1996;16:720–726 [DOI] [PubMed] [Google Scholar]
- 37.Costacou T, Fried L, Ellis D, Orchard TJ. Sex differences in the development of kidney disease in individuals with type 1 diabetes mellitus: a contemporary analysis. Am J Kidney Dis 2011;58:565–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chase HP, Marshall G, Garg SK, Harris S, Osberg I. Borderline increases in albumin excretion rate and the relation to glycemic control in subjects with type I diabetes. Clin Chem 1991;37:2048–2052 [PubMed] [Google Scholar]
- 39.Cho YH, Craig ME, Hing S, et al. Microvascular complications assessment in adolescents with 2- to 5-yr duration of type 1 diabetes from 1990 to 2006. Pediatr Diabetes 2011;12:682–689 [DOI] [PubMed] [Google Scholar]