Abstract
OBJECTIVE
The objective of this study was to evaluate the relationship between childhood and adult secondhand smoke and type 2 diabetes.
RESEARCH DESIGN AND METHODS
We conducted a prospective cohort study among 37,343 French women from the E3N-EPIC (Etude Epidémiologique auprès des femmes de la Mutuelle Générale de l’Education Nationale-European Prospective Investigation into Cancer and Nutrition) who never smoked and who were free of type 2 diabetes, cancer, or cardiovascular disease at baseline in 1992. Self-reported childhood secondhand smoke exposure was defined as having at least one parent who smoked. Adult secondhand smoke was defined as the sum of self-reported hours recorded at baseline of exposure to tobacco smoke from a spouse who smoked (or domestic close contact) and from outside the home.
RESULTS
Between 1992 and 2007, 795 cases of incident type 2 diabetes were identified and validated through a drug reimbursement dataset and a specific questionnaire. Women with at least one parent who smoked appeared to have an 18% higher rate of type 2 diabetes than women with parents who did not smoke (age-adjusted hazard ratio 1.18 [95% CI 1.02–1.36]). Adult secondhand smoke exposure (no exposure versus ≥4 h/day) was associated with an increased rate of type 2 diabetes (1.36 [1.05–1.77], P = 0.002 for trend) after adjusting for parental history of diabetes, education, body silhouette at age 8, childhood secondhand smoke exposure, physical activity, body mass index, hypertension, hypercholesterolemia, menopausal status and hormone use, alcohol intake, and processed red meat and coffee consumption.
CONCLUSIONS
This prospective analysis suggests that secondhand smoke exposure in childhood and adulthood are associated with a higher rate of type 2 diabetes.
It is estimated that 603,000 nonsmokers worldwide die of exposure to secondhand smoke (1). Even after evidence from the United States and Western Europe that smoke-free legislation lowers acute myocardial infarction rates (2,3), national comprehensive policies for smoke-free environments are still lacking in many countries. Twenty-two U.S. states and 13 European Union member states still do not have smoking bans in public places (4,5). In some U.S. states, up to 18% of children are regularly exposed to secondhand smoke (6), and even in countries where tobacco bans have been in place, secondhand smoke exposure among nonsmokers may be as high as 30% (7).
Prospective studies have consistently observed a direct relationship between tobacco smoking and type 2 diabetes (8). According to animal models, tobacco smoke exposure results in chronic pancreatic inflammation (9) and affects weight gain and glucose metabolism (10,11). In humans, secondhand smoke is associated with obesity (12) and insulin resistance (13) in children who were exposed early in life. Consequently, exposure to secondhand tobacco smoke may play a role in childhood obesity and in the 1 million deaths in North America and Europe attributable to type 2 diabetes (14). Thus, limiting secondhand smoke exposure in the population may have a positive impact on this worldwide epidemic.
Few studies evaluated the role of secondhand smoke on incident type 2 diabetes (15–19). These analyses had limited detail on exposure, did not account for important type 2 diabetes risk factors, or had insufficient follow-up. Therefore, we evaluated secondhand smoke exposure in relation to incident type 2 diabetes in a large prospective cohort of French women who were nonsmokers and who responded to a detailed questionnaire on childhood and current exposure to secondhand tobacco smoke and were followed for up to 15 years.
RESEARCH DESIGN AND METHODS
Study population
The Etude Epidémiologique auprès des femmes de la Mutuelle Générale de l’Education Nationale (E3N) is a French prospective cohort study of 98,995 female members of a health insurance plan covering mostly teachers and teacher-spouses that began in 1990 and is the French component of the EPIC (European Prospective Investigation into Cancer and Nutrition) study. Participants return mailed questionnaires to update health-related information every 2–3 years, and a drug reimbursement claims database has been available since 2004 from their medical insurance provider (MGEN [Mutuelle Générale de l’Education Nationale]). Average follow-up per questionnaire cycle has been 83%, and loss to follow-up is <3%. In 1992, 86,164 participants responded to a questionnaire that included detailed information on secondhand tobacco smoke exposure. We excluded current (n = 12,611) and past smokers (n = 27,061) and women with missing smoking status (n = 5,072); with prevalent type 2 diabetes, cancer, or cardiovascular disease (n = 3,161); and with no follow-up after 1992 (n = 916). The final study population was 37,343 nonsmoking women. All participants signed an informed consent letter to comply with the French National Commission for Computerized Data and Individual Freedom.
Secondhand tobacco smoke exposure assessment
In 1992, participants were asked about parental smoking during their childhood (yes, no, do not know). For frequency of exposure in childhood, participants were asked, “During childhood, how often did you remain in a room with tobacco smoke?” (never, do not know, rarely, occasionally [some hours per week], a few hours per day, many hours per day). Regarding their current exposure, participants were asked, “Currently, does your spouse (or the person you live with) smoke?” (no spouse; no; yes, occasionally; yes, regularly). Finally, participants were asked to estimate their daily secondhand tobacco smoke exposure at home and outside the home separately by summing the time spent during the day in a room while someone was smoking (18 response categories from 0 to ≥16 h/day).
Ascertainment of type 2 diabetes
As previously described (20), 3,496 type 2 diabetes cases were identified up to 2007 through information from several sources (Supplementary Fig. 1). First, potential cases were women who self-reported either type 2 diabetes, use of diabetic medications, or a hospitalization for type 2 diabetes in at least one of the eight follow-up questionnaires up to July 2005 (n = 4,289). Among them, 2,315 were found to have at least one reimbursement claim for diabetic medications (acarbose, carbutamide, glibenclamide, glibornuride, glicazide, glimepiride, glipizide, insulin, metformin, pioglitazone, repaglinide, or rosiglitazone) and were classified as confirmed cases. Of the 1,974 remaining, 342 were validated through a supplementary questionnaire that assessed diagnosis date, symptoms, fasting or random glucose concentrations at diagnosis, current therapy, and the most recent values for fasting glucose and HbA1c. Type 2 diabetes was confirmed if participants reported a glucose level at diagnosis above the World Health Organization recommendations (fasting ≥1.26 g/L, random glucose ≥2.00 g/L), being on drug therapy for type 2 diabetes, or having a recent fasting glucose level of ≥1.26 g/L or HbA1c ≥7%. Date of diagnosis was defined as the date of first report of type 2 diabetes. As a second validation strategy, we sent the supplementary questionnaire to 1,139 women who had filed at least once for reimbursement for diabetic medications between 1 January 2004 and 30 June 2007 and had not previously self-reported type 2 diabetes; we confirmed type 2 diabetes in 458. Among the 405 women who did not respond to the supplementary questionnaire, we confirmed type 2 diabetes in 338 who had filed for diabetic medication reimbursement two or more times.
Covariate assessment
In the baseline questionnaire (in 1990), we obtained information on parental history of diabetes, education, body silhouette at age 8, menopausal status, weight, height, physical activity, treated hypertension, and hypercholesterolemia. Weight and height were used to calculate the BMI (defined as weight [kg] divided by height squared [m2]). Regular moderate and vigorous physical activity were assessed with a validated physical activity questionnaire and transformed into weekly METs (21). Dietary data were collected in 1993 with a previously validated self-administered diet history questionnaire (22).
Statistical analysis
We classified participants as having no or at least one parent who smoked and assumed that participants who responded that they did not know the smoking status of their parents were not exposed. Participants were also classified into one of the following categories: never, rare, occasional, and regular childhood secondhand smoke exposure. Total daily adult secondhand smoke exposure was defined as the sum of secondhand smoke exposure at home and outside the home and was categorized as no exposure or <1, 1–1.9, 2–3.9, or ≥4 h/day, with no exposure to secondhand smoke used as the reference category. When one component was missing, exposure level was set to 0. For home exposure, participants who reported having no spouse or a nonsmoking spouse were considered unexposed. Person-time at risk was calculated from the date of completion of the 1992 questionnaire to the date of diagnosis of type 2 diabetes, death, mailing of the last follow-up questionnaire (2005), or date of last follow-up, whichever occurred first. Cox multivariate regression models with age as the time scale were fit to estimate hazard ratios (HRs) and 95% CIs with the SAS PHREG procedure (SAS Institute Inc., Cary, NC). For adult secondhand smoke analyses, multivariate models were adjusted for parental history of diabetes, education (less than high school, high school, and college or more), body silhouette at age 8 (1, 2, or ≥3), childhood secondhand smoke exposure, treated hypercholesterolemia and hypertension, BMI (continuously), menopausal hormone therapy (premenopausal, ever use, never use), and physical activity level (quartiles) at baseline. In an additional multivariate model, we included the following dietary factors: energy intake excluding alcohol (quartiles), alcohol (<5, 5–10, 10–15, and ≥15 g/day) and coffee (0, ≤ 1, 1–3, and ≥ 3 cups/day) intake, and processed red meat consumption (<1, 1–3, 3–5, and ≥ 5 servings/week). Variables to adjust for confounding were diabetes risk factors that could potentially cause or share a common cause with secondhand smoke exposure. To test for linear trend, the median value for each category was included as a continuous variable. We tested whether the relationship between secondhand smoke and type 2 diabetes risk differed by BMI (<25 or ≥25 kg/m2) by including a cross-product term of the median or ordinal value for each category of secondhand smoke exposure as a continuous variable and BMI as a dichotomous variable. We compared models with and without the cross-product term by log-likelihood test.
RESULTS
After a median follow-up of 13.4 years (466,133 person-years), we identified 795 incident cases of type 2 diabetes. Fifty-eight percent of participants reported exposure to parental smoking. Most of the exposure was paternal, with only 3% of participants reporting exposure to maternal smoking. Parental smoking was not strongly related to education and body type. Incidence was 155 per 100,000 person-years among participants who were not exposed to childhood secondhand smoke and 182 per 100,000 person-years among those with at least one parent who smoked, leading to an age-adjusted HR of 1.18 (95% CI 1.02–1.36). Adjusting for potential confounders that may also be intermediates (education and body silhouette at age 8) yielded a slightly attenuated estimate (multivariate HR 1.15, 0.99–1.33). In an additional analysis, we excluded 284 women who did not know or did not report parental smoking status and were considered unexposed. Results were unaltered. There was no association between intensity of childhood secondhand smoke exposure and type 2 diabetes risk. Relative to no or unknown exposure, multivariate HRs were 0.96 (0.81–1.14) for rare, 1.04 (0.85–1.27) for occasional, and 1.08 (0.87–1.33) for regular (a few hours and many hours per day) exposure (P = 0.45 for trend).
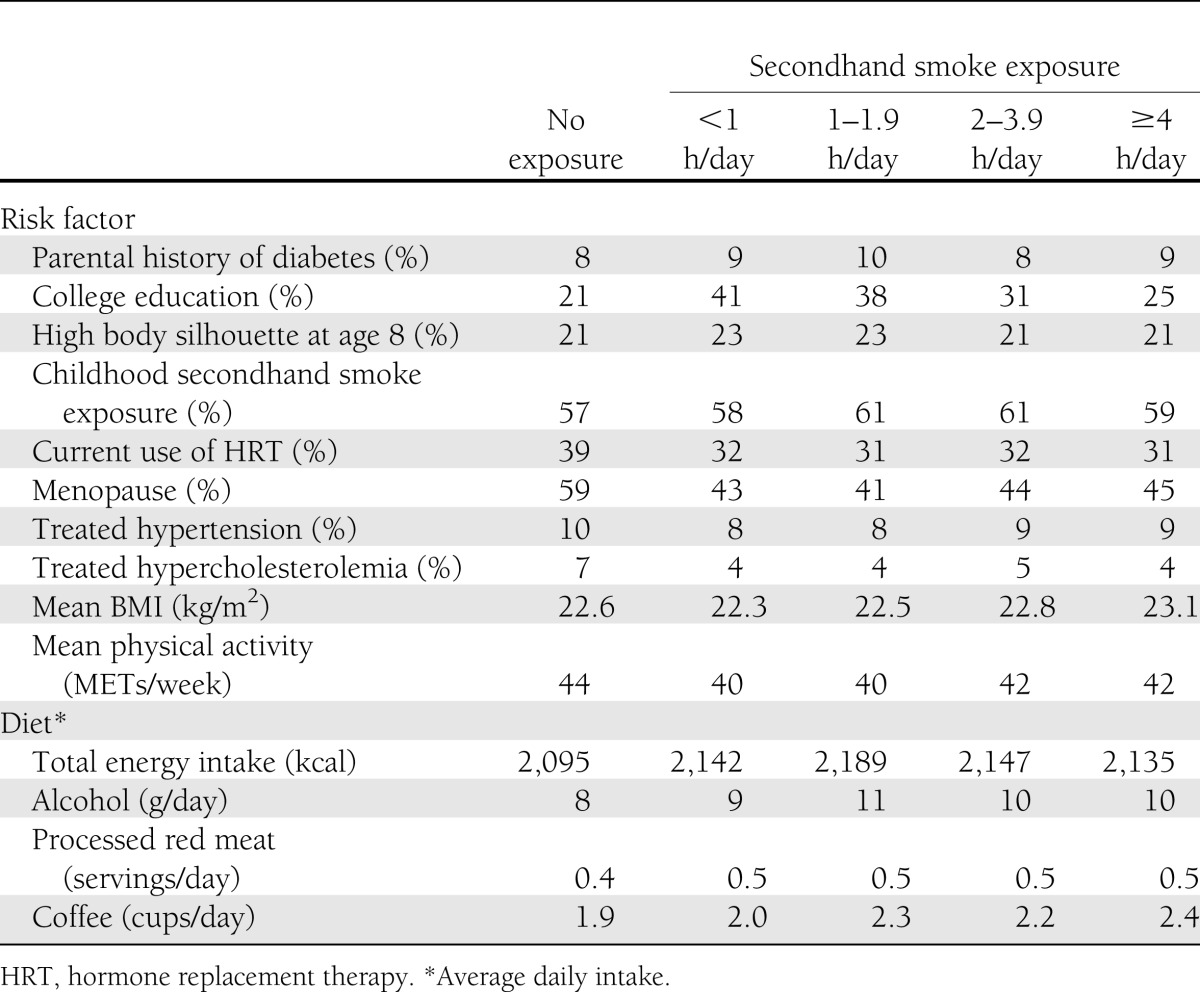
The mean ± SD adult secondhand smoke exposure was 0.9 ± 1.9 h/day (10th–90th percentile 0.0–2.5), home exposure was 1.6 ± 2.4 h/day (0.0–4.0), and exposure outside the home was 0.5 ± 1.3 h/day (0.0–1.0). BMI increased with increasing categories of total adult exposure to secondhand smoke, and higher educational attainment seemed to be more common among women who reported exposure than among those who identified themselves as unexposed; however, among the exposed participants, we observed an inverse relation (Table 1).
Table 1.
Age-standardized characteristics of the cohort
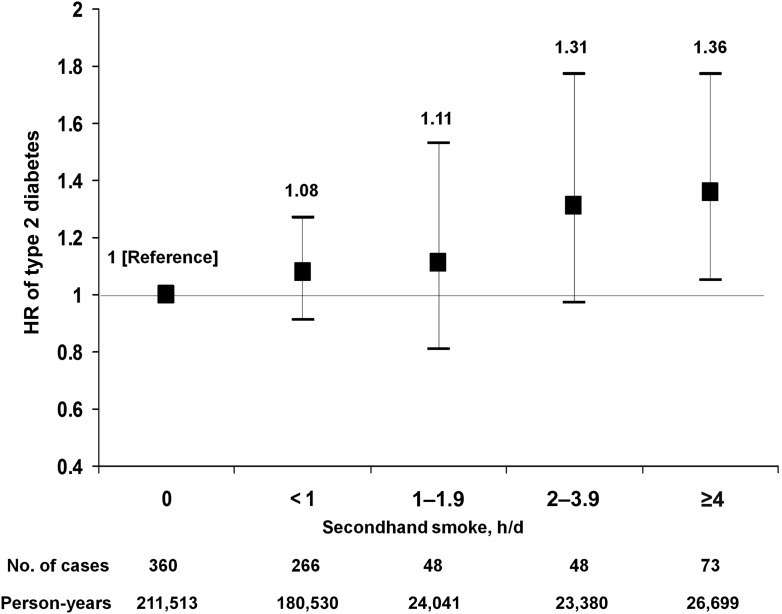
For adult secondhand smoke exposure, type 2 diabetes incidence was 170 per 100,000 person-years for both participants who reported no exposure and those who reported exposure. However, the age-adjusted HR comparing women with exposure to adult secondhand smoke compared with no exposure was 1.18 (95% CI 1.02–1.36) and the multivariate HR was 1.16 (1.00–1.34). When evaluating the dose of exposure, the age-adjusted HR was 1.89 (1.46–2.43, P < 0.0001 for trend) for women who reported ≥4 h/day of secondhand exposure compared with those who reported no exposure (Table 2). After adjusting for parental history of diabetes, education, body silhouette at age 8, childhood secondhand smoke exposure, BMI, physical activity, menopause, hormone replacement therapy, treated hypercholesterolemia and hypertension, and alcohol, coffee, and processed red meat consumption, participants who reported ≥4 h/day of secondhand smoke had a statistically significant 36% higher rate of type 2 diabetes than those who reported no exposure to secondhand smoke (1.36 [1.05–1.77]). There appeared to be a direct dose-response relationship between daily secondhand tobacco smoke exposure and the rate of type 2 diabetes in the multivariate model (P = 0.002 for trend) (Fig. 1). In a sensitivity analysis, we excluded 276 women who started smoking during follow-up to account for the possibility that women who are exposed to secondhand smoke may be more likely to start smoking. Results did not materially change (data not shown).
Table 2.
Rate of type 2 diabetes according to daily hours of exposure to secondhand smoke
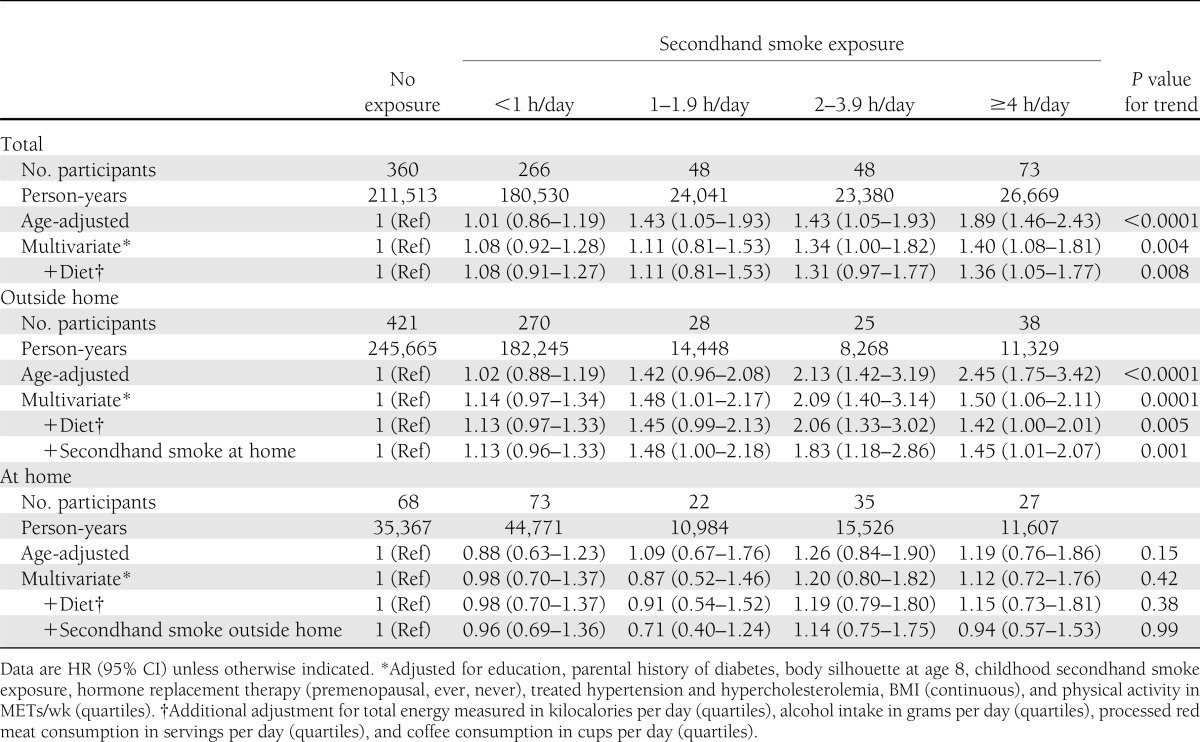
Figure 1.
Multivariate adjusted HRs for type 2 diabetes according to adult secondhand smoke exposure (P = 0.008 for trend). Error bars indicate 95% CIs. Secondhand smoke was calculated by summing exposure at home and outside the home and adjusted for parental history of diabetes, education, body silhouette at age 8, childhood secondhand smoke exposure, hormone replacement therapy (premenopausal, ever, never), treated hypertension and hypercholesterolemia, and BMI (continuous) and for physical activity measured in METs per week (quartiles), total energy in kilocalories per day (quartiles), alcohol intake in grams per day (quartiles), processed red meat consumption in servings per day (quartiles), and coffee consumption in cups per day (quartiles). d, day.
The association between type 2 diabetes risk and adult secondhand smoke seemed to be restricted to exposure outside the home. The multivariate HR comparing participants who reported ≥4 h/day of secondhand smoke exposure outside the home to no exposure was 1.45 (95% CI 1.01–2.07, P = 0.001 for trend). Women who lived with a person who smoked seemed to have a higher rate of type 2 diabetes than those who did not; however, results were not significant (1.10 [0.84–1.44] for living with an occasional smoker and 1.15 [0.95–1.39] for living with a regular smoker). The HR comparing women in the highest exposure category of daily hours of secondhand exposure at home compared with no exposure at home was 0.94 (0.57–1.53). Information on hours of secondhand smoke exposure at home was missing for 75% of the person-time. Estimates comparing extreme categories of exposure were slightly strengthened but nonsignificant when we conducted sensitivity analyses for total exposure restricted to participants for whom information was complete for both sources of exposure (1.52 [0.95–2.40]) and for secondhand exposure at home where missing values were considered 0 (1.20 [0.81–1.77]).
The observed association between secondhand smoke and type 2 diabetes differed slightly according to BMI. The HRs comparing extreme categories of exposure to total secondhand smoke exposure were 1.82 (95% CI 1.20–2.76, P = 0.001 for trend) for BMI <25 kg/m2 and 1.36 (0.99–1.89, P = 0.08 for trend) for BMI ≥25 kg/m2, but the test for heterogeneity was not statistically significant (P = 0.28). We also evaluated whether having had a parent who smoked resulted in differences in the association between secondhand smoke exposure in adulthood and type 2 diabetes. We found no evidence of a statistical interaction (P = 0.29 for the test for heterogeneity).
CONCLUSIONS
In a large prospective cohort of nonsmoking French women free of type 2 diabetes at baseline and followed for up to 15 years, type 2 diabetes rates increased with secondhand exposure in childhood and with increasing exposure to secondhand smoke in adulthood. For adult exposure, the association appeared to be restricted to exposure outside the home.
Different biological mechanisms may explain the observed associations. Rats exposed to nicotine early in life have higher fat mass, insulinemia, and increased leptin levels in adulthood than do unexposed rats (10). Thus, early nicotine exposure may program individuals for future adipocyte hypertrophy and insulin and leptin resistance. Additionally, exposure to environmental tobacco smoke results in chronic pancreatic inflammation in rats (9) and induces the production of interleukin-1β (23) in the lung. This cytokine is believed to govern the pancreatic inflammation observed in type 2 diabetes that may result in β-cell death and impair insulin production (24).
A meta-analysis of 25 prospective cohort studies found a 44% higher rate of type 2 diabetes among smokers than among nonsmokers (8). However, evidence for the role of secondhand smoke in type 2 diabetes risk is limited. In the present study, we observed a significant association between parental smoking and subsequent risk of type 2 diabetes. We evaluated the sensitivity of the results to adjustment by a proxy of parental social status, the participants’ education, and body size at age 8. The association became borderline statistically significant; however, these variables could potentially be intermediates. In cross-sectional analyses of children (12) and adolescents (25), individuals exposed to environmental tobacco were four times more likely to be overweight than those who were not exposed. In a group of 10-year-olds participating in two birth cohorts, having a mother who smoked during pregnancy and being exposed to secondhand tobacco smoke in childhood were associated with insulin resistance (13). It is unlikely that our observation is the result of maternal smoking during pregnancy or lactation because only 3% of the participants with a parent who smoked reported that the parent was their mother. Thus, the present results may only reflect childhood secondhand smoke exposure and not exposure in utero.
In a large prospective cohort of women with a 24-year follow-up in the United States, a borderline significant 16% higher rate of type 2 diabetes was observed among nonsmokers who were regularly exposed to secondhand cigarette smoke than among women who reported not being exposed (19). In a prospective study of young adults with a 15-year follow-up, passive never-smokers had a 35% higher rate of glucose intolerance relative to nonsmokers (17). In a much smaller cohort of 4,442 Korean never-smokers with a detailed assessment of frequency and duration of secondhand smoke at home and at work, there appeared to be a linear relationship between daily hours of exposure and incidence of type 2 diabetes (16). Compared with participants who declared not being exposed to secondhand tobacco smoke, the incidence of type 2 diabetes among those who declared exposure to 0–1, 1.1–2, 2.1–4, and >4 h/day was 34, 32, 44, and 96% higher, respectively. Our estimate for individuals exposed to ≥4 h/day was smaller than that observed in Korea (HR 1.36). Residual confounding by omitted lifestyle variables in the Korean study, like dietary factors, may partly explain the observed difference in estimates.
There are several explanations for the absence of an association between secondhand smoke exposure at home and type 2 diabetes in the present study. For a large proportion of participants, we were unable to assign secondhand exposure at home; thus, the analysis may be underpowered to detect an association, and given the limited number of cases in certain categories, estimates are very unstable. Alternatively, the intensity of exposure may be much higher outside the home than within the home. This explanation is consistent with a prior observation that secondhand smoke outside the home is associated with lung cancer, but no association was found with exposure at home (26).
The major strengths of this study are its prospective nature, detailed report of secondhand smoke exposure, large sample size, and availability of type 2 diabetes risk factor information. The study also has limitations, and the results should be interpreted with caution. One limitation was the use of a nonvalidated self-reported assessment of secondhand smoke as the main exposure measure. Because in many environments secondhand smoke is ubiquitous, misclassification of secondhand exposure is common. In a group of people with asthma, the correlation between reported exposure and urine cotinine levels was 0.47 (27). Among nonsmokers in a cancer screening clinic, 76% reported exposure to secondhand smoke, whereas 91% had detectable levels of cotinine (28). In addition, secondhand smoke exposure was evaluated only at baseline, whereas the exposure level may have changed over time. Nevertheless, it is likely that the error introduced is random and independent of the outcome, which would result in an attenuation of the observed associations and may explain the null results with regard to intensity of childhood and at-home exposure to secondhand smoke. Similarly, misclassification of type 2 diabetes status may have occurred. This error is also likely to be nondifferential with respect to exposure status. Additionally, we expected few, if any, false-positive results; therefore, the bias introduced was probably minimal. We cannot rule out the possibility of confounding by unmeasured factors and residual confounding by risk factors for type 2 diabetes that were measured with error. For example, parental social status may be a common cause of parental smoking and type 2 diabetes risk. Information on parental social status was unavailable. However, as mentioned previously, we were able to include in our analyses all the major lifestyle risk factors for type 2 diabetes, and we have previously shown the validity of these factors (21,22). Selection bias may have been introduced by excluding from our analyses 5,072 women (6.2% of the cohort) for whom information on smoking status at baseline was not available. Given this small proportion, this bias is likely to have been small. Finally, the results may not be generalizable to men or non-Caucasian populations. However, the results are similar to those reported in an Asian population comprising both sexes, indicating that the associations may not differ by race, ethnicity, or sex (16).
In conclusion, this prospective analysis suggests that secondhand smoke exposure in childhood and adulthood is associated with a higher rate of type 2 diabetes. Smoke-free environments have proven to be relatively easy to implement and effective in the control of cardiovascular disease (2,3). Curbing the worldwide type 2 diabetes epidemic requires extensive and lasting changes in public policy. Limiting secondhand smoke exposure by providing smoke-free environments and improving compliance with smoking bans may be an important strategy.
Acknowledgments
The E3N study was performed with the financial support of the Mutuelle Générale de l’Education Nationale, European Community, French League against Cancer, Gustave-Roussy Institute, French Institute of Health and Medical Research, and several general councils in France. The validation of potential type 2 diabetes cases was supported by the European Union (Integrated Project LSHM-CT-2006-037197 in the Framework Program 6 of the European Community) InterAct project. M.L. was supported by the National Council for Science and Technology (CONACYT, Mexico) and the Department of Epidemiology at the Harvard School of Public Health.
No potential conflicts of interest relevant to this article were reported.
M.L. wrote the analysis plan and drafted and revised the manuscript. L.T. cleaned and analyzed the data and revised the manuscript. G.F. designed the data collection tools and revised the manuscript. B.d.L.-G. designed the data collection tools, validated the cases, and revised the manuscript. M.-C.B.-R. monitored the data collection and revised the manuscript. F.C.-C. initiated the study, secured funding, designed and monitored the data collection, and revised the manuscript. M.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Results of this study were presented at the American Heart Association Epidemiology and Prevention/Nutrition, Physical Activity and Metabolism 2012 Scientific Sessions, San Diego, California, 13–16 March 2012.
The authors thank the participants in the E3N study for their continuing dedication and support.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2173/-/DC1.
A slide set summarizing this article is available online.
References
- 1.Oberg M, Jaakkola MS, Woodward A, Peruga A, Prüss-Ustün A. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet 2011;377:139–146 [DOI] [PubMed] [Google Scholar]
- 2.Pell JP, Haw S, Cobbe S, et al. Smoke-free legislation and hospitalizations for acute coronary syndrome. N Engl J Med 2008;359:482–491 [DOI] [PubMed] [Google Scholar]
- 3.Dove MS, Dockery DW, Mittleman MA, et al. The impact of Massachusetts’ smoke-free workplace laws on acute myocardial infarction deaths. Am J Public Health 2010;100:2206–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Cancer Institute Tobacco Control Research Branch. Smokefree.gov website. Available from http://smokefree.gov Accessed 9 January 2012
- 5.World Health Organization, WHO Report on the Global Tobacco Epidemic, 2011: Warning About the Dangers of Tobacco. Geneva, World Health Organization, 2011 [Google Scholar]
- 6.Singh GK, Siahpush M, Kogan MD. Disparities in children’s exposure to environmental tobacco smoke in the United States, 2007. Pediatrics 2010;126:4–13 [DOI] [PubMed] [Google Scholar]
- 7.Martínez-Sánchez JM, Gallus S, Zuccaro P, et al. Exposure to secondhand smoke in Italian non-smokers 5 years after the Italian smoking ban. Eur J Public Health 2012;22:707–712 [DOI] [PubMed] [Google Scholar]
- 8.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2007;298:2654–2664 [DOI] [PubMed] [Google Scholar]
- 9.Wittel UA, Pandey KK, Andrianifahanana M, et al. Chronic pancreatic inflammation induced by environmental tobacco smoke inhalation in rats. Am J Gastroenterol 2006;101:148–159 [DOI] [PubMed] [Google Scholar]
- 10.de Oliveira E, Moura EG, Santos-Silva AP, et al. Neonatal nicotine exposure causes insulin and leptin resistance and inhibits hypothalamic leptin signaling in adult rat offspring. J Endocrinol 2010;206:55–63 [DOI] [PubMed] [Google Scholar]
- 11.Ng SP, Conklin DJ, Bhatnagar A, Bolanowski DD, Lyon J, Zelikoff JT. Prenatal exposure to cigarette smoke induces diet- and sex-dependent dyslipidemia and weight gain in adult murine offspring. Environ Health Perspect 2009;117:1042–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raum E, Küpper-Nybelen J, Lamerz A, Hebebrand J, Herpertz-Dahlmann B, Brenner H. Tobacco smoke exposure before, during, and after pregnancy and risk of overweight at age 6. Obesity (Silver Spring) 2011;19:2411–2417 [DOI] [PubMed] [Google Scholar]
- 13.Thiering E, Brüske I, Kratzsch J, et al. GINIplus and LISAplus Study Groups Prenatal and postnatal tobacco smoke exposure and development of insulin resistance in 10 year old children. Int J Hyg Environ Health 2011;214:361–368 [DOI] [PubMed] [Google Scholar]
- 14.Roglic G, Unwin N. Mortality attributable to diabetes: estimates for the year 2010. Diabetes Res Clin Pract 2010;87:15–19 [DOI] [PubMed] [Google Scholar]
- 15.Hayashino Y, Fukuhara S, Okamura T, et al. HIPOP-OHP Research Group A prospective study of passive smoking and risk of diabetes in a cohort of workers: the High-Risk and Population Strategy for Occupational Health Promotion (HIPOP-OHP) study. Diabetes Care 2008;31:732–734 [DOI] [PubMed] [Google Scholar]
- 16.Ko KP, Min H, Ahn Y, et al. A prospective study investigating the association between environmental tobacco smoke exposure and the incidence of type 2 diabetes in never smokers. Ann Epidemiol 2011;21:42–47 [DOI] [PubMed] [Google Scholar]
- 17.Houston TK, Person SD, Pletcher MJ, Liu K, Iribarren C, Kiefe CI. Active and passive smoking and development of glucose intolerance among young adults in a prospective cohort: CARDIA study. BMJ 2006;332:1064–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kowall B, Rathmann W, Strassburger K, et al. Association of passive and active smoking with incident type 2 diabetes mellitus in the elderly population: the KORA S4/F4 cohort study. Eur J Epidemiol 2010;25:393–402 [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Curhan GC, Hu FB, Rimm EB, Forman JP. Association between passive and active smoking and incident type 2 diabetes in women. Diabetes Care 2011;34:892–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Lauzon-Guillain B, Fournier A, Fabre A, et al. Menopausal hormone therapy and new-onset diabetes in the French Etude Epidemiologique de Femmes de la Mutuelle Générale de l’Education Nationale (E3N) cohort. Diabetologia 2009;52:2092–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tehard B, Friedenreich CM, Oppert JM, Clavel-Chapelon F. Effect of physical activity on women at increased risk of breast cancer: results from the E3N cohort study. Cancer Epidemiol Biomarkers Prev 2006;15:57–64 [DOI] [PubMed] [Google Scholar]
- 22.van Liere MJ, Lucas F, Clavel F, Slimani N, Villeminot S. Relative validity and reproducibility of a French dietary history questionnaire. Int J Epidemiol 1997;26(Suppl. 1):S128–S136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castro P, Legora-Machado A, Cardilo-Reis L, et al. Inhibition of interleukin-1beta reduces mouse lung inflammation induced by exposure to cigarette smoke. Eur J Pharmacol 2004;498:279–286 [DOI] [PubMed] [Google Scholar]
- 24.Donath MY, Böni-Schnetzler M, Ellingsgaard H, Ehses JA. Islet inflammation impairs the pancreatic beta-cell in type 2 diabetes. Physiology (Bethesda) 2009;24:325–331 [DOI] [PubMed] [Google Scholar]
- 25.Weitzman M, Cook S, Auinger P, et al. Tobacco smoke exposure is associated with the metabolic syndrome in adolescents. Circulation 2005;112:862–869 [DOI] [PubMed] [Google Scholar]
- 26.Vineis P, Airoldi L, Veglia F, et al. Environmental tobacco smoke and risk of respiratory cancer and chronic obstructive pulmonary disease in former smokers and never smokers in the EPIC prospective study. BMJ 2005;330:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisner MD, Katz PP, Yelin EH, Hammond SK, Blanc PD. Measurement of environmental tobacco smoke exposure among adults with asthma. Environ Health Perspect 2001;109:809–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cummings KM, Markello SJ, Mahoney M, Bhargava AK, McElroy PD, Marshall JR. Measurement of current exposure to environmental tobacco smoke. Arch Environ Health 1990;45:74–79 [DOI] [PubMed] [Google Scholar]