Abstract
OBJECTIVE
To study the relationship between maternal circulating fuels and neonatal size and compare the relative effects of glucose and lipids.
RESEARCH DESIGN AND METHODS
The Pune Maternal Nutrition Study (1993–1996) investigated the influence of maternal nutrition on fetal growth. We measured maternal body size and glucose and lipid concentrations during pregnancy and examined their relationship with birth size in full-term babies using correlation and regression techniques.
RESULTS
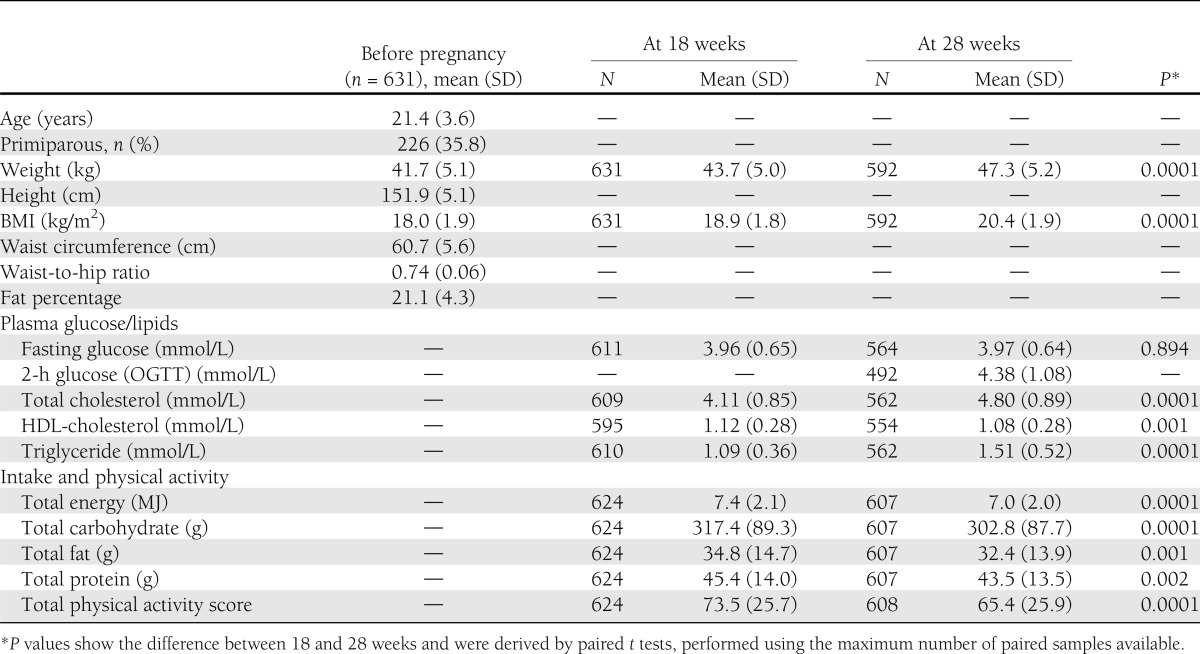
The mothers (n = 631) were young (mean age 21 years), short (mean height 151.9 cm), and thin (BMI 18.0 kg/m2) but were relatively more adipose (body fat 21.1%). Their diet was mostly vegetarian. Between 18 and 28 weeks’ gestation, fasting glucose concentrations remained stable, whereas total cholesterol and triglyceride concentrations increased and HDL-cholesterol concentrations decreased. The mean birth weight of the offspring was 2666 g. Total cholesterol and triglycerides at both 18 and 28 weeks and plasma glucose only at 28 weeks were associated directly with birth size. One SD higher maternal fasting glucose, cholesterol, and triglyceride concentrations at 28 weeks were associated with 37, 54, and 36 g higher birth weights, respectively (P < 0.05 for all). HDL-cholesterol concentrations were unrelated to newborn measurements. The results were similar if preterm deliveries also were included in the analysis (total n = 700).
CONCLUSIONS
Our results suggest an influence of maternal lipids on neonatal size in addition to the well-established effect of glucose. Further research should be directed at defining the clinical relevance of these findings.
In recent years there has been a resurgence of interest in studying factors influencing fetal growth because of the demonstration that fetal growth is related to the risk of developing type 2 diabetes and cardiovascular disease in later life (1–3). The mother’s nutrition and metabolism are major determinants of fetal growth (4,5). In clinical practice, glucose is considered the most important nutrient crossing the placenta (6,7) because of the well-known association between maternal diabetes and fetal overgrowth (macrosomia) and evidence of improved pregnancy and perinatal outcomes by controlling diabetes before and during pregnancy (8–10). Current recommendations are for universal screening for diabetes in pregnancy (10). Lipids and amino acids usually are not considered in the clinical management of pregnancy because there is less known about the role of these nutrients in the well-being of the pregnancy. Studies of nondiabetic animals and humans have shown a relationship between maternal triglyceride (11–13) and cholesterol concentrations and newborn weight and body composition and placental weight (14–16). A number of studies also have shown an association between maternal lipids (mostly triglycerides) and newborn weight in hyperglycemic women (8,12,17,18).
Pedersen (19) proposed that the transfer of excess maternal glucose in a diabetic pregnancy stimulates fetal islets to produce fetal hyperinsulinemia, which leads to macrosomia. Freinkel (20) proposed that a “mixture” of maternal nutrients (glucose, lipids, and amino acids) not only affects fetal growth and development but also influences risk of future obesity, diabetes, and neurocognitive development (“fuel-mediated teratogenesis”). The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study supported a role for maternal glucose in influencing newborn size in a continuous manner (21); maternal lipid concentrations were not reported. In mice, maternal hypercholesterolemia has been associated with atherosclerosis in offspring by influencing arterial gene expression (22). In humans, maternal hypercholesterolemia during pregnancy has been shown to be associated with fatty streaks in the fetal aorta (23).
There is little information from undernourished mothers in low- and middle-income countries on maternal glucose and lipid concentrations during pregnancy and their role in fetal growth, body composition, and future risk of disease. The Pune Maternal Nutrition Study (PMNS) was set up in six villages near Pune, India, to investigate the influence of maternal health and nutrition on fetal growth (4). This rural community depended on labor-intensive subsistence agriculture in a drought-prone region. We have reported that we found no associations of maternal dietary energy, carbohydrate, and protein intake with newborn size, whereas higher maternal fat intake was associated with greater neonatal length (4). Mothers who ate green leafy vegetables, milk, and fruit more frequently and had higher blood concentrations of folate and vitamin C gave birth to larger newborns. This highlighted the role of maternal micronutrients in fetal growth. In this article, we report the relationship between maternal circulating fuels (glucose, total and HDL-cholesterol, and triglycerides) and neonatal size and compare the relative effects of glucose and lipids.
RESEARCH DESIGN AND METHODS
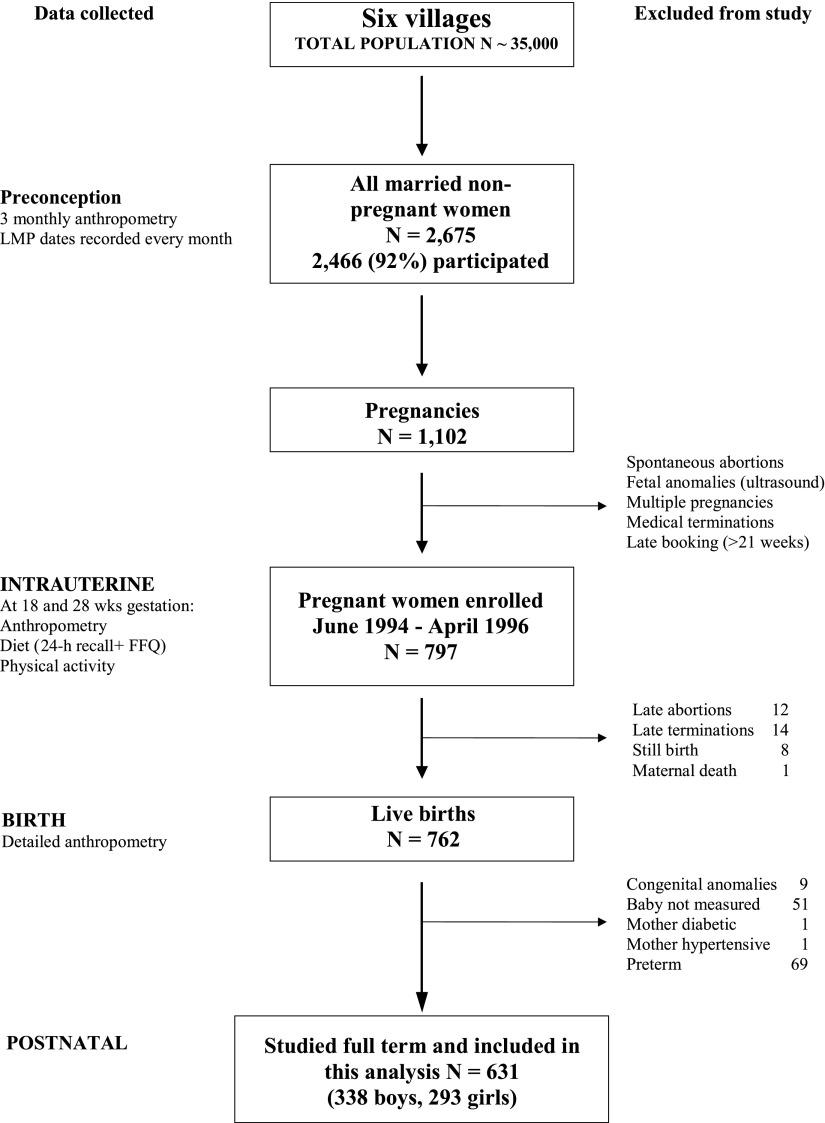
The design and methods of the PMNS have been described previously (4). In brief, we identified 2,675 married, nonpregnant women in six villages near Pune for possible enrolment in the study; 2,466 consented to take part (Fig. 1). Socioeconomic status (SES) was assessed using a standardized questionnaire (24), and a higher score represents higher status. Anthropometry (height, weight, circumferences, and skinfolds) was recorded every 3 months, and fat mass was calculated using four skinfolds (Durnin formula) (25). The women’s menstrual dates were recorded every month, and women missing two successive periods underwent an ultrasound examination to confirm pregnancy and assess gestational age (26). Pregnant women (n = 1,102) were identified and of these, women with a singleton pregnancy of <21 weeks’ gestation (n = 797) entered the study. Enrollment for the study started in September 1993, the first pregnancy was enrolled in June 1994, and the last delivery occurred in April 1996. Ethical permission for the study was granted by the KEM Hospital Ethics Committee.
Figure 1.
A flow diagram describing data collection and exclusions from the Pune Maternal Nutrition Study. LMP, last menstrual period; FFQ, food frequency questionnaire.
Maternal measurements during pregnancy
At 18 ± 2 and 28 ± 2 weeks’ gestation the following information was collected: anthropometry, dietary intakes using a semi-weighed 24-h recall method and food frequency questionnaire (4), and physical activity (the conventional 24-h recall method was modified and made more objective by incorporating information on portion sizes, most of which were weighed at mealtime by a trained field worker). Physical activity was assessed using a structured questionnaire to record the women’s daily workload, which mainly included farming and domestic activities (27). A weighted score was generated and was used in analysis; a higher score indicates higher physical activity. At 18 weeks’ gestation a fasting venous blood sample was collected. At 28 weeks, fasting and 2-h venous blood samples during an oral glucose tolerance test (OGTT; 75-g anhydrous glucose load) were collected. The following measurements were made using the fasting blood samples and standard enzymatic kits at both time points: plasma glucose, total cholesterol, HDL-cholesterol, and triglycerides. Glucose tolerance was classified by the then prevalent World Health Organization 1985 criteria (28) (diabetes mellitus: fasting plasma glucose ≥7.77 mmol/L and/or 2-h plasma glucose ≥11.1 mmol/L; impaired glucose tolerance [IGT]: fasting plasma glucose <7.77 mmol/L and 2-h plasma glucose between 7.77 and 11.0 mmol/L).
Neonatal anthropometry
Babies were measured by one of five trained fieldworkers within 72 h of birth. Birth weight was measured to the nearest 50 g using a Salter spring balance (Salter Abbey, Suffolk, U.K.); crown-heel length was measured to the nearest 0.1 cm using a portable Pedobaby baby meter (ETS JMB, Brussels, Belgium). Ponderal index (PI) was calculated using the formula: PI = [birth weight (g) × 100 / (crown heel length (cm))3]. Triceps and subscapular skinfold thicknesses were measured on the left side of the body to the nearest 0.2 mm using Harpenden skinfold calipers (CMS Instruments, London, U.K.). Occipito-frontal head circumference, mid-upper arm circumference (MUAC), and abdominal circumference (at the level of the umbilicus during expiration) were measured to the nearest 0.1 cm using fiberglass tapes (CMS Instruments). Placental weight was recorded to the nearest 5 g using Ishida scales after trimming the umbilical cord. Interobserver and intraobserver variation studies were conducted every 3 months to ensure the quality of these measurements.
Statistical methods
Data in tables are presented as mean ± SD. For statistical analysis, variables with skewed distributions (subscapular and triceps skinfolds) were log-transformed to satisfy assumptions of normality. Relationships between maternal size before pregnancy and maternal fuels (glucose and lipids) during pregnancy, as well as the interrelationships between maternal fuels, were tested using Pearson correlation coefficients adjusting for SES, parity, and maternal age. We performed regression analyses to study the univariate associations of Z-standardized maternal plasma glucose and lipid concentrations with neonatal measurements, adjusting for gestation at the time of measurements, sex, SES, parity, maternal age, and maternal BMI before pregnancy. We show the effect of a 1-SD change in each maternal “fuel” on the birth size measurement in original units. Finally, we constructed multivariate models in which glucose and lipids were included together to examine independent associations. Analyses were carried out using STATA version 11.2 (Stata Corp, College Station, TX).
Analysis sample
Of the total of 797 mothers enrolled in the study (Fig. 1), 12 had late abortions, 14 terminated their pregnancy, 8 babies were stillborn, and 1 mother died during pregnancy. Thus 762 mothers delivered during the study, of whom 9 had babies with congenital anomalies and 51 had babies who could not be measured within 72 h after birth. One mother had pregestational diabetes, one had pregnancy-induced hypertension, and 69 delivered preterm (<37 weeks’ gestation). They were excluded from the analysis. Thus, we included 631 full-term mother-baby pairs who had all the relevant data and were included in the analysis. Mothers who were excluded (n = 166) were of a similar age and had similar plasma glucose and lipid concentrations during pregnancy compared with those who were included in the study.
RESULTS
Measurements before pregnancy
The characteristics of the mothers are shown in Table 1. These rural mothers were young, short, and thin but had relatively high adiposity (body fat %). None of them smoked tobacco or drank alcohol, but one-fourth used non-smoked tobacco (for chewing or as a tooth powder). The majority of women (68%) belonged to subsistence farming families. One-third of the women were lacto-vegetarian and only 15% of women ate nonvegetarian foods more than once every alternate day. The portion sizes of nonvegetarian foods were small (<120 g/day for chicken, fish, and meat dishes and ∼60 g/day for eggs).
Table 1.
Maternal body size and biochemical characteristics
Measurements during pregnancy
Mean maternal weight gain from before pregnancy was 2.0 kg (SD 2.8 kg) up to 18 weeks’ gestation and 5.6 (SD 2.9) up to 28 weeks’ gestation. In 221 women we also had a weight measurement near delivery (35.1 [SD 1.1] weeks’ gestation), and the mean weight gain was 7.7 kg (SD 3.2 kg).
Dietary intake and physical activity
Mean daily maternal energy and protein intakes at 18 and 28 weeks’ gestation were 7.4 and 7.0 MJ and 45.4 and 43.5 g, respectively (4). The difference of 5.7% in the energy intake was statistically significant. These values were lower compared with the recommended daily allowances for Indian pregnant women given by the Indian Council of Medical Research at that time (29). Carbohydrates were the main energy source (72%), whereas 10 and 18% of energy was derived from protein and fat, respectively. These women had relatively high levels of physical activity; a woman with an average physical activity score (73.5) spent more than 8 h performing domestic activities that included cooking, washing clothes and utensils, sweeping the house, and fetching water and firewood (27). Two-thirds of women did farm work for more than 4 h/day.
Glycemia
Mean fasting plasma glucose concentrations were similar at 18 and 28 weeks (Table 1). At 28 weeks, OGTT results were available for 492 women. Using World Health Organization 1985 criteria (28), there were two women with impaired glucose tolerance and one with diabetes. They were given dietary advice and their subsequent glucose concentrations were normal; none required antidiabetic medication.
Lipids
Between 18 and 28 weeks’ gestation, plasma total cholesterol concentrations increased by 16.9% and triglyceride concentrations by 38.4%, whereas HDL-cholesterol concentrations decreased by 3.9%. Using the National Cholesterol Education Program criteria in the nonpregnant state (30), 10% of women had hypercholesterolemia (≥5.18 mmol/L) at 18 weeks’ and 34% at 28 weeks’ gestation, whereas 6% had hypertriglyceridemia (≥1.69 mmol/L) at 18 weeks’ and 31% at 28 weeks’ gestation. Low HDL-cholesterol concentrations (<1.29 mmol/L) were present in 74% of women at 18 weeks’ and 77% at 28 weeks’ gestation.
Interrelationships between glucose and lipids
Fasting plasma glucose concentrations were not significantly associated with lipid concentrations at 18 and 28 weeks. At 28 weeks’ gestation, 2-h plasma glucose was directly related to total cholesterol and triglyceride concentrations (r = 0.11; P = 0.01 for both). Total cholesterol and triglycerides were directly related (r = 0.34 and 0.31, respectively; P < 0.001 for both gestations).
Interrelationships between maternal weight, food intake, physical activity, and circulating glucose and lipids
Maternal BMI before pregnancy was inversely related to HDL-cholesterol concentrations (r = −0.11; P < 0.01 for both gestations) and directly related to triglycerides (r = 0.11; P = 0.008 at 28 weeks’ gestation). Waist circumference showed similar relationships as maternal BMI. Weight gain, macronutrient intake (total energy, protein, fat), and physical activity score at 18 weeks were unrelated to any of the metabolic measurements. Weight gain at 28 weeks’ gestation was directly related to plasma total cholesterol (r = 0.18; P = 0.001) and triglyceride concentrations (r = 0.11; P = 0.006). Macronutrient intakes were not associated with any fuels. Physical activity at 28 weeks was inversely associated with 2-h glucose (r = −0.14; P = 0.002) and total cholesterol (r = −0.12; P = 0.005) concentrations. All these associations were independent of maternal age, SES, and parity.
Birth measurements
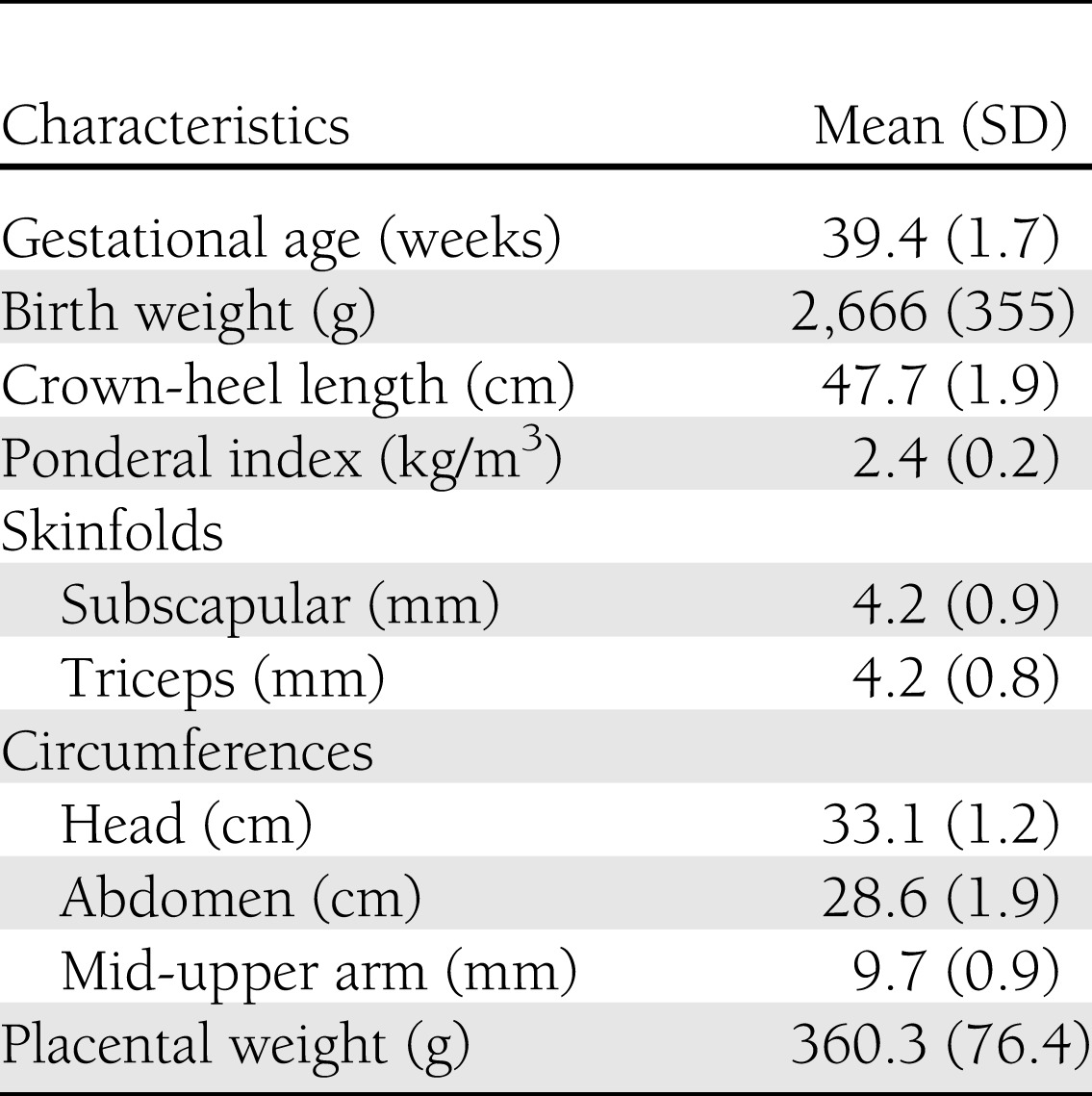
Mean birth weight was 2,666 g, mean length was 47.7 cm, and mean PI was 2.4 kg/m3 (Table 2). Twenty-eight percent of babies had a low birth weight (<2.5 kg). Mean placental weight was low at 360 g.
Table 2.
Characteristics of full-term babies (N = 631)
Relationships of maternal weight, glucose, and lipids during pregnancy with neonatal size
Weight at different time points during pregnancy was directly associated with all newborn measurements (r = 0.08–0.35; P < 0.05 for all). Weight gain up to 18 weeks was unrelated to any of the newborn measurements. Weight gain up to 28 weeks was directly related to all newborn measurements with the exception of placental weight (r = 0.09–0.16; P < 0.05 for all). The mean gestation was 39.4 (SD 1.7) weeks, and maternal fuels were unrelated to duration of pregnancy.
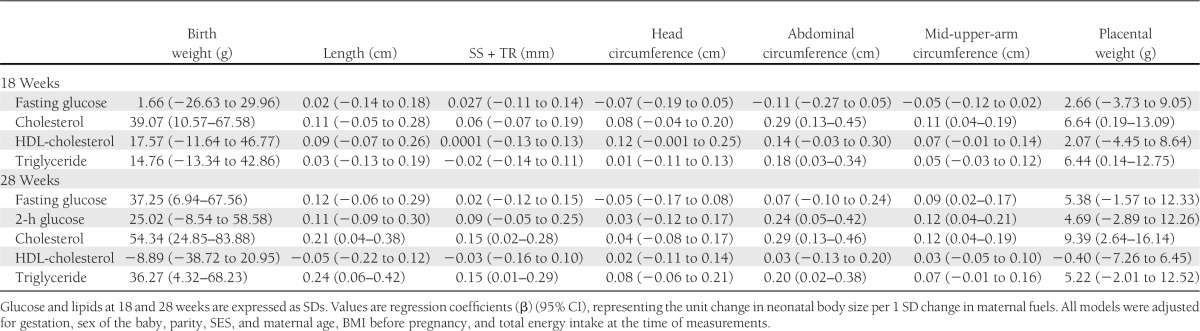
At 18 weeks’ gestation, plasma glucose and HDL-cholesterol concentrations were unrelated to newborn size. Maternal total cholesterol concentration was directly associated with birth weight, abdominal circumference and MUAC, and placental weight; a 1-SD-higher total cholesterol was associated with a 39-g-higher birth weight. Maternal triglyceride concentration was directly associated with abdominal circumference and placental weight (Supplementary Fig. 1).
At 28 weeks’ gestation, fasting plasma glucose concentration was directly associated with birth weight and MUAC; a 1-SD-higher fasting glucose concentration was associated with a 37-g-higher birth weight. Two-hour plasma glucose concentration was directly associated with abdominal circumference and MUAC. Plasma cholesterol concentration was directly associated with all newborn measurements except head circumference; a 1-SD-higher maternal cholesterol concentration was associated with a 54-g-higher birth weight. Maternal triglyceride concentration was directly associated with birth weight, birth length, skinfold thicknesses, and abdominal circumference; a 1-SD-higher maternal triglyceride concentration was associated with a 36-g-higher birth weight.
Although in general the effects of 28-week maternal measurements seemed to be stronger than those of 18-week measurements (Table 3), and although the strongest effects seemed to be with cholesterol, overlapping CIs indicate that the associations were broadly similar in magnitude at both gestational time points and that the associations of glucose, cholesterol, and triglycerides were of similar strength.
Table 3.
Univariate analyses of maternal glucose and lipids at 18 and 28 weeks’ gestation as predictors of neonatal size
Multivariate analysis
In a multivariate model with fasting glucose, total and HDL-cholesterol, and triglyceride concentrations at 18 weeks, maternal total cholesterol concentration was directly associated with birth weight, abdominal circumference, and MUAC (Table 4). There were no independent relationships with glucose, HDL-cholesterol, and triglyceride concentrations. In a multivariate model with 28-week fasting glucose, total and HDL-cholesterol, and triglyceride concentrations, fasting glucose was directly associated with birth weight and MUAC. Total cholesterol was directly associated with all newborn measurements except head circumference and skinfolds. There were no independent relationships with HDL-cholesterol and triglyceride concentrations. In a multivariate model with 28-week, 2-h glucose, glucose was directly associated with abdominal circumference and MUAC and total cholesterol was directly associated with birth weight and abdominal circumference.
Table 4.
Multivariate analyses of maternal glucose and lipids at 18 and 28 weeks’ gestation as predictors of neonatal size
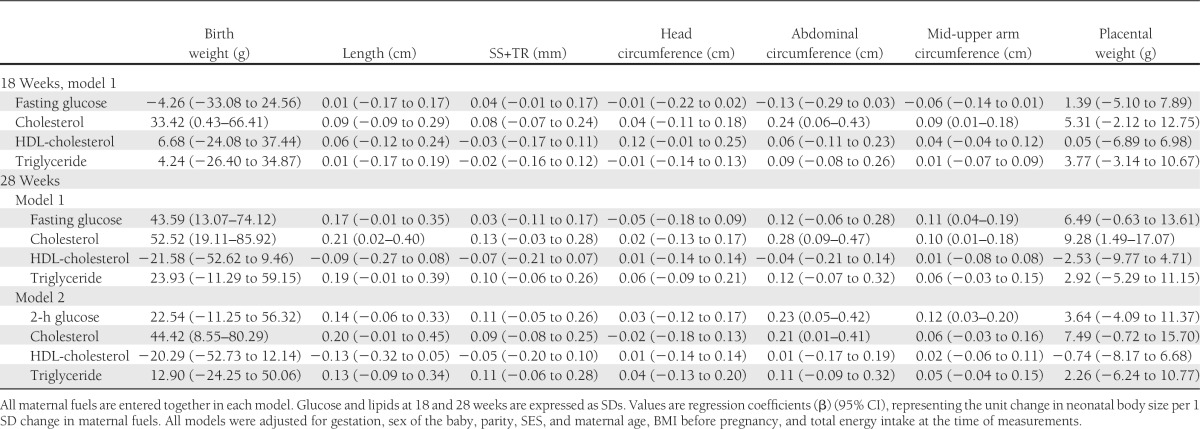
All the results were similar if preterm deliveries also were included in the analysis. One-SD-higher maternal fasting glucose, cholesterol, and triglyceride concentrations at 28 weeks were associated with 29-g-, 50-g-, and 31-g-higher birth weights, respectively, in univariate analyses (Supplementary Table 1).
CONCLUSIONS
We investigated the relationships of maternal glucose and lipid concentrations at 18 and 28 weeks’ gestation with newborn anthropometry in a population of undernourished, rural Indian women with a low prevalence of diabetes. Fasting glucose concentrations were normal and similar at 18 and 28 weeks’ gestation, whereas plasma total cholesterol and triglyceride concentrations increased and HDL-cholesterol concentrations decreased between the two visits. A third of the women had hypercholesterolemia and hypertriglyceridemia by nonpregnant criteria at 28 weeks’ gestation, whereas three-quarters had low HDL-cholesterol concentrations, reminiscent of the metabolic syndrome. Although plasma glucose and lipid concentrations were significantly correlated, the relationship accounted for only 1% of the variation. Higher BMI before pregnancy and higher gestational weight gain predicted higher concentrations of fuels during pregnancy, although the effect was small (r = 0.11–0.18). Maternal glucose, total cholesterol, and triglyceride concentrations were directly associated with newborn size, with the exception of neonatal head circumference. HDL-cholesterol concentrations were unrelated to newborn size. Only lipid concentrations at 18 weeks’ gestation, but glucose as well as lipid concentrations at 28 weeks’ gestation, predicted newborn size. The size of the effect of all these associations was broadly similar. Placental weight was predicted by plasma total cholesterol (18 and 28 weeks) and triglycerides (18 weeks) but not by glucose.
The dietary intake of our women was below that recommended by the Indian Council of Medical Research (29) and substantially lower than that reported in a contemporary Canadian cohort (31). In addition, there was a reduction in energy intake between 18 and 28 weeks’ gestation, which is probably attributable to the tradition of “eating down” during late pregnancy to facilitate easy delivery. It is noteworthy that weight gain in these women was lower than Institute of Medicine recommendations (32). The stable glucose concentrations probably are attributable to low energy intake, high physical activity, and continued transfer to the fetus. The rise in lipids could be ascribed to increasing catabolism of stored fat depots in the second half of pregnancy to match the increasing demands of the growing fetus (13,33). It would be interesting to perform “kinetic” studies to learn about metabolic adaptations during pregnancy in this population.
Pedersen (19) proposed a role for maternal glucose in the etiology of fetal macrosomia in diabetic pregnancies and Frienkel (20) suggested that “mixed nutrients” contributed to the “fuel-mediated teratogenesis,” which extended the role of maternal fuels from fetal growth to “programming” of subsequent obesity and diabetes. However, in clinical practice only glucose is measured, and the significance of maternal lipids during pregnancy has not been pursued. Animal and human studies have suggested an important role for maternal lipids in fetal growth. Higher maternal lipids (predominantly triglycerides) predict larger neonatal size (7,11,12,14,34). Maternal cholesterol, triglyceride, and nonesterified fatty acid concentrations rise during pregnancy (35,36). This is related to obesity before pregnancy and higher weight gain during pregnancy, and South Asian Indian women show a higher rise in triglyceride concentrations compared with Europeans despite a lower BMI (37). The rise in triglycerides is ascribed to an effect of estrogens on the maternal liver from early pregnancy, exaggerated by the increase in maternal insulin resistance as gestation progresses (13). Diabetic women have higher circulating lipid levels (18), and studies of Europeans have shown maternal triglycerides to be a stronger correlate of fetal size and body fat than maternal BMI, glucose, and insulin (18,38). HDL-cholesterol has been inversely related to birth weight in overweight and obese women but not in normal-weight women (34).
The majority of these studies were of well-nourished women in high-income countries with nonvegetarian food habits and predominantly diabetic pregnancies. In rural Indian mothers with very low BMI, fasting glucose concentration remained similar but total cholesterol and triglyceride concentrations rose by 16.9% and 38.4%, respectively, and they were as strong predictors of neonatal size as glucose concentrations, if not stronger. Thus, the pattern of associations in Indian undernourished rural women might indicate metabolic adjustments to maintain fetal growth. The placenta transfers glucose from mother to baby by both passive and facilitated diffusion (39). Cholesterol is directly transferred, although the mechanisms are poorly understood (15), while triglycerides are broken down into fatty acids and glycerol, transported across the placenta, and reconstituted before transfer to the fetus (7,40). Glucose and fatty acids are used for energy production by the placenta and the fetus. Cholesterol and fatty acids are used as structural elements of cell membranes and contribute to synthesis of hormones and other messengers by the fetus and placenta. Low concentrations of arachidonic acid precursors and high concentrations of trans fatty acids in both early and late pregnancy have been associated with restriction of fetal growth (41,42), thought to be especially important in high-risk pregnancies, such as adolescent pregnancies (43).
Thus, maternal fuels contribute to both structural and functional requirements of fetal growth, and our findings highlight the importance of maternal lipids in this relationship. Humans have the highest body fat percentage (15%) at birth of all mammals, including sea lions (5%) and pigs (∼2%), suggesting an evolutionary advantage of adiposity during the perinatal period (44): it contributes to energy stores, thermal insulation, and even attracting the mothers to feed (45). We have shown that these rural Indian babies, despite their low weights, are relatively more adipose compared with English babies (46). The relatively high adiposity of our rural mothers despite a low BMI also suggests an advantage of the “thin-fat” phenotype in reproduction. On the other hand, higher triglyceride concentrations during early pregnancy increase the risk of gestational diabetes and postpartum diabetes (40,47,48), and in an obese or diabetic pregnancy, maternal dyslipidemia contributes to fetal macrosomia. This suggests a continuum and the need for further research to decide on treatment strategies to achieve a balance between fetal growth and morbidity. Maternal hypercholesterolemia increases the risk of early atherogenesis in the fetus (fatty streaks in the aorta) both in animals and human beings (16,23). There is no information on the effect of treating these severe lipid abnormalities on the growth and future health of the fetus.
Ours is the first study relating maternal lipid concentrations to newborn size in normoglycemic, undernourished, rural Indian women with relatively low fat and cholesterol intakes and contributes a piece to solving a complex puzzle. The women were representative of the village population. Measurements of glucose and lipids were made twice, during mid- and late pregnancy, and included an OGTT in late pregnancy in 492 women. We also collected high-quality data on maternal food intake and physical activity and detailed newborn anthropometry, allowing us to construct multivariate models to examine the association between maternal fuels and newborn size and allowing for potential confounding factors. However, this is an observational study and therefore we cannot be sure of the causality of associations. We did not measure circulating fatty acids because of insufficient stored samples, and therefore we are unable to comment on the effect of individual fatty acids.
In summary, we demonstrate in rural, undernourished, normoglycemic Indian pregnant women a significant association between maternal circulating lipids and fetal growth, which was at least as strong as that of glucose. It will be important to study these associations in other populations with different rates of obesity and glycemic levels, for example, in the HAPO study. A study of future maternal and fetal risk associated with pregnancy lipids will help understand the importance of our findings over the life course. Such a study is now in progress.
Acknowledgments
This study was supported by The Wellcome Trust, London, U.K., and the U.K. Medical Research Council.
No potential conflicts of interest relevant to this article were reported.
S.R.K. performed statistical analysis and wrote the manuscript. K.K. wrote and edited the manuscript. S.R.R. collected data and edited the manuscript. S.D.C, T.M.D, A.J.B, V.A.S, and D.S.B collected data. C.H.D.F. and C.S.Y. planned the research, guided the analysis, and wrote and edited the manuscript. C.H.D.F. and C.S.Y. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors are grateful to the study participants for taking part in this study and Dr. Siddhi Hirve, KEM Hospital Research Center, Pune, India, for supervising the study. The authors thank the late Dr. B.J. Coyaji and Dr. V.N. Rao, KEM Hospital Research Center, Pune, India, for providing research facilities, and Himangi Lubree and Sonali Rege, Diabetes Unit, KEM Hospital Research Center, Pune, India, for collecting nutritional data. The authors also thank Charu Joglekar, Diabetes Unit, KEM Hospital Research Center, Pune, India, for helpful comments on the draft. The authors also acknowledge the support of SNEHA-India.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2445/-/DC1.
References
- 1.Barker DJP. Fetal origins of coronary heart disease. BMJ 1995;311:171–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huxley R, Owen CG, Whincup PH, et al. Is birth weight a risk factor for ischemic heart disease in later life? Am J Clin Nutr 2007;85:1244–1250 [DOI] [PubMed] [Google Scholar]
- 3.Yajnik CS. Early life origins of insulin resistance and type 2 diabetes in India and other Asian countries. J Nutr 2004;134:205–210 [DOI] [PubMed] [Google Scholar]
- 4.Rao SR, Yajnik CS, Kanade AS, et al. Intake of micronutrient-rich foods in rural Indian mothers is associated with the size of their babies at birth: Pune Maternal Nutrition Study. J Nutr 2001;131:1217–1224 [DOI] [PubMed] [Google Scholar]
- 5.Hoet JJ, Hanson MA. Intrauterine nutrition: its importance during critical periods for cardiovascular and endocrine development. J Physiol 1999;514:617–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lain KY, Catalano PM. Metabolic changes in pregnancy. Clin Obstet Gynecol 2007;50:938–948 [DOI] [PubMed] [Google Scholar]
- 7.Herrera E. Metabolic adaptations in pregnancy and their implications for the availability of substrates to the fetus. Eur J Clin Nutr 2000;54(Suppl. 1):S47–S51 [DOI] [PubMed] [Google Scholar]
- 8.Tieu J, Middleton P, Crowther CA. Preconception care for diabetic women for improving maternal and infant health. Cochrane Database Syst Rev 2010;12:CD007776. [DOI] [PubMed] [Google Scholar]
- 9.Mitanchez D. Foetal and neonatal complications in gestational diabetes: perinatal mortality, congenital malformations, macrosomia, shoulder dystocia, birth injuries, neonatal complications. Diabetes Metab 2010;36:617–627 [DOI] [PubMed] [Google Scholar]
- 10.Wendland EM, Torloni MR, Falavigna M, et al. Gestational diabetes and pregnancy outcomes—a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth 2012;31:12–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Cianni G, Miccoli R, Volpe L, et al. Maternal triglyceride levels and newborn weight in pregnant women with normal glucose tolerance. Diabet Med 2005;22:21–25 [DOI] [PubMed] [Google Scholar]
- 12.Kitajima M, Oka S, Yasuhi I, Fukuda M, Rii Y, Ishimaru T. Maternal serum triglyceride at 24--32 weeks’ gestation and newborn weight in nondiabetic women with positive diabetic screens. Obstet Gynecol 2001;97:776–780 [DOI] [PubMed] [Google Scholar]
- 13.Herrera E, Ortega H. Metabolism in normal pregnancy. In Textbook of Diabetes and Pregnancy. 2nd ed. Hod M, Jovanovic LG, Di Renzo GC, De Leiva A, Langer O, Eds. New York: CRC Press, 2008, p. 25–35 [Google Scholar]
- 14.Catalano PM, Drago NM, Amini SB. Maternal carbohydrate metabolism and its relationship to fetal growth and body composition. Am J Obstet Gynecol 1995;172:1464–1470 [DOI] [PubMed] [Google Scholar]
- 15.Plösch T, van Straten EM, Kuipers F. Cholesterol transport by the placenta: placental liver X receptor activity as a modulator of fetal cholesterol metabolism? Placenta 2007;28:604–610 [DOI] [PubMed] [Google Scholar]
- 16.Napoli C, de Nigris F, Welch JS, et al. Maternal hypercholesterolemia during pregnancy promotes early atherogenesis in LDL receptor-deficient mice and alters aortic gene expression determined by microarray. Circulation 2002;105:1360–1367 [DOI] [PubMed] [Google Scholar]
- 17.Knopp RH, Magee MS, Walden CE, Bonet B, Benedetti TJ. Prediction of infant birth weight by GDM screening tests. Importance of plasma triglyceride. Diabetes Care 1992;15:1605–1613 [DOI] [PubMed] [Google Scholar]
- 18.Schaefer-Graf UM, Graf K, Kulbacka I, et al. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care 2008;31:1858–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pedersen J. Weight and length at birth of infants of diabetic mothers. Acta Endocrinol (Copenh) 1954;16:330–342 [DOI] [PubMed] [Google Scholar]
- 20.Freinkel N. Banting Lecture 1980. Of pregnancy and progeny. Diabetes 1980;29:1023–1035 [DOI] [PubMed] [Google Scholar]
- 21.Metzger BE, Lowe LP, Dyer AR, et al. HAPO Study Cooperative Research Group Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002 [DOI] [PubMed] [Google Scholar]
- 22.Palinski W, Napoli C. The fetal origins of atherosclerosis: maternal hypercholesterolemia, and cholesterol-lowering or antioxidant treatment during pregnancy influence in utero programming and postnatal susceptibility to atherogenesis. FASEB J 2002;16:1348–1360 [DOI] [PubMed] [Google Scholar]
- 23.Napoli C, Glass CK, Witztum JL, Deutsch R, D’Armiento FP, Palinski W. Influence of maternal hypercholesterolaemia during pregnancy on progression of early atherosclerotic lesions in childhood: Fate of Early Lesions in Children (FELIC) study. Lancet 1999;354:1234–1241 [DOI] [PubMed] [Google Scholar]
- 24.Pareek U, Trivedi G. Reliability and variability of socioeconomic case. Ind J Appl Psychol 1964;1:34–40 [Google Scholar]
- 25.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr 1974;32:77–97 [DOI] [PubMed] [Google Scholar]
- 26.Hadlock FP. Sonographic estimation of fetal age and weight. Radiol Clin North Am 1990;28:39–50 [PubMed] [Google Scholar]
- 27.Rao SR, Kanade AS, Margetts BM, et al. Maternal activity in relation to birth size in rural India. The Pune Maternal Nutrition Study. Eur J Clin Nutr 2003;57:531–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. Report of a WHO consultation. Part 1: diagnosis and classification of diabetes mellitus [report online], 1999. Available from http://www.whqlibdoc.who/int/hg/1999/WHO_NCD_NCS_99.2.pdf Accessed 1 August 2012
- 29.Indian Council of Medical Research. Recommended Dietary Intakes for Indians New Delhi, India, Indian Council of Medical Research, 1987. [Google Scholar]
- 30.National Cholesterol Education Program. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Final report [report online], 2002. Available from http://www.nhlbi.nih.gov/guidelines/cholesterol/atp3full.pdf Accessed 26 March 2013
- 31.Snyder J, Gray-Donald K, Koski KG. Predictors of infant birth weight in gestational diabetes. Am J Clin Nutr 1994;59:1409–1414 [DOI] [PubMed] [Google Scholar]
- 32.Institute of Medicine for the National Academics. Weight gain during pregnancy: reexamining the Guidelines. [report online], 28 May 2009. Available from http://iom.edu/reports/2009/weight-gain-during-pregnancy-reexamining-the-guidelines.aspx Accessed 15 February 2013
- 33.Butte NF. Energy requirements during pregnancy and consequences of deviations from requirement on fetal outcome. Nestle Nutr Workshop Ser Pediatr Program 2005;55:49–67; discussion 67–71 [DOI] [PubMed] [Google Scholar]
- 34.Misra VK, Trudeau S, Perni U. Maternal serum lipids during pregnancy and infant birth weight: the influence of prepregnancy BMI. Obesity (Silver Spring) 2011;19:1476–1481 [DOI] [PubMed] [Google Scholar]
- 35.Warth MR, Arky RA, Knopp RH. Lipid metabolism in pregnancy. II. Altered lipid composition in intermediage, very low, low and high-density lipoprotein fractions. J Clin Endocrinol Metab 1975;41:649–655 [DOI] [PubMed] [Google Scholar]
- 36.Sattar N, Greer IA, Louden J, et al. Lipoprotein subfraction changes in normal pregnancy: threshold effect of plasma triglyceride on appearance of small, dense low density lipoprotein. J Clin Endocrinol Metab 1997;82:2483–2491 [DOI] [PubMed] [Google Scholar]
- 37.Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet 2011;378:169–181 [DOI] [PubMed] [Google Scholar]
- 38.Harmon KA, Gerard L, Jensen DR, et al. Continuous glucose profiles in obese and normal-weight pregnant women on a controlled diet: metabolic determinants of fetal growth. Diabetes Care 2011;34:2198–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herrera E. Implications of dietary fatty acids during pregnancy on placental, fetal and postnatal development–a review. Placenta 2002;23(Suppl. A):S9–S19 [DOI] [PubMed] [Google Scholar]
- 40.Ghio A, Bertolotto A, Resi V, Volpe L, Di Cianni G. Triglyceride metabolism in pregnancy. Adv Clin Chem 2011;55:133–153 [DOI] [PubMed] [Google Scholar]
- 41.Dirix CE, Kester AD, Hornstra G. Associations between neonatal birth dimensions and maternal essential and trans fatty acid contents during pregnancy and at delivery. Br J Nutr 2009;101:399–407 [DOI] [PubMed] [Google Scholar]
- 42.van Eijsden M, Hornstra G, van der Wal MF, Vrijkotte TG, Bonsel GJ. Maternal n-3, n-6, and trans fatty acid profile early in pregnancy and term birth weight: a prospective cohort study. Am J Clin Nutr 2008;87:887–895 [DOI] [PubMed] [Google Scholar]
- 43.Oliveira OR, Santana MG, Santos FS, et al. Composition of fatty acids in the maternal and umbilical cord plasma of adolescent and adult mothers: relationship with anthropometric parameters of newborn. Lipids Health Dis 2012;11:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuzawa CW. Adipose tissue in human infancy and childhood: an evolutionary perspective. Am J Phys Anthropol 1998;41(Suppl. 27):177–209 [DOI] [PubMed] [Google Scholar]
- 45.Hrdy SB. Mother Nature. London, UK, Vintage, 2000 [Google Scholar]
- 46.Yajnik CS, Fall CHD, Coyaji KJ, et al. Neonatal anthropometry: the thin-fat Indian baby. The Pune Maternal Nutrition Study. Int J Obes Relat Metab Disord 2003;27:173–180 [DOI] [PubMed] [Google Scholar]
- 47.Basaran A. Pregnancy-induced hyperlipoproteinemia: review of the literature. Reprod Sci 2009;16:431–437 [DOI] [PubMed] [Google Scholar]
- 48.Brisson D, Perron P, Guay SP, Gaudet D, Bouchard L. The “hypertriglyceridemic waist” phenotype and glucose intolerance in pregnancy. CMAJ 2010;182:E722–E725 [DOI] [PMC free article] [PubMed] [Google Scholar]