Abstract
OBJECTIVE
Hemopexin is a well-recognized permeability factor in the kidney, but its potential role in blood-retinal barrier (BRB) breakdown has not been explored. The main aims of this study were as follows: 1) to determine hemopexin expression in the retina and its content in the vitreous fluid from diabetic patients with diabetic macular edema (DME) and nondiabetic patients, 2) to evaluate the effect of hemopexin on BRB permeability, and 3) to determine whether dexamethasone prevents an eventual hemopexin-induced hyperpermeability.
RESEARCH DESIGN AND METHODS
Biological material included 1) retinas from 10 diabetic donors with nonproliferative retinopathy and from 10 nondiabetic donors and 2) vitreous fluid from 14 patients with DME and 14 nondiabetic patients. Hemopexin and hemopexin receptor mRNA levels were measured by quantitative RT-PCR and hemopexin concentrations by ELISA. The effect of hemopexin on permeability in culture was evaluated in human retinal pigment epithelial (ARPE)-19 cells and bovine retinal endothelial cells. The experiments were repeated in the presence of hemopexin-neutralizing antibodies and dexamethasone.
RESULTS
A higher expression of hemopexin was detected in the retinal pigment epithelium (RPE) from diabetic patients in comparison with nondiabetic control subjects. Intravitreal hemopexin concentration was higher in patients with DME than in nondiabetic subjects. Hemopexin significantly increased permeability in ARPE-19 cells, which was prevented by both hemopexin-neutralizing antibodies and dexamethasone.
CONCLUSIONS
Hemopexin is overexpressed in the RPE of diabetic patients with DME and induces the breakdown of RPE cells in vitro. Dexamethasone was able to prevent hemopexin-induced hyperpermeability. Our results suggest that hemopexin can be considered a new pathogenic candidate for DME.
Diabetic retinopathy remains the leading cause of preventable blindness among working-age individuals in developed countries (1). Whereas proliferative diabetic retinopathy (PDR) is the commonest sight-threatening lesion in type 1 diabetes, diabetic macular edema (DME) is the primary cause of poor visual acuity in type 2 diabetes. Because of the high prevalence of type 2 diabetes, DME is the main cause of visual impairment in diabetic patients (2). When clinically significant DME appears, laser photocoagulation is currently indicated. However, the optimal period of laser treatment is frequently passed and, moreover, is not uniformly successful in halting visual decline. In addition, photocoagulation is not without side effects, with visual field loss and impairment of either adaptation or color vision being the most frequent. Intravitreal corticosteroids have been successfully used in eyes with persistent DME and loss of vision after the failure of conventional treatment. However, reinjections are commonly needed, and there are substantial adverse effects such as infection, glaucoma, and cataract formation. Intravitreal anti–vascular endothelial growth factor (VEGF) agents have also found an improvement of visual acuity and decrease of retinal thickness in DME, even in nonresponders to conventional treatment (3). However, apart from local side effects such as endophthalmitis and retinal detachment, the response to treatment of DME by VEGF blockade is not prolonged and is subject to significant variability. For all these reasons, new pharmacological treatments based on the understanding of the pathophysiological mechanisms of DME are needed.
Vascular leakage due to the breakdown of the blood-retinal barrier (BRB) is the main event involved in the pathogenesis of DME (4). However, little is known regarding the molecules primarily involved in this event. By means of a proteomic analysis, we have found that hemopexin was significantly increased in the vitreous fluid of patients with DME in comparison with PDR and nondiabetic control subjects (5). Hemopexin is the best characterized permeability factor in steroid-sensitive nephrotic syndrome (6,7). In addition, hemopexin could be involved in either causing or perpetuating enhanced glomerular permeability in minimal change nephrotic syndrome (7). T cell–associated cytokines like tumor necrosis factor-α are able to enhance hemopexin production in mesangial cells in vitro, and this effect is prevented by corticosteroids (8). However, whether hemopexin also acts as a permeability factor in the BRB and its potential response to corticosteroids remains to be elucidated.
On these bases, the aims of the current study were 1) to compare hemopexin and hemopexin receptor (LDL receptor–related protein [LRP1]) levels in retina and in vitreous fluid from diabetic and nondiabetic patients, 2) to evaluate the effect of hemopexin on the permeability of outer and inner BRB in cell cultures, and 3) to determine whether anti-hemopexin antibodies and dexamethasone were able to prevent an eventual hemopexin-induced hyperpermeability.
RESEARCH DESIGN AND METHODS
Human retinas
Ten human postmortem eyes were obtained from diabetic donors (age 66.9 ± 5.4 years) with nonproliferative diabetic retinopathy (NPDR). Ten eye cups obtained from nondiabetic donors matched by age (age 67.2 ± 6.8 years) were used as the control group. The time elapsed from death to eye enucleation was <4 h. After enucleation, one eye of each donor was snap-frozen at –80°C and stored until assayed for mRNA and Western blot analyses.
Human vitreous fluid
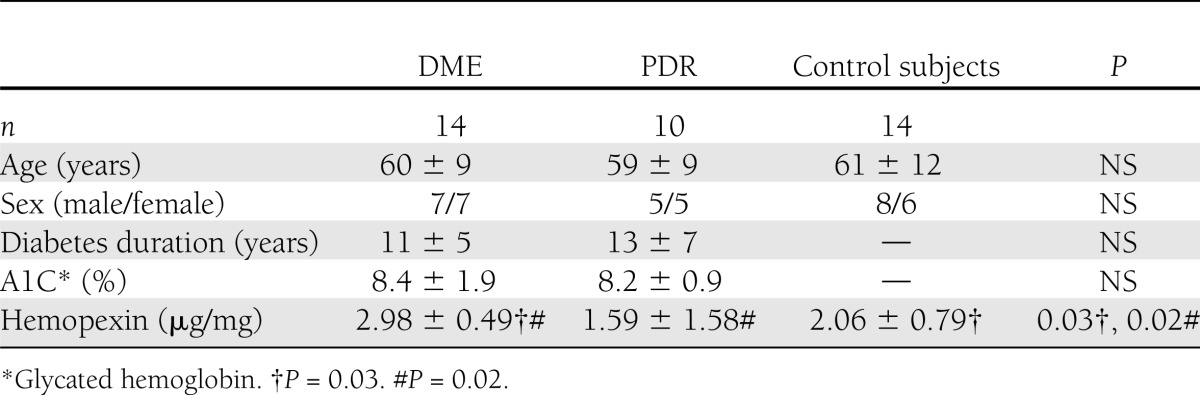
Samples of vitreous from 14 diabetic patients with DME without PDR, 10 diabetic patients with PDR without DME, and 14 nondiabetic subjects with idiopathic macular hole were selected from our vitreous bank. Subjects with macular hole were considered control subjects. All groups were closely matched by age. The exclusion criteria were 1) previous vitreoretinal surgery, 2) photocoagulation in the preceding 6 months, 3) recent vitreous hemorrhage (<3 months before vitrectomy) or intravitreous hemoglobin >5 mg/mL, 4) renal failure (creatinine ≥120 μmol/L), and 5) other chronic diseases apart from diabetes.
For diabetic retinopathy classification, we used the International Clinical Diabetic Retinopathy Severity Scale (9). The main characteristics of control subjects and patients with DME are summarized in Table 1. Details of vitrectomy and sample collection have previously been described elsewhere (10).
Table 1.
Main clinical features and intravitreal hemopexin concentrations of subjects included in the study
All ocular tissues were used in accordance with applicable laws and with the Declaration of Helsinki for research involving human tissue. In addition, this study was approved by the ethics committee of our hospital.
Assessment of hemopexin and its receptor in retina
mRNA isolation and cDNA synthesis.
Neuroretina and retinal pigment epithelium (RPE) were harvested under the microscopic dissection of isolated eye cups from donors. The neuroretina and RPE from each eye were ground to powder in liquid nitrogen using a mortar. Tissue was homogenized by QIAshredder spin column (Quiagen, Hilden, Germany), and mRNA was extracted from tissue using RNeasy Micro Kit (Quiagen) according to the manufacturer's instructions. mRNA concentration and integrity was determined by RNA nano Laboratory Chip kit Bioanalyzer (Agilent, Palo Alto, CA). One microgram of total mRNA was reverse transcribed using TaqMan Reverse Transcription Reagents (Applied Biosystems, Roche, NJ) following the manufacturer’s protocol for random hexanucleotide priming.
Quantitative RT real-time PCR.
Quantitative real-time PCR was performed using an ABI Prism 7000 Sequence Detection System (Perkin-Elmer Applied Biosystems, Madrid, Spain) according to the manufacturer’s protocol. Levels of hemopexin and LRP1 were assessed with the TaqMan Assays Hs00167197_m1 and Hs00233856_m1 (for HPX and LRP1 detection, respectively; Applied Biosystems). The 2−ΔΔCt method was used to estimate relative transcript levels with ACTB (Hs9999903_m1) as endogenous control. Relative quantification data were obtained with ABI Prism 7000 SDS software (Applied Biosystems). Each sample was studied in triplicate.
Measurement of hemopexin in vitreous fluid
Hemopexin was determined by using ELISA kit assay (Assaypro, Winfield, MO). The vitreous was diluted 1:5 in assay dilution buffer. The detection limit of the assay was 50 ng/mL. Intra-assay and interassay coefficients of variation were 4.9 and 7.3%, respectively.
Cell cultures
In vitro model of outer BRB: human RPE cell cultures.
Human RPE (ARPE)-19 cells (American Type Culture Collection, Manassas, VA), a spontaneously human immortalized cell line from RPE, was used in the experiments. Cell cultures were maintained in 75 cm2 cell flasks with DMEN Ham’s F-12 standard medium supplemented with 10% FBS (fetal bovine serum; Hyclone, Cultek, Barcelona, Spain) and antibiotics streptomycin (100 mg/mL) and penicillin (100 units/mL) (Invitrogen, Madrid, Spain) at 37°C and 5% CO2. The concentration of d-glucose in the medium was adjusted to 25 mmol/L to mimic the diabetic milieu. The medium was replaced every 3 days.
In vitro model of inner BRB: bovine retinal endothelial cells.
Bovine retinal endothelial cells (BRECs) were isolated from bovine eyes according to the Antonetti and Wolpert protocol (11). Briefly, retinas isolated from bovine eyeballs from a local farm were digested with collagenase, DNase, and pronase at 37°C for 30 min and filtered. Endothelial cells were plated in fibronectin (5 mg/mL; Sigma, Madrid, Spain) and grown until confluence in DMEN (PAA, Pasching, Austria) supplemented with 10% FBS serum (SBF; Hyclone, Cultek) and the antibiotics streptomycin (100 mg/mL) and penicillin (100 units/mL) (Invitrogen), ECGS (20 mg/mL; Sigma, Madrid, Spain), and heparin (100 mg/mL; Sigma).
Immunohistochemical analysis
Monolayers of ARPE-19 cells grown for 15 days at confluence on glass coverslips (Thermo Scientific; Menzel-Glaser, Braunschweig, Germany) were subject to appropriate treatments with plasmatic hemopexin (50 μg/mL; Sigma) or antibodies as described below. Then, cells were washed with PBS and fixed with cold methanol (−20°C) for 10 min. After blocking and permeabilization with PBS, 2% BSA, and 0.05% Tween overnight at 4°C, monolayers were incubated with a mouse anti-human ZO-1 primary antibody (diluted 1: 200; Zymed Laboratories, San Francisco, CA). After washing with PBS, monolayers were further incubated with the secondary antibody Alexa 594 anti-mouse (1:200; Invitrogen, San Diego, CA) for 1 h at room temperature. The slides were mounted with a mounting medium containing DAPI for fluorescence (Vectashiedl; Vector Laboratories, Burlingame, CA). Images were acquired with a FV1000 (Olympus, Hamburg, Germany) confocal laser microscope with a ×60 immersion objective.
Permeability studies
Outer BRB.
The permeability studies were performed following the methods previously described by our group (12). In summary, ARPE-19 cells were planted at a density of 400,000 cells/mL (80,000 RPE cells/well) into polystyerene inserts with a surface area of 0.33 cm2 (HTS-Transwells; Corning, Corning, NY). At this density, the cells formed a monolayer that was maintained in culture for 15 days, with the medium being changed every 3 days. On the 15th day, the medium of the apical part of the insert was replaced by deprived medium (1% SBF). Then, plasmatic hemopexin (50 µg/mL; Sigma) was included in the apical compartment. After 15 h, 70 kDa-FITC dextran (100 µg/mL; Sigma) was added to the apical compartment of the insert. Aliquots of 200 μL were taken from the basal compartment every 30 min and fluorescence read in a SpectraMax Gemini (Molecular Devices, Sunnyvale, CA) at a wavelength of excitation/emission 485/528 nm. Finally, the concentration of dextran was determined by extrapolation of the fluorescence read in a standard curve. Each condition was tested in quadruplicate, and the experiments were repeated three times.
Inner BRB.
For BREC cell permeability studies, cells were seeded in endothelial complete medium into transwells (HTS-Transwells; Costar; Corning) (100,000 cells/insert). Cells were maintained in monolayer for 7 days before treatments. Then, the apical compartment was deprived and treatments and procedures were performed as described above.
Neutralization assay
Hemopexin solution (50 µg/mL) was prepared in a deprived medium (1% SBF). Then, the required concentration of antibody was added to the solution and the tubs were briefly mixed by vortexing and incubated 1 h at 37°C. Finally, the different solutions were added to the cell monolayers. Three different anti-hemopexin antibodies obtained in goat, rabbit, and mouse (17D, 300 H, and ABS 013, respectively; Santa Cruz Biotechnology, Heidelberg, Germany) were used. The antibody 17D is directed against the NH2-terminal epitope of the protein, while 300 H is obtained against the COOH-terminal epitope region.
The effect of dexamethasone on hemopexin-induced RPE cell permeability was evaluated. ARPE-19 cells were treated with dexamethasone (1 µmol/L) 18 h before hemopexin treatment.
Cell counting and citotoxicity
Nuclei from seven fields of each condition were counted to determine the total number of cells and cells in division per field. Images, equivalent to an area of 0.57 mm2, were acquired at ×20 with a fluorescence microscope (BX61; Olympus).
Lactate dehydrogenase (LDH) was measured as an indicator of cell death by using a cytotoxicity detection kit (Roche; Applied Science, Barcelona, Spain). LDH activity was measured in a 96-well plate with two replicates for each condition at an absorbance of 490 nm. Results are expressed as percentage of cells showing cytotoxicity ± SD. Percent cytotoxicity = (experimental value − low control)/(high control − low control) × 100.
Statistical analysis
The Kolmogorov-Smirnov test was used to confirm the assumption of the normality of the variables. A Student t test was used to compare continuous variables that were expressed as mean ± SD. Levels of statistical significance were set at P < 0.05.
RESULTS
Hemopexin and LRP1 expression in the human retina
No differences were observed in β-actin mRNA expression between diabetic and nondiabetic retinas (P = not significant). Thus, we have calculated hemopexin mRNA gene expression after normalizing with β-actin.
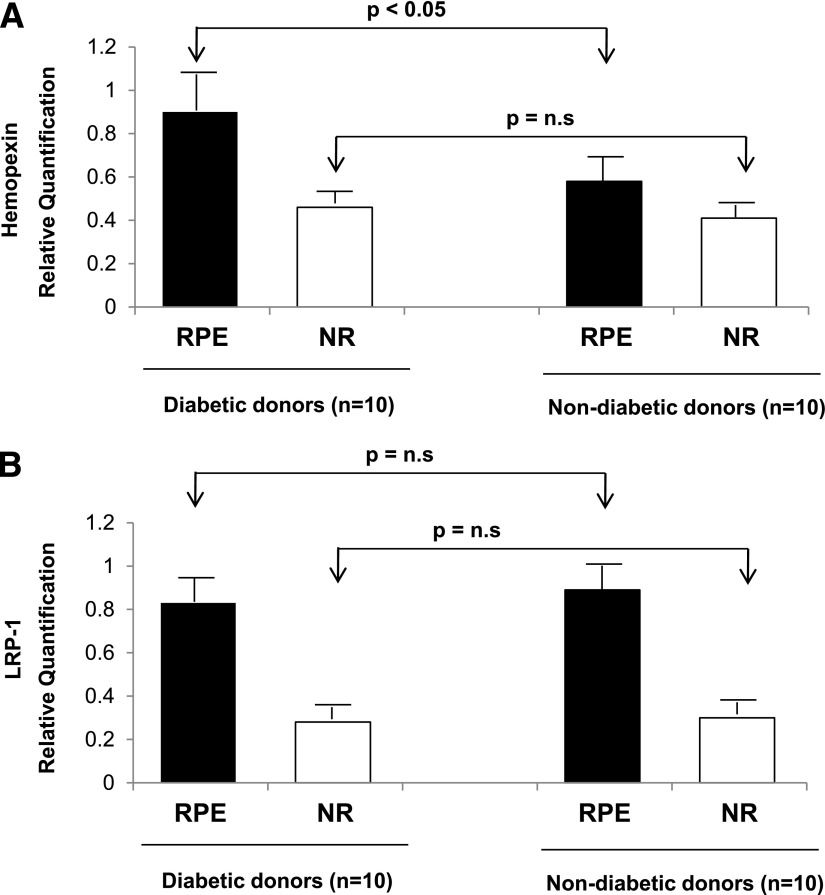
Significantly higher hemopexin mRNA expression was observed in RPE than in the neuroretina in both diabetic (0.85 ± 0.20 vs. 0.46 ± 0.16; P < 0.01) and nondiabetic (0.59 ± 0.11 vs. 0.40 ± 0.06; P < 0.05) donors. A higher expression of hemopexin mRNA was detected in the RPE from diabetic donors compared with nondiabetic donors (P < 0.05). No differences were observed in hemopexin expression in neuroretina between the two groups (Fig. 1A).
Figure 1.
A: Real-time quantitative RT-PCR analysis of hemopexin in RPE and neuroretina (NR) from diabetic donors and nondiabetic donors. B: Real-time quantitative RT-PCR analysis of LRP1 in RPE and neuroretina from diabetic donors and nondiabetic donors. Hemopexin and LRP1 gene expression data (ΔΔCt) were calculated after normalizing by β-actin. Results are presented as means ± SD.
A significantly higher level of mRNA LRP1 was observed in the RPE than in the neuroretina in both diabetic (0.83 ± 0.47 vs. 0.28 ± 0.07; P < 0.05) and nondiabetic (0.89 ± 0.26 vs. 0.30 ± 0.05; P < 0.01) donors. However, we did not find any difference between LRP1 mRNA levels in diabetic and nondiabetic donors in either the RPE or the neuroretina (Fig. 1B).
Hemopexin concentration in vitreous fluid
Intravitreous levels of hemopexin were significantly higher in the vitreous fluid obtained from patients with DME without PDR than from with PDR without DME and from nondiabetic subjects (Table 1).
Effect of hemopexin on permeability of BRB
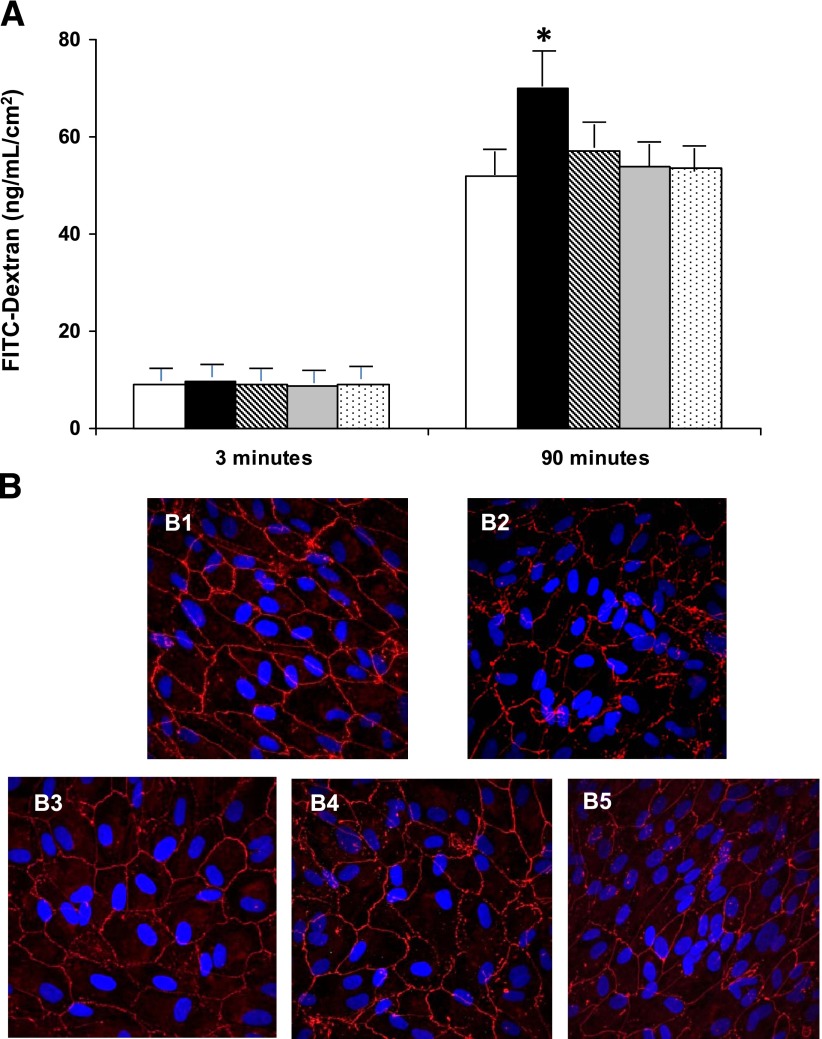
Hemopexin treatment (50 µg/mL) does not increase permeability in BREC. By contrast, hemopexin produced a clear disruption of the ARPE-19 cell monolayer, thus leading to a significant increase of dextran permeability (75 ± 8 vs. 43 ± 6 ng/mL/cm2; P < 0.05). It is worth mentioning that this effect was detected using a hemopexin dosage in the range detected in the vitreous fluid of diabetic patients with DME. Pretreatment with anti-hemopexin antibodies prevented the breakdown of the monolayer and the increase of permeability induced by hemopexin (Fig. 2A). The results of the immunohistochemistry of ZO-1 performed under the different conditions are shown in Fig. 2B.
Figure 2.
A: Results of 70 kDa dextran permeability in ARPE-19 cells cultured under 25 mmol/L d-glucose in the different conditions examined. The vertical axis is the concentration of dextran. Dextran permeability was measured at 3 and 90 min. Empty bars, control condition (25 mmol/L d-glucose); black bars, hemopexin (50 µg/mL); gray bars, hemopexin after anti-hemopexin antibody (0.75 µg/mL) obtained from goat; stripped bars, hemopexin after anti-hemopexin antibody (0.75 µg/mL) obtained from rabbit; dotted bars, hemopexin after anti-hemopexin antibody (0.75 µg/mL) obtained from mouse. Results are expressed as means; n = 4. *P < 0.05 in comparison with hemopexin treatment. B: Immunofluorescence images obtained by confocal microscopy showing the ARPE-19 monolayer. B1: Immunostaining for ZO-1 (red) in cells cultured under 25 mmol/L d-glucose showing a well-structured monolayer. B2: Disorganization of ZO-1 and breakdown of ARPE-19 monolayer induced by hemopexin. B3–B5: Prevention of the disruption of ARPE-19 monolayer with anti-hemopexin antibodies before hemopexin treatment. Nuclei stained with DAPI (blue). Bar: 10 μm. FITC, fluorescein isothiocyanate.
In order to rule out a potential bias in the results due to changes in cell proliferation, the total number of cells as well as cells in division was counted, and no significant differences were found between the different conditions. In addition, we did not observe any significant differences regarding cytotoxicity as measured by LDH assay.
Dexamethasone prevents hemopexin-induced RPE cell hypermeability
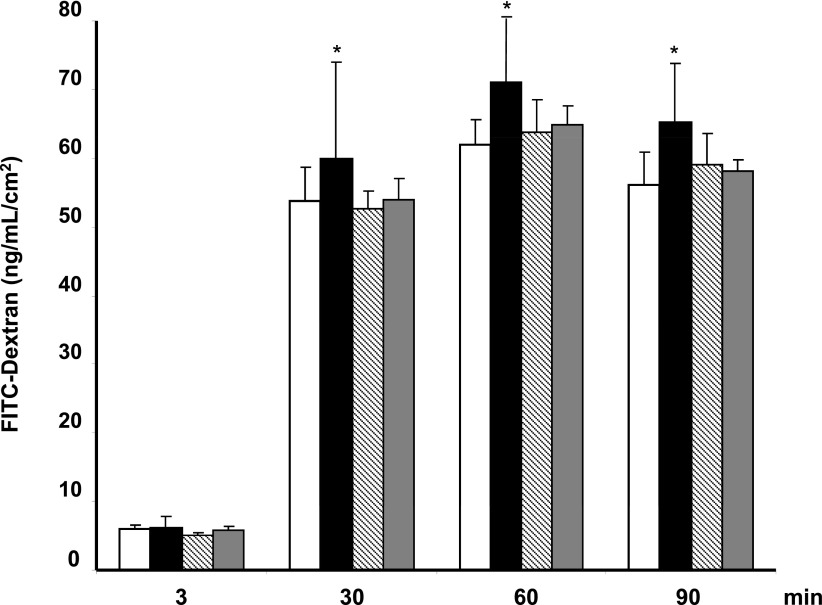
The effect of dexamethasone on hemopexin-induced RPE cell permeability is represented in Fig. 3. Dexamethasone (1 μmol/L) prevented the disruption of the ARPE-19 cell monolayer and significantly reduced the hyperpermeability induced by hemopexin (P < 0.05).
Figure 3.
Results of 70 kDa dextran permeability in ARPE-19 cells cultured under 25 mmol/L d-glucose in the different conditions examined. The vertical axis is the concentration of dextran. Dextran permeability was measured at 3, 30, 60, and 90 min. Empty bars, control condition (25 mmol/L d-glucose); black bars, hemopexin (50 µg/mL); striped bars, dexamethasone (1 μmol/L); gray bars, hemopexin (50 µg/mL) after dexamethasone (1 μmol/L). Results are expressed as means; n = 4. *P < 0.05 in comparison with hemopexin treatment. FITC, fluorescein isothiocyanate.
CONCLUSIONS
In the current study, we have confirmed our previous results obtained by a proteomic approach showing that hemopexin is higher in the vitreous fluid of diabetic patients with DME in comparison with diabetic patients with PDR and nondiabetic subjects. In addition, we provide the first evidence that hemopexin is overexpressed in diabetic eye. Furthermore, we have shown that hemopexin leads to the disruption of RPE cells, thus increasing permeability, and that this effect is prevented by dexamethasone.
Hemopexin is probably the primary specific carrier of plasma heme, having the highest binding affinity for heme (13,14). Hemopexin is expressed mainly in the liver but also in the central nervous system and peripheral nervous system (13). In humans, expression of hemopexin has been previously reported in neuroretina and glial cell (15,16). In the current study, we have found that hemopexin is also expressed in RPE. Furthermore, we have found that RPE is the part of the retina with the highest expression of hemopexin and is the main contributor to the increase of hemopexin that occurs in DME. At this point, a double-edged sword action of hemopexin at the retinal level could be postulated. Thus, under physiological conditions hemopexin could play a protective role against the toxic effects of iron, but its overexpression induced by diabetes could favor the DME.
The reason why hemopexin is overexpressed in the retina of diabetic patients with DME is beyond the scope of the present article. However, it should be noted that hemopexin belongs to the acute-phase proteins whose expression can be induced by various cytokines in a context of inflammatory processes (17). Interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor-α have been found to be elevated in the vitreous fluid of diabetic patients (18–21), and it has also been demonstrated that these proinflammatory cytokines can stimulate hemopexin production (8,17). In addition, proinflammtory cytokines play an essential role in the development of DME (22). Therefore, proinflammatory cytokines could be the reason why hemopexin is upregulated in the retinas from diabetic patients with DME. Furthermore, high hemopexin levels could be a mechanism by which proinflammatory cytokines lead to BRB disruption and in turn to DME.
In this study, we provide the first evidence that hemopexin is able to impair the sealing function of RPE, thus leading to an increase of permeability. Notably, this effect was observed using a hemopexin concentration within the range found in the vitreous fluid from patients with DME. In addition, we have provided evidence that hemopexin induces hyperpermeability in RPE cells but not in BRECs. This different behavior of hemopexin is similar to that described in the kidney. In the renal glomerulus, hemopexin causes increased permeability by injuring podocytes, which are cells of epithelial origin such as the RPE, but does not affect endothelial cells (23). Although it is generally assumed that the disruption of the inner BRB is the main cause of DME, there is growing evidence that the disruption of the outer limiting membrane and RPE significantly contributes to the pathogenesis of DME (24–29). Therefore, it could be postulated that any treatment addressed to block hemopexin action, although limited to the outer BRB, would be effective in preventing or arresting DME. However, specific studies on this issue are needed.
The only known receptor for the heme-hemopexin complex is the scavenger receptor LRP-1, which is expressed in most cell types (30–32). LRP-1, a member of the LDL receptor superfamily, is well-known for its classical role in scavenging chylomicron remnants in the liver (32,33). Subsequent studies, however, have demonstrated that LPR1 also acts as a multifunctional scavenging receptor by transporting a variety of ligands including proteases into endosomes in the cells. We have found that LRP1 expression was higher in RPE that in the neuroretina, but there were no significant differences between patients and control subjects. Given that LPR1 might bind to several ligands, its primary role in retinal physiopathology is still far from being understood. However, our finding that the RPE was the part of the retina with higher expression of both hemopexin and LPR1 suggests an autocrine/paracrine action.
Finally, we found that dexamethasone significantly inhibited hemopexin-induced leakage in RPE cells. Intravitreous steroidal anti-inflammatory drugs are currently used for the treatment of DME (34–37). Apart from their anti-inflammatory properties, it has been shown that one important mechanism accounting for the effectiveness of intravitreous steroidal drugs is the inhibition of both VEGF synthesis and downstream receptor signaling (36,38). The inhibition of hemopexin-induced permeability detected in the current study represents an additional mechanism accounting for the beneficial effects of corticosteroids on DME.
In conclusion, hemopexin is overexpressed in the retina of diabetic patients with DME and induces outer BRB disruption. This effect is abrogated by dexamethasone. Our findings suggest that hemopexin can be considered a new candidate in the pathogenesis of DME and a new therapeutic target.
Acknowledgments
This study was supported by grants from Ministerio de Economía y Competitividad (SAF2009-07408) and Generalitat de Catalunya (SGR2009-739) and by CIBERDEM. CIBERDEM is an initiative of Instituto de Salud Carlos III.
No potential conflicts of interest relevant to this article were reported.
C.H. designed the study, analyzed data, and is the first author of the manuscript. M.G.-R. collected and analyzed data and reviewed the manuscript. R.S. provided the study concept, obtained funding, supervised the study, and critically reviewed and edited the manuscript. All authors approved the final version of the manuscript. R.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet 2010;376:124–136 [DOI] [PubMed] [Google Scholar]
- 2.Lightman S, Towler HM. Diabetic retinopathy. Clin Cornerstone 2003;5:12–21 [DOI] [PubMed] [Google Scholar]
- 3.Simó R, Hernández C. Advances in the medical treatment of diabetic retinopathy. Diabetes Care 2009;32:1556–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joussen AM, Smyth N, Niessen C. Pathophysiology of diabetic macular edema. Dev Ophthalmol 2007;39:1–12 [DOI] [PubMed] [Google Scholar]
- 5.Hernández C, García-Ramírez M, Colomé N, et al. New pathogenic candidates for diabetic macular edema detected by proteomic analysis. Diabetes Care 2010;33:e92. [DOI] [PubMed] [Google Scholar]
- 6.Bakker WW, Baller JF, van Luijk WH. A kallikrein-like molecule and plasma vasoactivity in minimal change disease. Increased turnover in relapse versus remission. Contrib Nephrol 1988;67:31–36 [PubMed] [Google Scholar]
- 7.Cheung PK, Klok PA, Baller JF, Bakker WW. Induction of experimental proteinuria in vivo following infusion of human plasma hemopexin. Kidney Int 2000;57:1512–1520 [DOI] [PubMed] [Google Scholar]
- 8.Kapojos JJ, van den Berg A, van Goor H, et al. Production of hemopexin by TNF-alpha stimulated human mesangial cells. Kidney Int 2003;63:1681–1686 [DOI] [PubMed] [Google Scholar]
- 9.Wilkinson CP, Ferris FL, 3rd, Klein RE, et al. Global Diabetic Retinopathy Project Group Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003;110:1677–1682 [DOI] [PubMed] [Google Scholar]
- 10.Hernández C, Carrasco E, Casamitjana R, Deulofeu R, García-Arumí J, Simó R. Somatostatin molecular variants in the vitreous fluid: a comparative study between diabetic patients with proliferative diabetic retinopathy and nondiabetic control subjects. Diabetes Care 2005;28:1941–1947 [DOI] [PubMed] [Google Scholar]
- 11.Antonetti DA, Wolpert EB. Isolation and characterization of retinal endothelial cells. Methods Mol Med 2003;89:365–374 [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Ramírez M, Villarroel M, Corraliza L, Hernández C, Simó R. Measuring permeability in human retinal epithelial cells (ARPE-19): implications for the study of diabetic retinopathy. Methods Mol Biol 2011;763:179–194 [DOI] [PubMed] [Google Scholar]
- 13.Tolosano E, Altruda F. Hemopexin: structure, function, and regulation. DNA Cell Biol 2002;21:297–306 [DOI] [PubMed] [Google Scholar]
- 14.Kumar S, Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicol Lett 2005;157:175–188 [DOI] [PubMed] [Google Scholar]
- 15.Hunt RC, Hunt DM, Gaur N, Smith A. Hemopexin in the human retina: protection of the retina against heme-mediated toxicity. J Cell Physiol 1996;168:71–80 [DOI] [PubMed] [Google Scholar]
- 16.Chen W, Lu H, Dutt K, Smith A, Hunt DM, Hunt RC. Expression of the protective proteins hemopexin and haptoglobin by cells of the neural retina. Exp Eye Res 1998;67:83–93 [DOI] [PubMed] [Google Scholar]
- 17.Prowse KR, Baumann H. Interleukin-1 and interleukin-6 stimulate acute-phase protein production in primary mouse hepatocytes. J Leukoc Biol 1989;45:55–61 [DOI] [PubMed] [Google Scholar]
- 18.Hernández C, Segura RM, Fonollosa A, Carrasco E, Francisco G, Simó R. Interleukin-8, monocyte chemoattractant protein-1 and IL-10 in the vitreous fluid of patients with proliferative diabetic retinopathy. Diabet Med 2005;22:719–722 [DOI] [PubMed] [Google Scholar]
- 19.Demircan N, Safran BG, Soylu M, Ozcan AA, Sizmaz S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye (Lond) 2006;20:1366–1369 [DOI] [PubMed] [Google Scholar]
- 20.Gustavsson C, Agardh CD, Agardh E. Profile of intraocular tumour necrosis factor-α and interleukin-6 in diabetic subjects with different degrees of diabetic retinopathy. Acta Ophthalmol. 20 April 2012 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Simó-Servat O, Hernández C, Simó R. Usefulness of the vitreous fluid analysis in the translational research of diabetic retinopathy. Mediators Inflamm 2012;2012:872978 [DOI] [PMC free article] [PubMed]
- 22.Ehrlich R, Harris A, Ciulla TA, Kheradiya N, Winston DM, Wirostko B. Diabetic macular oedema: physical, physiological and molecular factors contribute to this pathological process. Acta Ophthalmol (Copenh) 2010;88:279–291 [DOI] [PubMed] [Google Scholar]
- 23.Lennon R, Singh A, Welsh GI, et al. Hemopexin induces nephrin-dependent reorganization of the actin cytoskeleton in podocytes. J Am Soc Nephrol 2008;19:2140–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinberger D, Fink-Cohen S, Gaton DD, Priel E, Yassur Y. Non-retinovascular leakage in diabetic maculopathy. Br J Ophthalmol 1995;79:728–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Do carmo A, Ramos P, Reis A, Proença R, Cunha-vaz JG. Breakdown of the inner and outer blood retinal barrier in streptozotocin-induced diabetes. Exp Eye Res 1998;67:569–575 [DOI] [PubMed] [Google Scholar]
- 26.Miyamoto N, de Kozak Y, Normand N, et al. PlGF-1 and VEGFR-1 pathway regulation of the external epithelial hemato-ocular barrier. A model for retinal edema. Ophthalmic Res 2008;40:203–207 [DOI] [PubMed] [Google Scholar]
- 27.Kowalczuk L, Touchard E, Omri S, et al. Placental growth factor contributes to micro-vascular abnormalization and blood-retinal barrier breakdown in diabetic retinopathy. PLoS ONE 2011;6:e17462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Runkle EA, Antonetti DA. The blood-retinal barrier: structure and functional significance. Methods Mol Biol 2011;686:133–148 [DOI] [PubMed] [Google Scholar]
- 29.Xu HZ, Le YZ. Significance of outer blood-retina barrier breakdown in diabetes and ischemia. Invest Ophthalmol Vis Sci 2011;52:2160–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith A, Farooqui SM, Morgan WT. The murine haemopexin receptor. Evidence that the haemopexin-binding site resides on a 20 kDa subunit and that receptor recycling is regulated by protein kinase C. Biochem J 1991;276:417–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moestrup SK, Gliemann J, Pallesen G. Distribution of the α 2-macroglobulin receptor/low density lipoprotein receptor-related protein in human tissues. Cell Tissue Res 1992;269:375–382 [DOI] [PubMed] [Google Scholar]
- 32.Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest 2001;108:779–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tolosano E, Fagoonee S, Morello N, Vinchi F, Fiorito V. Heme scavenging and the other facets of hemopexin. Antioxid Redox Signal 2010;12:305–320 [DOI] [PubMed] [Google Scholar]
- 34.Bandello F, Pognuz R, Polito A, Pirracchio A, Menchini F, Ambesi M. Diabetic macular edema: classification, medical and laser therapy. Semin Ophthalmol 2003;18:251–258 [DOI] [PubMed] [Google Scholar]
- 35.Jonas JB, Akkoyun I, Kreissig I, Degenring RF. Diffuse diabetic macular oedema treated by intravitreal triamcinolone acetonide: a comparative, non-randomised study. Br J Ophthalmol 2005;89:321–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brooks HL, Jr, Caballero S, Jr, Newell CK, et al. Vitreous levels of vascular endothelial growth factor and stromal-derived factor 1 in patients with diabetic retinopathy and cystoid macular edema before and after intraocular injection of triamcinolone. Arch Ophthalmol 2004;122:1801–1807 [DOI] [PubMed] [Google Scholar]
- 37.Campochiaro PA, Brown DM, Pearson A, et al.; FAME Study Group. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology 2011;118:626.e2–635.e2 [DOI] [PubMed]
- 38.Edelman JL, Lutz D, Castro MR. Corticosteroids inhibit VEGF-induced vascular leakage in a rabbit model of blood-retinal and blood-aqueous barrier breakdown. Exp Eye Res 2005;80:249–258 [DOI] [PubMed] [Google Scholar]