Abstract
OBJECTIVE
In vivo corneal confocal microscopy (IVCCM) has been proposed as a noninvasive technique to assess small nerve fiber structural morphology. We investigated the structure-function relationship of small fibers in diabetic sensorimotor polyneuropathy (DSP).
RESEARCH DESIGN AND METHODS
Ninety-six type 1 diabetic subjects with a spectrum of clinical DSP and 64 healthy volunteers underwent IVCCM examinations to determine corneal nerve structure, including corneal nerve fiber length (CNFL), fiber density (CNFD), branch density (CNBD), and fiber tortuosity (CNFT). Small nerve fiber function was assessed by cooling detection thresholds (CDTs), axon reflex–mediated neurogenic vasodilatation in response to cutaneous heating by laser Doppler imaging flare technique (LDIFLARE), and heart rate variability (HRV). Linear associations between structural and functional measures in type 1 diabetic subjects were determined using Spearman correlation coefficients and linear regression analysis.
RESULTS
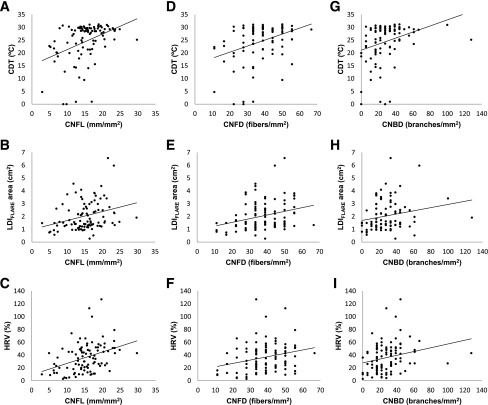
Of the type 1 diabetic subjects, with a mean age of 38.2 ± 15.5 years and a mean HbA1c of 7.9 ± 1.4%, 33 (34%) had DSP according to the consensus definition. Modest correlations were observed between CNFL, CNFD, and CNBD and all functional small-fiber tests (rs = 0.25 to 0.41; P ≤ 0.01 for all comparisons). For example, quantitatively every 1 mm/mm2 lower CNFL was associated with a 0.61°C lower CDT, a 0.07 cm2 lower LDIFLARE area, and a 1.78% lower HRV. No significant associations were observed for CNFT and the functional small-fiber measures.
CONCLUSIONS
Small nerve fiber structural morphology assessed by IVCCM correlated well with functional measures of small nerve fiber injury. In particular, CNFL, CNFD, and CNBD demonstrated clear structure-function relationships.
Diabetic sensorimotor polyneuropathy (DSP) is a progressive complication of type 1 diabetes with diffuse symmetrical and length-dependent injury affecting the small unmyelinated C fibers and thinly myelinated Aδ fibers (1). In addition to poor glycemic control, risk factors for DSP include elevated BMI and triglyceride levels, smoking, and hypertension (2). Early nerve injury in individuals with DSP appears to have a long subclinical latency period, which is difficult to diagnose by clinical examination or conventional testing because both of these methods primarily measure later-stage dysfunction of large myelinated nerve fibers. Thus, it is of critical importance to assess whether small-fiber function or structure tests can evaluate earlier stages of DSP in order to predict—and determine therapies to prevent—progressive morbidity that may involve pain, imbalance, foot deformities, infection, ulceration, and amputation (3,4).
Several candidates, each with their own inherent limitations, have become established as small-fiber functional tests in clinical practice and clinical research settings. These fundamental tests of small-fiber function include assessment of temperature fibers by way of thermal threshold testing such as determination of cooling detection thresholds (CDTs) (5–7), assessment of parasympathetic cardiac autonomic reflexes such as heart rate variability (HRV) with deep breathing (8,9), and assessment of the small-fiber axon reflex–mediated neurogenic vasodilatory response to cutaneous heating by way of the laser Doppler imaging flare technique (LDIFLARE) (10–12).
While these three tests have become accepted in practice and research, there are concerns regarding their diagnostic performance and specificity for small nerve fiber impairment. For example, CDTs are limited by the need for subjective patient responses, impaired reproducibility, and the possibility of poor specificity for small fibers (13). Though promising as a method to evaluate the small-fiber axon-reflex loop between temperature sensory fibers and those involved in cutaneous capillary vasodilatation, the LDIFLARE technique remains an investigational tool in research settings that requires further validation (10). Finally, though more objective by virtue of independence from the requirement of patient responses, HRV requires patient cooperation with deep breathing and has potential confounding influences such as glycemic variation, medication use, and caffeine consumption that may similarly impair its specificity to small fiber dysfunction (9,14). Despite the potential limitations of these three small-fiber functional tests, a novel putative structural test for small-fiber impairment must—at face value—correlate with their results in order to be considered a valid measure of the small-fiber impairment observed in the early stages of DSP.
In vivo corneal confocal microscopy (IVCCM) is a proposed noninvasive technique used to image the structure of the nerve plexus adjacent to the Bowman layer of the cornea (15). Loss of corneal nerve fibers in patients with diabetes has been linked to functional changes in the cornea such as a decrease in corneal sensitivity as well as vulnerability to corneal trauma: a process similar to loss of sensation and subsequent trauma in the lower limbs (16,17). Though IVCCM measures branches originating from the relatively short fifth cranial nerve rather than the longer spinal nerves classically evaluated by standard tests for DSP, IVCCM has been shown to be reproducible and diagnostically valid for the identification of DSP in type 1 diabetes (18,19). In this population, IVCCM is associated with large-fiber dysfunction; specifically, shorter corneal nerve fiber length (CNFL) is correlated with lower amplitude potentials and slower conduction velocities for both sural and peroneal nerves (18). Its face validity is reinforced by its apparent unconfounded relationship with peripheral nerve function in healthy volunteers (20,21) and also by the recovery of fibers in response to major glycemic improvement in those with diabetes who have undergone whole pancreas transplantation (22).
The structure-function relationship—which is fundamentally important to the face validity of IVCCM as a measure of small fibers—has not been firmly established in the literature. Specifically, one study showed a moderate association between thermal threshold testing as well as HRV that was unique to corneal nerve fiber density (CNFD) (23). In opposition, a recent study did not confirm the association of thermal thresholds with fiber density; yet, it reported a moderate association with fiber length (24). Additionally, not all studies show a complete structure-function relationship. This may be explained by error in experimental measurements or may relate to inconsistencies in the distribution of the nerves sampled such as deeper dermal nerves compared with terminal nerve endings observed in the epidermis (25). As these findings were not the primary objectives of the published studies, the structure-function relationship of corneal nerve parameters and function tests has not been reconciled.
In a cross-sectional study of a cohort of type 1 diabetic subjects who underwent deep phenotyping for peripheral nerve measures, we aimed to determine the linear association and the magnitude of correlation between accepted tests of small nerve fiber function and measures of their structure determined by IVCCM.
RESEARCH DESIGN AND METHODS
A total of 96 subjects with type 1 diabetes and, for comparison, 64 age- and sex-matched healthy volunteers were examined between November 2008 and February 2012 as a contemporary subset of an ongoing longitudinal cohort study funded by JDRF (operating grant no. 17-2008-715). This study was conducted through the Diabetes and Endocrinology Clinic and the Diabetic Neuropathy Clinic of the Toronto General Hospital and the University of Toronto. Subjects were included if they were ≥18 years of age and provided written informed consent. Subjects were excluded if they presented with a current eye infection, recent history of corneal abrasion, severe movement disorder, allergy to proparacaine, or neuropathy not related to diabetes as determined by detailed medical history, family history, history of toxin exposure, renal failure, or presence of abnormal B12, folate, and serum or urine protein electrophoresis. Subjects were not excluded from the study owing to current contact lens wear or a history of ocular surgery, including refractive surgery and surgery for retinopathy.
To ensure full representation of a broad spectrum of nerve injury by our study cohort, we used an accrual strategy using the 19-point Toronto Clinical Neuropathy Score (TCNS) (26). Type 1 diabetic subjects were accrued until ~20 participants were enrolled in each TCNS score quartile: no clinical impairment (n = 57), mild DSP (n = 18), and moderate-to-severe DSP (n = 21). Specific power calculations have previously been described (18). Briefly, ~20 subjects per strata (60 subjects in total) were required assuming a type 1 error (α-level) of 0.05 and 95% power.
All subjects underwent evaluation for classification of DSP according to clinical neurologic examination that included nerve conduction studies, the examination of small nerve fiber structure by IVCCM, and the examination of small nerve fiber function by CDT, LDIFLARE, and HRV. A comprehensive medical and neurologic evaluation of each participant was performed for the assessment of neuropathy-related symptoms, lifestyle factors and comorbidities, physical examination, and biochemical tests (HbA1c, cholesterol, and triglycerides) (9,18–20,27). The protocol and consent procedures were conducted in accordance with the World Medical Association’s Helsinki Declaration and approved by the research ethics board of the Toronto General Hospital Research Institute, University Health Network (Toronto, Ontario, Canada).
Defining DSP
Cases of DSP were defined according to established consensus criteria using nerve conduction studies (NCSs) and clinical examination (28,29). These case subjects had at least one abnormal nerve conduction parameter in both the sural and peroneal nerve and at least one neuropathic symptom or sign (28). As determined by the TCNS questionnaire, neuropathic symptoms included numbness, tingling, weakness, foot pain, or ataxia, and neuropathic signs included abnormal knee or ankle reflexes, temperature, light touch, monofilament, or vibration sensation (26).
NCSs were performed using the Sierra Wave instrument (Cadwell Laboratories, Kennewick, WA). Specific parameters measured included sural sensory nerve action potential amplitude and conduction velocity and peroneal compound muscle action potential amplitude, conduction velocity, and F wave latency. NCS values were age and height adjusted where applicable, and standard laboratory reference values were used in which NCS parameters greater >99th percentile or <1st percentile in a healthy population are generally considered abnormal (30).
Structural measurements of corneal nerve fibers
Bilateral corneal nerve fiber measurements of the subbasal corneal nerve plexus superficial to the Bowman layer were obtained by IVCCM using a 300 × 300 mm field of view lens on the Rostock Cornea Module of the Heidelberg Tomograph III (Heidelberg Engineering, Smithfield, RI). Corneal nerve images, seen in Fig. 1, were obtained and analyzed by methods previously described (18). In brief, topical anesthetic (proparacaine hydrochloride 0.5%; Alcan, Mississauga, Ontario, Canada) and a viscous gel (Tear-gel; Novartis Pharmaceuticals, Dorval, Quebec, Canada) were applied to the eye. The gel was necessary to applanate the surface of the cornea to the disposable cap covering the objective lens. To reduce saccadic eye movement during imaging, subjects focused their eyes on a target behind the microscope while the examiner used a side-view video camera to ensure that the central area of the cornea was scanned.
Figure 1.
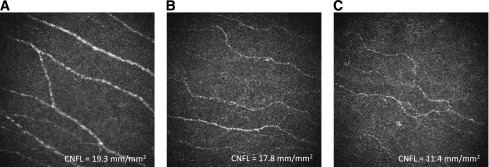
Representative IVCCM images according to membership in the following cohorts: healthy volunteers, type 1 diabetic control subjects without DSP, and type 1 diabetic case subjects with DSP. A: 26-year-old male healthy volunteer (TCNS = 0) with mean CNFL 19.3 mm/mm2. B: 26-year-old male with type 1 diabetes without clinical evidence of DSP (TCNS = 0) and mean CNFL 17.8 mm/mm2. C: 33-year-old male with type 1 diabetes and clinical evidence of mild DSP (TCNS = 6) and mean CNFL 11.4 mm/mm2.
The first image was obtained manually, and subsequent images were acquired automatically using the “volume scan” mode to capture a set of 40 contiguous images. This procedure was repeated twice per eye. From one image-set, the most in-focus, high-contrast image was selected for each eye by visual inspection. To select images adjacent to the Bowman layer, we excluded from analysis images with visible stromal cells, corneal folding, or other distortion artifacts. From each digital image, corneal nerve fiber parameters were determined using the CCMetrics Image Analysis Tools software, version 1.1 (provided by R. Malik and M. Dabbah, University of Manchester, Manchester, U.K.). CNFL was calculated by manually tracing nerve fibers and branches using a graphic pen tablet (Bamboo Pen; Wacom). The pixel-lengths traced were multiplied by 0.78125 µm (representing the height and width of each pixel) and divided by the area of the field (0.09 mm2) to produce a CNFL value in millimeters per millimeter squared. For ordinal variables, CNFD in fibers per millimeters squared and corneal nerve branch density (CNBD) in branches per millimeters squared, the number of fibers and branches traced by the examiner were divided by area of the image causing the distribution of the data to appear interrupted and not purely continuous. The analysis tool also estimated corneal nerve fiber tortuosity (CNFT) to assess nerve fiber curvature (31). All IVCCM parameters were calculated as means from the bilateral examination.
Functional tests of small nerve fibers
HRV was assessed by R-R interval variation (RRvar) using the algorithm for assessment of parasympathetic small nerve fiber function, arising from the moderately long vagus nerve, termed “RR4”. This method was developed by Stålberg and Nogués and later operationalized in the Dantec Keypoint Workstation (Natus Medical, San Carlos, CA) (8,9). Two surface electrodes were placed on the chest to obtain an electrocardiogram tracing, and a baseline was recorded at rest. RR4 values were recorded over 1 min during deep breathing at a frequency of 6 breaths/min, and R-R intervals versus time were obtained for maximum and minimum R-R intervals. Peaks with amplitudes >75% above or below the median RR interval were excluded to minimize the effect of ectopic beats and irregular breathing. The RRvar was calculated to be the difference between the shortest and the longest R-R intervals in 1 min given as a percentage of the mean of all peaks [(R-Rmax – R-Rmin)/R-Rmean × 100%].
CDT, a measure of small nerve fiber function in the peroneal nerve distribution—a nerve anatomically longer than the vagus nerve—was evaluated using the TSA-II NeuroSensory Analyzer (Medoc, Ramat-Yishai, Israel) (13). In brief, a stimulator was applied to the dorso-lateral aspect of the right foot. Initiated at 32°C, the temperature was gradually decreased to the first level perceived by the patient as being cooler than the preceding stimulus. Five test trials were performed and averaged to establish a mean CDT in degrees Celsius.
Slightly distal to where CDT was measured, C fiber function was assessed by LDIFLARE. A skin-heating probe was applied to the skin proximal to the first and second metatarsal heads on the dorsum of the right foot, and a temperature of 44°C was maintained for 20 min. The movement of blood in the dermal capillaries over a 6 cm × 6 cm area was measured using a laser Doppler imager that calculated LDIFLARE area in centimeters squared using MoorLDI software (version 3.11) (Moore Instruments, Devon, U.K.) as previously described (10,12).
Statistical analysis
Statistical analysis was performed using SAS (version 9.3 for Windows). Clinical characteristics were expressed as mean ± SD, except for the positive-skewed variable TCNS and the negative-skewed CDT variable, which were each expressed as median (interquartile range). Differences in categorical variables were assessed in two- and three-group comparisons using the χ2 test, while differences in continuous variables were assessed using ANOVA except in the case of TCNS and CDT in which the Kruskal-Wallis test was applied. The linear associations between IVCCM parameters and function measures were assessed using linear regression and correlation methods, and we report both linear regression line slope estimates and the correlation coefficients. Linear regression slope estimates as well as plots of this linear association are shown for descriptive purposes. As some of the structure-function variables showed deviations from normality, Spearman rank correlations were calculated for all structure-function relationships. We explored the possibility of nonlinear relationships by way of standard transformations (including powers, roots, logarithms, and polynomial), but none offered advantage over linear regression or Spearman rank correlations. Cronbach α was calculated to determine internal consistency among structural and functional parameters.
RESULTS
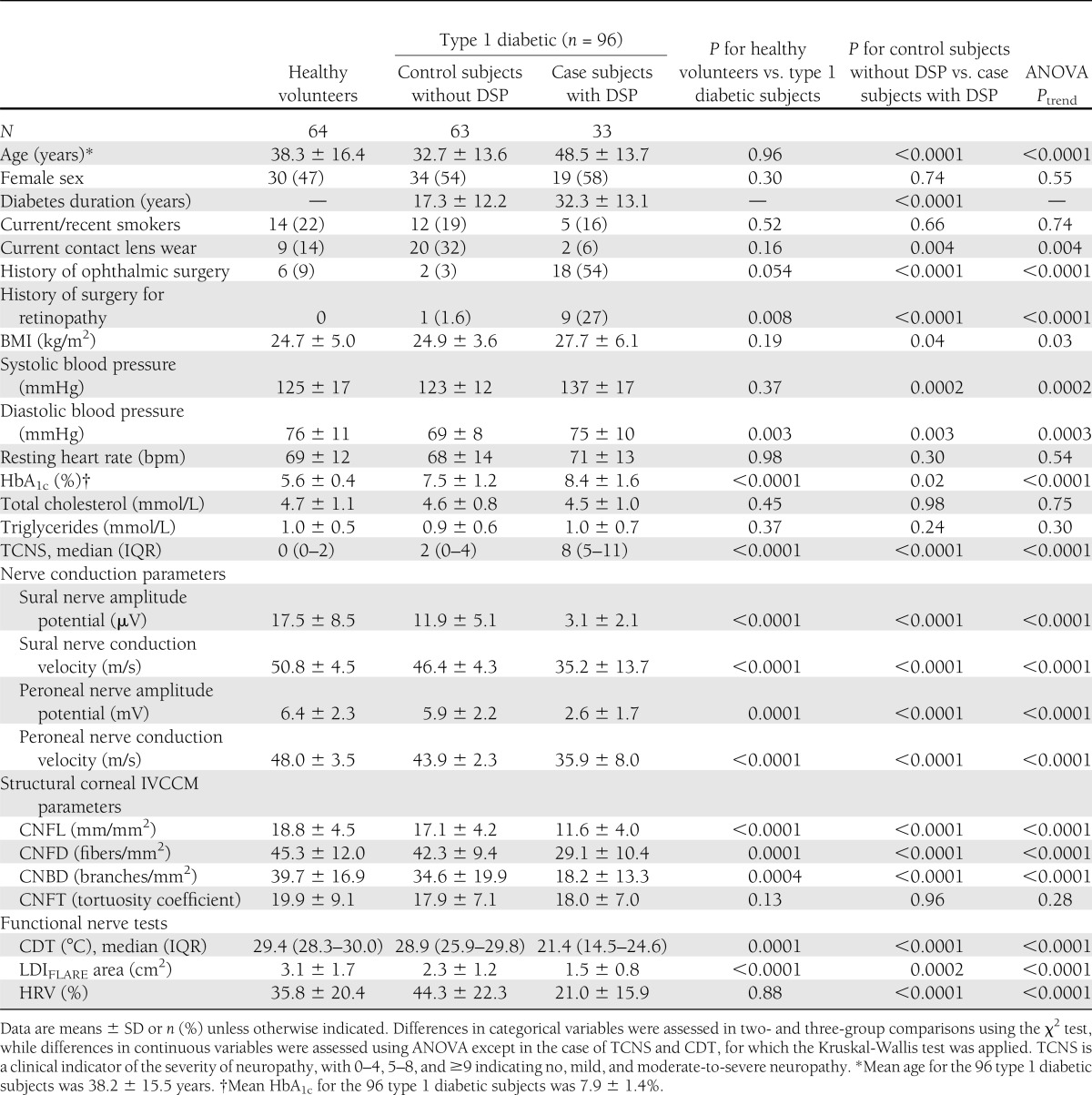
Clinical characteristics of the 96 type 1 diabetic subjects and 64 healthy volunteers are shown in Table 1. Type 1 diabetic subjects did not differ from healthy volunteers in age (38.2 ± 15.5 vs. 38.3 ± 16.4 years, P = 0.96) or sex (53 male [55%] vs. 30 female [47%], P = 0.30). Of the 96 type 1 diabetic subjects who had a mean HbA1c 7.9 ± 1.4%, 33 (34%) were classified as case subjects with DSP (28) and 63 (66%) as control subjects without DSP. Case subjects with DSP were significantly older (P < 0.0001) and had longer duration of diabetes (P < 0.0001) than control subjects without DSP. Case subjects had a higher BMI (P = 0.04), higher systolic (P = 0.0002) and diastolic (P = 0.003) blood pressures, and higher HbA1c values (P = 0.02). Cholesterol and triglycerides were not significantly different between case and control subjects. Additionally, the case subjects had a significantly higher TCNS score (P < 0.0001) and significantly lower sural and peroneal nerve amplitude potentials and conduction velocities than did control subjects (P < 0.0001 for all comparisons). Most importantly, the TCNS score and nerve conduction study parameter distributions had substantial variability indicating that the diabetic subjects represented individuals with a wide spectrum of clinical nerve injury. IVCCM parameters—measures of small nerve fiber structure—are also shown in Table 1. This analysis indicated that case subjects had significantly lower CNFL, CNFD, and CNBD than did control subjects (P < 0.0001 for all comparisons). Moreover, case subjects with DSP had significantly lower measures of small nerve fiber function with lower CDT values (P < 0.0001), smaller LDIFLARE areas (P = 0.0002), and lower HRV values (P < 0.0001) in comparison with control subjects.
Table 1.
Clinical characteristics of 64 healthy volunteers and 96 type 1 diabetic subjects according to the presence or absence of DSP
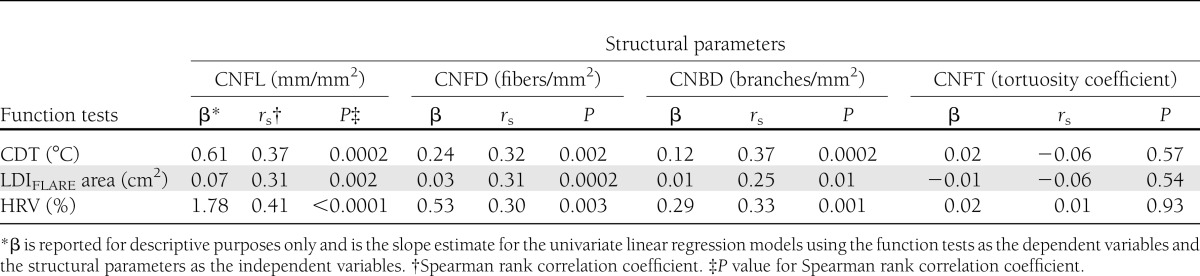
To further investigate this apparent relationship between structure and function, we plotted the results of the participants’ functional small-fiber tests against their structural IVCCM parameters (Fig. 2). We demonstrate these linear associations using correlation coefficients and present linear regression βs for descriptive purposes only (Table 2). Significant correlations were found between CNFL, CNFD, and CNBD and all small nerve fiber functional tests (rs = 0.25–0.41; P ≤ 0.01 for all comparisons). However, CNFT did not correlate with any of the functional tests (P > 0.05). Quantitatively, we found consistent linear associations between CNFL and all small nerve fiber functional tests. Specifically, for every 1 mm/mm2 lower CNFL measurement, CDT was also lower by 0.61°C (P < 0.0001). Moreover, associations were found with LDIFLARE area (linear regression β = 0.07 cm2, P = 0.003) and HRV (β = 1.78%, P = 0.0001), such that low CNFL was consistently associated with impaired small nerve fiber function tests. In addition, lower CNFD was associated with lower CDT (β = 0.24°C, P = 0.0003), LDIFLARE (β = 0.03 cm2, P = 0.004), and HRV (β = 0.53%, P = 0.01), and lower CNBD was associated with lower CDT (β = 0.12°C, P = 0.002), LDIFLARE (β = 0.01 cm2, P = 0.04), and HRV (β = 0.29%, P = 0.01). CNFT was not associated with any of the functional small-fiber tests.
Figure 2.
Plots showing linear associations between small-fiber function tests (CDT, LDIFLARE, and HRV) and CNFL (A–C), CNFD (D–F), and CNBD (G–I) for 96 subjects with type 1 diabetes.
Table 2.
Linear associations and correlations between small-fiber function tests (CDT, LDIFLARE, and HRV) and the small-fiber structural parameters measured by IVCCM in 96 type 1 diabetic subjects
We further inspected the relationship within the structural and functional categories as well as the relationship between these variables using Cronbach α test for internal consistency. Within the structural category, CNFL, CNFD, and CNBD show good consistency at 0.84 but questionable internal consistency with the addition of CNFT (0.64). The functional category, CDT, LDIFLARE, and HRV, performs poorly at 0.54 but shows acceptable (LDIFLARE = 0.77; HRV = 0.78) and good (CDT = 0.80) individual consistency together with CNFL, CNFD, and CNBD.
CONCLUSIONS
We examined a cohort of type 1 diabetic subjects with a broad spectrum of neuropathy to determine the structure-function relationship between the morphology of small corneal nerve fibers—sampled by IVCCM and originating from the trigeminal nerve—and peripheral nerve function evaluated by conventional small-fiber tests. Despite representing the morphology of fibers arising from a short cranial nerve, we showed a strong relationship between the structural measures of corneal nerve fibers, both length and density, and small-fiber function as assessed by three conventional methods: CDT, LDIFLARE, and HRV. Supporting previous findings (23), our results provide justification for the use of IVCCM as a valid measure of small nerve fiber damage in DSP.
In the natural history of DSP, it is hypothesized that early subclinical small-fiber damage precedes large-fiber impairment and the presentation of common clinical signs and symptoms. The gold standard for assessing small nerve fiber degeneration is the morphometry of intraepidermal nerve fiber density measured by skin punch biopsy, which is an invasive and painful technique (32). This quantitative relationship between severity of DSP and intraepidermal nerve fiber density is equivalently paralleled by corneal nerve fiber morphology, measured by IVCCM (23). In view of this, IVCCM has been targeted in research as a noninvasive alternative to skin biopsy with studies on practical aspects of its performance including reproducibility (19,33), concurrent validity for the diagnosis of DSP (18,34), assessment for confounding variables (2,20,21,35), and ability to track changes in nerve injury over time (22). Yet, despite all of these supportive findings, corneal nerve fibers arise from the relatively short fifth cranial nerve rather than the longer spinal nerves classically evaluated by standard tests for DSP. In light of this major concern, the current findings are of paramount importance. They demonstrate that the structural changes evaluated in the small fibers arising from the short trigeminal nerve are well reflected by conventional functional tests of the moderately long vagus nerve, for the assessment of HRV, and the longer nerves innervating the foot, where CDT and LDIFLARE are measured. These findings confirm that the IVCCM method measures parameters relevant to the biology of the natural history of small-fiber injury in DSP.
The use of IVCCM as a measure of small-fiber impairment in DSP, through the examination of corneal nerve structure, requires further research. The current reference definition for DSP is based primarily on large-fiber tests of the long nerves—the results of clinical evaluation and the presence of abnormal nerve conduction studies (28). Though not defined by the results of small fiber tests, in view of the prevailing concept of its natural history, DSP could be predicted prospectively by impairment in small-fiber structure. Although much work has been accomplished in cross-sectional studies to determine the role of IVCCM parameters—and even threshold values (18,34)—for the diagnosis of DSP, a major research gap is in determining its role in predicting the future onset of clinically relevant disease. This goal can only be accomplished by the longitudinal study of patients without DSP to determine which thresholds of IVCCM parameters are associated with future onset.
Our current findings show a strong association between the structure of the small nerve endings of the short trigeminal nerve and the function of the long nerves modulating HRV, as well as the even longer nerves innervating the ankle and foot. This study provides face validity for the use of IVCCM in prospective studies and helps to define the parameters most likely to be useful for incipient diagnosis of DSP. Specifically, we found that CNFL, CNFD, and CNBD correlated well with measures of small-fiber function, making these potential predictive biomarkers. Together with the findings of substantially better reproducibility (36) and concurrent validity (18,34) of CNFL for the identification of DSP, the current study highlights the role of CNFL as the preferred parameter for future research. As a dependable proxy to both the gold standard measures of small fiber structure and function in the natural history of DSP, CNFL could be integrated into clinical practice during regular retinopathy screening examinations. Additionally, in clinical research protocols CNFL could be used as an outcome measure in evaluating DSP disease-modifying therapies. However, these roles are dependent on the results of ongoing longitudinal research studies (37).
Although the data we present in this study arise from the systematic study of a cohort of individuals with type 1 diabetes with extensive small-fiber phenotyping and representing a broad spectrum of nerve injury, potential limitations remain. First, we only studied a limited number of structural and functional tests. We examined a single test for nerve fiber structure and did not include other techniques such as skin biopsy for the assessment of intraepidermal nerve fiber density (23). Additionally, we examined nerve function by three conventional tests when many alternative methods for the evaluation of small nerve impairment exist, such as corneal aesthesiometry (16), cutaneous thresholds for heating and heat pain (38), and sudomotor testing (39). Second, we only examined participants with type 1 diabetes; thus, further investigation must be performed to confirm whether this relationship is also found in type 2 diabetes. Third, we were not able to fully account for the potential confounding effect of age and diabetes duration on the relationship between structural and functional small-fiber measures because of the inherent relationship of these variables with DSP itself. Additionally, we did not exclude contact lens wearers or subjects with a history of refractive surgery from this study. Finally, we have only established a relationship between IVCCM structural parameters and functional tests cross-sectionally; to support this association, a prospective longitudinal study is necessary to definitively conclude that abnormal IVCCM is predictive of functional decline.
In this study, we have shown that the IVCCM parameters, which represent small nerve fiber structure, correlate consistently with small-fiber function. These results provide face validity for the use of IVCCM in longitudinal studies evaluating the predictive role of small fibers in predicting the onset of clinical DSP.
Acknowledgments
The work in this study was funded by JDRF (operating grant no. 17-2008-715). S.O. and A.W. were supported by Residency Research Electives from the University of Toronto Internal Medicine Program, and B.A.P. was a Canadian Diabetes Association Scholar.
No potential conflicts of interest relevant to this article were reported.
G.A.S. researched and performed statistical analysis of the data, prepared the first draft of the manuscript, and reviewed the manuscript for scholarly content and accuracy. E.M.H. researched data, prepared the final manuscript, and reviewed the manuscript for scholarly content and accuracy. L.E.L. researched and performed statistical analysis of the data and reviewed the manuscript for scholarly content and accuracy. A.W. researched data, prepared the manuscript, and reviewed the manuscript for scholarly content and accuracy. S.O. provided technical advice on HRV, data collection, and analysis and reviewed the manuscript for scholarly content and accuracy. V.B. contributed to the study design and reviewed the manuscript for scholarly content and accuracy. B.A.P. created the hypothesis and objective, designed the study, performed statistical analysis of the data, and reviewed the manuscript for scholarly content and accuracy. B.A.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 22nd Annual Meeting of the Diabetic Neuropathy Study Group of the European Association for the Study of Diabetes, Dresden, Germany, 27–30 September 2012.
References
- 1.Boulton AJM, Vinik AI, Arezzo JC, et al. American Diabetes Association Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005;28:956–962 [DOI] [PubMed] [Google Scholar]
- 2.Tesfaye S, Chaturvedi N, Eaton SE, et al. EURODIAB Prospective Complications Study Group Vascular risk factors and diabetic neuropathy. N Engl J Med 2005;352:341–350 [DOI] [PubMed] [Google Scholar]
- 3.Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care 1990;13:513–521 [DOI] [PubMed] [Google Scholar]
- 4.Partanen J, Niskanen L, Lehtinen J, Mervaala E, Siitonen O, Uusitupa M. Natural history of peripheral neuropathy in patients with non-insulin-dependent diabetes mellitus. N Engl J Med 1995;333:89–94 [DOI] [PubMed] [Google Scholar]
- 5.Dyck PJ, Bushek W, Spring EM, et al. Vibratory and cooling detection thresholds compared with other tests in diagnosing and staging diabetic neuropathy. Diabetes Care 1987;10:432–440 [DOI] [PubMed] [Google Scholar]
- 6.Gruener G, Dyck PJ. Quantitative sensory testing: methodology, applications, and future directions. J Clin Neurophysiol 1994;11:568–583 [PubMed]
- 7.Suarez G, Dyck PJ. Quantitative sensory assessment. In Diabetic Neuropathy. Dyck PJ, Thomas PK, Eds. Philadelphia, W.B. Saunders, 1999, p. 151–169 [Google Scholar]
- 8.Stålberg EV, Nogués MA. Automatic analysis of heart rate variation: I. Method and reference values in healthy controls. Muscle Nerve 1989;12:993–1000 [DOI] [PubMed] [Google Scholar]
- 9.Orlov S, Bril V, Orszag A, Perkins BA. Heart rate variability and sensorimotor polyneuropathy in type 1 diabetes. Diabetes Care 2012;35:809–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishnan ST, Rayman G. The LDIflare: a novel test of C-fiber function demonstrates early neuropathy in type 2 diabetes. Diabetes Care 2004;27:2930–2935 [DOI] [PubMed] [Google Scholar]
- 11.Krishnan ST, Quattrini C, Jeziorska M, Malik RA, Rayman G. Abnormal LDIflare but normal quantitative sensory testing and dermal nerve fiber density in patients with painful diabetic neuropathy. Diabetes Care 2009;32:451–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vas PRJ, Green AQ, Rayman G. Small fibre dysfunction, microvascular complications and glycaemic control in type 1 diabetes: a case-control study. Diabetologia 2012;55:795–800 [DOI] [PubMed] [Google Scholar]
- 13.Zinman LH, Bril V, Perkins BA. Cooling detection thresholds in the assessment of diabetic sensory polyneuropathy: comparison of CASE IV and Medoc instruments. Diabetes Care 2004;27:1674–1679 [DOI] [PubMed] [Google Scholar]
- 14.Tesfaye S, Boulton AJ, Dyck PJ, et al. Toronto Diabetic Neuropathy Expert Group Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33:2285–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malik RA, Kallinikos P, Abbott CA, et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia 2003;46:683–688 [DOI] [PubMed] [Google Scholar]
- 16.Tavakoli M, Kallinikos PA, Efron N, Boulton AJ, Malik RA. Corneal sensitivity is reduced and relates to the severity of neuropathy in patients with diabetes. Diabetes Care 2007;30:1895–1897 [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg ME, Tervo TM, Immonen IJ, Müller LJ, Grönhagen-Riska C, Vesaluoma MH. Corneal structure and sensitivity in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci 2000;41:2915–2921 [PubMed] [Google Scholar]
- 18.Ahmed A, Bril V, Orszag A, et al. Detection of diabetic sensorimotor polyneuropathy by corneal confocal microscopy in type 1 diabetes: a concurrent validity study. Diabetes Care 2012;35:821–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hertz P, Bril V, Orszag A, et al. Reproducibility of in vivo corneal confocal microscopy as a novel screening test for early diabetic sensorimotor polyneuropathy. Diabet Med 2011;28:1253–1260 [DOI] [PubMed] [Google Scholar]
- 20.Wu T, Ahmed A, Bril V, Orszag A, Ng E, Nwe P, Perkins BA. Variables associated with corneal confocal microscopy parameters in healthy volunteers: implications for diabetic neuropathy screening. Diabet Med 2012;29:e297–e303 [DOI] [PubMed]
- 21.Patel DV, Tavakoli M, Craig JP, Efron N, McGhee CN. Corneal sensitivity and slit scanning in vivo confocal microscopy of the subbasal nerve plexus of the normal central and peripheral human cornea. Cornea 2009;28:735–740 [DOI] [PubMed] [Google Scholar]
- 22.Mehra S, Tavakoli M, Kallinikos PA, et al. Corneal confocal microscopy detects early nerve regeneration after pancreas transplantation in patients with type 1 diabetes. Diabetes Care 2007;30:2608–2612 [DOI] [PubMed] [Google Scholar]
- 23.Quattrini C, Tavakoli M, Jeziorska M, et al. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes 2007;56:2148–2154 [DOI] [PubMed] [Google Scholar]
- 24.Edwards K, Pritchard N, Vagenas D, Russell A, Malik RA, Efron N. Utility of corneal confocal microscopy for assessing mild diabetic neuropathy: baseline findings of the LANDMark study. Clin Exp Optom 2012;95:348–354 [DOI] [PubMed] [Google Scholar]
- 25.Casanova-Molla J, Grau-Junyent JM, Morales M, Valls-Solé J. On the relationship between nociceptive evoked potentials and intraepidermal nerve fiber density in painful sensory polyneuropathies. Pain 2011;152:410–418 [DOI] [PubMed] [Google Scholar]
- 26.Bril V, Perkins BA. Validation of the Toronto Clinical Scoring System for diabetic polyneuropathy. Diabetes Care 2002;25:2048–2052 [DOI] [PubMed] [Google Scholar]
- 27.Nabavi Nouri M, Ahmed A, Bril V, et al. Diabetic neuropathy and axon reflex-mediated neurogenic vasodilatation in type 1 diabetes. PLoS ONE 2012;7:e34807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.England JD, Gronseth GS, Franklin G, et al. American Academy of Neurology. American Association of Electrodiagnostic Medicine. American Academy of Physical Medicine and Rehabilitation Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2005;64:199–207 [DOI] [PubMed] [Google Scholar]
- 29.Shin TM, Bril V, Orszag A, Ahmed A, Perkins BA. How sensitive is the case definition for diabetic sensorimotor polyneuropathy to the use of different symptoms, signs, and nerve conduction parameters in type 1 diabetes? Diabetes Res Clin Pract 2011;92:e16–e19 [DOI] [PubMed] [Google Scholar]
- 30.Oh SJ. Normal values for common nerve conduction tests. In Clinical Electromyography: Nerve Conduction Studies. Philadelphia, Lipincott Williams & Wilkins, 2003, p. 86–106 [Google Scholar]
- 31.Kallinikos P, Berhanu M, O’Donnell C, Boulton AJ, Efron N, Malik RA. Corneal nerve tortuosity in diabetic patients with neuropathy. Invest Ophthalmol Vis Sci 2004;45:418–422 [DOI] [PubMed] [Google Scholar]
- 32.England JD, Gronseth GS, Franklin G, et al. American Academy of Neurology Practice Parameter: evaluation of distal symmetric polyneuropathy: role of autonomic testing, nerve biopsy, and skin biopsy (an evidence-based review). Report of the American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and American Academy of Physical Medicine and Rehabilitation. Neurology 2009;72:177–184 [DOI] [PubMed] [Google Scholar]
- 33.Efron N, Edwards K, Roper N, et al. Repeatability of measuring corneal subbasal nerve fiber length in individuals with type 2 diabetes. Eye Contact Lens 2010;36:245–248 [DOI] [PubMed] [Google Scholar]
- 34.Tavakoli M, Quattrini C, Abbott C, et al. Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care 2010;33:1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 36.Vagenas D, Pritchard N, Edwards K, et al. Optimal image sample size for corneal nerve morphometry. Optom Vis Sci 2012;89:812–817 [DOI] [PubMed] [Google Scholar]
- 37.Ziegler D, Luff D. Clinical trials for drugs against diabetic neuropathy: can we combine scientific needs with clinical practicalities? Int Rev Neurobiol 2002;50:431–463 [DOI] [PubMed] [Google Scholar]
- 38.Magda P, Latov N, Renard MV, Sander HW. Quantitative sensory testing: high sensitivity in small fiber neuropathy with normal NCS/EMG. J Peripher Nerv Syst 2002;7:225–228 [DOI] [PubMed] [Google Scholar]
- 39.Gibbons CH, Illigens BM, Wang N, Freeman R. Quantification of sweat gland innervation: a clinical-pathologic correlation. Neurology 2009;72:1479–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]