Abstract
OBJECTIVE
We determined prevalence, risk factors, phenotype, and pathophysiological mechanism of new-onset diabetes after transplantation (NODAT) to generate strategies for optimal pharmacological management of hyperglycemia in NODAT patients.
RESEARCH DESIGN AND METHODS
Retrospective cohort study comparing demographics, laboratory data, and oral glucose tolerance test (OGTT)-derived metabolic parameters from kidney transplant recipients versus subjects not receiving transplants.
RESULTS
Among 1,064 stable kidney transplant recipients (≥6 months posttransplantation), 113 (11%) had a history of NODAT and 132 (12%) had pretransplant diabetes. In the remaining patients, randomly assigned OGTTs showed a high prevalence of abnormal glucose metabolism (11% diabetes; 32% impaired fasting glucose, impaired glucose tolerance, or both), predominantly in older patients who received tacrolimus as the primary immunosuppressant. Compared with 1,357 nontransplant subjects, stable kidney transplant recipients had lower basal glucose, higher glycated hemoglobin, lower insulin secretion, and greater insulin sensitivity in each of the three subgroups, defined by OGTT 2-h glucose (<140, 140–199, ≥200 mg/dL). These findings were reinforced in linear spline interpolation models of insulin secretion and sensitivity (all P < 0.001) and in another regression model in which the estimated oral glucose insulin sensitivity index was substantially higher (by 79–112 mL/min m2) for transplant versus nontransplant subjects despite adjustments for age, sex, and BMI (all P < 0.001).
CONCLUSIONS
Glucose metabolism differs substantially between kidney transplant recipients and nontransplant controls. Because impaired insulin secretion appears to be the predominant pathophysiological feature after renal transplantation, early therapeutic interventions that preserve, maintain, or improve β-cell function are potentially beneficial in this population.
More than 15,000 adults in the United States receive kidney transplants from deceased and living donors every year, and the majority will have improved quality of life and stable allograft function over the longer-term (1). By the end of the third posttransplant year, however, >40% of previously nondiabetic kidney transplant recipients develop new-onset diabetes after transplantation (NODAT) (2), which portends elevated risks for cardiovascular disease (3). NODAT is not mentioned in the American Diabetes Association position statement, but the American Diabetes Association experts emphasize that it is less important to label the particular type of diabetes than to understand the pathogenesis of hyperglycemia to treat it effectively (4). The pathophysiology underlying NODAT, however, is at present only poorly understood.
Type 2 diabetes mellitus (DM) in the general population ranges from predominantly insulin resistance with relative insulin deficiency to predominantly an insulin secretory defect with insulin resistance (4). In kidney transplant recipients with overt NODAT versus normal glucose tolerance (NGT), insulin resistance is increased and β-cell function is diminished (5), but the relative importance of either one component has been controversially reported (6–11). Calcineurin inhibitors, albeit standard initial maintenance immunosuppressive agents in the United States (1), are diabetogenic and inhibit a signaling pathway that is crucial for β-cell growth and function (12). Glucocorticoids are often used simultaneously and are well-known to decrease insulin sensitivity (13) but also might diminish insulin secretion (14).
In the current study, we aimed at assessing mechanisms of NODAT development in kidney transplant recipients. Our first goal was to determine prevalence, risk factors, and phenotype of a disturbed posttransplant glucose metabolism. Because previous treatment strategies for NODAT were suggested to follow type 2 DM (15), our second goal was to compare insulin secretion and insulin sensitivity between kidney transplant recipients and nontransplant individuals. In addition, we sought to validate results from stable kidney transplant recipients (≥6 months) against recent findings from patients studied as early as 3 months posttransplantation (16). We hypothesized that these pathomechanistic details, together, would help generate strategies for optimal management of hyperglycemia in NODAT patients.
RESEARCH DESIGN AND METHODS
Study participants and data retrieval
In stable kidney transplant recipients, glucose metabolism was analyzed using data from the Transplant-Associated Hyperglycemia (TAHG) study, an open, noninterventional, observational cohort study (Medical University of Vienna [MUV] Ethics Committee approval 566/2009). All outpatients ≥6 months after renal transplantation with one or more visits between March 2009 and March 2010 at MUV renal transplant clinic were eligible. Patients were pseudonymized, and their routinely recorded clinical data were extracted electronically or by chart review. As part of a routine screening program starting in March 2009, all kidney transplant recipients without history of pretransplantation DM (pretransplantation DM = type 1 DM and type 2 DM) or NODAT were randomly assigned to an oral glucose tolerance test (OGTT) using Glucoral 75 citron (Germania Pharmazeutika, Vienna, Austria) after an overnight fast. Three-hundred seven patients had their OGTT (fasting and 2-h glucose) completed by March 2010, and OGTT-derived data were included in the present analysis. A random 105 of these 307 OGTTs were performed with measurements of glucose and insulin at 0, 10, 20, 30, 60, 90, and 120 min, allowing the estimation of several metabolic parameters, as described.
In kidney transplant recipients during the early postoperative period, glucose metabolism was analyzed using data from the Treat-to-target Trial of Basal Insulin in Posttransplant Hyperglycemia (TIP) study, a recently completed randomized controlled clinical trial (16). In the control arm of the TIP study, 20 of 25 patients with standard-of-care antihyperglycemic management were not using antidiabetic pharmacotherapy 91 ± 6 days postoperatively and underwent an OGTT with sequential measurements of glucose and insulin (as noted). OGTT-derived metabolic results and basic demographic information, as listed in Table 3 and partially published previously (16), were used for comparison with patients from the TAHG study.
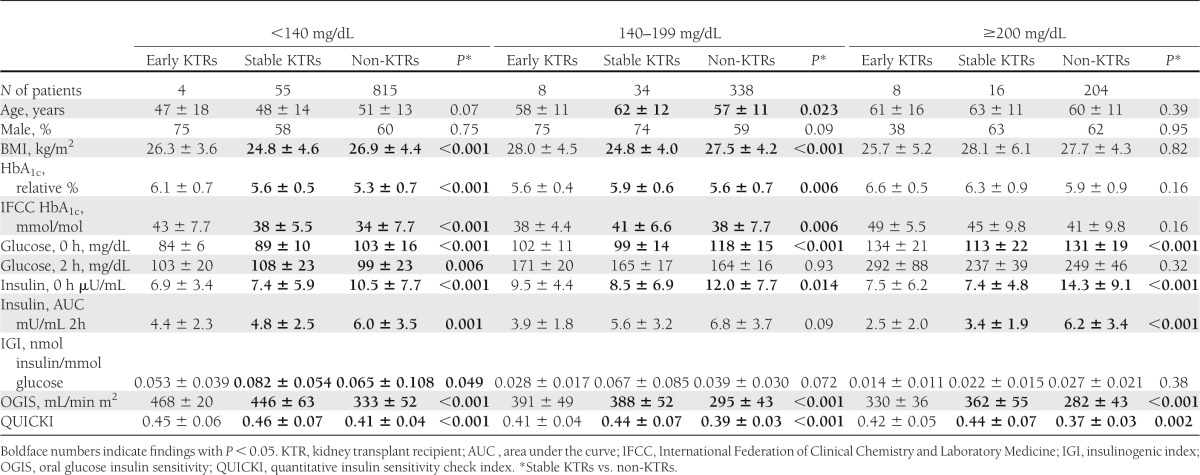
Table 3.
Baseline characteristics and OGTT-derived indices in previously nondiabetic kidney transplant recipients at 3 months (early KTRs) and ≥6 months posttransplantation (stable KTRs) at MUV, grouped by OGTT outcome, and compared with the nontransplant population (non-KTRs) at VGH
In nontransplant subjects, glucose metabolism was evaluated using unselected pseudonymized data from the Venice Regional General Hospital (VGH), Italy. By this hospital’s standard of care, patients who were admitted underwent a routine OGTT, regardless of the underlying medical problem. Only OGTTs from subjects without a known pathology affecting glucose metabolism are reported. OGTT-derived metabolic results and basic demographic information, as listed in Table 3, were used for comparison with patients from the TAHG study and TIP study. Parts of the study cohort have been published elsewhere (17). The majority (>90%) of nontransplant subjects and kidney transplant recipients were Caucasian. All data obtained from nontransplant subjects and kidney transplant recipients were analyzed retrospectively.
Laboratory measurements
At MUV, glucose was assessed by the hexokinase method, glycated hemoglobin A1c (HbA1c) was assessed by high-performance liquid chromatography separation of hemoglobin fractions (18), and insulin was assessed by chemiluminescence immunoassay (19). At VGH, glucose was assessed by glucose oxidase, HbA1c was assessed by high-performance liquid chromatography, and insulin was assessed by radioimmunoassay. The upper range of normal for fasting insulin in healthy individuals was 29 µU/mL at MUV and 22 µU/mL at VGH.
Definition of impaired glucose metabolism
NODAT was defined as need for antidiabetic treatment in patients without diabetes history before transplantation (15) or according to the OGTT result (2-h glucose ≥200 mg/dL; fasting glucose ≥126 mg/dL) (4,15). Impaired glucose tolerance (IGT) was defined as 2-h glucose 140–199 mg/dL during the OGTT, and impaired fasting glucose was defined as fasting glucose 100–125 mg/dL (4). Three subgroups of patients were formed according to the 2-h glucose level during the OGTT (<140, 140–199, ≥200 mg/dL).
Evaluation of insulin secretion, insulin sensitivity, and β-cell function
Insulin secretion during the OGTT was assessed from the area under the curve (AUC; trapezoidal rule) and β-cell function was assessed by insulinogenic index (IGI) using the ratio of suprabasal (dynamic) insulin AUC to the corresponding suprabasal glucose AUC (20). Insulin sensitivity was evaluated in dynamic conditions through the oral glucose insulin sensitivity index (OGIS), which describes glucose clearance per unit change of insulin concentration (21), and at fasting by the quantitative insulin sensitivity check index (QUICKI) (22). All these indices have been validated in the nontransplant population (23). Here, we also validated OGIS and QUICKI against the infusion rate of an euglycemic-hyperinsulinemic glucose clamp performed in 21 stable kidney transplant recipients from the TAHG study. Briefly, blood samples were drawn for glucose measurements from venous catheters at minutes −5, −3, −2, and 0. Thereafter, the euglycemic-hyperinsulinemic clamp was started with a primed continuous infusion of insulin (24) and a later rate of 40 mU·min−1⋅m−2 for 2 h; blood samples were drawn at minutes 4, 5, 8, and 10, and every 5 min thereafter. Whole-body insulin sensitivity (Mbw = glucose uptake, normalized to body weight) values were calculated from glucose infusion rates during the clamp tests, corrected for glucose space and urinary glucose loss as previously described (24).
Statistical analyses
Among descriptive statistics, we used counts and percentages and means ± SD, unless otherwise indicated. Because data reported in Table 1 were collected over the course of 1 year, we calculated patient age on 30 September 2009. If patients had multiple measurements of the same laboratory parameter, then we determined the patient median to calculate the mean of the group. In Tables 2 and 3, age and HbA1c are reported for the day of the OGTT.
Table 1.
Demographics and patient characteristics of all stable* kidney transplant recipients at MUV, by history of diabetes prevalence
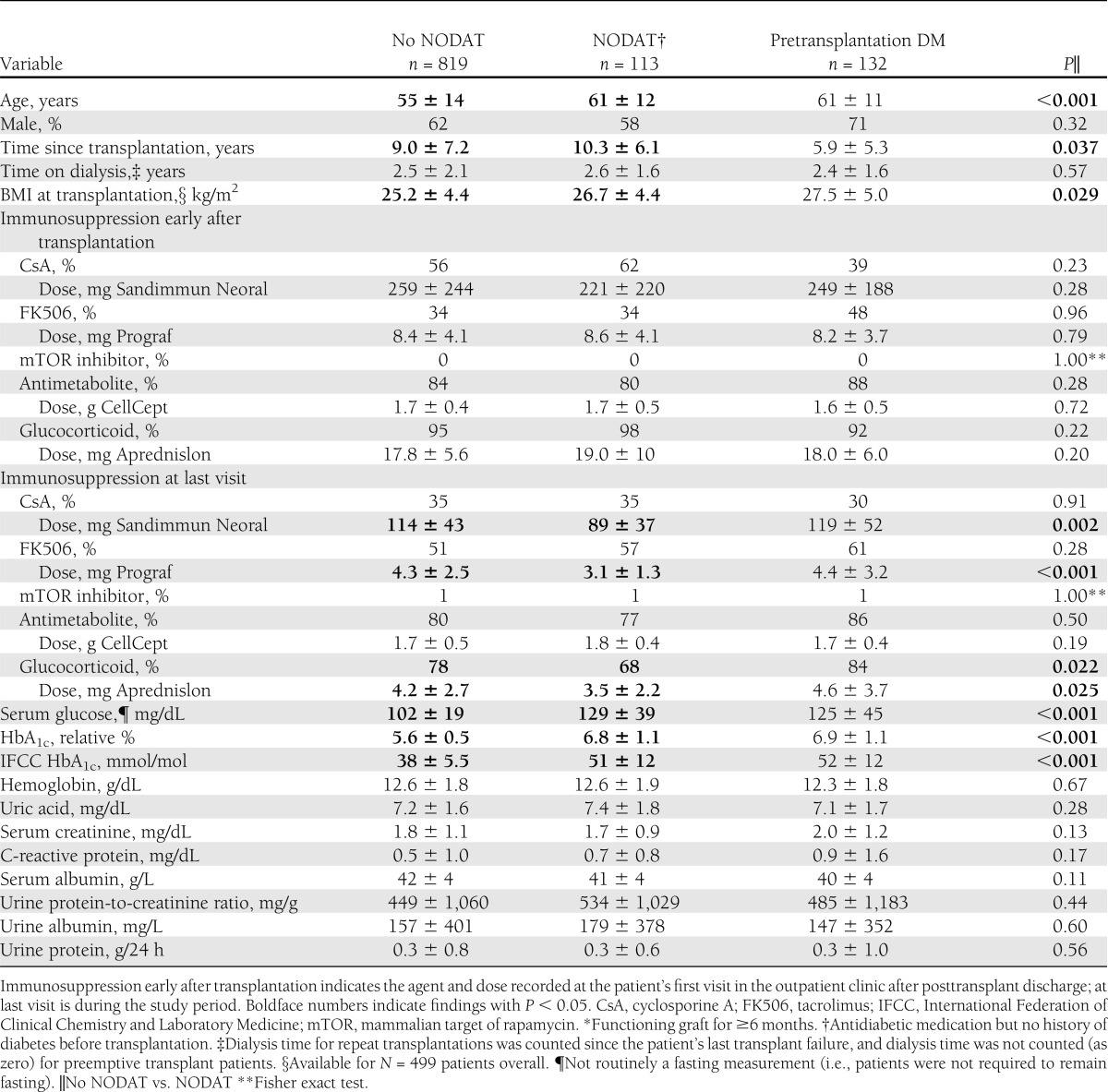
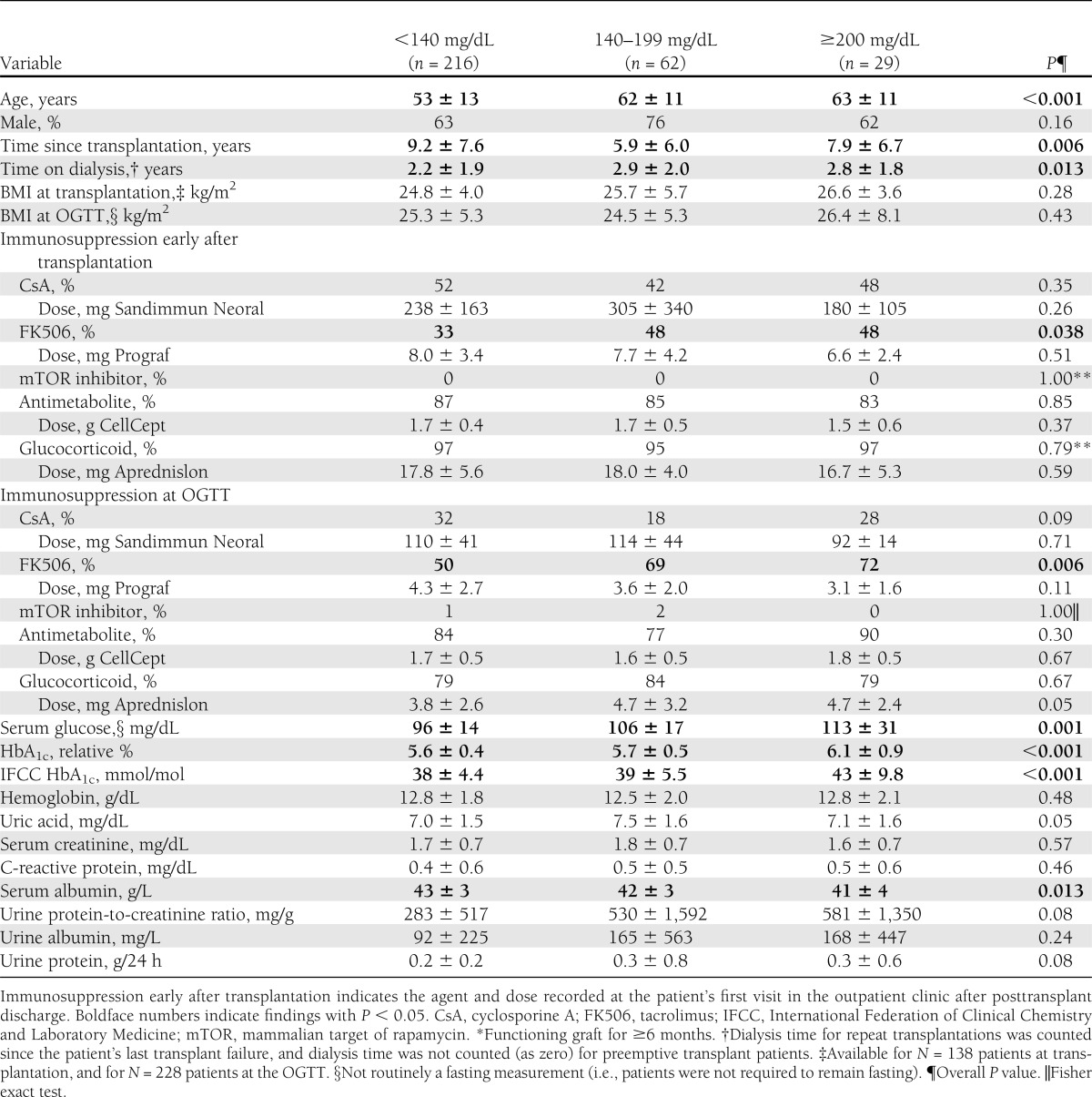
Table 2.
Characteristics of randomly selected, previously nondiabetic, stable* kidney transplant recipients at MUV undergoing an OGTT, by OGTT outcome
Demographic parameters, immunosuppression, and laboratory values in various patient groups were compared with the unpaired two-tailed Student t test for continuous variables, and the unadjusted χ2 test or Fisher exact test (when appropriate) was used for categorical variables. Analysis of variance was used for simultaneous comparisons of continuous variables between three groups.
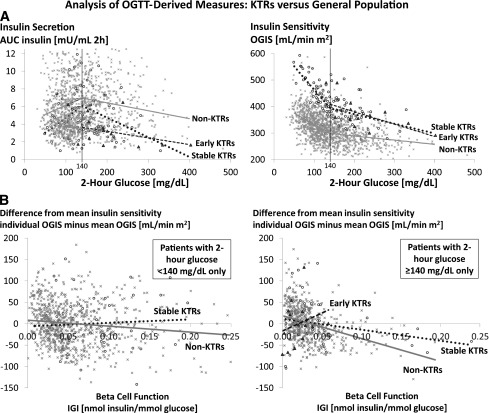
Ordinary least-squares regression models were used to analyze the association of 2-h glucose (independent variable) with both insulin AUC and OGIS (dependent variables). The 2-h glucose was modeled using a linear spline function with a single knot at 140 mg/dL (Fig. 1A). OGIS also was regressed on IGI by 2-h glucose and dichotomized at 140 mg/dL (Fig. 1B). Results were centered at the mean OGIS within each category of 2-h glucose. Model results for the subgroup of TIP patients with 2-h glucose <140 mg/dL were hidden in all figures because of insufficient sample size.
Figure 1.
Glucose metabolism after renal transplantation. OGTT-derived measures are shown from subjects at 3 months posttransplant (TIP study patients = early kidney transplant recipients [KTRs]) and ≥6 months posttransplant (TAHG study patients = stable KTRs) in comparison with the general population (VGH patients = non-KTRs). A: Insulin secretion by 2-h glucose (left) and insulin sensitivity by 2-h glucose (right). Model shows ordinary least-squares regression analysis of 2-h glucose (independent variable) against (left) insulin AUC and (right) OGIS (dependent variables). Within each of the three patient groups (TIP, TAHG, non-KTRs), 2-h glucose was modeled using a linear spline function with a single knot at 140 mg/dL. P for interaction testing slope difference between stable KTRs and non-KTRs was <0.001 (left) and <0.001 (right). P for interaction testing slope difference between early KTRs and non-KTRs was 0.016 (left) and <0.001 (right). B: Insulin sensitivity by β-cell function (left) in patients with 2-h glucose <140 mg/dL and (right) in patients with 2-h glucose ≥140 mg/dL. Model shows ordinary least-squares regression of IGI (independent variable) against OGIS (dependent variable). Results were centered at the mean OGIS within each category of 2-h glucose. P for interaction testing slope difference between stable KTRs and non-KTRs was 0.21 (left) and 0.05 (right). P for interaction testing slope difference between early KTRs and non-KTRs was 0.06 (right). A and B: Model results for the subgroup of TIP study patients with 2-h glucose <140 mg/dL were hidden because of insufficient sample size. x, Non-KTRs; ○, stable KTRs; ▲, early KTRs.
For OGIS and QUICKI validations, these parameters were regressed with Mbw from the euglycemic-hyperinsulinemic clamp. To consider a possible effect of individual patient characteristics on OGTT-derived measures, we modeled the association of transplant status (≥6 months posttransplant vs. nontransplant) with each OGTT-derived measure in each of three glucose strata (OGTT subgroups) and evaluated unadjusted and adjusted results. Each OGTT-derived measure (AUC insulin, IGI, OGIS, QUICKI) was regressed on glucose category, transplant status, and an interaction term to produce estimates in each glucose stratum, both unadjusted and adjusted for age, sex, and BMI (Supplementary Table 2). For calculations we used MS Excel 2003, SAS 9.2 for Windows (SAS Institute, Cary, NC), and Stata 9.0 (Stata, College Station, TX).
RESULTS
Data from all 1,064 stable kidney transplant recipients followed-up at MUV during the study period were available for analysis. Mean age was 56 ± 14 years, 63% were males, and the median time since transplantation was 8 years (interquartile range, 4–14 years).
Prevalence of impaired glucose metabolism and its association with the patient profile
By patient history, 12% of our stable renal transplant population had pretransplantation DM and 11% had overt NODAT. Serum glucose, HbA1c, and age were increased in kidney transplant recipients with pretransplantation DM and NODAT compared with those without diabetes history (Table 1). Kidney transplant recipients with pretransplantation DM and NODAT also had lower serum albumin and higher C-reactive protein levels. Glucocorticoid use in percent, glucocorticoid dosage, and calcineurin inhibitor dosage (cyclosporine A and tacrolimus) were significantly lower in NODAT patients at their last visit during the study period, but not at their first visit after postoperative discharge, which is possibly a sign of indication bias. Compared with patients without diabetes history, those with NODAT and pretransplantation DM also had higher BMI at transplantation and NODAT patients had their transplant for a longer time.
Among stable kidney transplant recipients without NODAT history, routinely performed OGTTs were completed in 307 patients during the study period; 11% of these OGTTs showed diabetes and 8% showed IGT, whereas impaired fasting glucose occurred in 12% and IGT plus impaired fasting glucose occurred in 12%. Throughout the three subgroups of OGTT outcome (normal toward diabetic), serum glucose, HbA1c, patient age, and tacrolimus use increased significantly, glucocorticoid dosage trended higher, and glucocorticoid use was similar (Table 2). Serum albumin was significantly lower in kidney transplant recipients with OGTTs showing IGT and diabetes, whereas urine protein, albumin, and protein-to-creatinine ratio trended higher (all P ≤ 0.24). Compared with patients with normal OGTT results, those with IGT and diabetes had spent longer time on dialysis before transplantation and time since transplantation was shorter, whereas BMI was not different.
OGTT-derived analysis of insulin secretion, insulin sensitivity, and β-cell function
Of all 307 OGTTs in the stable renal transplant population, 105 were performed with sequential measurements of glucose and insulin, which allowed calculations of insulin secretion, insulin sensitivity, and β-cell function. As a prerequisite, the OGTT-derived index of insulin sensitivity, OGIS, was validated against the clamp-derived Mbw, and showed a significant association (r2 = 0.38; P = 0.003). Throughout the three subgroups of OGTT outcome (normal toward diabetic), insulin secretion (basal and OGTT-derived AUC) and basal glucose were lower in kidney transplant recipients compared with 1,357 nontransplant controls, whereas insulin sensitivity and HbA1c were higher (Table 3). Thus, the glucose metabolism in kidney transplant recipients differed from the nontransplant control population.
In Fig. 1A, we provide every individual’s value for insulin secretion (AUC insulin) and insulin sensitivity (OGIS) by OGTT-derived 2-h glucose (on the y-axis). Up to 2-h glucose of 140 mg/dL insulin secretion increased, and from 2-h glucose of 140 mg/dL onwards insulin secretion decreased, whereas insulin sensitivity decreased throughout the whole range of 2-h glucose in all three patient populations. However, the graphs for insulin secretion in both renal transplant populations remained below that of the nontransplant control population (P < 0.001 and P = 0.016, respectively) and for insulin sensitivity remained consistently above that of the nontransplant control population (both P < 0.001).
We hypothesized that peripheral insulin sensitivity in kidney transplant recipients might be increased secondary to a decrease in β-cell function as described in early studies on pancreatogenic diabetes (25). Therefore, we evaluated the relationship between individual values for IGI (x-axis) versus OGIS (y-axis), relative to the mean OGIS of the population (Fig. 1B). In the nontransplant control population, as expected and previously shown (26), higher values for β-cell function were genuinely correlated with lower values for insulin sensitivity, i.e., with a negative slope. However, compared with the nontransplant population, the slope of the regression line was close to zero for stable kidney transplant recipients with 2-h glucose <140 mg/dL (P = 0.21), was positive for early postoperative transplant patients with 2-h glucose ≥140 mg/dL (P = 0.06), and was negative but much flatter for stable kidney transplant recipients with 2-h glucose ≥140 mg/dL (P = 0.05). These results indicated that the relationship between insulin sensitivity and β-cell function was altered in the transplant population.
Sensitivity analysis by BMI and adjusted analysis
Compared with kidney transplant recipients, BMI of the control population was significantly higher in those individuals with normal OGTT results and with OGTT results showing IGT, whereas age and sex distribution differed nondirectionally through the three subgroups of OGTT outcome (Table 3). However, a sensitivity analysis of baseline characteristics and OGTT-derived indices in transplant compared with nontransplant patients grouped by BMI <30 compared with ≥30 kg/m2 yielded results that were consistent with those reported in Table 3 (Supplementary Table 1). Moreover, when we adjusted insulin sensitivity estimates (OGIS and QUICKI) for age, sex and BMI, OGIS and QUICKI remained significantly higher in transplant patients, regardless of the subgroup of OGTT outcome (all P < 0.001; Supplementary Table 2). Specifically, adjusted OGIS was 82–104 mL/min/m2 higher for transplant versus nontransplant subjects.
CONCLUSIONS
This study shows cross-sectionally for the MUV transplant center that 11% of all stable kidney transplant recipients had a history of NODAT and that 43% of those without diabetes history had abnormal OGTT results (11% diabetic). Hence, an estimated 50% of stable kidney transplant recipients had impaired glucose metabolism, among them 22% had NODAT. Compared with patients with a normal OGTT outcome, the use of tacrolimus, patient age, and proteinuria was increased in the subgroups with IGT and diabetes. Compared with a nontransplant control population without previously known impairment of glucose metabolism, kidney transplant recipients had lower BMI and decreased insulin secretion, but increased insulin sensitivity. Even when results were adjusted for age, sex, and BMI, we found that kidney transplant recipients had lower insulin secretion but higher insulin sensitivity.
Several previous studies analyzed OGTT outcomes after renal transplantation (27–30). Overall, the reported prevalence of abnormal glucose metabolism was remarkably similar to the present results and ranged from 32 (28) to 51% (27). Our previous OGTT-based analysis of TIP study control patients at 3 months posttransplantation, however, showed 84% abnormal glucose metabolism, with 52% NODAT (16). Patients in the TIP study were older than patients in other reports, and all of them had received the more diabetogenic primary immunosuppressant tacrolimus (31) immediately after transplantation, whereas in the described analyses only 12% of patients in a United States cohort (30) and 15% of patients in a Norwegian study (29) received tacrolimus. Moreover, because patients with NODAT have an increased mortality risk (3,32), they could have been lost to cross-sectional analyses.
A study led by Ekstrand et al. (33) in 1992 has shown reduced insulin sensitivity in 10 kidney transplant recipients with NGT compared with 10 healthy control subjects. However, the transferability of these much older findings into the modern transplant era is questionable, because their kidney transplant recipients had received 0.40 mg/kg/day methylprednisolone on average (equivalent to 35 mg prednisolone in a 70-kg patient), as well as no tacrolimus (7 of 10 patients had received cyclosporine). Moreover, mean HbA1c in these kidney transplant recipients was higher than in the controls (by 0.7 relative % [7.7 mmol/mol]), perhaps indicating that glucose metabolism was more impaired, although both patient groups were in the category of NGT.
Ekstrand et al. (33) concluded from their additional study findings that both insulin resistance and insulin deficiency are necessary for NODAT to develop, but subsequent analyses (6,7,9,10) are supportive of our finding that insulin secretion rather than insulin resistance might be the principal problem. Even the study by Midtvedt et al. (8) and a more recent examination led by Hornum et al. (11) might not disagree with this concept. Specifically, Midtvedt et al. (8) identified a significant difference in insulin secretion between patients with NODAT compared with those with NGT. Hornum et al. (11) noted a significant decrease in insulin sensitivity from before to after transplantation, but they did not analyze insulin sensitivity separately within subgroups of patients with NGT, IGT, and diabetes, and the results also are in contrast to an earlier study by Nam et al. (7). In our experience, insulin resistance is perceived to be more important than insulin secretion, and this perhaps has to do with the title of the publication by Midtvedt et al. (8). Moreover, nephrologists are well aware of the risk associated with the metabolic syndrome (30) and they also know that glucocorticoids increase insulin resistance. On the other hand, nephrologists may not be equally concerned with insulin secretion, and the fact that this can potentially be preserved.
Attempting to clarify the mechanism of increased insulin sensitivity, we assumed that insulin sensitivity as a compensatory counter-regulation to decreased insulin secretion would be reflected by even higher OGIS values for lower IGI values in the renal transplant population compared with nontransplant subjects. However, such a relationship was not observed. Instead, the finding depicted in Fig. 1B indicated, if anything, that insulin sensitivity had been added, independent of β-cell function. In line with the latter hypothesis, recent data showed acutely improved insulin sensitivity after 5-h calcineurin inhibitor infusion in healthy human volunteers (34).
Because screening OGTTs at MUV are only performed in particular risk groups, nontransplant subjects from MUV could not serve as controls, which necessitated the use of a control group from a different city. Compared with a previously published large OGTT-based study from the German general population aged 55–74 years, which showed 7% previously undiagnosed diabetes and 26% prediabetes, respectively (35), our control group was younger and leaner but still had more diabetes (15%), possibly because subjects were recruited from the hospital setting. Our results therefore must be interpreted with caution, even though OGTT-derived measures showed consistency with the principal results when they were adjusted for age, sex, and BMI (Supplementary Table 2). Additional confirmation of our findings in other transplant and control populations is warranted.
In 84 nontransplant subjects with NGT from the German Diabetes Center (36) the average value of 447 mL/min/m2 for OGIS was almost identical, whereas average values for AUC insulin and β-cell function (IGI) during an OGTT were higher (data provided by M.R.) by 40 and 83%, respectively, than in stable kidney transplant recipients with NGT from the current study, as shown in Table 3. However, basal glucose and 2-h glucose were 15 and 17%, respectively, lower, indicating that the subjects were metabolically healthier. The finding of similar insulin sensitivity but increased insulin secretion and increased β-cell function in these control subjects with lower glucose values therefore is in agreement with an overall higher insulin sensitivity combined with lower insulin secretion identified in kidney transplant recipients compared with nontransplant controls.
In 191 nontransplant type 2 patients with diabetes receiving sulfonyl urea or metformin therapy alone (37), the average value for OGIS was 239 mL/min/m2, i.e., 34% lower than in stable diabetic kidney transplant recipients from the current study, as shown in Table 3 (data provided by G.P.). Basal glucose and 2-h glucose were higher, by 88 and 56%, respectively, indicating that these treated type 2 patients with diabetes were metabolically unhealthier than our stable diabetic transplant patients. Nevertheless, these data from an unrelated control cohort also suggest that kidney transplant recipients, when compared with nontransplant subjects, may have increased insulin sensitivity, thereby predominantly experiencing an insulin secretion problem.
For immunoassays, which were used to determine insulin concentration in serum, assay standardization is crucial and previously has been questioned (38). However, the normal range of the insulin assays was higher for the MUV than for the VGH laboratory, indicating, if anything, that insulin secretion in kidney transplant recipients might have been even lower than in the nontransplant population assessed at VGH. Moreover, if differences existed, then they would only have affected insulin AUC, whereas OGTT-derived index values for insulin sensitivity (calculated by relative differences during the OGTT) are expected to be adequate and comparable.
OGTTs in stable kidney transplant recipients were performed per the start of a routine screening program at MUV in March 2009. Although patients were randomly called in, those who suspected they had diabetes might have been more likely to attend the examination, which could have resulted in higher diabetes prevalence. This potential bias unlikely would have affected our comparison of OGTT-derived insulin sensitivity and secretion with the nontransplant control population, however, because this comparison was made by 2-h glucose (Table 3, Fig. 1). Moreover, our data from kidney transplant recipients during the early postoperative period, the control group of a randomized controlled trial (16), showed consistent results.
In conclusion, the current study found insulin secretion decreased and insulin sensitivity increased in kidney transplant recipients compared with >1,000 nontransplant control patients. In agreement with several previous studies but contrary to perhaps general belief, decreased insulin secretion rather than increased insulin resistance therefore seems to be the primary or causal factor for NODAT development. Based on these results, antidiabetic agents that preserve, maintain, or improve β-cell function, such as exogenous insulin, glucagon-like peptide 1 analogs, dipeptidyl-peptidase 4 inhibitors, and thiazolidinediones (39), may merit attention for the vulnerable (40) transplant population and early posttransplant period. Future research is, however, necessary to confirm our analyses and also should be aimed at elucidating the mechanism of the increase in insulin sensitivity we observed. Such research must further clarify whether our findings of lower fasting glucose but higher HbA1c at similar or higher 2-h glucose levels than in the nontransplant population are, as we would speculate, the direct consequence of the observed alteration in the insulin secretion/sensitivity axis. Ultimately, it should be determined if long-term outcomes in NODAT patients are similar or worse than those in the general population with the same HbA1c, for example, through higher glycemic variability.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
M.He. designed the study, performed OGTTs at MUV, collected and researched data, and wrote the manuscript. A.Kai. and A.Kar. researched data (statistics), contributed to the discussion, and reviewed the manuscript. J.W. designed the study, performed OGTTs at MUV, contributed to the discussion, and reviewed the manuscript. M.Ha. designed the study, performed OGTTs at MUV, collected data, contributed to the discussion, and reviewed the manuscript. D.D. performed OGTTs at MUV, collected data, and reviewed the manuscript. A.T. reviewed the manuscript and calculated OGTT-derived indices. W.H.H. and A.S. contributed to the discussion and reviewed the manuscript. M.W. reviewed the manuscript and organized and financed clamp analyses at MUV. M.R. researched data, contributed to the discussion, reviewed the manuscript, and provided data from an unrelated study cohort. E.M. organized OGTTs at VGH. G.P. contributed to the discussion, reviewed the manuscript, and calculated OGTT-derived indices. F.K.P. designed the study, contributed to the discussion, and reviewed the manuscript. M.D.S. designed the study, researched data, contributed to the discussion, and reviewed the manuscript. M.He. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented at the American Society of Nephrology Kidney Week 2012, San Diego, California, 30 October–4 November 2012.
The authors thank the nursing personnel at MUV for their help in performing the OGTTs, and Dr. Christian Bieglmayer, MUV, Department of Laboratory Medicine, for revising the manuscript and advising on the methodological aspects of glucose, HbA1c, and insulin measurements. Dr. Reinhard Kramar, Austrian Transplant Registry, kindly provided data on dialysis time and BMI.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2441/-/DC1.
References
- 1.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients. (SRTR) 2010 Annual Data Report, 2011. Available from http://www.srtr.org/annual_reports/2010/pdf/2010_SRTR_ADR.pdf Accessed 26 March 2012
- 2.U.S. Renal Data System. USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, 2012. Available from http://www.usrds.org/adr.aspx Accessed 18 January 2013
- 3.Hjelmesaeth J, Hartmann A, Leivestad T, et al. The impact of early-diagnosed new-onset post-transplantation diabetes mellitus on survival and major cardiac events. Kidney Int 2006;69:588–595 [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2012;35(Suppl. 1):S64–S71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hjelmesaeth J, Hagen M, Hartmann A, Midtvedt K, Egeland T, Jenssen T. The impact of impaired insulin release and insulin resistance on glucose intolerance after renal transplantation. Clin Transplant 2002;16:389–396 [DOI] [PubMed] [Google Scholar]
- 6.Hagen M, Hjelmesaeth J, Jenssen T, Morkrid L, Hartmann A. A 6-year prospective study on new onset diabetes mellitus, insulin release and insulin sensitivity in renal transplant recipients. Nephrol Dial Transplant 2003;18:2154–2159 [DOI] [PubMed] [Google Scholar]
- 7.Nam JH, Mun JI, Kim SI, et al. beta-Cell dysfunction rather than insulin resistance is the main contributing factor for the development of postrenal transplantation diabetes mellitus. Transplantation 2001;71:1417–1423 [DOI] [PubMed] [Google Scholar]
- 8.Midtvedt K, Hartmann A, Hjelmesaeth J, Lund K, Bjerkely BL. Insulin resistance is a common denominator of post-transplant diabetes mellitus and impaired glucose tolerance in renal transplant recipients. Nephrol Dial Transplant 1998;13:427–431 [DOI] [PubMed] [Google Scholar]
- 9.Shimizu M, Iino Y, Terashi A. [Improvement of insulin sensitivity after renal transplantation measured by a glucose clamp technique] [article in Japanese]. Nippon Ika Daigaku Zasshi 1998;65:50–54 [DOI] [PubMed] [Google Scholar]
- 10.Zelle DM, Corpeleijn E, Deinum J, et al. Pancreatic β-cell dysfunction and risk of new-onset diabetes after kidney transplantation. Diabetes Care 2013;36:1926–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hornum M, Jørgensen KA, Hansen JM, et al. New-onset diabetes mellitus after kidney transplantation in Denmark. Clin J Am Soc Nephrol 2010;5:709–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heit JJ, Apelqvist AA, Gu X, et al. Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature 2006;443:345–349 [DOI] [PubMed] [Google Scholar]
- 13.Qi D, Rodrigues B. Glucocorticoids produce whole body insulin resistance with changes in cardiac metabolism. Am J Physiol Endocrinol Metab 2007;292:E654–E667 [DOI] [PubMed] [Google Scholar]
- 14.van Raalte DH, Nofrate V, Bunck MC, et al. Acute and 2-week exposure to prednisolone impair different aspects of beta-cell function in healthy men. Eur J Endocrinol 2010;162:729–735 [DOI] [PubMed] [Google Scholar]
- 15.Davidson J, Wilkinson A, Dantal J, et al. International Expert Panel New-onset diabetes after transplantation: 2003 International consensus guidelines. Proceedings of an international expert panel meeting. Barcelona, Spain, 19 February 2003. Transplantation 2003;75(Suppl.):SS3–SS24 [DOI] [PubMed] [Google Scholar]
- 16.Hecking M, Haidinger M, Döller D, et al. Early basal insulin therapy decreases new-onset diabetes after renal transplantation. J Am Soc Nephrol 2012;23:739–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kautzky-Willer A, Brazzale AR, Moro E, et al. Influence of increasing BMI on insulin sensitivity and secretion in normotolerant men and women of a wide age span. Obesity (Silver Spring) 2012;20:1966–1973 [DOI] [PubMed] [Google Scholar]
- 18.Goldstein DE, Little RR, Wiedmeyer HM, England JD, McKenzie EM. Glycated hemoglobin: methodologies and clinical applications. Clin Chem 1986;32(Suppl.):B64–B70 [PubMed] [Google Scholar]
- 19.Vezzosi D, Bennet A, Fauvel J, Caron P. Insulin, C-peptide and proinsulin for the biochemical diagnosis of hypoglycaemia related to endogenous hyperinsulinism. Eur J Endocrinol 2007;157:75–83 [DOI] [PubMed] [Google Scholar]
- 20.Tura A, Kautzky-Willer A, Pacini G. Insulinogenic indices from insulin and C-peptide: comparison of beta-cell function from OGTT and IVGTT. Diabetes Res Clin Pract 2006;72:298–301 [DOI] [PubMed] [Google Scholar]
- 21.Mari A, Pacini G, Murphy E, Ludvik B, Nolan JJ. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care 2001;24:539–548 [DOI] [PubMed] [Google Scholar]
- 22.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000;85:2402–2410 [DOI] [PubMed] [Google Scholar]
- 23.Pacini G, Mari A. Methods for clinical assessment of insulin sensitivity and beta-cell function. Best Pract Res Clin Endocrinol Metab 2003;17:305–322 [DOI] [PubMed] [Google Scholar]
- 24.Anderwald C, Pfeiler G, Nowotny P, et al. Glucose turnover and intima media thickness of internal carotid artery in type 2 diabetes offspring. Eur J Clin Invest 2008;38:227–237 [DOI] [PubMed] [Google Scholar]
- 25.Nosadini R, del Prato S, Tiengo A, et al. Insulin sensitivity, binding, and kinetics in pancreatogenic and type I diabetes. Diabetes 1982;31:346–355 [DOI] [PubMed] [Google Scholar]
- 26.Weir GC, Bonner-Weir S. A dominant role for glucose in beta cell compensation of insulin resistance. J Clin Invest 2007;117:81–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharif A, Moore RH, Baboolal K. The use of oral glucose tolerance tests to risk stratify for new-onset diabetes after transplantation: An underdiagnosed phenomenon. Transplantation 2006;82:1667–1672 [DOI] [PubMed] [Google Scholar]
- 28.Delgado P, Diaz JM, Silva I, et al. Unmasking glucose metabolism alterations in stable renal transplant recipients: a multicenter study. Clin J Am Soc Nephrol 2008;3:808–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valderhaug TG, Jenssen T, Hartmann A, et al. Fasting plasma glucose and glycosylated hemoglobin in the screening for diabetes mellitus after renal transplantation. Transplantation 2009;88:429–434 [DOI] [PubMed] [Google Scholar]
- 30.Luan FL, Stuckey LJ, Ojo AO. Abnormal glucose metabolism and metabolic syndrome in non-diabetic kidney transplant recipients early after transplantation. Transplantation 2010;89:1034–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vincenti F, Friman S, Scheuermann E, et al. DIRECT (Diabetes Incidence after Renal Transplantation: Neoral C Monitoring Versus Tacrolimus) Investigators Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus [corrected in Am J Transplant 2008;8:908]. Am J Transplant 2007;7:1506–1514 [DOI] [PubMed] [Google Scholar]
- 32.Cosio FG, Pesavento TE, Kim S, Osei K, Henry M, Ferguson RM. Patient survival after renal transplantation: IV. Impact of post-transplant diabetes. Kidney Int 2002;62:1440–1446 [DOI] [PubMed] [Google Scholar]
- 33.Ekstrand AV, Eriksson JG, Grönhagen-Riska C, Ahonen PJ, Groop LC. Insulin resistance and insulin deficiency in the pathogenesis of posttransplantation diabetes in man. Transplantation 1992;53:563–569 [DOI] [PubMed] [Google Scholar]
- 34.Øzbay LA, Møller N, Juhl C, et al. Calcineurin inhibitors acutely improve insulin sensitivity without affecting insulin secretion in healthy human volunteers. Br J Clin Pharmacol 2012;73:536–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kowall B, Rathmann W, Heier M, et al. Categories of glucose tolerance and continuous glycemic measures and mortality. Eur J Epidemiol 2011;26:637–645 [DOI] [PubMed] [Google Scholar]
- 36.Kahl S, Livingstone N, Nowotny B, et al. Validation of indices predicting hepatic steatosis by 1H-magnetic resonance spectroscopy and their correlation with glucose tolerance. Diabetologia 2012;55(Suppl. 1):S135 [Google Scholar]
- 37.Roden M, Mariz S, Brazzale AR, Pacini G. Free fatty acid kinetics during long-term treatment with pioglitazone added to sulfonylurea or metformin in Type 2 diabetes. J Intern Med 2009;265:476–487 [DOI] [PubMed] [Google Scholar]
- 38.Staten MA, Stern MP, Miller WG, Steffes MW, Campbell SE, Insulin Standardization Workgroup Insulin assay standardization: leading to measures of insulin sensitivity and secretion for practical clinical care. Diabetes Care 2010;33:205–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nyalakonda K, Sharma T, Ismail-Beigi F. Preservation of beta-cell function in type 2 diabetes. Endocr Pract 2010;16:1038–1055 [DOI] [PubMed] [Google Scholar]
- 40.Sharif A. Should metformin be our antiglycemic agent of choice post-transplantation? Am J Transplant 2011;11:1376–1381 [DOI] [PubMed] [Google Scholar]